Chapter 25
Developmental Changes in Ion Channels
Chapter Outline
IIIE. Inward-Rectifier K+ Channels
IIIF. Voltage-Gated K+ Channels
I Summary
Action potentials (APs) and resting potentials (RPs) in excitable cells, such as cardiomyocytes, skeletal muscle fibers and neurons, are greatly altered during development. In general, the RP increases (becomes more negative), the AP rate of rise increases, overshoot increases and duration decreases. These electrophysiological alterations are mainly produced by developmental changes in the ion channels, i.e. by changes in the types, number and kinetic properties of the ion channels.
In cardiomyocytes, the RP at the early embryonic/fetal period is low and there is a gradual hyperpolarization during development. This increase in the RP during development can be accounted for by the increase in the density of inward-rectifier K+ current (IK1) and the resultant decrease in the permeability ratio for Na+ to K+ (PNa/PK ratio). In contrast, the hyperpolarization-activated inward current (Ih), which may affect automaticity, is dominant in early embryonic chick and mouse cardiomyocytes and disappears during development.
The Na+ current of cardiomyocytes increases markedly during development. This increase in number (density) of fast Na+ channels accounts for the large increase in the maximal rate of depolarization of the AP (max dV/dt) during development. The fast Na+ current in embryonic/fetal hearts has a significant sustained component. The sustained component decreases during development and this decrease contributes, at least in part, to the abbreviation of the AP duration.
Developmental change of Ca2+ channels varies with the species. The density of total Ca2+ current in chick cardiomyocytes decreases during the developmental period from fetal to neonate. In rat and mouse, however, it increases from the fetal to the neonatal period, followed by a substantial decrease in the adult. In contrast, the current density in the neonatal period is smaller than that in the adult in rabbit and guinea pig.
In fetal heart cells, the role of the sarcoplasmic reticulum (SR) increases with embryonic development. Therefore, Ca2+ influx through the Ca2+ channels is especially important for the excitation–contraction (E-C) coupling process of immature cardiomyocytes.
The density of the transient outward current (Ito) of cardiomyocytes increases during development. An increase in the density of the delayed-rectifier K+ current (IK) also occurs during early development and decreases during the postnatal period. The change of these voltage-gated outward currents acts to abbreviate the AP duration during development.
In skeletal muscle fibers, there is an overall trend toward an increase in the density of IK1 during the early developmental period. However, there is a transient period during which the current density actually decreases. This transient period parallels the increased incidence of spontaneous firings, presumably due to a less stable RP. The major voltage-gated inward currents, the Na+ and Ca2+ currents, are already present in the early embryonic skeletal myocytes in culture and they increase in intensity during development. These changes contribute to the increases in the AP rate of rise, overshoot and propagation velocity.
The nicotinic acetylcholine receptor/channel (nAChR), which is essential to transmission at the neuromuscular junction, is converted from fetal-type to adult-type and this conversion may be related to innervation of the muscles that occurs during development.
In neuronal cells, there is an overall trend that the ionic dependence of the AP is altered during development from being Ca2+-dependent (prolonged AP duration) to Na+-dependent (brief AP duration). The pattern of ion channel development varies among different types of neuronal cells, with faster development of Ca2+ channels occurring in some cells. Another important factor that determines the ionic dependence of the AP is the developmental increase in IK. Therefore, the change in the IK/ICa ratio during development is the major determinant of the conversion of the AP configuration and influences Ca2+ influx. The activities of ligand-gated Ca2+ permeable channels (such as NMDA receptor/channels and AMPA-gated receptor/channels) are also altered during development. Therefore, the Ca2+ influx through the voltage-gated and the ligand-gated Ca2+ channels, and the subsequent effects on intracellular Ca2+, may affect the structural changes of the developing neurons and help the establishment of the neuronal network.
II Introduction
Cellular functions and tissue structures change dramatically during development. Ion channels are responsible for cellular signaling and maintenance of the intracellular environment. For example, the Ca2+ channels allow Ca2+ influx into the cell, which acts as a second messenger that affects several structures: activation of enzymes; activation of some ion channels; and activation of the contractile proteins. The ion channels change in their types, number and kinetic properties during the embryonic/fetal period and the neonatal period. Particularly in excitable cells (i.e. cardiomyocytes, skeletal muscle fibers, neurons), their resting potential (RP) and action potentials (APs) are progressively altered during the developmental stages. For example, the RP increases in amplitude during development and large changes occur in the AP rate of rise, overshoot and duration. In general, the rate of rise increases markedly, the overshoot increases and the duration decreases during development. This chapter focuses primarily on the ion channels of cardiomyocytes, skeletal muscle fibers and neurons, where most is known about the developmental changes.
III Cardiomyocytes
IIIA Resting Potential
In the early embryonic period, the heart primordium (the so-called cardiac crescent) begins to contract before the appearance of a linear heart tube (Kobayashi et al., 2011). In the middle embryonic period, the heart tube twists and then the cardiac loop is constructed. In this period, the ventricular portion becomes distinguished from the atrial portion.
The electrophysiological properties are also altered during development. The RP of the ventricular cells in the early embryonic/fetal period is low (e.g. −40 to −55 mV) and there is a gradual hyperpolarization during development. Finally, in the late embryonic period, the RP becomes nearly equal to that of adult cells (around −80 mV) (Bernard, 1975). During this increase of the RP, a decrease in the permeability ratio for Na+ to K+ (PNa/PK ratio) occurs (Sperelakis and Shigenobu, 1972). The developmental changes in the RP cannot be accounted for by changes in the intracellular ion concentrations because [K+]i is already high in the early embryonic period. Although the Na+-K+ pump specific activity was found to be low in the early embryonic period (Sperelakis and Lee, 1971), the Na+-K+ pump is sufficient to maintain a high [K+]i and low [Na+]i because of the less leaky membrane (i.e. high in resistance) (Sperelakis and Shigenobu, 1972). Therefore, the developmental change in the RP is due to changes in membrane permeability (conductance) for the ions.
In the early embryonic period, the low RP of the ventricular cells is not stable, but exhibits a spontaneous depolarization, the pacemaker potential (phase 4 diastolic depolarization). During embryonic development, the maximum diastolic potential increases (hyperpolarized) and the slope of the pacemaker potential progressively decreases. When the RP has attained the adult level in the late embryonic period, the pacemaker potential disappears. Thus, automaticity of the ventricular cells is lost by the late embryonic period. One possible factor in the loss of automaticity is the decrease in the PNa/PK ratio and the resultant hyperpolarization. This factor is closely related to the increase in the inward-rectifier K+ current (IK1) and the loss of the hyperpolarization-activated inward current (Ih or If) (see Sections IIIE and IIIG).
IIIB Action Potential
The action potentials (APs) get larger and rise faster during embryonic development (Couch et al., 1969; Sperelakis and Shigenobu, 1972; Bernard, 1975; Kojima et al., 1990). These changes are accompanied by the hyperpolarization of the RP and by an increase in overshoot to about +30 mV. The maximal rate of depolarization (max dV/dt) progressively increases during development, from about 20 V/s to 200 V/s in the late embryonic stage, which is about the adult level. However, the time course of the increase in max dV/dt is not parallel to the increase in RP. The increase in RP precedes the increase in max dV/dt by several days. Therefore, this increase in max dV/dt is not simply due to the hyperpolarization, but is produced by a much greater number (density) of tetrodotoxin (TTX)-sensitive fast Na+ channels (see Section IIIC).
The duration of the AP (e.g. at 50% repolarization, APD50) hardly changes in the chick during development (Sperelakis and Shigenobu, 1972). The same is true of the guinea pig heart, as well as in many other mammalian species (Sanchez-Chapula et al., 1994; Kato et al., 1996). However, in human atrial cells, the APD50 is significantly shortened with development (Wang et al., 2003). The rat also shows a marked decrease in APD50 beginning in the late fetal period and extending through the first 3 weeks of the neonatal period, after which adult-like brief APs are attained (Kojima et al., 1990). Several factors contribute to this marked abbreviation of the AP in the rat, including increase in the transient outward current (Ito) and loss of the sustained component of the fast Na+ current (see Section IIIC).
IIIC Na+ Channels
Slow Ca2+ channel current makes a major contribution to the upstroke of the AP in the early embryonic period (Bernard, 1975). The fast Na+ current in ventricular cells increases markedly during development by a factor of about 10-fold in chick, rat and murine hearts (Fujii et al., 1988; Sada et al., 1995; 1988; Conforti et al., 1993; Davies et al., 1996). However, in rabbit and canine sinoatrial node cells, the fast Na+ current actually decreases during development (Baruscotti et al., 1996; Protas et al., 2010). Tetrodotoxin- (TTX, a specific blocker of the fast Na+ channels) binding studies indicated that a marked increase in density of the fast Na+ channel protein occurs during development of embryonic chick heart (Renaud et al., 1981, 1983). This increase in number (density) of fast Na+ channels accounts for the large increase in max dV/dt of the AP that occurs. Therefore, the contribution of fast Na+ channel current to the AP of ventricular cells progressively increases during development.
The heart greatly enlarges in size during development. Thus, the excitation wave must travel over longer distances in the larger hearts during the late embryonic and adult periods. A fast conduction velocity for excitation is required to allow a synchronized contraction of the ventricle, i.e. to allow the heart to serve as an effective pump. The increase in max dV/dt during development would contribute to the required increase in conduction velocity. Another factor involved in conduction velocity is that the cell size (i.e. diameter) becomes much greater. It is well known that conduction velocity is a function of the square root of cell diameter (see Chapter 18).
The TTX sensitivity of the fast Na+ current in avian cardiomyocytes (in the nanomolar range) is about 1000-fold greater than that for adult mammalian hearts (such as guinea pig), which are in the micromolar range (Sada et al., unpublished observation). It is not known whether the high TTX sensitivity of chick embryonic hearts is due to a different isoform of the channel. This finding is in agreement with previous reports of the high sensitivity of embryonic chick hearts to TTX (Iijima and Pappano, 1979; Marcus and Fozzard, 1981; Fujii et al., 1988).
The fast Na+ channels are completely blocked by 10 μM TTX in fetal rat cardiomyocytes (Conforti et al., 1993) and by 30 μM TTX in adult rat cardiomyocytes (Brown et al., 1981). Binding studies using TTX and saxitoxin (STX, also a specific blocker of the fast Na+ channels) revealed that fetal and newborn rat ventricular myocytes have only low-sensitivity Na+ channels. In contrast, adult rat ventricular myocytes have both high-sensitive and low-sensitive to (TTX/STX) Na+ channels, leading to the discovery of the low-sensitive “cardiac” isoform of the Na+ channel (Nav1.5) (Renaud et al., 1983; Rogart et al., 1989). In mouse heart, high TTX-sensitive Na+ channel isoforms (Nav1.1–Nav1.4) increase with development, although the “cardiac” isoform Na+ channel is always predominantly expressed during development (Haufe et al., 2005; Dominguez et al., 2008).
The fast Na+ current has a slow inactivating or sustained component. This component is small, but has a larger contribution in the embryonic period than in adult (Conforti et al., 1993). Sustained Na+ current, which is blocked by 10 μM TTX, is observed in the early embryonic period of chicks. Reopening of some of the fast Na+ channels during a long depolarizing clamp step is one explanation for the small sustained component (Josephson and Sperelakis, 1989). The sustained component may reflect the window current produced by a balance between the activating (m) gate and the inactivating (h) gate (Sada et al., 1995) (see Chapter 19). In rat heart cells, the fast Na+ current has a slow inactivating component and its time constant decreases in neonatal cells compared to fetal cells (Conforti et al., 1993). Although the slow component of the Na+ current is small, inward current produced by the slow component helps to maintain the longer duration of the AP plateau in the fetal period. TTX, which is specific for fast Na+ channels, shortens the AP duration in rat fetal cardiomyocytes (Fig. 25.1) (Conforti et al., 1993). A key factor that may contribute to the shortening of the AP duration during development of rat heart is the loss of the slow component of the Na+ current. However, in adult hearts, it appears that the sustained component of the Na+ current persists in the Purkinje fiber, because its AP plateau is substantially shortened by TTX (Morikawa et al., 1987). Another factor responsible is an increase in Ito carried primarily by K+ (see Section IIIF).

