 What is the most likely primary diagnosis for this patient?
What is the most likely primary diagnosis for this patient?A 30-year-old woman presents to your office with the chief complaint of a “yeast infection that I can’t seem to shake.” She also has noticed that she has been urinating more frequently, but thinks that it is related to her yeast infection. Over the last several years she has noticed that she has gained more than 40 lb. She has tried numerous diets, most recently a low-carbohydrate, high-fat diet. The patient’s only other pertinent history is that she was told to watch her diet during pregnancy because of excessive weight gain. Her baby had to be delivered by cesarean because he weighed more than 9 lb. Her family history is not known, as she was adopted. On physical examination, her blood pressure is 138/88 mm Hg, her pulse is 72 beats/min, and her respiratory rate is 16 breaths/min. Her height is 65 in and her weight is 190 lb (body mass index [BMI] = 31.6). Her physical examination reveals darkened skin that appears to be thickened on the back of her neck and moist, reddened skin beneath her breasts. Her pelvic examination reveals a thick, white, vaginal discharge. A wet preparation from the vaginal discharge reveals branching hyphae consistent with Candida. A urine dipstick is performed that is negative for leukocyte esterase, nitrites, protein, and glucose.
 What is the most likely primary diagnosis for this patient?
What is the most likely primary diagnosis for this patient?
 What physical findings does she have that are suggestive of the diagnosis and have implications for management?
What physical findings does she have that are suggestive of the diagnosis and have implications for management?
 What diagnostic studies should be ordered at this time?
What diagnostic studies should be ordered at this time?
Summary: A 30-year-old obese woman presents with a difficult-to-treat yeast infection and polyuria. She has gained 40 lb in spite of efforts to lose weight. She has a history of significant weight gain and having been told to “watch her diet” during a pregnancy. On examination she is found to have a BMI of 31.6, acanthosis nigri-cans, candidal vaginitis, but a negative urine dip.
• Most likely diagnosis: Type 2 diabetes mellitus.
• Significant physical findings: Obesity, acanthosis nigricans, blood pressure that is elevated for a diabetic (goal is <130/80 mm Hg), candidal vaginitis, and possibly candidal skin infection under her breasts.
• Diagnostic studies: Blood glucose measurement (random sugar can be checked in the office with a finger-stick sample); follow-up testing should include electrolytes, blood urea nitrogen (BUN), creatinine, fasting lipids, urine microalbumin: creatinine ratio, and hemoglobin A1c.
1. Know the diagnostic criteria for diabetes mellitus, including signs and symptoms, physical findings, and diagnostic studies.
2. Know the pathophysiologic and epidemiologic differences between type 1 and type 2 diabetes mellitus.
3. Learn the treatment options for diabetic patients.
4. Be aware of the acute emergencies that can occur to diabetics and how to manage them.
Diabetes mellitus is one of the most common medical problems encountered in medical practice. There are an estimated 26 million (~8%) diabetics in the United States and the number is increasing both in the United States and worldwide. Diabetes affects all ethnic groups, but there is a disproportionate burden of disease in African Americans, Native Americans, and Hispanics. The global epidemic of obesity has led to a dramatic increase in the number of type 2 diabetics presenting with disease in their teens and twenties.
The complications of diabetes are myriad. Diabetics are 6 to 10 times more likely than nondiabetics to be hospitalized for cardiovascular disease and 15 times more likely to be hospitalized for peripheral vascular diseases. It is the leading cause of blindness in working-age adults in the United States, most of which is preventable. It is also the leading cause for end-stage renal disease and nontraumatic amputations. In 2007, the direct and indirect cost related to diabetes mellitus was estimated to be $174 billion dollars.
Other complications that may be less well known to patients but that are attributable to diabetes include neuropathic, gastrointestinal, and immunologic changes. Peripheral neuropathy, leading to reduced sensation or pain, can lead to the development of ulcerations, infections, or injuries of the extremities. Gastroparesis can be a difficult-to-manage problem that makes diabetes more difficult to manage by impairing the patient’s ability to eat properly. Immunologic changes make diabetics more prone to opportunistic infections, such as fungal skin or genitourinary infections.
