Chapter 38
Visual Transduction
Chapter Outline
I Summary
The process of vertebrate vision begins with photon capture and visual transduction in the rods and cones. The rods are outstanding single photon detectors that are used in dim light, whereas the cones provide excellent visual acuity, movement detection and color vision in bright light. The first step in the response to light is the absorption of a photon by the chromophore, retinal, within a visual pigment molecule. This triggers activation of a G-protein cascade, causing hydrolysis of cyclic guanosine monophosphate (cGMP) and thereby closure of cyclic nucleotide-gated (CNG) cation channels. These channels are normally held open in the dark by cGMP, conducting the inward “dark current”. Closure of the channels hyperpolarizes the cell, decreasing neurotransmitter release onto other retinal neurons. There are elaborate mechanisms for shutting off the cascade and for light and dark adaptation. Although many of the fundamental molecular steps in visual transduction have been determined, numerous mysteries still remain.
II Introduction
The miracle of vision begins when our photoreceptors absorb light that is reflected by our surroundings. However, the photoreceptor’s duty does not end with photon capture. In addition, the photoreceptor must convert the energy of the absorbed photon into an electrochemical signal to be relayed to the visual cortex of the brain. This process of visual transduction involves a G-protein-mediated second messenger system that ultimately controls membrane potential and neurotransmitter release.
This chapter gives an overview of vertebrate visual transduction. Further information can be obtained from many excellent reviews on the subject (e.g. Burns and Baylor, 2001; Fu and Yau, 2007; Kawamura and Tachibanaki, 2008; Gross and Wensel, 2011; Lamb, 2011; MacLeish and Makino, 2011). Details of the cyclic nucleotide-gated ion channels that mediate the light response are covered in Chapter 35. Invertebrate visual transduction is not discussed here, but thorough descriptions and comparisons are available elsewhere, along with discussions of evolutionary links between invertebrate and vertebrate visual transduction (e.g. Katz and Minke, 2009; Lamb, 2009; Yau and Hardie, 2009; Fain et al., 2010). Interestingly, invertebrate photoreceptors use a different light-activated enzyme cascade than that found in rods and cones and their membranes hyperpolarize in response to light, whereas rods and cones depolarize. Finally, this chapter will deal only with image-forming vision. The retina also contains a very interesting pathway for light detection without image formation that is involved in circadian rhythms. This pathway was recently discovered to involve the photopigment melanopsin and a third class of photoreceptors in the retina: intrinsically photosensitive retinal ganglion cells, ipRGCs (reviewed in Do and Yau, 2010; Wong and Berson, 2011).
III Photoreceptor Cells
The two kinds of vertebrate photoreceptors – rods and cones – are specialized for use under different conditions. The rods are employed for vision in dim light, whereas the cones are used in moderate to bright light. Thus, while a rod can reliably detect a single photon in a darkened room, its sensing mechanism shuts down completely when normal room lights are switched on. Cones, on the other hand, are not sensitive enough to detect single photons, but give us color vision and high spatial and temporal resolution when light levels are sufficiently high.
Rods and cones also occupy different parts of the retina. Cones are concentrated in the center of the retina, the fovea, which receives light from the center of our visual field. The rods, however, are almost entirely excluded from the fovea and instead dominate the peripheral retina. However, a few cones are scattered throughout the peripheral retina and a few rods exist at the edge of the fovea. Thus, in daylight, our peripheral vision has much lower spatial resolution than our central vision because of the sparse distribution of peripheral cones. At night, our ability to locate a dim star is enhanced by looking “out of the corners of our eyes”, using the abundant rods in the peripheral retina.
As shown in Fig. 38.1, the vertebrate photoreceptor can be divided into three regions: the outer segment, the inner segment and the synaptic terminal. The outer segment is the region of the cell dedicated to photon capture and visual transduction. The inner segment, located closer to the front of the eye, contains the nucleus, abundant mitochondria and other general cell machinery. The synaptic terminal contains vesicles of the neurotransmitter glutamate for release onto the second-order retinal neurons (the bipolar cells).
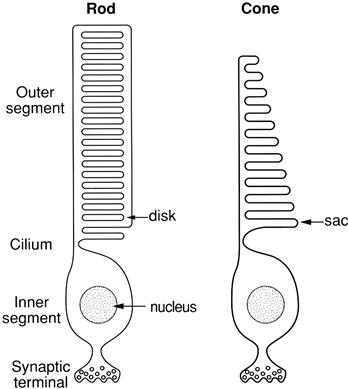
FIGURE 38.1 A simplified view of rod and cone structure. The outer segment is specialized for visual transduction. Cone photopigment molecules are located on infoldings of the plasma membrane, called sacs, rather than in the membranes of intracellular disks as in rods.
The anatomy of rods and cones is very similar, except that cones have a more conical shape and contain infoldings of the plasma membrane, called sacs (see Fig. 38.1), rather than the intracellular disks found in rods. The membranes of the disks and sacs contain photopigment molecules and various other proteins used in visual transduction. The disks or sacs are packed very tightly together: usually about 1000–2000 in a typical outer segment whose length is about 30–60 μm. This parallel array of membranes serves to align the photopigment molecules in the correct orientation for optimal absorption of light, which normally travels the length of the cell from the synaptic terminal toward the tip of the outer segment. The disks and sacs also serve to concentrate the photopigment molecules (e.g. about a hundred million in a typical rod) and to position the photopigment in close proximity to other important molecules in the visual transduction cascade.
