20 Essential Fatty Acids (Omega-3 and Omega-6)
Omega-3 and omega-6 fatty acids are polyunsaturated fatty acids (PUFAs), meaning they contain more than one cis double bond.1 In all omega-3 fatty acids, the first double bond is located between the third and fourth carbon atom counting from the methyl end of the fatty acid (n-3). Similarly, the first double bond in all omega-6 fatty acids is located between the sixth and seventh carbon atom from the methyl end of the fatty acid (n-6). Scientific abbreviations for fatty acids tell the reader something about their chemical structure. One scientific abbreviation for α-linolenic acid (ALA) is 18:3n-3. The first part (18:3) tells the reader that ALA is an 18-carbon fatty acid with three double bonds, while the second part (n-3) tells the reader that the first double bond is in the n-3 position, which defines it as an omega-3 fatty acid.
Although humans and other mammals can synthesize saturated fatty acids and some monounsaturated fatty acids from carbon groups in carbohydrates and proteins, they lack the enzymes necessary to insert a cis double bond at the n-6 or the n-3 position of a fatty acid.1 Consequently, omega-6 and omega-3 fatty acids are essential nutrients. The parent fatty acid of the omega-6 series is linoleic acid (LA; 18:2n-6), and the parent fatty acid of the omega-3 series is ALA (Fig. 20.1). Humans can synthesize long-chain (20 carbons or more) omega-6 fatty acids, such as dihomo-γ-linolenic acid (DGLA; 20:3n-6) and arachidonic acid (AA; 20:4n-6) from LA and long-chain omega-3 fatty acids, such as eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3) from ALA (see the Bioavailability and Metabolism section below). It has been estimated that the ratio of omega-6 to omega-3 fatty acids in the diet of early humans was 1:1,2 but the ratio in the typical Western diet is now almost 10:1 due to increased use of vegetable oils rich in LA, as well as reduced fish consumption.3 A large body of scientific research suggests that increasing the relative abundance of dietary omega-3 fatty acids may have several health benefits.
Bioavailability and Metabolism
Prior to absorption in the small intestine, fatty acids must be hydrolyzed from dietary fats (triglycerides, phospholipids, and cholesterol) by pancreatic enzymes.4 Bile salts must also be present in the small intestine to allow for the incorporation of fatty acids and other products of fat digestion into mixed micelles. Fat absorption from mixed micelles occurs throughout the small intestine and is 85%–95% efficient under normal conditions. Humans can synthesize longer omega-6 and omega-3 fatty acids from the essential fatty acids LA and ALA, respectively, through a series of desaturation (addition of a double bond) and elongation (addition of two carbon atoms) reactions (Fig. 20.2).5 LA and ALA compete for the same elongase and desaturase enzymes in the synthesis of longer polyunsaturated fatty acids, such as AA and EPA. Although ALA is the preferred substrate of the Δ6 desaturase enzyme, the excess of dietary LA compared with ALA results in greater net formation of AA (20:4n-6) than EPA (20:5n-3).6 The capacity for conversion of ALA to DHA is higher in women than men. Studies of ALA metabolism in healthy young men indicate that approximately 8% of dietary ALA is converted to EPA and 0%–4% is converted to DHA.7 In healthy young women, approximately 21% of dietary ALA is converted to EPA and 9% is converted to DHA.8 The better conversion efficiency of young women compared with men appears to be related to the effects of estrogen.6,9 Although ALA is considered the essential omega-3 fatty acid because it cannot be synthesized by humans, evidence that human conversion of EPA and particularly DHA is relatively inefficient suggests that EPA and DHA may also be essential under some conditions.10,11
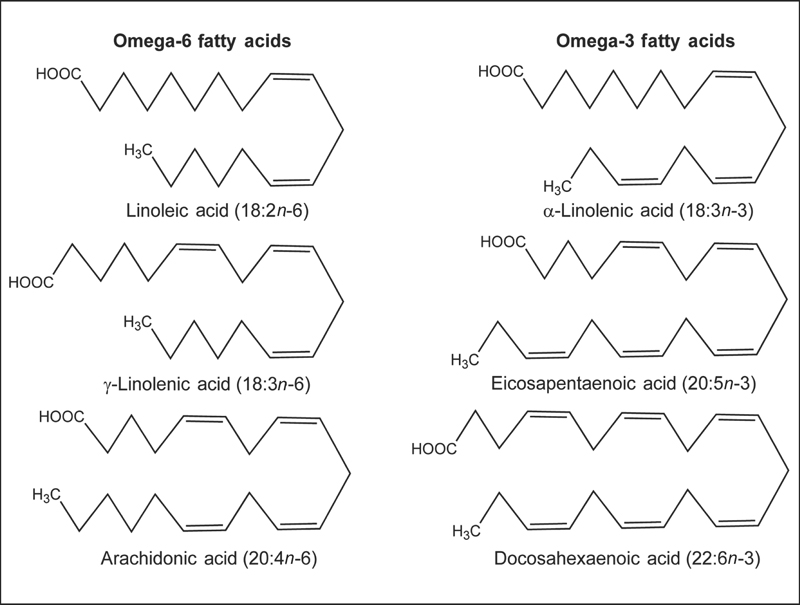
Fig. 20.1 Chemical structures of the omega-6 fatty acids, linoleic acid (LA, 18:2n-6), γ-linolenic acid (GLA, 18:3n-6), and arachidonic acid (AA, 20:4n-6), and the omega-3 fatty acids, α-linolenic acid (ALA, 18:3n-3), eicosapentaenoic acid (EPA, 20:5n-3), and docosahexaenoic acid (DHA, 22:6n-3).
Biological Activities
Membrane Structure and Function
Omega-6 and omega-3 PUFA are important structural components of cell membranes. When incorporated into phospholipids, they affect cell membrane properties such as fluidity, flexibility, permeability, and the activity of membrane-bound enzymes.12 DHA is selectively incorporated into retinal cell membranes and postsynaptic neuronal cell membranes, suggesting it plays important roles in vision and nervous system function.
Vision
DHA is found at very high concentrations in the cell membranes of the retina; the retina conserves and recycles DHA even when omega-3 fatty acid intake is low.13 Animal studies indicate that DHA is required for the normal development and function of the retina. Moreover, these studies suggest that there is a critical period during retinal development when inadequate DHA will result in permanent abnormalities in retinal function. Recent research indicates that DHA plays an important role in regeneration of the visual pigment rhodopsin, which plays a critical role in the visual transduction system that converts light hitting the retina to visual images in the brain.14
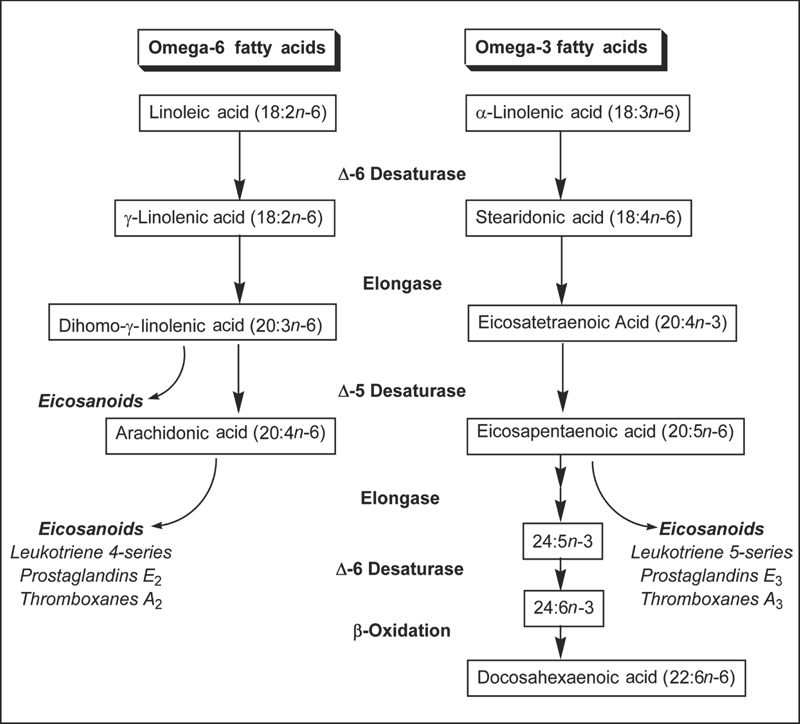
Fig. 20.2 Synthesis of long-chain omega-6 and omega-3 polyunsaturated fatty acids from the parent fatty acids, linoleic acid (LA, 18:2n-6) and α-linolenic acid (ALA, 18:3n-3), in humans.
Nervous System
The phospholipids of the brain's gray matter contain high proportions of DHA and AA, suggesting they are important to central nervous system function.15 Brain DHA content may be particularly important, since animal studies have shown that depletion of DHA in the brain can result in learning deficits. It is not clear how DHA affects brain function, but changes in the DHA content of neuronal cell membranes could alter the function of ion channels or membrane-associated receptors, as well as the availability of neurotransmitters.16
Eicosanoid Synthesis
Eicosanoids, derived from 20-carbon PUFA, are potent chemical messengers that play critical roles in immune and inflammatory responses. During an inflammatory response, DGLA, AA, and EPA in cell membranes can be metabolized by enzymes known as cyclooxygenases and lipoxygenases, to form prostaglandins and leukotrienes, respectively (Fig. 20.2). In those who consume typical Western diets, the amount of AA in cell membranes is much greater than the amount of EPA, resulting in the formation of more eicosanoids derived from AA than from EPA. However, increasing omega-3 fatty acid intake increases the EPA content of cell membranes, resulting in higher proportions of eicosanoids derived from EPA. Physiological responses to AA-derived eicosanoids differ from responses to EPA-derived eicosanoids. In general, eicosanoids derived from EPA are less potent inducers of inflammation, blood vessel constriction, and coagulation than eicosanoids derived from AA.3,17
Regulation of Gene Expression
The results of cell culture and animal studies indicate that omega-6 and omega-3 fatty acids can modulate the expression of several genes, including those involved with fatty acid metabolism and inflammation.17,18 Although the mechanisms require further clarification, omega-6 and omega-3 fatty acids may regulate gene expression by interacting with specific transcription factors, including peroxisome proliferator-activated receptors (PPARs) and liver X receptors (LXRs).19 Multiple mechanisms are involved in these regulatory schemes.20 In many cases, PUFAs act like hydrophobic hormones (e.g., steroid hormones) to control gene expression. In this case, PUFAs bind directly to receptors like PPARs. These receptors bind to the promoters of genes and function to increase/decrease transcription of genes. In other cases, PUFAs regulate the abundance of transcription factors inside the cell's nucleus.20 For these factors, the mechanism for PUFA control is less clear. Two examples include nuclear factor kappa B (NF-κB) and sterol regulatory element–binding protein 1 (SREBP-1). NF-κB is a transcription factor involved in regulating the expression of multiple genes involved in inflammation. Omega-3 PUFAs suppress NF-κB nuclear content, thus inhibiting the production of inflammatory eicosanoids and cytokines. SREBP-1 is a major transcription factor controlling fattyacid synthesis—both de novo lipogenesis and PUFA synthesis.21 Dietary PUFAs can suppress SREBP-1, which decreases the expression of enzymes involved in fatty acid synthesis and PUFA synthesis.22,23 In this way, dietary PUFAs function as feedback inhibitors of all fatty acid synthesis.
Deficiency
Essential Fatty Acid Deficiency
Clinical signs of essential fatty acid deficiency include a dry scaly rash, decreased growth in infants and children, increased susceptibility to infection, and poor wound healing.24 Omega-3, omega-6, and omega-9 fatty acids compete for the same desaturase enzymes. The desaturase enzymes show preference for the different series of fatty acids in the following order: omega-3 > omega-6 > omega-9. Consequently, synthesis of the omega-9 fatty acid eicosatrienoic acid (20:3n-9, mead acid, or 5,8,11-eicosatrienoic acid) increases only when dietary intakes of omega-3 and omega-6 fatty acids are very low; therefore, mead acid is one marker of essential fatty acid deficiency.25 A plasma eicosatrienoic acid:arachidonic acid (triene:tetraene) ratio greater than 0.2 is generally considered indicative of essential fatty acid deficiency.24,26 In patients who were given total parenteral nutrition containing fat-free glucose-amino acid mixtures, biochemical signs of essential fatty acid deficiency developed in as little as 7–10 days.27 In these cases, the continuous glucose infusion resulted in high circulating insulin levels, which inhibited the release of essential fatty acids stored in adipose tissue. When glucose-free amino acid solutions were used, parenteral nutrition up to 14 days did not result in biochemical signs of essential fatty acid deficiency. Essential fatty acid deficiency has also been found to occur in patients with chronic fat malabsorption28 and in patients with cystic fibrosis.29 Moreover, it has been proposed that essential fatty acid deficiency may play a role in the pathology of protein–energy malnutrition.25
Omega-3 Fatty Acid Deficiency
At least one case of isolated omega-3 fatty acid deficiency has been reported. A young girl who received intravenous lipid emulsions with very little ALA developed visual problems and sensory neuropathy; these conditions were resolved when she was administered an emulsion containing more ALA.30 Plasma DHA concentrations decrease when omega-3 fatty acid intake is insufficient, but no cutoff values have been established. Isolated omega-3 fatty acid deficiency does not result in increased plasma triene:tetraene ratios.1 Studies in rodents, however, have revealed significant impairment of learning and memory associated with n-3 PUFA deficiency.31,32 These studies have prompted clinical trials in humans to assess the impact of omega-3 PUFA on cognitive development and cognitive decline.
