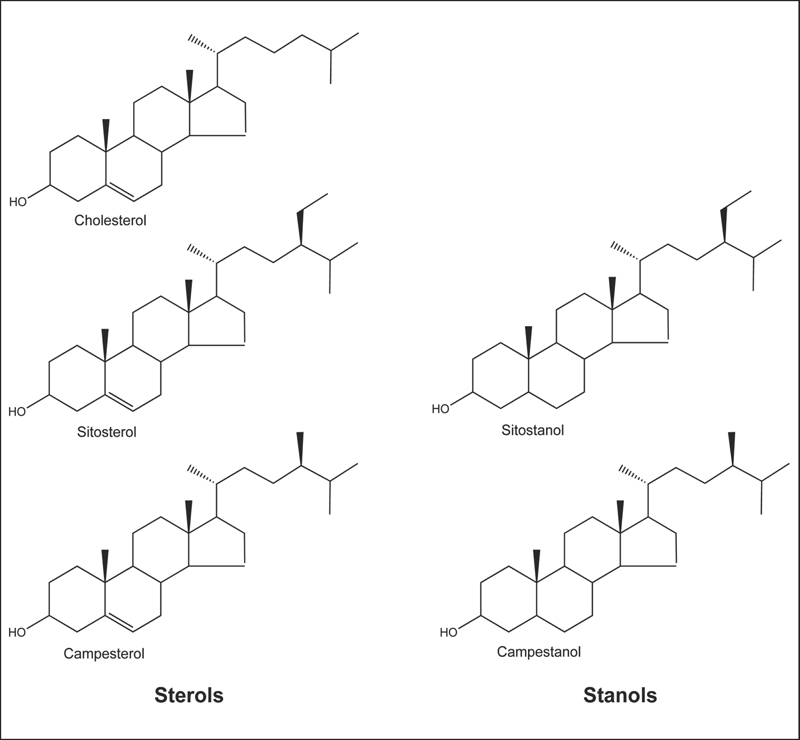
Fig. 18.1 Chemical structure of cholesterol compared with plant sterols (sitosterol and campesterol) and plant stanols (sitostanol and campestanol).
Throughout much of human evolution, it is likely that large amounts of plant foods were consumed.1 In addition to being rich in fiber and plant protein, the diets of our ancestors were also rich in phytosterols—plant-derived sterols that are similar in structure and function to cholesterol. There is increasing evidence that the reintroduction of plant foods providing phytosterols into the modern diet can improve serum lipid (cholesterol) profiles and reduce the risk of cardiovascular disease.2
Although cholesterol is the predominant sterol in animals, including humans, a variety of sterols are found in plants.3 Nutritionists recognize two classes of phytosterols: (1) sterols, which have a double bond in the sterol ring; and (2) stanols, which lack a double bond in the sterol ring (Fig. 18.1). The most abundant sterols in plants and the human diet are sitosterol and campesterol. Stanols are also present in plants, but they comprise only approximately 10% of total dietary phytosterols. Cholesterol in human blood and tissues is derived from the diet as well as endogenous cholesterol synthesis. In contrast, all phytosterols in human blood and tissues are derived from the diet because humans cannot synthesize phytosterols.4

Fig. 18.1 Chemical structure of cholesterol compared with plant sterols (sitosterol and campesterol) and plant stanols (sitostanol and campestanol).
Phytosterols. A collective term for plant-derived sterols and stanols.
Plant sterols or stanols. Terms generally applied to plant-derived sterols or stanols; these phytochemicals are added to foods or supplements.
Plant sterol or stanol esters. Plant sterols or stanols that have been esterified by creating an ester bond between a fatty acid and the sterol or stanol. Esterification occurs in intestinal cells and is also an industrial process. Esterification makes plant sterols and stanols more fat soluble so they are easily incorporated into fat-containing foods, including margarines and salad dressings. In this chapter, the weights of plant sterol and stanol esters are expressed as the equivalent weights of free (unesterified) sterols and stanols.
Dietary cholesterol must be incorporated into mixed micelles to be absorbed by the cells that line the intestine (enterocytes).5 Mixed micelles are mixtures of bile salts, lipids (fats), and sterols formed in the small intestine after a fat-containing meal is consumed. Inside the enterocyte, cholesterol is esterified and incorporated into triglyceride-rich lipoproteins known as chylomicrons, which enter the circulation.6 As circulating chylomicrons become depleted of triglycerides, they become chylomicron remnants, which are taken up by the liver. In the liver, cholesterol from chylomicron remnants may be repackaged into other lipoproteins for transport throughout the circulation or, alternatively, secreted into bile, which is released into the small intestine.
Although varied diets typically contain similar amounts of phytosterols and cholesterol, serum phytosterol concentrations are usually several hundred times lower than serum cholesterol concentrations in humans.7 Less than 10% of dietary phytosterols are systemically absorbed, in contrast to approximately 50%–60% of dietary cholesterol.8 Like cholesterol, phytosterols must be incorporated into mixed micelles before they are taken up by enterocytes. Once inside the enterocyte, systemic absorption of phytosterols is inhibited by the activity of efflux transporters, consisting of a pair of ATP-binding cassette (ABC) proteins known as ABCG5 and ABCG8.4 ABCG5 and ABCG8 each form one half of a transporter that secretes phytosterols and unesterified cholesterol from the enterocyte into the intestinal lumen. Phytosterols are secreted back into the intestine by ABCG5/G8 transporters at a much greater rate than cholesterol, resulting in much lower intestinal absorption of dietary phytosterols than of cholesterol. Within the enterocyte, phytosterols are not as readily esterified as cholesterol, so they are incorporated into chylomicrons at much lower concentrations. Those phytosterols that are incorporated into chylomicrons enter the circulation and are taken up by the liver. Once inside the liver, phytosterols are rapidly secreted into bile by hepatic ABCG5/G8 transporters. Although cholesterol may also be secreted into bile, the rate of phytosterol secretion into bile is much greater than the rate of cholesterol secretion.9 Thus, the low serum concentrations of phytosterols relative to cholesterol can be explained by decreased intestinal absorption and increased excretion of phytosterols into bile.