FIGURE 25.1 Effect of TTX on the Na+ current and the AP configuration recorded from a fetal rat cardiomyocyte. (A) Top panel: superimposed current traces showing the Na+ current recorded before (C, Control ) and after 90-s exposure to 1 and 10 μM TTX. Holding potential (HP) was −97 mV and the test potential was −7 mV. Bottom panel: time course of the TTX effect. Steady-state responses were attained at about 1–2 min. (B) Effect of TTX on the AP configuration of a fetal cell recorded in current-clamp mode. Top panel: superimposed traces (averaged from 10 consecutive records) showing APs before (C, Control) and after a 90-s exposure to 1 μM TTX. Bottom panel: time course of the change in APD50 produced by TTX. Arrows indicate points of introduction and washout (w) of TTX. (Reproduced with permission from Conforti et al., 1993.)
IIID Ca2+ Channels
Changes in the slow (L-type) Ca2+ current also occur during development of the heart. However, the direction of the change is opposite in avian versus mammalian hearts (Fig. 25.2). In rat hearts, the L-type Ca2+ current increases during development (Masuda et al., 1995), whereas in chick hearts it actually decreases (Tohse et al., 1992b). In the chick early embryonic period, the current density of the L-type channels is 8 μA/cm2 which is comparable to that in other adult animals (about 10 μA/cm2). The current density decreased during development to about 5 μA/cm2 in the late embryonic period (Fig. 25.2B). However, the current density of the L-type Ca2+ channels of rat cardiomyocytes increases through the middle fetal, late fetal and neonatal period (Masuda et al., 1995) (Fig. 25.2A). In mouse, the current density of L-type Ca2+ channels increases with development (Davies et al., 1996; Liu et al., 2002; Nguemo et al., 2007). Another investigation demonstrated that the current density in the neonatal period is actually larger than that in adult rat (Cohen and Lederer, 1988). That is, in the development of rat heart, the current density increases, followed by a decrease. In contrast, in rabbit (Osaka and Joyner, 1991; Huang et al., 2006) and guinea pig (Kato et al., 1996) cardiomyocytes, the current density in the neonatal period is smaller than that in adults. Thus, the changes in the L-type Ca2+ channel density that occur during development are complex and vary from one species to another.
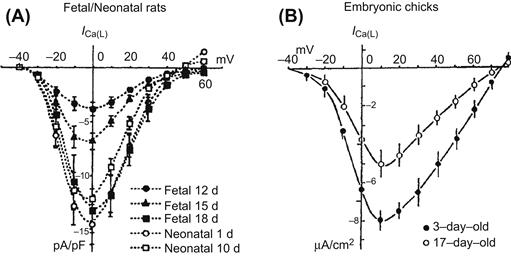
FIGURE 25.2 Developmental changes of ICa(L) in (A) fetal/neonatal rats and (B) embryonic chicks. (A) Ba2+ currents through L-type Ca2+ channels (ICa(L)) were elicited by depolarizing steps from an HP of −40 mV (22°C). Current–voltage curves (normalized as current density (in pA/pF)(mean ± SE)) are shown for the different developmental stages (from day 12 fetal to day 10 neonatal). (B) Changes in the density of ICa(L) in isolated embryonic chick heart cells; 1.8 mM [Ca2+]o, 35°C. ((A) modified with permission from Masuda et al. (1995). Long openings of calcium channels in fetal rat ventricular cardiomyocytes. Pflügers Arch. 429, 595–597, copyright Springer-Verlag. (B) Tohse et al. (1992b). Developmental changes in long-opening behavior of L-type Ca2+ channels in embryonic chick heart cell. Circ. Res. 71, 376–384. Reproduced with permission Circulation Research. Copyright 1992 American Heart Association.)
Other types of Ca2+ channels are also observed during development. In chick embryonic heart cells, it has been reported that the T-type channel is dominant in the early embryonic period, but that the L-type current is dominant in the late embryonic period (Kawano and DeHaan, 1991; Kitchens et al., 2003). However, other reports indicate that the L-type current is also dominant in the early chick embryonic period (Tohse and Sperelakis, 1990; Tohse et al., 1992b). In rat and mouse fetal cardiomyocytes, although L-type current is always dominant, T-type current is observed during the embryonic and postnatal periods and decreases with development (Leuranguer et al., 2000; Ferron et al., 2002; Niwa et al., 2004). However, another report shows that embryonic rat ventricle myocytes have a substantial fraction of the total Ca2+ current that is resistant to nifedipine (a relatively selective blocker of L-type Ca2+ channels) shown in Fig. 25.3 (Tohse et al., 1992a). This nifedipine-resistant current is not blocked by ω-conotoxin (N-type Ca2+ channel-blocker), tetramethrine (T-type Ca2+ channel-blocker) and is only partially inhibited by 30 μM Ni2+, which is a blocker of T-type Ca2+ channels. Therefore, this channel is called a fetal-type (F-type) Ca2+ channel. The F-type Ca2+ current is absent in adult heart cells. That is, in the fetal period, the total Ca2+ current has two main components: L-type current, which is blocked by nifedipine and the F-type current, which is not blocked by nifedipine.
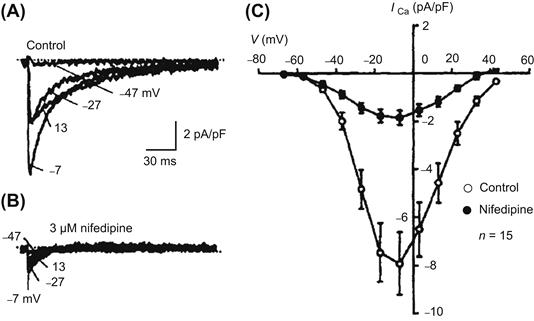
FIGURE 25.3 Presence of fetal-type Ca2+ channels in fetal (18-day) rat cardiomyocytes. (A) Currents elicited by 300-ms (only 150 ms shown) depolarizing pulses to −47, −27, −7 and 13 mV from an HP of −87 mV. (B) In the presence of 3 μM nifedipine, a significant inward current remained at each potential. (C) Current-voltage relationship; data points given as mean ± SE. Nifedipine did not completely block the Ca2+ current, indicating the presence of a nifedipine-resistant Ca2+ current. (Reproduced with permission from Tohse et al., 1992a.)
In chick embryonic heart, unit conductance of the L-type Ca2+ channel is 26 pS (using 50 mM Ba2+ in the pipette), which is comparable to that in adult heart cells (Tohse and Sperelakis, 1990). The single-channel activity of the L-type Ca2+ channel in the embryonic cells was completely blocked by nifedipine.
The kinetics of opening of the L-type Ca2+ channels in embryonic heart cells is different from that in adult heart cells. Long-lasting openings of the channels occur relatively frequently, in addition to the more usual brief bursting openings observed in adult heart cells (Fig. 25.4). These long-lasting openings are similar to the mode 2 openings produced by Ca2+ agonists, such as the dihydropyridine Bay-K-8644. For example, Fig. 25.4 shows long openings that persist over the entire duration of the clamp pulse (i.e. 300 ms); the long openings are sometimes punctuated by brief closures. As can be seen, in many sweeps, the open probability (P0) is close to 1.00. The long-lasting openings gradually disappear during development (Tohse et al., 1992b; Masuda et al., 1995).
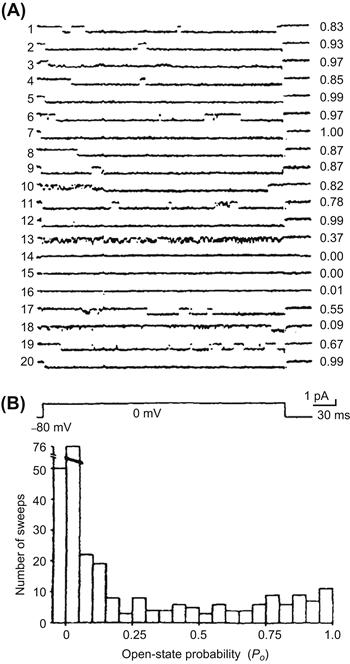
FIGURE 25.4 Presence of long openings of the slow (L-type) Ca2+ channels in young embryonic (3-day) chick heart cell. (A) Single-channel activity elicited by consecutive command pulses to 0 mV (from an HP of −80 mV) every 2 s. Sweep-to-sweep variations of the probability of the channel opening (P0) are given in the right-hand column. (B) A histogram of P0 data from nine cells (30 sweeps each). Note that many sweeps showed long openings and high P0. (Reproduced with permission from Tohse and Sperelakis, 1990.)
IIIE Inward-Rectifier K+ Channels
It has been demonstrated that the inward-rectifier K+ current (IK1 or IK(IR)) of ventricular cells increases markedly during development of rabbit (Huynh et al., 1992; Sanchez-Chapula et al., 1994) and embryonic chick heart (Josephson and Sperelakis, 1990). In rat ventricle, IK1 increases from the embryonic to neonatal period, whereas some gradual decrease (due to an increase in cell membrane capacitance) is evident in myocytes from neonate to adult period (Kilborn and Fedida, 1990; Masuda and Sperelakis, 1993; Xie et al., 1997; Nagashima et al., 2001). In mouse atrial and ventricular myocytes, IK1 substantially increases with development, although it is still controversial whether the current density of IK1 increases or not during the embryonic period (Davies et al., 1996; Trepanier-Boulay et al., 2004; Grandy et al., 2007; Liu et al., 2010).
The increase in IK1 is likely to be a major factor responsible for the increase in the RP (hyperpolarization) that occurs during development, concomitant with a decrease in membrane resistivity and in the membrane time constant (τm = RmCm). The increase in IK1 channels can also account for the decrease in the PNa/PK ratio that occurs during development (Sperelakis and Shigenobu, 1972); namely, it results in an increase in the K+ permeability (PK). Similar change in the PNa/PK ratio during development is shown in the skeletal muscle fibers (see Section IVA).
The increase in IK1 during development may be due to two factors: (1) an increase in the number of channel molecules and (2) an increase in single-channel conductance (Masuda and Sperelakis, 1993). The single-channel conductance in young fetal rats is much less than that in old fetuses and neonates (Fig. 25.5). However, the mean open time of the channels is longer in young fetal cells than in old fetal and neonatal cells. These observations suggest that the structure (i.e. a different isoform) of the inward-rectifier K+ channel changes dramatically during development. On the other hand, a later study (Xie et al., 1997) suggests that the small conductance events in young fetal heart are sublevels of the large conductance channels in old fetal hearts. The developmental changes of the molecular correlate of IK1 (Kir2.1–2.3) were evaluated in rat and mouse heart. During development of the rat embryonic ventricle, the expression of Kir 2.1 and 2.2 mRNA increases, whereas Kir2.3 is not detected (Nagashima et al., 2001). In mouse atrial and ventricular myocytes, it has been demonstrated that the expression of Kir 2.1 and 2.2 increase during development, whereas Kir2.3 decreases (Nakamura et al., 1999; Trepanier-Boulay et al., 2004; Grandy et al., 2007; Liu et al., 2010).
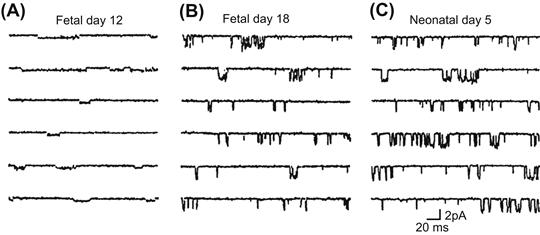
FIGURE 25.5 Developmental increase in IK1 in maturing rats. Single-channel activities illustrated for (A) 12-day fetal, (B) 18-day fetal and (C) 5-day neonatal ventricular myocytes. Single-channel activities were recorded at −80 mV. (Reproduced with permission from Masuda and Sperelakis, 1993.)
