Chapter 39
Gustatory and Olfactory Sensory Transduction
Chapter Outline
I Summary
Taste and olfaction share certain common features. Receptor cells for both senses are epithelial cells and part of a renewing population. Olfactory and taste sensory cells alike lie at the interface between two strikingly different environments – an external environment of volatile odors or water-borne tastants, respectively, versus a relatively constant milieu of interstitial fluid. Moreover, transduction mechanisms in both these chemical senses involves G-protein-coupled receptors, although transduction mechanisms in gustation also include ion channels through which certain taste stimuli pass. Transduction channels and integral membrane receptors appear to be concentrated on the exposed apical tips of both types of chemosensory receptor cells, although the basolateral membranes of taste cells may be additional sites for chemosensory transduction. In some regards, sensory transduction in olfaction is similar to that in photoreception. Photoreceptors and olfactory receptor neurons alike possess cyclic nucleotide-gated ion channels. Nonetheless there are key differences between olfaction and taste. Olfactory receptor neurons are optimized for low concentrations of chemical stimuli; gustatory sensory cells require much higher concentrations of stimuli to become activated. Current topics of intensive research in the peripheral transduction mechanisms for taste and olfaction include, among others, discovering structural details of, and ligand-binding pockets for, the receptor proteins, learning how an olfactory receptor neuron determines which one odorant receptor gene it will express from the several hundred in its genome; identifying transmitters and determining synaptic mechanisms that transmit signals between cells in the taste bud; and understanding how the output signals from the peripheral sensory organs of olfaction and gustation are encoded as patterns of nerve impulses (the sensory code) that are transmitted to the brain and produce odor and taste perceptions.
II Introduction
Peripheral sensory cells in olfaction, taste, hearing and vision are modified epithelial cells and share certain properties common to epithelial tissues. These properties include (1) an ongoing renewal of the cell population1 and (2) cellular polarity, manifest in epithelial cells as two distinctive membrane regions – apical versus basolateral surfaces, with a barrier of tight junctions to separate the superficial (apical) environment from the tissue spaces surrounding the rest of the cell. Apical and basolateral cell surfaces in epithelial cells and many sensory receptor cells are exposed to two vastly different ionic milieus. This is especially true in gustatory and olfactory receptor cells. The apical membrane tips in these sensory cells confront external chemical environments in the oral and nasal cavities, respectively, that can change profoundly during stimulation and that can often be quite harsh. In contrast, the cell bodies of gustatory and olfactory sensory cells are bathed in a protected, relatively unchanging medium – interstitial fluid.
Chemical stimuli are initially transduced into intracellular electrical signals at the exposed apical tips of taste and olfactory sensory cells. Tight junctions confine most chemical stimuli to the specialized apical membrane and prevent the stimuli from penetrating deeper into the sensory epithelium (although there may be exceptions in gustatory sensory cells, see Section III). This has two implications for gustation and olfaction. First, molecular receptors for signal transduction must reside in the apical membrane of the sensory cells where chemical stimuli contact the cells. Second, because chemosensory stimulation is usually associated with a receptor (or generator) current (flux of ions) across the apical chemosensitive membrane, the ionic environment at the exposed apical region can have a significant impact on transduction. For example, olfactory sensory cells possess a Cl− conductance that exploits the low external and high intracellular Cl− concentrations at the apical membrane of these cells to generate an inward current during odor stimulation (discussed in detail below). This Cl− current amplifies the sensory signal.
Olfactory receptor neurons possess axons and transmit their signals directly to the brain and, specifically, the olfactory bulb (Fig. 39.1). Odor signals are decoded and processed in the olfactory bulb and in higher brain centers. In sharp contrast, taste receptor cells transmit their signals via synapses onto primary sensory fibers innervating taste buds. The primary sensory fibers then communicate with the brain. Moreover, cells within a taste bud interact via chemical and electrical synapses to process gustatory signals via excitatory and inhibitory cell–cell interactions (Roper, 1992; Chaudhari and Roper, 2010). Thus, some degree of cross-talk and information processing occurs in the end organs of taste. The details and significance of these cell–cell interactions are only now emerging.
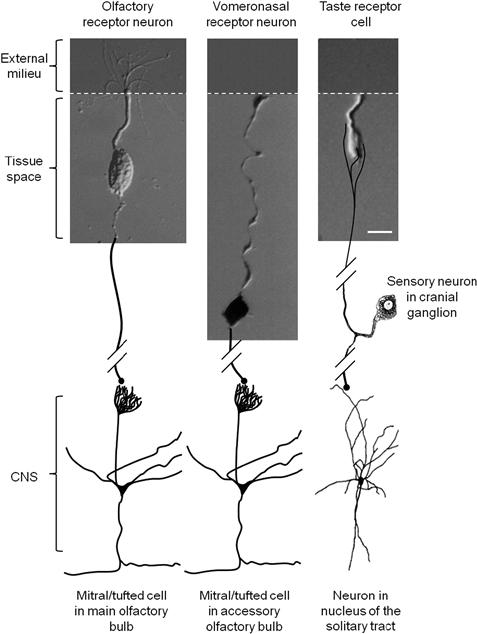
FIGURE 39.1 Schematic drawing comparing sensory cells experimentally isolated from olfactory epithelium, vomeronasal organs and taste buds. The apical tips of these cells are exposed to the external environment (dark shaded) where the initial events in chemosensory transduction occur. The basolateral membrane of the cells lies below the apical junctional complex (white dashed line) and is surrounded by interstitial fluid. Olfactory receptor neurons and vomeronasal sensory cells have axons (drawn in here) that project into the central nervous system (CNS) where they form synapses with mitral/tufted cells in the olfactory bulb. In contrast gustatory receptor cells form synapses in the taste bud with primary sensory fibers from cranial sensory ganglion neurons. Calibrations = 10 μm. (Olfactory receptor neuron modified from Kleene and Gesteland, 1981; vomeronasal receptor neuron modified from Ghiaroni et al., 2003; taste receptor neuron modified from Bigiani et al., 2002.)
This chapter describes events taking place in mammalian taste and olfactory sensory cells. Reference to other vertebrate species will occasionally be made, but chemosensory transduction in mammals will be the focus. Distinctly different molecules and mechanisms for taste and olfaction occur in invertebrates and these will not be described.
III Taste Receptor Cells
IIIA General Comments
Peripheral sensory organs for taste consist of clusters of receptor cells, the taste buds, found throughout the oral cavity. There are 2000–10 000 taste buds in humans. This number varies considerably from individual to individual. Each taste bud consists of 50–100 cells. Taste buds are embedded in the lingual epithelium in specialized protuberances, or papillae (fungiform, foliate and circumvallate papillae). Taste buds are also found on the soft palate, uvula, epiglottis, pharynx, larynx and have even been reported in the upper esophagus. Interestingly, certain cells in the walls of the stomach and intestine express proteins that previously were associated only with taste buds, indicating that chemosensing most likely occurs throughout the length of the digestive tract. However, cells in the stomach and intestines that have taste bud-like proteins are not gustatory sensory cells. They are not organized into collections resembling taste buds; they are not innervated by cranial gustatory nerves; and they are not known to generate taste perceptions.
Taste buds respond to a number of basic taste qualities, including sweet, sour, salty, bitter, umami (discussed later) and perhaps others such as fat. Early investigators tested whether particular regions of the tongue were specialized for these different qualities (Hänig, 1901). They found a somewhat greater sensitivity to bitter in the posterior tongue and to sweet at the tip (Fig. 39.2). However, these original findings were overinterpreted in the intervening years, leading to the popular misconception that there is a discrete and well-delineated “taste map” on the tongue. The truth is that all parts of the tongue sense the basic qualities, as shown in Fig. 39.2, and individual taste buds have been shown to respond to several different taste qualities.
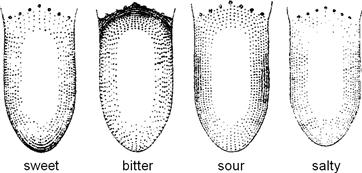
FIGURE 39.2 Regional distribution of sweet, bitter, sour and salty taste on the human tongue, as originally determined by D.P. Hänig (1901). The sensitivity for a particular taste quality is represented by the density of stippling. There is a slight tendency for the anterior tip of the tongue to be more sensitive to sweet, the lateral regions to be more sensitive to sour and the posterior to bitter. However, there is complete overlap for all these taste qualities. Also, see discussion in Lindemann, (1999).
Most of the cells in a taste bud are narrow elongate cells, extending nearly the full thickness of the lingual epithelium. The basal processes of taste bud cells reach to the basement membrane below the lingual epithelium and the apical processes extend up into a tiny cavity in the lingual surface at the top of the taste bud, the taste pore. The elongate, columnar morphology of gustatory sensory cells sets taste buds apart from the surrounding laminated (“stratified”) epithelium. That is, taste buds form tiny islands of simple columnar epithelium within the larger sheet of stratified squamous lingual epithelium. Such an arrangement positions taste cells directly across the electric field generated by lingual transepithelial ion transport currents. This may have implications for taste transduction.
There are three or more different types of cells in mammalian taste buds. Based on cytological features, histologists categorized taste cells as dark cells and light cells. Later, electron microscopists delineated types I, II and III cells based on ultrastructural features, including that only one of the cell types (type III) possesses synaptic connections. These morphological classifications have recently been tremendously clarified by combining molecular profiling with functional analyses on isolated taste cells. It is now recognized that type I cells are glial-like; type II cells express G-protein-coupled receptors for and respond to sweet, bitter and umami tastes; and type III cells express synaptic proteins associated with vesicular release, possess synaptic specializations and respond to sour (acid) taste (reviewed in Chaudhari and Roper, 2010). The taste cells responsible for salt (NaCl) taste have not yet been identified unambiguously, though there are suggestions that type I and III cells participate. In recognition of the functional roles of the cells comprising taste buds, type II cells have also been termed receptor cells and type III cells named presynaptic cells.
