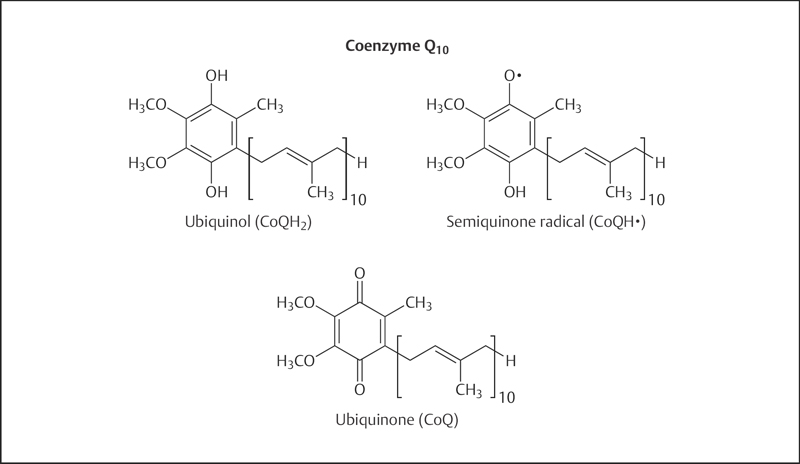
Fig. 22.1 Chemical structure of coenzyme Q10. Coenzyme Q10 can exist in three oxidation states: the fully reduced ubiquinol form (CoQH2), the radical semiquinone intermediate (CoQH·), and the fully oxidized ubiquinone form (CoQ).
Coenzyme Q10 is a member of the ubiquinone family of compounds. All animals, including humans, can synthesize ubiquinones, hence coenzyme Q10 cannot be considered a vitamin.1 The name ubiquinone refers to the ubiquitous presence of these compounds in living organisms and their chemical structure, which contains a functional group known as a benzoquinone. Ubiquinones are fat-soluble molecules with anywhere from one to 12 isoprene (5-carbon) units. The ubiquinone found in humans, ubidecaquinone or coenzyme Q10, has a “tail” of 10 isoprene units (a total of 50 carbon atoms) attached to its benzoquinone “head” (Fig. 22.1).2
Coenzyme Q10 is soluble in lipids (fats) and is found in virtually all cell membranes, as well as lipoprotein.2 The ability of the benzoquinone head group of coenzyme Q10 to accept and donate electrons is a critical feature in its biochemical functions. Coenzyme Q10 can exist in three oxidation states (Fig. 22.1): (1) the fully reduced ubiquinol form (CoQ10H2), (2) the radical semiquinone intermediate (CoQ10H·), and (3) the fully oxidized ubiquinone form (CoQ10).
The conversion of energy from carbohydrates and fats to adenosine triphosphate (ATP), the form of energy used by cells, requires the presence of coenzyme Q10 in the inner mitochondrial membrane. As part of the mitochondrial electron transport chain, coenzyme Q10 accepts electrons from reducing equivalents generated during fatty acid and glucose metabolism and then transfers them to electron acceptors. At the same time, coenzyme Q10 transfers protons outside the inner mitochondrial membrane, creating a proton gradient across that membrane. The energy released when the protons flow back into the mitochondrial interior is used to form ATP.2
Lysosomes are organelles within cells that are specialized for the digestion of cellular debris. The digestive enzymes within lysosomes function optimally at an acidic pH, meaning they require a permanent supply of protons. The lysosomal membranes that separate those digestive enzymes from the rest of the cell contain relatively high concentrations of coenzyme Q10. Research suggests that coenzyme Q10 plays an important role in the transport of protons across lysosomal membranes to maintain the optimal pH.2,3
In its reduced form, CoQ10H2 is an effective fat-soluble antioxidant. The presence of a significant amount of CoQ10H2 in cell membranes, along with enzymes that are capable of reducing oxidized CoQ10 back to CoQ10H2, supports the idea that CoQ10H2 is an important cellular antioxidant.2 CoQ10H2 has been found to inhibit lipid peroxidation when cell membranes and low-density lipoproteins (LDL) are exposed to oxidizing conditions outside the body (ex vivo). When LDL is oxidized ex vivo, CoQ10H2 is the first antioxidant consumed. Moreover, the formation of oxidized lipids and the consumption of α-tocopherol (α-TOH, biologically the most active form of vitamin E) are suppressed while CoQ10H2 is present.4 In isolated mitochondria, coenzyme Q10 can protect membrane proteins and DNA from the oxidative damage that accompanies lipid peroxidation.1 In addition to neutralizing free radicals directly, CoQ10H2 is capable of regenerating α-TOH from its one-electron oxidation product, α-tocopheroxyl radical (α-TO•).
α-Tocopherol (vitamin E) and coenzyme Q10 are the principal fat-soluble antioxidants in membranes and lipoproteins. When α-TOH neutralizes a free radical, such as a lipid peroxyl radical (LOO·), it becomes oxidized itself, forming α-TO·, which can promote the oxidation of lipoprotein lipids under certain conditions in the test tube. When the reduced form of coenzyme Q10 (CoQ10H2) reacts with α-TO·, α-TOH is regenerated and the semiquinone radical (CoQ10H·) is formed. It is possible for CoQ10H· to react with oxygen (O2) to produce superoxide anion radical (O2·–), which is a much less oxidizing radical than LOO·. However, CoQ10H· can also reduce α-TO· back to α-TOH, resulting in the formation of fully oxidized coenzyme Q10 (CoQ10), which does not react with O2 to form O2·– (Fig. 22.2).4,5

Fig. 22.1 Chemical structure of coenzyme Q10. Coenzyme Q10 can exist in three oxidation states: the fully reduced ubiquinol form (CoQH2), the radical semiquinone intermediate (CoQH·), and the fully oxidized ubiquinone form (CoQ).
Symptoms of coenzyme Q10 deficiency have not been reported in the general population, so it is generally assumed that normal biosynthesis and a varied diet provide sufficient coenzyme Q10 for healthy individuals.6 It has been estimated that dietary consumption contributes approximately 25% of plasma coenzyme Q10, but there are currently no specific dietary intake recommendations for coenzyme Q10 from the Institute of Medicine or other agencies.7 The extent to which dietary consumption contributes to tissue coenzyme Q10 levels is not clear.
Primary coenzyme Q10 deficiency is a rare, autosomal recessive disorder caused by genetic defects in coenzyme Q10 biosynthesis. The resultant low tissue levels of coenzyme Q10 severely compromise neuronal and muscular function. Oral coenzyme Q10 supplementation has been shown to improve neurological and muscular symptoms in some patients with primary coenzyme Q10 deficiency.8
Coenzyme Q10 levels have been found to decline gradually with age in several different tissues,1,9 but it is unclear whether this age-associated decline constitutes a deficiency (see the Disease Prevention section below). Decreased plasma levels of coenzyme Q10 have been observed in individuals with diabetes, cancer, and congestive heart failure (see the Disease Treatment section below). Lipid-lowering medications that inhibit the activity of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, a critical enzyme in both cholesterol and coenzyme Q10 biosynthesis, decrease plasma coenzyme Q10 levels (see the section on Drug Interactions below), although it remains unclear whether this has clinical or symptomatic implications.
Fig. 22.2 Potential interactions between coenzyme Q10 and α-tocopherol. When α-tocopherol (α-TOH) neutralizes a free radical, such as a lipid hydroperoxyl radical (LOO·), it becomes oxidized itself, forming the α-tocopheroxyl radical (α-TO·), which can promote the oxidation of lipoproteins under certain conditions in the test tube (Reaction 1). When the reduced form of coenzyme Q10 (CoQH2) reacts with α-TO·, α-TOH is regenerated and the semiquinone radical (CoQH·) is formed (Reaction 2). It is possible for CoQH· to react with oxygen (O2) to produce superoxide (O2·–), which is a much less-oxidizing radical than LOO· (Reaction 3a). However, CoQH· can also reduce α-TO· back to α-TOH, resulting in the formation of fully oxidized coenzyme Q10 (CoQ), which does not react with O2 to form O2·– (Reaction 3b).
