

It’s 6 a.m. and Alieyah has just left to get water for the day. Although she is only eight years old, Alieyah plays an essential role in her family’s daily life. She walks two miles every morning to the Ugulwara River to get freshwater, and then two miles back to her home in the village of Mbuma. She can’t carry much water, but her family depends on the water she is able to provide for the day. Freshwater is a rare and treasured resource in Togo, and thousands of children living in that poor country are a main source for that resource in villages around the country.
The Yakima River Valley of Washington State has long been called Apple Valley because of all the lush apple orchards there. Today, its residents are more likely to think of it as “grape valley,” because farmers are tearing out apple orchards and replacing them with grape vines. Why? Simple. Grapes take far less water to grow than do apples. And water is becoming a scarce resource in the Pacific Northwest.
Newspapers, magazines, television, and the Internet today carry endless numbers of stories about the world’s water crisis. Mbumba and Apple Valley are thousands of miles apart, but they are very similar in one important way: both areas are suffering from a shortage of clean water needed for the simplest activities of daily life as well as for the operation of agricultural, industrial, commercial, and other operations.
So what do people mean when they talk about “global water crisis”? This phrase refers to a number of different phenomena, such as:
One of the most famous literary commentaries on Earth’s water resources is found in a 1798 poem by English poet Samuel Taylor Coleridge, “The Rime of the Ancient Mariner.” In the poem, a sailing ship is becalmed near the Antarctic Sea, and its crew begins to fear for its survival. One member of the crew, the “ancient mariner,” reflects on their situation. From the deck of the ship, all that can be seen is the wide ocean; Earth might contain no land at all, for all their senses can tell. “Water, water, everywhere,” the ancient mariner observes.
And the mariner’s observation certainly makes sense, for Earth truly is “the blue planet” or “the water planet.” Had the mariner access to the modern technology used by the U.S. National Aeronautics and Space Administration and other research organizations, he might well have come to the same conclusion about the endless availability of water on the planet (see, e.g., Advancing the Science: Google Earth 2015). Alone among the planets that make up our solar system—and, in fact, all other known planets—Earth contains the water resources that appear to be necessary for the survival of most forms of life. Those resources occupy a total of about 332,500,000 cubic miles (1,386,000,000 cubic kilometers), or nearly three-quarters (70.9%) of the planet’s surface (Water Basics 2015). Water, water, everywhere. Indeed!
But the ancient mariner also made another keen observation immediately thereafter. He went on,
In other words, the vast extent of water visible to the mariner and his crew was of little value to them since it was not fresh water; they could not use the water to relieve their thirst.
And this fact is confirmed by modern estimates of the amount of fresh water available on Earth. Of the 332,500,000 cubic miles of water on Earth’s surface, about 96.5 percent is found in the oceans, with another 2.5 percent in the form of lakes, rivers, streams, and other freshwater (also called freshwater) resources, and less than 1 percent in the form of saline water. The term saline refers to water that contains dissolved salts, such as sodium chloride, potassium chloride, and magnesium chloride. Saline water can be further classified as slightly saline (1,000–3,000 parts per million [ppm]), moderately saline (3,000–10,000 ppm), and highly saline (10,000–35,000 ppm). By comparison, freshwater is usually defined as having less than 1,000 ppm of dissolved salts, and seawater as having more than 35,000 ppm (Saline Water 2015).
For many purposes, the statistic with which humans (e.g., the ancient mariner) are most interested is the amount of freshwater available on the planet. Of the approximately 8,312,000 cubic miles of freshwater on Earth, by far the greatest amount (68.7%) is stored in glaciers and ice caps, vast fields of frozen water that, for all practical purposes, are unavailable for human use. Another 30.1 percent occurs underground in the form of so-called groundwater. (Groundwater is generally defined as any water that occurs beneath the surface of the ground.) This leaves only 1.2 percent of all freshwater available in lakes, rivers, swamps, water stored in living organisms, soil moisture, and other sources (The World’s Water 2015). These data make it clear that, in spite of the vast amounts of water available on the planet, only a relatively small quantity is actually readily available for human use. The rest occurs in a form that is less convenient for use (saline water) or that is stored in inaccessible locations, such as underground or in glaciers and ice caps.
This summary reflects a fairly traditional method of calculating Earth’s water resources, so-called blue water resources. The term blue water is used to describe groundwater and surface water, as discussed in the preceding paragraph. Blue water can be thought of as rainwater that falls on Earth’s surface and then soaks downward to become groundwater or that runs across the ground and empties into rivers and lakes. Another form of water that has traditionally been ignored to some extent is so-called green water. Green water is rainwater that falls on the land and soaks into the ground, where it is available for growing plants. Current estimates suggest that twice as much rainwater ends up in the form of green water as in blue water. That is, for every 100 cubic feet of rainwater that falls on Earth’s surface, about 35 cubic feet eventually ends up in rivers and lakes, and the remaining 65 cubic feet ends up as green water that is then taken up by forests (about 41 cubic feet), grasslands (16 cubic feet), wetlands (1 cubic foot), and crops (7 cubic feet) (Ringersma, Batjes, and Dent 2003, Figure 1, page 2; estimates differ somewhat from study to study; see also, e.g., Hoekstra and Mekonnen 2011).
A third form of water resource is also sometimes identified, gray water. Gray water is defined as the water required to carry away the waste products of some industrial, municipal, agricultural, or other human activities, that is, polluted water. According to one recent survey of the total freshwater resources on Earth available between 1996 and 2005, about 74 percent of those resources could be classified as green water, 11 percent as blue water, and 15 percent as gray water (Hoekstra and Mekonnen 2011, 3232).
It probably goes without saying that the availability of freshwater is not even nearly distributed equally in various countries on Earth. For example, citizens of the Middle Eastern nation of Bahrain have available to them an estimated 3 m3 (cubic meters) of freshwater. By comparison, the residents of Iceland have an estimated 525,074 m3 of freshwater per person. Table 1.1 lists the amount of freshwater per capita in various nations around the world.
Table 1.1 Availability of Freshwater per Capita in Various Countries, 2010–2014


The interesting point about these data is that they do not necessarily reflect the likelihood that a particular nation is or is not experiencing (or likely to experience) water issues. The United States, for example, would appear to have a relatively large amount of water per capita available for a variety of uses. Yet, some people would argue that the United States faces water issues as severe in some respects as many other nations in the world, a point to be discussed in greater detail later in this book.
Note also that the location of a country on or near the oceans does not necessarily guarantee a ready supply of fresh water. The Bahamas, situated in the middle of the Caribbean Sea, ranks low in the amount of freshwater available to its residents, a striking contrast, for example, with landlocked Chad, in the middle of the continent of Africa, with more than 20 times as much as freshwater per capita as the Bahama Islands.
One of the questions that has long fascinated researchers is where and when Earth collected its current supply of water. The most common theory is that water did not appear on the planet until very long—perhaps hundreds of millions of years—after it was originally formed. Formation theories suggest that the young Earth was very hot, perhaps molten in some places, conditions that would not have allowed liquid water to remain on the planet. Water must have come, according to the most popular theories, from asteroids, comets, and other bodies that carried water within their structures and then released that water when they collided with the primordial Earth (Ball 2000).
In recent years, researchers have come closer to understanding the how and why of water formation on Earth. It now appears that water may have been present on the planet from almost the first moments of its formation and that the most likely source of the water was asteroids striking Earth, and not comets, as had previously been suspected (Beatty 2015; Sarafian, et al. 2014).
Whatever the origin of Earth’s water, one essential fact remains: that water is almost certainly a nonrenewable resource. That is, the amount of water that was present on the primordial Earth is probably almost the same as the amount available on the planet today and that is the amount humans have to live with for the foreseeable future.
Most people probably take it as a given that water is a nonrenewable resource. Yet, the testimony of one’s senses might easily raise questions about that fact. After all, rain falls from the sky, apparently adding water to the planet’s water resources, and lakes and rivers run dry, apparently depleting those resources. In fact, precipitation and evaporation are only two phases of an interconnected series of events through which all water passes at one time or another in its history.
The water cycle really has no beginning or no ending, but for purposes of description might be imagined as originating with water stored in the atmosphere. Water in the atmosphere makes up a vanishingly small amount of the total water on Earth (about 0.001%), as well as a very small amount of total freshwater on the planet (0.04%) (How Much Water Is There on, in, and above the Earth 2015). Water in the atmosphere can exist in any one of three states: solid (ice), liquid, or gas (water vapor), depending on ambient conditions (the conditions, such as temperature and pressure, at which the water exists). When water first reaches the atmosphere from Earth’s surface, it usually does so in the form of water vapor. As it rises to higher altitudes, it tends first to liquefy, forming liquid droplets of water, and then to freeze, forming tiny ice crystals.
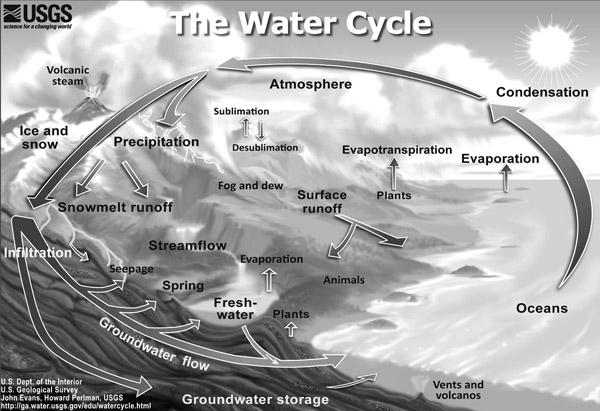
Figure 1.1 The Water Cycle. (United States Geological Survey. Available online at http://water.usgs.gov/edu/watercycle.html)
At times, water in the atmosphere is visible in the forms of clouds made up of liquid droplets or ice crystals. At other times, the water is so widely dispersed that it is invisible from the ground. Whatever the form in which it occurs, water remains in the atmosphere for periods of less than about two weeks, depending on whether it is situated over water (an average residence time of about 9 days) or over land (an average residence time of about 15 days) (Bice 2015). (Residence time is the average length of time water will remain in a specific part of the water cycle, such as the atmosphere.)
