CHAPTER TWO
Inside Living Cells
Over more than three billion years of evolution has honed cells into complex, efficient machines. Science has gazed inside those machines, cataloged each functioning part, and figured out exactly how the processes that sustain life actually work. There is tremendous diversity in the cellular world—but there are certain things that all cells have in common.
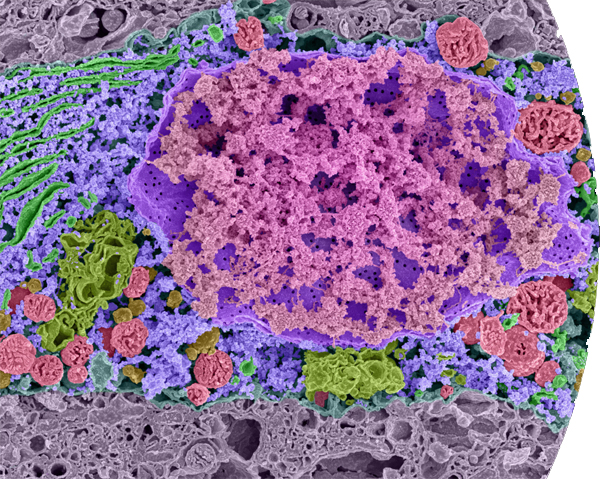
False color scanning electron micrograph of part of a single cell—a neuron in the brain of a mouse embryo. DNA is colored pink; the nuclear membrane is purple. Note the pores in the membrane: the endoplasmic reticulum is bright green; ribosomes, which manufacture proteins, are blue; Golgi complexes are colored dull green; mitochondria—the cell’s power plants—are red. This remarkable image was produced by removing water from the sample and freezing the sample in liquid nitrogen then breaking it with a razor blade.
Three domains, two types of cell
All living things on Earth fall into one of three main categories, or domains: Eukaryota, Bacteria, and Archaea. Eukaryota includes a wide range of organisms from single-celled amoeba and yeast to all plants and animals; “eukaryotic” cells are large and complex. Bacteria and Archaea are all simpler, single-celled organisms. Although there are some differences between them, their cells share certain features and are all classed as “prokaryotic.”
Eukaryotic and prokaryotic
The domain Eukaryota includes all animals, plants and fungi; humans are eukaryotes, as are falcons, chimpanzees, hydrangeas, and mushrooms. Eukaryota also includes some much smaller things, such as yeasts, amoebas, and algae. What unites this extraordinarily varied collection of organisms is their eukaryotic cells; all share certain features that differentiate them from the prokaryotic cells of Bacteria and Archaea.
The defining characteristic of eukaryotic cells is the presence of a nucleus. Every cell on Earth utilizes DNA to carry its genetic material; in eukaryotic cells, the DNA is housed inside a nucleus. Prokaryotic cells have no nucleus—their DNA is loose inside the cell. There are several other differences. For example, while every cell is enveloped in a double-layer fatty membrane, eukaryotic cells also have a sophisticated system of membranes inside. The internal membranes provide folded surfaces that participate in many of the cell’s functions and include the magnificently named Golgi apparatus and endoplasmic reticulum. There are also self-contained organelles, such as mitochondria and chloroplasts, with their own membranes. Prokaryotic cells lack these membrane-bound organelles and membranous structures.
One good analogy to help distinguish between the two types of cell is that a prokaryotic cell is like a village: perfectly complete and functioning but small and relatively simple. A eukaryotic cell, on the other hand, is like a city: larger and more complex, with more structures and more levels of organization. The analogy carries through to comparisons of size, too; eukaryotic cells are typically ten times the size of prokaryotic cells.
Compare and contrast
There are certain things—in addition to using DNA as the carrier of genetic information—that are common to both prokaryotic and eukaryotic cells. For example, all cells use the compound RNA to make copies of sections of DNA (genes), as templates for building proteins. And in both types of cell molecular machines called ribosomes “read” the RNA and actually construct the proteins. (The ribosomes in eukaryotic cells are slightly different from those in prokaryotic cells but the process is very similar.)
