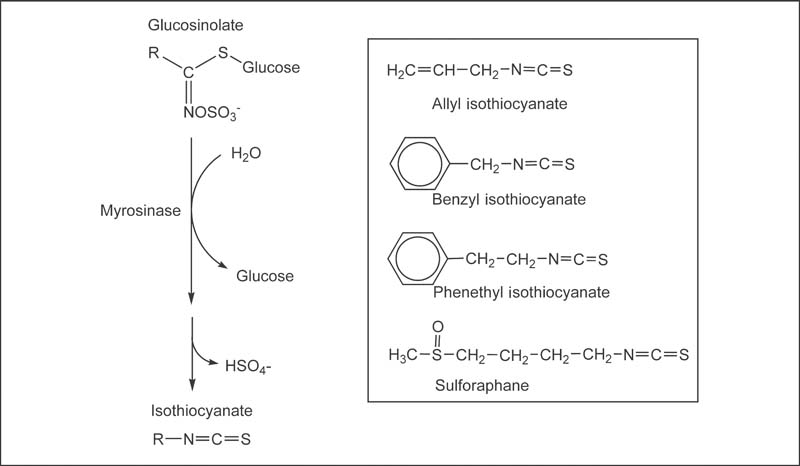
Fig. 13.1 Myrosinase-catalyzed hydrolysis of glucosinolates and the chemical structures of allyl isothiocyanate, benzyl isothiocyanate, phenethyl isothiocyanate, and sulforaphane.
Cruciferous vegetables, such as broccoli, cabbage, and kale, are rich sources of sulfur-containing compounds called glucosinolates. Isothiocyanates are biologically active hydrolysis (breakdown) products of glucosinolates. Cruciferous vegetables contain a variety of glucosinolates, each of which forms a different isothiocyanate when hydrolyzed (Fig. 13.1).1 For example, broccoli is a good source of glucoraphanin, the glucosinolate precursor of sulforaphane (SFN), and sinigrin, the glucosinolate precursor of allyl isothiocyanate (AITC).2 Watercress is a rich source of gluconasturtiin, the precursor of phenethyl isothiocyanate (PEITC), while garden cress is rich in glucotropaeolin, the precursor of benzyl isothiocyanate (BITC). At present, scientists are interested in the cancer-preventive activities of vegetables that are rich in glucosinolates (see Chapter 2), as well as individual isothiocyanates.3
Myrosinase, a class of enzymes that catalyzes the hydrolysis of glucosinolates, is physically separated from glucosinolates in intact plant cells.4 When cruciferous vegetables are chopped or chewed, myrosinase can interact with glucosinolates and release isothiocyanates from their precursors (Fig. 13.1). Thorough chewing of raw cruciferous vegetables increases glucosinolate contact with plant myrosinase and increases the amount of isothiocyanates absorbed.5 Even when plant myrosinase is completely inactivated by heat, the myrosinase activity of human intestinal bacteria allows for some formation and absorption of isothiocyanates.6 However, the absorption and excretion of isothiocyanates is substantially lower from cooked than from raw cruciferous vegetables5,7,8 (see the Food Sources section below). During metabolism, isothiocyanates are conjugated (bound) to glutathione, an activity that is promoted by a family of enzymes called glutathione S -transferases (GSTs), and further metabolized to mercapturic acids. These isothiocyanate metabolites can be measured in the urine and are highly correlated with dietary in-take of cruciferous vegetables.9 There is also some evidence that isothiocyanate metabolites contribute to the biological activity of isothiocyanates.3,10

Fig. 13.1 Myrosinase-catalyzed hydrolysis of glucosinolates and the chemical structures of allyl isothiocyanate, benzyl isothiocyanate, phenethyl isothiocyanate, and sulforaphane.