Taste cells, like the adjacent non-taste stratified epithelium, represent a renewing population. Taste bud cells have an estimated average life span of approximately 10 days in the mammal, longer than the surrounding non-sensory epithelium (3–4 days). There are early indications that the type I, II (receptor) and III (presynaptic) taste cells may have different longevities, but the data are only preliminary. Taste cells may be born from stem cells at the base of the taste bud. They may also originate from adjacent epithelial cells immediately surrounding taste buds. The precise origin of taste bud cells and whether the different cell types have a common lineage during development and in the adult are questions currently under investigation.
Recent investigations indicate that there are important cell–cell synaptic interactions in the taste bud, including feed forward and feedback signaling (reviewed in Roper, 2007; Herness and Zhao, 2009; Chaudhari and Roper, 2010). These interactions appear to shape the signals generated within the taste bud during taste excitation and may be critical in establishing a taste code that is transmitted to the brain for further processing. A discussion of signal processing in taste buds and how gustatory information is decoded in the central nervous system are topics beyond the scope of this chapter.
IIIB Nature of Taste Stimuli
Many, if not most water-soluble chemicals probably elicit a taste. Chemical stimuli have been grouped into five primary taste qualities: sweet, sour, salty, bitter and umami. There is ongoing debate whether there are additional taste qualities such as fatty and astringent, though these categories are blurred by somatosensory contributions (tactile, texture, etc). The existence of primary taste qualities argues there are distinct transduction mechanisms and signaling pathways for each of the basic tastes and this notion has guided taste research.
Chemicals that elicit taste usually do so only at high concentrations (millimolar to molar). However, certain bitter-tasting substances (such as quinine, caffeine, strychnine) and artificial sweeteners are relatively potent stimuli and elicit taste responses at μM concentrations. Nonetheless, it is clear that taste cells are relatively insensitive when compared with the exquisite responsiveness of photoreceptors (i.e. single photons) or olfactory and pheromone chemoreceptors (i.e. picomolar stimulus concentrations, possibly even single molecules). Primary functions of taste organs include regulating the intake of important nutrients and protecting against the ingestion of spoiled food and other harmful substances. Therefore, the generally low sensitivity of gustatory sensory cells to nutrients (carbohydrates, sweet; amino acids/protein, umami; Na+, salty) and high sensitivity to potentially detrimental compounds (toxins and rancid food, bitter) may represent an optimization for tastant concentrations that are physiologically relevant. That is, low taste sensitivity prevents receptors from becoming saturated and unresponsive to tastants that are required in abundance (nutrients); high bitter sensitivity assures that dangerous compounds will be detected and avoided even at low concentrations. (Many bitter-tasting chemicals are toxic and, conversely, many toxic chemicals elicit bitter taste.) In this light, the biological significance of sour taste (acids) is somewhat enigmatic. Sourness is an aversive taste to non-human animals and human infants but can be an acquired taste in adults. Further, rancid or spoiled food is often acidic.
Taste stimuli are dissolved in saliva to bathe the chemosensitive (apical) surface of gustatory sensory cells. Most chemical stimuli are prevented from gaining access to the basolateral regions of the taste bud by the tight junctions of the junctional complex at the taste pore. Notable exceptions are organic acids, such as acetic acid (vinegar) and citric acid, which elicit sour taste, and which rapidly breach the epithelial barrier. In their fully protonated form (i.e. neutral molecules), these acids penetrate into and throughout the epithelium, including into the cellular interior. Inside cells, the protonated molecules dissociate to release H+. This penetration of organic (“weak”) acids into the cytosol is believed to be an important contribution to sour taste stimulation (see below). Also, tight junctions between taste bud cells are somewhat leaky to certain ions, such as Na+, K+ and Cl− (Ye et al., 1993). These ions pass from the lingual surface into the intercellular spaces within the taste bud. It is possible that chemotransduction mechanisms for NaCl, KCl and possibly other stimuli exist on the basolateral membranes of taste receptor cells as well as on their exposed apical tips. Basolateral transduction mechanisms may explain intravascular taste, where blood-borne chemicals elicit gustatory sensations (Bradley, 1973). The bitter taste of some drugs injected intravenously and the sweet taste of intravenously-injected saccharin, once used to monitor blood circulation, are examples of intravascular taste.
IIIC Initiation of Taste Receptor Potentials
Taste cells are excitable and generate action potentials when depolarized sufficiently. This was first demonstrated with intracellular recordings from the large taste bud cells found in the amphibian, Necturus maculosus (Roper, 1983), but has subsequently been verified with patch-clamp recordings from mammalian taste cells (Fig. 39.3A, B). Specifically, taste bud cells (types II and III) possess inward, voltage-dependent, tetrodotoxin-sensitive Na+ currents and outward, TEA-sensitive K+ currents (Fig. 39.3C, D), typical of many neurons. Type III cells, but not type II cells, also have inward, voltage-dependent Ca2+ currents.
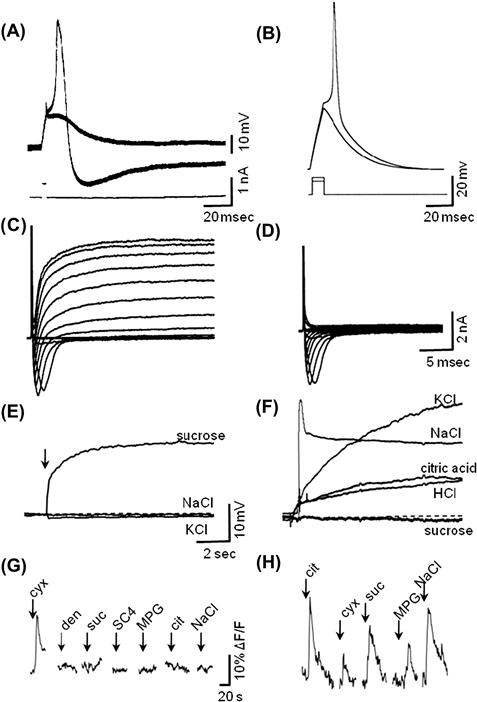
FIGURE 39.3 Taste receptor cells are excitable and respond to taste stimulation with depolarizing generator potentials and intracellular Ca2+ increases. (A) Intracellular recording from amphibian taste receptor cell showing responses to threshold current pulses passed through the recording electrode. (B) Patch electrode recording from mouse taste receptor cell, current clamp mode, showing responses to depolarizing current pulses at threshold. (C) Patch-clamp recording from mouse taste receptor cell showing inward and outward currents produced by current steps. (D) As in (C), but in presence of TEA to block outward K current. (E) Patch electrode recording of rat taste receptor cell, current clamp mode, showing a depolarizing response to sucrose applied to the apical, chemosensitive tip, but no responses to similarly applied solutions of NaCl or KCl. (F) As in (E), but showing another taste cell that was broadly sensitive to KCl, NaCl, citric acid and HCl, but not sucrose. (G) Mouse type II taste cell showing Ca2+ responses evoked by a series of different chemical stimuli applied to the apical chemosensitive tip. This cell responded only to one stimulus, a bitter taste (cycloheximide, cyx). (H) A mouse type III taste cell showing Ca2+ responses to several different stimuli, including sour (citric acid), bitter (cycloheximide, cyx), sweet (sucrose, suc), umami (monopotassium glutamate, MPG) and salty (NaCl). (G, H) were taken from calcium imaging experiments showing changes in fluorescence (ΔF) of an intracellular Ca2+-sensitive dye. ((A) Modified from Roper, 1983, (B, C, D) modified from Bigiani et al., 2002, (E, F) modified from Gilbertson et al., 2001, (G, H) modified from Tomchik et al., 2007.)
Gustatory stimuli generate receptor potentials and transient increases of [Ca2+] inside taste bud cells (Fig. 39.3E–H). The conversion of a gustatory chemical signal into an intracellular receptor potential or Ca2+ transient is termed taste transduction. The endpoint of taste transduction is the release of neurotransmitter(s) from the sensory cell.
Unlike signal transduction in most other sensory cells, there is no single unifying mechanism underlying taste transduction. The necessity of taste organs to sense a wide range of chemical substances, from simple ions to complex proteins, has led to the evolution of multiple taste transduction mechanisms (reviewed by Smith and Margolskee, 2001; Sugita, 2006; Roper, 2007; Chaudhari and Roper, 2010). Different taste bud cells possess distinct transduction mechanisms and respond to different taste stimuli. For instance, receptor (type II) taste cells respond specifically to sweet, bitter or umami taste compounds. Presynaptic (type III) taste cells respond to sour taste. Moreover, due to excitatory interactions between receptor and presynaptic cells, presynaptic cells also respond (indirectly) to sweet, bitter and umami (Tomchik et al., 2007). Presynaptic cells also are excited by salty stimuli, though whether this is due to direct stimulation or to indirect activation via intercellular communication from other taste bud cells remains unsolved.
Taste transduction can be divided into two primary mechanisms:
1. Ion permeation through specific channels. Certain taste stimuli, most notably Na+, enter taste cells through ion channels in the membrane, thereby generating transmembrane receptor currents and receptor potentials. Protons may also pass through ion channels and elicit sour taste responses.
2. Receptor-ligand binding. Interactions occur between chemical stimuli (ligands) and specific membrane bound receptors (in particular, G-protein-coupled receptors, GPCRs) that ultimately lead to receptor currents or the release of intracellular Ca2+, or both.
Parenthetically, some researchers believe that amphipathic stimuli, such as saccharin and quinine, may bypass ion channels and receptors and penetrate through the lipid plasma membrane to act directly on intracellular signaling cascades. Whether this occurs at physiologically relevant concentrations of the taste compounds, however, remains to be established.