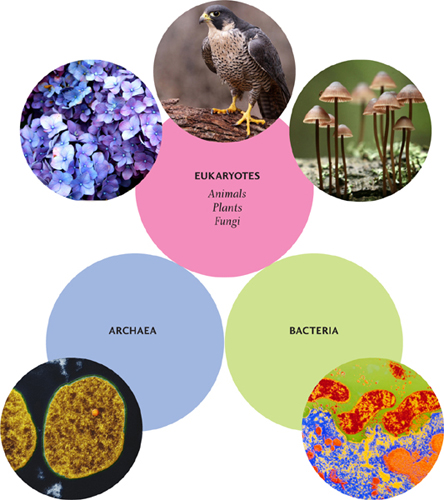
All prokaryotes are single-celled organisms. Eukaryotes generally have larger cells, because they have more components, or organelles. Most eukaryotic organisms are multicellular, but there are also single-celled eukaryotes, such as yeasts and many algae.
All cells also make a variety of motor proteins, which carry out a range of different tasks. In both types of cell, motor proteins might be found in flagella—the whipping tails that help waterborne, single-celled organisms get around. However, there is one important function of motor proteins that is found only in eukaryotes. Kinesin and dynein are remarkable motor proteins that drag other molecules or entire organelles around inside the cell, literally walking along tubular tracks called microtubules (see here)—for example, taking proteins manufactured by the cell up to the cell membrane to be released outside the cell. The microtubules are part of a dynamic system of ropes, poles, and tough fibers called the cytoskeleton, which is also found only in eukaryotic cells.
ANATOMY OF CELLS
Below are archetypical examples of an animal cell, a plant cell, and a fungal cell—all eukaryotes—and an archetypal prokaryotic cell. All prokaryotic organisms are single-celled (although not all single-celled organisms are prokaryotes).

Around the outside
If we were able to shrink down to the size of a typical cell and to peer inside a selection of different cells, what we would see as the outermost part of the cell would depend upon the kind of cell. All cells are enveloped by a translucent, fatty membrane, but with some cells, there are several layers of other material outside that.
Peeling back the layers
Bacteria are among the most protected of all cells. This might come as depressing news to anyone suffering from a bacterial infection—but fortunately, in most cases, the body is quite adept at killing the harmful ones anyway. On the outermost layer, the majority of bacterial cells have a capsule made of a selection of polymers. Polymers are compounds with relatively large molecules made up of repeating, interconnected smaller molecules. In the case of the bacterial cell capsule, the polymers are polysaccharides and the smaller molecules are sugars. The capsule can form a tough coat or a slimy coating; either way, it helps protect the bacterium from being consumed by other cells and helps it stick to surfaces (such as teeth, for example). Protruding out through the capsule are tiny protein fibers called fimbriae, which are also involved in making bacteria stick to surfaces.
Some bacteria have an outer membrane (in addition to the main cell membrane) directly underneath the capsule layer. This outer membrane is present on “gram-negative” bacteria (see here). It provides extra protection against the environment in which the bacterium lives but also has proteins embedded in it that allow only certain chemical compounds in and out. It keeps out harmful substances but allows in nutrients, for example. This selective permeability is something we will encounter again. If the bacterium we are examining has an outer membrane, by removing it we would come across a tough cell wall. Gram-negative bacteria have only a very thin cell wall—after all, they have that outer membrane. Gram-positive bacteria have a much thicker wall.
Bacterial cell walls are made of another polymer, called peptidoglycan. This compound’s long molecules are joined together by cross-links, making a very resilient structure. The links are constantly breaking and reforming; certain antibiotic medicines, including penicillin, work by preventing the formation of the cross-links, so that the wall quickly disintegrates.
Break down the wall
Once the bacterium’s capsule and outer membrane has been stripped away to reveal the cell wall, we can begin comparing bacterial cells with some other types of cell. Archaeal cells, fungal cells, and algal cells also have cell walls, and so do plant cells—but animal cells do not. The walls of archaeal cells are made of protein. The walls of fungal cells are made of a polymer called chitin. This polymer is found in all kinds of other places in the natural world, including a crab’s shell, a squid’s beak, and the external skeleton of a housefly.
GRAM-POSITIVE, GRAM-NEGATIVE
In 1884, German microbiologist Hans Gram invented a stain that would help show up bacteria. He found that bacterial cells fall into two different classes. Gram-negative bacteria, with a thin cell wall, stain pink; familiar examples are E. coli and Salmonella. Gram-positive bacteria, with a thicker cell wall, stain purple; examples are Bacillus anthracis, which causes anthrax, and the infamous methicillin-resistant Staphylococcus aureus (MRSA).