Disease Prevention
Visual and Neurological Development
Because the last trimester of pregnancy is a critical period for the accumulation of DHA in the brain and retina, preterm infants are thought to be particularly vulnerable to adverse effects of insufficient DHA on visual and neurological development.33 Human milk contains DHA in addition to ALA and EPA but, until recently, ALA was the only omega-3 fatty acid present in conventional infant formulas. Although preterm infants can synthesize DHA from ALA, they generally cannot synthesize enough to prevent declines in plasma and cellular DHA concentrations without additional dietary intake. Therefore, it was proposed that preterm infant formulas be supplemented with enough DHA to bring plasma and cellular DHA levels of formula-fed infants up to those of breast-fed infants.34 Although formulas enriched with DHA raise plasma and red blood cell DHA concentrations in preterm and term infants, the results of randomized controlled trials examining measures of visual acuity and neurological development in infants fed formulas with or without added DHA have been mixed.35–38 Although several controlled trials found that healthy preterm infants fed formulas with DHA added showed subtle but significant improvements in visual acuity at 2 months and 4 months of age compared with those fed DHA-free formulas,39 most randomized controlled trials found no differences in visual acuity between healthy preterm infants fed formulas with or without DHA added.36 Similarly, two randomized controlled trials that assessed general measures of infant development at 12 months and 24 months of age found no difference between preterm infants fed formula with or without DHA added.40,41 However, two randomized controlled trials assessing infant development at 18 months of age reported beneficial effects of DHA supplementation in preterm infants, but one of these trials found a significant effect only in boys.42,43 Infant formulas enriched with DHA are also commercially available for term infants, but the results of randomized controlled trials of these formulas on visual acuity and development in term infants have also been mixed.37,38,44–48 While DHA appears to be important for visual and neurological development, it is not yet clear whether feeding infants formula enriched with DHA enhances visual acuity or neurological development in preterm or term infants.49
Pregnancy and Lactation
Although infant requirements for DHA have been the subject of a great deal of research, there has been relatively little investigation of maternal requirements for omega-3 fatty acids, despite the fact that the mother is the sole source of omega-3 fatty acids for the fetus and exclusively breast-fed infant.50 The results of randomized controlled trials during pregnancy suggest that omega-3 fatty acid supplementation does not decrease the incidence of gestational diabetes, pregnancy-induced hypertension, or preeclampsia51–53 but may result in modest increases in the length of gestation, especially in women with low consumption of omega-3 fatty acid. In healthy Danish women, fish oil supplementation that provided 2.7 g/day of EPA + DHA increased the length of gestation by an average of 4 days.52 More recently, consumption of only 0.13 g/day of DHA from enriched eggs during the last trimester of pregnancy increased the length of gestation by an average of 6 days in a low-income population in the United States.53 A recent meta-analysis of six randomized controlled trials in women with low-risk pregnancies found that omega-3 PUFA supplementation during pregnancy resulted in an increased length of pregnancy by 1.6 days.54 In European women with high-risk pregnancies, fish oil supplementation, which provided 2.7 g/day of EPA + DHA during the last trimester of pregnancy, lowered the risk of premature delivery from 33% to 21%.55 However, a meta-analysis of randomized controlled trials in women with high-risk pregnancies found that supplementation with long-chain PUFAs did not affect the duration of pregnancy or the incidence of premature births but decreased the incidence of early premature births (<34 weeks of gestation).56 The World Association of Perinatal Medicine, the Early Nutrition Academy, and the Child Health Foundation recommend that pregnant and lactating women consume an average of at least 200 mg DHA daily (approx. one to two servings of fish weekly).57
The effect of long-chain PUFA supplementation during pregnancy and/or lactation on neurodevelopmental outcomes in the offspring is an area of active investigation. In Norway, children born to mothers who were given supplements of cod liver oil (2 g/day of EPA + DHA) during pregnancy and during the first 3 months of lactation scored higher on mental processing tests at 4 years of age when compared with the children whose mothers were not given the supplements.58 However, only 14% of the original study participants were available for testing when the children were aged 4 years. In a double-blind, randomized, placebo-controlled trial, children born to mothers who were given fish oil supplements (2.2 g DHA and 1.1 g EPA) during pregnancy (20 weeks’ gestation until delivery) displayed higher scores of eye and hand coordination at 2½ years of age, compared with children whose mothers were given olive oil supplements.59 A small trial that provided either DHA-containing cereal bars (300 mg DHA/bar, average of 5 bars/week) or placebo cereal bars to 29 pregnant women (from 24 weeks’ gestation until delivery) associated maternal DHA supplementation with improvements in infant problem-solving skills at 9 months of age.60 No differences in recognition memory tasks were observed between the two groups of infants. Results of randomized controlled trials assessing cognitive function in children whose mothers were provided omega-3 fatty acids only during lactation have been mixed.38 Although some results are promising, more studies are needed to determine whether long-chain PUFA supplementation during pregnancy and/or lactation has beneficial effects on long-term cognitive development in children. At present, the potential benefits associated with obtaining long-chain omega-3 fatty acids through moderate consumption of fish (e.g., one to two servings weekly) during pregnancy and lactation outweigh any risks of contaminant exposure, but fish with high levels of methylmercury should be avoided.61 For information about contaminants in fish and guidelines for fish consumption by women of childbearing age, see the Contaminants in Fish section below.
Cardiovascular Disease
Omega-6 Fatty Acids: Linoleic Acid
LA is the most abundant dietary PUFA. The results of prospective cohort studies examining the relationships between PUFA intake and the risk of coronary heart disease (CHD) have been somewhat inconsistent.62 Some, but not all, prospective cohort studies have found that higher PUFA intakes are associated with significant reductions in CHD risk63–65 or cardiovascular-related mortality.66 The largest prospective cohort study to examine the effects of dietary fat intake on CHD risk is the Nurses’ Health Study (NHS), which followed more than 78 000 women for 20 years. In that cohort, those with the highest intakes of total PUFA (7.4% of energy) and LA had a risk of CHD that was 25% lower than that of those with the lowest intakes of total PUFA (5% of energy) and LA.64 Although saturated fatty acid (SFA) intake was not associated with CHD risk, the ratio of PUFA:SFA intake was inversely associated with CHD risk. In controlled feeding trials, replacing dietary SFA with PUFA consistently lowers serum total and low-density lipoprotein (LDL) cholesterol concentrations.67 In fact, LA has been shown to be the most potent fatty acid for lowering serum total and LDL cholesterol when substituted for dietary SFA.68 Several dietary intervention trials have compared the effects of diets high in SFA (18%–19% of energy) with diets low in SFA (8%–9% of energy) and high in PUFA (14%–21% of energy) on morbidity (illness) and mortality from CHD.62 Although most of the increase in dietary PUFA was provided by LA, ALA intakes were also increased in these trials.67 Several dietary intervention trials in men found that replacing dietary SFA with PUFA reduced morbidity or mortality from CHD.69–72 However, two similar dietary intervention trials in women did not result in significant reductions in morbidity or mortality from CHD.73,74 In a recently released scientific advisory, the American Heart Association concluded that obtaining 5%–10% of total caloric intake from omega-6 PUFAs is associated with a reduced risk of CHD.75
Omega-3 Fatty Acids: α-Linolenic Acid
Several prospective cohort studies have examined the relationship between dietary ALA intake and CHD risk. In a cohort of more than 45 000 US men followed for 14 years, each 1 g/day increase in dietary ALA intake was associated with a 16% reduction in the risk of CHD.76 Moreover, in those who ate little or no seafood, each 1 g/day increase in dietary ALA intake was associated with a 47% reduction in the risk of CHD. In a cohort of more than 76 000 US women followed for 10 years, those with the highest ALA intakes (approx. 1.4 g/day) had a risk of fatal CHD that was 45% lower than in women with the lowest intakes (approx. 0.7 g/day).77 Interestingly, oil and vinegar salad dressing was an important source of dietary ALA in this population. Women who consumed oil and vinegar salad dressing five to six times weekly had a risk of fatal CHD that was 54% lower than that of those who rarely consumed it, even after adjusting the analysis for vegetable intake. In a smaller cohort of more than 6000 US men, those with the highest intakes of ALA had a risk of death from CHD over the next 10 years that was 40% lower than in those with the lowest intakes.78 In contrast, two studies in Europe found no association between dietary ALA intake and CHD risk.79,80 Additionally, in the NHS (76 763 women followed for 18 years), dietary intake of ALA was not associated with fatal CHD or nonfatal myocardial infarction (MI) but was inversely associated with sudden cardiac death (see the section on Sudden Cardiac Death below).81 Although not as consistent as the evidence supporting higher intakes of long-chain omega-3 fatty acids from seafood, the results of most prospective studies suggest that higher dietary ALA intakes (2–3 g/day) are associated with significant reductions in CHD risk, especially in populations with low levels of fish consumption.82 Unlike LA, the cardioprotective effects of higher ALA intakes do not appear to be related to changes in serum lipid profiles. A meta-analysis of 14 randomized controlled trials concluded that ALA supplementation had no effect on total cholesterol, LDL cholesterol, or triglyceride levels.83 However, several controlled clinical trials found that increasing ALA intake decreased serum concentrations of C-reactive protein, a marker of inflammation that is strongly associated with the risk of cardiovascular events such as MI and stroke.84–86
Long-Chain Omega-3 Fatty Acids: Eicosapentaenoic Acid and Docosahexaenoic Acid
Evidence is accumulating that increasing intakes of long-chain omega-3 fatty acids (EPA and DHA) can decrease the risk of cardiovascular disease by (1) preventing arrhythmias that can lead to sudden cardiac death, (2) decreasing the risk of thrombosis (a clot) that can lead to MI or stroke, (3) decreasing serum triglyceride levels, (4) slowing the growth of atherosclerotic plaque, (5) improving vascular endothelial function, (6) lowering blood pressure slightly, and (7) decreasing inflammation.87 A recent systematic review of randomized controlled trials found that consumption of EPA and DHA from fish or fish oil supplements was associated with reductions in all-cause mortality, cardiac death, and sudden death.88 Yet, another systematic review and meta-analysis of randomized controlled trials and prospective cohort studies concluded that long-chain omega-3 fatty acids do not significantly reduce the risk of total mortality or cardiovascular events.89
Coronary heart disease. Several prospective cohort studies have found that men who eat fish at least once weekly have lower mortality from CHD than men who do not eat fish.90–92 One such study followed 1822 men for 30 years and found that mortality from CHD was 38% lower in men who consumed an average of at least 35 g (1.2 oz) of fish daily than in men who did not eat fish, while mortality from MI was 67% lower in the group that ate fish.93 The cardioprotective effects of fish consumption may not be confined to those consuming a typical Western diet. A study in China that followed more than 18 000 men for 10 years found that those who consumed more than 200 g (approx. 7 oz) of fish or shellfish weekly had a risk of fatal MI that was 59% lower than that of men who consumed less than 50 g (approx. 2 oz) weekly.94 Less information is available regarding the effects of higher omega-3 fatty acid and fish intakes in women. In the NHS, which followed more than 84 000 women for 16 years, CHD mortality was 29%–34% lower in women who ate fish at least once a week compared with women who ate fish less than once a month.95 In a prospective study in 2445 Finnish women, those in the highest quintile of fish intake (≥41 g/day; mean 70 g/day) had a 41% lower risk of CHD compared with those in the lowest quintile (≤8 g/day; mean 4.2 g/day).96
A large prospective study in a cohort of 41 478 Japanese men and women found that higher intakes of fish are associated with further reductions in the risk of CHD. In this study, those who consumed fish eight times weekly had a 57% lower risk of nonfatal coronary events and a 56% lower risk of MI compared with those who consumed fish only once weekly.97 Yet, a smaller prospective study in 8879 Japanese men and women found that consumption of fish twice daily did not lower the risk of all-cause mortality or CHD mortality compared with eating fish once or twice weekly.98