IIIC1 Ion Permeation Through Specific Channels
ENaC Channels and Salt Taste
Sodium salts (“salty taste”) are believed to be transduced in a subset of taste cells by the direct permeation of Na+ through passive Na+-selective channels (as opposed to TTX-sensitive, voltage-dependent Na+ channels that underlie action potentials). Most of salt taste is transduced by a mechanism that is sensitive to the diuretic, amiloride. The most likely candidate Na taste transducer to date is the amiloride-sensitive epithelial Na+ channel (ENaC) found in renal tubules and elsewhere (Heck et al., 1984). Indeed, mRNA isolated from lingual epithelium and immunostaining of taste buds shows that all three ENaC subunits (α, β and γ) are expressed in taste cells (Kretz et al., 1999; Lin et al., 1999). Where it has been measured (in frog taste cells), taste Na+ channels have small unitary conductance (1–2 pS) and are blocked by low concentrations of amiloride (Ki = 0.2–1 μM), consistent with ENaC channels. Recent findings from mouse taste buds show that certain taste cells manifest ENaC-like ion currents (Vandenbeuch et al., 2008) and taste cells expressing ENaC respond to NaCl stimulation (Chandrashekar et al., 2010).
ENaC channels are open at rest and allow an ongoing influx of Na+, driven by this cation’s electrochemical gradient. The concentration of Na+ in human saliva varies from about 3 to 63 mM, depending on the rate of salivary secretion. This results in an equilibrium potential for Na+ (ENa) of about −30 to +50 mV (assuming [Na+]i is 10 mM) across the apical membrane of salt-sensitive taste cells. Thus, with a resting potential estimated to be −60 to −80 mV, there is likely to be a small steady-state influx of salivary Na+. During stimulation with NaCl when [Na+] in the oral cavity can reach concentrations up to a fraction of a mole (a typical soup, for instance, contains ≈0.25 M NaCl), the electrochemical gradient driving Na+ into the cells increases and Na+ influx depolarizes the cell. This current exits across the base of the cell forming a complete current loop via an extracellular (paracellular) pathway that includes the junctional complex near the taste pore. Consequently, any factors that affect the basolateral membrane conductance or the paracellular resistance, especially at the tight junctions near the taste pore, will also affect the magnitude of the receptor currents.
As mentioned previously, Na+ on the tongue surface may also partly penetrate the apical junctional complex of taste buds and raise [Na+]o in the interstitial spaces surrounding taste cells. If Na+ transduction channels are expressed on the basolateral as well as apical membrane of salt-sensitive taste cells, penetration of Na+ into the interior of the taste bud would provide another avenue for Na+ taste stimulation. Immunostaining for ENaC channels has suggested their presence on the basolateral membrane (Lindemann et al., 1998), but Na+ responses in mouse fungiform taste cells were unaffected when their basolateral surface was exposed to the ENaC antagonist amiloride (Yoshida et al., 2009a). This suggests that if basolateral Na+ transduction occurs, it is unlikely to be via ENaC channels.
Although the evidence is good in experimental animals, particularly rodents, a role for ENaC channels in human salt taste has been questioned. Amiloride appears to affect only the sourness, not saltiness, of NaCl solutions in psychophysical studies (Ossebaard and Smith, 1996). Furthermore, even in non-human species, not all salt taste can be explained by ENaC channels. A component of salt taste in experimental animals is unaffected by amiloride, especially in the posterior tongue. Mechanisms other than amiloride-sensitive (presumably ENaC) channels must exist.
Proton (H+) Channels and Sour Taste
How acids (sour taste) are transduced may differ from species to species and our understanding of sour transduction remains incomplete. In the amphibian, Necturus, protons block apical K+ channels and depolarize the cell. Additionally, protons themselves permeate the apical membrane, providing a depolarizing inward receptor current. Interestingly, protons permeate hamster taste cells via amiloride-sensitive Na+ channels, believed to be ENaC (Fig. 39.4A). Protons bind to the ion selectivity site in the ENaC channel more strongly than do Na+ ions. Consequently, H+ conductance of ENaC is less than that of Na+. This also means that protons interfere with the flux of Na+ through the channel. In contrast, protons permeate mouse taste cells via proton-gated channels that are unaffected by amiloride and thus are unlikely to be related to ENaC (Fig. 39.4B). In humans, psychophysical studies implicate a role for amiloride-sensitive Na+ channels in sour taste (Ossebaard and Smith, 1996). (The interference between Na+ and H+ permeation of amiloride-sensitive Na+ channels may help explain the culinary wisdom that acidifying food, for example by adding vinegar or lemon juice, reduces its salty taste.) In short, our understanding of roles for ion channels and proton influx in sour taste is fragmentary.
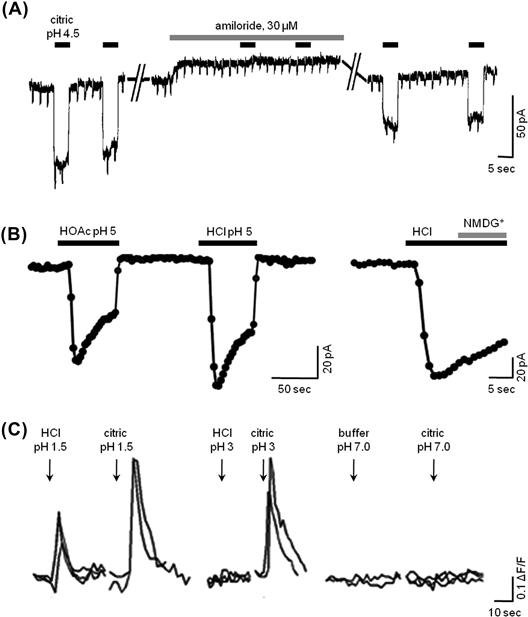
FIGURE 39.4 Sour taste transduction. (A) In hamsters, sour taste stimulation triggers an inward proton flux through amiloride-sensitive (presumably ENaC) channels. These traces are patch-clamp recordings of an isolated fungiform taste cell showing inward current responses evoked by 2.5 mM citric acid (pH 4.5) and their block by 30 μM amiloride (modified from Gilbertson et al., 1993). (B) In sour-sensitive taste cells from mice, acid stimulation also evokes inward proton currents. Unlike those in hamsters (A), these currents are not affected by amiloride (data not shown). Traces show patch-clamp recordings from an isolated mouse fungiform taste cell showing inward current responses evoked by 2 mM acetic acid (HOAc, pH 5) and 10 mM HCl (pH 5). Replacing Na+ with NMDG+ had no effect on the HCl-evoked response (final trace), consistent with the current being carried by protons, not Na+ (modified fromChang et al., 2010 ). (C) Sour stimuli also elicit Ca2+ transients in taste cells, recorded with functional imaging. The traces show Ca2+ influx in sour-sensitive circumvallate taste cells from a mouse. The responses were triggered by brief applications of acid stimuli applied focally to the taste pore in a lingual slice preparation. Citric acid was much more effective than HCl at equivalent pH values, (modified from Richter et al., 2003).
However, a major component of sour taste may be unrelated to the influx of protons and generation of a depolarizing H+ current. Namely, organic acids such as acetic acid (vinegar), citric acid (fruit) and tartaric acid (wine) are potent sour stimuli. In fact, at equal pH values, organic (“weak”) acids are more sour than mineral (“strong”) acids such as HCl. This cannot easily be explained by H+ influx through proton channels. At equal pH, the H+ concentration of an organic and a mineral acid stimulus, and hence the inward driving force for protons across the taste cell membrane, would be identical. Organic and mineral acids at the same pH would be expected to be equally sour, which is not the case. Psychophysical measurements of sourness, taste nerve responses to acid stimuli and responses of sour-sensitive taste cells consistently indicate that organic acids are more effective than mineral acids at a given pH (e.g. see Fig. 39.4C) (Richter et al., 2003; Huang et al., 2008). The explanation is that the protonated organic acid molecules readily pass through cell membranes and penetrate into the taste cell interior where they dissociate into proton(s) and anion(s) (see Roper, 2007). Permeation of organic acid molecules into the cell results in cytosolic acidification, which is believed to contribute markedly to sour taste transduction. That is, intracellular protons, not extracellular H+, appear to be a proximate stimulus for sour taste (Lyall et al., 2001). Accordingly, a cytosolic protein or the intracellular face of a membrane transducer likely binds protons and initiates the events leading to a signaling cascade for sour taste. One such candidate that has been shown to transduce acid-evoked nociception and that involves cytosolic acidification is the cation channel TRPA1 (Wang et al., 2011). However, to date, there is no strong evidence for the expression of TRPA1 in taste buds or TRPA1-mediated sour taste transduction. Other candidate intracellular targets that have been hypothesized include 2-pore domain K+ channels that are blocked by cytosolic acidification (Richter et al., 2004).
In all likelihood, sour taste transduction may eventually be explained by a combination of events – proton-gated channels triggered by extracellular H+ and cytosolic acidification leading to modification of intracellular proteins. The end result of sour taste transduction is depolarization of the acid-sensitive (presynaptic or type III) taste cells, influx of Ca2+ through voltage-gated Ca channels and release of transmitter(s), including serotonin (Huang et al., 2008).
IIIC2 G-Protein-Coupled Receptors for Taste
G-protein-coupled receptors (GPCRs) have been identified for three of the primary tastes – sweet, bitter and umami. These are the T1Rs (sweet, umami) and T2Rs (bitter) and their corresponding genes, Tas1Rs and Tas2Rs (Chandrashekar et al., 2000, 2006; Nelson et al., 2001, 2002; Max et al., 2001; Sainz et al., 2001). In humans and many other mammals, there are three members of the small family of T1R GPCRs – T1R1, T1R2 and T1R3. These GPCRs form heterodimers to create either a sweet (T1R2+T1R3) or an umami (T1R1+T1R3) receptor. (Interestingly, in frogs, there are no T1Rs, implying that these amphibia do not sense sweet or umami tastes, at least not transduced by T1Rs). The dimeric sweet receptor (T1R2+T1R3) responds to a variety of different sugars (sucrose, fructose, glucose, etc.) as well as to artificial sweeteners (e.g. saccharin, aspartame, acesulfame potassium, cyclamate) and sweet-tasting proteins (monellin, thaumatin, brazzein)2. The binding pockets on the dimeric T1R2+T1R3 differ for the different sweet ligands. For example, glucose, sucrose and aspartame bind to the extensive amino termini (“venus fly trap” domain) of T1R2 and T1R3; other artificial sweeteners, such as cyclamate, to a pocket in the transmembrane region of T1R3; and sweet proteins to a cysteine-rich domain that links the large extracellular amino terminus of T1R3 to its seven transmembrane domains. Nonetheless, ligand interactions with T1R2+T1R3, whether at one or more of these different binding sites, activate a common downstream effector pathway, described below.