Water remains in the atmosphere for only a relatively short period of time because individual water molecules, water droplets, and ice crystals tend to collide with each other, forming increasingly larger structures. Eventually these structures (larger water droplets and ice crystals) become heavy enough to start falling to Earth’s surface in the form or precipitation such as rain, snow, hail, sleet, or fog. An estimated 398 × 1015 kilograms of water per year falls on the oceans by this process, compared with an estimated 108 × 1015 kilograms of water falling on land (of which 99% is rain and less than 1%, snow) (Bice 2015).
The vast majority of the water that falls on the oceans (435 × 1015 kilograms of water per year) and on land (71 × 1015 kilograms per year) is returned to the atmosphere by the process of evaporation, in which liquid (and very rarely, solid) water changes back to the gaseous state and rises into the atmosphere. This return process tends to be very slow for ice, with a residence time of about 27,500 years in its Earth reservoirs, 3,110 years for ocean water, and about 2.57 years for surface water on land (such as rivers and lakes) (Bice 2015).
A relatively small amount of precipitation (34 × 1015 kilograms per year) runs off land by way of lakes, rivers, and steams into the oceans, after which it also evaporates to the atmosphere. The smallest fraction of precipitation (about 2 × 1015 kilograms per year) soaks into the ground (a process known as infiltration) and becomes a component of groundwater. Except for ice reservoirs, groundwater has the longest residence time of any water resource on Earth, a period of about 4,100 years. This number means that once water gets relatively deep into the earth beneath ground level, it tends to stay there for very long periods of time.
Groundwater is an extraordinarily important feature of Earth’s water resources and a key element in many of the problems associated with current global water shortages. Groundwater is supplied when precipitation falls on the surface of the ground and sinks into the ground. This water continues to sink further until it reaches a point at which the ground is permanently saturated. The upper boundary of that region is called the water table, and the region itself is referred to as an aquifer. The land above the water table remains unsaturated because water is able to flow out of it, across the water table, and into the aquifer. The openings between rocks, stones, gravel, sand, and other particles in the upper unsaturated zone are lined with, but not filled with, water. Within the aquifer, however, the spaces between particles, cracks in the rocks, and all other openings in the ground are filled with water. The aquifer is, therefore, like a huge sponge that soaks up large quantities of precipitation that fall on the land’s surface.
Water in an aquifer flows from higher to lower elevations, just as does surface water, but at a far more leisurely pace. The water in most rivers and streams flows at the rate of a few miles per hour; in comparison, groundwater may flow at the rate of a foot or so a day, a foot per year, or even a foot per decade (General Facts and Concepts about Ground Water 2015). The water stored in an aquifer is, therefore, relatively stable, accounting for typically high residence times of a few thousand years.
The water in an aquifer is relatively easy to access in many cases. If one sinks a well into an aquifer, the pressure exerted by water around the well will push it up the well pipe to a height above the water table and, in some cases, directly out of the well onto the land. If the water does not rise to that height on its own, one can then install a pump to raise the water the rest of the way to the surface of the ground, providing a simple and long-used method of extracting water from an aquifer. Vast agricultural projects around the world depend on such wells for the water they need to supply their crops.
Aquifers can truly be of enormous size and of unparalleled importance to the world’s water needs. Table 1.2 lists a dozen of the world’s largest aquifer systems, the most extensive of which is the West Siberian Basin aquifer system that underlies central Russia and covers an area estimated at about 3.1 million square kilometers with a maximum thickness of 6,000 meters. By comparison, the largest aquifer in the United States is the Northern Great Plains aquifer that lies beneath North Central United States and South Central Canada with an area of about 2.0 million square kilometers. The most famous American aquifer, and one of the best known and most thoroughly studied, is the Ogallala (or High Plains) aquifer that underlies parts of the states of Colorado, Kansas, Nebraska, New Mexico, Oklahoma, South Dakota, Texas, and Wyoming. The Ogallala is among the most frequently mentioned of all world aquifers because of its critical role in the agricultural system of the western United States. (For a map of the world’s largest aquifers, see http://www.whymap.org/whymap/EN/Downloads/Global_maps/whymap_largeaquifers_pdf.pdf?__blob=publicationFile&v=3. For a comprehensive list of aquifers in the United States, see https://water.usgs.gov/ogw/aquiferbasics/alphabetical.html.)
Table 1.2 Some of the World’s Largest Aquifers

It probably goes without saying that water is one of the most essential substances on Earth. Plants and animals—including humans—depend absolutely on a constant supply of freshwater for their very survival. Humans use water on a largely daily basis for drinking, washing, cleaning, growing of crops, and other essential activities. Over the centuries, its role in transportation, power production, and other activities has also grown substantially.
So it is hardly surprising that early human civilizations almost inevitably had their beginnings on the shores of dependable sources of freshwater, usually streams and rivers (Tvedt and Coopey 2010). Possibly the oldest human settlements of which we have any records are those that developed along the shores of the Nile River, in modern-day Egypt. Those settlements date to at least 5500 BCE, although they did not yet qualify as organized communities characteristic of later societies at that early date (Midant-Reynes 2000).
Credit for being the earliest true human civilizations usually goes to a series of settlements that evolved in the region that constitutes modern-day Iraq, at the confluence of the Tigris and Euphrates Rivers, the eastern portion of what is now known as the Fertile Crescent. In fact, that early civilization took its name from that location, Mesopotamia meaning “between the rivers.” Beginning almost simultaneously with the first Egyptian settlements on the Nile, the Mesopotamian cultures include those of Samarra, Akkadia, Ur, Babylonia, Minoa, Assyria, and the Hittites, all names familiar to any student of ancient history (Roaf 2008).
Civilizations of eastern Asia followed a similar pattern of development. By about 2600 BCE, the first human settlements were being organized in the region of modern-day India along the banks of the Indus River. Archaeologists now know of more than 1,500 such settlements constructed between about 2600 BCE and 1900 BCE, providing a detailed picture of the type of lives lived by residents of the area (Kenoyer 2011, 17). And in adjacent China, a similar process had begun by about 1700 BCE along the banks of the Yellow River (Nilsson 2015). Of course, even settlements that were located in coastal areas still had to be sited near rivers or streams, since the vast quantities of saltwater available to them from the seas were of no help in meeting the communities’ daily need for a dependable supply of freshwater.
In a few locations on Earth, early humans had to be especially resourceful in order to begin building settlements where freshwater was either entirely absent or in short supply. The modern-day nation of Saudi Arabia, for example, has no permanent rivers or lakes, and an annual rainfall of about 5.9 cm (2.3 in), environmental conditions that have existed in the Arabian Desert at least since the end of the Holocene epoch, about 12,000 years ago. The region would be completely uninhabitable were it not for the vast aquifers underlying the area. Early humans discovered that they could recover the freshwater they needed to survive either by seeking out natural seeps from such aquifers (oases) or by digging wells into an aquifer. One of the earliest settlements built on such a site, if not the earliest, is called Qaryat al-Faw, located on the western edge of the desert (Al Ansary 1981).
Desert settlements depend, therefore, entirely on the water stored in these aquifers, sometimes known as fossil water because it was deposited on Earth’s surface hundreds or thousands of years earlier. This fossil water is nonrenewable because once it is gone, it is gone forever; annual rainfall is not nearly sufficient to recharge the aquifer (Foster and Loucks 2006).
Water wells are among the earliest structures found to have been associated with ancient civilizations. As humans abandoned their nomadic lifestyle and settled into permanent communities, they may have found it necessary to find ways of accessing the water they needed for their daily activities. Apparently, one such method was simply digging into the ground until the water table was reached, after which groundwater would begin to flow into the well under artesian pressure.
Such wells were relatively simple to build in concept, but often required a somewhat specific set of skills to produce permanent and dependable structures. The crucial component of such structures was a lining to hold the well’s shape and allow water to collect in its lower levels. Archaeologists have found a number of systems for achieving this result, involving the use of wood, fiber, stone, metal, and other materials for the linings of water wells. The oldest well yet discovered, for example, was found in eastern Germany and dates to 5469–5098 BCE. Its inner lining is made of oak timbers that have survived sufficiently well to permit carbon dating of their age. The sophistication of the workmanship involved prompted the wells’ discoverers to suggest that the first farmers were also “the first carpenters” (Tegel, et al. 2012).
Accessing the water collected in a well was the second technical challenge facing early inventors. One of the simplest methods was to make the well large enough to allow the installation of steps leading down into the well, making it possible for a person to simply go down into the well and collect the water. Another straightforward method involved hanging a bucket on a windlass at the top of the well. Lowering the bucket into the well was a simple method of collecting the water, one that has been used until recent times on some agricultural facilities. With the development of pumps in the 15th century, a newer and easier method of drawing water from a well was made available, a system that now dominates the construction of nearly all modern water well systems in the world (Segrest 2015).
Today, water wells are often classified as one of three primary types: dug, driven, and drilled. These names come from the method by which they are made, by (often) hand-digging into the ground, by driving a pipe into the ground, or by drilling (“boring”) into the ground and then inserting a pipe. In each case, the fundamental problem is a simple one: extending the shove, pipe, or drill into the ground to a point at which it penetrates the water table, and then shoring up the hole produced to prevent it from caving in during use (Groundwater: Wells 2015). The deepest and most sophisticated water wells in use today are almost always produced by drilling.
