CHAPTER 2
What’s in Garlic
There are two special things about garlic. First, more than 160 of a garlic bulb’s constituents are bioactive, meaning they can affect our body. The amounts are tiny, but can nevertheless contribute to health, especially if consumed regularly. Second, garlic’s odour, flavour and tingle on the tongue are unique.
Here we’ll consider garlic’s contents, odour and flavour, as well as what’s in garlic supplements, and the reasons for the recommendations in Chapter 3 on storing, preparing and cooking garlic. Go directly to the next chapter if you prefer to skip the science.
The constituents most important to our health and our enjoyment of garlic are its sulfur compounds.
Sulfur Compounds
Compared weight for weight with onions, garlic is four times richer in sulfur-containing compounds. These include certain enzymes (such as glutathione) and amino acids (cysteine, cystine, methionine, taurine), as well as various cysteine derivatives. All these bioactive compounds are vital for our metabolism and can be obtained from various foods. But it is the uniquely high content of cysteine derivatives that is largely responsible for garlic’s scent, flavour and health-giving potential.
Cysteine derivatives
Fresh whole raw garlic cloves contain three groups of these water-soluble, odour-free sulfur compounds:
• Alliin and other cysteine sulfoxides. The amount of alliin (pronounced ‘alley-in’), for example, varies ten-fold, depending on the variety of bulb and the soil and climate in which it grew.
• Gamma-glutamyl cysteines. Storing garlic at a cool temperature slowly converts these into S-cysteines (see below).
• S-cysteines. S-allyl cysteine, for example, is arguably garlic’s most important sulfur compound.
Alliinase
Crushing, chopping or chewing raw garlic cloves sparks a cascade of activity by tearing open tiny compartments containing the enzyme alliinase. This exposes it to oxygen and water, enabling it to convert alliin into sulfenic acid. The more a clove is damaged, the more sulfenic acid is produced. Within a few seconds, sulfenic acid forms allicin and other pungent thiosulfinates. This time lag explains why, if you chew raw garlic, several seconds elapse before it tastes pungent. The sulfenic acid and allicin in newly crushed garlic can inflame or burn the cells of our skin, mouth, throat, stomach and eyes. Garlic produces these substances to deter invasion by soil fungi, bacteria and parasites.
Allicin production is steady for 6¼ minutes, then spurts for ½ minute. This cycle repeats every 6¾ minutes until 90 minutes, which is when the allicin level peaks. This has implications for cooks. The longer you wait up to 90 minutes before using newly crushed or chopped garlic, the more allicin is formed. Wait 7 minutes and you benefit from the first cycle. Wait 14 minutes, and you also benefit from the second. Wait 90 minutes, and you get the maximum yield.
However, alliinase is permanently deactivated (preventing the conversion of alliin to allicin) if crushed or chopped garlic is:
• cooked – because heat (depending on its degree and the cooking time) destroys alliinase;
• mixed with lemon juice or vinegar – because acids of pH 3 or below destroy alliinase; or
• eaten – because normal stomach acidity and body heat break down almost all the alliinase in swallowed garlic, leading to a 99 per cent loss of potential allicin production. Normal stomach juice has a pH from 1 (highly acid – for example, while eating a low-protein meal) to 5 (low acid – for example, on waking). If you have low stomach acid (for example, because of ageing, stress, antacid or acid-suppressant medication, or a diet low in vegetables), or if you dilute it by drinking water, or if you take an enteric-coated garlic supplement, more alliinase may get through the stomach. But most will then be inactivated by your intestinal juice and cells.
So the sooner you cook newly crushed or chopped garlic, mix it with something acidic, or eat it, the more alliin is retained and the less allicin is produced. Conversely, the longer you leave crushed or chopped garlic before you cook it, mix it with something acidic or eat it, the less alliin is retained and the more allicin is produced.