IIIF Voltage-Gated K+ Channels
Transient outward current (Ito) density, mainly carried by K+ ion, has been reported to increase during development. A substantial amount of Ito has been observed in early embryonic chick heart cells (Satoh, 1995). In rat ventricular myocytes, the density of Ito was reported to increase with development (Kilborn and Fedida, 1990; Wahler et al., 1994; Kobayashi et al., 2003). In mouse (Wang and Duff, 1997), rabbit (Sanchez-Chapula et al., 1994) and canine ventricular cells (Jeck and Boyden, 1992), a postnatal increase of Ito was also observed. This increase in Ito contributes to the abbreviation of the AP that occurs in the neonatal period.
In the mammalian ventricular myocytes, Ito is composed of at least two components; one is characterized by a relatively fast recovery from inactivation (Ito,f) and another by a slow recovery from inactivation (Ito,s). The molecular correlate of Ito,f is thought to be Kv4.2/4.3 and that of Ito,s is Kv1.4. In rat ventricular myocytes, Kv4.2 protein levels increase during postnatal development, whereas Kv1.4 decreases (Matsubara et al., 1993; Xu et al., 1996). Therefore, the developmental increase in Ito was thought to be associated with the increase in Ito,f. However, there is a mismatch in the expression of Kv4.2 and Ito between postnatal days 5 and 30 (Xu et al., 1996). Recently, it has been demonstrated that the expression of voltage-gated K+ channel-interacting proteins 2 (KChIP2) increases from embryonic day 12 to postnatal day 10, whereas Kv4.2 and Kv4.3 do not increase (Kobayashi et al., 2003). Therefore, KChIP2 is thought to be crucial to induce Ito,f during development.
Between postnatal days 8 and 20, rat plasma thyroid hormone (T3) level increases (Shimoni et al., 1997). T3 stimulation enhanced Kv4.2 and Kv4.3 expression and decreased Kv1.4 transcription, while KChIP2 remained unaffected (Wickenden et al., 1997; Guo et al., 1998; Gassanov et al., 2009). Therefore, it has been suggested that T3 plays a role in postnatal upregulation of Ito,f. In addition, basic fibroblast growth factor (bFGF), which has tyrosine kinase activity, may also promote the expression of Ito during development (Guo et al., 1995).
As Ito in neonatal rat ventricular cells is smaller than that in adult cells, an ultrarapidly activating delayed-rectifier K+ current (IKur) predominates Ito only during the early neonatal period (Guo et al., 1997b). In cultured neonatal rat ventricular cells, IGF-I promotes the expression of IKur as well as Kv1.5 protein (Guo et al., 1997a). IKur in ventricular cells decreases during postnatal development, which is associated with a reduction of the Kv1.5 expression. However, no substantial change in Kv1.5 protein is also reported from neonate to adult in the same preparation (Xu et al., 1996). Recently, it has been demonstrated that co-expression of KChIP2 decreases Kv1.5-encoded K+ currents (Li et al., 2005). Therefore, KChIP2 is thought to be crucial to the decreased IKur during development.
In mouse fetal ventricular cells, a rapidly activating delayed-rectifier K+ current (IKr) is the dominant component of delayed-rectifier IK, whereas a slowly activating delayed-rectifier K+ current (IKs) becomes dominant in early postnatal period (day one to three). However, both components disappear in adult mouse ventricular cells (Davies et al., 1996; Wang et al., 1996). Similarly, IKr in rat ventricular cells functions during the fetal period, but is negligible in adults. The main molecular correlates of IKr are thought to be ERG and MiRP1, whereas those of IKs are KvLQT1 and minK. ERG expression is observed in mouse ventricle in the embryonic period, whereas MiRP1 expression is not observed (Franco et al., 2001). Evaluating the expression of mRNAs of mouse heart during the embryonic and postnatal periods revealed that KvLQT1 decreases during development, whereas minK increases until late embryonic period and then decreases (Mai et al., 2004).
The density of IK (the sum of IKr and IKs) in guinea pig ventricles is smaller in fetal cells than in neonatal and adult cells, suggesting a developmental increase in IK (Kato et al., 1996). No substantial changes in the kinetics and voltage dependency of IK are observed during development.
IIIG Hyperpolarization-Activated Inward Current
The hyperpolarization-activated inward current (Ih or If), which is mainly carried by Na+ and K+ ions, is observed in sinoatrial node and early embryonic cardiomyocytes. In the chick ventricular myocytes, Ih progressively decreased during development and essentially disappears in the late embryonic period (Fig. 25.6) (Satoh and Sperelakis, 1993). In rabbit sinoatrial node, a slope conductance of Ih in neonatal cells is larger than that in the adult cells (Accili et al., 1997). In the rat ventricular myocytes, it is still controversial whether the current density of Ih decreases or not during the postnatal period (Robinson et al., 1997; Cerbai et al., 1999). In the mouse cardiomyocyte, Ih increases at the early embryonic period (between embryonic day 8.5 to 9.5) and then decreases (between embryonic day 9.5 and 18) (Yasui et al., 2001; Stieber et al., 2003).
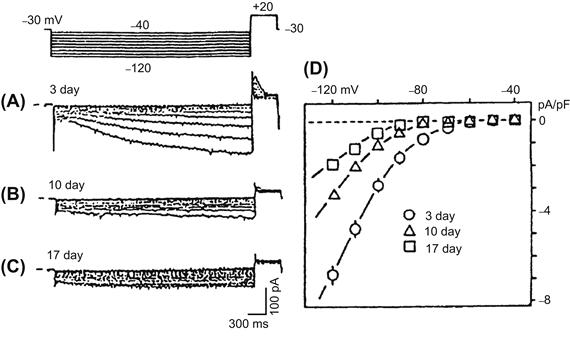
FIGURE 25.6 Developmental changes of the hyperpolarization-activated inward current (Ih) in young embryonic chick ventricular cells. Test pulses were applied between −40 mV and −120 mV, in 10-mV increments, from an HP of −30 mV. (A) A large inward current was slowly activated by hyperpolarization in a 3-day-old cell. (B) Smaller Ih in a 10-day-old cell. (C) Further reduced Ih in a 17-day-old cell. (D) Current-voltage relations for Ih current density at the three developmental stages (mean ± SE). (Reproduced from Satoh, H. and Sperelakis, N. (1993). Hyperpolarization-activated inward current embryonic chick cardiac myocytes: developmental changes and modulation by isoproterenol and carbachol. Eur J Pharmacol, 240, 283–290, Copyright 1993. With permission from Elsevier Science.)
The Ih is called the pacemaker current in adult cardiomyocytes. In Purkinje fibers, Ih plays a key role in pacemaker depolarization during the diastolic phase. In sinoatrial node cells, the contribution of Ih to pacemaker potential is still controversial for two reasons (Irisawa et al., 1993): the time course of activation of Ih is too slow to account for the high frequency of the pacemaker and the threshold potential for activation of Ih (close to −70 mV) is beyond the maximum diastolic potential (−60 to −70 mV) for the nodal cells. In chick embryonic cardiomyocytes, although the time course of decrease in Ih parallels the disappearance of the pacemaker potential, the contribution of Ih to the pacemaking may still be small (Satoh and Sperelakis, 1993).
Recently, it has been demonstrated that the Ih current is mediated by hyperpolarization-activated and cyclic nucleotide-gated (HCN) channels. In the early embryonic period, HCN4 mRNA is highly expressed in mouse ventricle and then the expression decreases with development (Yasui et al., 2001). Mice with a selective deletion of HCN4 in cardiomyocytes die between embryonic days 9.5 and 11.5 (Stieber et al., 2003). In mouse heart, although small Ih current could be detected in HCN4 knockout mice, it has been suggested that the HCN4 channel plays a key role in pacemaker depolarization during the diastolic phase.
IIIH Excitation–Contraction Coupling
Changes in the excitation–contraction coupling process also occur during development of the heart. In particular, the source of Ca2+ for producing contraction is altered during development (Fig. 25.7) (Nakanishi et al., 1988; Kitchens et al., 2003; Seki et al., 2003; Tohse et al., 2004; Kobayashi et al., 2011). In fetal heart cells, the role of the sarcoplasmic reticulum (SR) is none at first and increases with embryonic development. Therefore, most of the Ca2+ required for contraction of embryonic cells is derived from Ca2+ influx through the voltage-dependent Ca2+ channels (L-type and T-type), i.e. originates from the extracellular space. In neonatal heart cells, the SR matures and plays a main role as the source of Ca2+ for contraction. Therefore, the Ca2+-induced Ca2+ release from the SR compartment (Fabiato and Fabiato, 1978) becomes the more important system for contraction. That is, in adult heart cells, most of the Ca2+ for contraction comes from the internal SR stores. However, Ca2+ influx through the sarcolemma remains the determining factor for contractile force, because the Ca2+ influx controls the amount of Ca2+ released.
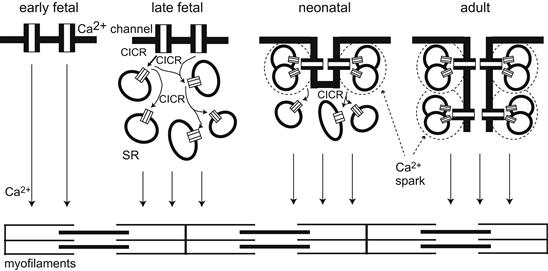
FIGURE 25.7 Schematic model of excitation–contraction (E-C) coupling during heart development. In the early fetal period at which the heart begins to contract, Ca2+ entering the cell through the sarcolemmal Ca2+ channels directly activates the myofilaments. During embryonic development, Ca2+ release from the sarcoplasmic reticulum (SR) through the ryanodine receptor (RyR) increases. This released Ca2+ from SR also stimulated another SR, as shown in “late fetal” panel. In the neonatal period, the formation of the T-tube system starts. A few L-type Ca2+ channels are close to RyRs and can evoke the Ca2+ spark in the periphery of cardiomyocytes. In the center of cardiomyocytes, the slow cascade of CICR still evokes. In the adult period, the T-tube system is established in all areas of cardioymyocytes. Each L-type Ca2+ channel is close to RyRs. The Ca2+ sparks simultaneously evoke in the periphery and centers of cells, producing fast and large Ca2+ transient in adulthood. (Reproduced in modified form, with permission, from Tohse et al., 2004.)
IV Skeletal Muscle Fibers
IVA Resting Potential and Action Potential
In skeletal muscle fibers, the RP also increases during development and differentiation from the individual myoblast stage to the multinucleated myotube stage to the mature fiber. (The myoblasts fuse end to end to form the myotubes which get progressively larger in length and diameter.) For example, the RP of cultured chick embryonic skeletal myocytes is low in the immature stage (short mononucleated myoblasts) and is dramatically hyperpolarized during differentiation (to long myotubes or mature fibers) (Fig. 25.8A) (Fischbach et al., 1971; Spector and Prives, 1977). The mature fibers in older cultures consist of multinucleated myotubes with cross-striations (i.e. aligned myofibrils). In the immature myoblasts, the RP generally is about −40 mV. However, the RP of the maturing myotubes is about −60 mV, which is approximately equal to that of adult skeletal muscles bathed in the same culture medium (Fig. 25.8A). A similar change in the RP occurs in rat skeletal myoblasts/myotubes during development (Ritchie and Fambrough, 1975). A progressive decrease in the PNa/PK ratio, which sets the RP closer to the equilibrium potential for K+ (EK), accounts for the developmental changes in the RP (Fig. 25.8B). The family of curves shown in Fig. 25.8B is similar to that reported in developing chick heart (Sperelakis and Shigenobu, 1972).