Impaired glucose tolerance or frank diabetes may be present for years prior to the diagnosis of type 2 diabetes. In the case presented, the history of excessive weight gain during pregnancy with a large baby and cautions on watching her diet may be a sign of a history of gestational diabetes. Women with gestational diabetes have an increased risk of developing nongestational diabetes.
As in the case presented, difficult-to-treat or recurrent fungal infections may be the initial presentation that leads to the diagnosis of diabetes. This patient has both vaginal and skin infections. Although, in this case the diagnosis is diabetes, other immune deficiency states must be considered when recurrent fungal infections are found. In the appropriate setting, HIV or other immunosuppressive conditions must be considered.
The symptom of polyuria should also lead to an increased suspicion for diabetes. High serum glucose levels function as an osmotic diuretic, resulting in frequent urination. This is often associated with polydipsia, a state of extreme thirst. Patients with type 1 diabetes also may present with polyphagia. Their lack of insulin prevents their food intake from being appropriately metabolized, resulting in a state of hunger for which they will frequently eat but not feel sated.
The absence of glucose in the urine dipstick does not exclude the diagnosis and should not delay a blood glucose measurement. Glucosuria occurs when the blood glucose level is greater than a renal “threshold” level, above which the glucose will spill into the urine. The lack of glucosuria only shows that the blood sugar level is not above this threshold level. Overt signs of insulin resistance (acanthosis nigricans, elevated blood pressure, obesity) also make the diagnosis of type 2 diabetes more likely.
General approach to managing diabetes is geared at secondary prevention of macrovascular (accelerated coronary artery disease, accelerated cerebral and peripheral vascular disease) and microvascular (retinopathy, nephropathy, and neuropathy) complications. Overall diabetic patient should be “controlled”: (1) glycemic control with a goal of hemoglobin A1c of 7% or less, (2) low-density lipoprotein (LDL) 70 to 100, (3) BP less than 130/80 mm Hg, and (4) life style modifications including a diet consisting of low carbohydrates and low saturated fat and physical activity counseling (at least 150 min/wk of moderate-intensity aerobic physical activity [50%-70% maximum heart rate] and resistance training [3 times/wk]).
DCCT: Diabetes Control and Complications Trial, a large, prospective, randomized controlled study of the advantages and disadvantages of “tight” versus “loose” diabetic control in type 1 diabetes.
UKPDS: United Kingdom Prospective Diabetes Study, a large, prospective, randomized controlled study of interventions and outcomes in type 2 diabetes.
Diabetes mellitus is a general term for several different diseases that result in high blood sugar levels and that eventually lead to microvascular and macrovascular complications. The major classifications of diabetes mellitus are type 1 diabetes, type 2 diabetes, and gestational diabetes.
Type 1 diabetes (previously called juvenile diabetes, juvenile-onset diabetes mellitus [JODM], or insulin-dependent diabetes mellitus [IDDM]) is a chronic disease of carbohydrate fat and protein metabolism due to a lack of insulin, resulting from autoimmune destruction of insulin-producing pancreatic β cells. The pathogenesis of type 1 diabetes mellitus is mediated by multiple risk factors that include viral infections, genetic susceptibility, and environmental risks.
Because of the lack of insulin, which is required for the metabolism of glucose, type 1 diabetics are prone to metabolize fats, with the resultant production of ketones. An extreme result of this process is diabetic ketoacidosis, a syndrome characterized by hyperglycemia, high levels of serum acetone, and an anion gap metabolic acidosis. This often occurs during times of physical stress, such as an infection or myocardial infarction, or when the patient does not use his or her insulin. Diabetic ketoacidosis is a medical emergency, requiring hospitalization, careful insulin management, correction of acidosis and electrolyte disturbances, and evaluation for the underlying cause of the condition.
Type 2 diabetes (previously called adult onset diabetes mellitus [AODM], non–insulin-dependent diabetes mellitus [NIDDM]) patients, in contrast to type 1 diabetics, in whom there is a lack of insulin, are typically hyperinsulinemic. Their disease results primarily from insulin resistance in the peripheral tissue and this resistance is often related to obesity. Type 2 diabetics often manifest signs of insulin resistance for many years prior to the diagnosis of overt diabetes. This type accounts for at least 90% of the diagnosed cases, and virtually all undiagnosed diabetes, in the United States.