The deepest hand-dug water well on record as of early 2016 is the Woodingdean Well, near Brighton, England, constructed from 1858 to 1862. It is 1,285 feet deep (Grant 2015). Woodingdean is not necessarily the largest water well, however, as it is exceeded in width by the Big Well of Greensburg, Kansas, which, only 109 feet deep, is 32 feet in diameter (World’s Largest Hand Dug Well 2015). Finally, the hand-dug water wells with the greatest total capacity appear to be to very old wells, the Well of Joseph in the Cairo Citadel and St. Patrick’s Well in Orvieto, Italy (Fisk 1822, 290; McGowan 2015).
Driven and drilled water wells are generally much deeper than hand-dug wells. The world’s current record for the deepest water well is apparently the Stensvad Well 11-W1 located in Rosebud County, Montana, with a depth of 7,320 feet. The well was originally drilled by the Great Northern Drilling Company (Sagmit and Soriano 1998, 194).
A discussion of water wells may seem like a somewhat mundane topic to the general observer. After all, a person or community wants access to freshwater not available from a nearby river, stream, or lake, so he or she or it decides to sink a well into a convenient aquifer and withdraws the water he or she or it needs for his or her or its everyday operations. But the freshwater obtained from aquifers is a very large component—often a majority—of all the freshwater collected and used by a community, a region, or a nation. Studies of groundwater withdrawal, which occurs almost entirely through systems of water wells, provide an excellent overview of the way communities and nations are collecting freshwater, the purposes for which that freshwater is used, and the ultimate consequences of removing the water from aquifers.
Probably the most comprehensive study of groundwater withdrawal, although now somewhat dated, is a study conducted for the International Hydrological Programme of the United Nations Educational, Scientific and Cultural Organization (now UNESCO) that has been in operation since 1975. The study, “Groundwater Resources of the World and Their Use,” provides a plethora of information about the location, extent, withdrawal, and use of groundwater resources in every region of the world (Zekster and Everett 2004; also see Foster and Loucks 2006). Perhaps the most important generalization that can be made about groundwater withdrawal resulting from the study is that it represents the largest single extraction process in the world, resulting in the release of somewhere between 600 and 700 billion m3 of freshwater every year. This water is used primarily for three purposes: drinking water (about 65% of all water removed), irrigation and livestock (about 20%), and industry and mining (about 15%) (Foster and Loucks 2006, 24).
No firm data are available for the number of water wells needed for this extraction process. But one source cites the data provided in Table 1.3 as estimates for these numbers.
These numbers are somewhat misleading, however, as the collection and use of groundwater vary widely from country to country around the world. According to the National Groundwater Association (NGWA), the country that removes the largest volume of freshwater for domestic use is India, which extracted about 251 cubic kilometers of groundwater in 2010. Of that amount, the vast majority of freshwater (89%) went for irrigation, 9 percent for domestic use, and 2 percent for industrial uses. Other nations that relied heavily on groundwater extraction are listed in Table 1.4.
Table 1.3 Estimated Number of Water Wells in Selected Countries, as of 2010

NGWA also announced that the country worldwide that depended most heavily on groundwater resources was Bahrain, which obtained all (100%) of its domestic and industrial water from groundwater sources and almost all (90%) of its agricultural water from that source. Other nations heavily dependent on groundwater resources are listed in Table 1.5. (An invaluable resource for detailed information on the location and nature of groundwater resources around the world is a pair of maps produced by the United Nations Educational, Scientific and Cultural Organization and the [German] Federal Institute for Geosciences and Natural Resources (BGR), “Groundwater Resources of the World,” released in 1999, and its latest update, “The Global Map of Groundwater Vulnerability to Floods and Droughts,” published in 2015. The maps can be found on a number of sites, including http://www.whymap.org/whymap/EN/Downloads/Global_maps/whymap_largeaquifers_pdf.pdf?__blob=publicationFile&v=3, for the former, and http://unesdoc.unesco.org/images/0023/002324/232431e.pdf, for the latter.)
Table 1.4 Nations with Largest Estimated Groundwater Extraction Rates, as of 2010

Table 1.5 Nations with Greatest Dependence on Groundwater Resources


Removing freshwater from underground aquifers is often only the first step in making use of this valuable resource. This water must then be distributed to those individuals, communities, companies, and other entities who will make use of it, such as purification plants, where it can be prepared for domestic use, or industrial plants, where it is employed for a vast variety of purposes. Perhaps the greatest challenge, however, has long been to move water from its source (e.g., a deep well) to the wide-flung agricultural fields where it is used for the growing of crops and watering of domestic animals. When did humans first develop methods for such systems of distribution, for irrigation systems?
Historical records suggest that the first irrigation systems were constructed in at least the sixth millennium BCE in Egypt. These systems took advantage of the country’s primary source of freshwater—the Nile River—as its major (and sometimes only) source of providing water for its crops. During flood stage, the Nile’s waters were diverted from the river itself into surrounding areas, where they flowed through sometimes complex systems of dams and canals to fields where the crops were grown. At the conclusion of the flood season, the excess water in the fields was then returned to the river (Irrigation Museum 2015).
Similar irrigation systems were developed throughout the Fertile Crescent (the region ranging from Egypt through the Middle East, which is considered to be the birthplace of human civilizations). The first laws dealing with the construction and use of irrigation systems are thought to date to about 1790 BCE as expressed in the legal code of King Hammurabi. The code described how irrigated water was to be distributed, what a landowner’s responsibility for maintaining the system was, and how the operation of the canal was to be administered (Law Code of Hammurabi (1780 B.C.), 53–56).
For at least four millennia, farmers relied entirely on surface waters (e.g., the Nile River) for their irrigation water. (According to the best estimates now available, about 61.3% of all irrigation systems still use surface water, rather than underground water [Siebert, et al. 2010, Table 2, 1868]). Then, in about 1700 BCE, someone invented the first device for extracting water from below ground and using it for irrigation, the shaduf (or shadoof). The shaduf is almost the simplest possible water-transferring machine that one can imagine, consisting of a long horizontal pole that pivots on a vertical post. A bucket hangs from one end of the horizontal pole, and a weight (e.g., a rock) at the opposite end. The bucket is lowered into a water well, filled with water, and then raised by pushing down on the opposite end. The horizontal pole can then be pivoted to move the bucket over an irrigation ditch, where it is emptied. The shaduf is still in use in parts of the world that do not have access to more sophisticated systems of groundwater transfer.
The greatest disadvantage of the shaduf, of course, was that it was able to transfer only a relatively small volume of water at a time, with considerable effort by the shaduf operator. An important breakthrough in the capture and transfer of groundwater occurred in about 550 BCE with the invention of the qanat. The qanat is a system that consists of a long tunnel dug underground into the water table, sloping downward with an outlet on the side of the hill. (For a video description of a qanat, see https://www.youtube.com/watch?v=ieBVMOPRYJ0.) The development of the qanat made possible for the first time the use of underground water as the primary source of the substance in regions where surface water was limited or nonexistent (Irrigation Museum 2015). As with the shaduf, qanats are still widely used and are essential to the existence of human settlements and agricultural projects in the driest parts of the world (Information Center of Qanat 2015).

Figure 1.2 A woman in Egypt collects water with a shaduf. (Library of Congress)
Nearly coincidental with the invention of the qanat was the invention of the sakia, or Persian waterwheel. The sakia was the first of many similar devices designed to move underground water to the surface by mechanical means, using animal, water, electrical, fossil fuel, solar, wind, or some other form of power. In that respect, the sakia is the grandfather of all systems for the use of groundwater for irrigation systems today. The type of pump for any given system is determined by a number of factors, including the volume of water to be transferred, access to power to operate the pump, and the ability of a user to pay for the pump system (Irrigation Handbook 2015, 47–49).
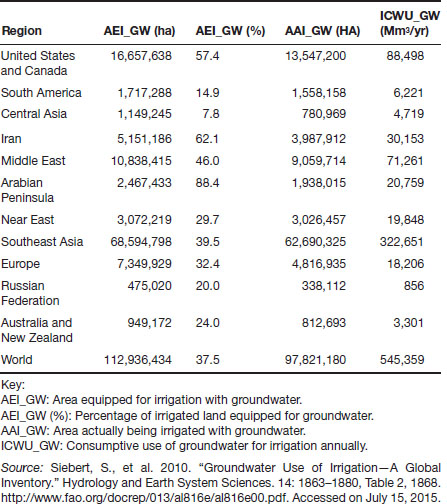
According to the most recent data available (2010), just more than 300 million hectares (740 million acres) of land are now equipped for irrigation of crops worldwide, of which about a third (113 million hectares; 279 million acres) is equipped for irrigation with groundwater. The amount of land actually being irrigated, however, is somewhat less, about 98 million hectares (242 acres), or about 87 percent of all land equipped for groundwater irrigation. These groundwater systems account for a total consumption of 543,359 Mm3/yr (cubic megameters per year). Table 1.6 provides a summary of these data for various regions of the world. (More complete, detailed, and recent data may be available on groundwater irrigation systems at Aquastat, http://www.fao.org/nr/water/aquastat/data/query/index.html?lang=en.)
Table 1.6 Groundwater Use for Irrigation

In many ways, surface irrigation systems are much easier to build and maintain than are groundwater irrigation systems. Collecting water from a river or lake and diverting it to an agricultural field can, at least in concept, be a relatively simple process. One can imagine that humans at a very early stage of agriculture simply dug a canal from the river or lake to the area where they wanted to plant their crops, and their water problem was solved. Of course, as agricultural systems grew larger and more complex, the surface irrigation systems they required also grew in complexity and size. One of the key elements that such advanced systems often required was a dam to force water into an artificial reservoir from which it was easier to collect.