Taste receptors responding to amino acids, particularly sodium glutamate (monosodium glutamate, MSG) and that elicit “umami” are constructed from the combination of T1R1+T1R3. Thus, T1R3 is common to sweet and umami taste receptors. Glutamate most likely binds to the large amino terminal, venus fly trap domain. Other binding sites have not been extensively explored to date. Taste receptors other than T1R1+T1R3 are believed to exist for umami, based on findings that mutant mice lacking T1R3 still detect, albeit less robustly, solutions of umami compounds. Additional candidate umami receptors include variants of G-protein-coupled synaptic glutamate receptors, mGluR4 and mGluR1.
Bitter taste is transduced by the T2R class of receptors. In contrast to the small number of T1Rs, T2R genes (Tas2R) comprise a larger family. The number of T2R genes varies from species to species. In humans, there are 25 functional T2R receptors; in frogs, 47; in zebrafish, 4. It is unclear whether T2Rs exist as monomers or oligomers. T2Rs lack the extensive N termini that characterize T1Rs. Bitter compounds are believed to bind in a single transmembrane pocket of a given T2R, with certain T2Rs being more selective and others more broadly responsive to multiple, related ligands.
Taste GPCRs (i.e. T1Rs and T2Rs) are expressed by the type II (receptor) taste cells. In general, a given receptor (type II) cell expresses only one type of taste receptor – either sweet, umami, or bitter receptors. Consistent with this finding, in physiological studies, receptor cells have been shown mainly to respond (i.e. are “tuned”) to single taste qualities, sweet, bitter or umami (Tomchik et al., 2007; Yoshida et al., 2009b). Receptor taste cells that respond to bitter compounds express small, overlapping subsets of four to 11 T2Rs of the full repertoire of 25 human T2Rs (Behrens et al., 2007). Thus, it is understandable that bitter-sensitive taste receptor cells, each expressing a subset of T2Rs, respond to multiple bitter compounds.
IIIC3 Downstream Effectors
Activating either T1Rs or T2Rs on receptor cells by sweet, umami or bitter compounds initiates a common signaling cascade, consisting of liberating Gβγ proteins (specifically Gβ3/Gγ13) bound to the taste GPCR, triggering a taste-specific phospholipase C (PLCβ2), generating IP3 and consequently stimulating IP3 receptors on intracellular Ca2+ stores (specifically, IP3R3), thereby releasing Ca2+ into the cytosol (reviewed by Chaudhari and Roper, 2010). Thus, the net effect of taste stimulation is to mobilize intracellular Ca2+ (see Fig. 39.3G, H). The increased [Ca2+]i has a dual effect. Intracellular Ca2+ opens taste-specific cation channels in the basolateral membrane of receptor cells, TRPM5, allowing Na+ influx and thereby producing a depolarizing current. Ca2+ also acts on pannexin 1 gap junction hemichannels embedded in the plasma membrane. Pannexin 1 hemichannels are triggered open by the combined action of TRPM5-mediated depolarization and IP3-mediated Ca2+ increases. Opening pannexin 1 channels allows the efflux of taste transmitter, ATP, from receptor cells (Huang et al., 2007; Romanov et al., 2007), the end result of taste stimulation for these cells (Fig. 39.5A).
In addition to Gβγ proteins released during taste GPCR activation, a taste-specific Gα protein, Gα gustducin (McLaughlin et al., 1992) is liberated from the receptor. Unlike the Gβγ pathway, Gα signaling in taste transduction is not as well characterized. Gene-knockout mice lacking Gα gustducin show deficits in their ability to sense bitter, sweet and umami tastes, but whether this taste-specific Gα protein is directly intercalated in the taste transduction pathway is disputed. Evidence suggests that Gα gustducin plays an indirect role. Gα gustducin is a type of Gi protein, related to transducin in photoreceptors. By stimulating a phosphodiesterase present in taste cells, Gα gustducin acts to lower cytosolic cAMP. Because cAMP inhibits PLCβ2/IP3/Ca2+ signaling, lowering cytosolic cAMP enhances the receptor cell’s sensitivity to taste compounds (Clapp et al., 2008). In mutant mice lacking Gα gustducin, cAMP accumulates in receptor cells and depresses sensitivity to sweet, umami and bitter taste stimuli.
Figure 39.5 summarizes the several transduction pathways for taste stimulation.
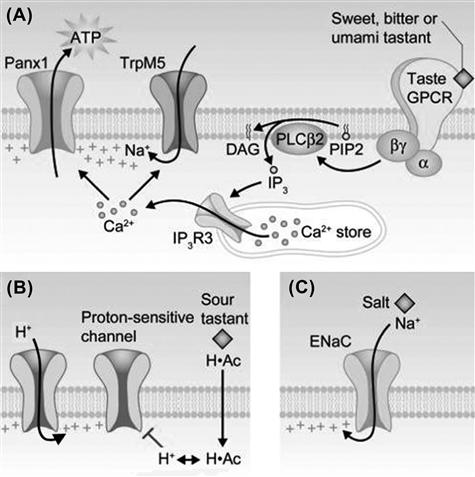
FIGURE 39.5 Taste transduction mechanisms for sweet, bitter, umami, sour and salty. (A) Sweet, bitter and umami compounds bind to G-protein-coupled taste receptors (shown at far right) on taste receptor (type II) cells and initiate a downstream cascade of Gβγ proteins, phospholipase C and intracellular Ca2+ release. (G-protein-coupled receptors for sweet and umami exist as heterodimers, not depicted in this cartoon.) Intracellular Ca2+ activates a cation channel, TrpM5, and a plasma membrane gap junction hemichannel, pannexin 1 (Panx1). When Panx1 opens, the taste neurotransmitter ATP is released into the interstitial spaces (Huang et al., 2007; Romanov et al., 2007). (B) Sour taste may involve proton influx through channels (left) and penetration of the sour tastant (shown here, acetic acid, H·Ac) across the plasma membrane to acidify the cytosol. Cytosolic acidification may block proton-sensitive K+ channels, as yet unidentified. (C) Salty taste can be evoked by Na+ influx through ENaC channels. This mechanism has been demonstrated in rodents but its generalization to humans has been questioned and remains to be established, (modified from Chaudhari and Roper, 2010).
IIID Termination of Taste Signals
Termination of chemostimulation in taste cells is probably due to two factors: diffusion of the stimulus away from the apical chemosensitive tips and receptor cell adaptation. Little is known about adaptation in gustatory sensory cells. It is plausible that the Ca2+-dependent Cl− conductance that exists in taste bud cells contributes to sensory adaptation in taste (Taylor and Roper, 1994; Herness and Sun, 1999). Ca2+ mobilization in receptor cells during taste transduction would be expected to activate a basolateral Ca2+-dependent Cl−-conductance, triggering an influx of Cl− from the interstitial fluid surrounding taste cells and generating an outward, repolarizing current.
IV Olfactory Receptor Cells
IVA General Comments
Receptor cells for odorants are distributed in specialized patches of olfactory sensory epithelium embedded within the nasal respiratory epithelium. These patches comprise about 1 cm2 of surface in each nostril in the human, or approximately 0.5–1% of the total nasal epithelium. The area of olfactory epithelium decreases with age, perhaps contributing to the decline of the sense of smell in the elderly.
Olfactory receptor cells are specialized neurons and are often termed olfactory receptor neurons (ORNs). Olfactory receptor neurons are elongate cells that extend from the base of the epithelium to the surface. Receptor neurons stand side by side with columnar supporting cells in the olfactory epithelium. The apical tips of ORNs consist of a tiny knob from which extend six to 12 long cilia (see Fig. 39.1). At their base, olfactory receptor neurons extend an axon that travels upward through perforations in the region of the cranium that overlies the olfactory epithelium (the cribriform plate) to reach the olfactory bulb of the CNS. Consequently, olfactory receptor neurons bypass any synaptic intervention in the periphery and transmit action potentials directly into the brain3.
Stem cells (horizontal and globose basal cells) reside at the base of the olfactory epithelium. These cells provide a reservoir of progenitors for the continuous renewal of the olfactory epithelium. Basal stem cells divide and differentiate into olfactory receptor neurons and supporting cells. The lifespan of olfactory receptor neurons was originally thought to be approximately 30 days (Graziadei and Monti Graziadei, 1978), but later findings indicate that ORNs may live for many months (Mackay-Sim and Kittel, 1991a,b).
Closely related to nasal olfactory epithelium is another peripheral chemosensory structure, the vomeronasal organ (VNO). The VNO detects a special category of volatile and non-volatile chemical stimuli which include pheromones. This sensory end organ is an invagination of the nasal epithelium in mammals4 or, in reptiles such as snakes, an opening in the oral cavity. Pheromones are chemical signals produced by one member of a species to communicate with other members of that same species (conspecific). Pheromones play a key role in endocrine responses and in shaping social and sexual interactions (e.g. territoriality, mating) among conspecifics, at least in non-human animals, and perhaps even in humans (Gelstein et al., 2011). Pheromone stimuli for VNO sensory neurons include airborne molecules (such as dihydro-exo-brevicomin, a volatile constituent of male mouse urine) and non-volatile molecules (such as aphrodisin, a component of vaginal secretions in hamsters). Animals also use their VNO to detect chemical stimuli such as predator odors from different species (heterospecific), as well as to identify food odors. Lastly, VNO sensory neurons may signal the health status of a conspecific (Riviere et al., 2009).