It is well established that high intakes of plant sterols or stanols can lower serum total and low-density lipoprotein (LDL) cholesterol concentrations in humans (see the Cardiovascular Disease section below).10,11 In the intestinal lumen, phytosterols displace cholesterol from mixed micelles and inhibit cholesterol absorption.12 In humans, the consumption of 1.5–1.8 g/day of plant sterols or stanols reduced cholesterol absorption by 30%–40%.13,14 At higher doses (2.2 g/day of plant sterols), cholesterol absorption was reduced by 60%.15 In response to decreased cholesterol absorption, tissue LDL-receptor expression was increased, resulting in increased clearance of circulating LDL.16 Decreased cholesterol absorption is also associated with increased cholesterol synthesis, and increasing phytosterol intake has been found to increase endogenous cholesterol synthesis in humans.13 Despite the increase in cholesterol synthesis induced by increasing phytosterol intake, the net result is a reduction in serum LDL cholesterol concentration.
Experiments in cell culture and animal models suggest that phytosterols may have biological activities unrelated to cholesterol lowering. However, their significance in humans is not yet known.
Cholesterol is an important structural component of mammalian cell membranes.17 Displacement of cholesterol with phytosterols has been found to alter the physical properties of cell membranes in vitro,18 which could potentially affect signal transduction or membrane-bound enzyme activity.19,20 Limited evidence from an animal model of hemorrhagic stroke suggested that very high intakes of plant sterols or stanols displaced cholesterol in red blood cell membranes, resulting in decreased deformability and potentially increased fragility.21,22 However, daily phytosterol supplementation (1 g/1000 kcal) for 4 weeks did not alter red blood cell fragility in humans.23
Limited evidence from animal studies suggests that very high phytosterol intakes can alter testosterone metabolism by inhibiting 5-α-reductase, a membrane-bound enzyme that converts testosterone to dihydrotestosterone, a more potent metabolite.24,25 It is not known whether phytosterol consumption alters testosterone metabolism in humans. No significant changes in free or total serum testosterone concentrations were observed in men who consumed 1.6 g/day of plant sterol esters for 1 year.26
Unlike normal cells, cancerous cells lose their ability to respond to death signals that initiate apoptosis (programmed cell death). Sitosterol has been found to induce apoptosis when added to cultured human prostate,27 breast,28 and colon cancer cells.29
Limited data from cell-culture and animal studies suggest that phytosterols may attenuate the inflammatory activity of immune cells, including macrophages and neutrophils.30,31
LDL cholesterol. Numerous clinical trials have found that daily consumption of foods enriched with free or esterified forms of plant sterols or stanols lowers concentrations of serum total and LDL cholesterol.10,32–35 A meta-analysis that combined the results of 18 controlled clinical trials found that the consumption of spreads providing an average of 2 g/day of plant sterols or stanols lowered serum LDL cholesterol concentrations by 9%–14%.36 More recently, a meta-analysis that combined the results of 23 controlled clinical trials found that the consumption of plant foods providing an average of 3.4 g/day of plant sterols or stanols decreased LDL cholesterol concentrations by approximately 11%.37 Another meta-analysis examined the results of 23 clinical trials of plant-sterol-enriched foods and 27 clinical trials of plant-stanol-enriched foods, separately.11 At doses of at least 2 g/day, both plant sterols and stanols decreased LDL cholesterol concentrations by approximately 10%. Doses higher than 2 g/day did not substantially improve the cholesterol-lowering effects of plant sterols or stanols. Most recently, a meta-analysis that analyzed the results of 59 randomized controlled trials found that reductions in LDL cholesterol are greater in those with higher baseline levels of LDL cholesterol.38 The results of studies providing lower doses of plant sterols or stanols suggest that 0.8–1.0 g/day is the lowest dose that results in clinically significant LDL cholesterol reductions of at least 5%.39–43 In general, trials that have compared the cholesterol-lowering efficacy of plant sterols with that of stanols have found them to be equivalent.44–46 Few of these studies lasted longer than 4 weeks, but at least two studies have found that the cholesterol-lowering effects of plant sterols and stanols last for up to 1 year.26,47 In addition to data from controlled clinical trials, a 5-year study that examined the customary use of phytosterol/stanol-enriched margarines under free-living conditions found beneficial effects on cholesterol levels.48 Recently, concerns have been raised that plant sterols are not as effective as stanols in maintaining long-term reductions in LDL cholesterol.49–51 Long-term trials that directly compare the efficacy of plant sterols and plant stanols are needed to address these concerns.11
Coronary heart disease risk. The effect of long-term use of foods enriched with plant sterols or stanols on coronary heart disease (CHD) risk is not known. The results of numerous intervention trials suggest that a 10% reduction in LDL cholesterol induced by medication or diet modification could decrease the risk of CHD by as much as 20%.52 The National Cholesterol Education Program (NCEP) Adult Treatment Panel III has included the use of plant sterol or stanol esters (2 g/day) as a component of maximal dietary therapy for elevated LDL cholesterol.53 The addition of plant-sterol- or stanol-enriched foods to a heart-healthy diet that is low in saturated fat and rich in fruits and vegetables, whole grains, and fiber offers the potential for additive effects in CHD risk reduction. For example, following a diet that substituted monounsaturated and polyunsaturated fats for saturated fat resulted in a 9% reduction in serum LDL cholesterol after 30 days, but the addition of 1.7 g/day of plant sterols to the same diet resulted in a 24% reduction.54 More recently, 1-month adherence to a diet providing a portfolio of cholesterol-lowering foods, including plant sterols (1 g/1000 kcal), soy protein, almonds, and viscous fibers lowered serum LDL cholesterol concentrations by an average of 30%—a decrease that was not significantly different from that induced by statin therapy (drugs that inhibit the enzyme, 3-hydroxy-3-methyl-glutaryl-coenzyme A [HMG-CoA] reductase).55 However, analysis of individuals on such a cholesterol-lowering diet for 1 year found that the average LDL cholesterol reduction was only 13%, but almost one-third of the participants experienced LDL cholesterol reductions that were greater than 20%.56 Plant sterols are the major component in this diet responsible for the observed reductions in cholesterol concentrations.57 The United States Food and Drug Administration (FDA) has authorized the use of health claims on food labels indicating that regular consumption of foods enriched with plant sterol or stanol esters may reduce the risk of heart disease.58
Clinical trials finding daily consumption of foods enriched with plant sterols or stanols can significantly lower LDL cholesterol concentrations do not account for naturally occurring phytosterols in the diet.59 Relatively few studies have considered the effects of dietary phytosterol intakes on serum LDL cholesterol concentrations. Dietary phytosterol intakes have been estimated to range from approximately 150 mg/day to 450 mg/day in various populations.60 Limited evidence suggests that dietary phytosterols may play an important role in decreasing cholesterol absorption. A cross-sectional study in the United Kingdom found that dietary phytosterol intakes were inversely related to serum total and LDL cholesterol concentrations, even after adjusting for saturated fat and fiber intake.61 Similarly, an analysis in a Swedish population found that dietary intake of phytosterols was inversely associated with total cholesterol in both men and women and with LDL cholesterol in women.62 In single-meal tests, removal of 150 mg of phytosterols from corn oil increased cholesterol absorption by 38%,63 and removal of 328 mg of phytosterols from wheat germ increased cholesterol absorption by 43%.64 Although more research is needed, these findings suggest that dietary intakes of phytosterols from plant foods could have an important impact on cardiovascular health.