Alliin and allicin (and, most importantly, allicin’s derivatives) each have a particular range of health benefits (see page 14). If you want garlic to contain relatively more alliin or allicin, leave crushed or chopped garlic for less or more time within the 90-minute timeframe, respectively.
Allicin is unstable and eventually converted by enzymes into oil-soluble allicin derivatives (see page 13). If you leave crushed or chopped garlic at a warm room temperature of 23°C/73°F, half its allicin is converted within 2½ days, the rest over a longer time. Its conversion takes longer in a cooler room.
Allicin’s conversion to its derivatives is faster if crushed or chopped garlic is:
• cooked – for example, 45 minutes of oven-roasting, or 1 minute of microwaving, converts all its allicin;
• mixed with vegetable oil – this converts half the allicin within 3 hours; also it converts relatively more into ajoene and vinyl dithiins than into allyl sulfides (see page 13); or
• mixed with warm water at 37°C/99°F – this converts half the allicin within 24 hours.
In addition, allicin’s conversion is slower if crushed or chopped garlic is:
• mixed with room-temperature or cool water – this converts half the allicin within 12 days at 23°C/73°F, 32 days at 15°C/59°F, 1 year at 4°C/39°F;
• mixed with alcohol – this converts half the allicin within 12 days;
• refrigerated – this slows allicin conversion 20-fold, so half might, for example, be converted within 60 days; or
• mixed with vinegar, lemon juice or wine: this converts all the allicin over 2 years.
Allicin derivatives include allyl sulfides (such as diallyl disulfide, diallyl trisulfide, allyl methyl sulfide), allyl mercaptan, methyl mercaptan, ajoene (pronounced ‘ah-ho-een’; a disulfide) and vinyl dithiins (pronounced ‘die-thigh-ins’). Their bioactivity and stability account for most of the health benefits previously attributed to allicin. They remain stable for a year or so at room temperature, or longer if refrigerated. Diallyl disulfide, ajoene and vinyl dithiins are the most stable.
What happens to garlic in the body
When you consume garlic, its water-soluble and oil-soluble compounds affect the body in different ways.
• Garlic’s water-soluble compounds (such as alliin, gammaglutamyl cysteine and S-allyl cysteine) have small molecules that easily diffuse from the intestine into the capillaries (tiny blood vessels) in the intestinal wall. These capillaries empty into the hepatic portal vein which takes blood directly to the liver. Gamma-glutamyl cysteine is converted into S-allyl cysteine, which contributes heavily to garlic’s health benefits and can be measured in the blood, liver and urine. The liver converts alliin into diallyl disulfide, which is subsequently converted into allyl mercaptan. Interestingly, the liver’s cytochrome P-450 enzyme can convert dialllyl disulfide back into allicin.
• Garlic’s oil-soluble compounds (allicin and allicin derivatives) have large molecules, so only very small amounts can diffuse from the intestine into the capillaries. Any allicin that enters the blood quickly reacts with substances such as the enzyme glutathione peroxidase, or breaks down into allicin derivatives, or enters cells.
However, the large molecules can diffuse into the lymph vessels in the intestinal wall, because the pressure in these vessels is very low. They then travel in lymph, where they may escape into cells, be used or broken down, or be emptied into the blood in the subclavian veins. When allyl sulfides, ajoene and vinyl dithiins reach the liver, they are either used or broken down into substances such as allyl methyl sulfide and allyl mercaptan.
What happens to garlic on the skin
When garlic is applied to the skin, some of its sulfur compounds penetrate it and enter the blood. So if garlic is rubbed on to the soles of the feet, for example, the person’s breath is scented with garlic within 10 minutes or so.