According to the free radical and mitochondrial theories of aging, oxidative damage of cell structures by reactive oxygen species (ROS) plays an important role in the functional declines that accompany aging.10 ROS are generated by mitochondria as a by-product of ATP production. If not neutralized by antioxidants, ROS may damage mitochondria over time, causing them to function less efficiently and to generate more damaging ROS in a self-perpetuating cycle. Coenzyme Q10 plays an important role in mitochondrial ATP synthesis and functions as an antioxidant in mitochondrial membranes. Moreover, tissue levels of coenzyme Q10 have been reported to decline with age.9 One of the hallmarks of aging is a decline in energy metabolism in many tissues, especially liver, heart, and skeletal muscle. It has been proposed that age-associated declines in tissue coenzyme Q10 levels may play a role in this decline.11 In recent studies, lifelong dietary supplementation with coenzyme Q10 increased tissue concentrations of coenzyme Q10 but did not increase the lifespans of rats or mice12,13; however, one study showed that coenzyme Q10 supplementation attenuates the age-related increase in DNA damage.14 Presently, there is no scientific evidence that coenzyme Q10 supplementation prolongs life or prevents age-related functional decline in humans.
Oxidative modification of LDL in arterial walls is thought to represent an early event leading to the development of atherosclerosis. Reduced coenzyme Q10 (CoQ10H2) inhibits the oxidation of LDL in the test tube (in vitro) and works together with α-TOH to inhibit LDL oxidation by reducing the α-TO· back to α-TOH. In the absence of a co-antioxidant, such as CoQ10H2 (or vitamin C), α-TOH can, under certain conditions, promote the oxidation of LDL in vitro.4 Supplementation with coenzyme Q10 increases the concentration of CoQ10H2 in human LDL.15 Studies in apolipoprotein-E-deficient mice, an animal model of atherosclerosis, found that coenzyme Q10 supplementation with suprapharmacological amounts of coenzyme Q10 significantly inhibited the formation of atherosclerotic lesions.16 Interestingly, co-supplementation of these mice with α-TOH and coenzyme Q10 was more effective in inhibiting atherosclerosis than supplementation with either α-TOH or coenzyme Q10 alone.17 Another important step in the development of atherosclerosis is the recruitment of immune cells known as monocytes into the blood vessel walls. This recruitment is dependent in part on monocyte expression of cell adhesion molecules (integrins). Giving supplements of 200 mg/day of coenzyme Q10 to 10 healthy men and women for 10 weeks resulted in significant decreases in monocyte expression of integrins, suggesting another potential mechanism for the inhibition of atherosclerosis by coenzyme Q10.18 Although coenzyme Q10 supplementation shows promise as an inhibitor of LDL oxidation and atherosclerosis, more research is needed to determine whether coenzyme Q10 supplementation can inhibit the development or progression of atherosclerosis in humans.
Mitochondrial encephalomyopathies represent a diverse group of genetic disorders resulting from numerous inherited abnormalities in the function of the mitochondrial electron transport chain. Coenzyme Q10 supplementation has resulted in clinical and metabolic improvement in some patients with various types of mitochondrial encephalomyopathies.19 Neuromuscular and widespread tissue coenzyme Q10 deficiencies have been found in a very small subpopulation of individuals with mitochondrial encephalomyopathies.20,21 In those rare individuals with genetic defects in coenzyme Q10 biosynthesis, coenzyme Q10 supplementation has resulted in substantial improvement.22,23 It is not clear whether coenzyme Q10 supplementation might have therapeutic benefit in patients with other mitochondrial disorders; a phase III clinical trial investigating that question is currently under way.24
Impairment of the heart's ability to pump enough blood for all of the body's needs is known as congestive heart failure. In coronary artery disease, accumulation of atherosclerotic plaque in the coronary arteries may prevent parts of the heart muscle from getting adequate blood supply, ultimately resulting in cardiac damage and impaired pumping ability. Myocardial infarction (MI) may also damage the heart muscle, leading to heart failure. Because physical exercise increases the demand on the weakened heart, measures of exercise tolerance are frequently used to monitor the severity of heart failure. Echocardiography is also used to determine the left ventricular ejection fraction, an objective measure of the heart's pumping ability.25 The finding that myocardial coenzyme Q10 levels were lower in patients with more severe versus milder heart failure led to several clinical trials of coenzyme Q10 supplementation in patients with heart failure.26 Several small intervention trials that administered supplemental coenzyme Q10 (100–300 mg/day of coenzyme Q10 for 1–3 months) to patients with congestive heart failure, in conjunction with conventional medical therapy, have demonstrated improvements in some cardiac function measures.27–29 However, other researchers have found that supplementing the diet with 100–200 mg/day of coenzyme Q10, along with conventional medical therapy, did not significantly improve left ventricular ejection fraction or exercise performance in patients with heart failure.30,31 A 2006 meta-analysis of 10 randomized controlled trials found that coenzyme Q10 supplementation (99–200 mg/day for 1–6 months) in patients with heart failure resulted in a significant, 3.7% improvement in left ventricular ejection fraction; the effect was stronger in patients not taking angiotensin-converting enzyme inhibitors.32 A slight increase in cardiac output (0.28 L/min) was also found with coenzyme Q10 supplementation, but this analysis only included two trials (60 mg/day for 1 month or 200 mg/day for 3 months).32 A recent study in 236 patients with heart failure found that lower plasma coenzyme Q10 levels were associated with a heightened risk of mortality;33 however, a larger study of 1191 patients with heart failure found that plasma coenzyme Q10 level was a biomarker of advanced heart disease and not an independent predictor of clinical outcomes in patients with heart failure.34 Although there is some evidence that coenzyme Q10 supplementation may be of benefit, large well-designed intervention trials are needed to determine whether coenzyme Q10 supplementation has value as an adjunct to conventional medical therapy in the treatment of congestive heart failure. One such large trial is presently being conducted.
The heart muscle may become oxygen deprived (ischemic) as the result of MI or during cardiac surgery. Increased generation of ROS when the heart muscle's oxygen supply is restored (reperfusion) is thought to be an important contributor to myocardial damage occurring during ischemia–reperfusion. Pretreatment of animals with coenzyme Q10 has been found to decrease myocardial damage due to ischemia–reperfusion.35 Another potential source of ischemia–reperfusion injury is aortic clamping during some types of cardiac surgery, such as coronary artery bypass graft (CABG) surgery. Three out of four placebo-controlled trials found that coenzyme Q10 pretreatment (100–300 mg/day for 7–14 days prior to surgery) provided some benefit in short-term outcome measures after CABG surgery.36,37 In the placebo-controlled trial that did not find preoperative coenzyme Q10 supplementation to be of benefit, patients were treated with 600 mg of coenzyme Q10 12 hours prior to surgery,38 suggesting that preoperative coenzyme Q10 treatment may need to commence at least 1 week prior to CABG surgery to realize any benefit. Although the results are promising, these trials have included relatively few people and have only examined outcomes shortly after CABG surgery.
Myocardial ischemia may also lead to chest pain known as angina pectoris. People with angina pectoris often experience symptoms when the demand for oxygen exceeds the capacity of the coronary circulation to deliver it to the heart muscle, for example, during exercise. Five small placebo-controlled studies have examined the effects of oral coenzyme Q10 supplementation (60–600 mg/day) in addition to conventional medical therapy in patients with chronic stable angina.28 In most of the studies, coenzyme Q10 supplementation improved exercise tolerance and reduced or delayed electrocardiographic changes associated with myocardial ischemia compared with placebo. However, only two of the studies found significant decreases in symptom frequency and nitroglycerin consumption with coenzyme Q10 supplementation. Presently, there is only limited evidence suggesting that coenzyme Q10 supplementation would be a useful adjunct to conventional angina therapy.
