
Fig. 6.43 Sites and nomenclature of intracranial (a) and spinal (b) infections.
Key Point
The CNS can be infected by bacteria, viruses, parasites, and other microorganisms. Different organisms tend to infect either the meninges or the brain substance itself. Thus, there are two main forms of intracranial infection, meningitis and encephalitis. Infectious diseases of the CNS can be classified, broadly speaking, into three basic clinical situations: a predominantly meningitic syndrome, which can be either acute or subacute to chronic, and a predominantly encephalitic syndrome. These three syndromes, and the organisms that cause them, will be discussed individually in this section. In addition, focal infections of the brain parenchyma can lead to the formation of brain abscesses, which will also be discussed.
Meningitis and encephalitis can also appear in mixed forms: a meningeal infection can spread to the brain (and/or spinal cord), or vice versa, causing meningo(myelo)encephalitis. The latter term is only used if the patient unequivocally manifests clinical signs of both meningeal and cerebral involvement.
The sites and nomenclature of infectious diseases of the CNS are summarized in ▶ Fig. 6.43.

Fig. 6.43 Sites and nomenclature of intracranial (a) and spinal (b) infections.
The general features of the meningitic syndrome are as follows:
Headache.
Fever (though elderly and immune-deficient patients are often afebrile).
Nausea and vomiting due to intracranial hypertension.
Meningismus, which, in severe cases, may be evident as a spontaneous extended posture of the neck, or opisthotonus.
Positive meningeal signs, with the patient examined in the supine position:
Lasègue sign: passive elevation of an extended leg to 45 degrees (at most) induces shooting pain into the leg.
Brudzinski sign: passive neck flexion induces reflexive knee flexion.
Kernig sign: passive elevation of the extended legs induces reflexive knee flexion.
The clinical aspects of individual types of meningitis depend on the pathogen and the immune state of the host.
Meningitis can be classified as shown in ▶ Table 6.19.
|
Syndrome |
Leukocyte count |
Protein |
Glucose |
Lactate |
|
Acute bacterial meningitis |
100 to 1,000, mainly multinucleated |
High |
Low or very low |
High |
|
Acute viral meningitis |
100, more mono- than multinucleated |
Normal or mildly elevated |
Normal or mildly diminished |
Normal |
|
Chronic meningitis |
100, mainly mononuclear |
High or very high |
Low or very low |
Normal or high |
The encephalitic, rather than meningitic, syndrome is characterized by focal neurologic and neuropsychological deficits as well as a variably severe impairment of consciousness. Encephalitis, like meningitis, can be of viral, bacterial, fungal, protozoal, or parasitic origin. Prion diseases are a special category of encephalitis. Encephalitis can also arise on an autoimmune or paraneoplastic basis; in such cases, it mainly affects the limbic system (see section ▶ 6.8.8).
The infectious processes that cause encephalitis often also involve other structures in the nervous system besides the brain (e.g., the peripheral nerves and plexuses, nerve roots, spinal cord, and meninges). In particular, the three important clinical varieties of spirochetal infection (syphilis, borreliosis, and leptospirosis) often present initially with meningitic or polyradiculitic/polyneuritic manifestations.
The general features of an encephalitic syndrome are as follows:
Fever.
Headache.
Impairment of consciousness.
Personality changes and neuropsychological abnormalities.
Epileptic seizures.
Focal neurologic deficits.
The diseases covered in this section are listed in ▶ Table 6.20.
|
Meninigitic syndrome |
Encephalitic syndrome |
|
Acute meningitis
Chronic meningitis
|
Viral encephalitis
Fungal, protozoal, and parasitic encephalitis (Meningo)encephalitis due to spirochetal infection
Encephalitis in prion disease
Encephalitis in slow viral disease Noninfectious encephalitis
|
|
Abbreviations: ESME, early summer meningoencephalitis; HIV, human immunodeficiency virus. |
|
History The following should be specifically asked about:
Contact with animals (e.g., certain viruses, leptospira, Coxiella burnetii).
Tick bites, fleas, mites (borreliosis, early summer meningoencephalitis, rickettsiae).
Swimming in ponds (amebiasis).
Consumption of unpasteurized dairy products (listeriosis, brucellosis).
Past medical history (immune deficiency, diabetes, surgery, endocarditis, or recent pneumonia, mumps, or measles). The patient’s past illnesses may predispose to CNS infection with certain types of pathogen.
Physical examination
Meningismus (abnormal response to passive movement of the neck), neurologic examination.
Inspection: skin (petechiae, e.g., in meningococcal sepsis), oral cavity and throat (pharyngitis, tonsillitis, teeth, and gums).
Palpation: lymph nodes, trigeminal exit points (sinusitis as the source of CNS infection).
Auscultation of the heart and lungs.
Further procedure If the clinical signs warrant, blood cultures should be drawn.
No intracranial hypertension or focal deficit: CSF is obtained by LP and treatment is begun with antibiotics (directed against the most likely pathogen) and glucocorticoids (dexamethasone). The antibiotic treatment is tailored later based on the specific pathogen detected.
Presence of intracranial hypertension and/or focal deficits: immediate initiation of treatment with antibiotics and glucocorticoids (dexamethasone), then head MRI. An LP should not be performed until the signs of intracranial hypertension have subsided.
Pathogens and routes of infection The bacteria that cause bacterial meningitis can reach the meninges by any of three routes:
Hematogenous spread (e.g., from a focus of infection in the nasopharynx).
Continuous extension (e.g., from the middle ear or paranasal sinuses).
Direct contamination (through an open wound or CSF fistula).
The organisms that most commonly cause acute, purulent meningitis are:
In neonates, Escherichia coli, group B streptococci, and Listeria monocytogenes.
In children, Haemophilus influenzae (HIB), pneumococci, and meningococci (Neisseria meningitidis).
In adults, pneumococci, meningococci, and, less commonly, staphylococci and Gram-negative enterobacteria.
Practical Tip
If meningitis is suspected, the patient’s vaccination status should be determined: infants have usually been vaccinated against HIB. The Centers for Disease Control and Prevention (CDC) in the United States and the Standing Committee on Vaccination (STIKO) in Germany recommend vaccination of all small children and high-risk adults against pneumococci and group C meningococci.
Clinical features
Note
The clinical onset of purulent meningitis is usually acute or hyperacute, and patients very quickly become severely ill, usually with high fever and vomiting. The initiation of antibiotic therapy as rapidly as possible is essential for a good outcome.
