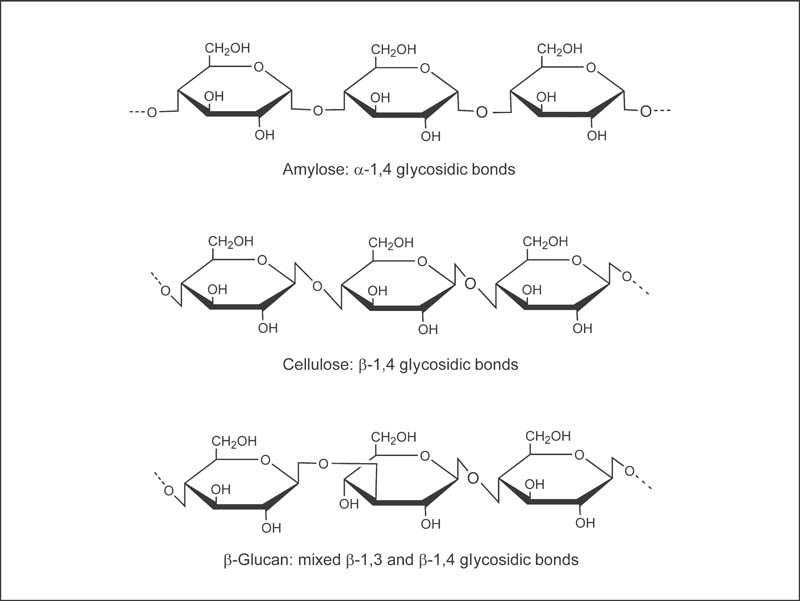
Fig. 16.1 Chemical structures of amylase (a digestible carbohydrate), cellulose (a fiber that is not digestible by human enzymes), and β-glucan (a fiber).7
All dietary fibers are resistant to digestion in the small intestine, meaning they arrive at the colon intact.1 Although most fibers are carbohydrates, one important factor that determines their susceptibility to digestion by human enzymes is the conformation of the chemical bonds between sugar molecules (glycosidic bonds). Humans lack digestive enzymes capable of hydrolyzing most β-glycosidic bonds, which explains why amylose, a glucose polymer with α-1,4 glycosidic bonds, is digestible by human enzymes, while cellulose, a glucose polymer with β-1,4 glycosidic bonds, is indigestible (Fig. 16.1).
Although nutritional scientists and clinicians generally agree that a healthy diet should include plenty of fiber-rich foods, agreement on the actual definition of fiber has been more difficult to achieve.2–4 In the 1970s, dietary fiber was defined as remnants of plant cells that are resistant to digestion by human enzymes.5 This definition includes a component of some plant cell walls called lignin, as well as indigestible carbohydrates found in plants. However, this definition omits indigestible carbohydrates derived from animal sources (e.g., chitin) and synthetic (e.g., fructooligosaccharides) and digestible carbohydrates that are inaccessible to human digestive enzymes (e.g., resistant starch).6 These compounds share many of the characteristics of fiber present in plant foods.

Fig. 16.1 Chemical structures of amylase (a digestible carbohydrate), cellulose (a fiber that is not digestible by human enzymes), and β-glucan (a fiber).7
Before establishing intake recommendations for fiber in 2001, a panel of experts convened by the Institute of Medicine developed definitions of fiber that made a distinction between fiber that occurs naturally in plant foods (dietary fiber) and isolated or synthetic fibers that may be added to foods or used as dietary supplements (functional fiber).4 However, these distinctions are controversial, and there are other classification systems for dietary fiber (see the section on Other Classification Systems below).
• Lignin: lignin is not a carbohydrate; rather, it is a polyphenolic compound with a complex three-dimensional structure that is found in the cell walls of woody plants and seeds.7
• Cellulose: cellulose is a glucose polymer with β-1,4 glycosidic bonds, found in all plant cell walls (Fig. 16.1).6
• β-Glucans: β-glucans are glucose polymers with a mixture of β-1,4 glycosidic bonds and β-1,3 glycosidic bonds (Fig. 16.1). Oats and barley are particularly rich in β-glucans.7
• Hemicelluloses: hemicelluloses are a diverse group of polysaccharides (sugar polymers) containing six-carbon sugars (hexoses) and five-carbon sugars (pentoses).6 Like cellulose, hemicelluloses are found in plant cell walls.
• Pectins: pectins are viscous polysaccharides that are particularly abundant in fruits and berries.4
• Gums: gums are viscous polysaccharides often found in seeds.4
• Inulin and oligofructose: inulin is a mixture of fructose chains that vary in length and often terminate with a glucose molecule.8 Oligofructose is a mixture of shorter fructose chains that may terminate in glucose or fructose. Inulin and oligofructose occur naturally in plants, such as onions and Jerusalem artichokes.
• Resistant starch: naturally occurring resistant starch is sequestered in plant cell walls and is therefore inaccessible to human digestive enzymes.4 Bananas and legumes are sources of naturally occurring resistant starch. Resistant starch may also be formed by food processing or by cooling and reheating.
According to the Institute of Medicine's definition, functional fiber “consists of isolated, nondigestible carbohydrates that have beneficial physiological effects in humans.”4 Functional fibers may be nondigestible carbohydrates that have been isolated or extracted from a natural plant or animal source, or they may be manufactured or synthesized. However, designation as a functional fiber by the Institute of Medicine requires the presentation of sufficient evidence of physiological benefit in humans. Fibers identified as potential functional fibers by the Institute of Medicine include:
• Isolated or extracted forms of the dietary fibers listed above.
• Psyllium: psyllium refers to viscous mucilage, which is isolated from the husks of psyllium seeds. The husks are usually isolated from the seeds of Plantago ovata or blond psyllium. Psyllium is also known as ispaghula husk.4
• Chitin and chitosan: chitin is a nondigestible carbohydrate extracted from the exoskeletons of crustaceans, such as crabs and lobsters. It is a long polymer of acetylated glucosamine units linked by β-1,4 glycosidic bonds. Deacetylation of chitin is used to produce chitosan, a nondigestible glucosamine polymer.9
• Fructooligosaccharides: fructooligosaccharides are short, synthetic fructose chains terminating with a glucose unit. They are used as food additives.8
• Polydextrose and polyols: polydextrose and polyols are synthetic polysaccharides used as bulking agents and sugar substitutes in foods.4
• Resistant dextrins: resistant dextrins, also called resistant maltodextrins, are indigestible polysaccharides formed when starch is heated and treated with enzymes. They are used as food additives.4
Total fiber is defined by the Institute of Medicine as “the sum of dietary fiber and functional fiber.”4
Some fibers form very viscous solutions or gels in water. This property is linked to the ability of some fibers to slow the emptying of the stomach, delay the absorption of some nutrients in the small intestine, and lower serum cholesterol. Viscous fibers include pectins, β-glucans, some gums (e.g., guar gum), and mucilages (e.g., psyllium). Cellulose, lignin, and some hemicelluloses are nonviscous fibers.6,7
Some fibers are readily fermented by bacteria that normally colonize the colon. In addition to increasing the amount of bacteria in the colon, fermentation results in the formation of short-chain fatty acids (acetate, propionate, and butyrate) and gases.1 Short-chain fatty acids can be absorbed and metabolized to produce energy. Interestingly, the preferred energy source for colonocytes (epithelial cells that line the colon) is butyrate. Pectins, β-glucans, guar gum, inulin, and oligofructose are readily fermented, while cellulose and lignin are resistant to fermentation in the colon.6,7 Foods that are rich in fermentable fibers include oats and barley, as well as fruits and vegetables. Cereal fibers that are rich in cellulose, such as wheat bran, are relatively resistant to bacterial fermentation.1
“Soluble fiber” originated as an analytical term.10 Soluble fibers are dispersible in water, while insoluble fibers are not. Originally, the solubility of fiber was thought to predict its physiological effects. For example, it was thought that soluble fibers were more likely to form viscous gels and were more easily fermented by colonic bacteria. Further research has revealed that solubility does not reliably predict the physiological effects of fiber. However, the terms “soluble” and “insoluble” fiber are still used by many nutrition and health-care professionals, as well as the US Food and Drug Administration (FDA) for nutrition labeling. β-glucans, gums, mucilages (e.g., psyllium), pectins, and some hemicelluloses are soluble fibers, while cellulose, lignin, some pectins, and some hemicelluloses are insoluble fibers.10 Oat products and legumes (dry beans, peas, and lentils) are rich sources of soluble fiber.