Type 2 diabetes has a stronger familial predisposition than type 1. Type 2 diabetics often have a family history of the disease. The genetic factors are multifactorial and have not been identified. It is strongly associated with obesity and its complications: metabolic syndrome, hyperinsulinemia, hypertension, dyslipidemia, hyperglycemia, and central obesity.
Uncontrolled type 2 diabetics can achieve extremely high blood sugars without developing ketosis and acidosis. This type is more prone to hyperosmolar states because of the high blood sugar levels. Nonketotic hyperosmolar syndrome occurs when blood sugar levels become highly elevated, often approaching 1000 mg/dL. This may be the presenting symptom of type 2 diabetes, or may result from a concurrent illness or failure to take medications. The serum osmolarity is elevated (>320 mOsm/kg) and the patient has a large fluid deficit (up to 9 L). In severe cases, coma or death can occur. This can be managed with hospitalization, rehydration, treatment of underlying illnesses, and, sometimes, the judicious use of insulin to overcome the acute glucose toxicity.
Gestational diabetes occurs in 3% to 10% of all pregnancies. Typically, women have 50% more insulin in their third trimester. Gestational diabetes is triggered by increased insulin resistance caused by elevated chorionic somatomammotropin, progesterone, and estrogens all of which act as insulin antagonist. Maternal and fetal complications are numerous. Maternal complications are hyperglycemia, diabetic ketoacidosis (DKA), increased urinary tract infection (UTI) risk, increased pregnancy-induced hypertension/preeclampsia, and retinopathy. Fetal effects are congenital malformations, macrosomia, respiratory distress syndrome, hypoglycemia, hyperbilirubinemia, hypocalcemia, polycythemia, and hydramnios. Women with gestational diabetes are more prone to develop non–pregnancy-related type 2 diabetes and should be screened with a glucose tolerance test postpartum.
Risk factors for gestational diabetes include age older than 25 years, member of a high-incidence racial group (Native American, African American, Hispanic American, South or East Asian, Pacific Islander), BMI of 25 or more, history of glucose intolerance, previous history of gestational diabetes, and history of diabetes in a first-degree family member.
The American College of Obstetricians and Gynecologists recommends screening all women for gestational diabetes. A 50-g 1-hour glucose challenge test (GCT) is administered to high-risk pregnant women at the initial visit and to rescreen at 24 to 28 weeks, and at 24 to 28 weeks for all pregnant women. If 1-hour glucose challenge is greater than 130 mg/dL, then 100-g 3-hour glucose challenge is needed. The 100-g glucose is administered orally with serum glucose drawn at fasting and at 1, 2, and 3 hours. Diagnosis is made based on greater than two abnormal results (95, 180, 155, 140 mg/dL, respectively). Gestational diabetes is treated with careful diet management via patient education and nutritional counseling and when necessary, insulin.
DIAGNOSIS
The diagnostic criteria for diabetes are:
1. A random glucose 200 mg/dL or more along with classic symptoms that include polydipsia, polyuria, polyphagia, frequent infections, and weight loss
2. A fasting glucose ≥126 mg/dL (no caloric intake for at least 8 hours)
3. A 2-hour plasma glucose 200 mg/dL or more after a 75-g glucose load
4. Hemoglobin A1c (HbA1c) of 6.5% or greater
A HbA1c <6% is considered normal and 6% to 6.5% is considered “pre-diabetes.” HbA1c is used to estimate the average glucose over the past 3 months in those who are diagnosed with diabetes. Fructosamine is used in patients with haemoglobinopathies, recent blood loss, or a recent change in diet or treatment and indicates average glucose levels over a 2- to 3-week period.