Limited data from animal studies suggest that very high intakes of phytosterols, particularly sitosterol, may inhibit the growth of breast and prostate cancer.65–67 Only a few epidemiological studies have examined associations between dietary phytosterol intakes and cancer risk in humans because databases providing information on the phytosterol content of commonly consumed foods have only recently been developed. A series of case–control studies in Uruguay found that dietary phytosterol intakes were lower in people diagnosed with stomach, lung, or breast cancer than in control groups of individuals who were cancer free.68–70 Case–control studies in the United States found that women diagnosed with breast or endometrial (uterine) cancer had lower dietary phytosterol intakes than women who did not have cancer.71,72 In contrast, another case–control study in the United States found that men diagnosed with prostate cancer had higher dietary campesterol intakes than men who did not have cancer, but total phytosterol consumption was not associated with prostate cancer risk.73 Although some epidemiological studies have found that higher intakes of plant foods containing phytosterols are associated with decreased cancer risk, it is not clear whether the protective factors are phytosterols or other compounds in plant foods.
Benign prostatic hyperplasia (BPH) is the term used to describe a noncancerous enlargement of the prostate. The enlarged prostate may exert pressure on the urethra, resulting in difficulty urinating. Plant extracts that provide a mixture of phytosterols (marketed as β-sitosterol) are often included in herbal therapies for urinary symptoms related to BPH. However, relatively few controlled studies have examined the efficacy of phytosterol supplements in men with symptomatic BPH. In a 6-month study of 200 men with symptomatic BPH, 60 mg/day of a β-sitosterol preparation improved symptom scores, increased peak urinary flow, and decreased post-void residual urine volume compared with placebo.74 A follow-up study reported that these improvements were maintained for up to 18 months in the 38 participants who continued β-sitosterol treatment.75 Similarly, in a 6-month study of 177 men with symptomatic BPH, 130 mg/day of a different β-sitosterol preparation improved urinary symptom scores, increased peak urinary flow, and decreased post-void residual urine volume compared with placebo.76 A systematic review that combined the results of these and two other controlled clinical trials found that β-sitosterol extracts increased peak urinary flow by an average of 3.9 mL/s and decreased post-void residual volume by an average of 29 mL.77 Although the results of a few clinical trials suggest that relatively low doses of phytosterols can improve lower urinary tract symptoms related to BPH, further research is needed to confirm these findings.78
Unlike the typical diet in most developed countries today, the diets of our ancestors were rich in phytosterols, likely providing as much as 1000 mg/day.1 Present-day dietary phytosterol intakes have been estimated to vary from 150 to 450 mg/day in different populations.3 Vegetarians, particularly vegans, generally have the highest intakes of dietary phytosterols.79 Phytosterols are found in all plant foods, but the highest concentrations are found in unrefined plant oils, including vegetable, nut, and olive oils.3 Nuts, seeds, whole grains, and legumes are also good dietary sources of phytosterols.5 The phytosterol contents of selected foods are presented in Table 18.1.
The majority of clinical trials that demonstrated a cholesterol-lowering effect used plant sterol or stanol esters solubilized in fat-containing foods, such as margarine or mayonnaise.11 More recent studies indicate that low-fat or even nonfat foods can effectively deliver plant sterols or stanols if they are adequately solubilized.10,59 Plant sterols or stanols added to low-fat yogurt,43,84–86 low-fat milk,87–89 low-fat cheese,90 dark chocolate,91 and orange juice92,93 have been reported to lower LDL cholesterol in controlled clinical trials. A variety of foods containing added plant sterols or stanols, including margarines, mayonnaises, vegetable oils, salad dressings, yogurt, milk, soy milk, orange juice, snack bars, and meats, are available in the United States, Europe, Asia, Australia, and New Zealand.10 A recent meta-analysis found that plant sterols/stanols added to fat spreads, mayonnaise, salad dressings, milk, or yogurt were more effective in reducing LDL cholesterol levels compared with plant sterols/stanols incorporated into other products, such as chocolate, orange juice, cheese, meats, and cereal bars.38 Available research indicates that the maximum effective dose for lowering LDL cholesterol is approximately 2 g/day11 and the minimum effective dose is 0.8–1.0 g/day.10 In the majority of clinical trials that demonstrated a cholesterol-lowering effect, the daily dose of plant sterols or stanols was divided among two or three meals, which may be more effective in lowering LDL cholesterol.38 However, consumption of the daily dose of plant sterols or stanols with a single meal has been found to lower LDL cholesterol in a few clinical trials.43,85,86,94,95
Table 18.1 Total phytosterol content of selected foods80–83
Food |
Serving |
Phytosterols (mg) |
Wheat germ |
½ cup |
197 |
Rice bran oil |
1 tbs |
162 |
Sesame oil |
1 tbs |
118 |
Corn oil |
1 tbs |
102 |
Canola oil |
1 tbs |
92 |
Peanuts |
1 oz |
62 |
Wheat bran |
½ cup |
58 |
Almonds |
1 oz |
39 |
Brussels sprouts |
½ cup |
34 |
Rye bread |
2 slices |
33 |
Macadamia nuts |
1 oz |
33 |
Olive oil |
1 tbs |
22 |
Take Controla spread |
1 tbs |
1650 mg plant sterol esters (1000 mg free sterols) |
Benecol spread |
1 tbs |
850 mg plant stanol esters (500 mg free stanols) |
Phytosterol supplements marketed as β-sitosterol are available without a prescription in the United States. Doses of 60–130 mg/day of β-sitosterol have been found to alleviate the symptoms of BPH in a few clinical trials (see Benign Prostatic Hyperplasia section above). Soft gel chews providing 0.5 g of plant stanols are being marketed for cholesterol lowering at a recommended dose of 2 g/day. Phytosterol supplements should be taken with meals that contain fat.