Sudden cardiac death. Sudden cardiac death (SCD) is the result of a fatal ventricular arrhythmia, which usually occurs in people with CHD. Studies in cell culture indicate that long-chain omega-3 fatty acids decrease the excitability of cardiac muscle cells (myocytes) by modulating ion-channel conductance.99 The results of epidemiological studies suggest that regular fish consumption is inversely associated with the risk of SCD. In a large prospective cohort study that followed more than 20 000 men for 11 years, those who ate fish at least once a week had a risk of SCD that was 52% lower than that of those who ate fish less than once a month.100 Plasma levels of EPA and DHA were also inversely related to the risk of SCD, supporting the idea that omega-3 fatty acids are at least partially responsible for the beneficial effect of fish consumption on SCD.101 More recently, a prospective study that followed more than 45 000 men for 14 years found that the risk of SCD was approximately 40%–50% lower in those who consumed an average of at least 250 mg/day of dietary EPA + DHA (the equivalent of 1–2 meals of oily fish weekly) than those who consumed less than 250 mg/day.76 Dietary EPA + DHA intake was not related to the risk of nonfatal MI or total CHD events, suggesting the antiarrhythmic effects of long-chain omega-3 fatty acids may be important at usual dietary intake levels. Thus, several observational studies and clinical trials have found that including fish or fish oils in the diet lowers the risk of SCD.102 Fewer studies have looked at whether consumption of ALA, a shorter-chain omega-3 fatty acid, affects the risk of SCD. In the NHS, which included 76 763 women, higher dietary intakes of ALA were associated with a 38%–40% lower risk of SCD.81 No association between dietary ALA intake and sudden death was found in the Health Professionals Follow-up Study, which included 45 722 men.76 It is also not clear whether omega-3 supplementation reduces the risk of ventricular arrhythmias. A recent meta-analysis of three clinical trials103–105 concluded that supplementation with fish oil did not help prevent ventricular arrhythmias in patients with implantable cardioverter defibrillators, but these patients had existing cardiac problems.106 More epidemiological and clinical research is needed to determine whether omega-3 fatty acid status influences the risk of ventricular arrhythmias.107
Stroke. Ischemic strokes, which comprise 87% of all strokes, are the result of insufficient blood flow to an area of the brain and may occur when an artery supplying the brain becomes occluded by a clot. Hemorrhagic strokes occur when a blood vessel ruptures and bleeds into the brain.108 Some prospective studies that have examined the relationship between fish or omega-3 fatty acid intake and total stroke incidence have found increased fish intake to be beneficial,109,110 while others have found no beneficial effect.111–113 More recently, two large prospective studies found that increased fish and omega-3 fatty acid intakes were associated with significantly lower risks of ischemic stroke but not hemorrhagic stroke. In a study that followed more than 79 000 women for 14 years, those who ate fish at least twice weekly had a risk of thrombotic (ischemic) stroke that was 52% lower than that of those who ate fish less than once a month.114 Similarly, in a study that followed more than 43 000 men for 12 years, those who ate fish at least once a month had a risk of ischemic stroke that was 43% lower than that of those who ate fish less than once a month.115 Although the effects of long-chain omega-3 fatty acid intake on the incidence of stroke have not been studied as thoroughly as the effects on CHD, a meta-analysis of available evidence suggests that increased fish intake may decrease the risk of ischemic stroke but not hemorrhagic stroke.116 Results of a recent study indicate that high-dose EPA supplementation may be beneficial in the secondary prevention of stroke, that is, preventing recurrent stroke in individuals with a prior history.117
Serum triglycerides. A meta-analysis of 17 prospective studies found hypertriglyceridemia (serum triglycerides >200 mg/dL) to be an independent risk factor for cardiovascular disease.118 Numerous controlled clinical trials in humans have demonstrated that increasing intakes of EPA and DHA significantly lower serum triglyceride concentrations.119 The triglyceride-lowering effects of EPA and DHA increase with dose,120 but clinically meaningful reductions in serum triglyceride concentrations have been demonstrated at doses of 2 g/day of EPA + DHA.3 In its recommendations regarding omega-3 fatty acids and cardiovascular disease (see the section on Intake Recommendations below), the American Heart Association indicates that an EPA + DHA supplement may be useful in patients with hypertriglyceridemia.87
Summary: Omega-3 and Omega-6 PUFA and Prevention of Cardiovascular Disease
The results of epidemiological studies and randomized controlled trials suggest that replacing dietary SFA with omega-6 and omega-3 PUFA lowers LDL cholesterol and decreases the risk of cardiovascular disease. Additionally, the results of epidemiological studies provide strong evidence that increasing dietary omega-3 fatty acid intake is associated with significant reductions in the risk of cardiovascular disease, through mechanisms other than lowering LDL cholesterol. In particular, increasing EPA and DHA intake from seafood has been associated with significant reductions in SCD, suggesting that long-chain omega-3 fatty acids have antiarrhythmic effects at intake levels equivalent to the amount in two small servings of oily fish per week. This amount of fish would provide approximately 400–500 mg/day of EPA + DHA.121 Thus, some researchers have proposed that the US Institute of Medicine should establish a dietary reference intake for EPA + DHA.122
Alzheimer Disease and Dementia
Alzheimer disease is the most common cause of dementia in older adults. It is characterized by the formation of amyloid plaque in the brain and nerve cell degeneration. Disease symptoms, including memory loss and confusion, worsen over time.123 Some epidemiological studies have associated high intake of fish with decreased risk of impaired cognitive function,124 dementia,125 and Alzheimer disease.125,126 DHA, the major omega-3 fatty acid in the brain, appears to be protective against Alzheimer disease.127 Observational studies have found that lower DHA status is associated with increased risk of Alzheimer disease,128–130 as well as other types of dementia.129 In a cohort of the Framingham Heart Study, men and women in the highest quartile of plasma phosphatidylcholine DHA content had a 47% decreased risk of developing all-cause dementia and a 39% decreased risk of developing Alzheimer disease when compared with those in the lower three quartiles.131 Individuals in the top quartile consumed an average of three servings of fish weekly (0.18 g/day of DHA).131 Thus, low DHA status may be a risk factor for Alzheimer disease, other types of dementia, and cognitive impairment associated with aging.
Disease Treatment
Coronary Heart Disease
Dietary Intervention Trials
Total mortality and fatal MI decreased by 29% in male MI survivors advised to increase their weekly intake of oily fish to 200–400 g (7–14 oz)—an amount estimated to provide an additional 500–800 mg/day of long-chain omega-3 fatty acids (EPA + DHA).132 In another dietary intervention trial, patients who survived a first MI were randomly assigned to usual care or advised to adopt a Mediterranean diet that was higher in omega-3 fatty acids (especially ALA) and lower in omega-6 fatty acids than the standard Westernstyle diet. After almost 4 years, those on the Mediterranean diet had a risk of cardiac death and nonfatal MI that was 38% lower than in the group that was assigned to usual care.133 Although higher plasma ALA levels were associated with better outcomes, the benefit of the Mediterranean diet cannot be attributed entirely to increased ALA intakes, since intakes of monounsaturated fatty acids and fruits and vegetables also increased. A recent intervention trial compared survival in MI survivors who followed a Mediterranean-style diet or a low-fat diet for an average of 46 months; total mortality and cardiovascular-related mortality did not differ between the two groups.134
Supplementation Trials
In the largest randomized controlled trial of supplemental omega-3 fatty acids to date, CHD patients who received supplements providing 850 mg/day of EPA + DHA for 3.5 years had a risk of sudden death that was 45% lower than that of those who did not take supplements; supplement users also experienced a 20% lower risk of death from all causes compared with non-supplement users.135 Interestingly, it took only 3 months of supplementation to demonstrate a significant decrease in total mortality and 4 months to demonstrate a significant decrease in sudden death.136 In another supplementation trial, patients admitted to hospital with an acute MI were randomized to receive capsules containing fish oil (1.8 g/day of EPA + DHA), mustard oil (2.9 g/day of ALA), or a placebo.137 After 1 year, total cardiac events, including nonfatal MI, were significantly lower in the groups that received fish oil or mustard oil compared with the groups that received a placebo. In contrast, patients with acute MI did not realize any additional benefit from supplementation with 3.5 g/day of EPA + DHA compared with corn oil in a region of Norway where fish intakes are relatively high.138 The results of a meta-analysis that pooled the findings of 11 randomized controlled trials of dietary or supplementary omega-3 fatty acids indicated that increased omega-3 fatty acid intakes significantly decreased overall mortality, mortality due to MI, and SCD in patients with CHD.139
Two randomized controlled trials have examined the effect of fish oil supplementation on the progression of coronary artery atherosclerosis measured by coronary angiography. Although a study of 59 patients with coronary artery disease found no benefit after 2 years of supplementation with fish oil, providing 6 g/day of EPA + DHA, compared with olive oil,140 a larger trial of 223 patients found that supplementation with 3.3 g/day of EPA + DHA for 3 months and 1.65 g/day for an additional 21 months resulted in a modest decrease in the progression of coronary atherosclerosis compared with a placebo.141 Numerous randomized controlled trials have examined the effect of fish oil supplementation on coronary artery restenosis after percutaneous transluminal coronary angioplasty. A meta-analysis that combined the results of 12 randomized controlled trials found that fish oil supplementation resulted in a 14% reduction of coronary restenosis, but this reduction did not quite reach statistical significance.142 Supplemental fish oil doses in the coronary artery restenosis trials ranged from 2.6 g/day to 6.0 g/day.
Summary: Long-Chain Omega-3 Fatty Acids in Treatment of Coronary Heart Disease
The results of randomized controlled trials in individuals with documented CHD suggest a beneficial effect of dietary and supplemental omega-3 fatty acids. Based on the results of these trials, the American Heart Association recommends that individuals with documented CHD consume approximately 1 g/day of EPA + DHA (see the section on Intake Recommendations below).143
Diabetes Mellitus
Cardiovascular diseases are the leading causes of death in individuals with diabetes mellitus (DM). Hypertriglyceridemia (serum triglycerides >200 mg/dL) is a common lipid abnormality in individuals with type 2 DM, and several randomized controlled trials have found that fish oil supplementation significantly lowers serum triglyceride levels in individuals with diabetes (see the section on serum triglycerides above).144 Although early, uncontrolled, studies raised concerns that fish oil supplementation adversely affected blood glucose (glycemic) control,145,146 randomized controlled trials have not generally found adverse effects of fish oil supplementation on long-term glycemic control.147 A systematic review that pooled the results of 18 randomized controlled trials, including more than 800 patients with diabetes, found that fish oil supplementation significantly lowered serum triglycerides, especially in those with hypertriglyceridemia.144 A meta-analysis that combined the results of 18 randomized controlled trials in individuals with type 2 DM or metabolic syndrome found that fish oil supplementation decreased serum triglycerides by 31 mg/dL compared with placebo but had no effect on serum cholesterol, fasting glucose, or hemoglobin A1c concentrations.147 A more recent meta-analysis of randomized controlled trials in patients with type 2 DM found that omega-3 fatty acid supplementation lowered serum triglyceride levels by 25%.148 However, fish oil supplementation has been associated with a slight increase in LDL cholesterol levels.144,148,149 Although few controlled trials have examined the effect of fish oil supplementation on cardiovascular disease outcomes in patients with diabetes, a prospective study that followed 5103 women diagnosed with type 2 DM, but free of cardiovascular disease or cancer at the start of the study, found that higher fish intakes were associated with significantly decreased risks of CHD over a 16-year follow-up period.150 Thus, increasing EPA and DHA intakes may be beneficial to individuals with diabetes, especially those with elevated serum triglycerides.151 Moreover, there is little evidence that daily EPA + DHA intakes of less than 3 g/day adversely affect long-term glycemic control in individuals with diabetes.144,152 The American Diabetes Association recommends that individuals with diabetes increase their consumption of omega-3 fatty acid, by consuming two to three 3-oz servings of fish weekly.153
Inflammatory Diseases
Rheumatoid Arthritis
Three meta-analyses of randomized controlled trials in patients with rheumatoid arthritis found that fish oil supplementation significantly decreased the number of painful and/or tender joints on physical examination.147,154,155 The most recent of these meta-analyses also associated omega-3 PUFA supplementation with improvements in the intensity of pain and the duration of morning stiffness.155 In general, clinical benefits were observed at a minimum dose of 2.7 g/day of EPA + DHA and were not apparent until at least 12 weeks from the start of supplementation.155 Two of these meta-analyses assessed the effect of fish-oil supplementation on erythrocyte sedimentation rate (ESR), a measure of inflammation.147,154 Neither found a significant effect of fish oil supplementation on ESR. Six out of seven studies that examined the effect of long-chain omega-3 fatty acid supplementation on nonsteroidal anti-inflammatory drug or corticosteroid use in patients with rheumatoid arthritis demonstrated a reduced requirement for anti-inflammatory medication.147
Inflammatory Bowel Disease
Clinical trials of long-chain omega-3 fatty acid supplementation have demonstrated beneficial effects less consistently in patients with inflammatory bowel disease than in patients with rheumatoid arthritis. Although two randomized controlled trials of fish oil supplementation in patients with Crohn disease reported no benefit,156,157 one randomized controlled trial found that a significantly higher proportion of patients with Crohn disease given supplements of 2.7 g/day of EPA + DHA remained in remission over a 12-month period than those given a placebo.158 A randomized controlled trial in 38 children (aged 5–16 years) with Crohn disease found that supplementation with omega-3 PUFA (1.2 g/day of EPA and 0.6 g/day of DHA), in addition to standard therapy with 5-aminosalicylic acid, significantly reduced the 1-year relapse rate.159 Three randomized controlled trials of EPA + DHA supplementation (4.2–5.4 g/day for 3–12 months) in patients with ulcerative colitis reported significant improvement in at least one outcome measure, including weight gain, decreased corticosteroid use, improved disease activity scores, and improved histology scores.160–162 In contrast, giving supplements of 5.1 g/day of EPA + DHA to patients with ulcerative colitis who were in remission did not significantly alter the incidence of relapse over a 2-year period.163 More research is necessary to determine whether long-chain omega-3 fatty acid supplementation has any therapeutic benefit in ulcerative colitis.164