Measurement of C-peptide and insulin levels can be used to distinguish type 2 from type 1 diabetes when the history, physical examination, and other tests, such as serum ketones and osmolality, are not enough. Other tests recommended by the American Diabetes Association are fasting lipid profiles (at the time of diagnosis and, at least, annually thereafter), serum creatinine, urinalysis, urine microalbumin: creatinine ratios (at time of diagnosis in type 2 diabetics and annually thereafter; in type 1 diabetics who have had disease for 5 years and annually thereafter), annual dilated eye examinations, regular foot examinations, ECG (in adults), and, in type 1 diabetics, thyroid disease screening with a thyroid-stimulating hormone (TSH).
The treatment for type 1 diabetes involves the use of insulin. In most cases, combination therapy using short-acting insulin prior to meals and an intermediate- or long-acting basal insulin is used. Insulin pump therapy, which provides a continuous subcutaneous infusion of short-acting insulin, is an alternative. Insulin management requires careful and frequent self-monitoring of glucose, often with adjustment of insulin dosage based on the glucose levels, amount of physical activity, and caloric/carbohydrate intake (Table 51–1).
Table 51–1 • INSULIN PREPARATIONS
Type 2 diabetics and those at risk of developing diabetes should be educated on the importance of diet and exercise as key components of their management. In some cases, that will be all that is needed to achieve appropriate control. An initial goal that is achievable by many is a 10% weight loss. When lifestyle changes alone do not result in adequate control, numerous oral agents are available. For severely obese patients, surgical gastric bypass may be considered, although long-term risk and benefits have not been fully explored.
Medications for prevention are currently not recommended but can be considered if lifestyle modification is unsuccessful. Several mediations are available for treating type 2 diabetes (Table 51–2). Metformin is the drug of choice to begin with unless contraindications are present.
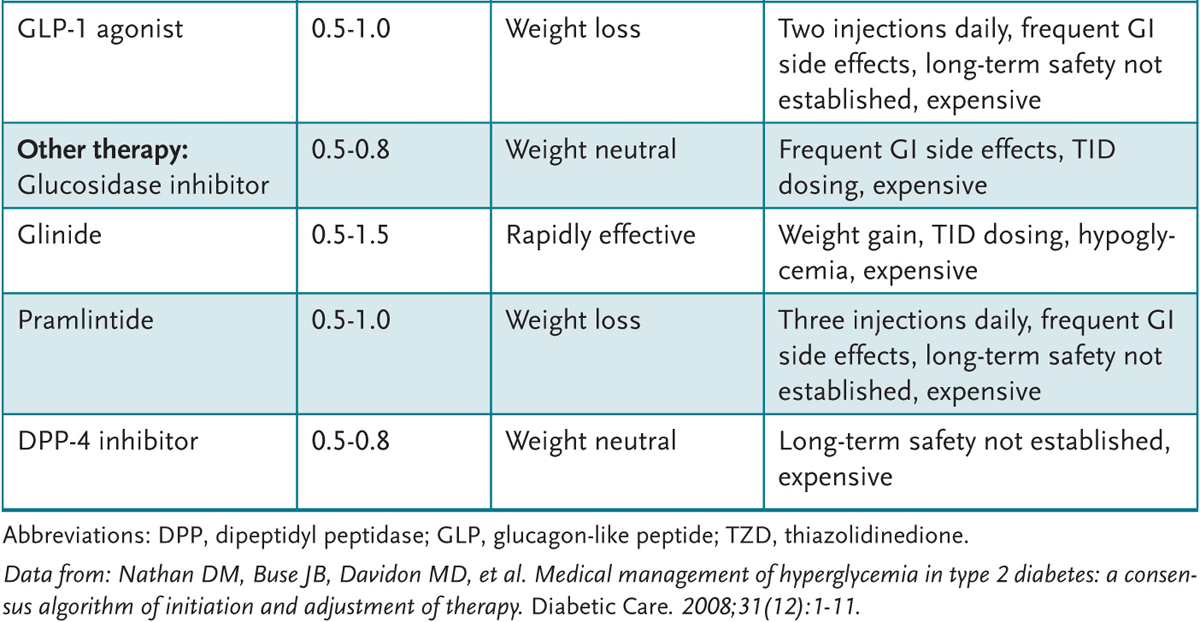
Table 51–2 • ORAL HYPOGLYCEMICS
Biguanides (metformin) act on the liver to decrease glucose output during gluconeogenesis. Secondary actions include improved insulin sensitivity in the liver and muscle and a hypothesized decrease in intestinal absorption of glucose. Metformin can lower the HbA1c by 1.5% to 2%. The UKPDS showed a significant reduction in cardiovascular events, diabetes-related deaths, and all causes of mortality in those who used metformin. Other advantages include no potential hypoglycemia, reduced insulin levels, a potential weight loss, and a reduction in triglycerides and LDL cholesterol. The efficacy, safety, and improved outcomes make it a popular first-line agent in type 2 diabetes.