Numerous controlled clinical trials have found that increasing the intake of viscous dietary fibers, particularly from legumes (beans, peas, and lentils)11–13 and oat products,14–19 decreases serum total and low-density lipoprotein (LDL) cholesterol. These findings led the FDA to approve health claims like the following on labels of foods containing at least 0.75 g/serving of soluble fiber from whole oats: “Soluble fiber from foods such as oat bran, as part of a diet low in saturated fat and cholesterol, may reduce the risk of heart disease.”20 Supplementation with viscous fibers, such as pectin, guar gum, and psyllium, has also been found to decrease total and LDL cholesterol levels when compared with low-fiber placebos.17,21–26 Although many of these studies examined relatively high fiber intakes, a meta-analysis that combined the results of 67 controlled trials found that even a modest, 10-g per day increase in viscous fiber intake resulted in reductions in LDL cholesterol averaging 22 mg/dL (0.57 mmol/L) and reductions in total cholesterol averaging 17 mg/dL (0.45 mmol/L).17
The addition of viscous dietary fiber27,28 and isolated viscous fibers29–32 to a carbohydrate-containing meal has been found to result in significant improvements in blood glucose and insulin responses in numerous controlled clinical trials.33 Large, rapid increases in blood glucose levels are potent signals to the β-cells of the pancreas to increase insulin secretion. Over time, recurrent elevations in blood glucose and excessive insulin secretion are thought to increase the risk of developing type 2 diabetes mellitus (DM), as well as cardiovascular disease (see the Disease Prevention section below). When the carbohydrate content of two meals is equal, the presence of fiber, particularly viscous fiber, generally results in smaller but more sustained increases in blood glucose and thus significantly lower insulin levels.33
Increasing intakes of dietary fibers and fiber supplements can prevent or ameliorate constipation by softening and adding bulk to stool and by speeding its passage through the colon.34 Wheat bran and fruits and vegetables are the fiber sources that have been most consistently found to increase stool bulk and shorten transit time.35 Fiber supplements that have been found to be effective in treating constipation include cellulose and psyllium.4 Sufficient fluid intake is also required to maximize the stool-softening effect of increased fiber intake.36 In addition to increasing fiber intake, drinking at least 64 oz (approx. 2 L) of fluid daily is usually recommended to help prevent and treat constipation.37
Observational studies that have identified associations between high fiber intakes and reductions in chronic disease risk have generally assessed only fiber-rich foods, rather than fiber itself, making it difficult to determine whether observed benefits are related to fiber or other nutrients and phytochemicals commonly found in fiber-rich foods. In contrast, intervention trials often use isolated fibers to determine whether a specific fiber component has beneficial health effects.
Prospective cohort studies have consistently found that high intakes of fiber-rich foods are associated with significant reductions in coronary heart disease (CHD) risk38–48 and cardiovascular-related mortality.48–51 A pooled analysis of 10 prospective cohort studies of dietary fiber intake in the United States and Europe found that each 10 g/day increase in total dietary fiber intake was associated with a 14% decrease in the risk of coronary events, such as myocardial infarction (MI), and a 24% decrease in deaths from CHD.51 This inverse association between fiber intake and CHD death was particularly high for cereal fiber and fruit fiber. Three large prospective cohort studies42,43,45 found that dietary fiber intakes of approximately 14 g per 1000 kcal of energy were associated with substantial (16%–33%) decreases in the risk of CHD; these results are the basis for the Institute of Medicine's adequate intake (AI) recommendation for fiber (see the Intake Recommendations section below).4
Although the cholesterol-lowering effect of viscous dietary fibers and fiber supplements probably contributes to the cardioprotective effects of dietary fiber, other mechanisms are likely to play a role. Beneficial effects of fiber consumption on blood glucose and insulin responses may also contribute to observed reductions in CHD risk.52 Low-fiber, high-glycemic-load diets are associated with higher serum triglyceride levels and lower high-density lipoprotein (HDL) cholesterol levels, two risk factors for cardiovascular disease.53,54 Fiber-rich diets that are rich in certain micronutrients like magnesium and potassium may also help lower blood pressure, another important risk factor for cardiovascular disease; some observational studies have found inverse associations between dietary fiber intake and blood pressure55 or hypertension.56 A meta-analysis of 24 randomized, placebo-controlled trials found that dietary fiber supplementation (average of 11.5 g/day) lowered systolic blood pressure (SBP) by 1.13 mmHg and diastolic blood pressure (DBP) by 1.26 mmHg.57 Similarly, another meta-analysis of 25 randomized controlled trials found that an increase in dietary fiber (median increase of 10.7 g/day compared with the control group) was associated with a 1.15 mmHg reduction in SBP and a 1.65 mmHg reduction in DBP.58 Both analyses reported that the reductions in DBP, but not SBP, were statistically significant. Additionally, recent studies have indicated that higher consumption of dietary fiber may lower levels of C-reactive protein,59,60 a biomarker of inflammation that is strongly associated with the risk of cardiovascular events, such as MI and stroke.61 Thus, several mechanisms might contribute to the cardioprotective effect of high-fiber foods. Although viscous dietary fibers and fiber supplements appear to be most effective in lowering LDL cholesterol levels, large epidemio-logical studies provide strong and consistent evidence that diets rich in all fiber from whole grains, legumes, fruits, and nonstarchy vegetables can significantly reduce CHD risk.62
Increasing intakes of refined carbohydrates and decreasing intakes of fiber in the United States have paralleled the increasing prevalence of type 2 DM to near epidemic proportions.63 Numerous prospective cohort studies have found that that diets rich in fiber, particularly cereal fiber from whole grains, are associated with significant reductions in the risk of developing type 2 DM.64–74 Although no intervention trials have evaluated the effect of increasing dietary fiber intake alone on type 2 DM prevention, two important intervention trials found that a combination of lifestyle modifications that included increasing fiber intake decreased the risk of developing type 2 DM in adults with impaired glucose tolerance.75,76 Although multiple factors, including obesity, inactivity, and genetics, increase the risk of developing type 2 DM, the results of observational studies and intervention trials indicate that fiber-rich diets improve glucose tolerance and decrease the risk of type 2 DM, particularly in high-risk individuals.
The majority of case–control studies conducted prior to 1990 found the incidence of colorectal cancer was lower in people with higher fiber in-takes.77,78 A recent nested case–control study found an inverse association between dietary fiber intake and risk of colorectal cancer when fiber intake was assessed by food diaries but not when assessed by food-frequency questionnaires.79 To date, most prospective cohort studies have not found significant associations between measures of dietary fiber intake and colorectal cancer risk.80–89 A pooled analysis of 13 prospective cohort studies, which analyzed data from 725 628 adults, did not find high dietary fiber in-take to be protective against colorectal cancer when other dietary factors were taken into account.90 However, the largest prospective study on diet and cancer to date, which included 519 978 men and women participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) study, found that dietary fiber from foods was protective against development of colon cancer.91 This EPIC study was not included in the earlier pooled analysis mentioned above that reported no association between dietary fiber intake and colorectal cancer.90
In addition, four controlled clinical trials have failed to demonstrate a protective effect of fiber consumption on the recurrence of colorectal adenomas (precancerous polyps). The rate of recurrence of colorectal adenomas over a 4-year period was not significantly different between those who consumed approximately 33 g/day of fiber from a fruit- and vegetable-rich, low-fat diet and those in a control group who consumed approximately 19 g/day of fiber.92 In another trial, there was no significant difference in the rate of colorectal adenoma recurrence over a 3-year period between those supplemented with 13.5 g/day of wheat-bran fiber and those supplemented with 2 g/day of wheat-bran fiber.93 More recently, a 4-year intervention trial found that supplementation with 7.5 g/day of wheat bran had no effect on colorectal adenoma recurrence.94 Surprisingly, in another intervention trial, supplementation with 3.5 g/day of psyllium for 3 years resulted in a significant increase in adenoma recurrence compared with placebo.95
The reasons for the discrepancies between the findings of case–control studies with those of most prospective cohort studies and recent intervention trials have generated considerable debate among scientists. Potential reasons for the lack of a protective effect of dietary fiber observed in these studies include the possibility that the type or the amount of fiber consumed by most people in these studies was inadequate to prevent colorectal cancer,4 or that other dietary factors like fat may interact with fiber, influencing its effects on colorectal cancer.1,96 The methods used to assess fiber intake in observational studies may also contribute to the disparate results.79 Clearly, more research is needed to sort out the complex effects of dietary fiber and fiber supplements on colorectal cancer risk and progression.