Asthma
Inflammatory eicosanoids (leukotrienes) derived from AA (20:4n-6) are thought to play an important role in the pathology of asthma.17 Since increasing omega-3 fatty acid intake has been found to decrease the formation of AA-derived leukotrienes, several clinical trials have examined the effects of supplementation with long-chain omega-3 fatty acid on asthma. Although there is some evidence that omega-3 fatty acid supplementation can decrease the production of inflammatory mediators in patients with asthma,165,166 evidence that omega-3 fatty acid supplementation decreases the clinical severity of asthma in controlled trials has been inconsistent.167 Three systematic reviews of randomized controlled trials of long-chain omega-3 fatty acid supplementation in adults and children with asthma found no consistent effects on clinical outcome measures, including pulmonary function tests, symptoms of asthma, medication use, or bronchial hyperreactivity.168–170
Immunoglobulin A Nephropathy
Immunoglobulin A (IgA) nephropathy is a kidney disorder that results from the deposition of IgA in the glomeruli of the kidney. The cause of IgA nephropathy is not clear, but progressive renal failure may eventually develop in 15%–40% of patients.171 Since glomerular IgA deposition results in increased production of inflammatory mediators, omega-3 fatty acid supplementation could potentially modulate the inflammatory response and preserve renal function. In a multicenter, randomized controlled trial, giving supplements of fish oil to patients with IgA nephropathy (1.8 g/day of EPA + 1.2 g/day of DHA) for 2 years significantly slowed declines in renal function.172 Over the 2-year treatment period, 33% of the placebo group experienced a 50% increase in serum creatinine (i.e., evidence of declining renal function) compared with only 6% in the group given fish oil supplements. These results were sustained over an average of 6 years of follow-up,173 but improvements were not observed with higher doses of fish oil.174 A much smaller 2-year trial found that a low dose of omega-3 fatty acids (0.85 g/day of EPA + 0.57 g/day of DHA) slowed the progression of renal disease in high-risk IgA nephropathy patients.175 In contrast, several studies have failed to find a significant benefit of omega-3 PUFA supplementation in patients with IgA nephropathy.176–179 Interestingly, fish-oil treatment (3 g/day of EPA + DHA) for 6 months did not decrease the urinary excretion of inflammatory mediators in patients with IgA nephropathy.180 Two meta-analyses of randomized controlled trials of fish oil supplementation did not find evidence of a statistically significant benefit in patients with IgA nephropathy overall.181,182 Due to the inconsistent results of available randomized controlled trials, it is not clear whether fish oil supplementation will prevent the progression of IgA nephropathy in children or adults.147
Major Depression and Bipolar Disorder
Data from ecological studies across different countries suggest an inverse association between seafood consumption and national rates of major depression183 and bipolar disorder.184 Several small studies have found omega-3 fatty acid concentrations to be lower in the plasma185–187 and adipose tissue (fat)188 of individuals suffering from depression compared with controls. Although it is not known how omega-3 fatty acid intake affects the incidence of depression, modulation of neuronal signaling pathways and eicosanoid production have been proposed as possible mechanisms.189 The results of randomized controlled trials examining the effect of supplementation with long-chain omega-3 fatty acids on depression have been mixed. Adding fish oil supplements (8 g/day) to existing therapy in people who were being treated for depression was not significantly more effective than adding the same amount of olive oil for 12 weeks.190 In patients with diagnosed major depression, fish oil supplementation (2.2 g/day of DHA + 0.6 g/day of EPA) for 4 months did not provide any therapeutic benefit beyond that associated with standard therapy.191 In patients with mild to moderate depression, a lower dose (0.85 g/day of DHA + 0.63 g of EPA) was not effective when taken for 12 weeks.192 Supplementation with 2 g/day of DHA for 6 weeks was not significantly more effective than a placebo in the treatment of major depression.193 However, a small randomized controlled trial in Chinese patients diagnosed with major depression found that supplementation with 6.6 g/day of EPA + DHA for 8 weeks improved scores on the Hamilton Rating Scale for Depression compared with placebo.194 Another small randomized controlled trial in 30 women diagnosed with borderline personality disorder found that the 20 women randomized to treatment with 1 g/day of ethyl-EPA for 8 weeks experienced less severe depressive symptoms than the 10 women randomized to treatment with a placebo.195 Additionally, results of a recent pilot study suggest that omega-3 fatty acid supplementation may have utility in treating children with major depression.196
Unipolar depression and bipolar disorder are considered distinct psychiatric conditions, although major depression occurs in both. A randomized controlled trial that assessed the effects of high doses of EPA (6.2 g/day) + DHA (3.4 g/day) in patients with bipolar disorder found that those given EPA + DHA supplements had a significantly longer period of remission than those on an olive oil placebo over a 4-month period.197 Patients who took the EPA + DHA supplements also experienced less depression than those who took the placebo. However, one study found that patients who took 6 g/day of ethyl-EPA for 4 weeks did not experience any relief from bipolar depression.198 Lower doses of EPA may be more efficacious in treating bipolar disorder. A small study found that patients taking 1.5 g/day or 2 g/day of EPA for 6 months had some relief of depression associated with bipolar disorder.199 A 12-week, double-blind, placebo-controlled trial in individuals with bipolar depression found those who took either 1 g/day or 2 g/day of ethyl-EPA experienced significant improvements in symptoms of depression, but measures of mania were not significantly different in either group compared with the placebo group.200 Further, some recent meta-analyses of randomized controlled trials have concluded that omega-3 PUFA supplementation is beneficial in treating unipolar and bipolar depressive disorders.201,202 However, another systematic review and meta-analysis concluded that there is little indication for using omega-3 PUFA supplementation in depression.203 Large, long-term randomized controlled trials are required to determine the efficacy of long-chain omega-3 fatty acid supplementation on major depression and bipolar disorder.
Schizophrenia
Findings of decreased levels of omega-3 fatty acid in the red blood cells204,205 and brains206 of a limited number of patients with schizophrenia, together with the results of uncontrolled supplementation studies,207 have created interest in the use of long-chain omega-3 fatty acid supplements as an adjunct to conventional regimens of antipsychotic therapy for schizophrenia. A pilot study in 45 patients with schizophrenia found that the addition of 2 g/day of EPA to standard antipsychotic therapy was superior to the addition of 2 g/day of DHA or placebo in decreasing residual symptoms.208 When EPA supplementation was used as the sole treatment for patients with schizophrenia experiencing a relapse, eight out of 14 patients given 2 g/day of EPA supplements required antipsychotic medication by the end of the 12-week study period, compared with 12 out of 12 of those on the placebo.208 Results of randomized controlled trials using ethyl-EPA as an adjunct to standard antipsychotic therapy in patients with schizophrenia have been somewhat contradictory. In one trial, the addition of 3 g/day of ethyl-EPA to standard antipsychotic treatment for 12 weeks improved symptom scores and decreased dyskinesia scores209; a similar 12-week trial found that 2 g/day of ethyl-EPA did not benefit patients with schizophrenia and dyskinesia.210 In another trial, supplementation with 3 g/day of ethyl-EPA for 16 weeks was not different than placebo in improving symptoms, mood, or cognition.211 In a placebo-controlled trial comparing the addition of 1, 2, or 4 g/day of ethyl-EPA to different medication regimens, ethyl-EPA supplementation improved the symptoms of patients with schizophrenia on the antipsychotic medication clozapine but not those on other medications.212 Although limited evidence suggests that EPA supplementation may be a useful adjunct to antipsychotic therapy in patients with schizophrenia, larger long-term studies addressing clinically relevant outcomes are needed.213
Alzheimer Disease and Dementia
Some epidemiological studies have associated decreased DHA status with Alzheimer disease and other types of dementia (see the Alzheimer Disease and Dementia section in the Disease Prevention section above). Although the results of studies in animal models have been promising,214 it is not known whether DHA supplementation can help treat Alzheimer disease in humans. Recently, a randomized, double-blind, placebo-controlled trial in 295 patients with mild to moderate Alzheimer disease found that 2 g/day of DHA for 18 months had no cognitive benefit compared with placebo.215
Sources
Food Sources
Omega-6 Fatty Acids
Linoleic acid. Food sources of LA include vegetable oils, such as soybean, safflower, and corn oil, nuts, seeds, and some vegetables. Dietary surveys in the United States indicate that the average adult intake of LA ranges from 12 g/day to 17 g/day for men and 9 g/day to 11 g/day for women.1 Some foods that are rich in LA are listed in Table 20.1.216
Arachidonic acid. Animals, but not plants, can convert LA to AA. Therefore, AA is present in small amounts in meat, poultry, and eggs.
Omega-3 Fatty Acids
α-Linolenic acid. Flaxseeds, walnuts, and their oils are among the richest dietary sources of ALA. Canola oil is also an excellent source of ALA. Dietary surveys in the United States indicate that average adult intakes for ALA range from 1.2 g/day to 1.6 g/day for men and from 0.9 g/day to 1.1 g/day for women.1 Some foods that are rich in ALA are listed in Table 20.2.216
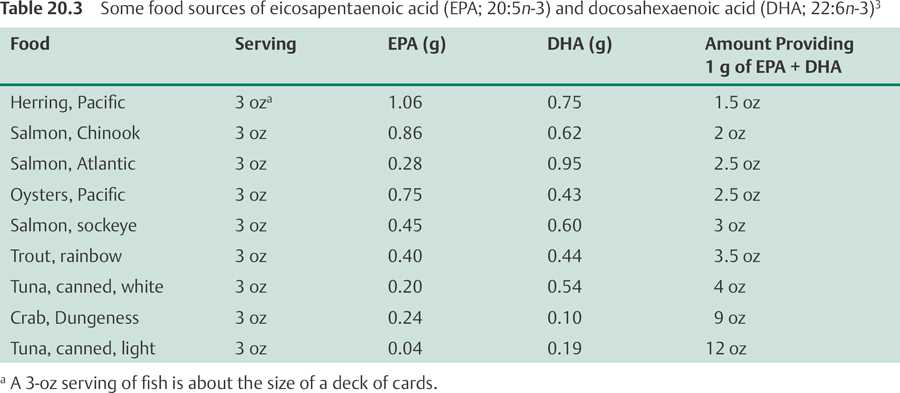
Eicosapentaenoic acid and docosahexaenoic acid. Oily fish are the major dietary source of EPA and DHA. Dietary surveys in the United States indicate that average adult intakes of EPA range from 0.04 g/day to 0.07 g/day and average adult intakes of DHA range from 0.05 g/day to 0.09 g/day.1 Eggs enriched with omega-3 fatty acid are also available in the United States. Some foods that are rich in EPA and DHA are listed in Table 20.3.216
Table 20.1 Some food sources of linoleic acid (18:2n-6)216
Food |
Serving |
Linoleic Acid (g) |
Safflower oil |
1 tbs |
10.1 |
Sunflower seeds, oil roasted |
1 oz |
9.7 |
Pine nuts |
1 oz |
9.4 |
Sunflower oil |
1 tbs |
8.9 |
Corn oil |
1 tbs |
7.3 |
Soybean oil |
1 tbs |
0.9 |
Pecans, oil roasted |
1 oz |
6.4 |
Brazil nuts |
1 oz |
5.8 |
Sesame oil |
1 tbs |
5.6 |
Biosynthesis
Humans can synthesize AA from LA and EPA and DHA from ALA through a series of desaturation and elongation reactions (see the Bioavailability and Metabolism section above).
Supplements
Omega-6 Fatty Acids
Borage seed oil, evening primrose oil, and blackcurrant seed oil are rich in γ-linolenic acid (GLA) and are often marketed as GLA or essential fatty acid (EFA) supplements.217
Omega-3 Fatty Acids
Flaxseed oil (also known as flax oil or linseed oil) is available as an ALA supplement. Several fish oils are marketed as omega-3 fatty acid supplements. Ethyl esters of EPA and DHA (ethyl-EPA and ethyl-DHA) are concentrated sources of long-chain omega-3 fatty acids. Since EPA and DHA content will vary in fish-oil and ethyl-ester preparations, it is necessary to read the label to determine the EPA and DHA content of a particular supplement. DHA supplements derived from algal and fungal sources are also available. All omega-3 fatty acid supplements are absorbed more efficiently with meals. Dividing one's daily dose into two or three smaller doses throughout the day will decrease the risk of gastrointestinal side effects (see the Safety section below). Cod liver oil is a rich source of EPA and DHA, but some cod liver oil preparations may contain excessive amounts of preformed vitamin A (retinol).217
Table 20.2 Some food sources of α-linolenic acid (18:3n-3)216
Food |
Serving |
α-Linolenic Acid (g) |
Flaxseed oil |
1 tbs |
7.3 |
Walnuts, English |
1 oz |
2.6 |
Flaxseeds, ground |
1 tbs |
1.6 |
Walnut oil |
1 tbs |
1.4 |
Canola oil |
1 tbs |
1.3 |
Soybean oil |
1 tbs |
6.9 |
Mustard oil |
1 tbs |
0.8 |
Tofu, firm |
½ cup |
0.7 |
Walnuts, black |
1 oz |
0.6 |
Infant Formula
In 2001, the US Food and Drug Administration (FDA) began permitting the addition of DHA and AA to infant formula in the United States.218 At present, manufacturers are not required to list the amounts of DHA and AA added to infant formula on the label. However, most manufacturers of infant formula provide this information. The amounts added to formulas in the United States range from 8 mg to 17 mg DHA/100 calories (5 fl oz) and from 16 mg to 34 mg AA/100 calories. For example, an infant drinking 20 fl oz of DHA-enriched formula daily would receive 32–68 mg/day of DHA and 64–136 mg/day of AA.
Safety
Adverse Effects
γ-Linolenic Acid (18:3n-6)
Supplemental GLA is generally well tolerated, and serious adverse side effects have not been observed at doses up to 2.8 g/day for 12 months.219 High doses of borage seed oil, evening primrose oil, or blackcurrant seed oil may cause gastrointestinal upset, loose stools, or diarrhea.217 Because of case reports that supplementation with evening primrose oil induced seizure activity in people with undiagnosed temporal lobe epilepsy,220 people with a history of seizures or seizure disorder are generally advised to avoid evening primrose oil and other GLA-rich oils.217
α-Linolenic Acid (18:3n-3)
Although flaxseed oil is generally well tolerated, high doses may cause loose stools or diarrhea.221 Allergic and anaphylactic reactions have been reported with ingestion of flaxseed and flaxseed oil.222
Eicosapentaenoic Acid (20:5n-3) and Docosahexaenoic Acid (22:6n-3)
Serious adverse reactions have not been reported in those using fish-oil or other EPA and DHA supplements. The most common adverse effect of fish oil or EPA and DHA supplements is a fishy aftertaste. Belching and heartburn have also been reported. Additionally, high doses may cause nausea and loose stools.