The most common side effects are gastrointestinal, such as nausea and diarrhea. These side effects are reduced by starting with lower doses and giving the medication with meals. The more dangerous side effect is the development of lactic acidosis. The risk of this potentially fatal side effect is increased by renal insufficiency. Metformin use is contraindicated in those with a creatinine more than 1.5 mg/dL in men and more than 1.4 mg/dL in women, hepatic insufficiency, or congestive heart failure. It is Category B in pregnancy and probably safe in nursing mothers. It is the oral agent of choice in type 2 diabetes in children older than age 10 years.
Sulfonylureas were the first oral agents available for type 2 diabetes. Their principal action is to function as insulin secretagogues that stimulate β cells in the pancreas to secrete insulin. Advantages include a potential 2% reduction in HbA1c, once- or twice-a-day dosing, and relatively low cost. Disadvantages are poor response in 20% of patients, a tendency of the users to gain weight, and a tendency for the medications to lose effectiveness over time. As insulin secretagogues, sulfonylureas carry a risk of causing hypoglycemia.
Sulfonylureas and insulin are considered the best validated second-line add-on therapy. The following medications are less well validated. Further studies are necessary to determine how these agents may play a role in the overall care of type 2 diabetics.
The principal action of thiazolidinediones (TZDs) is improving insulin sensitivity in muscle and adipose tissue. Secondary actions are decreased hepatic gluconeo-genesis and increased peripheral glucose utilization. Among their advantages is a decrease in triglyceride and increase in high-density lipoprotein (HDL) cholesterol levels. Because they are metabolized in the liver, they can be used in patients with renal impairment. They also do not, when used by themselves, cause hypoglycemia. Disadvantages include a slight weight gain and a slow onset of action. These agents may take up to 12 weeks to fully become effective. They cause water retention, which is of concern with renal compromise and congestive heart failure. There is some current controversy on whether the benefits of this class of medications outweigh the risks.
Meglitinides are short-acting secretagogues that increase insulin secretion from the pancreas. These medications are taken no more than 1 hour before meals because of the rapid onset and short duration of action. They are useful in patients whose blood sugars vary at mealtime but who have controlled fasting glucose levels. They reduce HbA1c levels from 0.5% to 2%. The disadvantages include a risk of hypoglycemia (especially if the medication is taken but no meal is then eaten) and expense. They should not be used in patients with hepatic dysfunction.
α-Glucosidase inhibitors delay carbohydrate absorption by inhibiting α-glucosidase in the small intestine, which decreases postprandial hyperglycemia. They reduce HbA1c levels by 0.7% to 1.0%. This class of medication may offer benefits to patients with erratic eating habits, as hypoglycemia will not occur if meals are skipped. The principal side effects are GI, including flatulence. These medications are contraindicated in ketoacidosis and in hepatic disorders.
Pramlintide is an amylinomimetic agent that has physiologic actions equivalent to those of human amylin (glucoregulatory hormone synthesized by pancreatic β cells and released with insulin in response to a meal). It inhibits inappropriately high glucagon secretion during episodes of hyperglycemia (eg, after a meal) in patients with type 1 or type 2 diabetes mellitus and does not impair normal glucagon response to hypoglycemia. It is a subcutaneous medication that does not require adjustments for renal or hepatic impairment. It reduces HbA1c levels by 0.5% to 1.0%. Known side effects are hypoglycemia, nausea, and diarrhea. Often dosing requires titration to balance hypoglycemia and preprandial glycemic control.