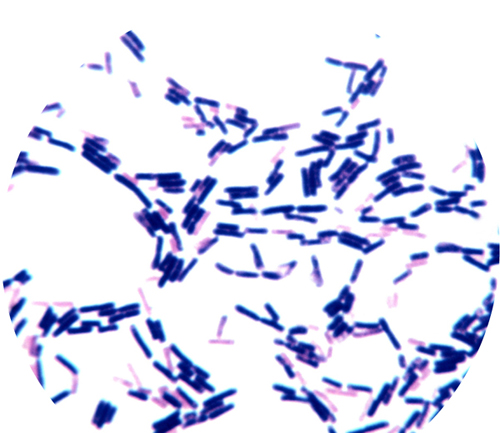
Light micrographof gram-positive bacteria (purplish), which have a thick cell wall, and gram-negative (pinkish) bacteria, which have a thinner cell wall.
Scanning electron micrograph showing intricate detail of a moth’s wing, which is made of chitin, the same material that forms the cell walls of fungi.
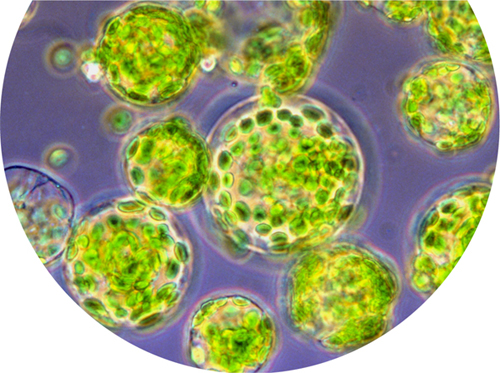
Light micrograph of plant cells with their cell walls removed—spherical globules called protoplasts.
Algal cell walls come in many flavors, as varied as algae themselves. Algae make up a diverse group of plant-like eukaryotes—everything from single-celled organisms floating free in lakes to seaweeds 54 yards (50 meters) in length. Some algal cell walls are made of proteins, while others are made of polymers of sugars, or “amino-sugars.” Some single-celled algae use minerals, such as silicon and calcium, to build an intricate, protective shell. There is more about those elaborate outer casings in chapter four.
The walls of plant cells have an outer covering made of a polymer called pectin (the substance used as a gelling agent in preserves). Pectin helps glue one cell to the next. Below that sticky layer is cellulose, another sugar-base polymer. Cellulose is indigestible to humans. It is the insoluble fiber we ingest from eating plants—and it is those fibers that are visible when a sheet of paper is torn. Some plant cells—those of woody plants, such as trees—also lay down a second cell wall inside the first one. This secondary wall is made mostly of lignin, a still tougher polymer.
Plants’ cell walls can remain long after the living cell inside has died. It was the thick cell walls of dead, empty cork cells that Robert Hooke saw down his microscope, when he came up with the name “cells” in 1663. A plant cell’s wall prevents the cell from bursting apart when it takes on water, and helps keep its structure when water is scarce.
Naked cells
Remove the cell walls from the bacterial, archaeal, fungal, algal, and plant cells … and we have naked cells. All that is left holding in the watery mixture (the cytoplasm) is a very thin fatty bag (the membrane). The scientific name for a naked cell is a protoplast. Since no animal cells have a cell wall, we can finally include them.
With no capsule, no secondary membrane, and no cell wall, on the face of it animal cells might seem vulnerable. But animals are multicellular, and the material that fills the space between them and holds them together—the extracellular matrix—offers them protection. Cells on the outer surface of the organism, exposed to the world at large, tend to have greater protection, often by secreting tough substances. Think of fish scales or bird feathers. The skin of many animals, including humans, is made of dead, tough, protein-rich cells that form a waterproof, infection-proof barrier. In the case of humans this layer is constantly replenished from below, and tens of thousands of old cells fall off the top layer every minute.