The course of purulent meningitis is characterized by the meningitic signs and symptoms listed earlier, as well as by:
Myalgia, back pain.
Photophobia.
Epileptic seizures (40%; these may occur if the infection is mainly located over the cerebral convexity with irritation of the underlying brain parenchyma).
Cranial nerve deficits (10–20%, sometimes permanent deafness, particularly after pneumococcal infection).
Variably severe impairment of consciousness.
In infection with N. meningitidis, there may be petechial cutaneous hemorrhages and hemorrhagic necrosis of the adrenal cortex due to endotoxic shock (Waterhouse–Friderichsen syndrome).
Diagnostic evaluation The most important and most urgent components of the diagnostic evaluation are blood culture (at least two sets of aerobic and anaerobic cultures from two different veins) and CSF culture after the CSF has been obtained by LP. Whenever acute meningitis is suspected, an LP should be performed at once, unless there is clinical evidence of intracranial hypertension.
Laboratory findings: the CRP and ESR are elevated, and the differential white blood count may reveal leukocytosis (with mainly segmented granulocytes).
CSF examination enables confirmation of the diagnosis of meningitis and, in two-thirds of patients, demonstration of bacteria by Gram stain and identification of the pathogen by CSF culture.
Note
Typical CSF findings in bacterial meningitis:
Turbid CSF.
Florid granulocytic pleocytosis with 1,000 to several thousand cells/mm3.
High protein concentration (>2,000 mg/L).
Low glucose concentration (ratio of CSF to serum glucose concentration < 0.5).
High lactate concentration (>3.5 mval/L).
Treatment The treatment begins with antibiotic therapy, with a single drug or multiple drugs, chosen for their effectiveness against the most likely causative organism(s) in the given clinical setting (= empiric treatment).
Previously well children and adults with community-acquired meningitis are treated empirically at first with a third-generation cephalosporin (e.g., ceftriaxone) and ampicillin (which also covers Listeria).
Nosocomial infections and infections in parts of the world where penicillin-resistant pneumococci are common are treated, for example, with a combination of ceftriaxone and vancomycin.
Corticosteroids (dexamethasone) are given as well, as they have been shown to improve the clinical course. Once the pathogen has been identified in the blood or CSF culture (with antibiogram), the empirically chosen antibiotics can be replaced with specifically tailored ones.
Note
The antibiotic treatment of bacterial meningitis must be started immediately after the blood draw for culture and the LP—sometimes even before these are done and before the CT or MRI. The elapsed time up to the initiation of treatment is the most important prognostic factor.
Course and Prognosis Bacterial meningitis can have severe neurologic and general medical complications, including the following:
Malresorptive hydrocephalus.
Brain infarction and sinus vein thrombosis.
Cerebral edema.
Brain abscess.
Cochlear damage resulting in hearing loss.
Cranial nerve deficits.
General medical complications: pneumonia, septic shock, consumption coagulopathy.
The prognosis depends on the pathogenic organism. The mortality in meningitis due to Streptococcus pneumoniae is over 50%. Survivors often suffer from neurologic deficits including deafness.
Pneumococci (S. pneumoniae) are Gram-positive diplococci; they are the commonest cause of bacterial meningitis in adults. The diagnosis is established by Gram stain, blood or CSF culture, or demonstration of the antigen in the blood or CSF. Vaccination is possible for infants as well as adults; it is recommended for persons with immune deficiency or chronic disease and for persons older than 60 years.
Meningococci (N. meningitidis) are Gram-negative diplococci; they are the commonest cause of bacterial meningitis in children and adolescents. The meningococcal antigen can be detected in the CSF or blood. In Germany, group B meningococci are the most common type (65–70%).
Note
The course of meningococcal infection can be complicated by the following:
Sepsis (35%).
Waterhouse–Friderichsen syndrome (15%), also known as adrenal apoplexy: fulminant meningococcal sepsis leading to adrenal dysfunction, petechiae (also called purpura), extensive skin necrosis, and disseminated intravascular coagulation. If untreated, patients with this condition die within hours.
Patients must be isolated for 24 hours after the beginning of antibiotic treatment, and their contacts should be given antibiotics prophylactically (e.g., rifampicin). Vaccination against group C meningococci is recommended for all infants, and vaccination against groups A and C is recommended for travelers to Africa (recommendations of the German vaccination authority [STIKO]). There is as yet no vaccine against group B. Meningococcal meningitis is a reportable illness: all cases (even suspected ones) and fatalities must be reported. Nasopharyngeal colonization with meningococci without any sign of illness is not reportable.
Pathogens and routes of entry Several viruses can cause so-called aseptic or lymphocytic meningitis. The more common ones are enteroviruses (polio- and Coxsackie viruses), arboviruses, and HIV; other, rarer ones include lymphocytic choriomeningitis virus, CMV, type II herpesvirus, and the mumps, Epstein–Barr, and influenza viruses.
Clinical features The illness begins acutely (less commonly, subacutely) after a nonspecific prodromal stage with flulike or gastrointestinal symptoms. The main clinical manifestations are headache, fever, meningismus (often mild), and general symptoms such as fatigue and myalgia.
Diagnostic evaluation The causative virus is identified by serologic testing.
Treatment and course The natural course of aseptic meningitis is usually favorable, provided the brain is not involved (i.e., provided there is no encephalitic component). Antiviral treatment is given if the causative virus is one for which an effective treatment exists (a virustatic agent, e.g., acyclovir for herpes simplex virus or varicella zoster virus). Residual neurologic deficits, such as deafness, are rare.
Chronic meningitis is caused by different organisms from the pus-forming bacteria that cause acute meningitis, and therefore takes a less acute and dramatic course, at least initially: the meningitic symptoms arise gradually, often fluctuate, and, depending on the causative organism, may progressively worsen over a long period of time. Fever and other clinical and laboratory signs of infection (elevated ESR and CRP, blood count abnormalities, general symptoms such as fatigue and myalgia) are common but may be absent. There may be variably severe neurologic deficits. The spectrum of causative organisms is very wide. By definition, chronic meningitis lasts longer than 4 months. Fortunately, it is a rare condition.
Etiology and route of infection Mycobacterium tuberculosis bacilli reach the meninges by hematogenous spread, either directly from a primary complex (early generalization) or from a focus of tuberculosis in an internal organ (late generalization). The site of origin may be clinically silent.