Health-promoting effects of garlic’s sulfur compounds
Scientists believe that alliin, gamma-glutamyl cysteine, S-allyl cysteine and allicin derivates such as allyl sulfides, ajoene and vinyl dithiins account for most of garlic’s health-promoting effects. Studies indicate that these and certain other sulfur compounds have various actions:
• Anti-cancer – test-tube studies show that S-allyl cysteine inhibits growth of prostate- and breast-cancer cells; S-allyl mercaptocysteine reduces cancer-cell multiplication; and certain sulfur compounds discourage the cancer-promoting effects of acrylamide (in cooked carbohydrates) and heterocyclic amines (in cooked meat). Alliin, S-allyl cysteine, allicin, diallyl disulfide, ajoene, vinyl dithiins, thiacremonone, allixin, sulfur amino acids, glutathione and sulforaphane (produced from glutathione when garlic is chopped or crushed) have antioxidant and possibly anti-cancer activity. Diallyl disulfide and diallyl trisulfide discourage the spread of cancer cells in lymph. Diallyl trisulfide discourages the spread of lung-cancer cells.
• Anti-clotting – allicin, ajoene, vinyl dithiins and diallyl disulfide are powerful anti-clotting agents in the test tube. Ajoene has equal potency to aspirin, but without its possible side effects.
• Anti-diabetic – allicin and allyl propyl disulfide may protect insulin by locking on to it so insulin-inactivating compounds found in people with diabetes can’t work. Early studies suggest that sulforaphane has anti-diabetic properties.
• Anti-microbial – vapour from crushed garlic can kill certain bacteria up to 20cm/8in away. Test-tube studies show that crushed garlic and certain garlic supplements inhibit the growth of certain bacteria, increase our natural killer cells’ anti-viral activity, and reduce the ‘stickability’ of fungi by altering the fat content of their cell membranes. Allicin acts against a wide range of bacteria and can break up biofilms (sheets of bacteria with high resistance to antibiotics and our immune-system’s defences; present in an estimated four in five infections). Bacteria don’t become resistant to allicin because, unlike many antibiotics, allicin penetrates them and inactivates their cysteine-protease enzymes. Allicin also acts against certain viruses, fungi and protozoa. It has been patented in the US for its antibiotic and anti-fungal effects. Various sulfides have anti-bacterial, anti-viral and anti-fungal actions; ajoenes have the highest anti-viral activity. Sulforaphane and allixin have antimicrobial action.
• Anti-obesity – laboratory studies suggest that allyl-sulfides discourage obesity by increasing adrenaline and noradrenaline, hormones that stimulate trialglycerol metabolism and are thought to boost heat production in brown fat (very metabolically active fat between the shoulder-blades). Ajoene can inactivate gastric lipase (an enzyme needed for fat digestion). And vinyl dithiins may discourage pro-inflammatory conditions that promote obesity.
• Antioxidant and anti-inflammatory – alliin, S-allyl cysteine, sulfenic acid, allicin, allyl disulfide, diallyl disulfide, ajoene, vinyl dithiins, allyl mercaptan, thiacremonone, sulfur amino acids, sulforaphane and glutathione have these effects in the test tube. Red blood cells convert diallyl disulfide and diallyl trisulfide to hydrogen sulfide, which increases certain anti-inflammatory factors (NFκB p65, Nrf2 and GLUT 4 and PPAR delta).
• Smooth-muscle-relaxing – laboratory studies show that gammaglutamyl cysteine acts like the ACE (angiotensin-converting enzyme) -inhibitor drugs used for high blood pressure, heart failure and strokes. Hydrogen sulfide (see above) expands arteries, which lowers blood pressure. Certain compounds stimulate the release of prostaglandins that relax smooth muscle and therefore expand arteries.
• Cholesterol-lowering – laboratory studies indicate that S-allyl cysteine reduces cholesterol production via a statin-like action (statins being cholesterol-reducing drugs). Gamma-glutamyl cysteine, diallyl disulfide, diallyltrisulfide, allyl mercaptan and vinyl dithiins may also have cholesterol-lowering action. And ajoene reduces cholesterol production. However, claims that garlic lowers cholesterol in people remain controversial.