Several early case–control studies found significant inverse associations between dietary fiber intake and breast cancer incidence,97–100 but many prospective cohort studies have not found dietary fiber intake to be associated with significant reductions in breast cancer risk.101–108 Three studies have reported a protective effect of dietary fiber on breast cancer risk. A prospective cohort study in the United Kingdom found that dietary fiber intake was inversely associated with risk of breast cancer in premenopausal women but not in postmenopausal women.109 Additionally, a prospective cohort study in Sweden found that postmenopausal women with the highest fiber intakes (averaging approximately 26 g/day) had a risk of breast cancer that was 40% lower than that of women with the lowest fiber intakes (averaging approximately 13 g/day).110 Women with the highest fiber and lowest fat intakes had the very lowest risk of breast cancer. More recently, a prospective study in a cohort of more than 185 000 postmenopausal women in the United States found that those with the highest intakes of dietary fiber (median, 26 g/day) had a 13% lower risk of all forms of breast cancer and a 44% lower risk of hormone receptor-negative tumors (ER–/PR–) compared with those with the lowest intakes of dietary fiber (median 11 g/day).111 A 2011 meta-analysis of 10 prospective cohort studies found a modest, 11% lower risk of breast cancer in women with the highest intakes of dietary fiber.112 The results of small, short-term intervention trials in premenopausal and postmenopausal women suggested that low-fat (10%–25% of energy), high-fiber (25–40 g/day) diets could decrease circulating estrogen levels by increasing the excretion of estrogens and by promoting the metabolism of estrogens to less estrogenic forms.113,114 However, it is not known whether fiber-associated effects on endogenous estrogen levels have a clinically significant impact on breast cancer risk.4 Overall, observational studies examining dietary fiber intake and breast cancer incidence have reported mixed results. In such studies, it is possible that the association may be confounded by consumption of fiber-rich foods, such as fruits and vegetables.
Some observational studies have associated high fiber intakes with a decreased risk of diverticulosis, a relatively common condition that is characterized by the formation of small pouches (diverticula) in the colon.115,116 However, a recent cross-sectional study of 2104 adults found that those with the highest fiber intakes, measured by food frequency questionnaires, had a higher prevalence of diverticula (assessed by colonoscopy) compared with those with the lowest fiber intakes.117 Although most people with diverticulosis experience no symptoms, approximately 15%–20% may develop pain or inflammation, known as diverticulitis.118 In a large prospective cohort study, men with the highest insoluble fiber intakes (median 22.7 g/day) had a 37% lower risk of developing symptomatic diverticular disease compared with men with the lowest insoluble fiber intakes (median 10.1 g/day). The protective effect of dietary fiber against diverticular disease was strongest for cellulose and lignin.119 More research is needed to clarify the association between dietary fiber and diverticular disease.
In addition to providing less energy, there is some evidence that higher fiber intakes can help to prevent weight gain or promote weight loss by extending the feeling of fullness after a meal (satiety).120 Observational studies have found that adults with higher intakes of dietary fiber are leaner121,122 and less likely to be obese than adults with low fiber intakes.123,124 One large prospective cohort study found that women whose in-take of high-fiber foods increased by an average of 9 g/day over a 12-year period were half as likely to experience a major weight gain of at least 55 lb (25 kg) than those whose intake of high-fiber foods decreased by an average of 3 g/day.125 The results of short-term clinical trials examining the effect of increased fiber intake on weight loss have been mixed. Overall, a systematic review of clinical trials conducted prior to 2001 found that increasing fiber intake from foods or supplements by 14 g/day resulted in a 10% decrease in energy intake and weight losses averaging approximately 4 lb (1.9 kg) over 4 months.120 However, some more recent clinical trials did not find fiber-rich cereal126 or fiber supplements127 enhanced weight loss. A 2011 systematic review of 61 randomized controlled trials examined the effect of different fiber types on body weight.128 This analysis found that dextrins and marine polysaccharides reduced body weight in all of the studies, while chitosan, arabinoxylans, and fructans reduced body weight in at least two-thirds of the studies. Average weight reductions were greatest for the fructans and marine polysaccharides groups (approximately 1.3 kg or 2.8 lb/4 weeks for a 79 kg person in both groups). For all fiber types combined, the average weight reduction was only 0.3 g (0.7 lb) per 4 weeks for a 79-kg person.128 Although people with higher intakes of fiber-rich foods, particularly whole grains, appear more likely to maintain a healthy body weight, the role of fiber alone in long-term weight control is not yet clear. Effects on body weight might depend on the specific type of dietary fiber.
Several prospective cohort studies have found higher intakes of dietary fiber to be associated with a lower risk of mortality from all causes. A recent report from the National Institutes of Health American Association of Retired Persons (NIH-AARP) Diet and Health Study, which followed 388 122 older adults for an average of 9 years, found that men and women in the highest quintiles of dietary fiber intake had a 22% lower risk of mortality when compared with the lowest quintiles of dietary fiber intake.50 Smaller prospective studies have also reported an inverse association between total fiber intake and all-cause mortality,44,48,129 and an inverse association between cereal fiber intake and all-cause mortality was found in the Nurses’ Health Study, which included more than 50 000 participants.130 However, no association was found between total fiber intake or soluble fiber intake and mortality from all causes in the National Health and Nutrition Examination Survey (NHANES) I Epidemiologic Follow-Up Study—a prospective study that assessed fiber intake by a single, 24-hour dietary recall method.46
Numerous controlled clinical trials in people who have type 1 or type 2 DM have found that increasing fiber intake from foods131,132 or viscous fiber supplements133–135 improves markers of glycemic control, particularly postprandial glucose levels and serum lipid profiles. A meta-analysis that combined the results of 23 clinical trials comparing the effects of high-fiber diets (>20 g/1000 kcal) with those of low-fiber diets (<10 g/1000 kcal) in patients with diabetes found that high-fiber diets lowered postprandial blood glucose concentrations by 13%–21%, serum LDL cholesterol concentrations by 8%–16%, and serum triglyceride concentrations by 8%–13%.136 Based on the evidence from this meta-analysis, the authors recommended a dietary fiber intake of 25–50 g/day (15–25 g/1000 kcal) for individuals with diabetes, which is consistent with the recommendations of many international diabetes organizations of at least 25–35 g/day.137–139 In general, the results of controlled clinical trials support recommendations that people with diabetes aim for high fiber intakes by increasing their consumption of whole grains, legumes, nuts, fruits, and nonstarchy vegetables. Since there is little evidence from clinical trials that increasing nonviscous fiber alone is beneficial,140 individuals with diabetes should avoid increasing fiber intake exclusively from nonviscous sources, such as wheat bran.136
Irritable bowel syndrome (IBS) is a functional disorder of the intestines, characterized by episodes of abdominal pain or discomfort associated with a change in bowel movements, such as constipation or diarrhea.141 Although people diagnosed with IBS are often encouraged by health-care providers to increase dietary fiber intake, the results of controlled clinical trials of psyllium, methylcellulose, and wheat bran have been mixed.142–144 A systematic review and meta-analysis of 12 randomized controlled trials found a beneficial effect of fiber that was limited to ispaghula husk (psyllium).143 More recently, a 3-month randomized, placebo-controlled trial in 275 patients with IBS found that supplementation with psyllium (10 g/day) improved symptoms of abdominal pain or discomfort in the first 2 months of supplementation and also improved symptom severity after 3 months’ supplementation.145 Compared with placebo, supplementation with insoluble bran fiber (10 g/day) improved abdominal pain or discomfort only after 3 months’ supplementation and had no effect on symptom severity.145 Additionally, a systematic review of 17 randomized controlled trials of fiber supplements in IBS patients found that supplementation with soluble fiber, mainly from psyllium, significantly improved a global measure of IBS symptoms, while supplementation with insoluble fiber, such as corn bran or wheat bran, did not improve IBS symptoms.146 In general, fiber supplements improved constipation in IBS patients but did not improve IBS-associated abdominal pain. Thus, the results of randomized controlled trials suggest that increasing soluble or viscous fiber intake gradually to 12–30 g/day may be beneficial for patients in whom constipation is the predominant symptom of IBS.147 However, fiber supplements could actually exacerbate symptoms in those in whom diarrhea predominates.148 A few clinical trials have found that partially hydrolyzed guar gum (5 g/day), a water-soluble, non-gelling fiber, may improve IBS symptoms in patients with diarrhea and in those with constipation-predominant IBS.149 IBS patients should be advised to increase fiber intake gradually, since increasing their intake of viscous, readily fermented fibers could increase gas production and bloating.
Table 16.1 Total fiber content of selected fiber-rich foods150
Food |
Serving |
Total Dietary Fiber (g) |
100% (wheat) bran cereal |
½ cup |
12.5 |
Navy beans, cooked from dried |
½ cup |
9.6 |
Split peas, cooked from dried |
½ cup |
8.1 |
Lentils, cooked from dried |
½ cup |
7.8 |
Kidney beans, canned |
½ cup |
6.8 |
Asian pear |
1 small |
4.4 |
Apple with skin |
1 medium |
4.4 |
English muffin, whole-wheat |
1 |
4.4 |
Bulgur, cooked |
½ cup |
4.1 |
Raspberries, raw |
½ cup |
4.0 |
Sweet potato, baked with peel |
1 medium |
3.8 |
Spinach, frozen, cooked |
½ cup |
3.5 |
Almonds |
1 oz |
3.5 |
Dietary fiber intakes in the United States average 16–18 g/day for men and 12–14 g/day for women—well below recommended intake levels (see the Intake Recommendations section below).4 Good sources of dietary fiber include legumes, nuts, whole grains, bran products, fruits, and nonstarchy vegetables. Legumes, whole grains, and nuts are generally more concentrated sources of fiber than fruits and vegetables. All plant-based foods contain mixtures of soluble and insoluble fiber.10 Oat products and legumes are rich sources of soluble and viscous fiber. Wheat bran and whole grains are rich sources of insoluble and nonviscous fiber. The total fiber content of some fiber-rich foods is presented in Table 16.1.150 Some strategies for increasing dietary fiber intake include increasing fruit and nonstarchy vegetable intake, increasing intake of legumes, eating whole-grain cereal or oatmeal for breakfast, substituting whole grains for refined grains, and substituting nuts or popcorn for less healthy snacks.