Biotransformation enzymes play important roles in the metabolism and elimination of a variety of chemicals, including drugs, toxins, and carcinogens. In general, phase I biotransformation enzymes catalyze reactions that increase the reactivity of hydrophobic (fat-soluble) compounds, preparing them for reactions catalyzed by phase II biotransformation enzymes. Reactions catalyzed by phase II enzymes generally increase water solubility and promote the elimination of the compound from the body.11
Some procarcinogens (carcinogen precursors) require biotransformation by phase I enzymes, such as those of the cytochrome P450 (CYP) family, to become active carcinogens that are capable of binding DNA and inducing mutations. Inhibition of specific CYP enzymes involved in carcinogen activation inhibits the development of cancer in animal models.3 Isothiocyanates, including PEITC and BITC, have been found in animal studies to inhibit carcinogen activation by CYP enzymes.12,13 Cell culture studies have also shown that SFN inhibits certain CYP enzymes.14 A small clinical trial in smokers found evidence that consumption of 170 g/day (6 oz/day) of watercress, which is rich in the glucosinolate precursor of PEITC, decreased the activation of a procarcinogen found in tobacco.15
Many isothiocyanates, particularly SFN, are potent inducers of phase II enzymes in cultured human cells.2,14 Phase II enzymes, including GSTs, uridine diphosphate (UDP)-glucuronosyl transferases (UGTs), quinone reductase, and γ-glutamatyl cysteine ligase, play important roles in protecting cells from DNA damage by carcinogens and reactive oxygen species.16 The genes for these and other phase II enzymes contain a specific sequence of DNA called an antioxidant response element (ARE). Isothiocyanates have been shown to increase phase II enzyme activity by increasing the transcription of genes that contain an ARE.17 Limited data from clinical trials suggest that glucosinolate-rich foods can increase phase II enzyme activity in humans. When smokers consumed 170 g/day (6 oz/day) of watercress, urinary excretion of glucuronidated nicotine metabolites increased significantly, suggesting UGT activity increased.18 Brussels sprouts are rich in several glucosinolates, including precursors of AITC and SFN. Consumption of 300 g/day (11 oz/day) of Brussels sprouts for a week significantly increased plasma and intestinal GST levels in nonsmoking men.19,20
After a cell divides, it passes through a sequence of stages known as the cell cycle, before dividing again. Following DNA damage, the cell cycle can be transiently arrested to allow for DNA repair or, if the damage cannot be repaired, activation of pathways leading to cell death (apoptosis).21 Defective cell-cycle regulation may result in the propagation of mutations that contribute to the development of cancer. Several isothiocyanates, including AITC, BITC, PEITC, and SFN, have been found to induce cell-cycle arrest in cultured cells.2
Unlike normal cells, cancer cells proliferate rapidly and lose the ability to respond to cell death signals that initiate apoptosis. Isothiocyanates have been found to inhibit proliferation and induce apoptosis in several cancer cell lines.3,22,23
In the nucleus of a cell, DNA is coiled around basic proteins called histones. In general, acetylation of histones by histone acetyl transferases pmakes DNA more accessible to transcription factors, which bind DNA and activate gene transcription. Deacetylation of histones by histone deacetylases restricts the access of transcription factors to DNA. Acetylation and deacetylation of nuclear histones is an important cellular mechanism for regulating gene transcription.24 However, the balance between histone acetyl transferase and histone deacetylase activities that exists in normal cells may be disrupted in cancer cells. Compounds that inhibit histone deacetylases can potentially suppress the development of cancer by inducing the transcription of tumor suppressor proteins that promote differentiation and apoptosis in transformed (precancerous) cells.25 SFN and AITC metabolites have been found to inhibit histone deacetylase activity in cultured cancer cells.10,26–28 Moreover, in vivo evidence for SFN inhibiting histone deacetylase came from a mouse model using prostate cancer xenografts.29 In humans, histone deacetylase activity was inhibited in blood cells following ingestion of 68 g (1 cup) of SFN-rich broccoli sprouts.29
Inflammation promotes cellular proliferation and inhibits apoptosis, increasing the risk of developing cancer.30 SFN and PEITC have been found to decrease the secretion of inflammatory signaling molecules by white blood cells; these compounds have also been shown to decrease DNA binding of nuclear factor kappa B (NF-κB), a pro-inflammatory transcription factor.31,32
Bacterial infection with Helicobacter pylori (H. pylori) is associated with a marked increase in the risk of gastric cancer.33 In the test tube and in tissue culture, purified SFN inhibited the growth and killed multiple strains of H. pylori, including antibiotic-resistant strains.34 In an animal model of H. pylori infection, SFN administration for 5 days eradicated H. pylori from eight out of 11 xenografts of human gastric tissue implanted in immune-compromised mice.35 However, in a small clinical trial, consumption of up to 56 g/day (2 oz/day) of glucoraphanin-rich broccoli sprouts for 1 week was associated with H. pylori eradication in only three out of nine patients with gastritis.36 Further research is needed to determine whether SFN or foods rich in its precursor, glucobrassicin, will be helpful in the treatment of H. pylori infection in humans.