Receptor neurons in the VNO, as in the olfactory epithelium, possess axons that travel to and innervate portions of the olfactory bulb. However, unlike ORNs, sensory neurons of the VNO possess apical microvilli, not cilia. The initial events of transduction are believed to occur on the microvilli. Moreover, VNO sensory neurons send their axons to a separate portion of the olfactory bulb (the accessory olfactory bulb) reserved for processing pheromone stimuli. Sensory mechanisms for transducing pheromones and odorants are believed to be quite dissimilar, as will be discussed next. This chapter will focus mainly on olfactory transduction, which has been studied much more extensively to date (see reviews by Frings, 2001; Ache and Young, 2005; Munger et al., 2009; DeMaria and Ngai, 2010).
IVB Nature of Olfactory Stimuli
Odorants for land-dwelling animals are volatile compounds, typically diluted in large volumes of air. Odorants are only poorly soluble in aqueous solutions, including the mucus layer overlaying the olfactory epithelium. ORNs and VNO sensory neurons are much more sensitive to chemical stimulation than are taste receptor cells. Odorants and pheromones in the range of subnanomolar to micromolar concentrations can be detected. Many animals have a keen sense of smell; for example, dogs have been reported to detect as few as 5000 to 10 000 molecules/cm3. Reports of extreme chemosensitivity, such as the ability of certain species (e.g. moths, dogs and sharks) to detect single molecules of pheromones or odorants may be overestimates based on theoretical calculations that assume uniform spread of the odors in air or water. The actual dispersion of odorant and pheromone molecules is more often in the form of irregular plumes rather than uniform diffusion. Within the plume, concentrations are much higher than predicted by simple diffusion. Nonetheless, olfactory and pheromone receptors are notoriously responsive to chemical stimuli. The acute sensitivity of these receptor neurons might be an optimization of the sensory neuron to the low concentrations of biologically important chemical signals that must be detected.
Volatile odoriferous compounds partition into the mucus layer covering the olfactory epithelial surface. A specialized carrier or transport protein has been identified in the mucus layer-odorant binding protein (OBP). It has been hypothesized that OBP facilitates the partitioning of odorants into the mucus. According to this hypothesis, odorants bound to OBP are carried and presented to the chemoreceptive surface on the cilia that protrude up into the mucus layer.
The low concentration of odorants imposes certain constraints on the possible receptor mechanisms for olfaction (pheromone transduction in VNO sensory neurons will be discussed later). Namely, amplifying second messenger cascades are involved in transduction. Additionally, ORNs have a substantial input resistance (up to 30 GΩ). Consequently, tiny receptor currents – some investigators believe even those generated by stimulation by a single odorant molecule – can produce measureable voltage changes in an olfactory receptor neuron. If sufficiently large, these currents depolarize an ORN to threshold and generate action potentials. Intracellular second messenger cascades and a high input resistance combine to increase the sensitivity of olfactory receptor neurons.
IVC Initiation of Olfactory Receptor Potentials
Olfactory transduction involves ligand (odorant) binding to specific receptors localized in the ciliary membrane of olfactory receptor neurons.
IVC1 Odorant Receptors (ORs)
The transduction pathways that have been characterized for olfaction to date involve GPCRs that are integral proteins of the ciliary membrane. When stimulated by an odor, odorant receptors (ORs) activate an olfactory-specific Gα protein, Golf, and increase cAMP inside the cilia (see below). Volatile compounds, being lipophilic, may also partition into the membrane. However, this is not believed to contribute prominently to olfactory transduction.
GPCRs for odorants have been cloned and sequenced (Buck and Axel, 1991; Reed, 1992; Raming et al., 1993). ORs are members of a huge GPCR superfamily. In rodents there are ≈1400 OR genes located in clusters throughout the genome, ≈1000 of which are functional (Zhang et al., 2004). In humans, there are perhaps 855 OR genes but only ≈400 are functional, the rest being pseudogenes (Hasin-Brumshtein et al., 2009). The total number of OR genes represents a sizeable fraction of the genome (the human genome having ≈30 000 genes). From the large number of possible OR genes, a given olfactory receptor neuron expresses but a single OR throughout its lifespan. How a receptor neuron selects which OR to express is a topic of intense research.
IVC2 G Proteins in Olfactory Receptor Neurons
Odorant binding to receptors triggers second messenger pathways, beginning with activation of G proteins. The canonical odorant transduction pathway involves Golf, a type of Gs protein that is enriched in olfactory epithelium. Ligand binding to ORs liberates Golf, which then activates type III adenylyl cyclase, also expressed selectively in olfactory epithelium, resulting in the formation of cAMP (Pace et al., 1985; Bakalyar & Reed, 1990). It has long been known that exposing isolated olfactory cilia to low concentrations of certain fruity, floral or herbaceous odors results in an increase in intracellular cAMP. These observations were key in establishing adenylyl cyclase in the intracellular pathway for signal transduction in olfaction, especially for what are considered pleasant odors. A less well-established G-protein pathway, believed by some to act as an intermediary for signaling in putrid and unpleasant odors, is discussed later.
IVC3 Cyclic AMP and Cyclic Nucleotide-Gated Channels in Olfactory Receptor Neurons
The ion channels that ultimately are activated by odorant binding and that produce a depolarizing flow of current into olfactory receptor neurons are triggered open by cAMP. Intracellular cAMP binds to and rapidly opens these non-selective cation (Na+, K+, Ca2+) channels, named cyclic nucleotide-gated (CNG) channels. CNG channels are distributed along the ciliary membrane of the olfactory receptor neurons. CNG channels were first demonstrated by applying cAMP and cGMP to isolated patches of membrane removed from olfactory cilia (Nakamura and Gold, 1987) or to isolated olfactory receptor neurons (Firestein et al., 1991). CNG channels have little or no voltage dependence and, under physiological conditions (i.e. in the presence of extracellular Ca2+ and Mg2+), have a small conductance, ≈100 fS. Inward depolarizing current (Fig. 39.6A) is carried principally by Ca2+ and Na+.
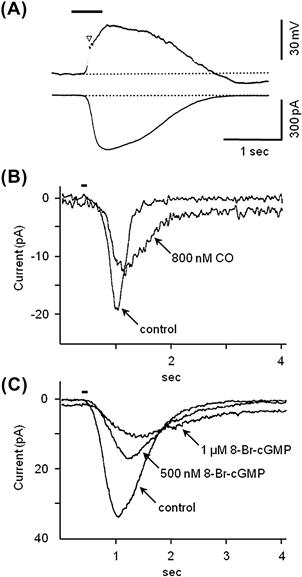
FIGURE 39.6 Patch-clamp recordings from olfactory receptor neurons. (A) Traces show the depolarizing receptor potential (top) and receptor current (bottom) elicited by applying an odorant (10 mM n-amyl acetate) to the olfactory cilia of isolated newt olfactory receptor neurons (bar above trace). Traces are from two different cells (modified fromKurahashi, 1989 ). (B) Inhibitory effects of carbon monoxide, CO and (C) of 8-Br-cGMP on receptor currents in salamander olfactory receptor neurons after brief applications of an odorant (100 pM cineole, bar above traces). CO stimulates the formation of cGMP during odorant stimulation, which ultimately decreases odor responses and may underlie long-lasting olfactory adaptation, (modified from Leinders-Zufall et al., 1996).
CNG channels from olfactory receptor neurons have been cloned and sequenced (Dhallan et al., 1990). Olfactory CNG channels are quite similar to CNG channels found in photoreceptors, though they differ in their sensitivity to cyclic nucleotides. CNG channels in olfactory receptor neurons respond to cAMP and cGMP alike, with cGMP being somewhat more potent. In contrast, cAMP is far less effective than cGMP in gating open photoreceptor CNG channels. It is an enigma that olfactory CNG channels are more sensitive to cGMP than cAMP because odorant stimulation is believed principally to elevate cAMP. A possible role for an indirect elevation of cGMP in olfactory adaptation (discussed later) may resolve this puzzle. Details of CNG channels are given in Chapter 35.
Calcium ion influx is a key component of the odorant-evoked current through olfactory CNG channels. The influx of Ca2+ has several important consequences in addition to supplying inward (depolarizing) current. First, Ca2+ influx activates a Ca2+-dependent Cl− conductance in the olfactory cilia5. This results in an outflow of Cl−, thereby producing an inward (depolarizing) current6. Inward current through the Ca2+-activated Cl− channels (i.e. efflux of Cl−) amplifies the ongoing inward current (Na+ and Ca2+ influx) through CNG channels. Indeed, in mouse olfactory receptor neurons, the Ca2+-activated Cl− current is even greater than the initial Na+ and Ca2+ current through CNG channels and can constitute up to 90% of the total inward current generated during odorant stimulation (Boccaccio and Menini, 2007). The combined action of the cAMP-gated cation channels and Ca2+-dependent Cl−-channels may exist to ensure a depolarizing receptor current even in the face of fluctuating extracellular (mucosal) cation concentrations7. Figure 39.7 summarizes this olfactory transduction pathway.
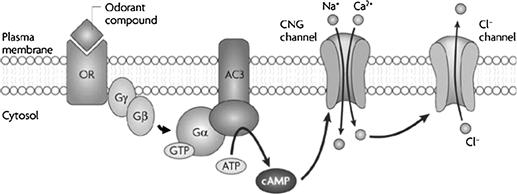
FIGURE 39.7 The canonical odorant transduction pathway. Binding of odorant compounds to an odorant receptor (OR far left) initiates a transduction cascade involving a G protein and activation of adenylate cyclase 3 (AC3) which, in turn, generates the second messenger cyclic AMP. cAMP binds to a cyclic nucleotide-gated (CNG) channel and results in the influx of cations (Na+ and Ca2+) which depolarize the cell membrane. Ca2+ also activates a Ca2+-dependent Cl− channel. Olfactory sensory neurons (OSNs) maintain a high intracellular Cl− concentration, such that this channel supports an efflux of negatively charged Cl−, producing a further depolarization of the cell membrane, (modified from Zou et al., 2009).