The history of dams is well studied, one that includes constant technological developments leading to larger, more efficient dams that have been increasingly effective at delivering water for (primarily) irrigation and power systems. The earliest known structure that qualifies as a dam is probably an earthen structure built at the ancient site of Jawa in Mesopotamia, now in modern-day Lebanon, dating to about 3000 BCE. The dam was about 30 feet high and about 3 feet wide, a part of a system of structures along the Rajil River. The system of dams around Jawa was supposedly designed to collect water in the dry season, as well as collecting surface runoff water (Helms 1977; Tanksley 2015; Violet 2010).
The Jawa dam was a gravity dam, one that remains in position because of the weight of the materials of which it is composed. This weight is greater than the force of the water behind the time (An Introduction to Gravity Dams 2015). Essentially, all dams built prior to Roman era were either gravity dams or embankment dams. An embankment dam is similar to a gravity dam in that it relies on the weight of the material of which it is made to resist the force of water stored behind the dam. The two dam types differ primarily in the type of material of which they are made: heavy materials, such as rock and stone for gravity dams, and lighter materials, such as sand and dirt for embankment dams. Today, gravity dams are usually defined as those structures that consist of concrete and other masonry materials, while embankment dams are defined as those made of earthy materials and rocks.
The first embankment dam of which scientists know is one built at the ancient Egyptian site of Sadd Al-Kafara in about 2700 BCE. It was about 45 feet in height and more than 370 feet in length. The dam was apparently designed to harness the severe, but infrequent, floods that struck the Sadd Al-Kafara valley. It was under construction for more than 10 years before being destroyed by the type of flood against which it was supposed to protect. It was then apparently never completed (Sadd Al-Kafara 2015; for a comparison of gravity and embankment dams, see Technology of Dams 2015).
The next technological development in dam building did not occur for nearly three millennia. In about the first century BCE, those master engineers, the Romans, invented a new type of dam known as the arch dam. The “arch” in an arch dam is a (usually) concrete structure that faces upward toward the water reservoir that it creates. When that water pushes against the walls of the arch dam, it compresses the material of which the dam is made, therefore increasing the dam’s resistance to water pressure.
The first arch dam was built in the Vallon de Baume in France sometime during the first century BCE. It was about 40 feet high and 60 feet long with a curvature of 73°. It consisted of two masonry walls separated by a narrow space about five feet wide (Agusta-Boularot, Sandrine, and Jean-Louis Paillet 1997; Key Developments in the History of Arch Dams 2015).
The fourth major type of dam to be developed was the buttress dam. The buttress dam gets its name from the use of heavy rectangular concrete structures attached to the downstream side of a dam to strengthen the resistance of the dam itself. The earliest buttress dams were built by Roman engineers in Spain to strengthen gravity or embankment dams they thought were not strong enough to survive on their own (in some cases of which they were correct, but not correct in others; see Key Developments in the History of Buttress Dams 2015). Partly because of the somewhat haphazard approach of Roman engineers to the construction of buttress dams, many authorities now claim that the earliest true dam of that type is one built in 1747 in northern Spain known as the Almendralejo (or Al-Mendralejo or Albuera de Feria or Feria) Dam (Key Developments in the History of Buttress Dams 2015).
Dam technology today is very sophisticated and makes use of all combinations of the four basic dam types, along with other modifications developed for the construction of dams in very specific locations. The largest dams in operation today exceed their earliest predecessors in size by very large factors. The largest dam currently in existence, measured by the volume of materials of which it is made, is the Syncrude Tailings Dam, an embankment dam near Fort McMurray, Alberta, Canada. This dam is just more than 290 feet tall with a length of more than 11 miles, and a volume of just more than 19,000 million cubic feet (Seminar on Safe Tailings Dam Construction 2001). By contrast, the largest dams in terms of their total height and their total reservoir capacity are, respectively, the Jinping-I Dam on the Yalong River in China, with a height of 1,001 feet, a concrete arch bridge, and the Kariba Dam (concrete arch), which forms Lake Kariba on the Zambezi River, in Zimbabwe, with a capacity of 43.3 cubic miles. The largest dams in the United States, by comparison, are (by volume of material) the Fort Peck Dam (embankment) in Fort Peck, Montana, with a materials volume of 3,390 million cubic feet, the Oroville Dam (embankment), in Oroville, California, with a height of 770 feet, and the Hoover Dam (concrete gravity arch), with a reservoir volume of 8.95 cubic miles (Dam, Hydropower, and Reservoir Statistics 2015).
One might be forgiven for thinking of ancient history as a period during which early human civilizations had access to and made use of pure water resources. With relatively small populations, no industry to speak of, and, in many cases, vast water resources, early humans might be expected to have more than enough pure water with which to conduct their lives. While that view might be accurate in a general sense, there is abundant archaeological evidence that those early humans also had to deal with problems of water impurities—water pollution—that are well known to today’s societies.
For example, some early Sanskrit manuscripts from the Indian subcontinent acknowledge the existence of impure water and suggest a number of techniques for remedying that problem. “It is good to keep water in copper vessels,” one such manuscript declares, “and filter it through charcoal.” Another remedy for impure water is to “heat foul water by boiling and exposing to sunlight and by dipping seven times into it a piece of hot copper, then to filter and cool in an earthen vessel” (Baker 1949, 1). It should be noted that during the period mentioned here—and for many centuries thereafter—no one had any idea as to what “impure” or “polluted” water might be. Awareness of non-pure water was generally related to a bad taste or smell, with little understanding that these properties might be indicators of more serious contamination of water. Nonetheless, they were apparent and important enough that humans used these signs as indications that a sample of water needed to be “purified” before being used.
A host of other methods and materials were developed down through the ages to deal with the problem of impure water. For example, the famous Greek physician, Hippocrates, a strong advocate of the curative properties of pure water, recommended the use of a device that has come to be known as Hippocrates sleeve for removing bad odors and tastes from impure water. The sleeve consisted of a cloth bag through which water could be poured. The bag captured the solids responsible for unpleasant odors and tastes and resulted in the release of (relatively) pure water (see an image of the Hippocrates sleeve at http://www.historicfood.com/Ypocras.htm, which shows its use for purifying both water and wine).
One of the earliest methods for dealing with a shortage of pure water was devised by Roman engineers in the third century BCE. During the earliest years of the Roman civilization, the city was able to obtain all the pure water it needed from local rivers and springs. But as the city grew in size and complexity, these sources of pure water proved to be inadequate. At the same time, the wastes generated by a growing city began to pollute those water resources. In response to this problem, the city devised a system of aqueducts, large bridge-like structures that brought clean, freshwater to the city from distant regions, the longest stretching more than 50 miles from source to the city. The first of these aqueducts was begun in the year 312 BCE. As the Roman Empire spread throughout the known world, so did the use of aqueducts until more than a thousand such structures were eventually built from Great Britain in the northwest of the empire to Turkey and the Middle Eastern region in the east (Hansen 2015a; Romaq 2015; one of the best single resource on the overall history of the development of water treatment systems currently available is Coffey and Reid 1976).
Aqueducts eventually proved to be a solution for obtaining pure water for many metropolitan areas around the world. New York City, for example, began construction on the High Bridge aqueduct in 1837 after a devastating outbreak of cholera in 1832 resulting from the use of contaminated water. The aqueduct was completed in 1848 and survives today in the form of a bridge connecting Manhattan and the Bronx (it was last remodeled and upgraded in 2015; Dwyer 2015).
From the earliest days of Christianity until the rise of the Renaissance, little new information or technology about water treatment was produced. Then, in about 1627, English scholar Sir Francis Bacon described a new way of thinking about the production of pure water: desalination. Desalination is the process by which seawater, or other saline water, is converted to pure water. In looking back over the history of the preceding millennium of scientific thought (such as it was), Bacon took note of the abundance of water in the oceans, generally unfit for human consumption, and one possible method of converting seawater to pure water that humans could use. He suggested that passing seawater through layers of sand, as occurs on beaches, might cause the removal of the salts that make seawater useless for most human uses. He devised an experiment for testing this hypothesis, an experiment that failed to confirm his hypothesis. In spite of this failure, however, Bacon brought the concept of desalination to the attention of future researchers (Bacon 1670, 1; Jesperson 1996, 7).
The realization that impure water—beyond its unpleasant odors and taste—might also possess the ability to cause disease had its origins in the historic research of Dutch microscopist Anton (or Antoni or Antony or Antonie) van Leeuwenhoek in the 1670s. Leeuwenhoek’s examination of droplets of water with the microscopes he invented showed that water was never completely pure; it always contained some number and variety of impurities, such as tiny “animalcules” that either skittered through the water or spun around in circles (van Leeuwenhoek 1677). As critical as van Leeuwenhoek’s research was to the understanding of water, a full explanation of the role of his tiny “animalcules” in the disease process had to await the research of Robert Koch, Louis Pasteur, and their colleagues 200 years later.
A comparable discovery of profound significance for water treatment theory and practice occurred nearly simultaneously to the development of the germ theory by Koch, Pasteur, and others. In 1854, the city of London was struck by one of the most serious outbreaks of cholera the nation had ever seen. Physician John Snow took upon himself the challenge of discovering how the outbreak had occurred and what could be done to contain it. By collecting data on the physical location of individuals who came down with the disease, he concluded that its origin was somehow associated with a pump that dispensed water from a public well on Broad Street. He proposed a simple solution to the problem: remove the pump handle. When that action was taken, citizens could no longer draw water from that well, and the cholera germs harbored (then unknown to anyone, including Snow) therein could no longer be transmitted through the population.
Snow recommended two procedures somewhat less dramatic than removing pump handles for further control of the disease. First, he said, water extracted from London wells should be passed through sand filters to remove whatever agent caused cholera. Second, all public water should be treated with chlorine, a substance known to kill bacteria and other microorganisms. With these procedures in place, the occurrence of cholera (and other waterborne diseases) was largely brought under control in London and other areas where they were introduced (Freichs 2015; Snow 1854).