Clinical features and pathogenesis Meningitic symptoms usually develop gradually. Febrile bouts and general symptoms are often but not always present. Because the infectious process typically centers on the base of the brain (so-called basal meningitis; ▶ Fig. 6.44), in contrast to bacterial meningitis, which is typically located around the cerebral convexities), cranial nerve palsies are common, particularly of the nerves of eye movement and the facial nerve. Moreover, arteritis of the cerebral vasculature may result in focal brain infarction. The protein concentration in CSF is typically markedly elevated, and gelatinous exudates in the subarachnoid space, including the basal cisterns, cause progressive fibrinous coating of the meninges and malresorptive hydrocephalus.

Fig. 6.44 Tuberculous meningitis. (a) This T1-weighted MR image shows the typical meningeal contrast enhancement along the course of the middle cerebral artery (→). (b) Typical contrast enhancement surrounding the brainstem (→).
Diagnostic evaluation The most important part of the evaluation is the detection of the pathogen in the CSF or other bodily fluids (sputum, tracheal secretions, gastric juice, urine). In the past, the detection of mycobacteria in the CSF often required weeks of culture; it can now be done relatively quickly with PCR (polymerase chain reaction). Occasionally, a Ziehl–Neelsen stain of the CSF will directly and immediately reveal acid-fast bacilli (mycobacteria).
Note
CSF findings in tuberculous meningitis:
Initially granulocytic, then lymphocytic pleocytosis.
High CSF protein (1,000–5,000 mg/L), low glucose, high lactate.
Neuroimaging (CT, MRI) reveals pathologic lesions of multiple types (e.g., tuberculomas, infarcts, hydrocephalus, coated basal meninges).
Treatment and prognosis The treatment generally begins with a combination of four tuberculostatic drugs (isoniazid, rifampicin, pyrazinamide, and myambutol), followed by a combination of three drugs, and then of two, for at least 12 months. Untreated tuberculous meningitis is lethal.
Tuberculosis (confirmed or suspected) is a reportable illness.
Several other organisms can rarely cause chronic meningitis, usually accompanied by variably severe encephalitis. These include:
Fungi, mainly but not exclusively in immunodeficient patients; the causative species include Cryptococcus neoformans, Candida albicans, and Aspergillus.
Protozoa: T. gondii.
Parasites: Cysticercus, Echinococcus.
The noninfectious causes of the chronic meningitic syndrome include sarcoidosis, which, like tuberculous meningitis, is mainly found around the base of the brain ( ▶ Fig. 6.45), and seeding of the meninges with metastatic carcinoma or sarcoma (carcinomatous or sarcomatous meningitis).
|
Stage |
Manifestations |
|
Local infection |
Erythema chronicum migrans: a red, annular rash that expands centrifugally around the site of the tick bite, clearing in the central area as it grows outward |
|
General symptoms (headache, fever, pain in the limbs, exhaustion) |
|
|
Dissemination |
General symptoms (fever, headache, diaphoresis) |
|
Bannwarth syndrome (= acute neuroborreliosis: meningopolyradiculitis, facial palsy) |
|
|
Lymphadenosis benigna cutis (hyperplasia of lymphatic cells, often visible as a reddish swelling of the earlobes) |
|
|
“Migratory” arthritis and myalgia (affecting different joints one after another) |
|
|
Late stagea |
Acrodermatitis chronica atrophicans of Herxheimer (ACA) |
|
Chronic, recurrent Lyme arthritis |
|
|
Chronic neuroborreliosis: polyneuropathy, meningitis, encephalitis or encephalomyelitis, vasculitis of the cerebral vessels |
|
|
Further inflammatory manifestations (e.g., heart, liver, sensory organs) |
|
|
aIf untreated; rare after antibiotic treatment. |
|
Etiology Borreliosis is called “Lyme disease” in North America (after the town of Lyme, Connecticut, in which an outbreak was described) and is caused there by Borrelia burgdorferi, a spirochete transmitted by bites of the tick Ixodes ricinus. Borrelia afzelii and Borrelia garinii cause borreliosis in Europe.
Clinical manifestations Borreliosis can involve the nervous system, joints, cardiovascular system, liver, and skin. Its clinical manifestations are equally varied and run through three stages ( ▶ Table 6.21). After transfer of the organism by a tick bite, some patients locally develop erythema chronicum migrans, a red, annular rash that expands centrifugally around the site of the tick bite, clearing in the central area as it grows outward. If the spirochetes are then disseminated systemically, headache, fever, arthralgia, and sometimes generalized lymphadenopathy will follow.

Fig. 6.45 Sarcoidosis. This MR image of a 31-year-old woman with sarcoidosis shows infiltration of the basal meninges. There is marked signal abnormality in the basal ambient cistern.
Fifteen percent of patients who reach the disseminated stage and do not receive treatment go on to develop neurologic manifestations, typically lymphocytic meningitis combined with radiculoneuritis, causing weakness, very unpleasant (often burning) dysesthesia, and severe pain in the distribution of the affected nerve roots (Bannwarth syndrome; ▶ Fig. 6.46). Cranial nerve involvement is also common and may cause facial diplegia, a condition that should always arouse suspicion of borreliosis. Less commonly, plexus neuritis, encephalitis, or myelitis can develop at this stage or later. Other possible complications of advanced borreliosis are vasculitis of the cerebral vessels and, outside the CNS, myopericarditis, acrodermatitis chronica atrophicans, arthralgia, and liver involvement. Arthralgia is typical in Lyme disease, which is therefore also known as “Lyme arthritis.”
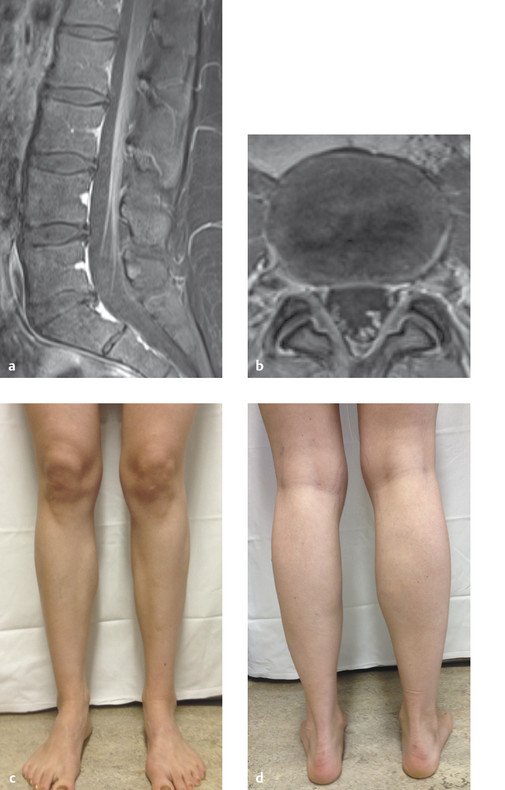
Fig. 6.46 Borrelia radiculitis. This 27-year-old woman complained of painful weakness of the left leg, mainly affecting the peroneal muscles. (a, b) T1-weighted MRI reveals contrast enhancement of the inflamed left lumbar and sacral nerve roots. (c, d) Atrophy of the left calf muscles as a late sequela.