In both rods and cones, the outer and inner segments are connected by a narrow region called the cilium. In some way that is not completely understood, the cilium segregates the inner and outer segment membrane proteins. For example, whereas the inner segment plasma membrane contains many types of ion channels (Bader et al., 1982; Barnes and Hille, 1989; reviewed in MacLeish and Makino, 2011), the outer segment plasma membrane contains essentially a pure population of light-regulated cyclic nucleotide-gated (CNG) ion channels (Baylor and Nunn, 1986). It is in the ciliary region that new disks or sacs are inserted during the outer segment regeneration process that occurs constantly. Recent evidence suggests that the formation of disks is accomplished by the repeated fusion of membrane vesicles containing the photopigment rhodopsin (reviewed in Sung and Chuang, 2010). Old disks or sacs are removed from the tip of the outer segment by phagocytosis by the retinal pigment epithelial (RPE) cells. These very opaque cells sit behind the retina, with finger-like cellular processes that hug the photoreceptor outer segments, reducing light scatter, as well as providing some of the molecules essential for visual transduction. Because the cilium is such a narrow structure, outer and inner segments often break apart (and their membranes reseal) during cell isolation from the retina. Thus, many studies of visual transduction are conducted on isolated outer segments, which are somewhat simpler preparations than whole cells.
IV Physiology of Visual Transduction
The physiological trademark of the vertebrate photoreceptor is its light-regulated “dark current” (Fig. 38.2) (reviewed in Burns and Baylor, 2001; Fu and Yau, 2007; MacLeish and Makino, 2011). This current circulates between the outer and inner segments in the dark and is reduced upon light absorption. The dark current is carried into the outer segment mainly by Na+ ions flowing through cyclic nucleotide-gated (CNG), non-selective cation channels (see Chapter 35) and out of the inner segment by K+ ions flowing through voltage-gated K+ channels. Because the CNG channels are non-selective cation channels, they allow the entry not only of Na+, but also of Ca2+ and Mg2+. These divalent cations have important consequences for visual transduction. First, Ca2+ regulates the light-dependent enzyme cascade (discussed below). Second, both Ca2+ and Mg2+ pass through CNG channels extremely slowly, reducing the effective single-channel conductance and thereby making the current–voltage relation for the dark current relatively flat in the physiological range of membrane voltages. Thus, under physiological conditions, the divalent cations make the dark current almost independent of voltage and, therefore, a faithful reporter of the light level, rather than of voltage changes induced by channel activity in the inner segment (Zimmerman and Baylor, 1992).
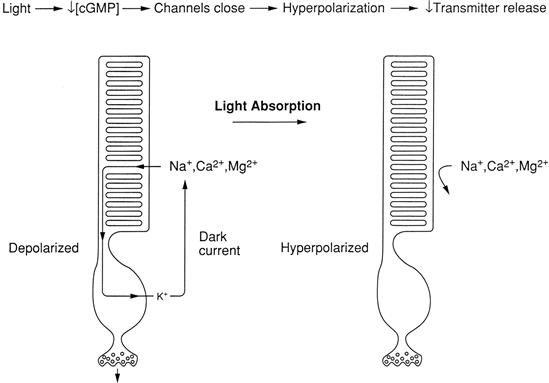
FIGURE 38.2 The absorption of light shuts down the dark current that circulates between the outer and inner segments of the photoreceptor. This reduction in current hyperpolarizes the cell, reducing the release of neurotransmitter.
Since the cell has a relatively high Na+ permeability in the dark, its resting potential is somewhat more positive than that for most cells (about −40 mV, instead of −70 mV). This resting depolarization tends to open voltage-gated Ca2+ channels in the synaptic terminal, allowing Ca2+ entry and vesicular release of neurotransmitter in the dark. When a photon is absorbed by a rhodopsin molecule in a disk membrane, a series of reactions leads to a reduction in the concentration of guanosine 3′, 5′-cyclic monophosphate (cGMP), which normally binds to, and opens, the outer segment CNG channels in the dark. Since there is less cGMP available to open the channels in the light, they close, decreasing the dark current (i.e. decreasing the Na+ permeability), causing a membrane hyperpolarization which, in turn, causes closure of the voltage-gated K+ and Ca2+ channels in the inner segment and synaptic terminal and, finally, a decrease in neurotransmitter release. The process is similar in cones.
The dark current and light response can be measured in a variety of ways, including a voltage-clamp method using intracellular microelectrodes, the whole-cell patch-clamp method and the suction electrode method. The relatively non-invasive suction electrode method (Fig. 38.3) works particularly well with mammalian photoreceptors, which are usually quite small and fragile. This method is much gentler than the other techniques, yet is able to measure the dark current without interference from inner segment currents.