Potential for excessive bleeding. The potential for high omega-3 fatty acid intakes, especially EPA and DHA, to prolong bleeding times has been well studied and may play a role in the cardioprotective effects of omega-3 fatty acids. Although excessively long bleeding times and increased incidence of hemorrhagic stroke have been observed in Greenland Eskimos with very high intakes of EPA + DHA (6.5 g/day), it is not known whether high intakes of EPA and DHA are the only factor responsible for these observations.1 The FDA has ruled that intakes up to 3 g/day of long-chain omega-3 fatty acids (EPA and DHA) are generally recognized as safe (GRAS) for inclusion in the diet, and available evidence suggests that intakes below 3 g/day are unlikely to result in clinically significant bleeding.3 Although the Institute of Medicine did not establish a tolerable upper intake level for omega-3 fatty acids, caution was advised with the use of supplemental EPA and DHA, especially in those who are at increased risk of excessive bleeding (see the Drug Interactions and Nutrient Interactions sections below).1
Potential for suppression of the immune system. Although the suppression of inflammatory responses resulting from increased omega-3 fatty acid intakes may benefit individuals with inflammatory or autoimmune diseases, anti-inflammatory doses of omega-3 fatty acids could decrease the potential of the immune system to destroy pathogens.223 Studies comparing measures of immune-cell function outside the body (ex vivo) at baseline and after giving supplements of omega-3 fatty acids, mainly EPA and DHA, have demonstrated immunosuppressive effects at doses as low as 0.9 g/day for EPA and 0.6 g/day for DHA.1 Although it is not clear if these findings translate to impaired immune responses in vivo, caution should be observed when considering omega-3 fatty acid supplementation in individuals with compromised immune systems.
Infant Formula
In early studies of DHA-enriched infant formula, EPA- and DHA-rich fish oil was used as a source of DHA. However, some preterm infants receiving formula enriched with fish oil had decreased plasma AA concentrations, which were associated with decreased growth.224 This effect was attributed to the potential for high concentrations of EPA to interfere with the synthesis of AA, which is essential for normal growth. Consequently, EPA was removed and AA was added to DHA-enriched formula. Currently available infant formulas in the United States contain only AA and DHA derived from algal or fungal sources, rather than from fish oil. Randomized controlled trials have not found any adverse effects on growth in infants fed formulas enriched with AA and DHA for up to 1 year.36,37
Pregnancy and Lactation
The safety of supplemental omega-3 and omega-6 fatty acids, including borage seed oil, evening primrose oil, blackcurrant seed oil, and flaxseed oil, has not been established in pregnant or lactating women.217 Studies of fish oil supplementation during pregnancy and lactation have not reported any serious adverse effects (see the Contaminants in Fish and Contaminants in Supplements sections below).
Contaminants in Fish
Some species of fish may contain significant levels of methylmercury, polychlorinated biphenyls (PCBs), or other environmental contaminants.61 In general, larger predatory fish, such as swordfish, tend to contain the highest levels of these contaminants. Removing the skin, fat, and internal organs of the fish prior to cooking and allowing the fat to drain from the fish while it cooks will decrease exposure to several fat-soluble pollutants, such as PCBs.225 However, methylmercury is found throughout the muscle of fish, so these cooking precautions will not reduce exposure to methylmercury. Organic mercury compounds are toxic, and excessive exposure can cause brain and kidney damage. The developing fetus, infants, and young children are especially vulnerable to the toxic effects of mercury on the brain. To limit their exposure to methylmercury, the US Department of Health and Human Services (DHHS) and Environmental Protection Agency (EPA) have made the following joint recommendations for women who may become pregnant, pregnant women, and breast-feeding women170:
1. Do not eat shark, swordfish, king mackerel, or tile fish (also known as golden bass or golden snapper) because they contain high methylmercury levels.
2. Eat up to 12 oz (two average meals) per week of a variety of fish that are lower in mercury.
a) The five most commonly consumed fish that are low in mercury include canned light tuna, shrimp, salmon, catfish, and pollock.
b) Limit the consumption of canned white (albacore) tuna and tuna steak to 6 oz (one average meal) per week.
3. Check local advisories regarding the safety of fish caught by friends or family in local lakes, rivers, and coastal areas.
When feeding fish to young children, the DHHS and the EPA advise following the above guidelines but serving smaller portions, such as 3 oz, for an average meal.
Contaminants in Supplements
Although concerns have been raised regarding the potential for omega-3 fatty acid supplements derived from fish oil to contain methylmercury, PCBs, and dioxins, several independent laboratory analyses in the United States have found commercially available omega-3 fatty acid supplements to be free of methylmercury, PCBs, and dioxins.226–228 The absence of methylmercury in omega-3 fatty acid supplements can be explained by the fact that mercury accumulates in the muscle, rather than the fat of fish.3 In general, fish body oils contain lower levels of PCBs and other fat-soluble contaminants than fish liver oils. Additionally, fish oils that have been more highly refined and deodorized also contain lower levels of PCBs.229 Pyrrolizidine alkaloids, potentially hepatotoxic and carcinogenic compounds, are found in various parts of the borage plant. People who take borage oil supplements should use products that are certified free of pyrrolizidine alkaloids.217
Drug Interactions
γ-Linolenic acid supplements, such as evening primrose oil or borage seed oil, may increase the risk of seizures in people on phenothiazines, such as chlorpromazine.220 High doses of blackcurrant seed oil, borage seed oil, evening primrose oil, flaxseed oil, and fish oil may inhibit platelet aggregation; therefore, these supplements should be used with caution in people on anticoagulant medications. In particular, people taking supplements of fish oil or long-chain omega-3 fatty acid (EPA and DHA) supplementation in combination with anticoagulant drugs, including aspirin, clopidogrel (Plavix), dalteparin (Fragmin), dipyridamole (Persantin), enoxaparin (Lovenox), heparin, ticlopidine (Ticlid), and warfarin (Coumadin), should have their coagulation status monitored using a standardized prothrombin time assay (international normalized ratio—INR). One small study found that 3 g/day or 6 g/day of fish oil did not affect INR values in 10 patients on warfarin over a 4-week period.230 However, a case report described an individual who required a reduction of her warfarin dose when she doubled her dose of fish oil from 1 g/day to 2 g/day.231
Nutrient Interactions
Vitamin E
Outside the body, PUFAs become rancid (oxidized) more easily than SFAs. Fat-soluble antioxidants, such as vitamin E, play an important role in preventing the oxidation of PUFAs. Inside the body, results of animal studies and limited data in humans suggest that the amount of vitamin E required to prevent lipid peroxidation increases with the amount of PUFA consumed.232 One widely used recommendation for vitamin E intake is 0.6 mg of α-tocopherol per gram of dietary PUFA. This recommendation was based on a small study in men and the ratio of α-tocopherol to LA in the US diet, and has not been verified in more comprehensive studies. Although EPA and DHA are easily oxidized outside the body, it is presently unclear whether EPA and DHA are more susceptible to oxidative damage within the body.233 High vitamin E intakes have not been found to decrease biomarkers of oxidative damage when EPA and DHA intakes are increased,234,235 but some experts believe that an increase in PUFA intake, particularly omega-3 PUFA intake, should be accompanied by an increase in vitamin E intake.1
Intake Recommendations
US Institute of Medicine
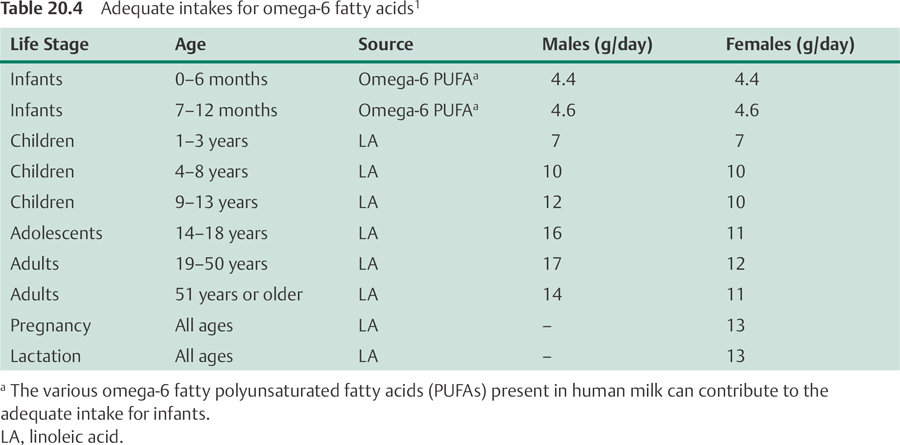
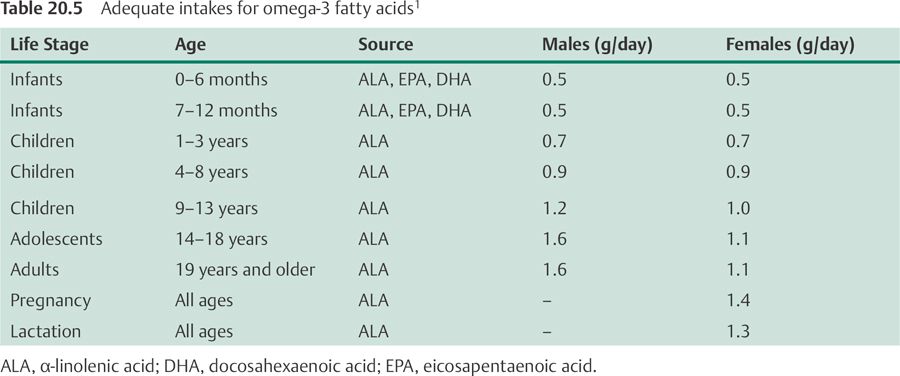
In 2002, the Food and Nutrition Board of the US Institute of Medicine established adequate intakes (AIs) for omega-6 and omega-3 fatty acids, which are listed in Tables 20.4 and 20.5, respectively.1
International Recommendations
The European Commission recommends an omega-6 fatty acid intake of 4%–8% of energy and an omega-3 fatty acid intake of 2 g/day of ALA and 200 mg/day of long-chain omega-3 fatty acids (EPA and DHA).236 The World Health Organization recommends an omega-6 fatty acid intake of 5%–8% of energy and an omega-3 fatty acid intake of 1%–2% of energy.147 However, the Japan Society for Lipid Nutrition has recommended that LA intake be reduced to 3%–4% of energy in Japanese people whose omega-3 fatty acid intakes average 2.6 g/day, including approximately 1 g/day of EPA + DHA.237
American Heart Association
The American Heart Association recommends that people without documented CHD eat a variety of fish (preferably oily) at least twice weekly, in addition to consuming oils and foods rich in ALA.143 Pregnant women and children should avoid fish that typically have higher levels of methylmercury (see the Contaminants in Fish section above). People with documented CHD are advised to consume approximately 1 g/day of EPA + DHA, preferably from oily fish, or to consider EPA + DHA supplements in consultation with a physician. Patients who need to lower their serum triglycerides may take 2–4 g/day of EPA + DHA supplements under a physician's care.143
Summary
• α-Linolenic acid (ALA), an omega-3 fatty acid, and linoleic acid (LA), an omega-6 fatty acid, are considered essential fatty acids because they cannot be synthesized by humans.
• The long-chain omega-6 fatty acid, arachidonic acid (AA), can be synthesized from LA.
• The long-chain omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), can be synthesized from ALA, but synthesis of EPA and DHA may be insufficient under certain conditions.
• Typical Western diets tend to be much higher in omega-6 fatty acids than omega-3 fatty acids.
• While DHA appears to be important for visual and neurological development, it is not yet clear whether feeding infants formula enriched with DHA and AA enhances visual acuity or neurological development in preterm or term infants.
• A large body of scientific research suggests that higher dietary omega-3 fatty acid intakes are associated with reductions in cardiovascular disease risk. Thus, the American Heart Association recommends that all adults eat fish, particularly oily fish, at least twice a week.
• The results of randomized controlled trials indicate that increasing omega-3 fatty acid intake can decrease the risk of myocardial infarction (heart attack) and sudden cardiac death in individuals with coronary heart disease.
• Low DHA status may be a risk factor for Alzheimer disease and other types of dementia, but it is not yet known whether DHA supplementation can help prevent or treat such cognitive disorders.
• Increasing EPA and DHA intake may be beneficial in individuals with type 2 diabetes, especially those with elevated serum triglycerides.
• Randomized controlled trials have found that fish oil supplementation decreases joint tenderness and reduces the requirement for anti-inflammatory medication in patients with rheumatoid arthritis.
• Although limited preliminary data suggest that omega-3 fatty acid supplementation may be beneficial in the therapy of depression, bipolar disorder, and schizophrenia, larger controlled clinical trials are needed to determine the therapeutic efficacy.