Diagnostic evaluation A clinical suspicion of neuroborreliosis can be supported, though not definitively confirmed, by the demonstration of specific immunoglobulin G (IgG) and, above all, IgM antibodies in the serum and CSF.
Note
Serologic testing for Borrelia is positive in at least 10% of asymptomatic persons. Thus, the demonstration of antibodies against Borrelia is no reason to ascribe an unclear neurologic condition to florid borreliosis.
The diagnosis of neuroborreliosis can only be made if there is an inflammatory CSF profile (elevated cell count and protein concentration, positive CSF Borrelia titer). A normal CSF profile makes the diagnosis questionable, even if the serologic tests are positive.
Treatment If borreliosis is suspected after a tick bite (overt erythema chronicum migrans, flulike symptoms), doxycycline, amoxicillin, or cefuroxime axetil is given orally. In all later stages of the disease, third-generation cephalosporins (e.g., ceftriaxone or cefotaxime) are given intravenously.
Etiology Syphilis is caused by the sexually transmitted spirochete, Treponema pallidum.
Clinical features Various neurologic changes arise in the secondary, tertiary, and quaternary phase of syphilis.
Secondary phase (6 weeks to 2 years after infection): hematogenous spread may lead to meningeal irritation or early syphilitic meningitis with cranial nerve palsies (basal meningitis).
Tertiary phase (usually 1 or 2 years after the primary infection and secondary seeding of treponemes): cerebrospinal syphilis (also called meningovascular syphilis) mainly affects the mesenchymal structures (blood vessels, meninges) of the brain and, often, the spinal cord. Inflammatory changes of vascular walls, particularly in the arteries of the skull base and the MCA, cause stenoses and recurrent ischemic strokes. Meningitis, mainly localized around the cranial base, presents with fluctuating headache and cranial nerve palsies. Occasionally, tertiary syphilis gives rise to polyneuropathic and polyradicular manifestations. In the rare gummatous variant of tertiary syphilis, large granulomatous masses (gummata) may form within the cranial cavity, producing mass effect and intracranial hypertension.
Quaternary phase (after a latency period of several years): the inflammatory process now involves the parenchyma of the brain and spinal cord, producing tabes dorsalis (spinal cord involvement) and/or progressive paralysis (chronic meningoencephalitis).
Tabes dorsalis appears in 7% of untreated syphilitics 8 to 12 years after the primary infection. It is characterized, above all, by progressive degeneration of the posterior columns and posterior roots. Its clinical manifestations include progressively severe ataxia, lancinating pains, bladder dysfunction, diminished reflexes, loss of pupillary reactivity (see section ▶ 12.3.6), diminished sensitivity to pain, hypotonia of the musculature, and joint deformities.
Progressive paralysis appears 10 to 15 years after the primary infection and is caused by parenchymal meningoencephalitis with formation of caseating granulomas. Its major clinical sign is progressive dementia, with typical features including impaired judgment, lack of social inhibition, and, in some patients, expansive agitation (megalomania, nonsensical and delusional ideas). In other cases, patients may develop flattening of drive and affect, become depressed, or manifest schizophreniform phenomena (hallucinations, paranoia).
The two late forms of neurosyphilis can also be present in combination.
Diagnostic evaluation The diagnosis of neurosyphilis is established by various serologic tests:
TPHA (T. pallidum) hemagglutinin test, a screening test).
FTA–ABS (fluorescent treponemal antibody absorption) test, a confirmatory test for the demonstration of previous contact with T. pallidum.
VDRL test for the assessment of current disease activity (this test detects anticardiolipin antibodies and is thus not specific for T. pallidum).
19-S-IgM–FTA–ABS test to demonstrate treponeme-specific IgM antibodies, which indicate an active or florid infection.
Neurosyphilis also causes an inflammatory CSF picture with elevated leukocyte count and protein concentration, a positive CFS VDRL test, and an elevated CSF concentration of treponeme-specific IgG.
MRI of the brain, and of the spinal cord as well if indicated, is a further component of the diagnostic workup. It is used to detect inflammatory changes, hydrocephalus, and infarcts, and to rule out other conditions in the differential diagnosis.
Treatment and prognosis All forms of neurosyphilis are treated with penicillin G; if the patient is allergic to penicillin, tetracycline, erythromycin, or a cephalosporin can be given instead. The success of treatment depends on its timing: improvement is less likely if the brain and spinal cord parenchyma are already damaged before treatment is begun. The prognosis of early syphilitic meningitis is good. In the later phases of neurosyphilis, progression can be prevented by appropriate treatment, but residual deficits are common.
Leptospirosis in its initial stage often causes acute lymphocytic meningitis. In a more advanced stage, there may be signs of encephalitis (epileptic seizures, delirium) or myelitis. The brain can also be damaged by vasculitis of the cerebral vessels. Outside the nervous system, leptospirosis can affect the liver (causing jaundice) and kidneys, and it can cause a bleeding diathesis.
Etiology Herpes simplex encephalitis is a serious infectious condition caused by the herpes simplex virus, type I.
Pathogenesis This disease is characterized by hemorrhagic–necrotic inflammation of the basal portions of the frontal and temporal lobes, combined with severe cerebral edema. The inflammatory foci are found in both hemispheres, but one is usually more strongly affected than the other.
Clinical features After a nonspecific prodromal phase with fever, headache, and other general symptoms, the disease presents overtly with the following:
Progressive impairment of consciousness.
Epileptic seizures (usually of complex partial type, with or without secondary generalization, because the infection affects the temporal lobe).
Focal neurologic and neuropsychological deficits, particularly impairment of memory and orientation.
Aphasia and hemiplegia may ensue.
Diagnostic evaluation CSF examination reveals up to 500 cells/mm3, mainly lymphocytes but also granulocytes; the CSF is sometimes bloody or xanthochromic. Viral DNA can be identified in the CSF by the PCR in the first few days of illness and, for at least 2 weeks, specific IgG for herpes simplex virus can be detected in the CSF as well.