By the time of Snow’s discovery, one of the essential developments on which he was to depend had already been put into place. The first municipal water treatment plant had been constructed in Paisley, Scotland, as early as 1804. The plant made use of a water filtering technology developed by John Gibb, owner of a bleachery plant in Paisley. In order to get the pure water he needed for his plant, Gibb invented a filtering system consisting of sand and charcoal over which polluted water from the River Cart was passed. Gibb then sold the excess purified water he could not use to local residents, who received their water shipments by donkey cart. Shortly after Gibb’s invention, a fellow resident of the city, Robert Thom, expanded Gibb’s ideas in the construction of a full-scale water treatment plant, the first of its kind in history, capable of purifying River Cart water for the whole community. The Gibb–Thom concept took hold quickly and began to spread to urban areas around the world in a short period of time, with the city of Paris completing a similar system for its water supply in 1806 (Galvis 1999, 11–12; Huisman and Wood 1974, 15).
The use of chlorine to purify water had its origins in the discovery of that element by Swedish chemist Carl Wilhelm Scheele in 1774. Its disinfectant properties, however, were poorly understood for more than a century. Beginning in the mid-19th century, researchers began studying a variety of possible water purifying agents and methods, including voltaic reactions, electrolysis, the use of various oxidizing agents, and chlorine. In the United States, for example, a Philadelphia physician named Robley Dunglison suggested adding gaseous chlorine or the salts of chlorine (chlorides) to impure water in order to make it potable. “It has been proposed,” he wrote, “to add to such water [marsh water] a small quantity of chlorine, or one of his chlorides, but a quantity sufficient to destroy the foulness of the fluid, can hardly fail, we should think, to communicate a taste and smell disagreeable to most individuals” (Dunglison 1835, 338). (Two technical points to be noted about Dunglison’s comments and the thinking of his peers: (1) the chemical, physical, physiological, and other properties of the element chlorine are very different from chloride compounds; and (2) the harmful biological effects of impure water are not necessarily the consequence of its unpleasant odors and tastes, so eliminating these odors and tastes does not guarantee that the treated water is yet safe to drink.)
Most of these efforts at devising methods for using chlorine (and other substances) for the purification of water were generally unsuccessful during the 19th century, although they did, of course, contribute to the general body of knowledge about water purification that was developing. By the end of the century, however, technology had become sufficiently developed that large-scale chlorination plants were being developed. Probably the first of those plants was constructed at Ostend, Belgium, in 1900, although it operated for only a short period of time. Two years later, however, another such plant opened at Middelkerke, Belgium, a facility that is generally recognized as the first successful use of chlorine to purify water in the world. At maximum capacity, the plant was able to produce 1,300,000 gallons of purified water per day (Race 1918, 9).
Chlorination of water was first studied experimentally in the United States at the Louisville (Kentucky) Experiment Station for a week or two in 1896. The first full-scale municipal application of the technology, however, was not put into operation until more than a decade later when Jersey City, New Jersey, began using sodium hypochlorite to purify its water on a continuous basis. The real breakthrough in municipal chlorination of water occurred, however, with the opening of the Boonton, New Jersey, water treatment plant in 1908. When operating at maximum capacity, the plant delivered 40 million gallons of water purified with liquid chlorine to Jersey City on a daily basis (A Public Health Giant Step 2015; Safe Drinking Water, Board on Toxicology and Environmental Health Hazards. Assembly of Life Sciences. National Research Council 1980, 17–18).
Water treatment facilities are ubiquitous throughout the developed world today, although they may be less available in some developing nations. Each individual water treatment plant differs on one way or another from other water treatment plants. One of the most common models of a water treatment plant consists of three main steps: coagulation and sedimentation, filtration, and chlorination and aeration. In the coagulation process, one or more chemicals (alum is the most common) are added to raw water, forming a sticky precipitate to which dirt particles and other small solids adhere. These particles, along with larger solid materials in the raw water, are then allowed to settle out to the bottom of a vat, from which they can be removed. In the next step, the semi-purified water is passed through filters made of sand, charcoal, and/or other materials. These filters remove very small solid particles suspended in the water, some of which are responsible for the unpleasant tastes and odors present in raw water. The final step in the sequence involves the addition of a chemical, such as chlorine or bromine or one of their compounds, or another type of treatment, such ultraviolet radiation, designed to kill pathogens present in the water. The water is then delivered to a storage area, from which it can be distributed to the community. (Water treatment is a far more complex process than can be described here. For more detailed information, see Virtual Tour of a Drinking Water Plant, http://water.epa.gov/drink/tour/.)
The collection of water for irrigation systems is by no means the only purpose for which dams are built. The other primary function of dams is as a source of power (hydropower) for operating mills and other types of machinery and the generation of hydroelectric power. The term hydroelectric power refers to electricity that is generated from the kinetic energy stored in running water.
“The Water Wheel,” according to one history of machines, “is probably the oldest power driven machine not operated by men or animals.” A waterwheel is a machine that converts the kinetic energy of running water into the kinetic energy of a rotating wheel and shaft. It can be used for a wide variety of purposes, such as grinding corn and other grains, crushing olives and grapes, tanning leather, making paper, forging iron, running textile machines, and operating bellows (Water Wheel 2015).
Water wheels were apparently not widely known in the earliest human civilizations of Mesopotamia and Egypt, largely because the rivers in those regions were slow-running, not possessed of enough energy to operate a waterwheel. One of the first definite mentions of a waterwheel, in fact, can be found in the writings of the Roman engineer Vitruvius (ca. 75 BCE–15 CE), who described such a device, but then noted that it was “rarely employed” by his colleagues (Hansen 2015b). The reason for this lack of interest, according to some observers, was that an abundance of human slaves and domestic animals was available for carrying out the type of work performed by the waterwheel.
Inventors faced with the challenge of designing a waterwheel that could operate on a slow-running river devised a simple solution. They constructed a dam along the river, allowing water to accumulate in the mill pond behind the dam. Water from the mill pond could then be released into channels leading to the waterwheel, whose power output was then considerably increased by the more rapidly running water (see, e.g., the demonstration diagrams at http://www.technologystudent.com/energy1/wtrwhl1.htm). Dam-waterwheel facilities of this type grew in popularity during the early Middle Ages. The survey taken for the Domesday Book in England in 1086, for example, found that at least 6,000 water mills were in operation, a striking figure that meant that there was one such machine for every 350 residents of the country. By 1300, that number had doubled, and it continued to rise until the middle of the 19th century, when a peak of 30,000 water mills were recorded in England. Similar numbers were reported in other countries, although the popularity of the waterwheel began to decline by 1850 as a result of the availability of other forms of power resulting from the Industrial Revolution (Smil 2008, 180–181, with many additional references at this source).
Future prospects for the development of water power in the mid-19th century—except for specialized geographic, topographic, social, economic, and other reasons—appeared dim because of the development of steam power during the Industrial Revolution. Such an approach turned out not to be the case, however, as a new and far more important use for hydropower appeared on the horizon: the generation of electricity. A key element in this new development was the discovery in 1831 and 1832 by English physicist Michael Faraday of the electric generator. The generator is a device for converting the mechanical motion of a wire within a magnetic field into an electric current. (For the principle of the electric generator, see http://www.tutorvista.com/content/physics/physics-ii/electricity/electric-generator.php.) Faraday’s discovery prompted inventors to find ways of using the kinetic energy of running water to turn a turbine (an oversized fan) that, in turn, could then be used to turn a metal shaft inside a magnetic field to produce electrical current. The concept of a hydroelectric power plant was created. (Many good models of hydroelectric plants are available. See, e.g., https://www.youtube.com/watch?v=rnPEtwQtmGQ.)
During the last quarter of the 19th century, a number of small hydroelectric systems were put into operation, primarily for the purpose of demonstrating the possibilities of the technology. Credit for the first such system often goes to a simple hydroelectric power system installed at the Cragside country house in Northumberland, England, which was capable of lighting a single lightbulb. Similar simple systems were later put into operation in Grand Rapids, Michigan (1880); Ottawa, Ontario (1881); Dolgeville, New York (1881); and Niagara Falls, New York (1881), all producing enough electrical power for homes or small businesses (A Brief History of Hydropower 2015).
The first commercial-scale hydroelectric plant began operations on September 30, 1882, on the Fox River, in Appleton, Wisconsin. The plant was built by paper manufacturer H. J. Rogers, and it supplied enough electricity to meet the needs of his own home, his paper plant, and one nearby building, equivalent to the electric current needed to light 250 lightbulbs (The World’s First Hydroelectric Power Plant 2015).
Over the next 20 years, hydropower saw an explosion of new facilities around the world, with more than 300 new plants going into operation over the period. The United States and Canada led the world in hydropower development, but significant new plants were being installed also in many other countries (A Brief History of Hydropower 2015). Construction of hydropower in the United States was motivated in particular by efforts to take advantage of the nation’s enormous water reserves and to develop the vast arid regions of the West. The first of these plants to come online was the Austin Dam, near Austin, Texas, the first such plant designed specifically for the generation of hydroelectric power. The Austin plant was followed by such now-famous plants as the Niagara Falls Hydropower Plant (1895–1896), Hoover Dam (1936), and Grand Coulee Dam (1942). During the same period, the federal government also created a number of regional agencies whose purpose it was to construct dams on various river systems in the United States, including the Tennessee Valley Authority, and specialized departments for the Colorado River (Bureau of Reclamation) and the Columbia River (U.S. Army Corps of Engineers) (Energy Timelines: Hydropower 2011; Hydroelectric Power 2003).
Today, the role played by hydropower in the generation of electricity varies widely from country to country, ranging from none to 100 percent. Table 1.7 lists some of the countries that are most dependent on hydroelectric power, along with others whose production numbers may be of special interest.