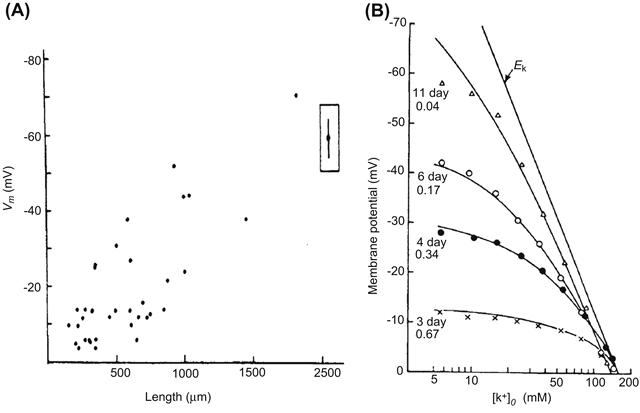
FIGURE 25.8 Developmental changes of resting potential in the skeletal myoblasts/myotubes from (A) embryonic chick and (B) fetal rat. (A) The relation between the RP and length of the chick skeletal myoblasts/myotubes in culture. The filled circle enclosed within the box is the mean (± 2SE) resting potential of 20 myotubes. (B) The relationship between the RP and external K+ ion concentration ([K+]o) for rat skeletal myotubes of different periods in culture. The Nernst equilibrium potential for K+ is indicated by the straight line labeled EK. The solid line for each myotube is the theoretical curve predicted by the Goldman equation for the PNa/PK ratios given at the left and the extrapolated [K+]i value. (Reproduced with permission from (A) Fischbach et al. (1971). J. Cell. Physiol. 78, 289–300. Copyright 1971 John Wiley & Sons, Inc. Reprinted by permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc. and (B) Ritchie and Fambrough, 1975. Reproduced from The Journal of General Physiology, by copyright permission of The Rockefeller University Press.)
In human skeletal muscle (from biopsy), the RP increases (hyperpolarization) during culture. Fetal and postnatal myocytes exhibit RPs of about −8 to −35 mV in the early period of culture and then hyperpolarize to about −50 to −74 mV in later culture (Iannaccone et al., 1987; Liu et al., 1998, 2003). This hyperpolarization, which is due to the sequential expression of IK and IK1, induces an increase in intracellular Ca2+ concentration, which is essential for myoblast differentiation and fusion to occur (Bijlenga et al., 2000; Fischer-Lougheed et al., 2001; Konig et al., 2006).
The AP configuration of skeletal muscle fibers also changes during development and differentiation. For example, in one study of chick embryonic myotubes, prolonged APs that persist for more than 500 ms, with prolonged contraction, were exhibited on day 5 in culture (Spector and Prives, 1977). By culture day 7, the myotubes exhibited primarily brief APs (less than 10 ms) and brief twitch contraction. (On day 5, small regions of the myotubes sometimes displayed brief APs with localized twitches in those regions.) Thus, the brief AP becomes more dominant during development that proceeds in culture.
A second study on cultured chick skeletal myoblast/myotubes is depicted in Fig. 25.9. As can be seen, at the early stage, the AP is small and does not overshoot (the zero membrane potential level) (Fig. 25.9A, upper record). In the myotubes formed by fusion of myoblasts after a few more days in culture (day 7 and 11), the amplitude and maximum rate of the spikelike APs increased markedly during development while in culture (Fig. 25.9A, middle and lower records, and Fig. 25.9B). A plot of max dV/dt versus the number of days in culture is given in Fig. 25.9B. Since the APs were blocked by TTX in almost all myotubes tested (Fig. 25.9A, right side, and Fig. 25.9B), differentiation of the spike-like APs is due to progressively increased intensity of inward current through fast Na+ channels (Kano and Yamamoto, 1977). That is, an increase in the number (density) of fast Na+ channels allows a regenerative AP to be produced, and the maximum rate of rise to become faster and faster.
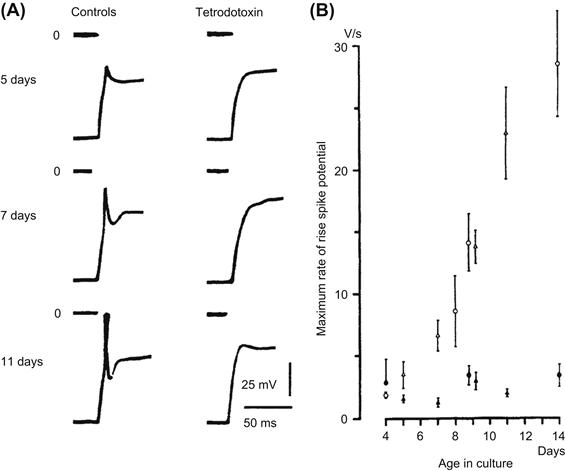
FIGURE 25.9 Spike-like APs and their maximum rate of rise in maturing skeletal myotubes from chick embryo. (A) Responses of three myotubes at different ages in culture: 5 days (upper), 7 days (middle) and 11 days (lower). Each pair of records is taken from the same myotube before (left) and after (right) application of TTX (10−7 M). Depolarizing current pulses were applied after the membrane potential was hyperpolarized to a standard level of −80 mV. The zero potential level is indicated. (B) Maximum rate of rise of spike potentials as a function of the age in culture (mean ± SE). Circles and triangles represent two different batches of culture; filled symbol indicates presence of TTX. (From Kano and Yamamoto (1977). Development of spike potentials in skeletal muscle cells differentiated in vitro from chick embryo. J. Cell. Physiol. 90, 439–444. Copyright © 1977 Journal of Cellular Physiology. Reprinted by permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.)
In myocytes of a marine tunicate (ascidian), AP duration abbreviates during the embryonic period (Greaves et al., 1996). There is a progressive decrease in AP duration from the middle embryonic to the late embryonic period and a corresponding increase in the rate of rise and fall of the AP. Spontaneous firing of APs is observed in most ascidian myocytes in the middle embryonic period. The automaticity progressively disappears during development.
IVB Inward-Rectifier K+ Channels
The RP of maturing myotubes gets closer to EK because the PNa/PK ratio is gradually decreased during development (see Fig. 25.8B). These characteristics are also observed in chick embryonic cardiomyocytes during development (Sperelakis and Shigenobu, 1972) and are produced by marked expression of the inward-rectifier K+ channels in the surface membrane of the myocytes (Shin et al., 1997). Therefore, it seems likely that the hyperpolarization of the RP of skeletal myocytes in culture is produced by a similar developmental change of inward-rectifier K+ channels.
The IK1 is present in skeletal muscle cells from early embryonic amphibian (Linsdell and Moody, 1995). Although there is a brief period (approximately 4 h) during which its density decreases, the overall trend is an increase during development.
In ascidian myocytes, IK1 exhibited dramatic changes during development (Fig. 25.10C) (Greaves et al., 1996). In the ascidian, the inward-rectifier K+ current is gained after fertilization of the egg. (The same change also occurs in other species.) When gastrulation ends (at 16 h after fertilization), the current density suddenly decreases from 4 to 0.5 pA/pF. After the tailbud stage (22 h after fertilization), the current density progressively increases again and reaches a value of 5 pA/pF before hatching. Because IK1 is one of the most important resting conductances (which stabilizes and helps to set the RP), this transient decrease and subsequent increase in the current density parallels the generation of spontaneous APs.
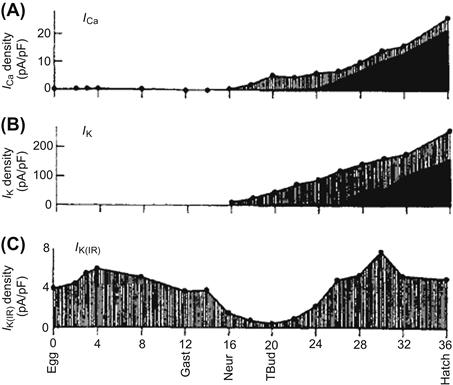
FIGURE 25.10 Development of Ca2+ and K+ currents in skeletal muscle of a marine tunicate (ascidian). The plots start at fertilization (0 h). (A) Total Ca2+ current density (filled circles), with inactivating (hatched) and sustained (solid) components. (B) Total K+ current density (filled circles), with voltage-dependent (hatched) and Ca2+-dependent (solid) components. (C) Inward-rectifier K+ current (IK1 or IK(IR)) density. Gast, Neur and Tbud indicate the stages of gastrula, neurula and tailbud, respectively. (Modified with permission from Greaves et al., 1996.)
Evaluating the human myoblast fusion revealed that the IK1 increases during differentiation (Liu et al., 1998). In addition, inhibition of Kir 2.1 expression with an antisense-Kir2.1-RNA reduced the endogenous IK1 and blocked fusion (Fischer-Lougheed et al., 2001). Therefore, it seems likely that the increase in IK1 is required for human muscle differentiation.
IVC Delayed-Rectifier K+ Channels
The delayed-rectifier K+ current (IK) in skeletal myocytes of early embryonic amphibian progressively increases during development in culture (Linsdell and Moody, 1995). A similar increase in IK occurs in ascidian myocytes after the neurula stage (16 h postfertilization) (see Fig. 25.10B) (Greaves et al., 1996). However, IK(Ca) progressively increases after 26 h postfertilization. The increase in IK(Ca) closely parallels the increase in sustained L-type Ca2+ current (see Figs. 25.10A,B). These developmental changes in K+ channels contribute to the abbreviation of AP duration during the late developmental period.
Evaluating the human myoblast fusion revealed that IK increases during differentiation (Bijlenga et al., 1998; Fischer-Lougheed et al., 2001). It seems likely that IK contributes to the RP, because the cells were depolarized by 5 μM dofetilide, a class III antiarrhythmic agent known selectively to inhibit ERG channels. However, it remains unknown whether IK is a prerequisite for myoblast fusion to occur, because the inhibition of IK accelerates the rate of human myoblast fusion (Liu et al., 2003). Further experiments are necessary to reveal the precise roles of IK during differentiation.
IVD Ca2+ Channels and Na+ Channels
High-voltage-activated Ca2+ channels have also been observed in the ascidian embryo (see Fig. 25.10A) (Greaves et al., 1996). These Ca2+ channels exhibit inactivation and may be an N-type Ca2+ channel because the current is blocked by conotoxin. The channels increase after the neurula stage (16 h after fertilization). After the tailbud stage, sustained Ca2+ channel activity (probably L-type) begins to increase and dominates at the time of hatching. Low voltage-activating, rapidly inactivating Ca2+ channels (T-type) are detected in about 50% of the cells at each stage of development. The contribution of the T-type channels to total Ca2+ influx is relatively small in comparison with L-type and N-type channels.
In early embryonic amphibian skeletal myocytes, substantial Ca2+ current and Na+ current appeared almost at the same time (after 10–12 h in culture) (Linsdell and Moody, 1995). These channel currents continued to increase steadily over an observation period of 10–28 h in culture.
In mouse skeletal muscle, L-type Ca2+ current increases during embryonic development, whereas T-type Ca2+ current increases until embryonic day 16 followed by a decrease until birth (Shimahara and Bournaud, 1991; Strube et al., 2000; Berthier et al., 2002). It seems likely that this developmental change of T-type Ca2+ current is involved in the early stages of muscle differentiation via the increase in the intracellular Ca2+ concentration, because the inhibition of T-type Ca2+ current by 100 μM amiloride, 200 μM Ni2+ or antisense oligonucleotides directed against the α1H subunit suppresses fusion of myoblasts (Bijlenga et al., 2000).
IVE Acetylcholine Receptor/Channel
The nicotinic acetylcholine receptor/channel (nAChR) is essential to transmission at the neuromuscular junction (see chapter on synaptic transmission). During development, the nAChR channels in embryonic muscles are converted to adult-type nAChR channels around the time of birth (Mishina et al., 1986). The fetal nAChR channel is composed of α-, β-, γ- and δ-subunits and, in the adult channel, the γ-subunit is substituted by an ε-subunit. In functional characteristics, the fetal channel exhibits a low conductance and long openings compared with those of the adult channel. This conversion of the nAChR channel may be related to innervation of the muscles that occurs during development, because the substitution of the fetal-type nAChR channels with an adult-type nAChR channels substantially alters the innervation pattern of mouse muscle by the motor nerve (Koenen et al., 2005).