Note
CSF findings in herpes simplex encephalitis:
Usually an initially lymphomonocytic pleocytosis.
Protein elevated, glucose normal.
The CT scan is usually normal at first but, within a few days, reveals temporal or frontal hypodense areas, which may contain foci of hemorrhage. MRI may reveal corresponding signal changes even earlier ( ▶ Fig. 6.47).
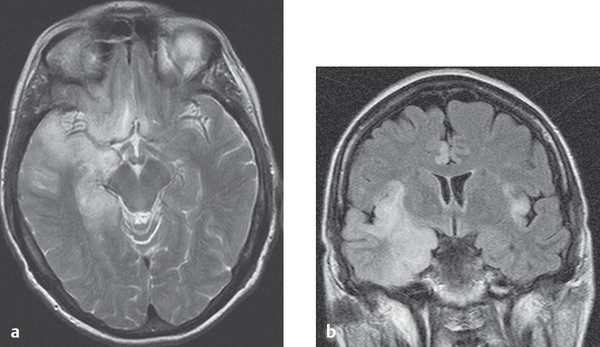
Fig. 6.47 Herpes simplex encephalitis. Herpes simplex encephalitis affecting both temporal lobes. (a) The axial T2-weighted MR image displays signal abnormalities in the right temporal lobe and in basal portions of both frontal lobes. (b) The coronal FLAIR image additionally shows typical bilateral involvement of the insular and cingulate cortex.
The EEG, in addition to nonspecific changes, may reveal characteristic focal findings over one or both temporal lobes.
Treatment Acyclovir is given intravenously. Corticosteroids are given to combat cerebral edema and anticonvulsants to prevent seizures.
Prognosis If untreated, herpes simplex encephalitis has a mortality of more than two-thirds, with neurologic damage in all surviving patients. Treatment with acyclovir lowers the mortality to 20%, but about half of the surviving patients have permanent deficits.
Note
Any one of the following findings is adequate grounds for suspicion of herpes simplex encephalitis:
Progressive impairment of consciousness, aphasia.
Epileptic seizures (particularly of the complex partial type).
An inflammatory CSF profile.
Uni- or bilateral frontotemporal signal changes in MRI.
Focal EEG abnormalities.
If any of these are present, intravenous acyclovir therapy must be started immediately.
Etiology and epidemiology This disease is caused by an arbovirus (arthropod-borne virus) of the flavivirus group and is transmitted by tick bites. In endemic areas (e.g., Austria and southern Germany), it affects 1 in every 100 to 1,000 tick-bite victims. The family Flaviviridae includes several tick-borne viruses affecting humans, such as the European or Western tick-borne encephalitis virus, the Siberian tick-borne encephalitis virus, and the Far Eastern tick-borne encephalitis virus. In the United States and Russia, another tick-borne flavivirus, Powassan virus, is responsible for encephalitis in humans.
Clinical features and course The course is biphasic: after an incubation period of 1 to 4 weeks, in which there is a nonspecific prodrome with fever and flulike or gastrointestinal symptoms, approximately 20% of patients develop headache, meningismus, and focal neurologic deficits referable to the brain and spinal cord. Peripheral nerve deficits may also appear some time later. When the patient has recovered from the acute illness, residual paresis and, less commonly, neuropsychological deficits may remain.
Diagnostic evaluation The essential diagnostic test is the demonstration of virus-specific IgM antibodies.
Therapy and prognosis Only symptomatic treatment is possible. The overall mortality is 1%. Children have a markedly better prognosis than adults. Persons who recover from meningoencephalomyelitis generally have permanent residual deficits.
Prophylaxis Early summer meningoencephalitis and other tick-borne flavivirus encephalitides can be effectively prevented by exposure prophylaxis (insect repellents and adequate clothing in endemic forest areas) and some also by active immunization (recommended for persons who are commonly exposed; boosters needed every 3–5 years).
Nearly 50% of persons infected with HIV have a clinically evident infection of the brain or other parts of the nervous system at some point in the course of their illness. The nervous system can be infected with HIV itself, other opportunistic pathogens, or both. In severe cases, patients may suffer from encephalitis, myelopathy, mono- and polyneuropathy, and/or myopathy. Encephalitis presents with neuropsychological abnormalities including delirium, personality change, and dementia.
HIV encephalopathy HIV encephalopathy arises when the HIV attacks the CNS, causing various neurologic deficits, primarily cognitive deficits (whence the term HIV-associated dementia). High viral titers are found in the CSF, and MRI reveals leukencephalopathy. The only effective treatment is properly administered HAART (highly active antiretroviral therapy).
Opportunistic infections The main AIDS-defining infections of the CNS are toxoplasmosis, cryptococcosis, tuberculosis, nocardiosis (usually as a brain abscess: Nocardia live in the soil, are part of the normal oral flora, and pose no danger to immunocompetent persons), CMV encephalitis, PML (progressive multifocal leukencephalopathy due to the JC virus, ▶ Fig. 6.48, ▶ Table 6.22), and EBV-associated lymphoma. HIV patients are also prone to develop herpes zoster encephalitis and neurosyphilis. Treatment consists of antiretroviral therapy along with treatment of the opportunistic pathogen(s), if available.