Ca2+ influx through CNG channels during odorant stimulation also exerts negative feedback onto the CNG channel itself, acting as a brake to retard further activity. Lastly, Ca2+ regulates the activity of a number of the enzymes involved in the second messenger cascades in olfactory signal transduction. These actions of Ca2+ are particularly important for signal adaptation (discussed later).
IVC4 TAAR Receptors
Another class of receptors found in olfactory sensory neurons and distinct from the OR superfamily was discovered in 2006 – trace amine-associated receptors (TAARs) (Liberles and Buck, 2006). These GPCRs are found in a small population of olfactory epithelial receptor neurons that do not express ORs. TAAR receptors comprise a much smaller family of GPCRs than ORs. Of the 15 TAAR receptor genes (mouse), at least 14 are expressed in ORNs (Liberles, 2009). In humans, there are six TAAR receptor genes (Fleischer et al., 2009). Ligands for TAARs are still being identified. In rodents, where they have been studied best, TAARs are stimulated by volatile amines, many of which are found in urine. This origin for TAAR ligands suggests that TAARs may function to detect social cues, akin to pheromones. Transduction mechanisms for olfactory TAARs are not known, though in rodent olfactory receptor neurons TAARs are co-expressed with Golf and in heterologous expression systems, stimulating TAARs increases cAMP. These data suggest a downstream pathway for TAARs similar to that for ORs.
IVC5 IP3 in Olfactory Receptor Neurons
There is suggestive evidence that a second messenger cascade other than the canonical pathway discussed above is activated in olfactory epithelial cells (reviewed in Ache, 2010). Albeit existence of this alternative pathway is controversial in mammals, there is little doubt that it exists in invertebrates. This alternative pathway may be triggered by a different set of odorants than those that are transduced by the canonical pathway involving Golf, adenylyl cyclase and cAMP. The main impetus to search for alternative signal transduction pathways stems from observations that certain odorants stimulate the rapid generation of inositol trisphosphate (IP3) in olfactory epithelium. According to this hypothesis, odorant receptors couple to Gαq/Gα11-like proteins, quite different from Golf. These G proteins are believed to activate membrane-bound enzyme phospholipase C (PLC). PLC hydrolyzes a phospholipid, phosphatidyl inositol 4,5-bisphosphate, in the plasma membrane. The resultant by-products, IP3 and diacylglycerol (DAG), are powerful bioactive compounds. IP3 in particular activates specific IP3 receptors on internal Ca2+ stores and triggers Ca2+ release into the cytoplasm. Recently, IP3 receptors have been localized only to a small population of microvillar cells within the olfactory epithelium (Hegg et al., 2010). These cells lack olfactory cilia and axons. They appear to be innervated and may represent a second, parallel chemosensory cell alongside olfactory receptor neurons. Little is currently understood about their function and the significance of an IP3 pathway in mammalian olfaction remains unresolved.
IVC6 Transduction in the Vomeronasal Organ (VNO)
Transduction pathways in VNO sensory neurons for pheromones and other chemical stimuli are presently under investigation. It appears that VNO sensory neurons utilize distinctive GPCRs, a different set of G proteins and different downstream effectors than ORNs. For example, VNO sensory neurons express two different families of GPCRs, V1Rs and V2Rs, that are unrelated to odorant receptors (Dulac and Axel, 1995). In the mouse, there are ≈240 functional V1R and 60 functional V2R genes; in humans there are only three to five functional V1R genes and no functional V2R genes (Rodriguez and Mombaerts, 2002; Shi and Zhang, 2007; Young et al., 2010). Parenthetically, because there is only a vestigial, if any, vomeronasal organ in adults, V1R in humans must be expressed elsewhere, such as in the olfactory epithelium. In non-human mammals, the two families of VNO receptors are expressed in anatomically separate regions of the VNO sensory epithelium and they send their axons to different regions of the olfactory bulb. Furthermore, V1Rs versus V2Rs respond to different types of stimuli and may mediate different behavioral responses. For instance, V1Rs are found in the more superficial (apical) layer of VNO sensory cells and respond to volatile compounds. In contrast, V2Rs are expressed in the more basal layer of the VNO epithelium and bind small water-soluble peptides. The behavioral consequences of activating V1Rs and V2Rs are still being scrutinized. To date, roles for V1Rs and V2Rs in gender discrimination, territoriality, aggression, social dominance and male/female sexual interactions have been implicated in non-human animals and perhaps in humans as well (Gelstein et al., 2011).
V1Rs and V2Rs initiate a downstream signaling pathway that markedly differs from that triggered by ORs in the olfactory epithelium. Instead of cAMP/CNG (or IP3/Ca2+ pathways), V1Rs and V2Rs activate a Ca-permeable ion channel, TRPC2 (canonical transient receptor potential channel 2, formerly called TRP2) that is highly expressed in vomeronasal sensory neurons8 (Liman et al., 1999). The complete signaling pathway for V1Rs/V2Rs and TRPC2 awaits elucidation. Indications to date are that activation of the vomeronasal V1 and V2 GPCRs leads to the formation of diacylglycerol (DAG) which stimulates TRPC2 and allows Ca2+ influx, thereby depolarizing the sensory cells and initiating action potentials.
In addition to the V1Rs and V2Rs, another small family of GPCRs was recently identified in vomeronasal sensory neurons – formyl peptide receptors (FPRs) (Riviere et al., 2009; Liberles et al., 2009). Five members of this family of receptors were identified. Interestingly, FPRs are also expressed on immune cells where they bind formyl peptides and lipids from microorganisms and signal the presence of pathogens. Expression of these FPR-like receptors in the vomeronasal organ may explain the ability of animals, especially rodents, to detect stimuli in urine and bodily secretions and recognize diseased conspecifics.
IVD Termination of Olfactory Signals
Olfactory receptor neuron excitation is terminated by a number of mechanisms. An obvious one is the unbinding and disappearance of the odorant from the chemoreceptive surface of the cilia, perhaps aided by odorant binding protein (OBP). The removal of odorants is complicated by the lipophilic nature of most odoriferous compounds; they will tend to partition into the plasma membrane and thus may linger in the vicinity of the receptors. Although the details are only now unfolding, one proposed mechanism for ridding the chemosensitive surfaces of odorants is the enzymatic modification of odorants by broad-spectrum biotransformation enzymes found in the mucus. These enzymes are similar to the detoxification enzymes found in the liver. Olfactory epithelial supporting cells adjacent to receptor neurons contain high concentrations of the detoxifying enzymes glutathione transferase, cytochrome P-450 and UDP gluconosyl transferase (Lazard et al., 1991). Indeed, the lipophilic nature of many odorants and their absorption into the sensory epithelium may necessitate the existence of such degradative mechanisms. Enzymatic biotransformation of volatiles in the mucosal layer occurs very quickly. In addition to terminating the actions of inhaled odorants, enzymatic biotransformation can yield olfactory stimuli that differ chemically and produce smells distinct from what had initially been present.
Besides disappearance of the odorant, sensory adaptation is also key in terminating olfactory signals. Adaptation in olfactory receptor neurons is explained by multiple mechanisms, many of which involve Ca2+. First, short-term adaptive mechanisms are believed to be set in motion by the Ca2+ that enters through CNG channels during odorant stimulation. Ca2+, acting via calmodulin inhibits CNG channels themselves (Chen & Yau, 1994; Kurahashi and Menini, 1997), a direct negative feedback on the olfactory signal. That is, an increase in Ca2+ in the ciliary cytosol will depress odorant responses by shutting down effector (CNG) channels.
Second, heightened intracellular Ca2+ also depresses the cascade of enzymes that are triggered during olfactory transduction. At high (μM) concentrations, Ca2+ inhibits adenylyl cyclase, thereby reducing the continued generation of cAMP during maintained stimulation. In olfactory receptor neurons, this inhibition appears to be mediated by Ca2+-calmodulin-induced phosphorylation via the enzyme Ca2+-calmodulin-dependent protein kinase II (CaMKII). However, at nM to pM concentrations, Ca2+ has the opposite effect, namely, it stimulates adenylyl cyclase. Consequently, the net effect of Ca2+ influx on adenylyl cyclase activity during odor stimulation is concentration dependent.
Additionally, Ca2+ influx stimulates phosphodiesterase (PDE), thereby accelerating the degradation of cAMP and reducing the odorant response.
Finally, Ca2+ influx activates Ca2+-dependent K+ channels that initiate an outward, repolarizing current. Counterbalancing this, Ca2+ also activates a Cl− conductance in the olfactory cilia that produces an inward depolarizing current, as described previously.