A fluid mosaic
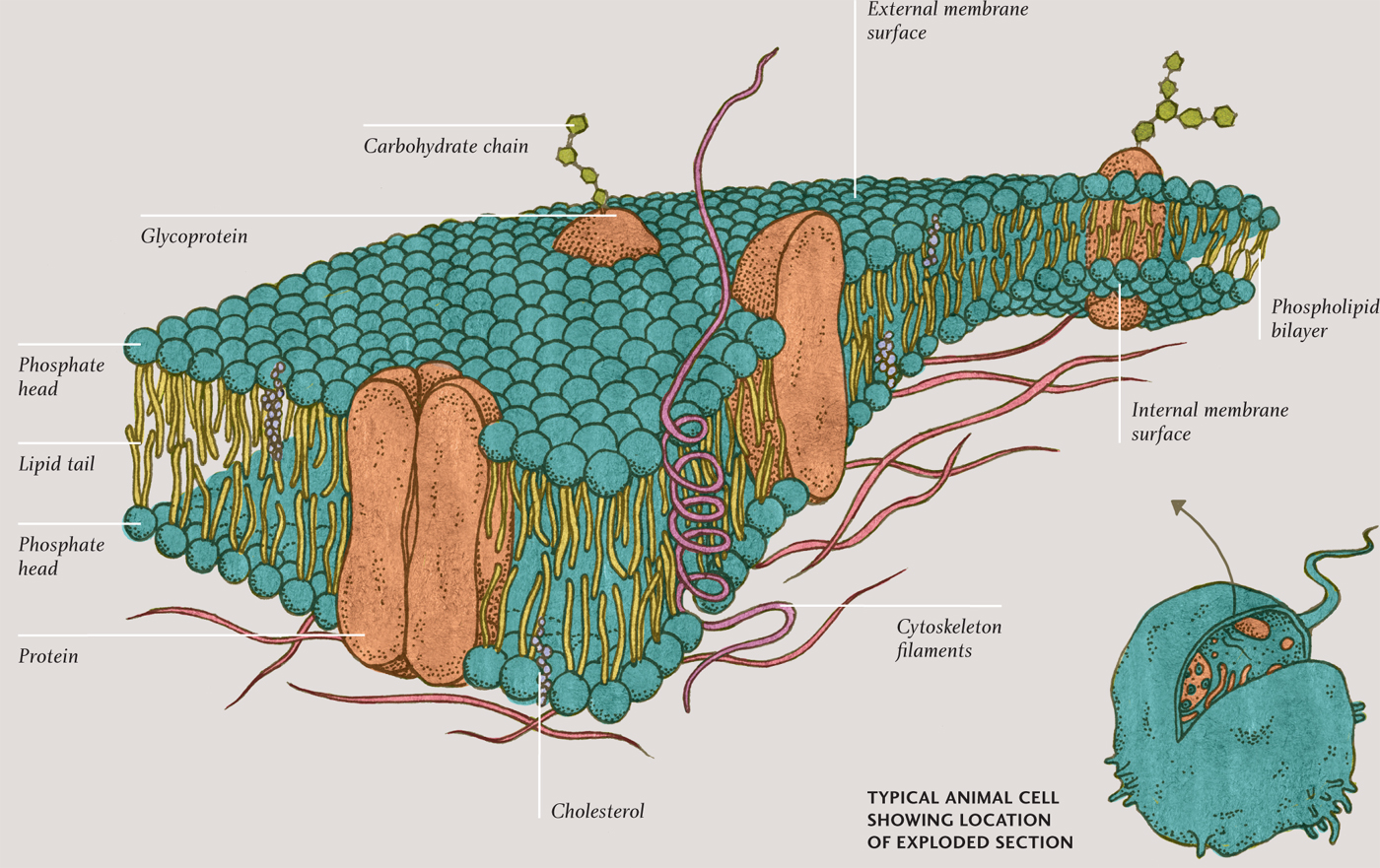
So now we have stripped-down bacterial, algal, fungal, and plant cells—and already naked animal cells. The cell membrane that surrounds these cells is a remarkable structure. Picture an ancient Roman mosaic floor: a pattern of small white tiles with some differently colored tiles interspersed here and there. Now, imagine the tiles shifting around within the floor, in constant motion, and that gives a pretty good idea of what a cell membrane is like. The white tiles represent molecules of phospholipids, the predominant compounds in the membrane. Mixed in are some off-white tiles, which represent molecules of cholesterol. Having cholesterol in the mix makes the membrane more robust.