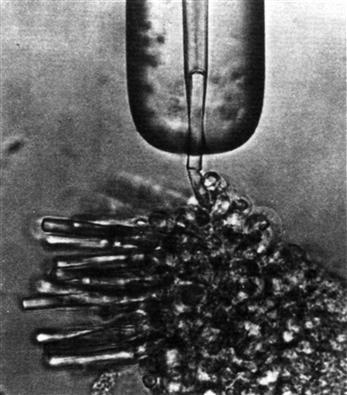
FIGURE 38.3 The use of a suction electrode to record the dark current flowing into the outer segment of a rod attached to a piece of toad retina. The horizontal line just below the suction electrode is the junction between the outer and inner segments. The electrode is filled with a physiological salt solution and connected to a sensitive amplifier. (Reproduced with permission from Baylor et al., 1979.)
Families of light responses recorded with a suction electrode from a monkey rod and cone are shown in Fig. 38.4. Light response amplitudes increase in a graded manner with light intensity until they reach a limiting value obtained with a “saturating light” that shuts off all the inward dark current (i.e. that closes all the CNG channels in the outer segment plasma membrane). Supersaturating light intensities cannot give larger responses, but instead give longer lasting ones. Cones are less sensitive than rods, requiring about a hundred times as many photons to shut down half the dark current; this seems to result at least in part from a lower amplification in the G-protein-mediated enzyme cascade described below (reviewed in Gross and Wensel, 2011). However, the cone responses are several times faster than those of rods, as evidenced by the shorter time to peak and faster recovery, and this gives them an advantage over rods in the detection of movement and rapid changes in illumination. The undershoots seen in the cone responses (but not in normal rod responses) are not yet fully understood, but appear to make the cone especially sensitive to changes in illumination (Schnapf et al., 1990).
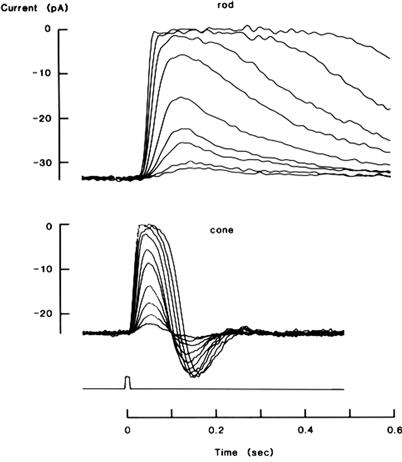
FIGURE 38.4 Families of photocurrents recorded by suction electrode from a rod and a cone of the monkey, Macaca fascicularis. Brief light flashes were given at time zero (rectangular pulse below the current recordings). Upward deflections indicate reductions in the inward dark current. From the bottom to the top of each family, flash strengths were increased by factors of 2. Expected numbers of photoisomerizations ranged from 2.9 to 860 for the rod and from 190 to 36 000 for the cone. Saturation of the responses (top traces in each family) occurred when all dark current was shut off. (Reproduced with permission from Baylor, 1987.)
Although single photon responses are too small to be measured in cones, they are about 1.0 pA in rods, and therefore have been studied in some detail there. Single photon responses are very reliable: they occur at least 80% of the time after absorption of a photon by a rhodopsin molecule and they almost never occur in the absence of photon absorption (reviewed in Baylor, 1987). A single absorbed photon gives a highly amplified response, shutting down 3–5% of the total dark current by closing a few hundred CNG channels in a narrow band (one to a few micrometers in width) of outer segment plasma membrane near the site of absorption. Like the dim flash responses in Fig. 38.4 (bottom traces of each set), the single photon response has a stereotypical waveform, with a slow, S-shaped rise and a very slow decay. These complex, slow kinetics reflect the complex enzyme cascade underlying the response.
When membrane voltage is recorded instead of outer segment current, the waveform resembles an inverted version of the current for dim lights but not for light levels near or beyond saturation. When many photons are absorbed, the voltage recordings (Fig. 38.5) show a characteristic “nose” and “plateau”. These features result from a shaping of the response by ion channels in the inner segment. Several types of channels contribute to the waveform (reviewed in MacLeish and Makino, 2011), but the dominant channel giving rise to this more complicated shape appears to be an inner segment, non-selective cation channel that is opened by hyperpolarization (the HCN1 channel). Thus, the closure of outer segment CNG channels gives the initial hyperpolarization (beginning of the nose). This hyperpolarization opens the inner segment HCN1 non-selective cation channels which, in turn, depolarize the membrane, giving the transition from nose to plateau. Eventually, these channels close because of the depolarization while, at the same time, the outer segment CNG channels reopen during recovery from the light response and the membrane potential returns to its initial, relatively depolarized, dark value.
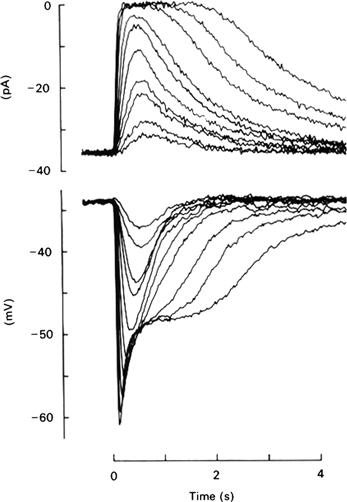
FIGURE 38.5 Comparison of light-evoked changes in outer segment current with those in membrane potential for a salamander rod. The currents were measured by a suction electrode, while the membrane potential was monitored by an intracellular microelectrode. The current and voltage responses to dim flashes have similar form, but a prominent “nose” and “plateau” are seen in the voltage responses to bright flashes. 500-nm, 11-ms flashes were given at t = 0 and photon densities increased by factors of about 2 between 1.5 and 430 photons μm−2. (Reproduced with permission from Baylor and Nunn, 1986.)