Thus, the actions of Ca2+ are complex. The net effect of Ca2+ entry through CNG channels during olfactory stimulation appears to be to reduce the responsiveness of olfactory receptor neurons perhaps as much as 20-fold, moving the stimulus–response relationship to a higher range of odorant concentrations. Longer-lasting adaption to a maintained odor stimulus involves another mechanism. A prolonged adaptation in olfactory receptor neurons, involving an intriguing role for the novel gaseous neurotransmitter carbon monoxide (CO), has been proposed (Leinders-Zufall et al., 1996) (see Fig. 39.6 B,C). CO, like the related neurotransmitter nitric oxide (NO), is a highly-diffusible substance and is generated during olfactory transduction via a mechanism that is not yet well characterized. CO, in turn, activates soluble guanylyl cyclase (sGC) and leads to the production of cGMP in the stimulated olfactory receptor neuron as well as in surrounding cells. As discussed previously, cGMP is a very effective ligand for olfactory CNG channels. At first glance, generating cGMP might seem contrary to adaptation and represent positive feedback during odorant stimulation. However, activation of olfactory CNG channels via the CO pathway produces only a low-level, subthreshold inward current. This maintains a persistent trickle of Ca2+ into the olfactory receptor neurons, chronically reducing CNG channel activity. The rapid transient influx of Ca2+ through CNG channels (the immediate effect of odorant stimulation), combined with a sustained low-level influx via cGMP activation of these channels (the CO pathway), overall leads to a long-lasting (minutes) reduction of the sensitivity of olfactory receptor neurons after the initial excitation.9
In addition to these sensory adaptation mechanisms, direct desensitization of ORs following odorant stimulation also plays a key role in terminating odorant responses (Schleicher et al., 1993). cAMP produced by odor stimulation opens CNG cation channels and secondarily activates protein kinase A (PKA). PKA and a specialized G-protein-coupled receptor kinase, GRK310, phosphorylate odorant-bond receptors (Fig. 39.8). These protein kinases, together with regulatory proteins, such as arrestin, render the odorant receptor inactive and quench the subsequent transduction cascade. Receptors are resensitized by the action of phosphatases. Thus, a cycle of phosphorylation/dephosphorylation controls the active state of odorant receptors and, ultimately, the responsiveness of olfactory receptor neurons.
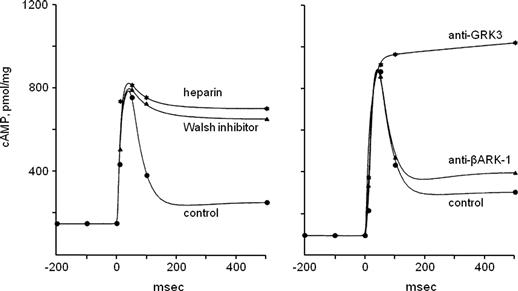
FIGURE 39.8 Desensitization of odorant receptors during prolonged stimulation. Olfactory second messenger signaling is quenched by phosphorylation cascades. Left, inhibiting protein kinase activity with Walsh inhibitor or heparin markedly prolongs increases in olfactory cAMP induced by odorant stimulation in cilia preparations from the rat. (•) odorant alone (1 μM citralva); ( ) odorant stimulation of cilia pretreated with Walsh inhibitor (3.8 μM); (∗) odorant stimulation of cilia pretreated with heparin (1 μM). Right, pretreating the cilia preparation with antibodies to GRK3 specifically prevents desensitization of odorant receptors and prolongs their activation (measured here as an increase in cAMP). (•) odorant alone (1 μM citralva); (
) odorant stimulation of cilia pretreated with Walsh inhibitor (3.8 μM); (∗) odorant stimulation of cilia pretreated with heparin (1 μM). Right, pretreating the cilia preparation with antibodies to GRK3 specifically prevents desensitization of odorant receptors and prolongs their activation (measured here as an increase in cAMP). (•) odorant alone (1 μM citralva); ( ) odorant stimulation of cilia pretreated with anti-βARK-1 antibodies (diluted 1:5000); (∗) odorant stimulation of cilia pretreated with anti-GRK3 antibodies (diluted 1:5000); abscissae, time in ms, (modified from Schleicher et al., 1993).
) odorant stimulation of cilia pretreated with anti-βARK-1 antibodies (diluted 1:5000); (∗) odorant stimulation of cilia pretreated with anti-GRK3 antibodies (diluted 1:5000); abscissae, time in ms, (modified from Schleicher et al., 1993).
Collectively, short-term adaptation, long-term adaptation and receptor desensitization act to shape and terminate olfactory responses to prolonged odor stimulation. Sensory adaptation in many sensory systems, including olfaction, serves a dual purpose. First, over time, a steady stimulus ceases to excite responses, i.e. the sensory organ adapts to the stimulus. In this sense, adaptation tends to accentuate signals from stimuli that fluctuate and emphasizes the dynamic aspects of sensory stimuli. Second, adaptation reduces responsivity to increasingly intense stimuli. This prevents the output from the sensory organs from becoming saturated by only moderately intense stimulus intensities while, at the same time, preserving the ability of the organ to respond well to very weak stimuli. That is, adaptation greatly extends the dynamic range of stimulus intensities over which a sensory organ can respond.
BIBLIOGRAPHY
1. Ache BW. Odorant-specific modes of signaling in mammalian olfaction. Chem Senses. 2010;35:533–539.
2. Ache BW, Young JM. Olfaction: diverse species, conserved principles. Neuron. 2005;48:417–430.
3. Bakalyar H, Reed RR. Identification of a specialized adenylyl cyclase that may mediate odorant detection. Science. 1990;250:1403–1406.
4. Behrens M, Foerster S, Staehler F, Raguse JD, Meyerhof W. Gustatory expression pattern of the human TAS2R bitter receptor gene family reveals a heterogenous population of bitter responsive taste receptor cells. J Neurosci. 2007;27:12630–12640.
5. Bigiani A, Cristiani R, Fieni F, Ghiaroni V, Bagnoli P, Pietra P. Postnatal development of membrane excitability in taste cells of the mouse vallate papilla. J Neurosci. 2002;22:493–504.
6. Boccaccio A, Menini A. Temporal development of cyclic nucleotide-gated and Ca2+ -activated Cl− currents in isolated mouse olfactory sensory neurons. J Neurophysiol. 2007;98:153–160.
7. Bradley RM. Electrophysiological investigations of intravascular taste using perfused rat tongue. Am J Physiol. 1973;224:300–304.
8. Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187.
9. Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294.
10. Chandrashekar J, Kuhn C, Oka Y, et al. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301.
11. Chandrashekar J, Mueller KL, Hoon MA, et al. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711.
12. Chang RB, Waters H, Liman ER. A proton current drives action potentials in genetically identified sour taste cells. Proc Natl Acad Sci USA. 2010;107:22320–22325.
13. Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190:285–296.
14. Chen TY, Yau KW. Direct modulation by Ca2+-calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature. 1994;368:545–548.
15. Clapp TR, Trubey KR, Vandenbeuch A, et al. Tonic activity of Galpha-gustducin regulates taste cell responsivity. FEBS Lett. 2008;582:3783–3787.
16. DeMaria S, Ngai J. The cell biology of smell. J Cell Biol. 2010;191:443–452.
17. Dhallan RS, Yau KW, Schrader KA, Reed RR. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990;347:184–187.
18. Dulac C, Axel R. A novel family of genes encoding putative pheromone receptors in mammals. Cell. 1995;83:195–206.
19. Firestein S, Darrow B, Shepherd GM. Activation of the sensory current in salamander olfactory receptor neurons depends on a G protein-mediated cAMP second messenger system. Neuron. 1991;6:825–835.
20. Fleischer J, Breer H, Strotmann J. Mammalian olfactory receptors. Front Cell Neurosci. 2009;3:9.
21. Frings S. Chemoelectrical signal transduction in olfactory sensory neurons of air-breathing vertebrates. Cell Mol Life Sci. 2001;58:510–519.
22. Gelstein. S, Yeshurun Y, Rozenkrantz L, et al. Human tears contain a chemosignal. Science. 2011;331:226–230.
23. Ghiaroni V, Fieni F, Tirindelli R, Pietra P, Bigiani A. Ion conductances in supporting cells isolated from the mouse vomeronasal organ. J Neurophysiol. 2003;89:118–127.
24. Gilbertson TA, Boughter Jr JD, Zhang H, Smith DV. Distribution of gustatory sensitivities in rat taste cells: whole-cell responses to apical chemical stimulation. J Neurosci. 2001;21:4931–4941.
25. Gilbertson TA, Roper SD, Kinnamon SC. Proton currents through amiloride-sensitive Na+ channels in isolated hamster taste cells: enhancement by vasopressin and cAMP. Neuron. 1993;10:931–942.
26. Graziadei PPC, Monti Graziadei GA. Continuous nerve cell renewal in the olfactory system. In: Jacobson M, ed. Handbook of Sensory Physiology. New York: Springer-Verlag; 1978;:55–82.
27. Hänig DP. Zur Psychophysik des Geschmackssinnes. Philosophische Studien. 1901;17:576–623.
28. Hasin-Brumshtein Y, Lancet D, Olender T. Human olfaction: from genomic variation to phenotypic diversity. Trends Genet. 2009;25:178–184.
29. Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984;223:403–405.
30. Hegg CC, Jia C, Chick WS, Restrepo D, Hansen A. Microvillous cells expressing IP3 receptor type 3 in the olfactory epithelium of mice. Eur J Neurosci. 2010;32:1632–1645.
31. Herness MS, Sun XD. Characterization of chloride currents and their noradrenergic modulation in rat taste receptor cells. J Neurophysiol. 1999;82:260–271.
32. Herness S, Zhao FL. The neuropeptides CCK and NPY and the changing view of cell-to-cell communication in the taste bud. Physiol Behav. 2009;97:581–591.
33. Huang YA, Maruyama Y, Stimac R, Roper SD. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J Physiol. 2008;586:2903–2912.
34. Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA. 2007;104:6436–6441.
35. Kaneko H, Putzier I, Frings S, Kaupp UB, Gensch T. Chloride accumulation in mammalian olfactory sensory neurons. J Neurosci. 2004;24:7931–7938.
36. Kleene SJ, Gesteland RC. Dissociation of frog olfactory epithelium with N-ethylmaleimide. Brain Res. 1981;229:536–540.
37. Kretz O, Barbry P, Bock R, Lindemann B. Differential expression of RNA and protein of the three pore-forming subunits of the amiloride-sensitive epithelial sodium channel in taste buds of the rat. J Histochem Cytochem. 1999;47:51–64.
38. Kurahashi T. Activation by odorants of cation-selective conductance in the olfactory receptor cell isolated from the newt. J Physiol. 1989;419:177–192.