|
Virus |
Route of infection |
Season of peak incidence |
Persons at risk |
Clinical features |
Special aspects of diagnostic evaluation |
|
Echovirus |
Fecal–oral |
Summer/fall |
Children and family members living with them |
M, rash, gastrointestinal symptoms |
Virology |
|
Coxsackie A virus |
Fecal–oral |
Summer/fall |
Children and family members living with them |
M, rash, gastrointestinal symptoms |
Virology |
|
Coxsackie B virus |
Fecal–oral |
Summer/fall |
Children and family members living with them |
M, rash, pleuritis, pericarditis, myocarditis, orchitis, gastrointestinal symptoms |
Virology |
|
Mumps virus |
Inhalation |
Late winter/spring |
Children, mainly boys |
M, parotitis, orchitis, oophoritis, pancreatitis |
Elevated amylase, CSF cell count, and CSF glucose |
|
Adenovirus |
Inhalation |
Infants and children |
M, pharyngitis, pneumonia |
||
|
Lymphocytic choriomeningitis virus |
Mice |
Late winter/spring |
Laboratory personnel |
M, pharyngitis, pneumonia |
|
|
Hepatitis viruses |
Fecal–oral, sexual intercourse, blood transfusion |
Mainly intravenous drug abusers, homo- and bisexuals, recipients of blood transfusions |
M, jaundice, arthritis |
Hepatic dysfunction |
|
|
Epstein–Barr virus (infectious mononucleosis) |
Oral |
Teenagers and young adults |
M, lymphadenopathy, pharyngitis, rash, splenomegaly |
Atypical lymphocytes, Paul–Bunnell reaction, hepatic dysfunction |
|
|
Echovirus |
M, enanthem and exanthem |
||||
|
ESME virus (early summer meningoencephalitis) |
Tick bite, cutaneous |
Early summer, fall |
Persons who go into a forest in an endemic area |
M, E, myelitis, meningoradiculitis |
Serology |
|
Varicella zoster virus |
Inhalation |
Children and persons who come in contact with them |
M, radiculitis; M, E, and myelitis: pain, vesicular eruption |
Demonstration of intrathecal antibodies, PCR |
|
|
CMV |
HIV-positive persons |
E, epileptic seizures, radiculitis |
Detection of HIV in the CSF or urine, PCR of CSF or EDTA blood, CMV-specific intrathecal IgG synthase, CMV retinitis |
||
|
HSV type I |
Person-to-person |
All year |
All persons |
E, focal neurologic deficits, epileptic seizures, impairment of consciousness |
MRI ( ▶ Fig. 6.38), virus detection, PCR of the CSF, EEG with periodic steep waves, intrathecal HSV-specific IgG synthesis |
|
HSV type II |
Person-to-person |
All year |
Neonates and children, rarely adults |
E in neonates; M in others |
|
|
Arboviruses (eastern equine, western equine, Venezuelan equine) |
Mosquitoes |
Children and adults in the Americas |
E, rash |
Virology |
|
|
HIV |
Sexual intercourse, blood transfusion |
All year |
Sexual partners of HIV-positive persons, mother–child, intravenous drug abusers, homosexuals |
E, AIDS dementia, myelopathy, polyneuropathy, myopathy, opportunistic infections |
Serology |
|
Papovaviruses (e.g., JC virus) |
All year |
Immunocompromized persons (AIDS, lymphoma, natalizumab treatment for multiple sclerosis) |
E, myelitis, clinical picture of progressive multifocal leukoencephalopathy ( ▶ Fig. 6.48) |
MRI with subcortical T2-hyperintensities, virology |
|
|
Abbreviations: CMV, cytomegalovirus; CSF, cerebrospinal fluid; E, predominantly encephalitic manifestations; EDTA, ethylenediaminetetraacetic acid; EEG, electroencephalogram; HSV, herpes simplex virus; HIV, human immunodeficiency virus; IgG, immunoglobulin G; M, predominantly meningitic manifestations; MRI, magnetic resonance imaging; PCR, polymerase chain reaction. |
|||||
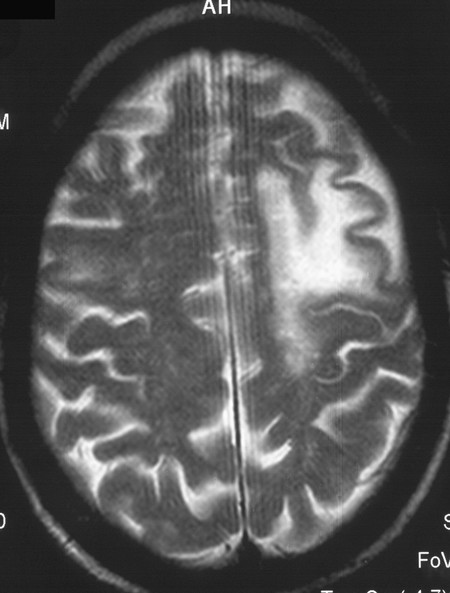
Fig. 6.48 Progressive multifocal leukencephalopathy (PML) due to JC virus in an immunosuppressed patient. The left frontal white matter is destroyed, with thinning of the overlying cortex. PML lesions generally do not take up contrast medium (except when there is immune reconstitution).
Herpes zoster encephalitis is accompanied by a segmental vesicular rash in the territory of a peripheral nerve (cranial nerve). CSF examination reveals lymphocytic pleocytosis up to 200 cells/mm3. The disease may appear in particularly severe form after a generalized herpes zoster infection. Herpes zoster oticus (involvement of the ear and auditory canal) and herpes zoster ophthalmicus (involvement of the dermatome of the ophthalmic nerve, in and around the eye) can cause severe neurologic complications, for example, postherpetic neuralgia.
Other, rarer viruses causing meningoencephalitis, some of which are specific to particular regions, are listed in ▶ Table 6.22 in addition to those already discussed.
▶ Fig. 6.48 illustrates a case of PML, caused by JC virus, in an immunosuppressed patient.
Etiology and clinical features Some of the fungi mentioned earlier as causes of meningitis can also cause encephalitis.
Practical Tip
Patients with neutropenia (mainly iatrogenic after bone marrow transplantation) are particularly susceptible to fungal infections.
In persons with normal immune competence, encephalitis can be caused by:
C. neoformans.
Coccidioides immitis.
Histoplasma capsulatum.
Blastomyces dermatitidis.
Persons with reduced immune competence due to disease or pharmacotherapy may develop encephalitis due to any of these or due to the following:
C. albicans (especially meningitis).
Aspergillus (cerebral aspergilloma and hemorrhagic stroke).
Zygomycetes (e.g., Mucorales, with diffuse cerebral spread and abscess formation; more common in diabetics).
Diagnostic evaluation The typical CSF findings of fungal infection are a lymphocytic pleocytosis (several hundred cells/µL) with elevated protein and lactate and low glucose concentrations. The fungi can be detected by direct microscopy of the CSF, by culture, or by capsular polysaccharides. Cryptococci can also be demonstrated by India ink staining.
Treatment Antimycotic drugs (e.g., amphotericin B) are given for 6 months, tailored to the particular pathogen; abscesses must be neurosurgically removed.
Parasites, particularly T. gondii, and various types of protozoa (amebae, plasmodia, trypanosomes, cysticerci [ ▶ Fig. 6.44], and echinococci) can also infect the brain.