Aside from the ion channels described earlier, two other types of ion transport protein are of obvious importance to the vertebrate photoreceptor. First, Na+-K+ pumps in the inner segment are responsible for expelling the Na+ that enters through the CNG channels in the outer segment. Second, Na+:Ca2+,K+ exchange carriers (NCKX; reviewed in Bauer, 2002; Schnetkamp, 2004) in the outer segment expel the Ca2+ that enters through the CNG channels and regulates the enzyme cascade. The Na+:Ca2+,K+ carriers are particularly important in light adaptation, where intracellular Ca2+ plays a large regulatory role.
The ability of the visual system to detect light depends partly on its degree of adaptation to background illumination. Although much of light and dark adaptation involves pupillary responses and processes occurring in the brain and non-photoreceptor layers of the retina, some aspects of adaptation clearly occur in the rods and cones themselves. In continuous light, there is a partial recovery of the dark current after its initial suppression when the light is switched on. This sag in the photoresponse is accompanied by a decrease in light sensitivity and an acceleration of response kinetics (although this acceleration is negligible in monkey cones; Schnapf et al., 1990). Thus, the response to a light flash during continuous background light is smaller and generally briefer than that to an equally bright flash occurring in the dark. To make the amplitude of the flash response equal to that obtained in the dark, one must increase the flash intensity (Fig. 38.6). This light adaptation allows the photoreceptor to respond over a much larger range of light intensities than it otherwise could. In effect, the photoreceptor uses a non-linear gain adjustment partially to overcome the response saturation that occurs as a result of having a finite number of CNG channels to close in the light. Cones light-adapt so well that it is essentially impossible to obtain saturation of their dark current in steady bright light. Saturation is only possible transiently when bright light is applied while the cone is in a very dark-adapted state (reviewed in Lamb, 2011).
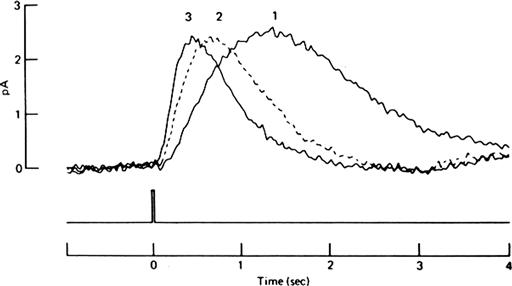
FIGURE 38.6 Decreased sensitivity and accelerated flash response kinetics characteristic of adaptation to background light in a rod. These outer segment currents were obtained by suction electrode recording from a toad rod that was given a test light flash in darkness (curve 1), with a dim background light (curve 2) and with a brighter background light (curve 3). In the presence of background light, the test flash intensity had to be increased to obtain responses of approximately the same amplitude. For example, the flash intensity used to obtain curve 2 was approximately five times the intensity used to obtain curve 1. (Reproduced with permission from Baylor et al., 1979.)
Photoreceptor dark adaptation is a rather complicated set of processes that has been most thoroughly studied in rods (reviewed in Lamb and Pugh, 2004). After exposure to a light that is bright enough to bleach all the photopigment, a rod ultimately cannot return to its dark state until non-excited photopigment molecules have been regenerated (including reinsertion of the chromophore, see following). However, when a rod has been exposed to only moderately bright light, which leaves some photopigment unbleached, it is able to respond to light again after a briefer period of adaptation. This period is characterized by a lingering suppression of the dark current for many minutes after the light is switched off, a decrease in light sensitivity similar to that found in the presence of background light and an increase in outer segment current noise (Fig. 38.7). These features limit visual detection and appear to derive from incomplete shutoff of the photopigment after loss of its chromophore. Ultimately, the speed with which the rod recovers from a bright light is limited by the rate at which 11-cis-retinal is delivered to opsin. Cones recover from a bright light so rapidly that it has been hypothesized that the shut-off reactions in their transduction enzyme cascade must be extremely fast (reviewed in Lamb, 2011).
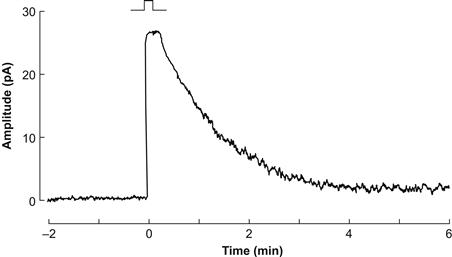
FIGURE 38.7 Prolonged dark current suppression and increased noise following a strong bleach. The rectangular pulse above the response indicates when the dark-adapted rod was given a bright flash of light that bleached about 0.7% of its rhodopsin (72×105 photons μm−2, calculated to isomerize 1.8×107 rhodopsins). Closer examination of the lingering noise revealed a strong resemblance to that produced by the absorption of single photons. (Reprinted with permission from Nature, Lamb, 1980. Copyright 1980 Macmillan Magazines Limited.)