Naturally occurring isothiocyanates and their metabolites have been found to inhibit the development of chemically induced cancers of the lung, liver, esophagus, stomach, small intestine, colon, and mammary gland (breast) in a variety of animal models.3,12 Although epidemiological studies provide some evidence that higher in-takes of cruciferous vegetables are associated with decreased cancer risk in humans,37 it is difficult to determine whether such protective effects are related to isothiocyanates or other factors associated with consumption of cruciferous vegetables (see Chapter 2). Investigators have attempted to calculate human isothiocyanate exposure based on assessments of cruciferous vegetable intake and measurements of the maximal amounts of isothiocyanates that can be liberated from various cruciferous vegetables in the laboratory.38 Case–control studies using this technique found that dietary isothiocyanate intakes were significantly lower in Chinese women39 and US men40 diagnosed with lung cancer than in cancer-free control groups.
Assessing dietary intake of cruciferous vegetables may not accurately measure an individual exposure to isothiocyanates, since other factors may alter the amount of isothiocyanates formed and absorbed (see the Bioavailability and Metabolism section above). Measuring urinary excretion of isothiocyanates and their metabolites may provide a better assessment of isothiocyanate exposure,9,41,42 but few studies have examined the relationships between urinary isothiocyanate excretion and cancer risk. In a prospective study, Chinese men with detectable levels of urinary isothiocyanates at baseline were at significantly lower risk of developing lung cancer over the next 10 years than men with undetectable levels.43 A case–control study found that urinary isothiocyanate excretion was significantly lower in Chinese women diagnosed with breast cancer than in a cancer-free control group.44 In contrast, cruciferous vegetable intake estimated from a food frequency questionnaire was not associated with breast cancer risk in the same study.
GSTs are a family of phase II biotransformation enzymes that promote the metabolism and elimination of isothiocyanates and other compounds from the body. Genetic variations (polymorphisms) that affect the activity of GST enzymes have been identified in humans. Null variants of the GSTM1 gene and GSTT1 gene contain large deletions, and individuals who inherit two copies of the GSTM1-null or GSTT1-null gene cannot produce the corresponding GST enzyme.45 Lower GST activity in such individuals could result in slower elimination and thus longer exposure to isothiocyanates after cruciferous vegetable consumption.9 In support of this idea, several epidemiological studies found that inverse associations between isothiocyanate intake from cruciferous vegetables and the risk of lung cancer39,40,43,46,47 or colon cancer48–50 were more pronounced in GSTM1-null or GSTT1-null individuals. These findings suggest a protective role for isothiocyanates that may be enhanced in individuals who eliminate them more slowly from the body.