Toxoplasmosis is transmitted mainly by contact with cat feces and raw meat; it can be asymptomatic once acquired and only become evident later through endogenous activation when the individual is immunosuppressed. The disease is mainly dangerous for unborn children. In immunosuppressed persons (e.g., persons with AIDS), it can cause multifocal necrotizing encephalitis leading to a wide variety of neurologic manifestations (paresis, aphasia, epileptic seizures). The CSF is often normal (mild pleocytosis). Contrast-enhanced imaging (CT, MRI) reveals ring-shaped lesions. Toxoplasmosis is treated with a combination of multiple antiparasitic drugs. Co-trimoxazole can be given prophylactically to persons at risk.
Man is a definitive host for Taenia solium (the pork tapeworm). Cysticerci that are ingested orally with food (typically incompletely cooked pork) migrate through the bowel wall and travel to various organs, including the CNS, where they can cause manifest symptoms (cysticercosis) after an incubation period of weeks to years. A chronic meningoencephalitis ensues, with headache, signs of intracranial hypertension, focal deficits, and seizures. The pathogens and antibodies against them can be found in CSF; CT and MRI reveal granulomas, cysts, and calcifications ( ▶ Fig. 6.49). Various antihelminthic drugs (e.g., albendazole) are given for treatment.
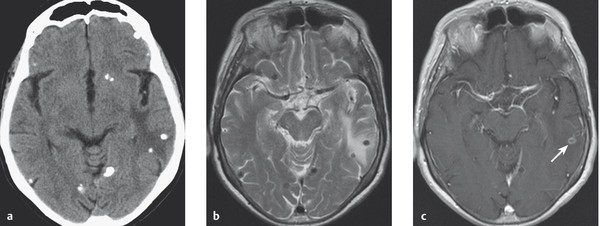
Fig. 6.49 Cysticercosis in a 74-year-old woman with epilepsy. (a) The CT shows multiple calcifications and a hypodensity in the left parietal lobe. (b) The T2-weighted MRI scan shown multiple nodular foci with low signal intensity in the area of the calcifications in the left parietal lobe. (c) A lesion within the edematous area takes up contrast medium (arrow), indicating an inflammatory reaction.
Man is an accidental intermediate host for the dog and fox tapeworms (Echinococcus granulosus and multilocularis). This organism affects the CNS, causing intracerebral cysts that can lead to neurologic abnormalities (focal deficits, epileptic seizures, signs of intracranial hypertension). It can be detected by serologic testing. CT reveals the lesions (E. granulosus: round cysts, E. multilocularis: infiltrative growth). Antihelminthic agents are given.
Pathogens and routes of infection Prions are infectious particles composed of protein that possess no genetic material (nucleic acids) of their own. They can arise in situ by mutation of the host’s genetic material or else reach the body from outside and incorporate themselves into its cells, where they replicate. The replicating proteins have an abnormal steric configuration compared with the prion proteins that are normally found in the body.
Pathogenesis Neurons in the brain that have been infected by prions may die after a latency period of years or even decades. The typical pathologic findings in prion infection are vacuolization and the formation of amyloid plaques (spongiform encephalopathy, SEP).
Epidemiology The main prion diseases are Creutzfeldt–Jakob disease (subacute SEP), kuru, Gerstmann–Sträussler–Scheinker syndrome, familial progressive subcortical gliosis, and familial fatal insomnia. Creutzfeldt–Jakob disease, the most common prion disease in Europe and North America, is nevertheless rare, with an incidence of about one case per million individuals per year.
Clinical features The disease presents initially with mental abnormalities, insomnia, and fatigability. Soon, progressive dementia develops, along with pyramidal tract signs, cerebellar signs, abnormalities of muscle tone, fasciculations, and myoclonus.
Diagnostic evaluation In about two-thirds of patients, the EEG reveals characteristic, periodic triphasic and tetraphasic theta and delta waves ( ▶ Fig. 6.50). T2-weighted MRI reveals hyperintensity of the basal ganglia and part of the occipital cortex ( ▶ Fig. 6.51). The 14–3–3 protein is found in the CSF.

Fig. 6.50 The progression of EEG changes over time in Creutzfeldt–Jakob disease (CJD). The diagnosis of CJD in this 57-year-old woman was later confirmed by autopsy. Six weeks after the onset of the prodromal phase (October 1, 1979), only a hint of periodic activity is seen. It is fully developed 1 month later (November 1, 1979) and slowly declines in amplitude in the ensuing months. TC, time constant in seconds.

Fig. 6.51 Diffusion-weighted MRI in a 68-year-old woman with Creutzfeldt–Jakob disease. The left occipital cortex appears hyperintense; the insular cortex does as well, but to a lesser extent.
Treatment, course, and prognosis Only symptomatic treatment is available. The disease progresses rapidly, leading to a decorticate state and death within months of onset.
Additional Information
A previously unknown variant of Creutzfeldt–Jakob disease attracted considerable attention when it broke out in the late 1990s, particularly in the United Kingdom. This variant was contracted by eating beef derived from cows with bovine spongiform encephalopathy (“mad cow disease”).
The slow virus diseases are characterized by extremely long incubation periods, a protracted, chronically progressive course, and little or no response to treatment.
Subacute sclerosing panencephalitis (SSPE) This disease usually arises in children who had measles in infancy. The virus persists in the CNS and, years later, gives rise to a disease of insidious onset and chronically progressive course, leading to death in 2 to 3 years. The initial presentation is with mental abnormalities such as irritability, fatigability, and impaired cognitive performance. Involuntary movements and noise-induced myoclonus appear a few weeks later. Finally, the child develops generalized spasticity and severe, progressive dementia. The EEG reveals periodic, high-amplitude slow waves. The illness is always fatal.
Rubella panencephalitis and other types of encephalitis Other illnesses that probably share pathogenetic mechanisms with SSPE are progressive rubella panencephalitis and various types of encephalitis that can arise a few days or weeks after an infectious illness (measles, mumps, chickenpox, rubella).
Note
Postvaccinial encephalitis: it has been hypothesized, but never proved, that encephalitis can rarely arise as a complication of vaccination against measles, rubella, or smallpox. This risk is no more than theoretical, but the associated anxiety in the general public is real and must be allayed by appropriately educating the parents of the children who are to be vaccinated. A child is at much greater risk of sustaining a severe complication from one of the supposedly trivial illnesses of childhood than from the vaccinations that prevent them. This is precisely the reason why the universal vaccination of infants and small children is recommended, and indeed required by law in many places.
Key Point
Brain abscesses are caused by focal infection of the brain parenchyma leading to tissue destruction and pus formation. They can be solitary or multiple. A special form is focal encephalitis, in which systemic sepsis or the embolization of infectious material into the CNS gives rise to multilocular, disseminated microabscesses.