In the United States, plant sterols and stanols added to a variety of food products are generally recognized as safe (GRAS) by the FDA.96 Additionally, the Scientific Committee on Foods of the European Union concluded that plant sterols and stanols added to various food products are safe for human use.97 However, this committee recommended that intakes of plant sterols and stanols from food products should not exceed 3 g/day because there is no evidence of health benefits at higher intakes and there might be undesirable effects at high intakes.
Few adverse effects have been associated with regular consumption of plant sterols or stanols for up to 1 year. People who consumed a plant-sterol-enriched spread providing 1.6 g/day did not report any more adverse effects than those consuming a control spread for up to 1 year,26 and people consuming a plant stanol-enriched spread providing 1.8–2.6 g/day for 1 year did not report any adverse effects.47 Consumption of up to 8.6 g/day of phytosterols in margarine for 3–4 weeks was well tolerated by healthy men and women and did not adversely affect intestinal bacteria or female hormone levels.98 Although phytosterols are usually well tolerated, nausea, indigestion, diarrhea, and constipation have occasionally been reported.74,76
Sitosterolemia, also known as phytosterolemia, is a very rare hereditary disease that results from inheriting a mutation in both copies of the ABCG5 or ABCG8 gene.99 Individuals who are homozygous for a mutation in either transporter protein have dramatically elevated serum phytosterol concentrations due to increased intestinal absorption and decreased biliary excretion of phytosterols. Although serum cholesterol concentrations may be normal or only mildly elevated, individuals with sitosterolemia are at high risk for premature atherosclerosis. People with sitosterolemia should avoid foods or supplements with added plant sterols.10 Two studies have examined the effect of plant sterol consumption in heterozygous carriers of sitosterolemia, a more common condition. Consumption of 3 g/day of plant sterols for 4 weeks by two heterozygous carriers,100 and consumption of 2.2 g/day of plant sterols for 6–12 weeks by 12 heterozygous carriers did not result in abnormally elevated serum phytosterols.101
Plant sterols or stanols added to foods or supplements are not recommended for pregnant or breast-feeding women because their safety has not been studied.10 At present, there is no evidence that high dietary intakes of naturally occurring phytosterols, such as those consumed by vegetarian women, adversely affect pregnancy or lactation.
The LDL-cholesterol-lowering effects of plant sterols or stanols may be additive to those of HMG-CoA reductase inhibitors (statins).102,103 The results of controlled clinical trials suggest that consumption of 2–3 g/day of plant sterols or stanols by individuals on statin therapy may result in an additional 7%–11% reduction in LDL cholesterol, an effect comparable to doubling the statin dose.50,104–106 Consumption of 4.5 g/day of stanol esters for 8 weeks did not affect prothrombin times (international normalized ratios—INRs) in patients on warfarin (Coumadin) for anticoagulation.107
Because plant sterols and stanols decrease cholesterol absorption and serum LDL cholesterol concentrations, their effects on fat-soluble vitamin status have also been studied in clinical trials. Plasma vitamin A (retinol) concentrations were not affected by plant stanol or sterol ester consumption for up to 1 year.11,26 Although the majority of studies found no changes in plasma vitamin D (25-hydroxyvitamin D3) concentrations, one placebo-controlled study in individuals consuming 1.6 g/day of sterol esters for 1 year observed a small (7%) but statistically significant decrease in plasma 25-hydroxyvitamin D3 concentrations.26 There is little evidence that plant sterol or stanol consumption adversely affects vitamin K status. Consumption of 1.6 g/day of sterol esters for 6 months was associated with a nonsignificant 14% decrease in plasma vitamin K1 concentrations, but carboxylated osteocalcin, a functional indicator of vitamin K status, was unaffected.26 In other studies of shorter duration, consumption of plant sterol and stanol esters did not significantly change plasma concentrations of vitamin K1108,109 or vitamin-K-dependent clotting factors.110 Consumption of plant sterol or stanol-enriched foods has been found to decrease plasma vitamin E (α-tocopherol) concentrations in several studies.11,109 However, those decreases generally do not persist when plasma α-tocopherol concentrations are standardized to LDL cholesterol concentrations. This suggests that observed reductions in plasma α-tocopherol are due in part to reductions in its carrier lipoprotein, LDL. In general, consumption of plant-sterol- and stanol-enriched foods at doses of 1.5 g/day or more have not been found to have adverse effects on fat-soluble vitamin status in well-nourished populations.
Dietary carotenoids are fat-soluble phytochemicals that circulate in lipoproteins. Several studies have observed 10%–20% reductions in plasma carotenoids after short-term and long-term consumption of plant-sterol- or stanol-enriched foods.11 Even when standardized to serum total or LDL cholesterol concentrations, decreases in α-carotene, β-carotene, and lycopene may persist, suggesting that phytosterols can inhibit the absorption of these carotenoids.111 It is not clear whether reductions in plasma carotenoid concentrations confer any health risks, but several studies have found that increasing intakes of carotenoid-rich fruits and vegetables can prevent phytosterol-induced decreases in plasma carotenoids.112 In one case, advice to consume five daily servings of fruits and vegetables, including one serving of carotenoid-rich vegetables, was enough to maintain plasma carotenoid levels in people consuming 2.5 g/day of plant sterol or stanol esters.113
• Phytosterols are plant-derived compounds that are similar in structure and function to cholesterol.
• Early human diets were rich in phytosterols, providing as much as 1 g/day; however, the typical Western diet today is relatively low in phytosterols.
• Phytosterols inhibit the intestinal absorption of cholesterol.
• Numerous clinical trials have demonstrated that daily consumption of foods enriched with at least 0.8 g of plant sterols or stanols lowers serum LDL cholesterol.
• Although some epidemiological studies have found that higher intakes of plant foods containing phytosterols are associated with decreased cancer risk, it is not clear whether phytosterols or other compounds in plant foods are the protective factors.
• The results of a few clinical trials suggest that phytosterol supplementation at relatively low doses can improve urinary tract symptoms related to benign prostatic hyperplasia, but further research is needed to confirm these findings.
• Foods rich in phytosterols include unrefined vegetable oils, whole grains, nuts, and legumes.
• Foods and beverages with added plant sterols or stanols are now available in many countries throughout the world, and many countries now allow health claims for such commercial products.