β-Glucans are viscous, easily fermented, soluble fibers found naturally in oats, barley, mushrooms, yeast, bacteria, and algae.151 β-Glucans extracted from oats, mushrooms, and yeast are available in a variety of nutritional supplements without a prescription.
Pectins are viscous fibers, most often extracted from citrus peels and apple pulp. Pectins are widely used as gelling agents in foods but are also available as dietary supplements without a prescription.9
Inulins and oligofructose, extracted from chicory root or synthesized from sucrose, are used as food additives.8 Isolated inulin is added to replace fat in products like salad dressing, while sweet-tasting oligofructose is added to products like fruit yogurts and desserts. Inulins and oligofructose are highly fermentable fibers that are also classified as prebiotics because of their ability to stimulate the growth of potentially beneficial Bifidobacteria species in the human colon.152 Encouraging the growth of Bifidobacteria could promote intestinal health by suppressing the growth of pathogenic bacteria known to cause diarrhea, or by enhancing the immune response.153 Although several dietary supplements containing inulins and oligofructose are marketed as prebiotics, the health benefits of prebiotics have not yet been convincingly demonstrated in humans.154,155
Guar gum is a viscous, fermentable fiber derived from the Indian cluster bean.4 It is used as a thickener or emulsifier in many food products. Dietary supplements containing guar gum have been marketed as weight-loss aids, but a meta-analysis that combined the results of 11 randomized controlled trials found that guar gum supplements were not effective in reducing body weight.156
Psyllium, a viscous, soluble fiber isolated from psyllium seed husks, is available without a prescription in laxatives, ready-to-eat cereals, and dietary supplements.9 The FDA has approved health claims like the following on the labels of foods containing at least 1.7 g/serving of soluble fiber from psyllium: “Diets low in saturated fat and cholesterol that include 7 g/day of soluble fiber from psyllium may reduce the risk of heart disease.”20
Chitosan is an indigestible glucosamine polymer derived from chitin. When administered with food in animal studies, chitosan decreased fat absorption.157 Consequently, chitosan has been marketed as a dietary supplement to promote weight loss and lower cholesterol. Controlled clinical trials in humans have not generally found chitosan supplementation to be more effective than placebo in promoting weight loss.158 While some clinical trials in humans have found chitosan supplementation to result in modest reductions in total and LDL cholesterol levels compared with placebo,159,160 others found no improvement.161,162 Chitosan is available as a dietary supplement without a prescription in the United States.
All fiber supplements should be taken with sufficient fluids. Most clinicians recommend taking fiber supplements with at least 8 oz (240 mL) of water and consuming a total of at least 64 oz (approx. 2 L) of fluid daily.163,164
Some people experience abdominal cramping, bloating, or gas when they abruptly increase their dietary fiber intakes.163,164 These symptoms can be minimized or avoided by increasing the intake of fiber-rich foods gradually and increasing fluid intake to at least 64 oz/day (approx. 2 L). There have been rare reports of intestinal obstruction related to large intakes of oat bran or wheat bran, usually in people with impaired intestinal motility or difficulty chewing.165–168 The Institute of Medicine has not established a tolerable upper intake level for dietary or functional fiber.4
Gastrointestinal symptoms. The following fibers have been found to cause gastrointestinal distress, including abdominal cramping, bloating, gas, and diarrhea: guar gum, inulin and oligofructose, fructooligosaccharides, polydextrose, resistant starch, and psyllium.4 Use of a guargum-containing supplement for weight loss has been associated with esophageal and small bowel obstruction.169 Additionally, several cases of intestinal obstruction by psyllium have been reported when taken with insufficient fluids or by people with impaired swallowing or gastrointestinal motility.170,171
Colorectal adenomas. One randomized controlled trial in patients with a history of colorectal adenomas (precancerous polyps) found that supplementation with 3.5 g/day of psyllium for 3 years resulted in a significant increase in colorectal adenoma recurrence compared with placebo (see the section on Colorectal Cancer above).95
Allergy and anaphylaxis. Since chitin and chitosan may be isolated from the exoskeletons of crustaceans, such as crabs and lobsters, people with shellfish allergies should avoid taking chitin or chitosan supplements.9 Anaphylaxis has been reported after intravenous administration of inulin,172 as well as ingestion of margarine containing inulin extracted from chicory.173 Anaphylaxis has also been reported after the ingestion of cereals containing psyllium, and asthma has occasionally been reported in people with occupational exposure to psyllium powder.174
Psyllium may reduce the absorption of lithium, carbamazepine (Tegretol), digoxin (Lanoxin), and warfarin (Coumadin) when taken at the same time.9 Guar gum may slow the absorption of digoxin, acetaminophen (paracetamol, Tylenol), and bumetanide (Bumex) and decrease the absorption of metformin (Glucophage), penicillin, and some formulations of glibenclamide (Glynase) when taken at the same time.175 Pectin may decrease the absorption of lovastatin (Mevacor) when taken at the same time.176 Concomitant administration of kaolin-pectin has been reported to decrease the absorption of clindamycin, tetracyclines, and digoxin, but it is not known whether kaolin or pectin is responsible for the interaction.9 In general, medications should be taken at least 1 hour before or 2 hours after fiber supplements.
The addition of cereal fiber to meals has generally been found to decrease the absorption of iron, zinc, calcium, and magnesium in the same meal, but this effect appears to be related to the phytate present in the cereal fiber rather than the fiber itself.177 In general, dietary fiber as part of a balanced diet has not been found to adversely affect the calcium, magnesium, iron, or zinc status of healthy people at recommended intake levels.4 Evidence from animal studies and limited research in humans suggests that inulin and oligofructose may enhance calcium absorption.178,179 The addition of pectin and guar gum to a meal significantly reduced the absorption of the carotenoids β-carotene, lycopene, and lutein from that meal.180,181
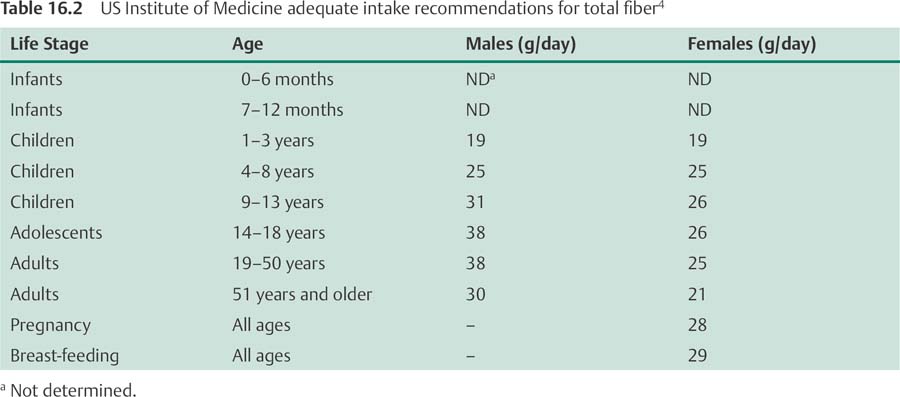
In light of consistent evidence from prospective cohort studies that fiber-rich diets are associated with significant reductions in cardiovascular disease risk, the Food and Nutrition Board of the US Institute of Medicine established its first recommended intake levels for fiber in 2001.4 The adequate intake (AI) recommendations for total fiber intake are based on the findings of several large prospective cohort studies that dietary fiber in-takes of approximately 14 g for every 1000 calories (kcal) consumed were associated with significant reductions in the risk of CHD,42,43,45 as well as type 2 DM.66,67 For adults aged 50 years and younger, the AI recommendation for total fiber intake is 38 g/day for men and 25 g/day for women. For adults aged over 50 years, the recommendation is 30 g/day for men and 21 g/day for women. The AI recommendations for males and females of all ages are presented in Table 16.2.4
• Dietary fiber is a diverse group of compounds, including lignin and complex carbohydrates, that cannot be digested by human enzymes in the small intestine.
• Although each class of fiber is chemically unique, scientists have tried to classify fibers on the basis of their solubility, viscosity, and fermentability, to better understand their physiological effects.
• Viscous fibers, such as those found in oat products and legumes, can lower serum low-density lipoprotein cholesterol levels and normalize blood glucose and insulin responses.
• High fiber intakes promote bowel health by preventing constipation and diverticular disease.