Table 13.1 Total glucosinolate content of selected cruciferous vegetables52
Vegetable (Raw) |
Serving |
Total Glucosinolates (mg) |
Brussels sprouts |
½ cup |
104 |
Garden cress |
½ cup |
98 |
Mustard greens |
½ cup |
79 |
Turnip |
½ cup |
60 |
Cabbage, Savoy |
½ cup |
35 |
Kale |
½ cup |
34 |
Cabbage, red |
½ cup |
29 |
Broccoli |
½ cup |
27 |
Bok choy (pak choi) |
½ cup |
19 |
Watercress |
½ cup |
16 |
Cruciferous vegetables, such as bok choy, broccoli, Brussels sprouts, cabbage, cauliflower, horseradish, kale, kohlrabi, mustard, radish, rutabaga, turnip, and watercress, are rich sources of glucosinolate precursors of isothiocyanates.51 Unlike some other phytochemicals, glucosinolates are present in relatively high concentrations in commonly consumed portions of cruciferous vegetables. For example ½ cup of raw broccoli might provide more than 25 mg of total glucosinolates. Total glucosinolate contents of selected cruciferous vegetables are presented in Table 13.1.52 Some cruciferous vegetables are better sources of specific glucosinolates (and isothiocyanates) than others. Vegetables that are relatively good sources of some of the isothiocyanates that are currently under study for their cancer-preventive properties are listed in Table 13.2. Amounts of isothiocyanates formed from glucosinolates in foods are variable and depend partly on food processing and preparation (see the Effects of Cooking section below). Consumption of five or more weekly servings of cruciferous vegetables has been associated with significant reductions in cancer risk in some prospective cohort studies.53–55
The amount of glucoraphanin, the precursor of SFN, in broccoli seeds remains more or less constant as those seeds germinate and grow into mature plants. Thus, 3-day-old broccoli sprouts are concentrated sources of glucoraphanin, which contain 10 to 100 times more glucoraphanin by weight than mature broccoli plants.56 Broccoli sprouts that are certified to contain at least 73 mg of glucoraphanin (also called sulforaphane glucosinolate) per 1-oz serving are available in some health food and grocery stores.
Table 13.2 Some food sources of selected isothiocyanates and their glucosinolate precursors12
Isothiocyanate |
Glucosinolate (Precursor) |
Food Sources |
Allyl isothiocyanate (AITC) |
Sinigrin |
Broccoli, Brussels sprouts, cabbage, horseradish, mustard, radish |
Benzyl isothiocyanate (BITC) |
Glucotropaeolin |
Cabbage, garden cress, Indian cress |
Phenethyl isothiocyanate (PEITC) |
Gluconasturtiin |
Watercress |
Sulforaphane (SFN) |
Glucoraphanin |
Broccoli, Brussels sprouts, cabbage |
Glucosinolates are water-soluble compounds that may be leached into cooking water. Boiling cruciferous vegetables for 9–15 minutes resulted in 18%–59% decreases in the total glucosinolate content of cruciferous vegetables.52 Cooking methods that use less water, such as steaming or microwaving, may reduce glucosinolate losses.57 However, some cooking practices, including boiling,5 steaming,7,58 and microwaving at high power (750–900 W),8,58,59 may inactivate myrosinase, the enzyme that catalyzes glucosinolate hydrolysis. Even in the absence of plant myrosinase activity, the myrosinase activity of human intestinal bacteria results in some glucosinolate hydrolysis.6 However, several studies in humans have found that inactivation of myrosinase in cruciferous vegetables substantially decreases the bio-availability of isothiocyanates.5,7,8
Dietary supplements containing extracts of broccoli sprouts, broccoli, and other cruciferous vegetables are available without a prescription. Some products are standardized to contain a minimum amount of glucosinolates and/or sulforaphane. However, the bioavailability of isothiocyanates derived from these supplements is not known.
No serious adverse effects of isothiocyanates in humans have been reported. The majority of animal studies have found that isothiocyanates inhibited the development of cancer when given prior to the chemical carcinogen (pre-initiation) However, very high intakes of PEITC or BITC (25–250 times higher than average human dietary isothiocyanate intakes) have been found to promote bladder cancer in rats when given after a chemical carcinogen (post-initiation).60 The relevance of these findings to human urinary bladder cancer is not clear, since at least one prospective cohort study found cruciferous vegetable consumption to be inversely associated with the risk of bladder cancer in men.55
Although high dietary intakes of glucosinolates from cruciferous vegetables are not known to have adverse effects during pregnancy or lactation, there is no information on the safety of purified isothiocyanates or supplements containing high doses of glucosinolates and/or isothiocyanates during pregnancy or lactation in humans.
Isothiocyanates are not known to interact with any drugs or medications. However, the potential for isothiocyanates to inhibit various isoforms of the CYP family of enzymes raises the potential for interactions with drugs that are CYP substrates.61
• Isothiocyanates are derived from the hydrolysis (breakdown) of glucosinolates—sulfur-containing compounds found in cruciferous vegetables.