V Molecular Mechanisms
VA Photopigment Activation and Shut-off
Visual pigments are integral membrane proteins called opsins that consist of apo-opsin and a ubiquitous light-absorbing chromophore, retinal (Fig. 38.8). Retinal is in a class of molecules called retinoids that also includes vitamin A (all-trans-retinol). There are many excellent reviews of visual pigments and rhodopsin in particular (e.g. Palczewski, 2006; Nickle and Robinson, 2007; Hofmann et al., 2009; Smith, 2010). Retinal is derived from vitamin A and comes in two forms in vertebrates: retinal1 and dehydroretinal, or retinal2 (reviewed in Nickle and Robinson, 2007). Human photoreceptors use retinal1. Interactions with different opsins change the spectral tuning characteristics of retinal. Thus, each type of photoreceptor has its own type of opsin, which results in a characteristic photopigment absorption spectrum and therefore a characteristic color sensitivity of the cell. Rods contain rhodopsin (Rh) and are therefore most sensitive to blue-green light (peak wavelength around 490 nm), whereas primate cones are of three types, with characteristic peak absorbances in the short-wavelength (peak about 430 nm), middle-wavelength (peak about 530 nm) and long-wavelength (peak about 560 nm) regions of the visible spectrum (Fig. 38.9). These three types of cone are sometimes referred to as blue-, green- and red-sensitive cones, respectively, although 560 nm actually corresponds to yellow, rather than red, light. The characteristic absorption spectra determine only the probability that a photon will be absorbed by the cell’s photopigment. The photoreceptor responds in exactly the same way to any absorbed photon, independent of its wavelength.
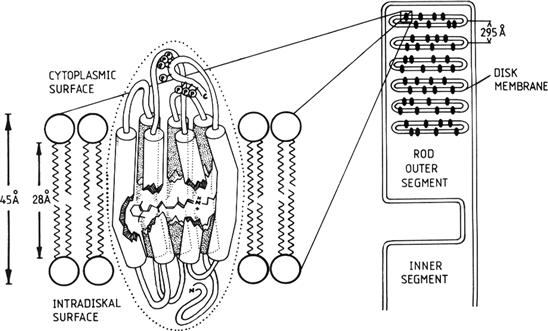
FIGURE 38.8 A model for the structure of rhodopsin in the disk membrane of a rod. The light-absorbing chromophore, retinal, sits in a binding pocket near the middle of the integral membrane protein, opsin. (Reproduced with permission from Dratz and Hargrave, 1983.)
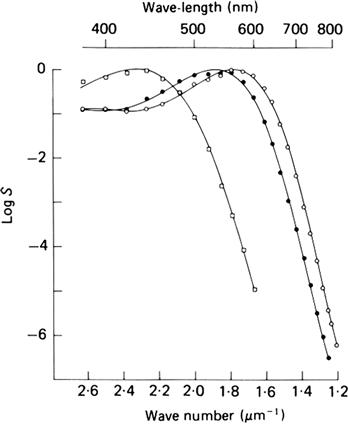
FIGURE 38.9 Cone spectral sensitivities, as measured by the suction electrode method. These spectra were obtained from blue-, green- and red-sensitive monkey cones (squares, filled circles, and open circles, respectively). The spectral sensitivity, S, was derived from the cone’s response to flashes of different color and it reflects the probability of absorption of a photon of that color (or wavelength). (Reproduced with permission from Baylor et al., 1987.)
Absorption of a photon causes isomerization of retinal from the 11-cis to the all-trans form (Fig. 38.10), breaking its covalent bond to the protein and destabilizing its position. Eventually all-trans-retinal hops out of its binding pocket within opsin, but a chain of conformational intermediates is generated before this dissociation occurs. For the best-studied pigment, rhodopsin, these intermediates are (in the order that follows photon absorption) photorhodopsin, bathorhodopsin, BSI (blue-shifted intermediate), lumirhodopsin, metarhodopsin I, metarhodopsin II and metarhodopsin III (reviewed in Palczewski, 2006; Smith, 2010). Similar transitions are thought to occur in other visual pigments. The metarhodopsin II (meta II) conformational state activates the G protein, transducin, which begins the cellular response to light. As all-trans-retinal leaves its binding pocket in opsin, it is released into the interior of the disk. It must then be transferred to the cytosolic side of the disk, apparently via diffusion through the plasma membrane and transport with phosphatidylethanolamine by the ABCA4 lipid carrier (reviewed in Molday and Zhang, 2010). On the cytosolic side, the aldehyde, all-trans-retinal, is converted by an enzyme (a retinol dehydrogenase) to the alcohol form, all-trans-retinol, which then travels through the plasma membrane into the extracellular space, where it is shuttled via retinoid binding proteins to the RPE and Müller cells. The process by which all-trans-retinal is transported and converted back into 11-cis-retinal for reloading into opsin is called the visual cycle (reviewed in Travis et al., 2007; Wang and Kefalov, 2011).
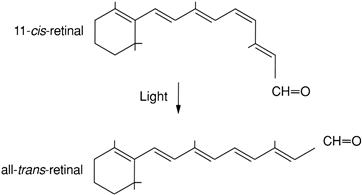
FIGURE 38.10 The light-induced isomerization of retinal1 from the 11-cis to the all-trans form. This is the first step in vertebrate visual transduction and the only one that is a direct action of light.