• Large prospective cohort studies provide strong and consistent evidence that diets rich in fiber from whole grains, legumes, fruits, and nonstarchy vegetables can reduce the risk of cardiovascular disease and type 2 diabetes.
• Although the results of case–control studies suggested that colorectal cancer was more prevalent in people with low-fiber intakes, more recent findings from large prospective cohort studies and four clinical intervention trials do not support an association between fiber intake and the risk of colorectal cancer.
• Observational studies on dietary fiber intake and breast cancer incidence have reported inconsistent findings.
• Numerous controlled clinical trials in people with type 1 and type 2 diabetes have found that increasing fiber intake improves glycemic control and serum lipid profiles.
• In 2001, the Food and Nutrition Board of the Institute of Medicine established an adequate intake (AI) recommendation for total daily fiber intake. For adults who are aged 50 years and younger, the AI recommendation for total fiber intake is 38 g/day for men and 25 g/day for women. For adults aged over 50 years, the recommendation is 30 g/day for men and 21 g/day for women.
1. Lupton JR. Microbial degradation products influence colon cancer risk: the butyrate controversy. J Nutr 2004;134(2):479–482
2. Ha MA, Jarvis MC, Mann JI. A definition for dietary fibre. Eur J Clin Nutr 2000;54(12):861–864
3. DeVries JW. On defining dietary fibre. Proc Nutr Soc 2003;62(1):37–43
4. Institute of Medicine. Dietary, Functional, and Total Fiber. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: National Academies Press; 2002:265–334
5. Trowell H. Dietary fibre, ischaemic heart disease and diabetes mellitus. Proc Nutr Soc 1973;32(3):151–157
6. Lupton JR, Turner ND. Dietary fiber. In: Stipanuk MH, ed. Biochemical and Physiological Aspects of Human Nutrition. Philadelphia: WB Saunders; 2000:143–154
7. Gallaher CM, Schneeman BO. Dietary fiber. In: Bowman BA, Russell RM, eds. Present Knowledge in Nutrition. 8th ed. Washington, DC: ILSI Press; 2001:83–91
8. Niness KR. Inulin and oligofructose: what are they? J Nutr 1999; 129(7, Suppl):1402S–1406S
9. Hendler SS, Rorvik DR, eds. PDR for Nutritional Supplements. 2nd ed. Montvale, NJ: Physicians’ Desk Reference Inc.; 2008
10. Marlett JA. Content and composition of dietary fiber in 117 frequently consumed foods. J Am Diet Assoc 1992;92(2):175–186
11. Bazzano LA, Thompson AM, Tees MT, Nguyen CH, Winham DM. Non-soy legume consumption lowers cholesterol levels: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis 2011; 21(2):94–103
12. Zhang Z, Lanza E, Kris-Etherton PM, et al. A high legume low glycemic index diet improves serum lipid profiles in men. Lipids 2010;45(9):765–775
13. Anderson JW, Major AW. Pulses and lipaemia, short-and long-term effect: potential in the prevention of cardiovascular disease. Br J Nutr 2002;88(Suppl 3): S263–S271
14. Wolever TM, Tosh SM, Gibbs AL, et al. Physicochemical properties of oat β-glucan influence its ability to reduce serum LDL cholesterol in humans: a randomized clinical trial. Am J Clin Nutr 2010;92(4):723–732
15. Naumann E, van Rees AB, Onning G, Oste R, Wydra M, Mensink RP. Beta-glucan incorporated into a fruit drink effectively lowers serum LDL-cholesterol concentrations. Am J Clin Nutr 2006;83(3):601–605
16. Ripsin CM, Keenan JM, Jacobs DR Jr, et al. Oat products and lipid lowering. A meta-analysis. JAMA 1992; 267(24):3317–3325
17. Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr 1999;69(1):30–42
18. Queenan KM, Stewart ML, Smith KN, Thomas W, Fulcher RG, Slavin JL. Concentrated oat beta-glucan, a fermentable fiber, lowers serum cholesterol in hyper-cholesterolemic adults in a randomized controlled trial. Nutr J 2007;6:6
19. Reyna-Villasmil N, Bermúdez-Pirela V, Mengual Moreno E, et al. Oat-derived beta-glucan significantly improves HDLC and diminishes LDLC and non-HDL cholesterol in overweight individuals with mild hypercholesterolemia. Am J Ther 2007;14(2):203–212
20. Food and Drug Administration, HHS. Food labeling: health claims; soluble dietary fiber from certain foods and coronary heart disease. Final rule. Fed Regist 2003;68(144):44207–44209
21. Wei ZH, Wang H, Chen XY, et al. Time- and dose-dependent effect of psyllium on serum lipids in mild-to-moderate hypercholesterolemia: a meta-analysis of controlled clinical trials. Eur J Clin Nutr 2009; 63(7):821–827
22. Pal S, Khossousi A, Binns C, Dhaliwal S, Ellis V. The effect of a fibre supplement compared to a healthy diet on body composition, lipids, glucose, insulin and other metabolic syndrome risk factors in overweight and obese individuals. Br J Nutr 2011;105(1):90–100
23. Anderson JW, Allgood LD, Lawrence A, et al. Cholesterol-lowering effects of psyllium intake adjunctive to diet therapy in men and women with hypercholesterolemia: meta-analysis of 8 controlled trials. Am J Clin Nutr 2000;71(2):472–479
24. Butt MS, Shahzadi N, Sharif MK, Nasir M. Guar gum: a miracle therapy for hypercholesterolemia, hyperglycemia and obesity. Crit Rev Food Sci Nutr 2007; 47(4):389–396
25. Theuwissen E, Mensink RP. Water-soluble dietary fibers and cardiovascular disease. Physiol Behav 2008;94(2):285–292
26. Bazzano LA. Effects of soluble dietary fiber on low-density lipoprotein cholesterol and coronary heart disease risk. Curr Atheroscler Rep 2008;10(6):473–477
27. Schäfer G, Schenk U, Ritzel U, Ramadori G, Leonhardt U. Comparison of the effects of dried peas with those of potatoes in mixed meals on postprandial glucose and insulin concentrations in patients with type 2 diabetes. Am J Clin Nutr 2003;78(1):99–103
28. Kabir M, Oppert JM, Vidal H, et al. Four-week lowglycemic index breakfast with a modest amount of soluble fibers in type 2 diabetic men. Metabolism 2002;51(7):819–826
29. Brand-Miller JC, Atkinson FS, Gahler RJ, Kacinik V, Lyon MR, Wood S. Effects of PGX, a novel functional fibre, on acute and delayed postprandial glycaemia. Eur J Clin Nutr 2010;64(12):1488–1493
30. Jenkins AL, Kacinik V, Lyon MR, Wolever TM. Reduction of postprandial glycemia by the novel viscous polysaccharide PGX, in a dose-dependent manner, independent of food form. J Am Coll Nutr 2010; 29(2):92–98
31. Williams JA, Lai CS, Corwin H, et al. Inclusion of guar gum and alginate into a crispy bar improves post-prandial glycemia in humans. J Nutr 2004;134(4):886–889
32. Sierra M, Garcia JJ, Fernández N, Diez MJ, Calle AP, Sahagún AM. Effects of ispaghula husk and guar gum on postprandial glucose and insulin concentrations in healthy subjects. Eur J Clin Nutr 2001;55(4):235–243
33. Wolever TM, Jenkins DA. Effect of dietary fiber and foods on carbohydrate metabolism. In: Spiller GA, ed. CRC Handbook of Dietary Fiber in Human Nutrition. 3rd ed. Boca Raton, FL: CRC Press; 2001:321–360
34. Marlett JA, McBurney MI, Slavin JL; American Dietetic Association. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc 2002;102(7):993–1000
35. Cummings JH. The effect of dietary fiber on fecal weight and composition. In: Spiller GA, ed. Fiber in Human Nutrition. 3rd ed. Boca Raton, FL: CRC Press; 2001:183–252
36. Anti M, Pignataro G, Armuzzi A, et al. Water supplementation enhances the effect of high-fiber diet on stool frequency and laxative consumption in adult patients with functional constipation. Hepatogastroenterology 1998;45(21):727–732
37. American Academy of Family Physicians. Constipation. Available at: http://familydoctor.org/037.xml. Accessed May 3, 2012
38. Fraser GE, Sabaté J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Arch Intern Med 1992;152(7):1416–1424
39. Humble CG, Malarcher AM, Tyroler HA. Dietary fiber and coronary heart disease in middle-aged hypercholesterolemic men. Am J Prev Med 1993;9(4):197–202
40. Jacobs DR Jr, Meyer KA, Kushi LH, Folsom AR. Whole-grain intake may reduce the risk of ischemic heart disease death in postmenopausal women: the Iowa Women's Health Study. Am J Clin Nutr 1998; 68(2):248–257
41. Khaw KT, Barrett-Connor E. Dietary fiber and reduced ischemic heart disease mortality rates in men and women: a 12-year prospective study. Am J Epidemiol 1987;126(6):1093–1102