1. Jenkins DJ, Kendall CW, Marchie A, et al. The Garden of Eden—plant based diets, the genetic drive to conserve cholesterol and its implications for heart disease in the 21st century. Comp Biochem Physiol A Mol Integr Physiol 2003;136(1):141–151
2. Kendall CW, Jenkins DJ. A dietary portfolio: maximal reduction of low-density lipoprotein cholesterol with diet. Curr Atheroscler Rep 2004;6(6):492–498
3. Ostlund RE Jr. Phytosterols in human nutrition. Annu Rev Nutr 2002;22:533–549
4. Sudhop T, Lütjohann D, von Bergmann K. Sterol transporters: targets of natural sterols and new lipid lowering drugs. Pharmacol Ther 2005;105(3):333–341
5. de Jong A, Plat J, Mensink RP. Metabolic effects of plant sterols and stanols (Review). J Nutr Biochem 2003;14(7):362–369
6. Plat J, Mensink RP. Plant stanol and sterol esters in the control of blood cholesterol levels: mechanism and safety aspects. Am J Cardiol 2005; 96(1A, Suppl):15D–22D
7. von Bergmann K, Sudhop T, Lütjohann D. Cholesterol and plant sterol absorption: recent insights. Am J Cardiol 2005; 96(1A, Suppl):10D–14D
8. Ostlund RE Jr, McGill JB, Zeng CM, et al. Gastrointestinal absorption and plasma kinetics of soy Delta (5)-phytosterols and phytostanols in humans. Am J Physiol Endocrinol Metab 2002;282(4):E911–E916
9. Sudhop T, Sahin Y, Lindenthal B, et al. Comparison of the hepatic clearances of campesterol, sitosterol, and cholesterol in healthy subjects suggests that efflux transporters controlling intestinal sterol absorption also regulate biliary secretion. Gut 2002;51(6):860–863
10. Berger A, Jones PJ, Abumweis SS. Plant sterols: factors affecting their efficacy and safety as functional food ingredients. Lipids Health Dis 2004;3(1):5
11. Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R; Stresa Workshop Participants. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc 2003; 78(8):965–978
12. Nissinen M, Gylling H, Vuoristo M, Miettinen TA. Micellar distribution of cholesterol and phytosterols after duodenal plant stanol ester infusion. Am J Physiol Gastrointest Liver Physiol 2002;282(6):G1009–G1015
13. Jones PJ, Raeini-Sarjaz M, Ntanios FY, Vanstone CA, Feng JY, Parsons WE. Modulation of plasma lipid levels and cholesterol kinetics by phytosterol versus phytostanol esters. J Lipid Res 2000;41(5):697–705
14. Normén L, Dutta P, Lia A, Andersson H. Soy sterol esters and beta-sitostanol ester as inhibitors of cholesterol absorption in human small bowel. Am J Clin Nutr 2000;71(4):908–913
15. Richelle M, Enslen M, Hager C, et al. Both free and esterified plant sterols reduce cholesterol absorption and the bioavailability of beta-carotene and alpha-tocopherol in normocholesterolemic humans. Am J Clin Nutr 2004;80(1):171–177
16. Plat J, Mensink RP. Effects of plant stanol esters on LDL receptor protein expression and on LDL receptor and HMG-CoA reductase mRNA expression in mononuclear blood cells of healthy men and women. FASEB J 2002;16(2):258–260
17. Mouritsen OG, Zuckermann MJ. What's so special about cholesterol? Lipids 2004;39(11):1101–1113
18. Halling KK, Slotte JP. Membrane properties of plant sterols in phospholipid bilayers as determined by differential scanning calorimetry, resonance energy transfer and detergent-induced solubilization. Biochim Biophys Acta 2004;1664(2):161–171
19. Awad AB, Chen YC, Fink CS, Hennessey T. beta-Sitosterol inhibits HT-29 human colon cancer cell growth and alters membrane lipids. Anticancer Res 1996; 16(5A):2797–2804
20. Leikin AI, Brenner RR. Fatty acid desaturase activities are modulated by phytosterol incorporation in microsomes. Biochim Biophys Acta 1989;1005(2):187–191
21. Ratnayake WM, L'Abbé MR, Mueller R, et al. Vegetable oils high in phytosterols make erythrocytes less deformable and shorten the life span of stroke-prone spontaneously hypertensive rats. J Nutr 2000;130(5): 1166–1178
22. Ratnayake WM, Plouffe L, L'Abbé MR, Trick K, Mueller R, Hayward S. Comparative health effects of margarines fortified with plant sterols and stanols on a rat model for hemorrhagic stroke. Lipids 2003;38(12): 1237–1247
23. Jones PJ, Raeini-Sarjaz M, Jenkins DJ, et al. Effects of a diet high in plant sterols, vegetable proteins, and viscous fibers (dietary portfolio) on circulating sterol levels and red cell fragility in hypercholesterolemic subjects. Lipids 2005;40(2):169–174
24. Awad AB, Hartati MS, Fink CS. Phytosterol feeding induces alteration in testosterone metabolism in rat tissues. J Nutr Biochem 1998;9(12):712–717
25. Cabeza M, Bratoeff E, Heuze I, Ramírez E, Sánchez M, Flores E. Effect of beta-sitosterol as inhibitor of 5 alpha-reductase in hamster prostate. Proc West Pharmacol Soc 2003;46:153–155
26. Hendriks HF, Brink EJ, Meijer GW, Princen HM, Ntanios FY. Safety of long-term consumption of plant sterol esters-enriched spread. Eur J Clin Nutr 2003; 57(5):681–692
27. von Holtz RL, Fink CS, Awad AB. beta-Sitosterol activates the sphingomyelin cycle and induces apoptosis in LNCaP human prostate cancer cells. Nutr Cancer 1998;32(1):8–12
28. Awad AB, Roy R, Fink CS. Beta-sitosterol, a plant sterol, induces apoptosis and activates key caspases in MDA-MB-231 human breast cancer cells. Oncol Rep 2003;10(2):497–500
29. Choi YH, Kong KR, Kim YA, et al. Induction of Bax and activation of caspases during beta-sitosterol-mediated apoptosis in human colon cancer cells. Int J Oncol 2003;23(6):1657–1662
30. Awad AB, Toczek J, Fink CS. Phytosterols decrease prostaglandin release in cultured P388D1/MAB macrophages. Prostaglandins Leukot Essent Fatty Acids 2004;70(6):511–520