39. Kurahashi T, Menini A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature. 1997;385:725–729.
40. Lazard D, Zupko K, Poria Y, et al. Odorant signal termination by olfactory UDP glucuronosyl transferase. Nature. 1991;349:790–793.
41. Leinders-Zufall T, Shepherd GM, Zufall F. Modulation by cyclic GMP of the odour sensitivity of vertebrate olfactory receptor cells. Proc Biol Sci. 1996;263:803–811.
42. Liberles SD. Trace amine-associated receptors are olfactory receptors in vertebrates. Ann NY Acad Sci. 2009;1170:168–172.
43. Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650.
44. Liberles SD, Horowitz LF, Kuang D, et al. Formyl peptide receptors are candidate chemosensory receptors in the vomeronasal organ. Proc Natl Acad Sci USA. 2009;106:9842–9847.
45. Liman ER, Corey DP, Dulac C. TRP2: a candidate transduction channel for mammalian pheromone sensory signaling. Proc Natl Acad Sci USA. 1999;96:5791–5796.
46. Lin W, Finger TE, Rossier BC, Kinnamon SC. Epithelial Na+ channel subunits in rat taste cells: localization and regulation by aldosterone. J Comp Neurol. 1999;405:406–420.
47. Lindemann B. Receptor seeks ligand: on the way to cloning the molecular receptors for sweet and bitter taste. Nat Med. 1999;5:381–382.
48. Lindemann B, Barbry P, Kretz O, Bock R. Occurrence of ENaC subunit mRNA and immunocytochemistry of the channel subunits in taste buds of the rat vallate papilla. Ann NY Acad Sci. 1998;855:116–127.
49. Lyall V, Alam RI, Phan DQ, et al. Decrease in rat taste receptor cell intracellular pH is the proximate stimulus in sour taste transduction. Am J Physiol Cell Physiol. 2001;281:C1005–C1013.
50. Mackay-Sim A, Kittel P. Cell dynamics in the adult mouse olfactory epithelium: a quantitative autoradiographic study. J Neurosci. 1991a;11:979–984.
51. Mackay-Sim A, Kittel PW. On the life span of olfactory receptor neurons. Eur J Neurosci. 1991b;3:209–215.
52. Max M, Shanker YG, Huang L, et al. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet. 2001;28:58–63.
53. McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–569.
54. Meredith M. Human vomeronasal organ function: a critical review of best and worst cases. Chem Senses. 2001;26:433–445.
55. Munger SD, Leinders-Zufall T, Zufall F. Subsystem organization of the mammalian sense of smell. Annu Rev Physiol. 2009;71:115–140.
56. Nakamura T, Gold GH. A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature. 1987;325:442–444.
57. Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416:199–202.
58. Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390.
59. Ossebaard CA, Smith DV. Amiloride suppresses the sourness of NaCl and LiCl. Physiol Behav. 1996;60:1317–1322.
60. Pace U, Hanski E, Salomon Y, Lancet D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature. 1985;316:255–258.
61. Pifferi S, Dibattista M, Sagheddu C, et al. Calcium-activated chloride currents in olfactory sensory neurons from mice lacking bestrophin-2. J Physiol. 2009;587:4265–4279.
62. Raming K, Krieger J, Strotmann J, et al. Cloning and expression of odorant receptors. Nature. 1993;361:353–356.
63. Reed RR. Signaling pathways in odorant detection. Neuron. 1992;8:205–209.
64. Richter TA, Caicedo A, Roper SD. Sour taste stimuli evoke Ca2+ and pH responses in mouse taste cells. J Physiol. 2003;547:475–483.
65. Richter TA, Dvoryanchikov GA, Chaudhari N, Roper SD. Acid-sensitive two-pore domain potassium (K2P) channels in mouse taste buds. J Neurophysiol. 2004;92:1928–1936.
66. Riviere S, Challet L, Fluegge D, Spehr M, Rodriguez I. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature. 2009;459:574–577.
67. Rodriguez I, Mombaerts P. Novel human vomeronasal receptor-like genes reveal species-specific families. Curr Biol. 2002;12:R409–R411.
68. Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–667.
69. Roper S. Regenerative impulses in taste cells. Science. 1983;220:1311–1312.
70. Roper SD. The microphysiology of peripheral taste organs. J Neurosci. 1992;12:1127–1134.
71. Roper SD. Signal transduction and information processing in mammalian taste buds. Pflügers Arch. 2007;454:759–776.
72. Sainz E, Korley JN, Battey JF, Sullivan SL. Identification of a novel member of the T1R family of putative taste receptors. J Neurochem. 2001;77:896–903.
73. Schleicher S, Boekhoff I, Arriza J, Lefkowitz RJ, Breer H. A beta-adrenergic receptor kinase-like enzyme is involved in olfactory signal termination. Proc Natl Acad Sci USA. 1993;90:1420–1424.
74. Shi P, Zhang J. Comparative genomic analysis identifies an evolutionary shift of vomeronasal receptor gene repertoires in the vertebrate transition from water to land. Genome Res. 2007;17:166–174.
75. Smith DV, Margolskee RF. Making sense of taste. Sci Am. 2001;284:32–39.
76. Sugita M. Taste perception and coding in the periphery. Cell Mol Life Sci. 2006;63:2000–2015.
77. Taylor R, Roper S. Ca(2+)-dependent Cl conductance in taste cells from Necturus. J Neurophysiol. 1994;72:475–478.
78. Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 2007;27:10840–10848.
79. Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci. 2008;9:1.
80. Wang YY, Chang RB, Allgood SD, Silver WL, Liman ER. A TRPA1-dependent mechanism for the pungent sensation of weak acids. J Gen Physiol. 2011;137:493–505.
81. Ye Q, Heck GL, DeSimone JA. Voltage dependence of the rat chorda tympani response to Na+ salts: implications for the functional organization of taste receptor cells. J Neurophysiol. 1993;70:167–178.
82. Yoshida R, Horio N, Murata Y, Yasumatsu K, Shigemura N, Ninomiya Y. NaCl responsive taste cells in the mouse fungiform taste buds. Neuroscience. 2009a;159:795–803.
83. Yoshida R, Miyauchi A, Yasuo T, et al. Discrimination of taste qualities among mouse fungiform taste bud cells. J Physiol. 2009b;587:4425–4439.
84. Young JM, Massa HF, Hsu L, Trask BJ. Extreme variability among mammalian V1R gene families. Genome Res. 2010;20:10–18.
85. Zhang X, Rodriguez I, Mombaerts P, Firestein S. Odorant and vomeronasal receptor genes in two mouse genome assemblies. Genomics. 2004;83:802–811.
86. Zou DJ, Chesler A, Firestein S. How the olfactory bulb got its glomeruli: a just so story?. Nat Rev Neurosci. 2009;10:611–618.
1 This epithelial property is not shared, however, with inner ear hair cells (auditory and vestibular) or, strictly speaking, with retinal photoreceptors. These sensory cells do not renew and are not replaced if damaged. Admittedly, retinal photoreceptors undergo continuous recycling of their photoreceptive components (opsin-containing disk membranes are shed and renewed), but the somata remain stable.
2 The human T1R2+T1R3 dimer responds to monellin and thaumatin, but the equivalent rodent sweet receptor dimer does not. This is explained by sequence differences between human and rodent sweet taste receptors.
3 This raises the intriguing possibility that certain agents, such as drugs or toxins, can be taken up by ORNs and transported along their axons directly into the brain. That is, the olfactory epithelium represents a “window” into the brain that might be exploited for pharmaceutical therapies or may be at the root of certain central nervous system disorders that are linked to environmental contaminants.
4 In humans, the VNO consists of shallow bilateral pits located in the nasal cavity, anterior to the olfactory epithelium. Arguably, the VNO may only be a vestigial structure in humans. If humans sense pheromones, these stimuli may be transduced by sensory cells lying in the olfactory epithelium. Meredith (2001) summarizes evidence for and against the existence of human vomeronasal organs.
5 Initially, this conductance was believed to be generated by the bestrophin-2 (Best2) channels that are present in olfactory cilia. However, olfactory sensory neurons from mutant mice lacking Best2 channels show normal Ca2+-activated Cl− current (Pifferi et al., 2009), dispelling the notion that Best2 channels underlie this amplifying inward current.
6 Olfactory cilia are suspended in a mucus that has a Cl− concentration of about 55 mM. Cl− concentration inside the apical tips of olfactory receptor neurons appears to be maintained at a remarkably high level (≈50 mM, Kaneko et al., 2004). This ratio of [Cl]o/[Cl]i yields an ≈0 mV Cl−-equilibrium potential across the apical tips and explains why opening Cl− channels produces a Cl− efflux (i.e. inward, depolarizing current).
7 Activating the Ca2+-dependent anion conductance in gustatory sensory cells results in the opposite effect than at the cilia of olfactory receptor neurons. Namely, in taste bud cells this conductance produces an influx of Cl− (i.e. a repolarizing, outward current). This is because the basolateral membrane of taste cells – where the Cl− conductance channels are situated – is exposed to interstitial fluid. Furthermore, [Cl−]i in taste cells is believed to be low. Consequently, in taste cells, the equilibrium potential for Cl− is likely to be near the resting potential. Opening Cl− channels when the cell is depolarized will allow Cl− to enter the taste cell and repolarize the membrane.
8 In humans, TRPC2 is a pseudogene and thus no functional channels are expressed. This fact, combined with the severely limited number of V1Rs and absence of V2Rs in humans, underscores the lack of definitive evidence for human pheromones (but see Gelstein et al., 2011).
9 Previously, it was believed that NO played this role in olfactory adaptation. However, data utilizing blockers of CO synthesis now implicate CO, not NO, as the diffusible intra- and intercellular controller of CNG channel activity in olfactory receptor neurons.
10 GRK3 was formerly named β-adrenergic receptor kinase 2, or βARK-2.