42. Pietinen P, Rimm EB, Korhonen P, et al. Intake of dietary fiber and risk of coronary heart disease in a cohort of Finnish men. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Circulation 1996;94(11):2720–2727
43. Rimm EB, Ascherio A, Giovannucci E, Spiegelman D, Stampfer MJ, Willett WC. Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men. JAMA 1996;275(6):447–451
44. Todd S, Woodward M, Tunstall-Pedoe H, Bolton-Smith C. Dietary antioxidant vitamins and fiber in the etiology of cardiovascular disease and all-causes mortality: results from the Scottish Heart Health Study. Am J Epidemiol 1999;150(10):1073–1080
45. Wolk A, Manson JE, Stampfer MJ, et al. Long-term in-take of dietary fiber and decreased risk of coronary heart disease among women. JAMA 1999;281(21): 1998–2004
46. Bazzano LA, He J, Ogden LG, Loria CM, Whelton PK; National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Dietary fiber intake and reduced risk of coronary heart disease in US men and women: the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Intern Med 2003;163(16):1897–1904
47. Mozaffarian D, Kumanyika SK, Lemaitre RN, Olson JL, Burke GL, Siscovick DS. Cereal, fruit, and vegetable fiber intake and the risk of cardiovascular disease in elderly individuals. JAMA 2003;289(13):1659–1666
48. Streppel MT, Ocké MC, Boshuizen HC, Kok FJ, Kromhout D. Dietary fiber intake in relation to coronary heart disease and all-cause mortality over 40 y: the Zutphen Study. Am J Clin Nutr 2008;88(4):1119–1125
49. Eshak ES, Iso H, Date C, et al; JACC Study Group. Dietary fiber intake is associated with reduced risk of mortality from cardiovascular disease among Japanese men and women. J Nutr 2010;140(8):1445–1453
50. Park Y, Subar AF, Hollenbeck A, Schatzkin A. Dietary fiber intake and mortality in the NIH-AARP diet and health study. Arch Intern Med 2011;171(12):1061–1068
51. Pereira MA, O'Reilly E, Augustsson K, et al. Dietary fiber and risk of coronary heart disease: a pooled analysis of cohort studies. Arch Intern Med 2004; 164(4):370–376
52. Liu S, Willett WC. Dietary glycemic load and atherothrombotic risk. Curr Atheroscler Rep 2002;4(6):454–461
53. Ford ES, Liu S. Glycemic index and serum high-density lipoprotein cholesterol concentration among US adults. Arch Intern Med 2001;161(4):572–576
54. Liu S, Manson JE, Stampfer MJ, et al. Dietary glycemic load assessed by food-frequency questionnaire in relation to plasma high-density-lipoprotein cholesterol and fasting plasma triacylglycerols in postmenopausal women. Am J Clin Nutr 2001;73(3):560–566
55. Ascherio A, Hennekens C, Willett WC, et al. Prospective study of nutritional factors, blood pressure, and hypertension among US women. Hypertension 1996;27(5):1065–1072
56. Ascherio A, Rimm EB, Giovannucci EL, et al. A prospective study of nutritional factors and hypertension among US men. Circulation 1992;86(5):1475–1484
57. Streppel MT, Arends LR, van't Veer P, Grobbee DE, Geleijnse JM. Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials. Arch Intern Med 2005;165(2):150–156
58. Whelton SP, Hyre AD, Pedersen B, Yi Y, Whelton PK, He J. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J Hypertens 2005;23(3):475–481
59. King DE, Egan BM, Woolson RF, Mainous AG III, AlSolaiman Y, Jesri A. Effect of a high-fiber diet vs a fiber-supplemented diet on C-reactive protein level. Arch Intern Med 2007;167(5):502–506
60. Ma Y, Griffith JA, Chasan-Taber L, et al. Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr 2006;83(4):760–766
61. Patel VB, Robbins MA, Topol EJ. C-reactive protein: a ‘golden marker’ for inflammation and coronary artery disease. Cleve Clin J Med 2001;68(6):521–524, 527–534
62. Lupton JR, Turner ND. Dietary fiber and coronary disease: does the evidence support an association? Curr Atheroscler Rep 2003;5(6):500–505
63. Gross LS, Li L, Ford ES, Liu S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr 2004;79(5):774–779
64. Wannamethee SG, Whincup PH, Thomas MC, Sattar N. Associations between dietary fiber and inflammation, hepatic function, and risk of type 2 diabetes in older men: potential mechanisms for the benefits of fiber on diabetes risk. Diabetes Care 2009; 32(10):1823–1825
65. Hopping BN, Erber E, Grandinetti A, Verheus M, Kolonel LN, Maskarinec G. Dietary fiber, magnesium, and glycemic load alter risk of type 2 diabetes in a multi-ethnic cohort in Hawaii. J Nutr 2010;140(1):68–74
66. Salmerón J, Ascherio A, Rimm EB, et al. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 1997;20(4):545–550
67. Salmerón J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 1997;277(6):472–477
68. Meyer KA, Kushi LH, Jacobs DR Jr, Slavin J, Sellers TA, Folsom AR. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 2000;71(4):921–930
69. Stevens J, Ahn K, Juhaeri, Houston D, Steffan L, Couper D. Dietary fiber intake and glycemic index and incidence of diabetes in African-American and white adults: the ARIC study. Diabetes Care 2002; 25(10):1715–1721
70. Montonen J, Knekt P, Järvinen R, Aromaa A, Reunanen A. Whole-grain and fiber intake and the incidence of type 2 diabetes. Am J Clin Nutr 2003;77(3):622–629
71. Schulze MB, Liu S, Rimm EB, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr 2004;80(2):348–356
72. Ventura E, Davis J, Byrd-Williams C, et al. Reduction in risk factors for type 2 diabetes mellitus in response to a low-sugar, high-fiber dietary intervention in over-weight Latino adolescents. Arch Pediatr Adolesc Med 2009;163(4):320–327
73. Krishnan S, Rosenberg L, Singer M, et al. Glycemic index, glycemic load, and cereal fiber intake and risk of type 2 diabetes in US black women. Arch Intern Med 2007;167(21):2304–2309
74. Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H. Fiber and magnesium in-take and incidence of type 2 diabetes: a prospective study and meta-analysis. Arch Intern Med 2007; 167(9):956–965
75. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346(6):393–403
76. Tuomilehto J, Lindström J, Eriksson JG, et al; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344(18):1343–1350
77. Trock B, Lanza E, Greenwald P. Dietary fiber, vegetables, and colon cancer: critical review and meta-analyses of the epidemiologic evidence. J Natl Cancer Inst 1990;82(8):650–661
78. Howe GR, Benito E, Castelleto R, et al. Dietary intake of fiber and decreased risk of cancers of the colon and rectum: evidence from the combined analysis of 13 case-control studies. J Natl Cancer Inst 1992; 84(24):1887–1896
79. Dahm CC, Keogh RH, Spencer EA, et al. Dietary fiber and colorectal cancer risk: a nested case-control study using food diaries. J Natl Cancer Inst 2010;102(9):614–626
80. Steinmetz KA, Kushi LH, Bostick RM, Folsom AR, Potter JD. Vegetables, fruit, and colon cancer in the Iowa Women's Health Study. Am J Epidemiol 1994; 139(1):1–15
81. Kato I, Akhmedkhanov A, Koenig K, Toniolo PG, Shore RE, Riboli E. Prospective study of diet and female colorectal cancer: the New York University Women's Health Study. Nutr Cancer 1997;28(3):276–281
82. Pietinen P, Malila N, Virtanen M, et al. Diet and risk of colorectal cancer in a cohort of Finnish men. Cancer Causes Control 1999;10(5):387–396
83. Terry P, Giovannucci E, Michels KB, et al. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst 2001;93(7):525–533
84. Mai V, Flood A, Peters U, Lacey JV Jr, Schairer C, Schatzkin A. Dietary fibre and risk of colorectal cancer in the Breast Cancer Detection Demonstration Project (BCDDP) follow-up cohort. Int J Epidemiol 2003;32(2):234–239
85. Lin J, Zhang SM, Cook NR, et al. Dietary intakes of fruit, vegetables, and fiber, and risk of colorectal cancer in a prospective cohort of women (United States). Cancer Causes Control 2005;16(3):225–233
86. Michels KB, Fuchs CS, Giovannucci E, et al. Fiber in-take and incidence of colorectal cancer among 76,947 women and 47,279 men. Cancer Epidemiol Biomarkers Prev 2005;14(4):842–849