31. Navarro A, De las Heras B, Villar A. Anti-inflammatory and immunomodulating properties of a sterol fraction from Sideritis foetens Clem. Biol Pharm Bull 2001;24(5):470–473
32. St-Onge MP, Jones PJ. Phytosterols and human lipid metabolism: efficacy, safety, and novel foods. Lipids 2003;38(4):367–375
33. Moruisi KG, Oosthuizen W, Opperman AM. Phytosterols/stanols lower cholesterol concentrations in familial hypercholesterolemic subjects: a systematic review with meta-analysis. J Am Coll Nutr 2006; 25(1):41–48
34. Ellegård LH, Andersson SW, Normén AL, Andersson HA. Dietary plant sterols and cholesterol metabolism. Nutr Rev 2007;65(1):39–45
35. Van Horn L, McCoin M, Kris-Etherton PM, et al. The evidence for dietary prevention and treatment of cardiovascular disease. J Am Diet Assoc 2008;108(2):287–331
36. Law M. Plant sterol and stanol margarines and health. BMJ 2000;320(7238):861–864
37. Chen JT, Wesley R, Shamburek RD, Pucino F, Csako G. Meta-analysis of natural therapies for hyperlipidemia: plant sterols and stanols versus policosanol. Pharmacotherapy 2005;25(2):171–183
38. AbuMweis SS, Barake R, Jones P. Plant sterols/stanols as cholesterol lowering agents: A meta-analysis of randomized controlled trials. Food Nutr Res 2008; 52:doi:10.3402/fnr.v52i0.1811
39. Hendriks HF, Weststrate JA, van Vliet T, Meijer GW. Spreads enriched with three different levels of vegetable oil sterols and the degree of cholesterol lowering in normocholesterolaemic and mildly hypercholesterolaemic subjects. Eur J Clin Nutr 1999; 53(4): 319–327
40. Miettinen TA, Vanhanen H. Dietary sitostanol related to absorption, synthesis and serum level of cholesterol in different apolipoprotein E phenotypes. Atherosclerosis 1994;105(2):217–226
41. Pelletier X, Belbraouet S, Mirabel D, et al. A diet moderately enriched in phytosterols lowers plasma cholesterol concentrations in normocholesterolemic humans. Ann Nutr Metab 1995;39(5):291–295
42. Sierksma A, Weststrate JA, Meijer GW. Spreads enriched with plant sterols, either esterified 4,4-dimethylsterols or free 4-desmethylsterols, and plasma total- and LDL-cholesterol concentrations. Br J Nutr 1999;82(4):273–282
43. Volpe R, Niittynen L, Korpela R, et al. Effects of yoghurt enriched with plant sterols on serum lipids in patients with moderate hypercholesterolaemia. Br J Nutr 2001;86(2):233–239
44. Hallikainen MA, Sarkkinen ES, Gylling H, Erkkilä AT, Uusitupa MI. Comparison of the effects of plant sterol ester and plant stanol ester-enriched margarines in lowering serum cholesterol concentrations in hypercholesterolaemic subjects on a low-fat diet. Eur J Clin Nutr 2000;54(9):715–725
45. Vanstone CA, Raeini-Sarjaz M, Parsons WE, Jones PJ. Unesterified plant sterols and stanols lower LDL-cholesterol concentrations equivalently in hypercholesterolemic persons. Am J Clin Nutr 2002;76(6):1272–1278
46. Weststrate JA, Meijer GW. Plant sterol-enriched margarines and reduction of plasma total- and LDL-cholesterol concentrations in normocholesterolaemic and mildly hypercholesterolaemic subjects. Eur J Clin Nutr 1998;52(5):334–343
47. Miettinen TA, Puska P, Gylling H, Vanhanen H, Vartiainen E. Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesterolemic population. N Engl J Med 1995;333(20):1308–1312
48. Wolfs M, de Jong N, Ocké MC, Verhagen H, Monique Verschuren WM. Effectiveness of customary use of phytosterol/-stanol enriched margarines on blood cholesterol lowering. Food Chem Toxicol 2006; 44(10):1682–1688
49. Miettinen TA, Gylling H. Plant stanol and sterol esters in prevention of cardiovascular diseases. Ann Med 2004;36(2):126–134
50. O'Neill FH, Brynes A, Mandeno R, et al. Comparison of the effects of dietary plant sterol and stanol esters on lipid metabolism. Nutr Metab Cardiovasc Dis 2004; 14(3):133–142
51. O'Neill FH, Sanders TA, Thompson GR. Comparison of efficacy of plant stanol ester and sterol ester: short-term and longer-term studies. Am J Cardiol 2005; 96(1A):29D–36D
52. National Cholesterol Education Program. Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Bethesda, MD: National Heart Lung and Blood Institute, National Institutes of Health; 2002. Available at: http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3_rpt.htm. Accessed May 7, 2012
53. Grundy SM. Stanol esters as a component of maximal dietary therapy in the National Cholesterol Education Program Adult Treatment Panel III report. Am J Cardiol 2005; 96(1A, Suppl):47D–50D
54. Jones PJ, Ntanios FY, Raeini-Sarjaz M, Vanstone CA. Cholesterol-lowering efficacy of a sitostanol-containing phytosterol mixture with a prudent diet in hyperlipidemic men. Am J Clin Nutr 1999;69(6):1144–1150
55. Jenkins DJ, Kendall CW, Marchie A, et al. Direct comparison of a dietary portfolio of cholesterol-lowering foods with a statin in hypercholesterolemic participants. Am J Clin Nutr 2005;81(2):380–387
56. Jenkins DJ, Kendall CW, Faulkner DA, et al. Assessment of the longer-term effects of a dietary portfolio of cholesterol-lowering foods in hypercholesterolemia. Am J Clin Nutr 2006;83(3):582–591
57. Jenkins DJ, Kendall CW, Nguyen TH, et al. Effect of plant sterols in combination with other cholesterol-lowering foods. Metabolism 2008;57(1):130–139
58. Food and Drug Administration. Federal Register 65-FR 54685-54739, September 8, 2000. Food Labeling: Health Claims; Plant Sterol/Stanol Esters and Coronary Heart Disease; Interim Final Rule. Available at: http://www.fda.gov/Food/LabelingNutrition/LabelClaims/HealthClaimsMeetingSingnificantScientificAgreementSSA/ucm/74747.htm
59. Ostlund RE Jr. Phytosterols and cholesterol metabolism. Curr Opin Lipidol 2004;15(1):37–41