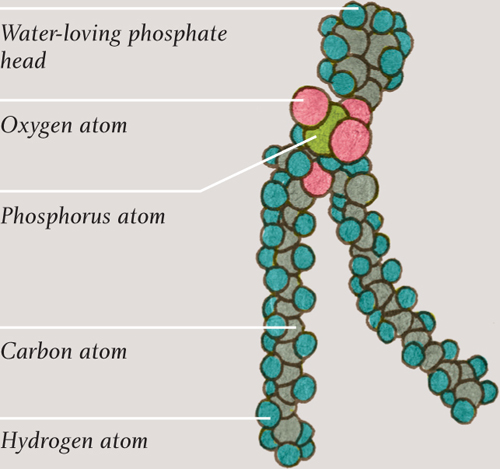
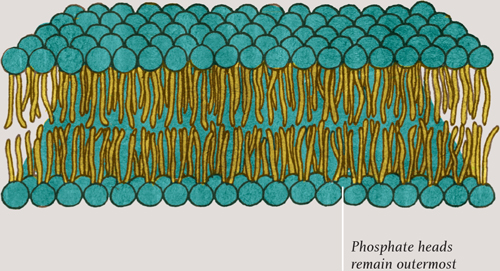
Each phospholipid molecule has two long lipid tails—the same tails are present in molecules of fat—and a round head containing a phosphate group (see here). As might be expected, the fatty tails do not mix with water but the phosphate head does. Put phospholipid molecules in water and they spontaneously arrange themselves tail-to-tail, either in small globules or in double-layered sheets. In the sheets, the phosphate heads point away from each other, making contact with the water. The tails come together, away from the water, staying firmly between the two layers. The sheets have no edges—they wrap around and enclose whatever is inside them, like fluid-filled bubbles.
Illustration showing a phospholipid molecule (see here) and a small section of a cell membrane, showing how phospholipid molecules self-arrange into a sheet, with their hydrophobic (water-hating) tails pointing away from the watery inside and outside of the cell.
PHOSPHOLIPID MOLECULE
PHOSPHOLIPID BILAYER SHEET
Two-way traffic
Although the phospholipid bilayer membrane has to keep the cell contents confined, it is by no means impenetrable. If it were, the cell would take in no nutrients and would be unable to get rid of any waste—it would soon die. However, the phospholipid bilayer membrane cannot just let anything in and out; it needs to be selective about what gets through. Water is essential—although it is important that not too much or too little passes through, for obvious reasons. Water can cross the membrane directly but most of it passes—in molecular single file—through tiny holes in ring-shaped protein molecules. These proteins, called aquaporins, straddle the two layers of the membrane (in the Roman mosaic analogy, they are among the colored tiles, interspersed among the white tiles).
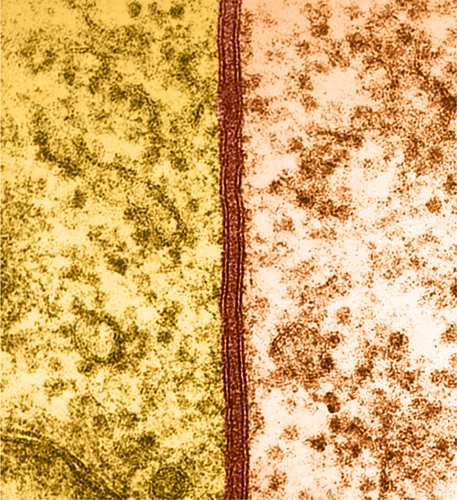
The cell membrane is about 10 nanometers thick (0.00001 mm) but is clearly visible in this coloured transmission electron micrograph.
Most cells are aerobic. They depend upon oxygen for the energy-releasing cellular respiration reactions that keep the cell ticking over (cells that do not rely on oxygen are known as anaerobic). Carbon dioxide is a waste product of cellular respiration and must leave the cell. Fortunately, these two small molecules, O2 and CO2, are fat-soluble; they pass through the fatty membrane relatively easily in a process called diffusion. They pass in both directions through the membrane at all times, but there will be more crossings from the side that has more molecules—so that, on balance, both oxygen and carbon dioxide pass from a higher to a lower concentration.
THE CELL MEMBRANE
Molecular guest list
Most cells also need glucose, the fuel for cellular respiration. Glucose cannot diffuse through the phospholipid bilayer as oxygen molecules do. There are, however, transfer proteins that only let through glucose molecules, just as the aquaporin proteins allow through only water. There are similar transfer proteins that let other molecules through, each one specific to a particular compound.
Also embedded in the fluid mosaic of the cell membrane are receptor proteins. Each of these proteins allows a specific compound or ion—a ligand—to attach to it. Most ligands are proteins that have been released by other cells, such as hormones and neurotransmitters, and which, therefore, act as a signaling mechanism. There are, for example, special cells in the pancreas that release the hormone insulin when blood sugar is high.