• Detoxifying – sulfates detoxify substances such as paracetamol (acetaminophen), estrogen and adrenaline by a process called sulfation. Allyl sulfides are potent detoxifiers. Glutathione can detoxify several potentially dangerous substances, including paracetamol. And sulforaphane can detoxify certain carcinogens and toxins. Garlic’s sulfur compounds can also help to remove heavy metals such as arsenic, lead and mercury from the body.
• Digestive-juice-stimulating – garlic encourages normal stomach contractions and promotes the flow of stomach juice.
• Immunity-enhancing – our immune system uses diallyl disulfide to make antibodies. And test-tube studies indicate that garlic can boost the performance of natural killer cells – white cells that help to destroy cancer cells and virus-infected cells.
Other Constituents of Garlic
Besides sulfur compounds, garlic cloves contain water (which accounts for over half their weight), proteins, free amino acids, oils, fats, carbohydrates, vitamins, other minerals and other constituents.
Proteins and amino acids
The proteins include certain enzymes (such as alliinase, peroxidase and myrosinase) and lectins (sugar-binding proteins that are currently being investigated for their anti-cancer potential).
The 17 free amino acids in garlic include arginine (an antioxidant that helps to maintain healthy blood pressure and is being studied for anti-cancer potential) and four sulfur-containing amino acids: cysteine, cystine, methionine and taurine (essential for our body’s structural proteins, including keratin in nails and hair, and collagen in joints).
Oils and fats
Garlic’s essential oil, found mainly in its cell membranes, consists of free fatty acids, fats called glycolipids and phospholipids, and terpenes. In cut, chopped or crushed garlic, these are joined by oil-soluble allicin and allicin derivatives.
Carbohydrates
These include:
• Mucilage – a sticky substance that forms nearly half a clove’s weight.
• Sugars – 17 sorts, including glucose, oligosaccharides, fructose and fructans, which may enhance our immunity (fermentation of fructans by bowel bacteria discourages gastro-enteritis, constipation and, perhaps, high blood pressure, high cholesterol and cancer.
• Pectin – found in garlic cloves’ papery skins and extracted for commercial use
Vitamins
Garlic’s main vitamins are B6 and C; others include A, B1, B2, B3, biotin (B7) and E. The S-allyl group common to many of garlic’s sulfur compounds aids absorption of vitamin B1.
Minerals
Garlic is rich in phosphorus, potassium, selenium, sulfur and the trace elements germanium and manganese.
Garlic is the richest in selenium of all vegetables. Interestingly, it can extract selenium even when the concentration of this mineral in soil is poor. While a large clove contains only 1.6 per cent of the US Recommended Dietary Allowance (RDA) for selenium, a large clove grown in selenium-enriched soil can contain four times this RDA. Garlic’s sulfur content enhances the antioxidant power of its selenium.
Garlic is the richest in sulfur of all vegetables, containing at least four times as much as onions and cabbage, for example.
Our body’s sulfur is distributed among certain enzymes and bile acids, as well as insulin, biotin, collagen, elastin, proteoglycans (‘mucopolysaccharides’, in connective tissues and joint fluid), coenzyme A, lipoic acid and the antioxidant enzyme glutathione.
Studies suggest that the average diet in the US, for example, lacks sufficient sulfur. Deficiency often goes undetected as it causes no clearly defined symptoms. So the average person might do well to eat more sulfur-rich foods. Meat, fish and eggs are the richest sources, followed by pulses, but garlic is useful, too, even though the amounts are small.
Other
Garlic also includes:
• Flavonoids – including myricetin and apignin (being investigated for its anti-cancer effects). Many, notably quercetin, allixin and anthocyanins, are powerful antioxidants. Quercetin also has anti-histamine and anti-viral properties, can lower blood sugar by stimulating insulin production, and can strengthen capillaries. Allixin helps to prevent the formation of prostaglandins that encourage inflammation, blood clotting and cancer growth; it also has anti-microbial effects and enhances nerve growth.