The results of several small, uncontrolled studies in humans suggest that coenzyme Q10 supplementation could be beneficial in the treatment of hypertension.37 More recently, two short-term placebo-controlled trials found that coenzyme Q10 supplementation resulted in moderate decreases in blood pressure in hypertensive individuals. The addition of 120 mg/day of coenzyme Q10 to conventional medical therapy for 8 weeks in patients with hypertension and coronary artery disease decreased systolic blood pressure by an average of 12 mmHg and diastolic blood pressure by an average of 6 mmHg, in comparison to a placebo containing B-complex vitamins.39 In patients with isolated systolic hypertension, supplementation with both coenzyme Q10 (120 mg/day) and vitamin E (300 IU/day) for 12 weeks resulted in an average decrease of 17 mmHg in systolic blood pressure compared with 300 IU/day of vitamin E alone.40 A 2007 meta-analysis of 12 clinical trials, including 362 hypertensive patients, found that supplemental coenzyme Q10 reduces systolic blood pressure by 11–17 mmHg and diastolic blood pressure by 8–10 mmHg.41 The four randomized controlled trials included in this meta-analysis used doses of 100–120 mg/day of coenzyme Q10.
Normal function of the inner lining of blood vessels, known as the vascular endothelium, plays an important role in preventing cardiovascular diseases.42 Atherosclerosis is associated with impairment of vascular endothelial function, thereby compromising the ability of blood vessels to relax and permit normal blood flow. Endothelium-dependent blood vessel relaxation (vasodilation) is impaired in individuals with elevated serum cholesterol levels as well as in patients with coronary artery disease or diabetes. One placebo-controlled trial found that coenzyme Q10 supplementation (200 mg/day) for 12 weeks improved endothelium-dependent vasodilation in patients with diabetes and abnormal serum lipid profiles, although it did not restore vasodilation to levels seen in individuals who did not have diabetes.43 Another placebo-controlled study in 23 individuals with type 2 diabetes taking statins (HMG-CoA reductase inhibitors) found that 200 mg/day of coenzyme Q10 for 12 weeks improved flow-mediated dilatation, but not nitrate-mediated dilatation, of the brachial artery.44 However, a placebo-controlled trial in 80 individuals with type 2 diabetes found that this supplementation protocol did not improve endothelial function.45
In a study of 12 individuals with high serum cholesterol levels and endothelial dysfunction who were otherwise healthy, supplementation with 150 mg/day of coenzyme Q10 did not affect endothelium-dependent vasodilation.46 A prospective, randomized crossover study of 25 men with endothelial dysfunction found that coenzyme Q10 supplementation (150 mg/day) significantly improved endothelial function, similar to that of a lipid-lowering medication.47 Yet, it is important to mention that this study was not placebo-controlled and, importantly, the authors reported that the subjects’ mean baseline for flow-mediated vasodilation was below zero. A randomized, double-blind, placebo-controlled trial in 22 patients with coronary artery disease found that 300 mg/day of coenzyme Q10 for 1 month improved endothelium-dependent vasodilation.48 Another randomized, double-blind, placebo-controlled trial in 56 patients with ischemic left ventricular systolic dysfunction reported that 300 mg/day of coenzyme Q10 for 8 weeks significantly improved measures of endothelial dysfunction.49 A 2011 meta-analysis examining the results of five randomized controlled trials, including 194 subjects, found that supplemental coenzyme Q10 (150–300 mg/day for 4–12 weeks) resulted in a clinically significant, 1.7% increase in flow-dependent endothelial-mediated dilatation.50 Large-scale studies are needed to further elucidate the therapeutic role of coenzyme Q10 in endothelial dysfunction.
Diabetes mellitus is a condition of increased oxidative stress and impaired energy metabolism. Plasma levels of reduced coenzyme Q10 (CoQ10H2) have been found to be lower in patients with diabetes than in healthy controls, when normalized to plasma cholesterol levels.51,52 However, supplementation with 100 mg/day of coenzyme Q10 for 3 months neither improved glycemic (blood glucose) control nor decreased insulin requirements in patients with type 1 (insulin-dependent) diabetes compared with placebo.53 Similarly, 200 mg/day of coenzyme Q10 supplementation for 12 weeks or 6 months did not improve glycemic control or serum lipid profiles in individuals with type 2 (noninsulin-dependent) diabetes.44,54 Because coenzyme Q10 supplementation did not influence glycemic control in either study, the authors of both studies concluded that coenzyme Q10 supplements could be used safely in patients with diabetes as adjunct therapy for cardiovascular diseases.
Maternally inherited diabetes mellitus and deafness is the result of a mutation in mitochondrial DNA, which is inherited exclusively from one's mother. Although mitochondrial diabetes accounts for less than 1% of all diabetes, there is some evidence that long-term supplementation with coenzyme Q10 (150 mg/day) may improve insulin secretion and prevent progressive hearing loss in these patients.55,56
Parkinson disease is a degenerative neurological disorder characterized by tremors, muscular rigidity, and slow movements. It is estimated to affect approximately 1% of Americans over the age of 65 years. Although the causes of Parkinson disease are not all known, decreased activity of complex I of the mitochondrial electron transport chain and increased oxidative stress in a part of the brain called the substantia nigra are thought to play a role. Coenzyme Q10 is the electron acceptor for complex I as well as an antioxidant, and decreased ratios of reduced to oxidized coenzyme Q10 have been found in platelets of individuals with Parkinson disease.57,58 One study also found higher concentrations of oxidized coenzyme Q10 in the cerebrospinal fluid of patients with untreated Parkinson disease compared with healthy controls.59 Additionally, a study of coenzyme Q10 levels in postmortem Parkinson disease patients found lower levels of total coenzyme Q10 in the cortex region of the brain compared with age-matched controls, but no differences were seen in other brain areas, including the striatum, substantia nigra, and cerebellum.60 A 16-month randomized placebo-controlled trial evaluated the safety and efficacy of 300, 600, or 1200 mg/day of coenzyme Q10 in 80 people with early Parkinson disease.61 Coenzyme Q10 supplementation was well tolerated at all doses and was associated with slower deterioration of function in patients with Parkinson disease compared with placebo. However, the difference was statistically significant only in the group taking 1200 mg/day. A smaller placebo-controlled trial showed that oral administration of 360 mg/day of coenzyme Q10 for 4 weeks moderately benefited patients with Parkinson disease.62 More recently, a randomized, double-blind, placebo-controlled trial in 106 patients with midstage Parkinson disease reported that 300 mg/day of nanoparticular coenzyme Q10 for 3 months had no therapeutic benefit.63 Another trial found that 2400 mg/day of coenzyme Q10 for 12 months was not effective in early Parkinson disease.64 A phase III clinical trial of coenzyme Q10 (1200–2400 mg/day) and vitamin E (1200 IU/day) supplementation in patients with Parkinson disease was recently terminated because it was unlikely that such a treatment was effective in treating Parkinson disease.65
Huntington disease is an inherited neurodegenerative disorder characterized by selective degeneration of nerve cells known as striatal spiny neurons. Symptoms, such as movement disorders and impaired cognitive function, typically develop in the fourth decade of life and progressively deteriorate over time. Animal models indicate that impaired mitochondrial function and glutamate-mediated neurotoxicity may play roles in the pathology of Huntington disease. Coenzyme Q10 supplementation has been found to decrease brain lesion size in animal models of Huntington disease and to decrease brain lactate levels in patients with Huntington disease.66,67 Feeding a combination of coenzyme Q10 (0.2% of diet) and remacemide (0.007% of diet) to transgenic mice that express the Huntington disease protein (HDN171-82Q mice) resulted in improved motor performance and/or survival.68,69 Remacemide is an antagonist of the neuronal receptor that is activated by glutamate.