When ligands “dock” on the part of the receptor sticking out of the membrane, the part of the receptor molecule inside the cell can change shape, start an enzyme reaction, or release compounds. These cause the cell to change its behavior. Human muscle cells and fat cells have insulin receptors in their membranes; when insulin docks into the receptors, the receptors directly affect the glucose transfer proteins. That encourages the transfer proteins to let more glucose into these cells for storage as glycogen or fat. The effect of all this is to reduce blood sugar level and to stock up on energy for times when food is scarce. It is a remarkably robust system, although it doesn’t work in people with diabetes. In type-1 diabetes, the cells in the pancreas fail to produce enough insulin; in type-2 diabetes, the receptors stop responding adequately to the presence of insulin.
Like the bubbles in a glass of champagne
There is another way that substances can get in and out of a (eukaryotic) cell, a method perhaps even more remarkable than protein gatekeepers. Molecules that the cell needs can be “swallowed.” When the desired molecules are next to the membrane, an indentation forms in the membrane; the indentation grows deeper and eventually part of the membrane completely surrounds the molecule. That portion of membrane snips off, forming a tiny delivery bubble, which is then subsumed down into the cell. Imagine a video showing a bubble rising to the surface of a glass of champagne and bursting … played in reverse. Once inside, the bubble melds into internal membranes, and the precious compound is released and made available.
The opposite also happens. Now play that champagne video forward to show bubbles bursting at the surface. Certain compounds manufactured inside the cell, mostly proteins, cannot pass directly through the membrane. Instead, they are carried up to the membrane inside a fatty bubble; this pops open at the surface, releasing the contents outside the cell. This is how insulin is released from those pancreatic cells; the compounds that build cell walls are also released this way. The fatty delivery bubbles originating from inside the cell are called “secretory vesicles” (there are other types of vesicle, see here). The precious protein cargo they carry is manufactured deep inside the cell, according to instructions held in the DNA in the nucleus. The process by which those instructions are copied and carried out is another incredible series of molecular events.
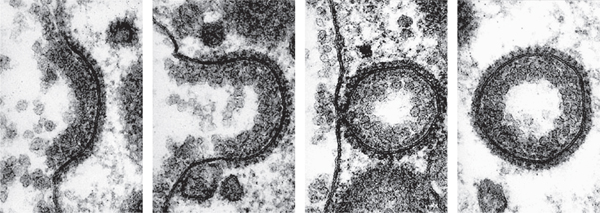
Series of transmission electron micrographs that show the process of endocytosis—when a cell absorbs substances across its cell membrane. Receptor proteins in the membrane change shape when a target compound is present, forming an indentation and eventually a vesicle that surrounds the compound. The vesicle carries the compound into the cytoplasm.
Protein factory
Proteins have many vital roles in living things. Some proteins are enzymes, which enable essential chemical reactions; some are structural materials inside or outside the cell; some are used as signaling molecules; and some as receptors. Then there are carrier proteins, such as the oxygen-carrying hemoglobin. All proteins are manufactured inside cells by incredible molecular machines called ribosomes, which follow instructions held in DNA.
Words of life
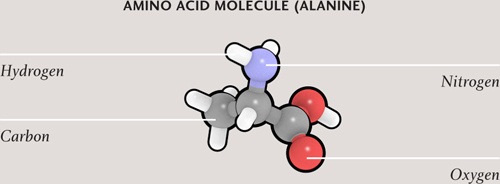
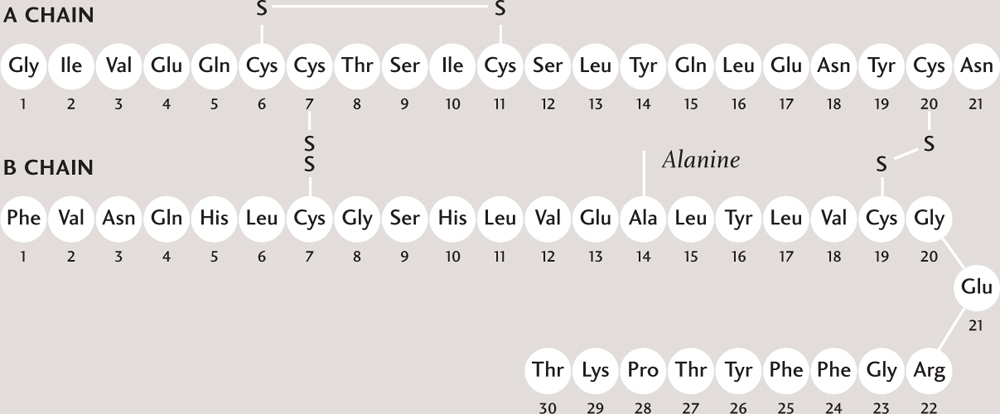
Proteins are made up of smaller molecules—molecules of compounds called amino acids. A protein molecule is not really a polymer, however, because the amino acids are not connected in a repeating pattern. Instead, they attach together one after another, like letters in a long word. The hormone insulin (in humans) is a protein consisting of a sequence of 51 amino acids. That’s relatively short; the average length of a protein manufactured in human cells is 470 amino acids. The sequence is dictated by the protein-building instructions in DNA. Just as there is a fixed pool of letters in the alphabet from which to make words, so there are only 21 amino acids available to build proteins. That’s more than enough, however, to build tremendous variety into the world of proteins. The number of possible combinations in a string of just ten amino acids is in the tens of trillions; most proteins are hundreds or thousands of amino acids long. Protein biochemists have identified around one million different proteins so far.