GLP-1 agonist (glucagon-like peptide-1 mimetic). Exenatide is an incretin mimetic which belongs to this class. It is a synthetic peptide that stimulates insulin release. Adjunctive therapy for type 2 diabetics with inadequate glycemic control while on either metformin, sulfonylurea, and/or thiazolidinedione (glitazone). It reduces HbA1c levels by 0.5% to 1.0%. Side effects include hypoglycemia when added to sulfonylurea (but not when added to metformin), nausea, vomiting, diarrhea, and acute pancreatitis.
DPP-4 inhibitor (dipeptidyl peptidase-4 inhibitor). Sitagliptin, saxagliptin, and others pending approval inhibit dipeptidyl peptidase-4 (DPP-4), an enzyme that inactivates incretin hormones glucagon-like peptide (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). GIP and GLP-1 stimulate insulin synthesis and release from pancreatic β cells in a glucose-dependent manner. GLP-1 also decreases glucagon secretion from pancreatic α cells in a glucose-dependent manner, leading to reduced hepatic glucose production. Used as monotherapy and as an adjunct to diet and exercise for management of type 2 diabetes mellitus in patients whose hyperglycemia cannot be controlled by diet and exercise alone. Used in combination with metformin, a sulfonylurea, or a thiazolidinedione as second-line therapy for management of type 2 diabetes mellitus in patients who do not achieve adequate glycemic control with diet, exercise, and metformin, sulfonylurea, or thiazolidinedione monotherapy. It reduces HbA1c levels by 0.5% to 0.8%. The principal side effects are upper respiratory symptoms and severe hypersensitivity (ie, anaphylaxis and/or angioedema).
The goal of diabetic management is to safely lower the blood sugar so as to reduce the risk of macrovascular and microvascular complications. Both the UKPDS and DCCT showed lower rates of complications in controlled diabetes. The goal of treatment is to achieve a HbA1c of less than 7%, although some authorities advocate 6.5% as a goal. Goal fasting blood sugar is 70 to 130 mg/dL and 1- to 2-hour postprandial sugar is less than 180 mg/dL.
Other treatments are equally important to tight glucose control in the effort to reduce adverse events, such as heart attacks and strokes. The UKPDS clearly showed that tight blood pressure control is effective in reducing cardiovascular events. The blood pressure goal in diabetes is less than 130/80 mm Hg. Diabetes is considered a coronary heart disease risk equivalent for decisions regarding lipid management. The goal LDL cholesterol level is less than 100 mg/dL. All diabetics should be advised to be immunized with the pneumococcal vaccine and to get annual influenza vaccination.
Hypoglycemic symptoms are related to the brain and the sympathetic nervous system. Decreased levels of glucose lead to deficient cerebral glucose availability that can manifest as confusion, difficulty with concentration, irritability, hallucinations, focal impairments (eg, hemiplegia), and eventually, coma and death. Stimulation of the sympatho-adrenal nervous system leads to sweating, palpitations, tremulousness, anxiety, and hunger.
Causes of hypoglycemia include fasting, exogenous insulin, autoimmunity, sulfonylurea abuse, and hormonal deficiency (hypoadrenalism, hypopituitary, glucagon deficiency).
When hypoglycemia is suspected and the patient is conscious and cooperative, juice, soda, candy, or some other sugar-containing product can rapidly alleviate the symptoms. If the person is not able to take something by mouth, rapid administration of IM glucagon can be effective. In a hospital setting, or when IV access is available, an injection of 50% dextrose (D50) rapidly corrects the problem. Following any of these therapies, the patient should be closely watched, as the hypoglycemia may recur (especially if the patient uses a long-acting insulin or oral hypoglycemic agent).
51.1 A 16-year-old adolescent girl has had an increased craving for sweets. She often consumes two to three ice cream sundaes and four large sodas a day, but has still managed to maintain her weight. Friends often notice her using the bathroom more frequently but she denies any episodes of purging and states that she just has to urinate after drinking so much cola. On physical examination she is 5 ft 8 in and 110 lb and thyroid is nonpalpable. Which of the following test results is diagnostic of diabetes mellitus?