• Cruciferous vegetables contain a variety of glucosinolates, each of which forms a different isothiocyanate when hydrolyzed.
• Isothiocyanates, such as sulforaphane, may help prevent cancer by promoting the elimination of potential carcinogens from the body and by enhancing the transcription of tumor suppressor proteins.
• Epidemiological studies provide some evidence that human exposure to isothiocyanates through cruciferous vegetable consumption may decrease cancer risk, but the protective effects may be influenced by individual genetic variation in the metabolism and elimination of isothiocyanates from the body.
• Glucosinolates are present in relatively high concentrations in cruciferous vegetables, but cooking, particularly boiling and microwaving at high power, may decrease the bioavailability of isothiocyanates.
1. Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001;56(1):5–51
2. Zhang Y. Cancer-preventive isothiocyanates: measurement of human exposure and mechanism of action. Mutat Res 2004;555(1–2):173–190
3. Hecht SS. Chemoprevention by isothiocyanates. In: Kelloff GJ, Hawk ET, Sigman CC, eds. Promising Cancer Chemopreventive Agents, Vol 1: Cancer Chemopreventive Agents. Totowa, NJ: Humana Press; 2004:21–35
4. Holst B, Williamson G. A critical review of the bio-availability of glucosinolates and related compounds. Nat Prod Rep 2004;21(3):425–447
5. Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer Epidemiol Biomarkers Prev 2001;10(5):501–508
6. Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev 1998;7(12):1091–1100
7. Conaway CC, Getahun SM, Liebes LL, et al. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr Cancer 2000;38(2):168–178
8. Rouzaud G, Young SA, Duncan AJ. Hydrolysis of glucosinolates to isothiocyanates after ingestion of raw or microwaved cabbage by human volunteers. Cancer Epidemiol Biomarkers Prev 2004;13(1):125–131
9. Seow A, Shi CY, Chung FL, et al. Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiol Biomarkers Prev 1998;7(9):775–781
10. Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase. Cancer Res 2004;64(16):5767–5774
11. Lampe JW, Peterson S. Brassica, biotransformation and cancer risk: genetic polymorphisms alter the preventive effects of cruciferous vegetables. J Nutr 2002;132(10):2991–2994
12. Conaway CC, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr Drug Metab 2002;3(3):233–255
13. Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev 2000;32(3–4):395–411
14. Fimognari C, Hrelia P. Sulforaphane as a promising molecule for fighting cancer. Mutat Res 2007;635(2–3):90–104
15. Hecht SS, Chung FL, Richie JP Jr, et al. Effects of water-cress consumption on metabolism of a tobacco-specific lung carcinogen in smokers. Cancer Epidemiol Biomarkers Prev 1995;4(8):877–884
16. Kensler TW, Talalay P. Inducers of enzymes that protect against carcinogens and oxidants: drug- and food-based approaches with dithiolethiones and sulforaphane. In: Kelloff GJ, Hawk ET, Sigman CC, eds. Promising Cancer Chemopreventive Agents, Vol 1: Cancer Chemopreventive Agents. Totowa, NJ: Humana Press; 2004:3–20
17. Dinkova-Kostova AT, Holtzclaw WD, Cole RN, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A 2002;99(18):11908–11913
18. Hecht SS, Carmella SG, Murphy SE. Effects of water-cress consumption on urinary metabolites of nicotine in smokers. Cancer Epidemiol Biomarkers Prev 1999;8(10):907–913
19. Nijhoff WA, Grubben MJ, Nagengast FM, et al. Effects of consumption of Brussels sprouts on intestinal and lymphocytic glutathione S-transferases in humans. Carcinogenesis 1995;16(9):2125–2128
20. Nijhoff WA, Mulder TP, Verhagen H, van Poppel G, Peters WH. Effects of consumption of brussels sprouts on plasma and urinary glutathione S-transferase class-alpha and -pi in humans. Carcinogenesis 1995;16(4):955–957