In the early embryonic period, nAChR channels are present at a moderate level throughout the myotube surface. In adult muscle, nAChR channels are highly concentrated in the neuromuscular junction (Sanes and Lichtman, 2001). A recent study has revealed the coordinated activities of Wnt3 and agrin, which are both secreted by motoneurons, in clustering nAChR channels at the neuromuscular junction (Henriquez et al., 2008).
IVF Regulation of Expression of Ion Channels
It has been well analyzed that several humoral factors regulate expression of ion channels during development. Activin, a member of the TGFβ family, has proved to be a particularly potent inducing agent to form different mesodermal cell types from animal cap cells. In vitro induction of animal cap Xenopus cells under culturing with activin triggers a whole cascade of developmental events, resulting in the differentiation of skeletal muscle (Currie and Moody, 1999). The developmental pattern of ion channel expression in muscle induced in vitro by activin is close to that of normal muscle. First, the same currents (IK, IK1, INa, IA, ICa) are expressed over a similar time course during differentiation. The sequence in which the currents are expressed is also maintained, with IK and IK1 being expressed first, followed by INa, IA and ICa at slightly later stages. This study indicates that activin is very important for development of electrical activity in skeletal muscle.
V Neurons
VA Action Potential
The ionic dependence of the neuronal AP is altered during the early stages of embryonic development (Spitzer and Baccaglini, 1976; Spitzer et al., 1994). Initially, the AP exhibits a prominent Ca2+ dependence (i.e. Ca2+-dependent AP) and its duration is prolonged. Later in development, the AP duration becomes brief and most of the inward current during the depolarizing phase is carried by Na+ (i.e. Na+-dependent AP). The Na+-dependent APs continue until maturation of the neuron. For example, in embryonic amphibian neurons in vivo, the AP is prolonged and the rate of rise is slow at a relatively early stage (Fig. 25.11A). Removal of Na+ ion does not affect the AP configuration at this stage, whereas it is almost abolished by Co2+ ion, an inorganic blocker of voltage-dependent Ca2+ channels. In the late embryonic stage, the AP becomes greatly abbreviated and loses the shoulder on its falling phase (Fig. 25.11B). This AP is completely blocked by removal of Na+ ion (or by TTX), but is unaffected by Co2+ (Spitzer and Baccaglini, 1976).
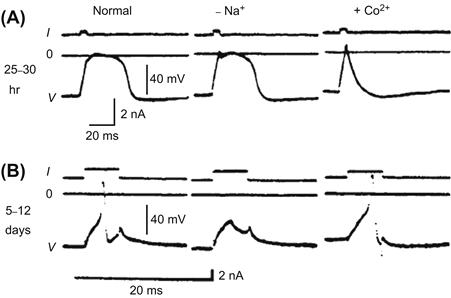
FIGURE 25.11 Developmental changes in APs of embryonic amphibian neurons at two different stages: (A) 25–30 h and (B) 5–12 days after fertilization of the egg. Depolarizing current (I) is applied to evoke the AP (V). The zero potential is shown by a solid line (0). (A) The AP is of long duration and the rate of rise is slow at this early stage. This AP is little affected by removal of Na+, but is abolished by Co2+. (B) The AP at this late stage is brief and its amplitude is large. This AP is blocked by removal of Na+ and is unaffected by Co2+. (Modified from Spizer, N.C. and Baccaglini, P.I. (1976). Development of the action potential in embryo amphibian neurons in vivo, Brain Res. 107, 610–616, Copyright 1976, with permission from Elsevier Science.)
In rat spinal motoneurons at embryonic day 16, the AP upstroke may be dependent upon Na+ ion, although maximum rate of rise of the AP is slow (about 20 V/s) (Gao and Ziskind-Conhaim, 1998). Duration of the AP at day 16 is prolonged (about 10 ms). At postnatal day 3, the AP upstroke is very fast (about 70 V/s) and duration of the AP is very short (2–3 ms).
In mouse cortical neurons at embryonic day 14, the AP duration is prolonged. During development, the AP duration becomes brief. This developmental change is mainly due to the increase in Na+ current density, while outward K+ current density remains almost unchanged (Picken Bahrey and Moody, 2003).
VB Ca2+ Transient
Spontaneous transient elevation of intracellular Ca2+ is observed in developing neurons. The spontaneous Ca2+ transient (recorded by use of fluorescent dyes, e.g. FURA-2, fluo-3, indo-1, etc.) is exclusively dependent on Ca2+ influx because it is abolished either by removal of extracellular Ca2+ or by agents that block Ca2+ channels (Holliday and Spitzer, 1990; Spitzer, 1994). Therefore, the observed intracellular Ca2+ transient is due to Ca2+ influx from the extracellular space.
Two classes of spontaneous Ca2+ transients have been detected: rapid events, termed Ca2+ spikes (Gu et al., 1994) and slow events, termed Ca2+ waves. The incidence of these Ca2+ transients changes during development in culture. Ca2+ spikes in the cell body (soma) are triggered by spontaneous APs and are rapidly propagated to the growth cone. Ca2+ spikes also use the intracellular Ca2+ store because depletion of the store with caffeine substantially reduces their amplitude. Ca2+ spikes may be required for the normal appearance of the transmitter γ-aminobutyric acid (GABA), since blocking of Ca2+ spikes by a Ca2+ channel-blocker prevents the acquisition of GABA immunoreactivity (Spitzer et al., 1993). The normal developmental increase in the activation kinetics of K+ currents is also prevented by the blocking of Ca2+ spikes.
Ca2+ waves occur often (about 10/h) in the growth cone and they are not generally propagated to the soma. The Ca2+ waves in the soma occur at a lower frequency (about 2/h). Therefore, the Ca2+ waves are local and occur independently in separate growth cones of the same neuron. Because there seems to be some relation between external Ca2+ and the length of the neurite, Ca2+ waves in growth cones are likely to regulate neurite extension (Gu et al., 1994; Spitzer, 1994; Spitzer et al., 1994).
The recent evaluation of Ca2+ signaling in gastrula stage embryos revealed that not only Ca2+ transients (Ca2+ spikes and Ca2+ waves) but also a slow increase in [Ca2+]i was observed during early gastrulation of Xenopus and zebrafish (Gilland et al., 1999; Leclerc et al., 2000; Webb et al., 2005). Inhibition of these Ca2+ signalings decreases the expression of neuralising genes, Zic3 and geminin, and proto-oncogene, c-fos and FOS-related protein (Leclerc et al., 1999, 2000). Furthermore, the activators of L-type Ca2+ channel (ConA and Bay-K-8644) trigger neural induction (Moreau et al., 1994). Although the precise downstream targets of the Ca2+ signaling cascades remain to be elucidated, Ca2+ might be a key central regulator in the process of neural induction (Webb et al., 2005).
VC Voltage-Gated Ion Channels
In mature excitable cell membranes, the major inward currents consist of two ions, Na+ and Ca2+, which are carried through voltage-gated Na+ channels and Ca2+ channels, respectively. Na+ and Ca2+ channels exhibit two patterns of development (Gottmann et al., 1988; O’Dowd et al., 1988). In the first pattern, Ca2+ channels appear earlier and develop faster than Na+ channels. This explains, at least in part, the conversion of the Ca2+-dependent AP to the Na+-dependent one discussed earlier.
In the second pattern, Ca2+ channels and Na+ channels become expressed almost at the same time. For example, in cultured embryonic amphibian neurons, Ca2+ currents are large even at the early stage of development and the peak current density does not change from the early stage to the late stage (Fig. 25.12A). Na+ currents are also present, but the current density is small at the early stage. The peak density of the Na+ current approximately doubles between the early and late stages of development in culture (Fig. 25.12B).
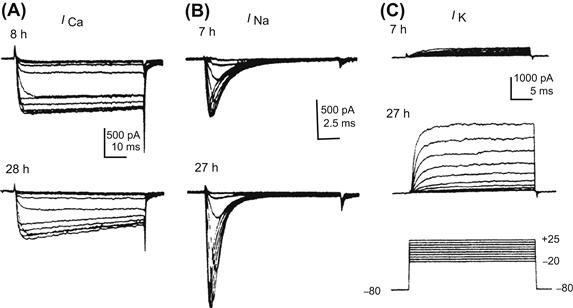
FIGURE 25.12 Developmental changes in ionic currents of cultured maturing neurons of embryonic amphibian. Records from cells in the early stage (7–8 h in culture)(upper traces) and the late stage (27–28 h)(lower traces). Records in (A) and (B) were obtained in presence of Cs+ (internal solution) and TEA (external solution) to block outward K+ currents. (A) Ca2+ current (ICa) recorded in Na+-free solution. The amplitude of ICa was nearly equal at early and late stages. (B) Na+ current (INa) recorded in presence of Co2+ to ICa block. INa was smaller at the early stage than at the late stage. (C) K+ current (IK) recorded in the presence of Co2+ and TTX to block the inward currents (ICa and INa). IK is small at the early stage and gets larger in amplitude and faster in rate of activation at the late stage. Pulse protocols for (A), (B) and (C) are given at the bottom of (C). (Modified with permission from O’Dowd et al. (1988). Development of voltage-dependent calcium, sodium and potassium currents in Xenopus spinal neuron. J. Neurosci. 8, 792–805, Copyright 1988 by the Society for Neuroscience.)
In ascidian embryos, Na+ channels in neural cells dramatically change during the early developmental stage (Takahashi and Okamura, 1998). In unfertilized eggs, a small amount of Na+ current (type A) is already observed. However, the type A Na+ current is almost abolished after fertilization. After fertilization, fast-activating and inactivating Na+ current (type C) with slow-inactivating Na+ current (type B) is markedly increased. However, in the blastomere, which develops into neurons, the type B current is abolished in a moment. Finally, the type C current, corresponding to neural-specific TuNa I gene, increases further and becomes responsible for the neural Na+ spike.
Patch-clamp experiments with acute sliced preparation of brain revealed that Na+ current densities of neurons increase during development of mouse cortical plate of neocortex (Picken Bahrey and Moody, 2003), mouse cerebellum (Fry, 2006) and rat spine (Gao and Ziskind-Conhaim, 1998). On the other hand, Na+ current densities remain almost unchanged in the intermediate zone of mouse neocortex (Picken Bahrey and Moody, 2003) and layers III–V of rat neocortex (Wang et al., 2009).
As described earlier, the Ca2+ channel allows Ca2+ influx and is responsible for the spontaneous Ca2+ transient in developing neurons. However, regulation of Ca2+ influx during development is not always due to change in the density of the Ca2+ channels, because some neurons show no increase in the Ca2+ current density (Salgado et al., 2005). The ratio of the K+ current to the Ca2+ current (IK/ICa) affects the configuration of the APs, because the inward ICa is deactivated earlier when the outward IK is augmented, resulting in a decrease in the net inward current. In other words, any outward (K+) current subtracts from the inward (Ca2+) current to give a lower net inward current. In addition, the change in the amplitude and the kinetics of IK that occurs during development greatly affects the AP configuration and apparent ionic dependence (Barish, 1986; Lockery and Spitzer, 1992). Therefore, the IK/ICa ratio determines whether Ca2+-independent APs are exhibited as well as influences the Ca2+ influx.
It is well known that Ca2+ channels are classified by their threshold voltage (low-voltage activated, LVA; high-voltage activated, HVA) or specific blockers (T-, L- and N-type). In rat cortical neurons of neocortex and phrenic motoneurons of cervical spine, the major Ca2+ channel subtype shifts from the LVA (T-type) Ca2+ current to the HVA Ca2+ current during development (Tarasenko et al., 1998; Martin-Caraballo and Greer, 2001). In mouse brainstem neurons, the density of LVA Ca2+ currents increases during the first postnatal week, while the density of HVA Ca2+ currents increases after the first postnatal week (Zhang et al., 1999).