60. Ostlund RE Jr, Racette SB, Stenson WF. Effects of trace components of dietary fat on cholesterol metabolism: phytosterols, oxysterols, and squalene. Nutr Rev 2002;60(11):349–359
61. Andersson SW, Skinner J, Ellegård L, et al. Intake of dietary plant sterols is inversely related to serum cholesterol concentration in men and women in the EPIC Norfolk population: a cross-sectional study. Eur J Clin Nutr 2004;58(10):1378–1385
62. Klingberg S, Ellegård L, Johansson I, et al. Inverse relation between dietary intake of naturally occurring plant sterols and serum cholesterol in northern Sweden. Am J Clin Nutr 2008;87(4):993–1001
63. Ostlund RE Jr, Racette SB, Okeke A, Stenson WF. Phytosterols that are naturally present in commercial corn oil significantly reduce cholesterol absorption in humans. Am J Clin Nutr 2002;75(6):1000–1004
64. Ostlund RE Jr, Racette SB, Stenson WF. Inhibition of cholesterol absorption by phytosterol-replete wheat germ compared with phytosterol-depleted wheat germ. Am J Clin Nutr 2003;77(6):1385–1389
65. Ju YH, Clausen LM, Allred KF, Almada AL, Helferich WG. Beta-sitosterol, beta-sitosterol glucoside, and a mixture of beta-sitosterol and beta-sitosterol glucoside modulate the growth of estrogen-responsive breast cancer cells in vitro and in ovariectomized athymic mice. J Nutr 2004;134(5):1145–1151
66. Awad AB, Fink CS, Williams H, Kim U. In vitro and in vivo (SCID mice) effects of phytosterols on the growth and dissemination of human prostate cancer PC-3 cells. Eur J Cancer Prev 2001;10(6):507–513
67. Awad AB, Downie A, Fink CS, Kim U. Dietary phytosterol inhibits the growth and metastasis of MDAMB-231 human breast cancer cells grown in SCID mice. Anticancer Res 2000;20(2A):821–824
68. De Stefani E, Boffetta P, Ronco AL, et al. Plant sterols and risk of stomach cancer: a case-control study in Uruguay. Nutr Cancer 2000;37(2):140–144
69. Mendilaharsu M, De Stefani E, Deneo-Pellegrini H, Carzoglio J, Ronco A. Phytosterols and risk of lung cancer: a case-control study in Uruguay. Lung Cancer 1998;21(1):37–45
70. Ronco A, De Stefani E, Boffetta P, Deneo-Pellegrini H, Mendilaharsu M, Leborgne F. Vegetables, fruits, and related nutrients and risk of breast cancer: a case-control study in Uruguay. Nutr Cancer 1999; 35(2): 111–119
71. McCann SE, Freudenheim JL, Marshall JR, Brasure JR, Swanson MK, Graham S. Diet in the epidemiology of endometrial cancer in Western New York (United States). Cancer Causes Control 2000;11(10):965–974
72. McCann SE, Freudenheim JL, Marshall JR, Graham S. Risk of human ovarian cancer is related to dietary intake of selected nutrients, phytochemicals and food groups. J Nutr 2003;133(6):1937–1942
73. Strom SS, Yamamura Y, Duphorne CM, et al. Phytoestrogen intake and prostate cancer: a case-control study using a new database. Nutr Cancer 1999; 33(1):20–25
74. Berges RR, Windeler J, Trampisch HJ, Senge T; Beta-sitosterol Study Group. Randomised, placebo-controlled, double-blind clinical trial of beta-sitosterol in patients with benign prostatic hyperplasia. Lancet 1995;345(8964):1529–1532
75. Berges RR, Kassen A, Senge T. Treatment of symptomatic benign prostatic hyperplasia with beta-sitosterol: an 18-month follow-up. BJU Int 2000;85(7):842–846
76. Klippel KF, Hiltl DM, Schipp B. A multicentric, placebo-controlled, double-blind clinical trial of beta-sitosterol (phytosterol) for the treatment of benign prostatic hyperplasia. German BPH-Phyto Study group. Br J Urol 1997;80(3):427–432
77. Wilt TJ, MacDonald R, Ishani A. beta-sitosterol for the treatment of benign prostatic hyperplasia: a systematic review. BJU Int 1999;83(9):976–983
78. Dreikorn K. The role of phytotherapy in treating lower urinary tract symptoms and benign prostatic hyperplasia. World J Urol 2002;19(6):426–435
79. Nair PP, Turjman N, Kessie G, et al. Diet, nutrition intake, and metabolism in populations at high and low risk for colon cancer. Dietary cholesterol, beta-sitosterol, and stigmasterol. Am J Clin Nutr 1984; 40(4, Suppl):927–930
80. US Department of Agriculture, Agricultural Research Service. USDA Nutrient Database for Standard Reference, Release 20. 2007. Available at: http://www.nal.usda.gov/fnic/foodcomp/Data/SR20/nutrlist/sr20w430.pdf. Accessed July 24, 2008
81. Normén L, Bryngelsson S, Johnsson M, et al. The phytosterol content of some cereal foods commonly consumed in Sweden and in the Netherlands. J Food Compost Anal 2002;15(6):693–704
82. Normén L, Johnsson M, Andersson H, van Gameren Y, Dutta P. Plant sterols in vegetables and fruits commonly consumed in Sweden. Eur J Nutr 1999; 38(2):84–89
83. Phillips KM, Ruggio DM, Toivo JI, Swank MA, Simpkins AH. Free and esterified sterol composition of edible oils and fats. J Food Compost Anal 2002;15(2):123–142
84. Mensink RP, Ebbing S, Lindhout M, Plat J, van Heugten MM. Effects of plant stanol esters supplied in low-fat yoghurt on serum lipids and lipoproteins, non-cholesterol sterols and fat soluble antioxidant concentrations. Atherosclerosis 2002;160(1):205–213
85. Plana N, Nicolle C, Ferre R, et al; DANACOL group. Plant sterol-enriched fermented milk enhances the attainment of LDL-cholesterol goal in hypercholesterolemic subjects. Eur J Nutr 2008;47(1):32–39
86. Doornbos AM, Meynen EM, Duchateau GS, van der Knaap HC, Trautwein EA. Intake occasion affects the serum cholesterol lowering of a plant sterol-enriched single-dose yoghurt drink in mildly hypercholesterolaemic subjects. Eur J Clin Nutr 2006;60(3):325–333
87. Noakes M, Clifton PM, Doornbos AME, Trautwein EA. Plant sterol ester-enriched milk and yoghurt effectively reduce serum cholesterol in modestly hyper-cholesterolemic subjects. Eur J Nutr 2005;44(4):214–222