21. Stewart ZA, Westfall MD, Pietenpol JA. Cell-cycle dys-regulation and anticancer therapy. Trends Pharmacol Sci 2003;24(3):139–145
22. Nakamura Y, Miyoshi N. Cell death induction by isothiocyanates and their underlying molecular mechanisms. Biofactors 2006;26(2):123–134
23. Zhang Y, Yao S, Li J. Vegetable-derived isothiocyanates: anti-proliferative activity and mechanism of action. Proc Nutr Soc 2006;65(1):68–75
24. Mei S, Ho AD, Mahlknecht U. Role of histone deacetylase inhibitors in the treatment of cancer (Review). Int J Oncol 2004;25(6):1509–1519
25. Marks PA, Richon VM, Miller T, Kelly WK. Histone deacetylase inhibitors. Adv Cancer Res 2004;91:137–168
26. Lea MA, Rasheed M, Randolph VM, Khan F, Shareef A, desBordes C. Induction of histone acetylation and inhibition of growth of mouse erythroleukemia cells by S-allylmercaptocysteine. Nutr Cancer 2002;43(1):90–102
27. Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E. Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis 2006;27(4):811–819
28. Pledgie-Tracy A, Sobolewski MD, Davidson NE. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol Cancer Ther 2007;6(3):1013–1021
29. Myzak MC, Tong P, Dashwood WM, Dashwood RH, Ho E. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Exp Biol Med (Maywood) 2007;232(2):227–234
30. Steele VE, Hawk ET, Viner JL, Lubet RA. Mechanisms and applications of non-steroidal anti-inflammatory drugs in the chemoprevention of cancer. Mutat Res 2003;523–524:137–144
31. Gerhäuser C, Klimo K, Heiss E, et al. Mechanism-based in vitro screening of potential cancer chemo-preventive agents. Mutat Res 2003;523–524:163–172
32. Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhäuser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem 2001;276(34):32008–32015
33. Normark S, Nilsson C, Normark BH, Hornef MW. Persistent infection with Helicobacter pylori and the development of gastric cancer. Adv Cancer Res 2003;90:63–89
34. Fahey JW, Haristoy X, Dolan PM, et al. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci U S A 2002;99(11):7610–7615
35. Haristoy X, Angioi-Duprez K, Duprez A, Lozniewski A. Efficacy of sulforaphane in eradicating Helicobacter pylori in human gastric xenografts implanted in nude mice. Antimicrob Agents Chemother 2003;47(12): 3982–3984
36. Galan MV, Kishan AA, Silverman AL. Oral broccoli sprouts for the treatment of Helicobacter pylori infection: a preliminary report. Dig Dis Sci 2004;49(7–8):1088–1090
37. Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev 1996;5(9):733–748
38. Jiao D, Yu MC, Hankin JH, Low SH, Chung FL. Total isothiocyanate contents in cooked vegetables frequently consumed in Singapore. J Agric Food Chem 1998; 46(3):1055–1058
39. Zhao B, Seow A, Lee EJ, et al. Dietary isothiocyanates, glutathione S-transferase -M1, -T1 polymorphisms and lung cancer risk among Chinese women in Singapore. Cancer Epidemiol Biomarkers Prev 2001; 10(10):1063–1067
40. Spitz MR, Duphorne CM, Detry MA, et al. Dietary in-take of isothiocyanates: evidence of a joint effect with glutathione S-transferase polymorphisms in lung cancer risk. Cancer Epidemiol Biomarkers Prev 2000; 9(10):1017–1020
41. Fowke JH, Hebert JR, Fahey JW. Urinary excretion of dithiocarbamates and self-reported Cruciferous vegetable intake: application of the ‘method of triads’ to a food-specific biomarker. Public Health Nutr 2002; 5(6):791–799
42. Kristensen M, Krogholm KS, Frederiksen H, Bügel SH, Rasmussen SE. Urinary excretion of total isothiocyanates from cruciferous vegetables shows high dose-response relationship and may be a useful biomarker for isothiocyanate exposure. Eur J Nutr 2007; 46(7):377–382