• Anthocyanins – flavonoid derivatives and phenolic compounds that include caffeic acid, ferulic acid and tannins (which have antioxidant properties).
• Terpenes – odoriferous compounds in garlic’s oil, including citral (lemon-scented), geraniol (rose-scented), linalool (with a sweet, woody, lavender scent) and phellandrenes (with a peppery, minty or citrus scent).
• Plant hormones – for example, glucokinin (‘plant insulin’), which is thought to lower blood sugar and so protect the pancreas from exhausting itself by having to produce a large amount of insulin.
• Saponins – steroid-like substances thought to have cholesteroland blood-pressure-lowering actions and to encourage healthy proportions of micro-organisms in the bowel. They are also thought to help protect against bowel cancer by breaking down the cholesterol-rich membranes of its cells. Certain saponins have anti-inflammatory, anti-microbial and anti-parasitic activities.
Garlic’s Odour and Flavour
These arise mainly from volatile oil-soluble thiosulfinates such as allicin (which has a slightly sweet and piquant garlicky scent, and makes the tongue tingle) and its derivatives (such as diallyl disulfide, allyl methyl disulfide, allyl mercaptan and methyl mercaptan). They also come from certain water-soluble substances, including alliin, methiin and other cysteine sulfoxides. The scents of garlic’s terpenes (see page 20) add to the complexity of its odour and flavour.
Differences in the variety of garlic, the climatic conditions, the soil’s sulfur content and how garlic is stored account for variations in odour and flavour. Garlic and onion smell and taste different mainly because they contain differing amounts of cysteine sulfoxides; for example, there is more isoalliin in onions, and more alliin in garlic.
The absence of garlic odour in dishes containing garlic implies they contain little in the way of volatile oil-soluble sulfur compounds, probably because crushed garlic was used before its alliinase could release allicin.
Garlic breath
Chewing and swallowing garlic releases sulfur compounds that immediately scent saliva, and mucus in the throat. This scents breath with garlic’s ‘primary odour’.
Certain sulfur compounds, including allyl methyl sulfide, are excreted from the lungs and in sweat for several hours after eating garlic. This scents breath and sweat with garlic’s ‘secondary odour’.
Sulfur compounds can also scent other body fluids, including urine and breast milk. Interestingly, babies spend longer breastfeeding (nursing) when their mothers have eaten garlic, implying they like the taste.
Our nose is so sensitive to the scent of garlic’s volatile sulfur compounds on the breath that it can detect less than one part in a billion. However, the nose’s olfactory sensors soon tire of the scent on another person’s breath, especially if you have eaten garlic yourself.
See page 41 for suggestions on dealing with garlic breath.
Garlic Supplements
The four main commercially produced garlic supplements are garlic powder, aged garlic extract, garlic oil and garlic oil-macerate. They are available in pharmacies, online and in certain supermarkets and health-food shops.
Which you choose (see page 85) will depend on the health benefits you are seeking, since different production methods result in different ranges and levels of bioactive compounds. While the composition of garlic powder is closer to that of raw garlic than any other supplement, garlic powder may nevertheless contain less than half the allicin present in the equivalent amount of fresh garlic.
The amounts and potency of the compounds in garlic supplements often remain unclear. Also, the amounts of a particular compound in different brands of a particular type of supplement can be very different. In addition, some products are not standardized for any particular bioactive compound, so you cannot be sure how much of that compound they contain.
Some manufacturers add herbs such as mistletoe or hawthorn to their garlic supplements. Some add parsley in the probably forlorn hope that it will overcome secondary garlic odour, and others add other substances, such as cayenne (to expand arteries) and selenium or vitamins (hoping this might help to prevent or fight cancer).
Lastly, pure allicin is available in various formulations.
Garlic powder
Commercially produced garlic-powder supplements usually come as capsules, but are also available as tablets or ointment.
Garlic powder is made by slicing, drying and grinding fresh raw garlic cloves. It takes 1–1.2kg/2lb 4oz–2lb 6oz to produce 450g/1lb garlic powder.