Table 1.7 Percentage of a Country’s Electricity Obtained from Hydroelectric Sources

Give the essential role of water in human society, it is hardly surprising that laws associated with its ownership and use date to the earliest days of human civilization. Most of those laws fall into two general categories: ownership rights and environmental controls. By the term water rights, one means how the ownership of a particular water is determined. By environmental controls, one refers to the actions that can or must be taken or not taken to maintain a water resource in some particular condition, usually pure enough for a variety of human uses such as drinking, washing, swimming, irrigating, or using in industry.
Who owns the natural resources found on Earth? This question can be relatively simple for some natural resources, such as land and mineral rights. A person can purchase a parcel of land, and then he or she can be said to own that land (except in countries and/or at times when a nation declared that it owned all land within its boundaries; then one could hold “hold” or “rent” the land). The same might be said for the purchase of mineral resources buried underground. One might be able to purchase the rights to those resources and then do with them as one wishes.
But water resources are a very different matter. In the first place, water resources are often impermanent. Rivers, streams, and lakes flow freely at some times of the year or in some years, but are dry at other times. Also, water resources often have a variety of users, from people who float by and “use” part of a river for only a few minutes or hours to industries and other operations that actually remove water from a resource, which they may or may not return in the same or less quantity and quality.
For reasons such as these, rulers and governments were making laws about the ownership of water resources very early in human history. The first person to make an effort to codify previous formal and informal declarations about water rights was the Roman emperor Justinian, who, in 528 CE, ordered the publication of a compilation of all known water laws from the preceding 13 centuries. This compilation became part of the Justinian Code, one of the first comprehensive legal doctrines of modern civilization.
One of the most significant features of the Justinian Code’s section on water rights was the doctrine of riparian rights. The term riparian rights comes from the Latin term ripa, for “bank,” as in “river bank.” The doctrine is of enormous historical significance because it has defined the way water can and cannot be used in a host of societies since Roman days, including modern water law in the United States and most other countries of the world.
With regard to riparian rights, the Justinian Code said that the water in a river, stream, lake, or ocean was part of the “public trust,” in the same way that air is part of the public trust. No one can “own” these natural resources; they are and must always be available for use by the general public (What Is Public Trust? 2015). This doctrine is not quite so simple as it sounds, however. While no one could “own” part of a river or lake or seashore, one could certainly own the bank of that river, lake, or seashore and, in some instances, could own the land under the water up to a certain distance from the shore. According to the code:
The public use of the banks of a river is part of the law of nations, just as is that of the river itself. All persons, therefore, are as much at liberty to bring their vessels to the bank, to fasten ropes to the trees growing there, and to place any part of their cargo there, as to navigate the river itself But the banks of a river are the property of those whose land they adjoin; and consequently the trees growing on them are also the property of the same persons.
The public use of the seashore, too, is part of the law of nations, as is that of the sea itself; and, therefore, any person is at liberty to place on it a cottage, to which he may retreat, or to dry his nets there, and haul them from the sea; for the shores may be said to be the property of no man, but are subject to the same law as the sea itself, and the sand or ground beneath it. (The Institutes of Justinian, 535 A.D. 2015)
The evolution of water rights laws following publication of the Justinian Code is far too complex to be considered in detail here. (For good discussions of this history, see Cech 2010, chapter 8; Getzler 2004; Hodgson 2004, chapter 4; Narasimhan 2008.) In general, the doctrine laid out by the code was largely adopted intact or with some modifications by other societies as they began to duplicate Justinian’s efforts to codify a history of their country’s laws on a variety of topics. For example, in 1256, King Alfonso X of Castile (“The Wise”) directed a compilation of Spanish laws similar to that of Justinian that was eventually published in 1263 as Las Siete Partidas (The Seven-Part Code or Seven Books of Law). Book 3 of the code dealt with issues of property ownership and reflected, to a remarkable degree, the doctrine expressed in the Justinian Code. At one point, for example, it states that
The things that belong to all creatures who live on earth are the following, air, rain, water, and the shore of the sea. For each living person may make use of each of these things according to his need. Therefore each man may make use of the sea and its shore, fishing or sailing and doing all the things which he deems to be for his benefit. (As cited in Stone 1995, 286)
Las Siete Partidas is actually of more than historical interest in current discussions over water issues in the United States. As noted in the article just cited earlier, the legal doctrine controlling water rights issues in the southwestern United States has long been strongly influenced by the views expressed in Alfonso’s document more than five centuries earlier (Stone 1995, 286–291).
Even more influential to water rights disputes in the United States has been the development of common law doctrine about water rights laws. The term common law refers to legal doctrines that have been established as a result of court decision rather than specific laws that have been passed by a legislature (which are known as statutory law). As with many features of the American legal system, the nation’s current legal policies toward water use are ground to a considerable extent in the history of English common law.
Probably the best single explication of that long and complex history is an article written by T. E. Lauer, then at the University of Missouri School of Law, “The Common Law Background of the Riparian Doctrine” (Lauer 1963). In that article, Lauer traces the intricate ins and outs of the development of common law doctrine on riparian rights from what seems to be its first mention in 1187 to its impact on the American legal system in 1826. In that year, a case came before the U.S. Circuit Court for the District of Rhode Island, where it was heard before Justice Joseph Story. The case, Tyler v. Wilkinson, involved a complaint by a group of millowners on the Pawtucket River that an upstream competitor was diverting water from the river in such a way as to cause damage to their own operations. Justice Story wrote a long opinion that set an important precedent regarding water rights in the United States. He pointed out that simply because one owner had precedence in water use, that is, had an operation in place before his competitors, he was not allowed to withdraw enough water to disturb the operations of those competitors. “Each riparian,” he ruled, “had a right to reasonable use of the water” (Lauer 1963, 60; Water Law 2015). With that decision, the first step in establishing the riparian rights of landowners in the United States was established.
Justice Story’s decision in Tyler v. Wilkinson seemed to suggest that the legal status of water rights in the United States would essentially continue to reflect those that had been followed in Europe for nearly two millennia. Such was not, however, to be the case for very long. One of the major events that altered that scenario was the gold rush that took place in the America West (especially in California) beginning in 1848. In the frenzy that accompanied the search for gold, some traditional legal niceties were ignored. One of those “niceties” was the riparian doctrine. Gold miners began to decide on their own that the person who owned the rights to a body of water, such as a stream where he was searching for gold, was the person who got there first and started to work the stream. The new water rights doctrine soon earned the formal name of prior appropriation. One formal definition for the term is that “the first person who physically takes water from a stream (or underground aquifer) and places that water to some type of beneficial use” becomes the owner of that water resource. The prior appropriation doctrine has earned the slogan of “first in time, first in right” (Prior Appropriation Law 2015).
Prior appropriation allows for more than one owner of a water resource, but does not guarantee that all “owners” will actually be able to use water from a resource. For example, suppose that three individuals have prior appropriation rights to a water resource, the first with the right to the use of 10 cubic feet per second, the second with the right to 5 cubic feet per second, and the third with the right to 2 cubic feet per second. As long as the source provides 17 cubic feet per second or more, all owners will be satisfied. But suppose that the source begins to dry up and produces on 10 cubic feet per second. In that case, the second and third owners are “out of luck” and will get no water from the source.
The legitimacy of the prior appropriation doctrine of water rights was confirmed by a now-famous court case, Irwin v. Phillips, settled in 1855. In that case, the California Supreme Court ruled that
however much the policy of the State, as indicated by her legislation, has conferred the privilege to work the mines, it has equally conferred the right to divert the streams from their natural channels, and as these two rights stand upon an equal footing, when they conflict, they must be decided by the fact of priority, upon the maxim of equity, qui prior est in tempore potior est in jure. [“who is there first in time is first in law.”] (Hess 1917, 146)
The prior appropriation doctrine gained acceptance rapidly in the western states. Colorado became the first state to officially adopt the policy in 1872, followed by Alaska, Arizona, California, Hawaii, Idaho, Kansas, Montana, Nebraska, Nevada, New Mexico, North Dakota, Oklahoma, Oregon, South Dakota, Utah, Washington, and Wyoming (State Water Withdrawal Regulations 2015). Thus, the United States today has two very different legal systems for deciding on water rights, the riparian doctrine in the eastern and central part of the country and the prior appropriation doctrine in the western states.
Human civilizations have probably always been accompanied by some level of air, water, and solid waste pollution. As societies became more technologically advanced (as during the Industrial Revolution), that level of pollution increased, often by significant amounts. Yet, relatively few examples of laws dealing with environmental pollution prior to the 20th century exist. One of the earliest laws sometimes cited as an example of the first water pollution law was actually written to deal with air pollution, “For punishing nuisances which cause corruption of the air near cities and great towns,” passed in 1388. This law took note of the fact that
For that so much dung and filth of the garbage and entrails as well of beasts killed, as of other corruptions, be cast and put into ditches, rivers and other waters, and also many other places, within, about, and nigh unto divers cities, boroughs, and towns of the realm and the suburbs of them, that the air there is greatly corrupt and infect, and many maladies and other intolerable diseases do daily happen, as well to the inhabitants and those that are conversant in the said cities, boroughs, towns and suburbs, as to others repairing and traveling thither, to the great annoyance, damage, and peril of the inhabitants, dwellers, repairers, and travellers aforesaid … and if any do he shall be called before the chancellor … and shall be punished after the discretion of the Chancellor. (Tomlins 1811, 120–122)
Early laws such as these were extremely rare and generally largely ineffective (Newson 1992, 17–20). In fact, it was not well into the Industrial Revolution before water pollution laws became significantly more common and sometimes more effective. As just one example, the Salmon Fisheries Act of 1861 was adopted in response to pollution of many rivers and streams by a variety of industrial operations that so badly contaminated the water that downstream fishing was essentially no longer possible. The 1861 act made a number of provisions requiring industries to refrain from polluting or, at least, cleaning up the pollution of the waterways they used. This act, like many other similar to it, was also largely ineffective (Newson 1992, 19; The Salmon Fisheries Act 1861).