It was recently shown that the R6/2 mouse model of Huntington disease exhibits a progressive decline in behavioral and neurological symptoms similar to that of the human condition.70 Thus, R6/2 mice may be an ideal model to investigate potential therapies for Huntington disease. Some, but not all, studies employing these mice have shown that dietary supplementation with coenzyme Q10 (0.2% of diet) improves motor performance and overall survival and helps prevent loss of body weight; coenzyme Q10 supplementation has also been associated with reductions in the various hallmarks of Huntington disease (i.e., brain atrophy, ventricular enlargement, and striatal neuronal atrophy).68,71 Interestingly, co-administration of coenzyme Q10 with remacemide, the antibiotic minocycline, or creatine has been shown to result in even greater improvements in most measured parameters.68,71,72
To date, only one clinical trial has examined whether coenzyme Q10 might be efficacious in human patients with Huntington disease. A 30-month, randomized, placebo-controlled trial of coenzyme Q10 (600 mg/day), remacemide, or both in 347 patients with early Huntington disease found that neither coenzyme Q10 nor remacemide significantly altered the decline in total functional capacity, although coenzyme Q10 supplementation (with or without remacemide) resulted in a nonsignificant 13% decrease in the decline.73 A recent 20-week pilot trial examined the safety and tolerability of increasing dosages of coenzyme Q10 (1200 mg/day, 2400 mg/day, and 3600 mg/day) in eight healthy subjects and in 20 patients with Huntington disease; 22 of the subjects completed the study.74 All dosages were generally well tolerated, with gastrointestinal symptoms being the most frequently reported adverse effect. Blood levels of coenzyme Q10 at the end of the study were not higher than the levels resulting from the intermediate dose, suggesting that the 2400 mg/day dose effectively maximizes blood coenzyme Q10 levels and potentially avoids any side effects with higher dosages.74 A phase III clinical trial administering 2400 mg/day of coenzyme Q10 or placebo for 5 years is currently recruiting participants with Huntington disease.75 At present, there is insufficient evidence to recommend coenzyme Q10 supplements to patients with Huntington disease.
Friedreich ataxia (FRDA) is an inherited, autosomal recessive neurodegenerative disease caused by mutations in the gene that encodes frataxin, a protein of unknown function that is primarily located in the mitochondria. Decreased expression of frataxin is associated with accumulation of iron within the mitochondria, thereby resulting in increased oxidative stress; imbalances in iron–sulfur proteins, including mitochondrial aconitase; and reduced activities of the mitochondrial respiratory chain.76 Clinically, FRDA is a progressive disease characterized by limb ataxia and abnormalities of the central nervous system that result from sensory nerve degeneration.77,78 In addition, FRDA patients experience symptoms of hypertrophic cardiomyopathy and diabetes.79 A pilot study administering coenzyme Q10 (200 mg/day) and vitamin E (2100 IU/day) to 10 FDRA patients found that energy metabolism of cardiac and skeletal muscle was improved after only 3 months of therapy.80 Follow-up assessments at 47 months indicated that cardiac and skeletal muscle improvements were maintained and that patients with FRDA showed significant increases in fractional shortening, a measure of cardiac function. Moreover, the therapy was effective at preventing the progressive decline of neurological function.81 A recent study reported that deficiencies of both coenzyme Q10 and vitamin E are quite common among FRDA patients and that cosupplementation with both compounds, at doses as low as 30 mg/day of coenzyme Q10 and 4 IU/day of vitamin E, may improve disease symptoms.82 Large-scale, randomized clinical trials are necessary to determine whether coenzyme Q10, in conjunction with vitamin E, has therapeutic benefit in FRDA.
Interest in coenzyme Q10 as a potential therapeutic agent in cancer was stimulated by an observational study that found that individuals with lung, pancreas, and especially breast cancer were more likely to have low plasma coenzyme Q10 levels than healthy controls.83 Although a few case reports and an uncontrolled trial suggest that coenzyme Q10 supplementation may be beneficial as an adjunct to conventional therapy for breast cancer,84 the lack of controlled clinical trials makes it impossible to determine the effects, if any, of coenzyme Q10 supplementation in patients with cancer.
Although coenzyme Q10 supplementation has improved exercise tolerance in some individuals with mitochondrial encephalomyopathies (see the Deficiency section above),19 there is little evidence that it improves athletic performance in healthy individuals. At least seven placebo-controlled trials have examined the effects of 100–150 mg/day of coenzyme Q10 supplementation for 3–8 weeks on physical performance in trained and untrained men. Most found no significant differences between groups taking coenzyme Q10 and groups taking placebos with respect to measures of aerobic exercise performance, such as maximal oxygen consumption and exercise time to exhaustion.85–89 One study found the maximal cycling workload to be slightly (4%) increased after 8 weeks of coenzyme Q10 supplementation compared with placebo, although measures of aerobic power were not increased.90 Two studies actually found significantly greater improvement in measures of anaerobic86 and aerobic85 exercise performance after supplementation with a placebo compared with coenzyme Q10. Studies on the effect of supplementation on physical performance in women are lacking, but there is little reason to suspect a sex difference in the response to coenzyme Q10 supplementation.
Coenzyme Q10 is synthesized in most human tissues. The biosynthesis of coenzyme Q10 involves three major steps: (1) synthesis of the benzoquinone structure from either tyrosine or phenylalanine, two amino acids; (2) synthesis of the isoprene side chain from acetyl-coenzyme A (CoA) via the mevalonate pathway; and (3) the joining or condensation of these two structures. The enzyme HMG-CoA reductase plays a critical role in the regulation of coenzyme Q10 synthesis, as well as the regulation of cholesterol synthesis.1,6
The first step in benzoquinone biosynthesis (the conversion of tyrosine to 4-hydroxyphenylpyruvic acid) requires vitamin B6 in the form of pyridoxal 5′-phosphate. Thus, adequate vitamin B6 nutrition is essential for coenzyme Q10 biosynthesis. A pilot study in 29 patients and healthy volunteers found significant positive correlations between blood levels of coenzyme Q10 and measures of vitamin B6 nutritional status.91 However, further research is required to determine the clinical significance of this association.
Based on food frequency studies, the average dietary intake of coenzyme Q10 in Denmark was estimated to be 3–5 mg/day.6,7 Most people probably have a dietary intake of less than 10 mg/day of coenzyme Q10. Rich sources of dietary coenzyme Q10 include mainly meat, poultry, and fish. Other relatively rich sources include soybean and canola oils, and nuts. Fruits, vegetables, eggs, and dairy products are moderate sources of coenzyme Q10. Approximately 14%–32% of coenzyme Q10 was lost during frying of vegetables and eggs, but the coenzyme Q10 content of these foods did not change when they were boiled. Some relatively rich dietary sources and their coenzyme Q10 content in milligrams are listed in Table 22.1.92–94
Coenzyme Q10 is available without a prescription as a dietary supplement in the United States. Supplemental doses for adults range from 30–100 mg/day, which is considerably higher than normal dietary coenzyme Q10 intake. Therapeutic doses for adults generally range from 100–300 mg/day, although doses as high as 3000 mg/day have been used to treat early Parkinson disease under medical supervision.95 Absorption of coenzyme Q10 decreases with increasing supplemental dose; total intestinal absorption is likely less than 10% in humans. Coenzyme Q10 is fat soluble and best absorbed with fat in a meal. Doses higher than 100 mg/day are generally divided into two or three doses throughout the day.7,96
Table 22.1 Coenzyme Q10 content of selected foods92–94
Food |
Serving |
Coenzyme Q10 (mg) |
Beef, fried |
3 oza |
2.6 |
Herring, marinated |
3 oz |
2.3 |
Chicken, fried |
3 oz |
1.4 |
Soybean oil |
1 tbsp |
1.3 |
Canola oil |
1 tbsp |
1.0 |
Rainbow trout, steamed |
3 oz |
0.9 |
Peanuts, roasted |
1 oz |
0.8 |
Sesame seeds, roasted |
1 oz |
0.7 |
Pistachio nuts, roasted |
1 oz |
0.6 |
Broccoli, boiled |
½ cup, chopped |
0.5 |
Cauliflower, boiled |
½ cup, chopped |
0.4 |
Orange |
1 medium |
0.3 |
Strawberries |
½ cup |
0.1 |
Egg, boiled |
1 medium |
0.1 |
a A 3-oz serving of meat or fish is about the size of a deck of cards.