The range of constituents in a well-produced garlic-powder supplement is as in whole raw garlic. But the processing can alter their amounts and proportions.
The alliinase in cloves remains safe but temporarily inactive (because of the lack of water), provided the temperature stays below 50–60°C/122–140°F. Rehydrating a garlic-powder supplement with water activates its alliinase, so it converts alliin to allicin. But alliinase is destroyed by normal stomach acidity. So if you particularly want allicin to be produced and to survive your stomach acid (so you can benefit from allicin and its derivatives), you need to do one of the following:
• Empty garlic powder from a capsule into a glass of water, stir and leave to stand: the longer you leave it, up to 90 minutes, the more allicin is produced. When you drink the garlic-powder-containing water, the water can dilute your stomach acid.
• Drink a glass of water to dilute your stomach acid, then swallow an enteric-coated product (see below).
Note that some manufacturers treat garlic powder with sulfur dioxide to reduce discoloration, and with hydrogen peroxide, salt solution or irradiation to eradicate bacterial contamination. These processes may alter the range and amounts of sulfur compounds.
Enteric-coated tablets
The majority of garlic-powder tablets are enteric-coated – meaning their manufactured ‘shell’ protects their contents from stomach acid. Historically, the expectation was that such a coating would dissolve only when the tablet reached the intestine, and that the garlic powder would then rehydrate so its alliinase could act on alliin to produce allicin. This gave rise to claims for a product’s ‘allicin potential’. But the reality is almost always different, because:
• too little alliinase may be present in the garlic powder;
• an enteric coating may dissolve early, allowing stomach acid to destroy the alliinase;
• an enteric coating may not dissolve in the intestine;
• intestinal juice inhibits allicin production by 40 per cent; and
• intestinal cells may break down allicin.
Test-tube experiments that mimic conditions in the stomach can predict allicin release, but few manufacturers provide such information. Also, this figure does not reflect what happens in real life: one study found that three in four types of enteric-coated product released less than 15 per cent of the expected amount of allicin.
Sometimes garlic powder or oil is put into gelatine capsules. However, stomach acid breaks down gelatine and prevents alliinase from converting alliin into allicin.
Garlic extracts
These are usually made by soaking whole or sliced garlic cloves in alcohol and water. The solution is then concentrated and can be dried, ground into a powder and formed into tablets.
Garlic extracts mainly contain water-soluble sulfur compounds, such as S-allyl cysteine and S-allyl mercaptocysteine. These two compounds are the least odorous of garlic’s sulfur compounds. Garlic extracts may also contain allixin, S-1-propenyl cysteine and fructosyl arginine – a potent antioxidant not present in raw or cooked garlic.
Garlic extracts can be standardized according to their content of S-allyl cysteine.
Aged garlic extract is made by soaking garlic cloves in alcohol and water for up to 20 months. This allows the ongoing conversion of gamma-glutamyl cysteines to S-allyl cysteine and S-allyl mercaptocysteine. Aged garlic extract is also rich in flavonoids such as allixin, and in fructosyl arginine. It also contains the antioxidant enzymes catylase and glutathione peroxidase.
However, it contain extremely little alliin, no active alliinase, little allicin, and only traces of allicin derivatives. Indeed, its total content of sulfur compounds is only one tenth of that of fresh or cooked garlic.
Manufacturers sometimes enrich aged garlic extract with other health-promoting compounds, such as vitamins B or E, co-enzyme Q, cayenne, hawthorn or fish oil or its acids (EPA and DHA), yeast, kelp, whey or digestive enzymes.
Steam-distilled garlic oil
This is the oldest type of commercial garlic supplement and is usually sold in capsules called perles. To produce garlic oil, steam is passed through crushed fresh raw garlic cloves, and the oil-rich steam condensed to form a reddish-brown oil.