The developmental increase in K+ currents and change in their activation kinetics have been investigated in amphibian neurons in intact (isolated) spinal cord (Desarmenien et al., 1993) and in dissociated cell culture (see Fig. 25.12C) (Barish, 1986; O’Dowd et al., 1988). The general rule regarding the order of appearance of ionic conductances in developing neurons is that K+ currents appear first before functional expression of the voltage-dependent inward currents (Spitzer et al., 1994). However, the types of K+ currents regulating the AP duration and the order of their maturation vary among different neuronal cells at early stages of development. In some neurons, delayed-rectifier K+ current precedes the appearance of the transient outward K+ current (IA or Ito). In contrast, in other neurons, the inactivating K+ current (IA) precedes expression of the delayed-rectifier K+ current (Bader et al., 1985; Aguayo, 1989; Beck et al., 1992).
The Ca2+-activated K+ channels play a pivotal role for afterhyperpolarization and regulation of repetitive firing in neurons. In rat spinal motoneurons, the Ca2+-activated K+ current is markedly increased from the embryonic period to the postnatal period (Gao and Ziskind-Conhaim, 1998). The increase in this current shortens the duration of the action potential and produces the afterhyperpolarization. In addition, the Ca2+-activated K+ current reduces the frequency of action potential repetitive firing after birth. In rat Purkinje cells of cerebellum, the large-conductance Ca2+-activated K+ channels (BK) increase during development, whereas the small-conductance Ca2+-activated K+ channels (SK) decrease (Muller et al., 1998; Muller and Yool, 1998; Cingolani et al., 2002).
VD Ligand-Gated Channels
Ionotropic glutamate receptors (iGluRs) are heteromeric ligand-gated ion channels. Based on their pharmacological and electrophysiological properties, iGluRs can be subdivided into three families: AMPA (α-amino-3-hydroxymethyl-4-isoxazolepropionic acid) receptor; NMDA (N-methyl-D-aspartate) receptor and KA (kinate) receptor.
In Purkinje and granule cells of cerebellum, NMDA receptors are expressed transiently during early stages of development, resulting in the increase in intracellular Ca2+ which could lead to neuronal differentiation (Garthwaite et al., 1987).
AMPA receptors in immature neurons produce Ca2+ influx and elevation of intracellular Ca2+. The Ca2+ permeability of iGluRs is governed by their subunit composition as well as by single amino acid substitutions generated by RNA editing; both factors may be developmentally regulated (Hume et al., 1991; Burnashev et al., 1992). It has been reported that AMPA receptors express in the early stage of differentiation of Xenopus spinal neurons. Ca2+ influx to regulate the neural differentiation mainly flows through AMPA receptors soon after neurite initiation and before expression of NMDA receptors (Gleason and Spitzer, 1998).
The evaluation of long-term potentiation (LTP) in hippocampus revealed an increase in the ratio of current carried by AMPA receptors relative to NMDA receptors during development (Hsia et al., 1998; Abrahamsson et al., 2008). It may be presumed that such developmental change is due to, not only the developmental change in the expression of iGluRs subunits, but also to the change of molecules that associate/regulate iGluRs subunits (Petralia et al., 2005; Hall and Ghosh, 2008).
VI Concluding Remarks
Action potentials (APs) and resting potentials (RPs) in excitable cells, such as cardiomyocytes, skeletal muscle fibers and neurons, are greatly altered during development. In general, the RP increases (becomes more negative), the AP rate of rise increases, overshoot increases and duration decreases. These electrophysiological alterations are mainly produced by developmental changes in the ion channels, i.e. by changes in the types, number and kinetic properties of the ion channels.
In cardiomyocytes, the density of inward-rectifier K+ currents (IK1) usually increases during development. This increase may result from changes in the single-channel conductance, as well as in the number and open probability of the channel, because the conductance of early fetal myocytes is much smaller than those of neonatal myocytes. The hyperpolarization of the RP during development can be accounted for by the increase in the density of IK1 and the resultant decrease in the PNa/PK ratio. In contrast, the hyperpolarization-activated inward current (Ih), which may affect automaticity, is dominant in early embryonic chick and mouse cardiomyocytes and disappears during development.
The Na+ current also increases markedly during development. That is, there are few or no functional fast Na+ channels present at the earliest stages, and the density of these channels increases progressively during development. The fast Na+ current is responsible for the increase in the AP rate of rise (independent of the hyperpolarization of RP). The max dV/dt increases dramatically during development, e.g. in chick heart, from 20 to 200 V/s.
The fast Na+ current in embryonic/fetal hearts has a significant sustained (i.e. slow-inactivating or steady-state) component. The sustained component of the embryonic/fetal Na+ current decreases during development and this decrease contributes, at least in part, to the abbreviation of the AP duration.
Development of Ca2+ channels seems more complex. The density of total Ca2+ current in chick cardiomyocytes decreases during the developmental period from fetal to neonate. In rat and mouse, however, it increases from the fetal to the neonatal period, followed by a substantial decrease in the adult. In rabbit and guinea pig cardiomyocytes, the current density in the neonatal period is smaller than that in the adult. The total Ca2+ current is composed of currents through several different types of channels: L-type, T-type, and F-type. (1) The proportion of the T-type Ca2+ channel current in immature cells is generally more than that in mature cells and it may actually disappear in adults. That is, the L-type Ca2+ channel current becomes more dominant in mature cells. (2) A nifedipine-resistant F-type Ca2+ channel current is also present in early fetal cardiomyocytes of rats. (3) Long-lasting openings of the L-type Ca2+ channels are relatively frequently observed in embryonic chick and fetal rat cardiomyocytes, but are quite unusual in adult cells.
Ca2+ influx through the Ca2+ channels is especially important for the excitation–contraction coupling process of fetal cardiomyocytes. This is because the SR function and/or the essential component for Ca2+ release from the SR is immature and so Ca2+ influx from the extracellular space is the main source of Ca2+ for contraction.
The density of the transient outward current (Ito) increases during development. An increase in the density of the delayed-rectifier K+ current (IK) also occurs during early development and decreases during the postnatal period. The change of these voltage-gated outward currents (i.e. Ito and IK) helps to abbreviate the AP duration during development.
In skeletal muscle fibers, there is an overall trend toward an increase in the density of IK1 during the early developmental period. However, there is a transient period during which the current density decreases. This transient period parallels the increased incidence of spontaneous firings, presumably due to a less stable RP. The major voltage-gated inward currents, the Na+ and Ca2+ currents, are already present in the early embryonic skeletal myocytes in culture and they increase in intensity during development. These changes contribute to the increases in the AP rate of rise, overshoot and propagation velocity. Prolonged APs and brief APs can be elicited in chick embryonic skeletal myotubes, depending on the stage of development. The prolonged APs are associated with long-lasting contractions of the myotubes. The brief APs become more dominant during development in culture and are associated with myotube twitches. The nicotinic acetylcholine receptor/channel, which is essential to transmission at the neuromuscular junction, is converted from fetal-type to adult-type and this conversion may be related to innervation of the muscles that occurs during development. Activin, a member of the TGFβ family, is important for development of ion channels in skeletal muscles. Activin stimulates the expression of the same ion channels (IK, IK1, INa, IA, ICa) in cultured skeletal myoblasts. The sequence in which currents are expressed is also maintained (IK and IK1 are expressed first).
In neuronal cells, there is an overall trend that the ionic dependence of the AP is altered from Ca2+-dependent (prolonged AP duration) to Na+-dependent (brief AP duration) during development. The pattern of ion channel development varies among different types of neuronal cells, with faster development of Ca2+ channels in some cells. Another important factor that determines the ionic dependence of the AP is the developmental increase in IK. Therefore, the IK/ICa ratio is the major determinant of the conversion of the AP configuration and influences Ca2+ influx during development. Two types of Ca2+ transients, Ca2+ spikes and Ca2+ waves, are present in developing neurons. Ca2+ spikes in the cell body (soma) are generated by spontaneous APs and propagate to the growth cone. The incidence of these Ca2+ transients changes in developing neurons in culture. In addition to Ca2+ transients, a slow increase in [Ca2+]i is observed during neural induction. The activities of ligand-gated Ca2+-permeable channels (such as NMDA receptor/channels and AMPA-gated receptor channels) are also altered during development. Therefore, the Ca2+ influx through the voltage-gated (LVA or HVA channels) and the ligand-gated Ca2+ channels, and the subsequent effects on intracellular Ca2+, may affect the structural changes of the developing neurons and help the establishment of the neuronal network.
As described earlier, ion channels exhibit dramatic changes during development in their type, structure, function and distribution. These dynamic alterations are controlled by expression of genes coding ion channels and may be essential to cellular growth and differentiation by affecting intracellular Ca2+ concentration and excitability. Thus, molecular biology, combined with electrophysiology, has enabled large advances in our understanding of cell function.
BIBLIOGRAPHY
1. Abrahamsson T, Gustafsson B, Hanse E. AMPA silencing is a prerequisite for developmental long-term potentiation in the hippocampal CA1 region. J Neurophysiol. 2008;100:2605–2614.
2. Accili EA, Robinson RB, DiFrancesco D. Properties and modulation of If in newborn versus adult cardiac SA node. Am J Physiol. 1997;272:H1549–H1552.
3. Aguayo LG. Post-natal development of K+ currents studied in isolated rat pineal cells. J Physiol. 1989;414:283–300.
4. Bader CR, Bertrand D, Dupin E. Voltage-dependent potassium currents in developing neurones from quail mesencephalic neural crest. J Physiol. 1985;366:129–151.
5. Barish ME. Differentiation of voltage-gated potassium current and modulation of excitability in cultured amphibian spinal neurones. J Physiol. 1986;375:229–250.
6. Baruscotti M, DiFrancesco D, Robinson RB. A TTX-sensitive inward sodium current contributes to spontaneous activity in newborn rabbit sino-atrial node cells. J Physiol. 1996;492:21–30.
7. Beck H, Ficker E, Heinemann U. Properties of two voltage-activated potassium currents in acutely isolated juvenile rat dentate gyrus granule cells. J Neurophysiol. 1992;68:2086–2099.
8. Bernard C. Establishment of Ionic Permeabilities of the Myocardial Membrane during Embryonic Development of the Rat Developmental and Physiological Correlates of Cardiac Muscle. New York: L.M. and S. T. Raven Press; 1975.
9. Berthier C, Monteil A, Lory P, Strube C. Alpha(1H) mRNA in single skeletal muscle fibres accounts for T-type calcium current transient expression during fetal development in mice. J Physiol. 2002;539:681–691.
10. Bijlenga P, Liu JH, Espinos E, et al. T-type alpha 1H Ca2+ channels are involved in Ca2+ signaling during terminal differentiation (fusion) of human myoblasts. Proc Natl Acad Sci USA. 2000;97:7627–7632.
11. Bijlenga P, Occhiodoro T, Liu JH, et al. An ether -a-go-go K+ current, Ih-eag, contributes to the hyperpolarization of human fusion-competent myoblasts. J Physiol. 1998;512:317–323.
12. Brown AM, Lee KS, Powell T. Sodium current in single rat heart muscle cells. J Physiol. 1981;318:479–500.
13. Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198.
14. Cerbai E, Pino R, Sartiani L, Mugelli A. Influence of postnatal-development on I(f) occurrence and properties in neonatal rat ventricular myocytes. Cardiovasc Res. 1999;42:416–423.
15. Cingolani LA, Gymnopoulos M, Boccaccio A, Stocker M, Pedarzani P. Developmental regulation of small-conductance Ca2+-activated K+ channel expression and function in rat Purkinje neurons. J Neurosci. 2002;22:4456–4467.
16. Cohen NM, Lederer WJ. Changes in the calcium current of rat heart ventricular myocytes during development. J Physiol. 1988;406:115–146.