Oral supplementation with coenzyme Q10 is known to increase blood and lipoprotein concentrations of coenzyme Q10 in humans.2,12,15 However, it is not clear whether oral supplementation increases coenzyme Q10 concentrations in other tissues of individuals with normal endogenous coenzyme Q10 biosynthesis. Oral coenzyme Q10 supplementation of young healthy animals has not generally resulted in increased tissue concentrations, other than in the liver, spleen, and blood vessels.97,98 Giving supplements of 120 mg/day for 3 weeks to healthy men did not increase skeletal muscle concentrations of coenzyme Q10.99 However, supplementation may increase coenzyme Q10 levels in tissues that are deficient. For example, oral supplementation of aged rats increased brain coenzyme Q10 concentrations,100 and a study of 24 older adults given supplements of 300 mg/day of coenzyme Q10 or placebo for at least 7 days prior to cardiac surgery found that the coenzyme Q10 content of atrial tissue was significantly increased in those taking coenzyme Q10, especially in those aged over 70 years.36 Additionally, in a study of patients with left ventricular dysfunction, supplementation with 150 mg/day of coenzyme Q10 for 4 weeks before cardiac surgery increased coenzyme Q10 levels in the heart but not in skeletal muscle.101 Clearly, this is an area of research that requires further investigation.
There have been no reports of significant adverse side effects of oral coenzyme Q10 supplementation at doses as high as 1200 mg/day for up to 16 months61 and 600 mg/day for up to 30 months.73 In fact, 1200 mg/day has recently been proposed as the observed safe level (OSL) for coenzyme Q10.102 Some people have experienced gastrointestinal symptoms, such as nausea, diarrhea, appetite suppression, heartburn, and abdominal discomfort. These adverse effects may be minimized if daily doses higher than 100 mg are divided into two or three daily doses. Because controlled safety studies in pregnant and lactating women are not available, the use of coenzyme Q10 supplements by pregnant or breast-feeding women should be avoided.96,103
Concomitant use of warfarin (Coumadin) and coenzyme Q10 supplements has been reported to decrease the anticoagulant effect of warfarin in at least four cases.104 An individual on warfarin should not begin taking coenzyme Q10 supplements without consulting the health-care provider who is managing his or her anticoagulant therapy. If warfarin and coenzyme Q10 are to be used concomitantly, blood tests to assess clotting time (prothrombin time international normalized ratio [INR]) should be monitored frequently, especially in the first 2 weeks.
HMG-CoA reductase is an enzyme that plays a critical role in the regulation of cholesterol synthesis, as well as coenzyme Q10 synthesis, although it is now recognized that there are additional rate-limiting steps in the biosynthesis of cholesterol and coenzyme Q10. HMG-CoA reductase inhibitors, also known as statins, are widely used cholesterol-lowering medications that may also decrease the endogenous synthesis of coenzyme Q10. Therapeutic use of statins, including simvastatin (Zocor), pravastatin (Pravachol), lovastatin (Mevacor, Altocor, Altoprev), rosuvastatin (Crestor), and atorvastatin (Lipitor), has been shown to decrease blood plasma or serum levels of coenzyme Q10.105–114 However, it has been suggested that blood coenzyme Q10 concentrations should be reported only after normalizing to total lipid or cholesterol levels, because coenzyme Q10 circulates with lipoproteins and levels of coenzyme Q10 are highly dependent upon levels of circulating lipids.115,116 Given the lipid-lowering effects of statins, it is therefore unclear whether these drugs actually decrease coenzyme Q10 levels independent of a reduction in circulating lipids. Also, very few studies have examined coenzyme Q10 content in target organs; thus, it is not clear whether statin therapy affects coenzyme Q10 concentrations in the body's tissues.111,113,117 At present, more research is needed to determine whether coenzyme Q10 supplementation might be beneficial for those taking HMG-CoA reductase inhibitors.
• Coenzyme Q10 is a fat-soluble compound primarily synthesized by the body and also consumed in the diet.
• Coenzyme Q10 is required for mitochondrial ATP synthesis and functions as an antioxidant in cell membranes and lipoproteins.
• Endogenous synthesis and dietary intake appear to provide sufficient coenzyme Q10 to prevent deficiency in healthy people, although tissue levels of coenzyme Q10 decline with age.
• Oral supplementation of coenzyme Q10 increases plasma, lipoprotein, and blood vessel levels, but it is unclear whether tissue coenzyme Q10 levels are increased, especially in healthy individuals.
• Coenzyme Q10 supplementation has resulted in clinical and metabolic improvement in some patients with hereditary mitochondrial disorders.
• Although coenzyme Q10 supplementation may be a useful adjunct to conventional medical therapy for congestive heart failure, additional research is needed.
• Roles for coenzyme Q10 supplementation in cardiovascular diseases, neurodegenerative diseases, cancer, and diabetes require further research.
• Coenzyme Q10 supplementation does not appear to improve athletic performance.
• Although coenzyme Q10 supplements are relatively safe, they may decrease the anticoagulant efficacy of warfarin.
• Although use of the cholesterol-lowering medications known as HMG-CoA reductase inhibitors (statins) decreases circulating levels of coenzyme Q10, it is unclear whether coenzyme Q10 supplementation provides any health benefit to patients taking these drugs.
1. Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta 1995;1271(1):195–204
2. Crane FL. Biochemical functions of coenzyme Q10. J Am Coll Nutr 2001;20(6):591–598
3. Nohl H, Gille L. The role of coenzyme Q in lysosomes. In: Kagan VEQ, Quinn PJ, eds. Coenzyme Q: Molecular Mechanisms in Health and Disease. Boca Raton, FL: CRC Press; 2001:99–106
4. Thomas SR, Stocker R. Mechanisms of antioxidant action of ubiquinol-10 for low-density lipoprotein. In: Kagan VE, Quinn PJ, eds. Coenzyme Q: Molecular Mechanisms in Health and Disease. Boca Raton, FL: CRC Press; 2001:131–150
5. Kagan VE, Fabisak JP, Tyurina YY. Independent and concerted antioxidant functions of coenzyme Q. In: Kagan VE, Quinn PJ, eds. Coenzyme Q: Molecular Mechanisms in Health and Disease. Boca Raton, FL: CRC Press; 2001:119–130
6. Overvad K, Diamant B, Holm L, Holmer G, Mortensen SA, Stender S. Coenzyme Q10 in health and disease. Eur J Clin Nutr 1999;53(10):764–770
7. Weber C. Dietary intake and absorption of coenzyme Q. In: Kagan VE, Quinn PJ, eds. Coenzyme Q: Molecular Mechanisms in Health and Disease. Boca Raton, FL: CRC Press; 2001:209–215
8. Rustin P, Munnich A, Rötig A. Mitochondrial respiratory chain dysfunction caused by coenzyme Q deficiency. Methods Enzymol 2004;382:81–88
9. Kalén A, Appelkvist EL, Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids 1989;24(7):579–584
10. Beckman KB, Ames BN. Mitochondrial aging: open questions. Ann N Y Acad Sci 1998;854:118–127
11. Alho H, Lonnrot K. Coenzyme Q supplementation and longevity. In: Kagan VE, Quinn PJ, eds. Coenzyme Q: Molecular Mechanisms in Health and Disease. Boca Raton, FL: CRC Press; 2001:371–380
12. Singh RB, Niaz MA, Kumar A, Sindberg CD, Moesgaard S, Littarru GP. Effect on absorption and oxidative stress of different oral Coenzyme Q10 dosages and intake strategy in healthy men. Biofactors 2005;25(1–4):219–224
13. Sohal RS, Kamzalov S, Sumien N, et al. Effect of coenzyme Q10 intake on endogenous coenzyme Q content, mitochondrial electron transport chain, antioxidative defenses, and life span of mice. Free Radic Biol Med 2006;40(3):480–487
14. Quiles JL, Ochoa JJ, Battino M, et al. Life-long supplementation with a low dosage of coenzyme Q10 in the rat: effects on antioxidant status and DNA damage. Biofactors 2005;25(1–4):73–86
15. Mohr D, Bowry VW, Stocker R. Dietary supplementation with coenzyme Q10 results in increased levels of ubiquinol-10 within circulating lipoproteins and increased resistance of human low-density lipoprotein to the initiation of lipid peroxidation. Biochim Biophys Acta 1992;1126(3):247–254
16. Witting PK, Pettersson K, Letters J, Stocker R. Anti-atherogenic effect of coenzyme Q10 in apolipoprotein E gene knockout mice. Free Radic Biol Med 2000;29(3–4):295–305
17. Thomas SR, Leichtweis SB, Pettersson K, et al. Dietary cosupplementation with vitamin E and coenzyme Q (10) inhibits atherosclerosis in apolipoprotein E gene knockout mice. Arterioscler Thromb Vasc Biol 2001;21(4):585–593
18. Turunen M, Wehlin L, Sjöberg M, et al. beta2-Integrin and lipid modifications indicate a non-antioxidant mechanism for the anti-atherogenic effect of dietary coenzyme Q10. Biochem Biophys Res Commun 2002;296(2):255–260
19. Shoffner JM. Oxidative phosphorylation diseases. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The metabolic and molecular bases of inherited disease. 8th ed. New York: McGraw-Hill; 2001:2367–2392
20. Rötig A, Appelkvist EL, Geromel V, et al. Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet 2000;356(9227):391–395
21. Boitier E, Degoul F, Desguerre I, et al. A case of mitochondrial encephalomyopathy associated with a muscle coenzyme Q10 deficiency. J Neurol Sci 1998;156(1):41–46
22. Munnich A, Rotig A, Cormier-Daire V, Rustin P. Clinical presentation of respiratory chain deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The metabolic and molecular bases of inherited disease. 8th ed. New York: McGraw-Hill; 2001:2261–2274
23. Horvath R, Gorman G, Chinnery PF. How can we treat mitochondrial encephalomyopathies? Approaches to therapy. Neurotherapeutics 2008;5(4):558–568
24. National Institutes of Health. Phase III Trial of Coenzyme Q10 in Mitochondrial Disease. ClinicalTrials. gov. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00432744?term=coenzyme+Q10+AND+mitochondrial&rank=1. Accessed May 9, 2012
25. Trupp RJ, Abraham WT. Congestive heart failure. In: Rakel RE, Bope ET, eds. Conn's Current Therapy 2002. 54th ed. New York: WB Saunders Company; 2002:306–313
26. Folkers K, Vadhanavikit S, Mortensen SA. Biochemical rationale and myocardial tissue data on the effective therapy of cardiomyopathy with coenzyme Q10. Proc Natl Acad Sci U S A 1985;82(3):901–904
27. Belardinelli R, Muçaj A, Lacalaprice F, et al. Coenzyme Q10 and exercise training in chronic heart failure. Eur Heart J 2006;27(22):2675–2681
28. Tran MT, Mitchell TM, Kennedy DT, Giles JT. Role of coenzyme Q10 in chronic heart failure, angina, and hypertension. Pharmacotherapy 2001;21(7):797–806
29. Belardinelli R, Muçaj A, Lacalaprice F, et al. Coenzyme Q10 improves contractility of dysfunctional myocardium in chronic heart failure. Biofactors 2005;25(1–4):137–145
30. Khatta M, Alexander BS, Krichten CM, et al. The effect of coenzyme Q10 in patients with congestive heart failure. Ann Intern Med 2000;132(8):636–640
31. Watson PS, Scalia GM, Galbraith A, Burstow DJ, Bett N, Aroney CN. Lack of effect of coenzyme Q on left ventricular function in patients with congestive heart failure. J Am Coll Cardiol 1999;33(6):1549–1552
32. Sander S, Coleman CI, Patel AA, Kluger J, White CM. The impact of coenzyme Q10 on systolic function in patients with chronic heart failure. J Card Fail 2006;12(6):464–472
33. Molyneux SL, Florkowski CM, George PM, et al. Coenzyme Q10: an independent predictor of mortality in chronic heart failure. J Am Coll Cardiol 2008;52(18): 1435–1441
34. McMurray JJ, Dunselman P, Wedel H, et al; CORONA Study Group. Coenzyme Q10, rosuvastatin, and clinical outcomes in heart failure: a pre-specified substudy of CORONA (controlled rosuvastatin multinational study in heart failure). J Am Coll Cardiol 2010;56(15):1196–1204
35. Lönnrot K, Tolvanen JP, Pörsti I, Ahola T, Hervonen A, Alho H. Coenzyme Q10 supplementation and recovery from ischemia in senescent rat myocardium. Life Sci 1999;64(5):315–323
36. Rosenfeldt FL, Pepe S, Linnane A, et al. The effects of ageing on the response to cardiac surgery: protective strategies for the ageing myocardium. Biogerontology 2002;3(1–2):37–40
37. Langsjoen PH, Langsjoen AM. Overview of the use of CoQ10 in cardiovascular disease. Biofactors 1999; 9(2–4):273–284
38. Taggart DP, Jenkins M, Hooper J, et al. Effects of short-term supplementation with coenzyme Q10 on myocardial protection during cardiac operations. Ann Thorac Surg 1996;61(3):829–833
39. Singh RB, Niaz MA, Rastogi SS, Shukla PK, Thakur AS. Effect of hydrosoluble coenzyme Q10 on blood pressures and insulin resistance in hypertensive patients with coronary artery disease. J Hum Hypertens 1999;13(3):203–208