A roughly similar pattern for water pollution laws holds true for the United States. The first such law was the Rivers and Harbors Act of 1899, whose purpose it was to keep the nation’s waterways clear of materials that would interfere with the navigation of ships. Interestingly enough, two sections of that law are still being used in lawsuits filed to promote clean waterways today, sections 10 and 13 that deal with obstructions in waterways and the dumping of materials into waterways, respectively (Kenney 2006).
A half century passed before the federal government enacted additional legislation protecting the nation’s waterway, the Federal Water Pollution Control Act (FWPCA) of 1948 (sometimes referred to as The Clean Water Act). This act did not actually provide for federal action in the maintenance of water quality in lakes, streams, and other bodies of water, focusing instead on various mechanisms to encourage the states to take such action as they felt was necessary. The FWPCA was amended a number of times, in 1961, 1966, 1970, 1972, 1977, and 1987, in attempts to improve its effectiveness in reducing pollution of the nation’s waterways (Digest of Federal Resource Laws 2015).
Possibly the most significant of the many amendments made to the FWPCA were those adopted in 1972, a group of provisions now known as the Clean Water Act of 1972. One of the motivating forces driving legislators to approve this act was the series of dramatic fires that had been breaking out on Cleveland’s Cuyahoga River from the early 1950s to the late 1960s. The nearly unimaginable pollution that allowed a large flowing river to catch fire inspired many ordinary citizens as well as state and federal legislators to begin calling for more aggressive federal action for the protection of the nation’s waterways, one major result being the 1972 Clean Water Act (Scott 2009).
As with previous legislation, the Clean Water Act of 1972 has been updated and revised a number of times since its adoption. It continues to provide the fundamental basis for the nation’s policies regarding the maintenance of water quality in U.S. rivers, streams, lakes, and other waterways.
Earth’s water resources are so enormous that one wonders how there could ever be a shortage of water. But this review makes clear that there have been many times and places in human history when sufficient quantities of pure water were not readily available for human use. Over the past half century, that problem has become even more serious with a host of troublesome issues now facing the world’s governments. Chapter 2 will discuss some of the most important of these issues and some of the solutions that have been proposed for those issues.
“Advancing the Science: Google Earth.” Marine Conservation Biology Institute. http://mcbi.marine-conservation.org/what/googleearth.htm. Accessed on July 11, 2015.
Agusta-Boularot, Sandrine, and Jean-Louis Paillet. 1997. “Le Barrage et L’aqueduc Occidental De Glanum: Le Premier Barragevoûte de L’histoire Des Techniques?” Revue Archéologique. 1: 27–78.
Al Ansary, A. R. 1981. Qaryat Al-fau: A Portrait of PreIslamic Civilisation in SaudiArabia. London: Croom Helm.
Bacon, Sir Francis. 1670. Sylva Sylvarum or a Natural History in Ten Centuries, 9th ed. London: Printed by J.R. for William Lee. https://archive.org/stream/sylvasylvarumorn00baco#page/n7/mode/2up. Accessed on July 17, 2015.
Baker, M. N. 1949. “The Quest for Pure Water: The History of Water Purification from the Earliest Records to the Twentieth Century.” New York: American Water Works Association. Available online at http://babel.hathitrust.org/cgi/pt?id=mdp.39015007372272;view=1up;seq=1. Accessed on July 16, 2015.
Ball, Philip. 2000. Life’s Matrix: A Biography of Water. New York: Farrar, Straus, and Giroux.
Beatty, Kelly. 2015. “Give-and-Take Origin for Earth’s Water?” Sky and Telescope. http://www.skyandtelescope.com/astronomy-news/origin-of-earths-water-01022015/. Accessed on July 11, 2015.
Bice, Dave. “Modeling the Global Water Cycle.” Exploring the Dynamics of Earth Systems. http://www3.geosc.psu.edu/~dmb53/DaveSTELLA/Water/global%20water/global_water.htm. Accessed on July 12, 2015.
“A Brief History of Hydropower.” International Hydropower Association. http://www.hydropower.org/a-brief-history-of-hydropower. Accessed on July 19, 2015.
Cech, Thomas V. 2010. Principles of Water Resources: History, Development, Management, and Policy, 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc.
Coffey, Kay, and G. W. Reid. 1976. “Historic Implication for Developing Countries of Developed Countries’ Water and Wastewater Technology.” Agency for International Development. http://pdf.usaid.gov/pdf_docs/pnaad288.pdf. Accessed on July 18, 2015.
“Dam, Hydropower, and Reservoir Statistics.” 2015. United States Society on Dams. http://www.ussdams.org/uscold_s.html. Accessed on July 16, 2015.
“Digest of Federal Resource Laws of Interest to the U.S. Fish and Wildlife Service.” 2015. https://www.fws.gov/laws/lawsdigest/FWATRPO.HTML. Accessed on July 23, 2015.
Dunglison, Robley. 1835. On the Influence of Atmosphere and Locality: Change of Air and Climate, Seasons, Food, Clothing, Bathing, Exercise, Sleep, Corporeal and Intellectual Pursuits, &C. &C. On Human Health : Constituting Elements of Hygiène. Philadelphia: Carey, Lea & Blanchard.
Dwyer, Jim. 2015. “A Stunning Link to New York’s Past Makes a Long-Awaited Return.” The New York Times. http://www.nytimes.com/2015/06/05/nyregion/a-stunning-link-to-new-yorks-past-makes-a-long-awaited-return.html. Accessed on July 17, 2015.
“Energy Timelines: Hydropower.” 2011. http://www.docstoc.com/docs/106654584/Energy-Timelines-Hydropower. Accessed on July 19, 2015.
Fisk, Pliny. 1822. “Egypt.” Religious Intelligencer. 7(19): 289–293.
Foster, Stephen, and Daniel P. Loucks, eds. 2006. “Non-Renewable Groundwater Sources.” Paris: United Nations Educational, Scientific and Cultural Organization. Available online at http://unesdoc.unesco.org/images/0014/001469/146997e.pdf. Accessed on July 13, 2015.
Freichs, Ralph R. 2015. “Removal of the Pump Handle.” UCLA Department of Epidemiology. School of Public Health. http://www.ph.ucla.edu/epi/snow/removal.html. Accessed on July 17, 2015.
Galvis, Gerardo. 1999. “Development and Evaluation of Multistage Filtration Plants.” Centre for Environmental Health Engineering. School of Engineering in the Environment. University of Surrey. http://www.bvsde.paho.org/bvsacg/i/fulltext/galvis/galvis.pdf. Accessed on July 17, 2015.
“General Facts and Concepts about Ground Water.” U.S. Geological Survey. http://pubs.usgs.gov/circ/circ1186/html/gen_facts.html. Accessed on July 12, 2015.
Getzler, Joshua. 2004. A History of Water Rights at Common Law. Oxford; New York: Oxford University Press.
Grant, Roy. 2015. “Woodingdean Well.” Brighton and Hove. http://www.mybrightonandhove.org.uk/page_id__6948.aspx. Accessed on July 14, 2015.
“Groundwater: Wells.” U.S. Geological Survey. http://water.usgs.gov/edu/earthgwwells.html. Accessed on July 14, 2015.
Hansen, Roger D. 2015a. “Water and Wastewater Systems in Imperial Rome.” http://www.waterhistory.org/histories/rome/t1.html#REF. Accessed on July 17, 2015.
Hansen, Roger D. 2015b. “Water Wheels.” http://www.waterhistory.org/histories/rome/t1.html#REF. Accessed on July 19, 2015.
Helms, S. W. 1977. “Jawa Excavations 1975: Third Preliminary Report.” Levant. 9(1): 21–35.
Hess, R. H. 1917. “California Irrigation Right.” California Law Review. 5(2): 142–159. http://scholarship.law.berkeley.edu/cgi/viewcontent.cgi?article=4084&context=californialawreview. Accessed on July 20, 2015.
Hodgson, S. 2004. “Land and Water—The Rights Interface.” Food and Agricultural Organization of the United Nations. http://www.fao.org/3/a-y5692e.pdf. Accessed on July 20, 2015.
Hoekstra, Arjen Y., and Mesfin M. Mekonnen. 2011. “The Water Footprint of Humanity.” PNAS. 109(9): 3232–3237.
“How Much Water Is There on, in, and above the Earth?” U.S. Geological Survey. http://water.usgs.gov/edu/earthhowmuch.html. Accessed on July 12, 2015.
Huisman, L., and W. E. Wood. 1974. “Slow Sand Filtration.” Geneva: World Health Organization. http://www.who.int/water_sanitation_health/publications/ssf9241540370.pdf. Accessed on July 17, 2015.
“Hydroelectric Power.” 2003. Dictionary of American History. http://www.encyclopedia.com/topic/hydroelectric_power.aspx#1. Accessed on July 19, 2015.
“Information Center of Qanat.” 2015. Internet Archive Wayback Machine. http://web.archive.org/web/20050205102218/http://qanat.info/en/index.php. Accessed on July 15, 2015.
“The Institutes of Justinian, 535 A.D.” 2015. http://faculty.cua.edu/pennington/Law508/InstitutesofJustinian.htm. Accessed on July 20, 2015.
“An Introduction to Gravity Dams.” 2015. Sim Science. http://simscience.org/cracks/advanced/grav_char1.html. Accessed on July 16, 2015.
“Irrigation Handbook.” 2015. Grundfos Water Services. https://us.grundfos.com/content/dam/GPU/Products/DME/irrigation-handbook-220165.pdf. Accessed on July 15, 2015.
“Irrigation Museum.” 2015. Irrigation Association. http://www.irrigationmuseum.org/exhibit2.aspx. Accessed on July 15, 2015.
Jesperson, Kathy. 1996. “Search for Clean Water Continues.” On Tap. Summer 1996: 6–8.