87. Shin A, Li H, Shu XO, Yang G, Gao YT, Zheng W. Dietary intake of calcium, fiber and other micronutrients in relation to colorectal cancer risk: Results from the Shanghai Women's Health Study. Int J Cancer 2006;119(12):2938–2942
88. Otani T, Iwasaki M, Ishihara J, Sasazuki S, Inoue M, Tsugane S; Japan Public Health Center-Based Prospective Study Group. Dietary fiber intake and subsequent risk of colorectal cancer: the Japan Public Health Center-based prospective study. Int J Cancer 2006;119(6):1475–1480
89. Schatzkin A, Mouw T, Park Y, et al. Dietary fiber and whole-grain consumption in relation to colorectal cancer in the NIH-AARP Diet and Health Study. Am J Clin Nutr 2007;85(5):1353–1360
90. Park Y, Hunter DJ, Spiegelman D, et al. Dietary fiber intake and risk of colorectal cancer: a pooled analysis of prospective cohort studies. JAMA 2005;294 (22):2849–2857
91. Bingham SA, Day NE, Luben R, et al; European Prospective Investigation into Cancer and Nutrition. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet 2003;361(9368):1496–1501
92. Schatzkin A, Lanza E, Corle D, et al; Polyp Prevention Trial Study Group. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. N Engl J Med 2000;342(16):1149–1155
93. Alberts DS, Martínez ME, Roe DJ, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians’ Network. N Engl J Med 2000;342(16): 1156–1162
94. Ishikawa H, Akedo I, Otani T, et al. Randomized trial of dietary fiber and Lactobacillus casei administration for prevention of colorectal tumors. Int J Cancer 2005;116(5):762–767
95. Bonithon-Kopp C, Kronborg O, Giacosa A, Räth U, Faivre J; European Cancer Prevention Organisation Study Group. Calcium and fibre supplementation in prevention of colorectal adenoma recurrence: a randomised intervention trial. Lancet 2000;356(9238): 1300–1306
96. Giovannucci E, Stampfer MJ, Colditz G, Rimm EB, Willett WC. Relationship of diet to risk of colorectal adenoma in men. J Natl Cancer Inst 1992;84(2):91–98
97. Van't Veer P, Kolb CM, Verhoef P, et al. Dietary fiber, beta-carotene and breast cancer: results from a case-control study. Int J Cancer 1990;45(5):825–828
98. Baghurst PA, Rohan TE. High-fiber diets and reduced risk of breast cancer. Int J Cancer 1994;56(2):173–176
99. Yuan JM, Wang QS, Ross RK, Henderson BE, Yu MC. Diet and breast cancer in Shanghai and Tianjin, China. Br J Cancer 1995;71(6):1353–1358
100. Ronco A, De Stefani E, Boffetta P, Deneo-Pellegrini H, Mendilaharsu M, Leborgne F. Vegetables, fruits, and related nutrients and risk of breast cancer: a case-control study in Uruguay. Nutr Cancer 1999;35(2): 111–119
101. Belle FN, Kampman E, McTiernan A, et al. Dietary fiber, carbohydrates, glycemic index, and glycemic load in relation to breast cancer prognosis in the HEAL cohort. Cancer Epidemiol Biomarkers Prev 2011;20(5):890–899
102. Graham S, Zielezny M, Marshall J, et al. Diet in the epidemiology of postmenopausal breast cancer in the New York State Cohort. Am J Epidemiol 1992;136(11):1327–1337
103. Terry P, Jain M, Miller AB, Howe GR, Rohan TE. No association among total dietary fiber, fiber fractions, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 2002;11(11):1507–1508
104. Cho E, Spiegelman D, Hunter DJ, Chen WY, Colditz GA, Willett WC. Premenopausal dietary carbohydrate, glycemic index, glycemic load, and fiber in relation to risk of breast cancer. Cancer Epidemiol Bio-markers Prev 2003;12(11 Pt 1):1153–1158
105. Holmes MD, Liu S, Hankinson SE, Colditz GA, Hunter DJ, Willett WC. Dietary carbohydrates, fiber, and breast cancer risk. Am J Epidemiol 2004;159(8):732–739
106. Lajous M, Boutron-Ruault MC, Fabre A, Clavel Chapelon F, Romieu I. Carbohydrate intake, glycemic index, glycemic load, and risk of postmenopausal breast cancer in a prospective study of French women. Am J Clin Nutr 2008;87(5):1384–1391
107. Wen W, Shu XO, Li H, et al. Dietary carbohydrates, fiber, and breast cancer risk in Chinese women. Am J Clin Nutr 2009;89(1):283–289
108. Maruti SS, Lampe JW, Potter JD, Ready A, White E. A prospective study of bowel motility and related factors on breast cancer risk. Cancer Epidemiol Bio-markers Prev 2008;17(7):1746–1750
109. Cade JE, Burley VJ, Greenwood DC; UK Women's Cohort Study Steering Group. Dietary fibre and risk of breast cancer in the UK Women's Cohort Study. Int J Epidemiol 2007;36(2):431–438
110. Mattisson I, Wirfält E, Johansson U, Gullberg B, Olsson H, Berglund G. Intakes of plant foods, fibre and fat and risk of breast cancer—a prospective study in the Malmö Diet and Cancer cohort. Br J Cancer 2004;90(1):122–127
111. Park Y, Brinton LA, Subar AF, Hollenbeck A, Schatzkin A. Dietary fiber intake and risk of breast cancer in postmenopausal women: the National Institutes of Health-AARP Diet and Health Study. Am J Clin Nutr 2009;90(3):664–671
112. Dong JY, He K, Wang P, Qin LQ. Dietary fiber intake and risk of breast cancer: a meta-analysis of prospective cohort studies. Am J Clin Nutr 2011; 94(3):900–905
113. Rock CL, Flatt SW, Thomson CA, et al. Effects of a high-fiber, low-fat diet intervention on serum concentrations of reproductive steroid hormones in women with a history of breast cancer. J Clin Oncol 2004;22(12):2379–2387
114. Kasim-Karakas SE, Almario RU, Gregory L, Todd H, Wong R, Lasley BL. Effects of prune consumption on the ratio of 2-hydroxyestrone to 16alpha-hydroxyestrone. Am J Clin Nutr 2002;76(6):1422–1427
115. Korzenik JR. Case closed? Diverticulitis: epidemiology and fiber. J Clin Gastroenterol 2006;40(Suppl 3):S112–S116
116. Bogardus ST Jr. What do we know about diverticular disease? A brief overview. J Clin Gastroenterol 2006;40(Suppl 3):S108–S111
117. Peery AF, Barrett PR, Park D, et al. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology 2012;142(2):266–272.e1
118. Farrell RJ, Farrell JJ, Morrin MM. Diverticular disease in the elderly. Gastroenterol Clin North Am 2001;30(2):475–496
119. Aldoori WH, Giovannucci EL, Rockett HR, Sampson L, Rimm EB, Willett WC. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr 1998;128(4):714–719
120. Howarth NC, Saltzman E, Roberts SB. Dietary fiber and weight regulation. Nutr Rev 2001;59(5):129–139
121. Du H, van der A DL, Boshuizen HC, et al. Dietary fiber and subsequent changes in body weight and waist circumference in European men and women. Am J Clin Nutr 2010;91(2):329–336
122. Appleby PN, Thorogood M, Mann JI, Key TJ. Low body mass index in non-meat eaters: the possible roles of animal fat, dietary fibre and alcohol. Int J Obes Relat Metab Disord 1998;22(5):454–460
123. Miller WC, Niederpruem MG, Wallace JP, Lindeman AK. Dietary fat, sugar, and fiber predict body fat content. J Am Diet Assoc 1994;94(6):612–615
124. Davis JN, Hodges VA, Gillham MB. Normal-weight adults consume more fiber and fruit than their ageand height-matched overweight/obese counterparts. J Am Diet Assoc 2006;106(6):833–840
125. Liu S, Willett WC, Manson JE, Hu FB, Rosner B, Colditz G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am J Clin Nutr 2003;78(5):920–927
126. Saltzman E, Moriguti JC, Das SK, et al. Effects of a cereal rich in soluble fiber on body composition and dietary compliance during consumption of a hypo-caloric diet. J Am Coll Nutr 2001;20(1):50–57
127. Howarth NC, Saltzman E, McCrory MA, et al. Fermentable and nonfermentable fiber supplements did not alter hunger, satiety or body weight in a pilot study of men and women consuming self-selected diets. J Nutr 2003;133(10):3141–3144
128. Wanders AJ, van den Borne JJ, de Graaf C, et al. Effects of dietary fibre on subjective appetite, energy intake and body weight: a systematic review of randomized controlled trials. Obes Rev 2011;12(9):724–739
129. Lubin F, Lusky A, Chetrit A, Dankner R. Lifestyle and ethnicity play a role in all-cause mortality. J Nutr 2003;133(4):1180–1185
130. Baer HJ, Glynn RJ, Hu FB, et al. Risk factors for mortality in the nurses’ health study: a competing risks analysis. Am J Epidemiol 2011;173(3):319–329
131. Giacco R, Parillo M, Rivellese AA, et al. Long-term dietary treatment with increased amounts of fiber-rich low-glycemic index natural foods improves blood glucose control and reduces the number of hypoglycemic events in type 1 diabetic patients. Diabetes Care 2000;23(10):1461–1466
132. Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med 2000;342(19):1392–1398
133. Groop PH, Aro A, Stenman S, Groop L. Long-term effects of guar gum in subjects with non-insulin-dependent diabetes mellitus. Am J Clin Nutr 1993; 58(4):513–518
134. Sierra M, García JJ, Fernández N, Diez MJ, Calle AP. Therapeutic effects of psyllium in type 2 diabetic patients. Eur J Clin Nutr 2002;56(9):830–842
135. Anderson JW, Allgood LD, Turner J, Oeltgen PR, Daggy BP. Effects of psyllium on glucose and serum lipid responses in men with type 2 diabetes and hyper-cholesterolemia. Am J Clin Nutr 1999;70(4):466–473
136. Anderson JW, Randles KM, Kendall CW, Jenkins DJ. Carbohydrate and fiber recommendations for individuals with diabetes: a quantitative assessment and meta-analysis of the evidence. J Am Coll Nutr 2004;23(1):5–17
137. American Diabetes Association Task Force for Writing Nutrition Principles and Recommendations for the Management of Diabetes and Related Complications. American Diabetes Association position statement: evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. J Am Diet Assoc 2002;102(1):109–118
138. Nutrition Subcommittee of the British Diabetic Association's Professional Advisory Committee. Dietary recommendations for people with diabetes: an update for the 1990s. Diabet Med 1992;9(2):189–202
139. National Nutrition Committee. Canadian Diabetes Association. Guidelines for the Nutritional Management of Diabetes Mellitus in the New Millennium: A Position Statement by the Canadian Diabetes Association. Can J Diabetes Care 1999;23(3):56–69
140. Jenkins DJ, Kendall CW, Augustin LS, et al. Effect of wheat bran on glycemic control and risk factors for cardiovascular disease in type 2 diabetes. Diabetes Care 2002;25(9):1522–1528
141. Horwitz BJ, Fisher RS. The irritable bowel syndrome. N Engl J Med 2001;344(24):1846–1850
142. Jailwala J, Imperiale TF, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized, controlled trials. Ann Intern Med 2000;133(2):136–147
143. Ford AC, Talley NJ, Spiegel BM, et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ 2008;337:a2313
144. Quartero AO, Meineche-Schmidt V, Muris J, Rubin G, de Wit N. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2005; (2):CD003460
145. Bijkerk CJ, de Wit NJ, Muris JW, Whorwell PJ, Knottnerus JA, Hoes AW. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ 2009;339: b3154
146. Bijkerk CJ, Muris JW, Knottnerus JA, Hoes AW, de Wit NJ. Systematic review: the role of different types of fibre in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 2004;19(3):245–251
147. Viera AJ, Hoag S, Shaughnessy J. Management of irritable bowel syndrome. Am Fam Physician 2002; 66(10):1867–1874
148. Mertz HR. Irritable bowel syndrome. N Engl J Med 2003;349(22):2136–2146
149. Giannini EG, Mansi C, Dulbecco P, Savarino V. Role of partially hydrolyzed guar gum in the treatment of irritable bowel syndrome. Nutrition 2006;22(3):334–342
150. US Department of Agriculture, Agricultural Research Service. National Nutrient Database for Standard Reference. Release 24. Available at: http://ndb.nal.usda.gov/ndb/foods/list. Accessed May 3, 2012
151. Hendler SS, Rorvik DR, eds. PDR for Nutritional Supplements. Montvale, NJ: Medical Economics Company, Inc; 2001
152. Gibson GR, Beatty ER, Wang X, Cummings JH. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 1995;108(4):975–982
153. Kolida S, Tuohy K, Gibson GR. Prebiotic effects of inulin and oligofructose. Br J Nutr 2002;87(Suppl 2): S193–S197
154. Cummings JH, Macfarlane GT. Gastrointestinal effects of prebiotics. Br J Nutr 2002;87(Suppl 2):S145–S151
155. Duggan C, Penny ME, Hibberd P, et al. Oligofructose-supplemented infant cereal: 2 randomized, blinded, community-based trials in Peruvian infants. Am J Clin Nutr 2003;77(4):937–942
156. Pittler MH, Ernst E. Guar gum for body weight reduction: meta-analysis of randomized trials. Am J Med 2001;110(9):724–730
157. Gallaher CM, Munion J, Hesslink R Jr, Wise J, Gallaher DD. Cholesterol reduction by glucomannan and chitosan is mediated by changes in cholesterol absorption and bile acid and fat excretion in rats. J Nutr 2000;130(11):2753–2759
158. Pittler MH, Ernst E. Dietary supplements for bodyweight reduction: a systematic review. Am J Clin Nutr 2004;79(4):529–536
159. Bokura H, Kobayashi S. Chitosan decreases total cholesterol in women: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr 2003;57(5): 721–725
160. Wuolijoki E, Hirvelä T, Ylitalo P. Decrease in serum LDL cholesterol with microcrystalline chitosan. Methods Find Exp Clin Pharmacol 1999;21(5):357–361
161. Metso S, Ylitalo R, Nikkilä M, Wuolijoki E, Ylitalo P, Lehtimäki T. The effect of long-term microcrystalline chitosan therapy on plasma lipids and glucose concentrations in subjects with increased plasma total cholesterol: a randomised placebo-controlled double-blind crossover trial in healthy men and women. Eur J Clin Pharmacol 2003;59(10):741–746
162. Ho SC, Tai ES, Eng PH, Tan CE, Fok AC. In the absence of dietary surveillance, chitosan does not reduce plasma lipids or obesity in hypercholesterolaemic obese Asian subjects. Singapore Med J 2001;42(1):6–10
163. American Academy of Family Physicians. Fiber: How to Increase the Amount in Your Diet. Available at: http://familydoctor.org/familydoctor/en/prevention-wellness/food-nutrition/nutrients/fiber-how-to-increase-the-amount-in-your-diet.html. Accessed May 3, 2012
164. Papazian R. Bulking Up Fiber's Healthful Reputation. Food and Drug Administration. Available at: http://www.fda.gov/fdac/features/1997/597_fiber.html. Accessed Sep 14, 2004
165. Rosario PG, Gerst PH, Prakash K, Albu E. Dentureless distention: oat bran bezoars cause obstruction. J Am Geriatr Soc 1990;38(5):608
166. Miller DL, Miller PF, Dekker JJ. Small-bowel obstruction from bran cereal. JAMA 1990;263(6):813–814
167. Cooper SG, Tracey EJ. Small-bowel obstruction caused by oat-bran bezoar. N Engl J Med 1989; 320(17):1148–1149
168. McClurken JB, Carp NZ. Bran-induced small-intestinal obstruction in a patient with no history of abdominal operation. Arch Surg 1988;123(1):98–100
169. Lewis JH. Esophageal and small bowel obstruction from guar gum-containing “diet pills”: analysis of 26 cases reported to the Food and Drug Administration. Am J Gastroenterol 1992;87(10):1424–1428
170. Agha FP, Nostrant TT, Fiddian-Green RG. “Giant colonic bezoar:” a medication bezoar due to psyllium seed husks. Am J Gastroenterol 1984;79(4):319–321
171. Schneider RP. Perdiem causes esophageal impaction and bezoars. South Med J 1989;82(11):1449–1450
172. Chandra R, Barron JL. Anaphylactic reaction to intravenous sinistrin (Inutest). Ann Clin Biochem 2002; 39(Pt 1):76
173. Gay-Crosier F, Schreiber G, Hauser C. Anaphylaxis from inulin in vegetables and processed food. N Engl J Med 2000;342(18):1372
174. Khalili B, Bardana EJ Jr, Yunginger JW. Psyllium-associated anaphylaxis and death: a case report and review of the literature. Ann Allergy Asthma Immunol 2003;91(6):579–584
175. Fugh-Berman A. Herb-drug interactions. Lancet 2000;355(9198):134–138
176. Richter WO, Jacob BG, Schwandt P. Interaction between fibre and lovastatin. Lancet 1991;338(8768): 706
177. Greger JL. Nondigestible carbohydrates and mineral bioavailability. J Nutr 1999; 129(7, Suppl):1434S–1435S
178. Bosscher D, Van Caillie-Bertrand M, Van Cauwenbergh R, Deelstra H. Availabilities of calcium, iron, and zinc from dairy infant formulas is affected by soluble dietary fibers and modified starch fractions. Nutrition 2003;19(7–8):641–645
179. Scholz-Ahrens KE, Schrezenmeir J. Inulin, oligofructose and mineral metabolism - experimental data and mechanism. Br J Nutr 2002;87(Suppl 2):S179–S186
180. Rock CL, Swendseid ME. Plasma beta-carotene response in humans after meals supplemented with dietary pectin. Am J Clin Nutr 1992;55(1):96–99
181. Riedl J, Linseisen J, Hoffmann J, Wolfram G. Some dietary fibers reduce the absorption of carotenoids in women. J Nutr 1999;129(12):2170–2176