17. Conforti L, Tohse N, Sperelakis N. Tetrodotoxin-sensitive sodium current in rat fetal ventricular myocytes – contribution to the plateau phase of action potential. J Mol Cell Cardiol. 1993;25:159–173.
18. Couch JR, West TC, Hoff HE. Development of the action potential of the prenatal rat heart. Circ Res. 1969;24:19–31.
19. Currie DA, Moody WJ. Time course of ion channel development in Xenopus muscle induced in vitro by activin. Dev Biol. 1999;209:40–51.
20. Davies MP, An RH, Doevendans P, et al. Developmental changes in ionic channel activity in the embryonic murine heart. Circ Res. 1996;78:15–25.
21. Desarmenien MG, Clendening B, Spitzer NC. In vivo development of voltage-dependent ionic currents in embryonic Xenopus spinal neurons. J Neurosci. 1993;13:2575–2581.
22. Dominguez JN, de la Rosa A, Navarro F, Franco D, Aranega AE. Tissue distribution and subcellular localization of the cardiac sodium channel during mouse heart development. Cardiovasc Res. 2008;78:45–52.
23. Fabiato A, Fabiato F. Calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cells from adult human, dog, cat, rabbit, rat, and frog hearts and from fetal and new-born rat ventricles. Ann NY Acad Sci. 1978;307:491–522.
24. Ferron L, Capuano V, Deroubaix E, Coulombe A, Renaud JF. Functional and molecular characterization of a T-type Ca(2+) channel during fetal and postnatal rat heart development. J Mol Cell Cardiol. 2002;34:533–546.
25. Fischbach GD, Nameroff M, Nelson PG. Electrical properties of chick skeletal muscle fibers developing in cell culture. J Cell Physiol. 1971;78:289–299.
26. Fischer-Lougheed J, Liu JH, Espinos E, et al. Human myoblast fusion requires expression of functional inward rectifier Kir2.1 channels. J Cell Biol. 2001;153:677–686.
27. Franco D, Demolombe S, Kupershmidt S, et al. Divergent expression of delayed rectifier K(+) channel subunits during mouse heart development. Cardiovasc Res. 2001;52:65–75.
28. Fry M. Developmental expression of Na+ currents in mouse Purkinje neurons. Eur J Neurosci. 2006;24:2557–2566.
29. Fujii S, Ayer Jr RK, DeHaan RL. Development of the fast sodium current in early embryonic chick heart cells. J Membr Biol. 1988;101:209–223.
30. Gao BX, Ziskind-Conhaim L. Development of ionic currents underlying changes in action potential waveforms in rat spinal motoneurons. J Neurophysiol. 1998;80:3047–3061.
31. Garthwaite G, Yamini Jr B, Garthwaite J. Selective loss of Purkinje and granule cell responsiveness to N-methyl-D-aspartate in rat cerebellum during development. Brain Res. 1987;433:288–292.
32. Gassanov N, Er F, Michels G, et al. Divergent regulation of cardiac KCND3 potassium channel expression by the thyroid hormone receptors alpha1 and beta1. J Physiol. 2009;587:1319–1329.
33. Gilland E, Miller AL, Karplus E, Baker R, Webb SE. Imaging of multicellular large-scale rhythmic calcium waves during zebrafish gastrulation. Proc Natl Acad Sci USA. 1999;96:157–161.
34. Gleason EL, Spitzer NC. AMPA and NMDA receptors expressed by differentiating Xenopus spinal neurons. J Neurophysiol. 1998;79:2986–2998.
35. Gottmann K, Dietzel ID, Lux HD, Huck S, Rohrer H. Development of inward currents in chick sensory and autonomic neuronal precursor cells in culture. J Neurosci. 1988;8:3722–3732.
36. Grandy SA, Trepanier-Boulay V, Fiset C. Postnatal development has a marked effect on ventricular repolarization in mice. Am J Physiol Heart Circ Physiol. 2007;293:H2168–H2177.
37. Greaves AA, Davis AK, Dallmanm JE, Moody WJ. Co-ordinated modulation of Ca2+ and K+ currents during ascidian muscle development. J Physiol. 1996;497:39–52.
38. Gu X, Olson EC, Spitzer NC. Spontaneous neuronal calcium spikes and waves during early differentiation. J Neurosci. 1994;14:6325–6335.
39. Guo W, Kada K, Kamiya K, Toyama J. IGF-I regulates K(+)-channel expression of cultured neonatal rat ventricular myocytes. Am J Physiol. 1997a;272:H2599–H2606.
40. Guo W, Kamiya K, Hojo M, Kodama I, Toyama J. Regulation of Kv4.2 and Kv1.4 K+ channel expression by myocardial hypertrophic factors in cultured newborn rat ventricular cells. J Mol Cell Cardiol. 1998;30:1449–1455.
41. Guo W, Kamiya K, Liu W, Toyama J. Developmental changes of the ultrarapid delayed rectifier K+ current in rat ventricular myocytes. Pflügers Arch. 1997b;433:442–445.
42. Guo W, Kamiya K, Toyama J. bFGF promotes functional expression of transient outward currents in cultured neonatal rat ventricular cells. Pflügers Arch. 1995;430:1015–1017.
43. Hall BJ, Ghosh A. Regulation of AMPA receptor recruitment at developing synapses. Trends Neurosci. 2008;31:82–89.
44. Haufe V, Camacho JA, Dumaine R, et al. Expression pattern of neuronal and skeletal muscle voltage-gated Na+ channels in the developing mouse heart. J Physiol. 2005;564:683–696.
45. Henriquez JP, Webb A, Bence M, et al. Wnt signaling promotes AChR aggregation at the neuromuscular synapse in collaboration with agrin. Proc Natl Acad Sci USA. 2008;105:18812–18817.
46. Holliday J, Spitzer NC. Spontaneous calcium influx and its roles in differentiation of spinal neurons in culture. Dev Biol. 1990;141:13–23.
47. Hsia AY, Malenka RC, Nicoll RA. Development of excitatory circuitry in the hippocampus. J Neurophysiol. 1998;79:2013–2024.
48. Huang J, Xu L, Thomas M, et al. L-type Ca2+ channel function and expression in neonatal rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol. 2006;290:H2267–H2276.
49. Hume RI, Dingledine R, Heinemann SF. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science. 1991;253:1028–1031.
50. Huynh TV, Chen F, Wetzel GT, Friedman WF, Klitzner TS. Developmental changes in membrane Ca2+ and K+ currents in fetal, neonatal, and adult rabbit ventricular myocytes. Circ Res. 1992;70:508–515.
51. Iannaccone ST, Li KX, Sperelakis N. Transmembrane electrical characteristics of cultured human skeletal muscle cells. J Cell Physiol. 1987;133:409–413.
52. Iijima T, Pappano AJ. Ontogenetic increase of the maximal rate of rise of the chick embryonic heart action potential Relationship to voltage, time, and tetrodotoxin. Circ Res. 1979;44:358–367.
53. Irisawa H, Brown HF, Giles W. Cardiac pacemaking in the sinoatrial node. Physiol Rev. 1993;73:197–227.
54. Jeck CD, Boyden PA. Age-related appearance of outward currents may contribute to developmental differences in ventricular repolarization. Circ Res. 1992;71:1390–1403.
55. Josephson IR, Sperelakis N. Tetrodotoxin differentially blocks peak and steady-state sodium channel currents in early embryonic chick ventricular myocytes. Pflügers Arch. 1989;414:354–359.
56. Josephson IR, Sperelakis N. Developmental increases in the inwardly-rectifying K+ current of embryonic chick ventricular myocytes. Biochim Biophys Acta. 1990;1052:123–127.
57. Kano M, Yamamoto M. Development of spike potentials in skeletal muscle cells differentiated in vitro from chick embryo. J Cell Physiol. 1977;90:439–444.
58. Kato Y, Masumiya H, Agata N, Tanaka H, Shigenobu K. Developmental changes in action potential and membrane currents in fetal, neonatal and adult guinea-pig ventricular myocytes. J Mol Cell Cardiol. 1996;28:1515–1522.
59. Kawano S, DeHaan RL. Developmental changes in the calcium currents in embryonic chick ventricular myocytes. J Membr Biol. 1991;120:17–28.
60. Kilborn MJ, Fedida D. A study of the developmental changes in outward currents of rat ventricular myocytes. J Physiol. 1990;430:37–60.
61. Kitchens SA, Burch J, Creazzo TL. T-type Ca2+ current contribution to Ca2+-induced Ca2+ release in developing myocardium. J Mol Cell Cardiol. 2003;35:515–523.
62. Kobayashi T, Maeda S, Ichise N, et al. The beginning of the calcium transient in rat embryonic heart. J Physiol Sci. 2011;61:141–149.
63. Kobayashi T, Yamada Y, Nagashima M, et al. Contribution of KChIP2 to the developmental increase in transient outward current of rat cardiomyocytes. J Mol Cell Cardiol. 2003;35:1073–1082.
64. Koenen M, Peter C, Villarroel A, Witzemann V, Sakmann B. Acetylcholine receptor channel subtype directs the innervation pattern of skeletal muscle. EMBO Rep. 2005;6:570–576.
65. Kojima M, Sada H, Sperelakis N. Developmental changes in beta-adrenergic and cholinergic interactions on calcium-dependent slow action potentials in rat ventricular muscles. Br J Pharmacol. 1990;99:327–333.
66. Konig S, Beguet A, Bader CR, Bernheim L. The calcineurin pathway links hyperpolarization (Kir2.1)-induced Ca2+ signals to human myoblast differentiation and fusion. Development. 2006;133:3107–3114.
67. Leclerc C, Duprat AM, Moreau M. Noggin upregulates Fos expression by a calcium-mediated pathway in amphibian embryos. Dev Growth Differ. 1999;41:227–238.
68. Leclerc C, Webb SE, Daguzan C, Moreau M, Miller AL. Imaging patterns of calcium transients during neural induction in Xenopus laevis embryos. J Cell Sci. 2000;113:3519–3529.
69. Leuranguer V, Monteil A, Bourinet E, Dayanithi G, Nargeot J. T-type calcium currents in rat cardiomyocytes during postnatal development: contribution to hormone secretion. Am J Physiol Heart Circ Physiol. 2000;279:H2540–H2548.
70. Li H, Guo W, Mellor RL, Nerbonne JM. KChIP2 modulates the cell surface expression of Kv 1.5-encoded K(+) channels. J Mol Cell Cardiol. 2005;39:121–132.
71. Linsdell P, Moody WJ. Electrical activity and calcium influx regulate ion channel development in embryonic Xenopus skeletal muscle. J Neurosci. 1995;15:4507–4514.
72. Liu A, Tang M, Xi J, et al. Functional characterization of inward rectifier potassium ion channel in murine fetal ventricular cardiomyocytes. Cell Physiol Biochem. 2010;26:413–420.
73. Liu JH, Bijlenga P, Fischer-Lougheed J, et al. Role of an inward rectifier K+ current and of hyperpolarization in human myoblast fusion. J Physiol. 1998;510:467–476.
74. Liu JH, Konig S, Michel M, et al. Acceleration of human myoblast fusion by depolarization: graded Ca2+ signals involved. Development. 2003;130:3437–3446.
75. Liu W, Yasui K, Opthof T, et al. Developmental changes of Ca(2+) handling in mouse ventricular cells from early embryo to adulthood. Life Sci. 2002;71:1279–1292.
76. Lockery SR, Spitzer NC. Reconstruction of action potential development from whole-cell currents of differentiating spinal neurons. J Neurosci. 1992;12:2268–2287.
77. Mai W, Janier MF, Allioli N, et al. Thyroid hormone receptor alpha is a molecular switch of cardiac function between fetal and postnatal life. Proc Natl Acad Sci USA. 2004;101:10332–10337.
78. Marcus NC, Fozzard H. Tetrodotoxin sensitivity in the developing and adult chick heart. J Mol Cell Cardiol. 1981;13:335–340.