88. Thomsen AB, Hansen HB, Christiansen C, Green H, Berger A. Effect of free plant sterols in low-fat milk on serum lipid profile in hypercholesterolemic subjects. Eur J Clin Nutr 2004;58(6):860–870
89. Seppo L, Jauhiainen T, Nevala R, Poussa T, Korpela R. Plant stanol esters in low-fat milk products lower serum total and LDL cholesterol. Eur J Nutr 2007; 46(2):111–117
90. Jauhiainen T, Salo P, Niittynen L, Poussa T, Korpela R. Effects of low-fat hard cheese enriched with plant stanol esters on serum lipids and apolipoprotein B in mildly hypercholesterolaemic subjects. Eur J Clin Nutr 2006;60(11):1253–1257
91. Allen RR, Carson L, Kwik-Uribe C, Evans EM, Erdman JW Jr. Daily consumption of a dark chocolate containing flavanols and added sterol esters affects cardiovascular risk factors in a normotensive population with elevated cholesterol. J Nutr 2008;138(4):725–731
92. Devaraj S, Jialal I, Vega-López S. Plant sterol-fortified orange juice effectively lowers cholesterol levels in mildly hypercholesterolemic healthy individuals. Arterioscler Thromb Vasc Biol 2004;24(3):e25–e28
93. Devaraj S, Autret BC, Jialal I. Reduced-calorie orange juice beverage with plant sterols lowers C-reactive protein concentrations and improves the lipid profile in human volunteers. Am J Clin Nutr 2006;84(4):756–761
94. Matvienko OA, Lewis DS, Swanson M, et al. A single daily dose of soybean phytosterols in ground beef decreases serum total cholesterol and LDL cholesterol in young, mildly hypercholesterolemic men. Am J Clin Nutr 2002;76(1):57–64
95. Plat J, van Onselen EN, van Heugten MM, Mensink RP. Effects on serum lipids, lipoproteins and fat soluble antioxidant concentrations of consumption frequency of margarines and shortenings enriched with plant stanol esters. Eur J Clin Nutr 2000;54(9):671–677
96. Food and Drug Administration. Agency Response Letter. GRAS Notice No. GRN 000112. 2003. Available at: http://www.fda.gov/ohrms/dockets/dockets/95s0316/95s-0316-rpt000343-043-appx-ERef-31-GRAS-vol268.pdf. Accessed May 7, 2012
97. Scientific Committee on Food. Opinion on Applications for Approval of a Variety of Plant Sterol-Enriched Foods. Brussels: European Commission; 2003. Available at: http://ec.europa.eu/food/fs/sc/scf/out174_en.pdf. Accessed May 7, 2012
98. Ayesh R, Weststrate JA, Drewitt PN, Hepburn PA. Safety evaluation of phytosterol esters. Part 5. Faecal short-chain fatty acid and microflora content, faecal bacterial enzyme activity and serum female sex hormones in healthy normolipidaemic volunteers consuming a controlled diet either with or without a phytosterol ester-enriched margarine. Food Chem Toxicol 1999;37(12):1127–1138
99. Berge KE. Sitosterolemia: a gateway to new knowledge about cholesterol metabolism. Ann Med 2003;35(7):502–511
100. Stalenhoef AF, Hectors M, Demacker PN. Effect of plant sterol-enriched margarine on plasma lipids and sterols in subjects heterozygous for phytosterolaemia. J Intern Med 2001;249(2):163–166
101. Kwiterovich PO Jr, Chen SC, Virgil DG, Schweitzer A, Arnold DR, Kratz LE. Response of obligate heterozygotes for phytosterolemia to a low-fat diet and to a plant sterol ester dietary challenge. J Lipid Res 2003;44(6):1143–1155
102. Normén L, Holmes D, Frohlich J. Plant sterols and their role in combined use with statins for lipid lowering. Curr Opin Investig Drugs 2005;6(3):307–316
103. Thompson GR. Additive effects of plant sterol and stanol esters to statin therapy. Am J Cardiol 2005; 96(1A, Suppl):37D–39D
104. Blair SN, Capuzzi DM, Gottlieb SO, Nguyen T, Morgan JM, Cater NB. Incremental reduction of serum total cholesterol and low-density lipoprotein cholesterol with the addition of plant stanol ester-containing spread to statin therapy. Am J Cardiol 2000;86(1):46–52
105. Neil HA, Meijer GW, Roe LS. Randomised controlled trial of use by hypercholesterolaemic patients of a vegetable oil sterol-enriched fat spread. Atherosclerosis 2001;156(2):329–337
106. Simons LA. Additive effect of plant sterol-ester margarine and cerivastatin in lowering low-density lipoprotein cholesterol in primary hypercholesterolemia. Am J Cardiol 2002;90(7):737–740
107. Nguyen TT, Dale LC. Plant stanol esters and vitamin K. Mayo Clin Proc 1999;74(6):642–643
108. Raeini-Sarjaz M, Ntanios FY, Vanstone CA, Jones PJ. No changes in serum fat-soluble vitamin and carotenoid concentrations with the intake of plant sterol/stanol esters in the context of a controlled diet. Metabolism 2002;51(5):652–656
109. Korpela R, Tuomilehto J, Högström P, et al. Safety aspects and cholesterol-lowering efficacy of low fat dairy products containing plant sterols. Eur J Clin Nutr 2006;60(5):633–642
110. Plat J, Mensink RP. Vegetable oil based versus wood based stanol ester mixtures: effects on serum lipids and hemostatic factors in non-hypercholesterolemic subjects. Atherosclerosis 2000;148(1):101–112
111. Plat J, Mensink RP. Effects of diets enriched with two different plant stanol ester mixtures on plasma ubiquinol-10 and fat-soluble antioxidant concentrations. Metabolism 2001;50(5):520–529
112. Ntanios FY, Duchateau GS. A healthy diet rich in carotenoids is effective in maintaining normal blood carotenoid levels during the daily use of plant sterol-enriched spreads. Int J Vitam Nutr Res 2002; 72(1):32–39
113. Noakes M, Clifton P, Ntanios F, Shrapnel W, Record I, McInerney J. An increase in dietary carotenoids when consuming plant sterols or stanols is effective in maintaining plasma carotenoid concentrations. Am J Clin Nutr 2002;75(1):79–86