Kenney, Robyn. 2006. “Rivers and Harbors Act of 1899, United States.” The Encyclopedia of Earth. http://www.eoearth.org/view/article/155764/. Accessed on July 23, 2015.
Kenoyer, Johnathan M. 2011. Ancient Cities of the Indus Valley Civilization. Karachi, Pakistan: Oxford University Press; Islamabad: American Institute of Pakistan Studies.
“Key Developments in the History of Arch Dams.” 2015. Cracking Dams. http://www.simscience.org/cracks/advanced/arch_hist1.html. Accessed on July 16, 2015.
“Key Developments in the History of Buttress Dams.” 2015. Cracking Dams. http://www.simscience.org/cracks/advanced/butt_hist1.html. Accessed on July 16, 2015.
Lauer, T. E. 1963. “The Common Law Background of the Riparian Doctrine.” Missouri Law Review. 1: 60–107.
“Law Code of Hammurabi (1780 B.C.).” 2015. http://mcadams.posc.mu.edu/txt/ah/assyria/hammurabi.html. Accessed on July 15, 2015.
McGowan, Joe. 2015. “A Visit to St. Patrick’s Well.” Irish Culture and Customs. http://www.irishcultureandcustoms.com/ACalend/StPatWell.html. Accessed on July 14, 2015.
Midant-Reynes, Béatrix. 2000. The Prehistory of Egypt: From the First Egyptians to the First Pharaohs. Oxford, UK; Malden, MA: Blackwell Publishers.
Narasimhan, T. N. 2008. “Water, Law, Science.” Journal of Hydrology. 349(1): 125–138. http://www.osti.gov/scitech/servlets/purl/924856-9Okl1w/. Accessed on July 20, 2015.
Newson, Malcolm David. 1992. Land, Water and Development: River Basin Systems and Their Sustainable Management. London; New York: Routledge.
Nilsson, Jan-Erik. 2015. “Chronology and History of China.” Gotheborg.com. http://gotheborg.com/chronology/index-chronology.htm. Accessed on July 13, 2015.
“Prior Appropriation Law.” 2015. Division of Water Resources. Department of Natural Resources. Colorado. http://water.state.co.us/SurfaceWater/SWRights/Pages/PriorApprop.aspx. Accessed on July 20, 2015.
“A Public Health Giant Step: Chlorination of U.S. Drinking Water.” 2015. Water Quality and Health. http://www.waterandhealth.org/drinkingwater/chlorination_history.html. Accessed on July 18, 2015.
Race, Joseph. 1918. Chlorination of Water. New York: J. Wiley & Sons, Inc. Available online at http://www.gutenberg.org/files/37389/37389-h/37389-h.htm#Fn1_14. Accessed on July 18, 2015.
Ringersma, Jacquelijn, Niels Batjes, and David Dent. 2003. “Green Water: Definitions and Data for Assessment. Wageningen, Netherlands. http://www.isric.org/isric/webdocs/docs/ISRICGreenwater%20ReviewFebr2004_.pdf. Accessed on January 17, 2016.
Roaf, Michael. 2008. Cultural Atlas of Mesopotamia and the Ancient Near East. New York: Facts on File.
“Romaq: The Atlas Project of Roman Aqueducts.” 2015. http://www.romaq.org/. Accessed on July 17, 2015.
“Sadd Al-Kafara … the Oldest Dam in the World.” 2015. Hydria Project. http://www.hydriaproject.net/en/egypt-sadd-al-kafara-dam/relevance9. Accessed on July 16, 2015.
Safe Drinking Water Committee. Board on Toxicology and Environmental Health Hazards. Assembly of Life Sciences. National Research Council. 1980. Drinking Water and Health, vol. 2. Washington, DC: National Academy Press.
Sagmit, Rosario S., and Nora N. Soriano. 1998. Geography in the Changing World. Manila, Philippines: Rex Book Store.
“Saline Water.” U.S. Geological Survey. http://water.usgs.gov/edu/saline.html. Accessed on July 10, 2015.
“The Salmon Fisheries Act.” 1861. London: “The Field” [sic] Office. Available online at http://babel.hathitrust.org/cgi/pt?id=hvd.hwg58z;view=1up;seq=9. Accessed on July 23, 2015.
Sarafian, Adam, et al. 2014. “Early Accretion of Water in the Inner Solar System from a Carbonaceous Chondrite-like Source.” Science. 346(6209): 623–626.
Scott, Michael. 2009. “Cuyahoga River Fire Galvanized Clean Water and the Environment as a Public Issue.” Cleveland.com. http://www.cleveland.com/science/index.ssf/2009/04/cuyahoga_river_fire_galvanized.html. Accessed on July 23, 2015.
Segrest, Michelle. 2015. “The History of Pumps through the Years.” Pumps and Systems. http://www.pumpsandsystems.com/topics/pumps/pumps/history-pumps-through-years. Accessed on July 13, 2015.
“Seminar on Safe Tailings Dam Constructions.” 2001. Swedish Mining Association, et al. http://ec.europa.eu/environment/waste/mining/pdf/mining_dams_seminar.pdf. Accessed on July 16, 2015.
Siebert, S., et al. 2010. “Groundwater Use of Irrigation—A Global Inventory.” Hydrology and Earth System Sciences. 14: 1863–1880. http://www.fao.org/docrep/013/al816e/al816e00.pdf. Accessed on July 15, 2015.
Smil, Vaclav. 2008. Energy in Nature and Society: General Energetics of Complex Systems. Cambridge, MA: MIT Press.
Snow, John. 1854. “Mode of Communication of Cholera.” UCLA Department of Epidemiology. School of Public Health. http://www.ph.ucla.edu/epi/snow/snowbook0a.html. Accessed on July 17, 2015.
Snyder, Laura J. 2015. Eye of the Beholder: Johannes Vermeer, Antoni Van Leeuwenhoek, and the Reinvention of Seeing. New York: W. W. Norton & Company.
“State Water Withdrawal Regulations.” 2015. National Conference of State Legislatures. http://www.ncsl.org/research/environment-and-natural-resources/state-water-withdrawal-regulations.aspx. Accessed on July 20, 2015.
Stone, Marilyn. 1995. “Las Siete Partidas in America: Problems of Cultural Transmission in the Translation of Legal Signs.” In Morris, Marshall, ed. Translation and the Law. Amsterdam; Philadelphia: John Benjamins.
Tanksley, Arlene Kim. 2015. “Jawa Dam.” Bayt.com. http://www.bayt.com/en/specialties/q/148613/jawa-dam-is-featured-an-originally-9m-high-and-1m-wide-stone-wall-supported-by-a-50m-wide-earth-rampart-in-which-country-was-this-earliest-known-dam/. Accessed on July 16, 2015.
“Technology of Dams.” 2015. International Commission on Large Dams. http://www.icold-cigb.org/GB/Dams/technology_of_dams.asp. Accessed on July 16, 2015.
Tegel Willy, et al. 2012. “Early Neolithic Water Wells Reveal the World’s Oldest Wood Architecture.” PLoS ONE. 7(12): e51374. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0051374. Accessed on July 13, 2015.
Tomlins, Thomas Edlyne, ed. 1811. The Statutes at Large of England and Great Britain, vol. 2. London: George Eyre and Andrew Strahan.
Tvedt, Terje, and Richard Coopey. 2010. Rivers and Society: From Early Civilizations to Modern Times. London: I. B. Tauris.
Van Leeuwenhoek, Antony van. 1677. “Observations Communicated to the Publisher by Mr. Antony van Leeuwenhoek, in a Dutch Letter of the 9th of October 1676. Here English’d: Concerning Little Animals by Him Observed in Rain-Well-Sea. and Snow Water; as Also in Water Wherein Pepper Had Lain Infused.” Philosophical Transactions. 12: 821–831. http://rstl.royalsocietypublishing.org/content/12/133-142/821.full.pdf. Accessed on July 17, 2015.
Violet, Pierre-Louis. 2010. “Water Engineering and Management in Early Bronze Age Civilizations.” In Cabrera, Enrique, and Francisco Arregui, eds. Water Engineering and Management through Time: Learning from History. Boca Raton, FL: CRC Press, Chapter 2.
“Water Basics.” U.S. Geological Survey. http://water.usgs.gov/edu/mwater.html. Accessed on July 10, 2015.
“Water Law.” 2015. http://ppc.uiowa.edu/sites/default/files/uploads/water_law_senior_college_class_2_classic_and_modern_cases.pdf. Accessed on July 20, 2015.
“Water Wheel.” 2015. Machine-History.com. http://www.machine-history.com/node/575. Accessed on July 19, 2015.
“What Is Public Trust?” 2015. Flow: For Love of Water. http://flowforwater.org/public-trust-solutions/what-is-public-trust/. Accessed on July 20, 2015.
“The World’s First Hydroelectric Power Plant Began Operation September 30, 1882.” 2015. America’s Story. http://www.americaslibrary.gov/jb/gilded/jb_gilded_hydro_1.html. Accessed on July 19, 2015.
“World’s Largest Hand Dug Well.” 2015. The Big Well. Greensburg, Kansas. http://www.bigwell.org/. Accessed on July 14, 2015.
“The World’s Water.” U.S. Geological Survey. http://water.usgs.gov/edu/gallery/global-water-volume.html. Accessed on July 11, 2015.
Zekster, Igor S., and Lorne G. Everett, eds. 2004. “Groundwater Resources of the World and Their Uses.” Paris: United Nations Educational, Scientific and Cultural Organization. http://unesdoc.unesco.org/images/0013/001344/134433e.pdf. Accessed on August 8, 2015.

People in developing nations often have no access to safe, fresh water. They may have to travel miles and/or wait for long periods to get the water they need for daily needs, such as drinking, cooking, and cleaning. (Sjors737/Dreamstime.com)