For the photoreceptor to reset to its dark condition after a light is switched off, the molecular players in the light response also must be switched off. Most of what we know about photopigment shut-off comes from work on rhodopsin (depicted in the top right loop of Fig. 38.11). The shut-off of rhodopsin’s ability to activate transducin begins even before all-trans-retinal has left the protein. Rhodopsin kinase phosphorylates photoexcited rhodopsin at multiple serine and threonine residues near the C-terminal. A 48-kDa protein called arrestin then binds phosphorylated rhodopsin, reducing its interaction with transducin. There are recent hints for still other players in the shut-off process (e.g. RGS9), but all the details have not yet been resolved (reviewed in Gross and Wensel, 2011). Once all-trans-retinal has separated from opsin, the photopigment cannot be reset to its photon-receptive dark state until 11-cis-retinal has been reinserted, arrestin has been released and a phosphatase (serine/threonine phosphatase type 2A) has removed the phosphates from the C-terminal residues. This process takes several minutes. The new 11-cis-retinal that is inserted into opsin is made in the RPE cells and transported into the rods. However, cones obtain new 11-cis-retinal by a different mechanism: the surrounding Müller cells make the alcohol form, 11-cis-retinol, which is transferred to the cones, where it is converted to the aldehyde, 11-cis-retinal (Mata et al., 2002; reviewed in Travis et al., 2007; Wang and Kefalov, 2011). Interestingly, in cones, there is much greater activity of the kinase that phosphorylates their pigments and this may partly explain why cones recover from a light response much more quickly than do rods (reviewed in Gross and Wensel, 2011). Furthermore, in cones there is a less stable interaction between 11-cis-retinal and the visual pigment, leading to a greater concentration of apo-opsin, which itself stimulates transducin (reviewed in Kawamura and Tachibanaki, 2008).
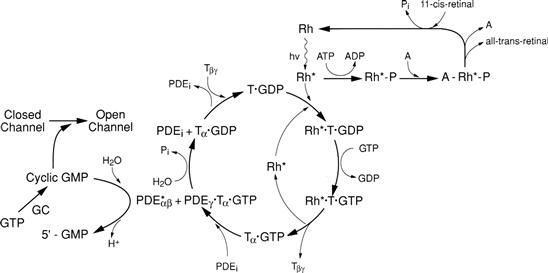
FIGURE 38.11 The enzyme cascade controlling visual transduction in rods. Abbreviations: A: arrestin; channel: cyclic nucleotide-gated (CNG) outer-segment ion channel; GC: guanylate cyclase; PDE: cGMP-specific phosphodiesterase (∗, active form); Rh: rhodopsin (∗, photoexcited, active form); T: the G protein, transducin. There is increasing evidence that, in spite of its complexity, this diagram is quite incomplete. (Modified with permission from Stryer, 1991 and drawing on information from Palczewski, 2006; Nickle and Robinson, 2007; Hofmann et al., 2009; Smith, 2010.)
VB Cyclic Nucleotide Cascade
A cyclic nucleotide enzyme cascade is used to translate the message of photon absorption into the language of the brain – electrical and chemical signals. The key second messenger is cGMP, with another second messenger, Ca2+, involved in more subtle aspects of visual transduction. Most of our information on the molecular mechanism of visual transduction derives from studies on rods, but the process appears to be similar in cones. A large body of biochemical and physiological evidence suggests that absorption of a single photon by rhodopsin initiates the following chain of events (diagrammed in Fig. 38.11):
1. Photoexcited rhodopsin (Rh∗) catalyzes the replacement of GDP by GTP on the G protein, transducin (T). Both Rh∗ and the peripheral membrane protein, T, are able to diffuse laterally in the disk membrane, where they find each other and interact. Before Rh∗ is shut off, it activates about 500 Ts, representing the first amplification step in the transduction process.
2. The α subunit of T, now with GTP bound, dissociates from the rest of T (the βγ subunit complex) and binds to and removes the γ inhibitory subunit of another peripheral disk membrane protein, cGMP-specific phosphodiesterase (PDE). Each Tα can activate only one PDE since the catalytic portion of PDE, PDEαβ, is only free to work when PDEγ is bound to Tα (and therefore unable to reassociate with PDEαβ).
3. Once disinhibited, one PDE molecule (PDEαβ) hydrolyzes about a million cGMP molecules, leading to the closure of hundreds of CNG ion channels and thereby halting entry of over a million Na+ ions during the time course of the single photon response.
The recovery of the dark current after photon absorption is accomplished by many processes, including these:
1. Shut-off of rhodopsin, as discussed above.
2. Inherent GTPase activity of Tα, converting Tα back to the GDP-bound, inactive form that reassociates with Tβγ.
3. Reassociation of PDEγ with PDEαβ, returning PDE to its more inhibited, dark state.
4. Synthesis of new cGMP from GTP by guanylate cyclase (GC).
5. Reopening of CNG ion channels, as more cGMP becomes available.
Although these processes are the most established ones, there is recent evidence that other processes and factors may be involved in regulating the cGMP cascade, mostly through feedback mechanisms. Some of the factors that may play a role are: regulatory cGMP-binding sites on PDE; auxiliary retinoid binding sites on opsin (Heck et al., 2003; Makino et al., 2010); calcium-binding proteins and other regulatory proteins that affect guanylate cyclase, PDE, rhodopsin, transducin and/or the CNG channel (e.g. guanylate cyclase activating protein, or GCAP; reviewed in Gross and Wensel, 2011); members of the inositol phosphate system whose function has not yet been established; and various kinases and phosphatases whose functions are also not yet clear. The field of regulation of the photoreceptor cGMP cascade is currently very unsettled and very intriguing.