Pathogens and routes of infection Brain abscesses are caused by one or more pathogens, mainly streptococci and staphylococci and, less commonly, Pseudomonas, Actinomyces, and fungi. Like the organisms that cause bacterial meningitis, these pathogens can reach the brain through local extension of infection (especially mastoiditis, sinusitis, and otitis), hematogenous dissemination from a distant infectious focus (usually pulmonary infections or endocarditis), or direct contamination (open brain injury). Immunocompromized patients are at increased risk.
Clinical features A large brain abscess exerts mass effect and typically causes fever, leukocytosis, and rapidly progressive intracranial hypertension. Marked perifocal edema generally adds to the mass effect.
Note
The typical manifestations of a brain abscess are: signs and symptoms of intracranial hypertension, including papilledema and impaired consciousness, along with hemiparesis or other focal neurologic signs, and/or epileptic seizures. Fever, leukocytosis, and elevation of the erythrocyte sedimentation rate and the C-reactive protein level may be seen but are sometimes absent.
Alternatively, there may be a subdural empyema between the dura mater and the arachnoid, or an epidural abscess between the dura mater and the inner table of the skull. These processes usually arise as a complication of sinusitis or otitis, less commonly after trauma. Fever, headache, and meningismus, accompanied by neurologic deficits, are their clinical hallmarks. The course of subdural empyema is often fulminant and life-threatening, while that of epidural abscess is usually more protracted.
Diagnostic evaluation The diagnosis is suspected on the basis of the typical clinical findings and relevant aspects of the past medical history (such as traumatic brain injuries, known lung or heart disease, and immune suppression or diseases of the immune system).
Laboratory findings: serum inflammatory parameters (particularly the C-reactive protein level) are usually elevated.
CSF examination may reveal inflammatory changes (predominantly granulocytic pleocytosis, elevation of total protein).
Imaging studies: CT or MRI scanning reveals a ring-shaped zone of contrast enhancement (abscess wall) surrounding the hypodense interior of the abscess ( ▶ Fig. 6.52). In the early stage of abscess formation (“cerebritis”), the enhancement may be diffuse rather than annular.
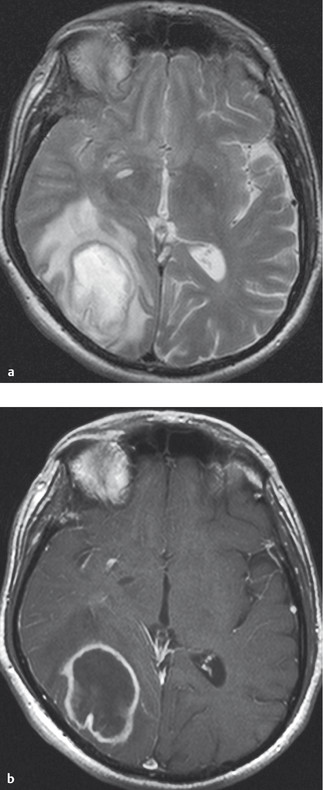
Fig. 6.52 Brain abscess (MRI). (a) The T2-weighted image reveals a right parieto-occipital mass with surrounding edema. (b) The T1-weighted image reveals ring-shaped contrast enhancement. (Reproduced from Mattle H, Mumenthaler M. Neurologie. Stuttgart: Thieme; 2013.)
Treatment Neurosurgical removal of the abscess (followed by microbiologic study of the abscess material) is the preferred form of treatment in most patients; antibiotic therapy is initiated immediately after surgery (in some cases, even beforehand) and continued thereafter for at least 6 weeks. A highly effective empirical combination of antibiotics consists of a third-generation cephalosporin (e.g., ceftriaxone), metronidazole, and an agent that is effective against staphylococci, such as flucloxacillin, rifampicin, or vancomycin. Cerebritis and abscesses measuring less than 3 cm in diameter can be treated with antibiotics alone. If clinically significant brain edema is present, it can be treated simultaneously with corticosteroids.
Practical Tip
It is important to find the source of the infection, that is, the original site from which bacteria made their way to the brain. The treatment of the original focus of infection is an important determinant of treatment success.
Pathogenesis Focal encephalitis consists of multilocular foci of infection in the brain parenchyma ( ▶ Fig. 6.53), which generally arise by one of two routes:
Metastatic focal encephalitis, that is, seeding of the brain with bacteria in generalized sepsis, possibly arising from a single focus of purulent infection anywhere in the body.
Embolic focal encephalitis, that is, the embolization of multiple infectious microthrombi into the cerebral vasculature. The latter is usually a complication of subacute bacterial endocarditis, which, in turn, is most often caused by Streptococcus viridans.
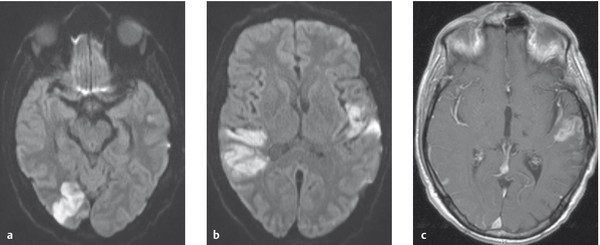
Fig. 6.53 MRI showing multiple cerebral infarcts in a patient with endocarditis. The diffusion-weighted images (a, b) show multiple fresh infarcts. The T1-weighted image (c) shows contrast enhancement in the left temporal infarct, but not in the right temporoparietal infarct; this implies that the two infarcts are of different ages, having arisen several days apart.
Streptococcal and staphylococcal infections are the usual causes.
Clinical features The typical findings include signs of generalized sepsis (high fever, shaking chills) combined with focal brain signs, impaired consciousness, and, not uncommonly, psychosis. The neurologic and psycho-organic signs fluctuate in severity. They manifest themselves in bouts, with remissions in between.
Note
A septic illness accompanied by fluctuating neurologic or psychiatric manifestations should raise suspicion of focal encephalitis.
Diagnostic evaluation The diagnosis is suspected from the clinical findings and, possibly, inflammatory CSF changes, and confirmed by a CT and/or MRI scan demonstrating multiple focal lesions in the brain. Clinical evidence of endocarditis should always be sought (including listening for a heart murmur). Blood cultures may reveal the responsible pathogen; blood should be drawn for culture during the upward phase of the fever curve and during shaking chills.
Treatment As in the treatment of brain abscesses, antibiotic therapy is indicated and should be tailored to the sensitivity profile of the responsible organism, if it can be identified. Infected heart valves may need to be surgically replaced.