A. Single glucose of 150 mg/dL
B. A 2-hour oral glucose tolerance test greater than 200 mg/dL with a 100-g glucose load
C. A random glucose greater than 200 mg/dL with symptoms such as polydipsia or polyuria
D. A HbA1c of 6.3%
51.2 A 7-year-old is brought to the office with symptoms of polydipsia, polyphagia, polyuria, and weight loss of 8 lb. For the past 24 hours he has had abdominal pain and vomiting. An urinalysis done in the office shows the presence of glucose and ketones. A finger-stick blood glucose is more than 500 mg/dL. Which of the following is the most appropriate management?
A. Prescription for oral metformin and referral to a nutritionist
B. Hospitalization with institution of insulin and IV fluids
C. Prescription for insulin to be started at home, with follow-up in 24 hours
D. Treatment for acute gastroenteritis and referral to an endocrinologist
51.3 An 83-year-old man was diagnosed with type 2 diabetes 3 months ago. He has altered his diet and tries to walk at least half a mile in the evenings. He drinks a glass of wine with lunch and dinner. For the past week he has felt dizzy upon standing and has fallen on two occasions, but he never lost consciousness. After the last episode he presented to the local ER where his blood pressure was found to be 155/76 mm Hg, HR 74 beats/min, and RR 16 breaths/min. A finger stick showed a glucose level of 84. Which of the following classes of medications has the lowest incidence of causing hypoglycemia when given as single-agent therapy?
A. Biguanide
B. Insulin
C. Sulfonylurea
D. Meglitinide
51.4 A 38-year-old G1P0 woman who is a new patient presents to the office at 10-week gestation. She is known to have type 2 diabetes. She currently takes metformin and her last HbA1c was 10.4%. Her urine dip is negative for ketones, protein, and leukocytes. She has no other medical problems and does not drink or smoke. On physical examination she is 5 ft 4 in and weighs 202 lb. She inquires about the risk to her fetus. As compared to gestational diabetes, this patient is at increased risk for which of the following?
A. Fetal malformations
B. Fetal macrosomia
C. Polyhydramnios
D. Shoulder dystocia
51.1 C. Diabetes mellitus can be defined by measurement of an 8-hour fasting glucose more than 125 mg/dL; a random glucose of 200 mg/dL or more with classic symptoms or a 2-hour glucose tolerance test of 200 mg/dL or more after a 75-g glucose load. Recently the American Diabetes Association (ADA) recommended that a HbA1c of ≥6.5% can be used for diagnosing diabetes.
51.2 B. This is a typical presentation of diabetic ketoacidosis, a medical emergency. This is a common initial presentation of type 1 diabetes. This child requires immediate hospitalization, IV fluids, and insulin.
51.3 A. Biguanides (metformin) are effective medications for the treatment of type 2 diabetes; they do not cause hypoglycemia when given as monotherapy. Insulin and insulin secretagogues carry a risk of hypoglycemia as a complication of therapy.
51.4 A. Gestational diabetes is more likely to lead to fetal macrosomia and polyhydramnios. Both gestational and pregestational diabetes are associated with shoulder dystocia. Pregestational diabetes is associated with greater fetal malformations due to the higher serum glucose levels during organogenesis (5- to 10-week gestational age), whereas gestational diabetes tends to be associated with hyperglycemia after 20-week gestation, when the fetal organs have already formed. Preterm labor occurs at same frequency in diabetics as nondiabetics.
American Diabetes Association (ADA) consensus statement on managing preexisting diabetes for pregnancy. Diabetes Care. 2008;31(5):1060.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34 (Suppl 1):S62-S69.
American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2002;25(Suppl 1):S33-S49.
Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. NEJM. 2005;352(24):2477-2486.
Knowler WC, Barret-Connor E, Fowler SE, et al. Reduction in the incident of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm of initiation and adjustment of therapy. Diabetic Care. 2008;31(12):1-11.
Powers AC. Diabetes mellitus. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill; 2008:2275-2305.