An interesting set of molecular interactions that seems to regulate visual transduction, and even rod outer segment morphogenesis, occurs at the interface between the rod disks and the plasma membrane. The rod CNG channel is a heterotetramer consisting of three α (CNGA1) subunits and one β (CNGB1) subunit (see Chapter 35). But this channel does not stand alone. Instead, its α subunits are linked to Na+:Ca2+, K+ exchange carriers in the plasma membrane and these carriers expel the calcium ions that enter the cell through the CNG channel. Furthermore, the β subunit of the CNG channel is physically linked, via its cytosolic GARP (glutamic acid rich protein) region, to a molecular complex in the disk rim. This disk rim complex consists of an oligomer of Peripherin-2 and Rom-1, which also interacts with other GARPs that associate with the cytosolic side of the disks (reviewed in Sung and Chuang, 2010). Interestingly, a genetic knockout of the CNG channel’s β subunit and the GARPs disrupts the orderly array of disks in the rod outer segment, produces shorter outer segments and reduces the light sensitivity of the rod (Zhang et al., 2009). Thus, these molecular complexes are vital to both visual transduction and rod morphogenesis.
One aspect of visual transduction that is controlled by feedback regulation is light adaptation (reviewed in Lamb, 2011) and a large body of evidence supports involvement of Ca2+ in this process. Coupled with calcium-binding proteins, Ca2+ has been found to inhibit guanylate cyclase (GC), to stimulate PDE via inhibition of Rh phosphorylation and to inhibit the CNG channels (see Chapter 35). All three types of Ca2+ feedback appear to occur in both rods and cones, but with different molecular details and rates. For example, inhibition of the CNG channels by Ca2+ is much greater in cones than in rods (Rebrik and Korenbrot, 2004). Calcium feedback in rods and cones is thought to involve the following steps. In the dark, when the cGMP concentration is relatively high, Ca2+ enters the cell through the CNG channels, raising the intracellular concentration of Ca2+ ([Ca2+]i). When the channels close following photon absorption, [Ca2+]i decreases, because its influx through the channels is decreased while its extrusion by Na+:Ca2+, K+ exchangers continues. The decreased [Ca2+]i would be expected to stimulate guanylate cyclase and to inhibit PDE, leading to a subsequent partial recovery of the cGMP level and a reopening of some of the channels. The feedback mechanisms involving Ca2+ are mediated by several calcium binding proteins (reviewed in Gross and Wensel, 2011), but the most significant Ca2+ feedback effect on visual transduction appears to be that mediated by guanylate cyclase activating proteins, or GCAPs (e.g. Burns et al., 2002; reviewed in Gross and Wensel, 2011). GCAPs are Ca2+ binding proteins that bind to guanylate cyclase. The GCAPs stimulate guanylate cyclase to make more cGMP but, when Ca2+ binds to a GCAP, the GCAP is less stimulatory, so guanylate cyclase makes less cGMP, resulting in closure of CNG channels and thereby reducing Ca2+ entry. This same negative feedback system works in reverse in the light: fewer CNG channels are open, so less Ca2+ enters; then lower intracellular [Ca2+] produces stimulation of guanylate cyclase by GCAPs, resulting in higher [cGMP], more channel opening and therefore more Ca2+ entry. The accumulated knowledge of these feedback processes and the associated enzyme cascade has made vertebrate visual transduction a model system for understanding G-protein-mediated signal transduction in general.
BIBLIOGRAPHY
1. Bader CR, Bertrand D, Schwartz EA. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol. 1982;331:253–284.
2. Barnes S, Hille B. Ionic channels of the inner segment of tiger salamander cone photoreceptors. J Gen Physiol. 1989;94:718–743.
3. Bauer PJ. Binding of the retinal rod Na+/Ca2+-K+ exchanger to the cGMP-gated channel indicates local Ca2+-signaling in vertebrate photoreceptors. Ann NY Acad Sci. 2002;476:325–334.
4. Baylor DA. Photoreceptor signals and vision. Invest Ophthalmol Visual Sci. 1987;28:34–49.
5. Baylor DA, Nunn BJ. Electrical properties of the lightsensitive conductance of rods of the salamander Ambystoma tigrinum. J Physiol London. 1986;371:115–145.
6. Baylor DA, Lamb TD, Yau K-W. The membrane current of single rod outer segments. J Physiol London. 1979;288:589–611.
7. Baylor DA, Nunn BJ, Schnapf JL. Spectral sensitivity of cones of the monkey Macaca fascicularis. J Physiol London. 1987;390:145–160.