40. Burke BE, Neuenschwander R, Olson RD. Randomized, double-blind, placebo-controlled trial of coenzyme Q10 in isolated systolic hypertension. South Med J 2001;94(11):1112–1117
41. Rosenfeldt FL, Haas SJ, Krum H, et al. Coenzyme Q10 in the treatment of hypertension: a meta-analysis of the clinical trials. J Hum Hypertens 2007;21(4):297–306
42. Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999;340(2):115–126
43. Watts GF, Playford DA, Croft KD, Ward NC, Mori TA, Burke V. Coenzyme Q (10) improves endothelial dysfunction of the brachial artery in Type II diabetes mellitus. Diabetologia 2002;45(3):420–426
44. Hamilton SJ, Chew GT, Watts GF. Coenzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes Care 2009;32(5):810–812
45. Lim SC, Lekshminarayanan R, Goh SK, et al. The effect of coenzyme Q10 on microcirculatory endothelial function of subjects with type 2 diabetes mellitus. Atherosclerosis 2008;196(2):966–969
46. Raitakari OT, McCredie RJ, Witting P, et al. Coenzyme Q improves LDL resistance to ex vivo oxidation but does not enhance endothelial function in hypercholesterolemic young adults. Free Radic Biol Med 2000;28(7):1100–1105
47. Kuettner A, Pieper A, Koch J, Enzmann F, Schroeder S. Influence of coenzyme Q (10) and cerivastatin on the flow-mediated vasodilation of the brachial artery: results of the ENDOTACT study. Int J Cardiol 2005;98(3): 413–419
48. Tiano L, Belardinelli R, Carnevali P, Principi F, Seddaiu G, Littarru GP. Effect of coenzyme Q10 administration on endothelial function and extracellular superoxide dismutase in patients with ischaemic heart disease: a double-blind, randomized controlled study. Eur Heart J 2007;28(18):2249–2255
49. Dai YL, Luk TH, Yiu KH, et al. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic left ventricular systolic dysfunction: a randomized controlled trial. Atherosclerosis 2011; 216(2):395–401
50. Gao L, Mao Q, Cao J, Wang Y, Zhou X, Fan L. Effects of coenzyme Q10 on vascular endothelial function in humans: A meta-analysis of randomized controlled trials. Atherosclerosis 2012;221(2):311–316
51. McDonnell MG, Archbold GP. Plasma ubiquinol/cholesterol ratios in patients with hyperlipidaemia, those with diabetes mellitus and in patients requiring dialysis. Clin Chim Acta 1996;253(1–2):117–126
52. Lim SC, Tan HH, Goh SK, et al. Oxidative burden in prediabetic and diabetic individuals: evidence from plasma coenzyme Q (10). Diabet Med 2006;23(12): 1344–1349
53. Henriksen JE, Andersen CB, Hother-Nielsen O, Vaag A, Mortensen SA, Beck-Nielsen H. Impact of ubiquinone (coenzyme Q10) treatment on glycaemic control, insulin requirement and well-being in patients with Type 1 diabetes mellitus. Diabet Med 1999;16(4):312–318
54. Eriksson JG, Forsén TJ, Mortensen SA, Rohde M. The effect of coenzyme Q10 administration on metabolic control in patients with type 2 diabetes mellitus. Biofactors 1999;9(2–4):315–318
55. Alcolado JC, Laji K, Gill-Randall R. Maternal transmission of diabetes. Diabet Med 2002;19(2):89–98
56. Suzuki S, Hinokio Y, Ohtomo M, et al. The effects of coenzyme Q10 treatment on maternally inherited diabetes mellitus and deafness, and mitochondrial DNA 3243 (A to G) mutation. Diabetologia 1998; 41 (5):584–588
57. Götz ME, Gerstner A, Harth R, et al. Altered redox state of platelet coenzyme Q10 in Parkinson's disease. J Neural Transm 2000;107(1):41–48
58. Shults CW, Haas RH, Passov D, Beal MF. Coenzyme Q10 levels correlate with the activities of complexes I and II/III in mitochondria from parkinsonian and nonparkinsonian subjects. Ann Neurol 1997;42(2): 261–264
59. Isobe C, Abe T, Terayama Y. Levels of reduced and oxidized coenzyme Q-10 and 8-hydroxy-2′-deoxyguanosine in the cerebrospinal fluid of patients with living Parkinson's disease demonstrate that mitochondrial oxidative damage and/or oxidative DNA damage contributes to the neurodegenerative process. Neurosci Lett 2010;469(1):159–163
60. Hargreaves IP, Lane A, Sleiman PM. The coenzyme Q10 status of the brain regions of Parkinson's disease patients. Neurosci Lett 2008;447(1):17–19
61. Shults CW, Oakes D, Kieburtz K, et al; Parkinson Study Group. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol 2002;59(10):1541–1550
62. Müller T, Büttner T, Gholipour AF, Kuhn W. Coenzyme Q10 supplementation provides mild symptomatic benefit in patients with Parkinson's disease. Neurosci Lett 2003;341(3):201–204
63. Storch A, Jost WH, Vieregge P, et al; German Coenzyme Q (10) Study Group. Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q (10) in Parkinson disease. Arch Neurol 2007;64(7):938–944
64. NINDS NET-PD Investigators. A randomized clinical trial of coenzyme Q10 and GPI-1485 in early Parkinson disease. Neurology 2007;68(1):20–28
65. National Institutes of Health. Effects of Coenzyme Q10 (CoQ) in Parkinson Disease (QE3). ClinicalTrials. gov. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00740714?term=coenzyme+Q&rank=2. Accessed May 9, 2012
66. Koroshetz WJ, Jenkins BG, Rosen BR, Beal MF. Energy metabolism defects in Huntington's disease and effects of coenzyme Q10. Ann Neurol 1997;41(2):160–165
67. Beal MF. Coenzyme Q10 as a possible treatment for neurodegenerative diseases. Free Radic Res 2002; 36(4):455–460
68. Ferrante RJ, Andreassen OA, Dedeoglu A, et al. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington's disease. J Neurosci 2002;22(5):1592–1599
69. Schilling G, Coonfield ML, Ross CA, Borchelt DR. Coenzyme Q10 and remacemide hydrochloride ameliorate motor deficits in a Huntington's disease transgenic mouse model. Neurosci Lett 2001;315(3):149–153
70. Stack EC, Kubilus JK, Smith K, et al. Chronology of behavioral symptoms and neuropathological sequela in R6/2 Huntington's disease transgenic mice. J Comp Neurol 2005;490(4):354–370
71. Stack EC, Smith KM, Ryu H, et al. Combination therapy using minocycline and coenzyme Q10 in R6/2 transgenic Huntington's disease mice. Biochim Biophys Acta 2006;1762(3):373–380
72. Yang L, Calingasan NY, Wille EJ, et al. Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson's and Huntington's diseases. J Neurochem 2009;109(5):1427–1439
73. Huntington Study Group. A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington's disease. Neurology 2001;57(3):397–404
74. Hyson HC, Kieburtz K, Shoulson I, et al; Huntington Study Group Pre2CARE Investigators. Safety and tolerability of high-dosage coenzyme Q10 in Huntington's disease and healthy subjects. Mov Disord 2010;25(12):1924–1928
75. National Institutes of Health. Coenzyme Q10 in Huntington's Disease (HD) (2CARE). ClinicalTrials.gov. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00608881?term=coenzyme+Q10+and+huntington&rank=1 Accessed 2/15/12. Accessed May 9, 2012
76. Gatchel JR, Zoghbi HY. Diseases of unstable repeat expansion: mechanisms and common principles. Nat Rev Genet 2005;6(10):743–755
77. Cooper JM, Schapira AH. Friedreich's Ataxia: disease mechanisms, antioxidant and Coenzyme Q10 therapy. Biofactors 2003;18(1–4):163–171