References
1. Food and Nutrition Board, Institute of Medicine. Dietary Fats: Total Fat and Fatty Acids. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: National Academies Press; 2002: 422–541
2. Simopoulos AP, Leaf A, Salem N Jr. Workshop statement on the essentiality of and recommended dietary intakes for Omega-6 and Omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids 2000; 63(3):119–121
3. Kris-Etherton PM, Harris WS, Appel LJ; American Heart Association. Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002;106(21):2747–2757
4. Lichtenstein AH, Jones PJ. Lipids: absorption and transport. In: Bowman BA, Russel RM, eds. Present Knowledge in Nutrition. 8th ed. Washington, DC: ILSI Press; 2001:93–103
5. Nakamura MT, Nara TY. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu Rev Nutr 2004;24:345–376
6. Burdge G. Alpha-linolenic acid metabolism in men and women: nutritional and biological implications. Curr Opin Clin Nutr Metab Care 2004;7(2):137–144
7. Burdge GC, Jones AE, Wootton SA. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men*. Br J Nutr 2002;88(4):355–363
8. Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr 2002;88(4):411–420
9. Giltay EJ, Gooren LJ, Toorians AW, Katan MB, Zock PL. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am J Clin Nutr 2004;80(5):1167–1174
10. Muskiet FA, Fokkema MR, Schaafsma A, Boersma ER, Crawford MA. Is docosahexaenoic acid (DHA) essential? Lessons from DHA status regulation, our ancient diet, epidemiology and randomized controlled trials. J Nutr 2004;134(1):183–186
11. Cunnane SC. Problems with essential fatty acids: time for a new paradigm? Prog Lipid Res 2003;42(6):544–568
12. Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids 2003;126(1):1–27
13. Jeffrey BG, Weisinger HS, Neuringer M, Mitchell DC. The role of docosahexaenoic acid in retinal function. Lipids 2001;36(9):859–871
14. SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res 2005;24(1):87–138
15. Innis SM. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J Pediatr 2003; 143(4, Suppl):S1–S8
16. Chalon S, Vancassel S, Zimmer L, Guilloteau D, Durand G. Polyunsaturated fatty acids and cerebral function: focus on monoaminergic neurotransmission. Lipids 2001;36(9):937–944
17. Calder PC. Dietary modification of inflammation with lipids. Proc Nutr Soc 2002;61(3):345–358
18. Price PT, Nelson CM, Clarke SD. Omega-3 polyunsaturated fatty acid regulation of gene expression. Curr Opin Lipidol 2000;11(1):3–7
19. Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of gene expression. Nutr Rev 2004;62(9): 333–339
20. Jump DB. Fatty acid regulation of gene transcription. Crit Rev Clin Lab Sci 2004;41(1):41–78
21. Jump DB, Botolin D, Wang Y, Xu J, Demeure O, Christian B. Docosahexaenoic acid (DHA) and hepatic gene transcription. Chem Phys Lipids 2008;153(1):3–13
22. Jump DB. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr Opin Lipidol 2008; 19(3):242–247
23. Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr 2005;135(11):2503–2506
24. Jeppesen PB, Høy CE, Mortensen PB. Essential fatty acid deficiency in patients receiving home parenteral nutrition. Am J Clin Nutr 1998;68(1):126–133
25. Smit EN, Muskiet FA, Boersma ER. The possible role of essential fatty acids in the pathophysiology of malnutrition: a review. Prostaglandins Leukot Essent Fatty Acids 2004;71(4):241–250
26. Mascioli EA, Lopes SM, Champagne C, Driscoll DF. Essential fatty acid deficiency and home total parenteral nutrition patients. Nutrition 1996;12(4):245–249
27. Stegink LD, Freeman JB, Wispe J, Connor WE. Absence of the biochemical symptoms of essential fatty acid deficiency in surgical patients undergoing protein sparing therapy. Am J Clin Nutr 1977;30(3):388–393
28. Jeppesen PB, Høy CE, Mortensen PB. Deficiencies of essential fatty acids, vitamin A and E and changes in plasma lipoproteins in patients with reduced fat absorption or intestinal failure. Eur J Clin Nutr 2000; 54(8):632–642
29. Lepage G, Levy E, Ronco N, Smith L, Galéano N, Roy CC. Direct transesterification of plasma fatty acids for the diagnosis of essential fatty acid deficiency in cystic fibrosis. J Lipid Res 1989;30(10):1483–1490
30. Holman RT, Johnson SB, Hatch TF. A case of human linolenic acid deficiency involving neurological abnormalities. Am J Clin Nutr 1982;35(3):617–623
31. Fedorova I, Hussein N, Baumann MH, Di Martino C, Salem N Jr. An n-3 fatty acid deficiency impairs rat spatial learning in the Barnes maze. Behav Neurosci 2009;123(1):196–205
32. Fedorova I, Salem N Jr. Omega-3 fatty acids and rodent behavior. Prostaglandins Leukot Essent Fatty Acids 2006;75(4–5):271–289
33. Uauy R, Hoffman DR, Peirano P, Birch DG, Birch EE. Essential fatty acids in visual and brain development. Lipids 2001;36(9):885–895
34. Larque E, Demmelmair H, Koletzko B. Perinatal supply and metabolism of long-chain polyunsaturated fatty acids: importance for the early development of the nervous system. Ann N Y Acad Sci 2002;967:299–310
35. Uauy R, Hoffman DR, Mena P, Llanos A, Birch EE. Term infant studies of DHA and A.R.A supplementation on neurodevelopment: results of randomized controlled trials. J Pediatr 2003; 143(4, Suppl):S17–S25
36. Simmer K, Patole S. Longchain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database Syst Rev 2004; (1):CD000375
37. Simmer K. Longchain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst Rev 2001; (4):CD000376
38. Eilander A, Hundscheid DC, Osendarp SJ, Transler C, Zock PL. Effects of n-3 long chain polyunsaturated fatty acid supplementation on visual and cognitive development throughout childhood: a review of human studies. Prostaglandins Leukot Essent Fatty Acids 2007;76(4):189–203
39. SanGiovanni JP, Parra-Cabrera S, Colditz GA, Berkey CS, Dwyer JT. Meta-analysis of dietary essential fatty acids and long-chain polyunsaturated fatty acids as they relate to visual resolution acuity in healthy preterm infants. Pediatrics 2000;105(6):1292–1298
40. O'Connor DL, Hall R, Adamkin D, et al; Ross Preterm Lipid Study. Growth and development in preterm infants fed long-chain polyunsaturated fatty acids: a prospective, randomized controlled trial. Pediatrics 2001;108(2):359–371
41. Fewtrell MS, Morley R, Abbott RA, et al. Double-blind, randomized trial of long-chain polyunsaturated fatty acid supplementation in formula fed to preterm infants. Pediatrics 2002;110(1 Pt 1):73–82
42. Fewtrell MS, Abbott RA, Kennedy K, et al. Randomized, double-blind trial of long-chain polyunsaturated fatty acid supplementation with fish oil and borage oil in preterm infants. J Pediatr 2004;144(4):471–479
43. Clandinin MT, Van Aerde JE, Merkel KL, et al. Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. J Pediatr 2005;146(4):461–468
44. Birch EE, Castañeda YS, Wheaton DH, Birch DG, Uauy RD, Hoffman DR. Visual maturation of term infants fed long-chain polyunsaturated fatty acid-supplemented or control formula for 12 mo. Am J Clin Nutr 2005;81(4):871–879
45. Auestad N, Scott DT, Janowsky JS, et al. Visual, cognitive, and language assessments at 39 months: a follow-up study of children fed formulas containing long-chain polyunsaturated fatty acids to 1 year of age. Pediatrics 2003;112(3 Pt 1):e177–e183
46. Gibson RA, Chen W, Makrides M. Randomized trials with polyunsaturated fatty acid interventions in preterm and term infants: functional and clinical outcomes. Lipids 2001;36(9):873–883
47. Birch EE, Garfield S, Castañeda Y, Hughbanks-Wheaton D, Uauy R, Hoffman D. Visual acuity and cognitive outcomes at 4 years of age in a double-blind, randomized trial of long-chain polyunsaturated fatty acid-supplemented infant formula. Early Hum Dev 2007;83(5):279–284
48. McCann JC, Ames BN. Is docosahexaenoic acid, an n-3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am J Clin Nutr 2005;82(2):281–295
49. Koo WW. Efficacy and safety of docosahexaenoic acid and arachidonic acid addition to infant formulas: can one buy better vision and intelligence? J Am Coll Nutr 2003;22(2):101–107
50. Makrides M, Gibson RA. Long-chain polyunsaturated fatty acid requirements during pregnancy and lactation. Am J Clin Nutr 2000; 71(1, Suppl):307S–311S
51. Onwude JL, Lilford RJ, Hjartardottir H, Staines A, Tuff-nell D. A randomised double blind placebo controlled trial of fish oil in high risk pregnancy. Br J Obstet Gynaecol 1995;102(2):95–100
52. Olsen SF, Sørensen JD, Secher NJ, et al. Randomised controlled trial of effect of fish-oil supplementation on pregnancy duration. Lancet 1992;339(8800):1003–1007
53. Smuts CM, Huang M, Mundy D, Plasse T, Major S, Carlson SE. A randomized trial of docosahexaenoic acid supplementation during the third trimester of pregnancy. Obstet Gynecol 2003;101(3):469–479
54. Szajewska H, Horvath A, Koletzko B. Effect of n-3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2006;83(6):1337–1344
55. Olsen SF, Secher NJ, Tabor A, Weber T, Walker JJ, Gluud C; Fish Oil Trials In Pregnancy (FOTIP) Team. Randomised clinical trials of fish oil supplementation in high risk pregnancies. BJOG 2000;107(3):382–395
56. Horvath A, Koletzko B, Szajewska H. Effect of supplementation of women in high-risk pregnancies with long-chain polyunsaturated fatty acids on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Br J Nutr 2007;98(2):253–259
57. Koletzko B, Lien E, Agostoni C, et al; World Association of Perinatal Medicine Dietary Guidelines Working Group. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med 2008;36(1):5–14
58. Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's I.Q. at 4 years of age. Pediatrics 2003;111(1):e39–e44
59. Dunstan JA, Simmer K, Dixon G, Prescott SL. Cognitive assessment of children at age 2(1/2) years after maternal fish oil supplementation in pregnancy: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2008;93(1):F45–F50
60. Judge MP, Harel O, Lammi-Keefe CJ. Maternal consumption of a docosahexaenoic acid-containing functional food during pregnancy: benefit for infant performance on problem-solving but not on recognition memory tasks at age 9 mo. Am J Clin Nutr 2007; 85(6):1572–1577
61. Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 2006;296(15):1885–1899
62. Kris-Etherton PM, Hecker KD, Binkoski AE. Polyunsaturated fatty acids and cardiovascular health. Nutr Rev 2004;62(11):414–426
63. Shekelle RB, Shryock AM, Paul O, et al. Diet, serum cholesterol, and death from coronary heart disease. The Western Electric study. N Engl J Med 1981; 304(2):65–70
64. Oh K, Hu FB, Manson JE, Stampfer MJ, Willett WC. Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the nurses’ health study. Am J Epidemiol 2005;161(7):672–679
65. Ascherio A, Rimm EB, Giovannucci EL, Spiegelman D, Stampfer M, Willett WC. Dietary fat and risk of coronary heart disease in men: cohort follow up study in the United States. BMJ 1996;313(7049):84–90
66. Laaksonen DE, Nyyssönen K, Niskanen L, Rissanen TH, Salonen JT. Prediction of cardiovascular mortality in middle-aged men by dietary and serum linoleic and polyunsaturated fatty acids. Arch Intern Med 2005; 165(2):193–199
67. Sacks FM, Katan M. Randomized clinical trials on the effects of dietary fat and carbohydrate on plasma lipoproteins and cardiovascular disease. Am J Med 2002;113(Suppl 9B):13S–24S
68. Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb 1992;12(8):911–919
69. Lewis B, Krikler D. Controlled trial of soya-bean oil in myocardial infarction. Lancet 1968;2(7570):693–699
70. Dayton S, Pearce ML, Goldman H, et al. Controlled trial of a diet high in unsaturated fat for prevention of atherosclerotic complications. Lancet 1968;2(7577): 1060–1062
71. Leren P. The Oslo diet-heart study. Eleven-year report. Circulation 1970;42(5):935–942
72. Turpeinen O, Karvonen MJ, Pekkarinen M, Miettinen M, Elosuo R, Paavilainen E. Dietary prevention of coronary heart disease: the Finnish Mental Hospital Study. Int J Epidemiol 1979;8(2):99–118
73. Frantz ID Jr, Dawson EA, Ashman PL, et al. Test of effect of lipid lowering by diet on cardiovascular risk. The Minnesota Coronary Survey. Arteriosclerosis 1989;9(1):129–135
74. Miettinen M, Turpeinen O, Karvonen MJ, Pekkarinen M, Paavilainen E, Elosuo R. Dietary prevention of coronary heart disease in women: the Finnish mental hospital study. Int J Epidemiol 1983;12(1):17–25
75. Harris WS, Mozaffarian D, Rimm E, et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 2009;119(6):902–907
76. Mozaffarian D, Ascherio A, Hu FB, et al. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation 2005;111(2):157–164
77. Hu F.B., Stampfer MJ, Manson J.E., et al. Dietary intake of alpha-linolenic acid and risk of fatal ischemic heart disease among women. Am J Clin Nutr 1999;69(5): 890–897
78. Dolecek TA. Epidemiological evidence of relationships between dietary polyunsaturated fatty acids and mortality in the multiple risk factor intervention trial. Proc Soc Exp Biol Med 1992;200(2):177–182
79. Pietinen P, Ascherio A, Korhonen P, et al. Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Epidemiol 1997;145(10):876–887
80. Oomen CM, Ocké MC, Feskens EJ, Kok FJ, Kromhout D. alpha-Linolenic acid intake is not beneficially associated with 10-y risk of coronary artery disease incidence: the Zutphen Elderly Study. Am J Clin Nutr 2001;74(4):457–463