79. Martin-Caraballo M, Greer JJ. Voltage-sensitive calcium currents and their role in regulating phrenic motoneuron electrical excitability during the perinatal period. J Neurobiol. 2001;46:231–248.
80. Masuda H, Sperelakis N. Inwardly rectifying potassium current in rat fetal and neonatal ventricular cardiomyocytes. Am J Physiol. 1993;265:H1107–H1111.
81. Masuda H, Sumii K, Sperelakis N. Long openings of calcium channels in fetal rat ventricular cardiomyocytes. Pflügers Arch. 1995;429:595–597.
82. Matsubara H, Suzuki J, Inada M. Shaker-related potassium channel, Kv1.4, mRNA regulation in cultured rat heart myocytes and differential expression of Kv1.4 and Kv1.5 genes in myocardial development and hypertrophy. J Clin Invest. 1993;92:1659–1666.
83. Mishina M, Takai T, Imoto K, et al. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986;321:406–411.
84. Moreau M, Leclerc C, Gualandris-Parisot L, Duprat AM. Increased internal Ca2+ mediates neural induction in the amphibian embryo. Proc Natl Acad Sci USA. 1994;91:12639–12643.
85. Morikawa Y, Rosen MR, Meiri H, Robinson RB. Developmental changes in the response of cardiac Purkinje fibers to SC-72-14. Am J Physiol. 1987;252:H771–H776.
86. Muller YL, Yool AJ. Increased calcium-dependent K+ channel activity contributes to the maturation of cellular firing patterns in developing cerebellar Purkinje neurons. Brain Res Dev Brain Res. 1998;108:193–203.
87. Muller YL, Reitstetter R, Yool AJ. Regulation of Ca2+-dependent K+ channel expression in rat cerebellum during postnatal development. J Neurosci. 1998;18:16–25.
88. Nagashima M, Tohse N, Kimura K, et al. Alternation of inwardly rectifying background K+ channel during development of rat fetal cardiomyocytes. J Mol Cell Cardiol. 2001;33:533–543.
89. Nakamura TY, Lee K, Artman M, Rudy B, Coetzee WA. The role of Kir2.1 in the genesis of native cardiac inward-rectifier K+ currents during pre- and postnatal development. Ann NY Acad Sci. 1999;868:434–437.
90. Nakanishi T, Seguchi M, Takao A. Development of the myocardial contractile system. Experientia. 1988;44:936–944.
91. Nguemo F, Fleischmann BK, Schunkert H, Hescheler J, Reppel M. Functional expression and inactivation of L-type Ca2+ currents during murine heart development – implications for cardiac Ca2+ homeostasis. Cell Physiol Biochem. 2007;20:809–824.
92. Niwa N, Yasui K, Opthof T, et al. Cav3.2 subunit underlies the functional T-type Ca2+ channel in murine hearts during the embryonic period. Am J Physiol Heart Circ Physiol. 2004;286:H2257–H2263.
93. O’Dowd DK, Ribera AB, Spitzer NC. Development of voltage-dependent calcium, sodium, and potassium currents in Xenopus spinal neurons. J Neurosci. 1988;8:792–805.
94. Osaka T, Joyner RW. Developmental changes in calcium currents of rabbit ventricular cells. Circ Res. 1991;68:788–796.
95. Petralia RS, Sans N, Wang YX, Wenthold RJ. Ontogeny of postsynaptic density proteins at glutamatergic synapses. Mol Cell Neurosci. 2005;29:436–452.
96. Picken Bahrey HL, Moody WJ. Early development of voltage-gated ion currents and firing properties in neurons of the mouse cerebral cortex. J Neurophysiol. 2003;89:1761–1773.
97. Protas L, Oren RV, Clancy CE, Robinson RB. Age-dependent changes in Na current magnitude and TTX-sensitivity in the canine sinoatrial node. J Mol Cell Cardiol. 2010;48:172–180.
98. Renaud JF, Kazazoglou T, Lombet A, et al. The Na+ channel in mammalian cardiac cells Two kinds of tetrodotoxin receptors in rat heart membranes. J Biol Chem. 1983;258:8799–8805.
99. Renaud JF, Romey G, Lombet A, Lazdunski M. Differentiation of the fast Na+ channel in embryonic heart cells: interaction of the channel with neurotoxins. Proc Natl Acad Sci USA. 1981;78:5348–5352.
100. Ritchie AK, Fambrough DM. Electrophysiological properties of the membrane and acetylcholine receptor in developing rat and chick myotubes. J Gen Physiol. 1975;66:327–355.
101. Robinson RB, Yu H, Chang F, Cohen IS. Developmental change in the voltage-dependence of the pacemaker current, if, in rat ventricle cells. Pflügers Arch. 1997;433:533–535.
102. Rogart RB, Cribbs LL, Muglia LK, Kephart DD, Kaiser MW. Molecular cloning of a putative tetrodotoxin-resistant rat heart Na+ channel isoform. Proc Natl Acad Sci USA. 1989;86:8170–8174.
103. Sada H, Ban T, Fujita T, Ebina Y, Sperelakis N. Developmental change in fast Na channel properties in embryonic chick ventricular heart cells. Can J Physiol Pharmacol. 1995;73:1475–1484.
104. Sada H, Kojima M, Sperelakis N. Fast inward current properties of voltage-clamped ventricular cells of embryonic chick heart. Am J Physiol. 1988;255:H540–H553.
105. Salgado H, Tecuapetla F, Perez-Rosello T, et al. A reconfiguration of CaV2 Ca2+ channel current and its dopaminergic D2 modulation in developing neostriatal neurons. J Neurophysiol. 2005;94:3771–3787.
106. Sanchez-Chapula J, Elizalde A, Navarro-Polanco R, Barajas H. Differences in outward currents between neonatal and adult rabbit ventricular cells. Am J Physiol. 1994;266:H1184–H1194.
107. Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805.
108. Satoh H. Identification of and developmental changes in transient outward current in embryonic chick cardiomyocytes. Reprod Fertil Dev. 1995;7:1369–1374.
109. Satoh H, Sperelakis N. Hyperpolarization-activated inward current in embryonic chick cardiac myocytes: developmental changes and modulation by isoproterenol and carbachol. Eur J Pharmacol. 1993;240:283–290.
110. Seki S, Nagashima M, Yamada Y, et al. Fetal and postnatal development of Ca2+ transients and Ca2+ sparks in rat cardiomyocytes. Cardiovasc Res. 2003;58:535–548.
111. Shimahara T, Bournaud R. Barium currents in developing skeletal muscle cells of normal and mutant mice foetuses with ‘muscular dysgenesis’. Cell Calcium. 1991;12:727–733.
112. Shimoni Y, Fiset C, Clark RB, et al. Thyroid hormone regulates postnatal expression of transient K+ channel isoforms in rat ventricle. J Physiol. 1997;500:65–73.
113. Shin KS, Park JY, Kwon H, Chung CH, Kang MS. A possible role of inwardly rectifying K+ channels in chick myoblast differentiation. Am J Physiol. 1997;272:C894–C900.
114. Spector I, Prives JM. Development of electrophysiological and biochemical membrane properties during differentiation of embryonic skeletal muscle in culture. Proc Natl Acad Sci USA. 1977;74:5166–5170.
115. Sperelakis N, Lee EC. Characterization of (Na+, K+)-ATPase isolated from embryonic chick hearts and cultured chick heart cells. Biochim Biophys Acta. 1971;233:562–579.
116. Sperelakis N, Shigenobu K. Changes in membrane properties of chick embryonic hearts during development. J Gen Physiol. 1972;60:430–453.
117. Spitzer NC. Spontaneous Ca2+ spikes and waves in embryonic neurons: signaling systems for differentiation. Trends Neurosci. 1994;17:115–118.
118. Spitzer NC, Baccaglini PI. Development of the action potential in embryo amphibian neurons in vivo. Brain Res. 1976;107:610–616.
119. Spitzer NC, Debaca RC, Allen KA, Holliday J. Calcium dependence of differentiation of GABA immunoreactivity in spinal neurons. J Comp Neurol. 1993;337:168–175.
120. Spitzer NC, Gu X, Olson E. Action potentials, calcium transients and the control of differentiation of excitable cells. Curr Opin Neurobiol. 1994;4:70–77.
121. Stieber J, Herrmann S, Feil S, et al. The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci USA. 2003;100:15235–15240.
122. Strube C, Tourneur Y, Ojeda C. Functional expression of the L-type calcium channel in mice skeletal muscle during prenatal myogenesis. Biophys J. 2000;78:1282–1292.
123. Takahashi K, Okamura Y. Ion channels and early development of neural cells. Physiol Rev. 1998;78:307–337.
124. Tarasenko AN, Isaev DS, Eremin AV, Kostyuk PG. Developmental changes in the expression of low-voltage-activated Ca2+ channels in rat visual cortical neurones. J Physiol. 1998;509:385–394.
125. Tohse N, Masuda H, Sperelakis N. Novel isoform of Ca2+ channel in rat fetal cardiomyocytes. J Physiol. 1992a;451:295–306.
126. Tohse N, Meszaros J, Sperelakis N. Developmental changes in long-opening behavior of L-type Ca2+ channels in embryonic chick heart cells. Circ Res. 1992b;71:376–384.
127. Tohse N, Seki S, Kobayashi T, et al. Development of excitation-contraction coupling in cardiomyocytes. Jap J Physiol. 2004;54:1–6.
128. Tohse N, Sperelakis N. Long-lasting openings of single slow (L-type) Ca2+ channels in chick embryonic heart cells. Am J Physiol. 1990;259:H639–H642.
129. Trepanier-Boulay V, Lupien MA, St-Michel C, Fiset C. Postnatal development of atrial repolarization in the mouse. Cardiovasc Res. 2004;64:84–93.
130. Wahler GM, Dollinger SJ, Smith JM, Flemal KL. Time course of postnatal changes in rat heart action potential and in transient outward current is different. Am J Physiol. 1994;267:H1157–H1166.
131. Wang K, Cui J, Cai Y, et al. Critical roles of voltage-dependent sodium channels in the process of synaptogenesis during the postnatal cortical development of rats. Cell Mol Neurobiol. 2009;29:1131–1142.
132. Wang L, Duff HJ. Developmental changes in transient outward current in mouse ventricle. Circ Res. 1997;81:120–127.
133. Wang L, Feng ZP, Kondo CS, Sheldon RS, Duff HJ. Developmental changes in the delayed rectifier K+ channels in mouse heart. Circ Res. 1996;79:79–85.
134. Wang Y, Xu H, Kumar R, et al. Differences in transient outward current properties between neonatal and adult human atrial myocytes. J Mol Cell Cardiol. 2003;35:1083–1092.
135. Webb SE, Moreau M, Leclerc C, Miller AL. Calcium transients and neural induction in vertebrates. Cell Calcium. 2005;37:375–385.
136. Wickenden AD, Kaprielian R, Parker TG, Jones OT, Backx PH. Effects of development and thyroid hormone on K+ currents and K+ channel gene expression in rat ventricle. J Physiol. 1997;504:271–286.
137. Xie LH, Takano M, Noma A. Development of inwardly rectifying K+ channel family in rat ventricular myocytes. Am J Physiol. 1997;272:H1741–H1750.
138. Xu H, Dixon JE, Barry DM, et al. Developmental analysis reveals mismatches in the expression of K+ channel alpha subunits and voltage-gated K+ channel currents in rat ventricular myocytes. J Gen Physiol. 1996;108:405–419.
139. Yasui K, Liu W, Opthof T, et al. I(f) current and spontaneous activity in mouse embryonic ventricular myocytes. Circ Res. 2001;88:536–542.
140. Zhang W, Elsen F, Barnbrock A, Richter DW. Postnatal development of GABAB receptor-mediated modulation of voltage-activated Ca2+ currents in mouse brain-stem neurons. Eur J Neurosci. 1999;11:2332–2342.