8. Burns ME, Baylor DA. Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu Rev Neurosci. 2001;24:779–805.
9. Burns ME, Mendez A, Chen J, Baylor DA. Dynamics of cyclic GMP synthesis in retinal rods. Neuron. 2002;36:81–91.
10. Do MTH, Yau K-W. Intrinsically photosensitive retinal ganglion cells. Physiol Rev. 2010;90:1547–1581.
11. Dratz EA, Hargrave PA. The structure of rhodopsin and the rod outer segment disk membrane. Trends Biochem Sci. 1983;8:128–131.
12. Fain GL, Hardie R, Laughlin SB. Phototransduction and the evolution of photoreceptors. Curr Biol. 2010;20:R114–R124.
13. Fu Y, Yau K-W. Phototransduction in mouse rods and cones. Pflügers Arch. 2007;454:805–819.
14. Gross AK, Wensel TG. Biochemical cascade of phototransduction. In: Kaufman PL, Alm A, Levin LL, Nilsson SFE, Ver Hoeve JN, Wu SM, eds. Adler’s Physiology of the Eye. Elsevier 2011;:394–410.
15. Heck M, Schädel SA, Maretzki D, Hofmann KP. Secondary binding sites of retinoids in opsin: characterization and role in regeneration. Vis Res. 2003;43:3003–3010.
16. Hofmann KP, Scheerer P, Hildebrand PW, et al. A G protein-coupled receptor at work: the rhodopsin model. Trends Biochem Sci. 2009;34:540–552.
17. Katz B, Minke B. Drosophila photoreceptors and signaling mechanisms. Front Cell Neurosci. 2009;3:1–18.
18. Kawamura S, Tachibanaki S. Rod and cone photoreceptors: molecular basis of the difference in their physiology. Comp Biochem Physiol Part A. 2008;150:369–377.
19. Lamb TD. Spontaneous quantal events induced in toad rods by pigment bleaching. Nature. 1980;287:349–351.
20. Lamb TD. Evolution of vertebrate retinal photoreception. Phil Trans R Soc B. 2009;364:2911–2924.
21. Lamb TD. Light adaptation in photoreceptors. In: Kaufman PL, Alm A, Levin LL, Nilsson SFE, Ver Hoeve JN, Wu SM, eds. Adler’s Physiology of the Eye. Elsevier 2011;:429–442.
22. Lamb TD, Pugh Jr EN. Dark adaptation and the retinoid cycle of vision. Prog Ret Eye Res. 2004;23:307–380.
23. MacLeish PR, Makino CL. Photoresponses of rods and cones. In: Kaufman PL, Alm A, Levin LL, Nilsson SFE, Ver Hoeve JN, Wu SM, eds. Adler’s Physiology of the Eye. Elsevier 2011;:411–428.
24. Makino CL, Riley CK, Looney J, Crouch RK, Okada T. Binding of more than one retinoid to visual opsins. Biophys J. 2010;99:2366–2373.
25. Mata NL, Radu RA, Clemmons RS, Travis GH. Isomerization and oxidation of Vitamin A in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80.
26. Molday RS, Zhang K. Defective lipid transport and biosynthesis in recessive and dominant Stargardt macular degeneration. Prog Lipid Res. 2010;49:476–492.
27. Nickle B, Robinson PR. The opsins of the vertebrate retina: insights from structural, biochemical, and evolutionary studies. Cell Molec Life Sci. 2007;64:2917–2932.
28. Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–767.
29. Rebrik TI, Korenbrot JI. In intact mammalian photoreceptors, Ca2+-dependent modulation of cGMP-gated ion channels is detectable in cones but not in rods. J Gen Physiol. 2004;123:63–75.
30. Schnapf JL, Nunn BJ, Meister M, Baylor DA. Visual transduction in cones of the monkey Macaca fascicularis. J Physiol London. 1990;427:681–713.
31. Schnetkamp PPM. The SLC24 Na+/Ca2+-K+ exchanger family: vision and beyond. Pflügers Arch Eur J Physiol. 2004;447:683–688.
32. Smith SO. Structure and activation of the visual pigment rhodopsin. Annu Rev Biochem. 2010;39:309–328.
33. Stryer L. Visual excitation and recovery. J Biol Chem. 1991;266:10711–10714.
34. Sung C-H, Chuang J-Z. The cell biology of vision. J Cell Biol. 2010;190:953–963.
35. Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharm Toxicol. 2007;47:469–512.
36. Wang J-S, Kefalov VJ. The cone-specific visual cycle. Prog Ret Eye Res. 2011;30:115–128.
37. Wong KY, Berson DM. Ganglion-cell photoreceptors and non-image-forming vision. In: Kaufman PL, Alm A, Levin LL, Nilsson SFE, Ver Hoeve JN, Wu SM, eds. Adler’s Physiology of the Eye. Elsevier 2011;:526–544.
38. Yau K-W, Hardie RC. Phototransduction motifs and variations. Cell. 2009;139:246–264.
39. Zhang Y, Molday LL, Molday RS, et al. Knock out of GARPs and the β-subunit of the rod cGMP-gated channel disrupts disk morphogenesis and rod outer segment structural integrity. J Cell Sci. 2009;122:1192–1200.
40. Zimmerman AL, Baylor DA. Cation interactions within the cyclic GMP-activated channel of retinal rods from the tiger salamander. J Physiol London. 1992;449:759–783.