43. London SJ, Yuan JM, Chung FL, et al. Isothiocyanates, glutathione S-transferase M1 and T1 polymorphisms, and lung-cancer risk: a prospective study of men in Shanghai, China. Lancet 2000;356(9231):724–729
44. Fowke JH, Shu XO, Dai Q, et al. Urinary isothiocyanate excretion, brassica consumption, and gene polymorphisms among women living in Shanghai, China. Cancer Epidemiol Biomarkers Prev 2003;12(12):1536–1539
45. Coles BF, Kadlubar FF. Detoxification of electrophilic compounds by glutathione S-transferase catalysis: determinants of individual response to chemical carcinogens and chemotherapeutic drugs? Biofactors 2003;17(1–4):115–130
46. Lewis S, Brennan P, Nyberg F, et al. Re: Spitz, M. R., Duphorne, C. M., Detry, M. A., et al. Dietary intake of isothiocyanates: evidence of a joint effect with glutathione S-transferase polymorphisms in lung cancer risk. Cancer Epidemiol. Biomark. Prev., 9: 1017–1020, 2000. Cancer Epidemiol Biomarkers Prev 2001;10(10):1105–1106
47. Brennan P, Hsu CC, Moullan N, et al. Effect of cruciferous vegetables on lung cancer in patients stratified by genetic status: a mendelian randomisation approach. Lancet 2005;366(9496):1558–1560
48. Seow A, Yuan JM, Sun CL, Van Den Berg D, Lee HP, Yu MC. Dietary isothiocyanates, glutathione S-transferase polymorphisms and colorectal cancer risk in the Singapore Chinese Health Study. Carcinogenesis 2002;23(12):2055–2061
49. Slattery ML, Kampman E, Samowitz W, Caan BJ, Potter JD. Interplay between dietary inducers of GST and the GSTM-1 genotype in colon cancer. Int J Cancer 2000;87(5):728–733
50. Turner F, Smith G, Sachse C, et al. Vegetable, fruit and meat consumption and potential risk modifying genes in relation to colorectal cancer. Int J Cancer 2004;112(2):259–264
51. Fenwick GR, Heaney RK, Mullin WJ. Glucosinolates and their breakdown products in food and food plants. Crit Rev Food Sci Nutr 1983;18(2):123–201
52. McNaughton SA, Marks GC. Development of a food composition database for the estimation of dietary intakes of glucosinolates, the biologically active constituents of cruciferous vegetables. Br J Nutr 2003;90(3):687–697
53. Feskanich D, Ziegler RG, Michaud DS, et al. Prospective study of fruit and vegetable consumption and risk of lung cancer among men and women. J Natl Cancer Inst 2000;92(22):1812–1823
54. Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of cruciferous vegetables and prostate cancer. Cancer Epidemiol Biomarkers Prev 2003;12(12):1403–1409
55. Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC, Giovannucci EL. Fruit and vegetable in-take and incidence of bladder cancer in a male prospective cohort. J Natl Cancer Inst 1999;91(7):605–613
56. Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A 1997;94(19):10367–10372
57. Song L, Thornalley PJ. Effect of storage, processing and cooking on glucosinolate content of Brassica vegetables. Food Chem Toxicol 2007;45(2):216–224
58. Rungapamestry V, Duncan AJ, Fuller Z, Ratcliffe B. Changes in glucosinolate concentrations, myrosinase activity, and production of metabolites of glucosinolates in cabbage (Brassica oleracea Var. capitata) cooked for different durations. J Agric Food Chem 2006;54(20):7628–7634
59. Verkerk R, Dekker M. Glucosinolates and myrosinase activity in red cabbage (Brassica oleracea L. var. Capitata f. rubra DC.) after various microwave treatments. J Agric Food Chem 2004;52(24):7318–7323
60. Okazaki K, Umemura T, Imazawa T, Nishikawa A, Masegi T, Hirose M. Enhancement of urinary bladder carcinogenesis by combined treatment with benzyl isothiocyanate and N-butyl-N-(4-hydroxybutyl)nitrosamine in rats after initiation. Cancer Sci 2003;94(11):948–952
61. Natural Medicines Comprehensive Database. Sulforaphane. Available at: http://naturaldatabase.com. Accessed May 4, 2012