78. Taroni F, DiDonato S. Pathways to motor incoordination: the inherited ataxias. Nat Rev Neurosci 2004; 5(8):641–655
79. Lodi R, Tonon C, Calabrese V, Schapira AH. Friedreich's ataxia: from disease mechanisms to therapeutic interventions. Antioxid Redox Signal 2006;8(3–4):438–443
80. Lodi R, Hart PE, Rajagopalan B, et al. Antioxidant treatment improves in vivo cardiac and skeletal muscle bioenergetics in patients with Friedreich's ataxia. Ann Neurol 2001;49(5):590–596
81. Hart PE, Lodi R, Rajagopalan B, et al. Antioxidant treatment of patients with Friedreich ataxia: fouryear follow-up. Arch Neurol 2005;62(4):621–626
82. Cooper JM, Korlipara LV, Hart PE, Bradley JL, Schapira AH. Coenzyme Q10 and vitamin E deficiency in Friedreich's ataxia: predictor of efficacy of vitamin E and coenzyme Q10 therapy. Eur J Neurol 2008; 15(12): 1371–1379
83. Folkers K, Osterborg A, Nylander M, Morita M, Mellstedt H. Activities of vitamin Q10 in animal models and a serious deficiency in patients with cancer. Biochem Biophys Res Commun 1997;234(2):296–299
84. Hodges S, Hertz N, Lockwood K, Lister R. CoQ10: could it have a role in cancer management? Biofactors 1999;9(2–4):365–370
85. Laaksonen R, Fogelholm M, Himberg JJ, Laakso J, Salorinne Y. Ubiquinone supplementation and exercise capacity in trained young and older men. Eur J Appl Physiol Occup Physiol 1995;72(1–2):95–100
86. Malm C, Svensson M, Ekblom B, Sjödin B. Effects of ubiquinone-10 supplementation and high intensity training on physical performance in humans. Acta Physiol Scand 1997;161(3):379–384
87. Weston SB, Zhou S, Weatherby RP, Robson SJ. Does exogenous coenzyme Q10 affect aerobic capacity in endurance athletes? Int J Sport Nutr 1997;7(3):197–206
88. Porter DA, Costill DL, Zachwieja JJ, et al. The effect of oral coenzyme Q10 on the exercise tolerance of middle-aged, untrained men. Int J Sports Med 1995; 16(7):421–427
89. Braun B, Clarkson PM, Freedson PS, Kohl RL. Effects of coenzyme Q10 supplementation on exercise performance, VO2max, and lipid peroxidation in trained cyclists. Int J Sport Nutr 1991;1(4):353–365
90. Bonetti A, Solito F, Carmosino G, Bargossi AM, Fiorella PL. Effect of ubidecarenone oral treatment on aerobic power in middle-aged trained subjects. J Sports Med Phys Fitness 2000;40(1):51–57
91. Willis R, Anthony M, Sun L, Honse Y, Qiao G. Clinical implications of the correlation between coenzyme Q10 and vitamin B6 status. Biofactors 1999;9(2–4):359–363
92. Mattila P, Kumpulainen J. Coenzymes Q9 and Q10: Contents in foods and dietary intake. J Food Compost Anal 2001;14(4):409–417
93. Kamei M, Fujita T, Kanbe T, et al. The distribution and content of ubiquinone in foods. Int J Vitam Nutr Res 1986;56(1):57–63
94. Weber C, Bysted A, Hølmer G. Coenzyme Q10 in the diet—daily intake and relative bioavailability. Mol Aspects Med 1997;18(Suppl):S251–S254
95. Shults CW, Flint Beal M, Song D, Fontaine D. Pilot trial of high dosages of coenzyme Q10 in patients with Parkinson's disease. Exp Neurol 2004;188(2):491–494
96. Hendler SS, Rorvik DR, eds. PDR for Nutritional Supplements. Montvale, NJ: Medical Economics Company, Inc; 2001
97. Lönnrot K, Holm P, Lagerstedt A, Huhtala H, Alho H. The effects of lifelong ubiquinone Q10 supplementation on the Q9 and Q10 tissue concentrations and life span of male rats and mice. Biochem Mol Biol Int 1998;44(4):727–737
98. Zhang Y, Aberg F, Appelkvist EL, Dallner G, Ernster L. Uptake of dietary coenzyme Q supplement is limited in rats. J Nutr 1995;125(3):446–453
99. Svensson M, Malm C, Tonkonogi M, Ekblom B, Sjödin B, Sahlin K. Effect of Q10 supplementation on tissue Q10 levels and adenine nucleotide catabolism during high-intensity exercise. Int J Sport Nutr 1999; 9(2):166–180
100. Matthews RT, Yang L, Browne S, Baik M, Beal MF. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc Natl Acad Sci U S A 1998;95(15):8892–8897
101. Keith M, Mazer CD, Mikhail P, Jeejeebhoy F, Briet F, Errett L. Coenzyme Q10 in patients undergoing CABG: Effect of statins and nutritional supplementation. Nutr Metab Cardiovasc Dis 2008;18(2):105–111
102. Hathcock JN, Shao A. Risk assessment for coenzyme Q10 (Ubiquinone). Regul Toxicol Pharmacol 2006; 45(3):282–288
103. Jellin JM. Natural Medicines Comprehensive Database. Therapeutic Research Faculty. Available at: http://www.naturaldatabase.com. Accessed May 9, 2012
104. Heck AM, DeWitt BA, Lukes AL. Potential interactions between alternative therapies and warfarin. Am J Health Syst Pharm 2000;57(13):1221–1227, quiz 1228–1230
105. Folkers K, Langsjoen P, Willis R, et al. Lovastatin decreases coenzyme Q levels in humans. Proc Natl Acad Sci U S A 1990;87(22):8931–8934
106. Colquhoun DM, Jackson R, Walters M, et al. Effects of simvastatin on blood lipids, vitamin E, coenzyme Q10 levels and left ventricular function in humans. Eur J Clin Invest 2005;35(4):251–258
107. Mabuchi H, Higashikata T, Kawashiri M, et al. Reduction of serum ubiquinol-10 and ubiquinone-10 levels by atorvastatin in hypercholesterolemic patients. J Atheroscler Thromb 2005;12(2):111–119
108. Bargossi AM, Battino M, Gaddi A, et al. Exogenous CoQ10 preserves plasma ubiquinone levels in patients treated with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Int J Clin Lab Res 1994;24(3):171–176
109. Watts GF, Castelluccio C, Rice-Evans C, Taub NA, Baum H, Quinn PJ. Plasma coenzyme Q (ubiquinone) concentrations in patients treated with simvastatin. J Clin Pathol 1993;46(11):1055–1057
110. Ghirlanda G, Oradei A, Manto A, et al. Evidence of plasma CoQ10-lowering effect by HMG-CoA reductase inhibitors: a double-blind, placebo-controlled study. J Clin Pharmacol 1993;33(3):226–229
111. Laaksonen R, Jokelainen K, Laakso J, et al. The effect of simvastatin treatment on natural antioxidants in low-density lipoproteins and high-energy phosphates and ubiquinone in skeletal muscle. Am J Cardiol 1996;77(10):851–854
112. Laaksonen R, Ojala JP, Tikkanen MJ, Himberg JJ. Serum ubiquinone concentrations after short- and long-term treatment with HMG-CoA reductase inhibitors. Eur J Clin Pharmacol 1994;46(4):313–317
113. Elmberger PG, Kalén A, Lund E, et al. Effects of pravastatin and cholestyramine on products of the mevalonate pathway in familial hypercholesterolemia. J Lipid Res 1991;32(6):935–940
114. Ashton E, Windebank E, Skiba M, et al. Why did high-dose rosuvastatin not improve cardiac remodeling in chronic heart failure? Mechanistic insights from the UNIVERSE study. Int J Cardiol 2011;146(3):404–407
115. Hughes K, Lee BL, Feng X, Lee J, Ong CN. Coenzyme Q10 and differences in coronary heart disease risk in Asian Indians and Chinese. Free Radic Biol Med 2002;32(2):132–138
116. Hargreaves IP, Duncan AJ, Heales SJ, Land JM. The effect of HMG-CoA reductase inhibitors on coenzyme Q10: possible biochemical/clinical implications. Drug Saf 2005;28(8):659–676
117. Laaksonen R, Jokelainen K, Sahi T, Tikkanen MJ, Himberg JJ. Decreases in serum ubiquinone concentrations do not result in reduced levels in muscle tissue during short-term simvastatin treatment in humans. Clin Pharmacol Ther 1995;57(1):62–66