81. Albert CM, Oh K, Whang W, et al. Dietary alpha-linolenic acid intake and risk of sudden cardiac death and coronary heart disease. Circulation 2005;112(21): 3232–3238
82. Mozaffarian D. Does alpha-linolenic acid intake reduce the risk of coronary heart disease? A review of the evidence. Altern Ther Health Med 2005;11(3):24–30, quiz 31, 79
83. Wendland E, Farmer A, Glasziou P, Neil A. Effect of alpha linolenic acid on cardiovascular risk markers: a systematic review. Heart 2006;92(2):166–169
84. Bemelmans WJ, Lefrandt JD, Feskens EJ, et al. Increased alpha-linolenic acid intake lowers C-reactive protein, but has no effect on markers of atherosclerosis. Eur J Clin Nutr 2004;58(7):1083–1089
85. Rallidis LS, Paschos G, Liakos GK, Velissaridou AH, Anastasiadis G, Zampelas A. Dietary alpha-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients. Atherosclerosis 2003;167(2):237–242
86. Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr 2004;134(11):2991–2997
87. Kris-Etherton PM, Harris WS, Appel LJ; AHA Nutrition Committee. American Heart Association. Omega-3 fatty acids and cardiovascular disease: new recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol 2003;23(2):151–152
88. Wang C, Harris WS, Chung M, et al. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr 2006;84(1):5–17
89. Hooper L, Thompson RL, Harrison RA, et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ 2006; 332(7544):752–760
90. Kromhout D, Bosschieter EB, de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med 1985;312(19):1205–1209
91. Kromhout D, Feskens EJ, Bowles CH. The protective effect of a small amount of fish on coronary heart disease mortality in an elderly population. Int J Epidemiol 1995;24(2):340–345
92. Dolecek TA, Granditis G. Dietary polyunsaturated fatty acids and mortality in the Multiple Risk Factor Intervention Trial (MRFIT). World Rev Nutr Diet 1991;66:205–216
93. Daviglus ML, Stamler J, Orencia AJ, et al. Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med 1997;336(15):1046–1053
94. Yuan JM, Ross RK, Gao YT, Yu MC. Fish and shellfish consumption in relation to death from myocardial infarction among men in Shanghai, China. Am J Epidemiol 2001;154(9):809–816
95. Hu FB, Bronner L, Willett WC, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA 2002;287(14):1815–1821
96. Järvinen R, Knekt P, Rissanen H, Reunanen A. Intake of fish and long-chain n-3 fatty acids and the risk of coronary heart mortality in men and women. Br J Nutr 2006;95(4):824–829
97. Iso H, Kobayashi M, Ishihara J, et al; JPHC. Study Group. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: the Japan Public Health Center-Based (JPHC.) Study Cohort I. Circulation 2006;113(2):195–202
98. Nakamura Y, Ueshima H, Okamura T, et al; NIPPON DATA80 Research Group. Association between fish consumption and all-cause and cause-specific mortality in Japan: NIPPON DATA80, 1980–99. Am J Med 2005;118(3):239–245
99. Leaf A, Xiao YF, Kang JX, Billman GE. Prevention of sudden cardiac death by n-3 polyunsaturated fatty acids. Pharmacol Ther 2003;98(3):355–377
100. Albert C.M., Hennekens C.H., O'Donnell C.J., et al. Fish consumption and risk of sudden cardiac death. JAMA 1998;279(1):23–28
101. Albert C.M., Campos H, Stampfer MJ, et al. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med 2002;346(15):1113–1118
102. Mozaffarian D. Fish and n-3 fatty acids for the prevention of fatal coronary heart disease and sudden cardiac death. Am J Clin Nutr 2008;87(6):1991S–1996S
103. Leaf A, Albert CM, Josephson M, et al; Fatty Acid Antiarrhythmia Trial Investigators. Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation 2005;112(18):2762–2768
104. Raitt MH, Connor WE, Morris C, et al. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: a randomized controlled trial. JAMA 2005;293(23):2884–2891
105. Brouwer IA, Zock PL, Camm AJ, et al; SOFA Study Group. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: the Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized trial. JAMA 2006;295(22):2613–2619
106. Jenkins DJ, Josse AR, Beyene J, et al. Fish-oil supplementation in patients with implantable cardioverter defibrillators: a meta-analysis. CMAJ 2008; 178(2): 157–164
107. London B, Albert C, Anderson ME, et al. Omega-3 fatty acids and cardiac arrhythmias: prior studies and recommendations for future research: a report from the National Heart, Lung, and Blood Institute and Office Of Dietary Supplements Omega-3 Fatty Acids and their Role in Cardiac Arrhythmogenesis Workshop. Circulation 2007;116(10):e320–e335
108. American Stroke Association. Types of Stroke. Available at: http://www.strokeassociation.org/STROKEORG/AboutStroke/Types-of-Stroke_UCM_308531_SubHomePage.jsp. Accessed May 8, 2012
109. Keli SO, Feskens EJ, Kromhout D. Fish consumption and risk of stroke. The Zutphen Study. Stroke 1994;25(2):328–332
110. Gillum R.F., Mussolino ME, Madans JH; The NHANES I Epidemiologic Follow-up Study (National Health and Nutrition Examination Survey). The relationship between fish consumption and stroke incidence. Arch Intern Med 1996;156(5):537–542
111. Morris MC, Manson JE, Rosner B, Buring JE, Willett WC, Hennekens CH. Fish consumption and cardiovascular disease in the physicians’ health study: a prospective study. Am J Epidemiol 1995;142(2):166–175
112. Orencia AJ, Daviglus ML, Dyer AR, Shekelle RB, Stamler J. Fish consumption and stroke in men. 30-year findings of the Chicago Western Electric Study. Stroke 1996;27(2):204–209
113. Myint PK, Welch AA, Bingham SA, et al. Habitual fish consumption and risk of incident stroke: the European Prospective Investigation into Cancer (EPIC)-Norfolk prospective population study. Public Health Nutr 2006;9(7):882–888
114. Iso H, Rexrode KM, Stampfer MJ, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA 2001;285(3):304–312
115. He K, Rimm EB, Merchant A, et al. Fish consumption and risk of stroke in men. JAMA 2002;288(24):3130–3136
116. He K, Song Y, Daviglus ML, et al. Fish consumption and incidence of stroke: a meta-analysis of cohort studies. Stroke 2004;35(7):1538–1542
117. Tanaka K, Ishikawa Y, Yokoyama M, et al; JELIS Investigators, Japan. Reduction in the recurrence of stroke by eicosapentaenoic acid for hypercholesterolemic patients: subanalysis of the JELIS trial. Stroke 2008;39(7):2052–2058
118. Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol 1998;81(4A):7B–12B
119. Harris WS. n-3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr 1997; 65(5, Suppl):1645S–1654S
120. Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis 2006;189(1):19–30
121. Harris WS, Kris-Etherton PM, Harris KA. Intakes of long-chain omega-3 fatty acid associated with reduced risk for death from coronary heart disease in healthy adults. Curr Atheroscler Rep 2008;10(6): 503–509
122. Harris WS, Mozaffarian D, Lefevre M, et al. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J Nutr 2009; 139(4):804S–819S
123. Maccioni RB, Muñoz JP, Barbeito L. The molecular bases of Alzheimer's disease and other neurodegenerative disorders. Arch Med Res 2001;32(5):367–381
124. Kalmijn S, van Boxtel MP, Ocké M, Verschuren WM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology 2004;62(2):275–280
125. Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol 1997;42(5):776–782
126. Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol 2003;60(7):940–946
127. van Marum RJ. Current and future therapy in Alzheimer's disease. Fundam Clin Pharmacol 2008; 22(3):265–274
128. Kyle DJ, Schaefer E, Patton G, Beiser A. Low serum docosahexaenoic acid is a significant risk factor for Alzheimer's dementia. Lipids 1999;34(Suppl):S245
129. Conquer JA, Tierney MC, Zecevic J, Bettger WJ, Fisher RH. Fatty acid analysis of blood plasma of patients with Alzheimer's disease, other types of dementia, and cognitive impairment. Lipids 2000;35(12):1305–1312
130. Tully AM, Roche HM, Doyle R, et al. Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer's disease: a case-control study. Br J Nutr 2003;89(4):483–489
131. Schaefer EJ, Bongard V, Beiser AS, et al. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol 2006;63(11):1545–1550
132. Burr ML, Fehily AM, Gilbert JF, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 1989;2(8666):757–761
133. de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation 1999;99(6):779–785
134. Tuttle KR, Shuler LA, Packard DP, et al. Comparison of low-fat versus Mediterranean-style dietary intervention after first myocardial infarction (from The Heart Institute of Spokane Diet Intervention and Evaluation Trial). Am J Cardiol 2008;101(11):1523–1530
135. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet 1999;354(9177):447–455
136. Marchioli R, Barzi F, Bomba E, et al; GISSI-Prevenzione Investigators. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation 2002;105(16):1897–1903
137. Singh RB, Niaz MA, Sharma JP, Kumar R, Rastogi V, Moshiri M. Randomized, double-blind, placebo-controlled trial of fish oil and mustard oil in patients with suspected acute myocardial infarction: the Indian experiment of infarct survival—4. Cardiovasc Drugs Ther 1997;11(3):485–491
138. Nilsen DW, Albrektsen G, Landmark K, Moen S, Aarsland T, Woie L. Effects of a high-dose concentrate of n-3 fatty acids or corn oil introduced early after an acute myocardial infarction on serum triacylglycerol and HDL cholesterol. Am J Clin Nutr 2001;74(1):50–56
139. Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med 2002;112(4):298–304
140. Sacks FM, Stone PH, Gibson CM, Silverman DI, Rosner B, Pasternak RC; HARP Research Group. Controlled trial of fish oil for regression of human coronary atherosclerosis. J Am Coll Cardiol 1995;25(7):1492–1498
141. von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. The effect of dietary omega-3 fatty acids on coronary atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1999;130(7):554–562
142. Balk E, Chung M, Lichtenstein A, et al. Effects of omega-3 fatty acids on cardiovascular risk factors and intermediate markers of cardiovascular disease. Evid Rep Technol Assess (Summ) 2004; (93):1–6
143. Lichtenstein AH, Appel LJ, Brands M, et al; American Heart Association Nutrition Committee. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006;114(1):82–96
144. Montori VM, Farmer A, Wollan PC, Dinneen SF. Fish oil supplementation in type 2 diabetes: a quantitative systematic review. Diabetes Care 2000;23(9): 1407–1415
145. Glauber H, Wallace P, Griver K, Brechtel G. Adverse metabolic effect of omega-3 fatty acids in non-insulin-dependent diabetes mellitus. Ann Intern Med 1988;108(5):663–668
146. Friday KE, Childs MT, Tsunehara CH, Fujimoto WY, Bierman EL, Ensinck JW. Elevated plasma glucose and lowered triglyceride levels from omega-3 fatty acid supplementation in type I.I. diabetes. Diabetes Care 1989;12(4):276–281
147. MacLean CH, Mojica WA, Morton SC, et al. Effects of omega-3 fatty acids on lipids and glycemic control in type II diabetes and the metabolic syndrome and on inflammatory bowel disease, rheumatoid arthritis, renal disease, systemic lupus erythematosus, and osteoporosis. Evid Rep Technol Assess (Summ) 2004; (89):1–4
148. Hartweg J, Farmer AJ, Perera R, Holman RR, Neil HA. Meta-analysis of the effects of n-3 polyunsaturated fatty acids on lipoproteins and other emerging lipid cardiovascular risk markers in patients with type 2 diabetes. Diabetologia 2007;50(8):1593–1602
149. Farmer A, Montori V, Dinneen S, Clar C. Fish oil in people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2001; (3):CD003205
150. Hu FB, Cho E, Rexrode KM, Albert CM, Manson JE. Fish and long-chain omega-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation 2003;107(14):1852–1857
151. Nettleton JA, Katz R. n-3 long-chain polyunsaturated fatty acids in type 2 diabetes: a review. J Am Diet Assoc 2005;105(3):428–440
152. Friedberg CE, Janssen MJ, Heine RJ, Grobbee DE. Fish oil and glycemic control in diabetes. A meta-analysis. Diabetes Care 1998;21(4):494–500
153. Franz MJ, Bantle JP, Beebe CA, et al; American Diabetes Association. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care 2003;26(Suppl 1):S51–S61
154. Fortin PR, Lew RA, Liang MH, et al. Validation of a meta-analysis: the effects of fish oil in rheumatoid arthritis. J Clin Epidemiol 1995;48(11):1379–1390
155. Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain 2007;129(1–2):210–223
156. Lorenz R, Weber PC, Szimnau P, Heldwein W, Strasser T, Loeschke K. Supplementation with n-3 fatty acids from fish oil in chronic inflammatory bowel disease—a randomized, placebo-controlled, double-blind cross-over trial. J Intern Med Suppl 1989;731: 225–232
157. Lorenz-Meyer H, Bauer P, Nicolay C, et al; Study Group Members (German Crohn's Disease Study Group). Omega-3 fatty acids and low carbohydrate diet for maintenance of remission in Crohn's disease. A randomized controlled multicenter trial. Scand J Gastroenterol 1996;31(8):778–785
158. Belluzzi A, Brignola C, Campieri M, Pera A, Boschi S, Miglioli M. Effect of an enteric-coated fish-oil preparation on relapses in Crohn's disease. N Engl J Med 1996;334(24):1557–1560