Steam-distilled garlic oil differs in several ways from garlic’s essential oil (see ‘Ether-extracted garlic oil’, page 26). It consists mainly of allicin-derived sulfides, many of which have a strong odour. In particular, it is rich in diallyl disulfide and diallyl trisulfide. While the amounts of these substances are vastly greater than in raw garlic or garlic powder, studies suggest that they are not as bioactive.
It takes about 450g/1lb garlic to produce 1g/just over 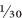 OZ steam-distilled oil. It is therefore very expensive. Supplement manufacturers usually sell it in capsules, perhaps diluted with a cheaper vegetable oil. The concentration of garlic oil in the average finished product is often as low as 1 per cent.
OZ steam-distilled oil. It is therefore very expensive. Supplement manufacturers usually sell it in capsules, perhaps diluted with a cheaper vegetable oil. The concentration of garlic oil in the average finished product is often as low as 1 per cent.
Ether-extracted garlic oil
Extracting garlic’s oil with ether produces an oil that contains volatile oil-soluble sulfur compounds formed by the conversion of allicin and other thiosulfinates. Compared with steam-distilled garlic oil, this oil contains nine times as much of the vinyl dithiins and allyl sulfides and four times as much ajoene.
A medium (4g/just under 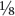 oz) clove of fresh raw garlic produces only about 4mg of this oil.
oz) clove of fresh raw garlic produces only about 4mg of this oil.
Garlic oil-macerate
Garlic oil-macerate is made by chopping or crushing (macerating) garlic, mixing it with vegetable oil, then leaving the mixture for 24 hours at room temperature before straining out the pieces of garlic. The resulting garlic oil-macerate is put into capsules to create garlic perles.
During manufacture, some of the garlic’s alliin is converted to allicin. This quickly converts into other sulfur compounds such as sulfides, ajoene and vinyl dithiins. Garlic oil-macerate is the only garlic supplement that contains significant amounts of ajoene and vinyl dithiins, and much of its bioactivity is thought to be due to the vinyl dithiins.
However, garlic oil-macerate contains much lower levels of diallyl disulfide and diallyl trisulfide than does steam-distilled garlic oil.
Low-odour or odour-free garlic tablets or capsules
Enteric-coated products are ‘low-odour’ in that they prevent primary garlic breath but not the secondary sort.
Odour-free garlic is produced by preventing alliinase from working, so that allicin and its derivatives cannot be produced. This can be done by:
• exposing garlic to fumaric acid;
• physically separating alliinase from garlic;
• freeze-drying garlic mixed with cyclodextrin;
• heating garlic;
• producing garlic tablets without an enteric coating, so gastric acid destroys alliinase; or
• mixing crushed garlic with yeasts that break down allicin into non-smelly sulfur compounds.
However, such products mostly lack the health benefits of allicin and its derivatives.
Timed-release garlic tablets or capsules
These are created in a way that delays the release of their contents. The aim is for each dose to have a longer and steadier effect.
Allicin
Purified stablilized allicin is available as capsules, liquid and cream.
The suggested daily dose of powdered allicin in capsules is 3.6–5.4mg, which equates to the potential allicin yield from one medium clove of garlic.
Other garlic products
These include garlic syrup (see recipe on page 40), garlic tincture and garlic juice.
For information on which garlic supplement is best suited for which common ailment, see page 90.
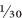 OZ steam-distilled oil. It is therefore very expensive. Supplement manufacturers usually sell it in capsules, perhaps diluted with a cheaper vegetable oil. The concentration of garlic oil in the average finished product is often as low as 1 per cent.
OZ steam-distilled oil. It is therefore very expensive. Supplement manufacturers usually sell it in capsules, perhaps diluted with a cheaper vegetable oil. The concentration of garlic oil in the average finished product is often as low as 1 per cent.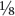 oz) clove of fresh raw garlic produces only about 4mg of this oil.
oz) clove of fresh raw garlic produces only about 4mg of this oil.