159. Romano C, Cucchiara S, Barabino A, Annese V, Sferlazzas C. Usefulness of omega-3 fatty acid supplementation in addition to mesalazine in maintaining remission in pediatric Crohn's disease: a double-blind, randomized, placebo-controlled study. World J Gastroenterol 2005;11(45):7118–7121
160. Aslan A, Triadafilopoulos G. Fish oil fatty acid supplementation in active ulcerative colitis: a double-blind, placebo-controlled, crossover study. Am J Gastroenterol 1992;87(4):432–437
161. Hawthorne AB, Daneshmend TK, Hawkey CJ, et al. Treatment of ulcerative colitis with fish oil supplementation: a prospective 12 month randomised controlled trial. Gut 1992;33(7):922–928
162. Stenson WF, Cort D, Rodgers J, et al. Dietary supplementation with fish oil in ulcerative colitis. Ann Intern Med 1992;116(8):609–614
163. Loeschke K, Ueberschaer B, Pietsch A, et al. n-3 fatty acids only delay early relapse of ulcerative colitis in remission. Dig Dis Sci 1996;41(10):2087–2094
164. De Ley M, de Vos R, Hommes DW, Stokkers P. Fish oil for induction of remission in ulcerative colitis. Cochrane Database Syst Rev 2007; (4):CD005986
165. Hodge L, Salome CM, Hughes JM, et al. Effect of dietary intake of omega-3 and omega-6 fatty acids on severity of asthma in children. Eur Respir J 1998; 11(2):361–365
166. Okamoto M, Mitsunobu F, Ashida K, et al. Effects of dietary supplementation with n-3 fatty acids compared with n-6 fatty acids on bronchial asthma. Intern Med 2000;39(2):107–111
167. Wong KW. Clinical efficacy of n-3 fatty acid supplementation in patients with asthma. J Am Diet Assoc 2005;105(1):98–105
168. Schachter HM, Reisman J, Tran K, et al. Health effects of omega-3 fatty acids on asthma. Evid Rep Technol Assess (Summ) 2004; (91):1–7
169. Woods RK, Thien FC, Abramson MJ. Dietary marine fatty acids (fish oil) for asthma in adults and children. Cochrane Database Syst Rev 2002;(3):CD001283
170. Reisman J, Schachter HM, Dales RE, et al. Treating asthma with omega-3 fatty acids: where is the evidence? A systematic review. BMC Complement Altern Med 2006;6:26
171. Donadio JV, Grande JP. IgA nephropathy. N Engl J Med 2002;347(10):738–748
172. Donadio JV Jr, Bergstralh EJ, Offord KP, Spencer DC, Holley KE; Mayo Nephrology Collaborative Group. A controlled trial of fish oil in IgA nephropathy. N Engl J Med 1994;331(18):1194–1199
173. Donadio JV Jr, Grande JP, Bergstralh EJ, Dart RA, Larson TS, Spencer DC; Mayo Nephrology Collaborative Group. The long-term outcome of patients with IgA nephropathy treated with fish oil in a controlled trial. J Am Soc Nephrol 1999;10(8):1772–1777
174. Donadio JV Jr, Larson TS, Bergstralh EJ, Grande JP. A randomized trial of high-dose compared with lowdose omega-3 fatty acids in severe IgA nephropathy. J Am Soc Nephrol 2001;12(4):791–799
175. Alexopoulos E, Stangou M, Pantzaki A, Kirmizis D, Memmos D. Treatment of severe IgA nephropathy with omega-3 fatty acids: the effect of a “very low dose” regimen. Ren Fail 2004;26(4):453–459
176. Bennett WM, Walker RG, Kincaid-Smith P. Treatment of IgA nephropathy with eicosapentanoic acid (EPA): a two-year prospective trial. Clin Nephrol 1989; 31(3):128–131
177. Cheng IK, Chan PC, Chan MK. The effect of fish-oil dietary supplement on the progression of mesangial IgA glomerulonephritis. Nephrol Dial Transplant 1990;5(4):241–246
178. Pettersson EE, Rekola S, Berglund L, et al. Treatment of IgA nephropathy with omega-3-polyunsaturated fatty acids: a prospective, double-blind, randomized study. Clin Nephrol 1994;41(4):183–190
179. Hogg RJ, Lee J, Nardelli N, et al; Southwest Pediatric Nephrology Study Group. Clinical trial to evaluate omega-3 fatty acids and alternate day prednisone in patients with IgA nephropathy: report from the Southwest Pediatric Nephrology Study Group. Clin J Am Soc Nephrol 2006;1(3):467–474
180. Branten AJ, Klasen IS, Wetzels JF. Short-term effects of fish oil treatment on urinary excretion of high-and low-molecular weight proteins in patients with IgA nephropathy. Clin Nephrol 2002;58(4):267–274
181. Dillon JJ. Fish oil therapy for IgA nephropathy: efficacy and interstudy variability. J Am Soc Nephrol 1997;8(11):1739–1744
182. Strippoli GF, Manno C, Schena FP. An “evidence-based” survey of therapeutic options for IgA nephropathy: assessment and criticism. Am J Kidney Dis 2003;41(6):1129–1139
183. Hibbeln JR.. Fish consumption and major depression. Lancet 1998;351(9110):1213
184. Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry 2003;160(12):2222–2227
185. Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Meltzer HY. Lowered omega3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res 1999; 85(3):275–291
186. Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry 1998;43(5):315–319
187. Tiemeier H, van Tuijl HR, Hofman A, Kiliaan AJ, Breteler MM. Plasma fatty acid composition and depression are associated in the elderly: the Rotterdam Study. Am J Clin Nutr 2003;78(1):40–46
188. Mamalakis G, Tornaritis M, Kafatos A. Depression and adipose essential polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids 2002; 67(5):311–318
189. Locke CA, Stoll AL. Omega-3 fatty acids in major depression. World Rev Nutr Diet 2001;89:173–185
190. Silvers KM, Woolley CC, Hamilton FC, Watts PM, Watson RA. Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins Leukot Essent Fatty Acids 2005; 72(3):211–218
191. Grenyer BF, Crowe T, Meyer B, et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31(7):1393–1396
192. Rogers PJ, Appleton KM, Kessler D, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr 2008;99(2):421–431
193. Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HF, Puryear LJ. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry 2003;160(5):996–998
194. Su KP, Huang SY, Chiu CC, Shen WW. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol 2003;13(4):267–271
195. Zanarini MC, Frankenburg FR. omega-3 Fatty acid treatment of women with borderline personality disorder: a double-blind, placebo-controlled pilot study. Am J Psychiatry 2003;160(1):167–169
196. Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry 2006;163(6):1098–1100
197. Stoll AL, Severus WE, Freeman MP, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry 1999;56(5):407–412
198. Keck PE Jr, Mintz J, McElroy SL, et al. Double-blind, randomized, placebo-controlled trials of ethyleicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry 2006;60(9):1020–1022
199. Osher Y, Bersudsky Y, Belmaker RH. Omega-3 eicosapentaenoic acid in bipolar depression: report of a small open-label study. J Clin Psychiatry 2005; 66(6):726–729
200. Frangou S, Lewis M, McCrone P. Efficacy of ethyleicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry 2006;188:46–50
201. Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry 2007;68(7):1056–1061
202. Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry 2006; 67(12):1954–1967
203. Appleton KM, Hayward RC, Gunnell D, et al. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials. Am J Clin Nutr 2006;84(6):1308–1316
204. Assies J, Lieverse R, Vreken P, Wanders RJ, Dingemans PM, Linszen DH. Significantly reduced docosahexaenoic and docosapentaenoic acid concentrations in erythrocyte membranes from schizophrenic patients compared with a carefully matched control group. Biol Psychiatry 2001;49(6):510–522
205. Kemperman RF, Veurink M, van der Wal T, et al. Low essential fatty acid and B-vitamin status in a subgroup of patients with schizophrenia and its response to dietary supplementation. Prostaglandins Leukot Essent Fatty Acids 2006;74(2):75–85
206. Horrobin DF, Manku MS, Hillman H, Iain A, Glen M. Fatty acid levels in the brains of schizophrenics and normal controls. Biol Psychiatry 1991;30(8):795–805
207. Laugharne JD, Mellor JE, Peet M. Fatty acids and schizophrenia. Lipids 1996;31(Suppl):S163–S165
208. Peet M, Brind J, Ramchand CN, Shah S, Vankar GK. Two double-blind placebo-controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Schizophr Res 2001;49(3):243–251
209. Emsley R, Myburgh C, Oosthuizen P, van Rensburg SJ. Randomized, placebo-controlled study of ethyleicosapentaenoic acid as supplemental treatment in schizophrenia. Am J Psychiatry 2002;159(9):1596–1598
210. Emsley R, Niehaus DJ, Koen L, et al. The effects of eicosapentaenoic acid in tardive dyskinesia: a randomized, placebo-controlled trial. Schizophr Res 2006;84(1):112–120
211. Fenton WS, Dickerson F, Boronow J, Hibbeln JR, Knable M. A placebo-controlled trial of omega-3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. Am J Psychiatry 2001; 158(12):2071–2074
212. Peet M, Horrobin DF; E-E Multicentre Study Group. A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res 2002; 36(1):7–18
213. Joy CB, Mumby-Croft R, Joy LA. Polyunsaturated fatty acid (fish or evening primrose oil) for schizophrenia. Cochrane Database Syst Rev 2003; (2):CD001257
214. Hooijmans CR, Kiliaan AJ. Fatty acids, lipid metabolism and Alzheimer pathology. Eur J Pharmacol 2008;585(1):176–196
215. Quinn JF, Raman R, Thomas RG, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA 2010;304(17):1903–1911
216. US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 21. 2008. Available at: http://www.nal.usda.gov/fnic/foodcomp/search/. Accessed May 8, 2012
217. Hendler SS, Rorvik DR, eds. PDR for Nutritional Supplements. Montvale, NJ: Medical Economics Company, Inc; 2001
218. US Food and Drug Administration, Center for Food Safety and Applied Nutrition. Agency Response Letter: GRAS Notice No. GRN 000080. 2001. Available at: http://www.fda.gov/ohrms/dockets/dockets/95s0316/95s-0316-rpt0354-061-Ref-F-FDA-Response-Ltr-g80-vol273.pdf. Accessed May 8, 2012
219. Zurier RB, Rossetti RG, Jacobson EW, et al. gamma-Linolenic acid treatment of rheumatoid arthritis. A randomized, placebo-controlled trial. Arthritis Rheum 1996;39(11):1808–1817
220. Vaddadi KS. The use of gamma-linolenic acid and linoleic acid to differentiate between temporal lobe epilepsy and schizophrenia. Prostaglandins Med 1981;6(4):375–379
221. Nordström DC, Honkanen VE, Nasu Y, Antila E, Friman C, Konttinen YT. Alpha-linolenic acid in the treatment of rheumatoid arthritis. A double-blind, placebo-controlled and randomized study: flaxseed vs. safflower seed. Rheumatol Int 1995;14(6):231–234
222. Alonso L, Marcos ML, Blanco JG, et al. Anaphylaxis caused by linseed (flaxseed) intake. J Allergy Clin Immunol 1996;98(2):469–470
223. Harbige LS. Fatty acids, the immune response, and autoimmunity: a question of n-6 essentiality and the balance between n-6 and n-3. Lipids 2003;38(4):323–341
224. Carlson SE, Cooke RJ, Werkman SH, Tolley EA. First year growth of preterm infants fed standard compared to marine oil n-3 supplemented formula. Lipids 1992;27(11):901–907
225. Environmental Protection Agency. Fish Consumption Advisories. Available at: http://www.epa.gov/waterscience/fish/. Accessed May 8, 2012
226. Omega-3 oil: fish or pills? Consum Rep 2003; 68(7):30–32
227. ConsumerLab. Product Review: Omega-3 Fatty Acids (EPA and DHA) from Fish, Algae, and Krill. 2010. Available at: http://www.consumerlab.com/results/omega3.asp. Accessed May 8, 2012
228. Melanson SF, Lewandrowski EL, Flood JG, Lewandrowski KB. Measurement of organochlorines in commercial over-the-counter fish oil preparations: implications for dietary and therapeutic recommendations for omega-3 fatty acids and a review of the literature. Arch Pathol Lab Med 2005;129(1):74–77
229. Hilbert G, Lillemark L, Balchen S, Højskov CS. Reduction of organochlorine contaminants from fish oil during refining. Chemosphere 1998;37(7):1241–1252
230. Bender NK, Kraynak MA, Chiquette E, Linn WD, Clark GM, Bussey HI. Effects of marine fish oils on the anticoagulation status of patients receiving chronic warfarin therapy. J Thromb Thrombolysis 1998;5(3): 257–261
231. Buckley MS, Goff AD, Knapp WE. Fish oil interaction with warfarin. Ann Pharmacother 2004;38(1):50–52
232. Valk EE, Hornstra G. Relationship between vitamin E requirement and polyunsaturated fatty acid intake in man: a review. Int J Vitam Nutr Res 2000;70(2):31–42
233. Higdon JV, Liu J, Du SH, Morrow JD, Ames BN, Wander RC. Supplementation of postmenopausal women with fish oil rich in eicosapentaenoic acid and docosahexaenoic acid is not associated with greater in vivo lipid peroxidation compared with oils rich in oleate and linoleate as assessed by plasma malondialdehyde and F (2)-isoprostanes. Am J Clin Nutr 2000;72(3):714–722
234. Wander RC, Du SH, Ketchum SO, Rowe KE. alpha-Tocopherol influences in vivo indices of lipid peroxidation in postmenopausal women given fish oil. J Nutr 1996;126(3):643–652
235. Wander RC, Du SH. Oxidation of plasma proteins is not increased after supplementation with eicosapentaenoic and docosahexaenoic acids. Am J Clin Nutr 2000;72(3):731–737
236. European Commission Directorate General for Health and Consumer Protection. Eurodiet: Nutrition and Diet for Healthy Lifestyles in Europe. 2001. Available at: http://ec.europa.eu/health/archive/ph_determinants/life_style/nutrition/report01_en.pdf. Accessed May 8, 2012
237. Hamazaki T, Okuyama H. The Japan Society for Lipid Nutrition recommends to reduce the intake of linoleic acid. A review and critique of the scientific evidence. World Rev Nutr Diet 2003;92:109–132