Unlike a word printed on a page a long chain of amino acids is rarely straight; most protein molecules bend and fold back on themselves. The shape of a protein molecule is often very important to its function. Signaling proteins, such as hormones, for example, fit into receptor proteins as keys do into locks. Many proteins change shape, or undergo conformational changes, when their circumstances change—for example, when certain other molecules are nearby or when the acidity or temperature changes. This is an important part of how cells respond to their environment.
FROM AMINO ACIDS TO PROTEIN
SCHEMATIC CHAIN OF AMINO ACIDS
THREE-DIMENSIONAL MOLECULAR MODEL OF INSULIN
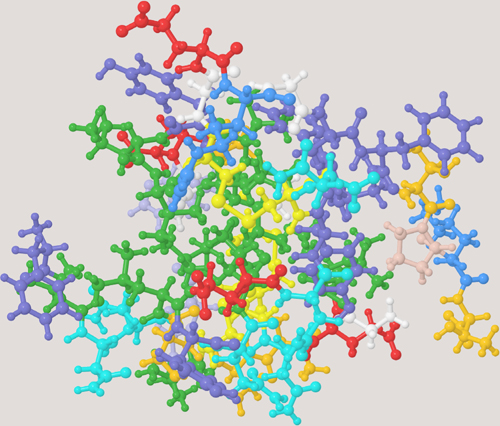
Amino acids are made up of carbon, oxygen, nitrogen, and hydrogen atoms. A sequence or chain of amino acids join together to make a protein. In the model, each amino acid is shown in a different color. The protein is folded and, here, cross-linked by sulfur atoms (S), and the resulting shape is important.
FROM NUCLEOBASES TO DNA
DNA

NUCLEOTIDES
NUCLEOBASES
The DNA double helix is made of nucleotides, each of which is a nucleobase plus a deoxyribose sugar and a phosphate. The sugar and phosphate groups form the twisting “backbones” of the DNA helix, while the nucleobases form the “rungs” that join the backbones.
Protein recipes
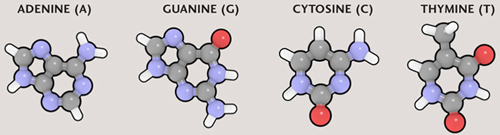
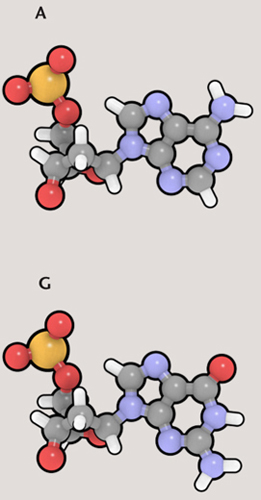
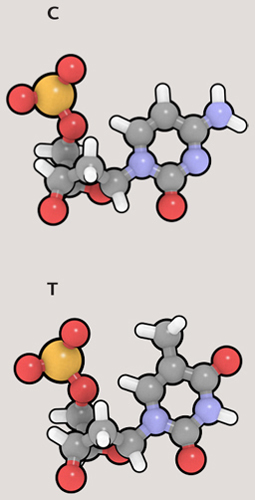
A gene is a particular section of DNA that represents the “recipe” for a particular protein. Genes exist one after another along the length of DNA, and each gene is “written” in code, as a sequence of nucleobases. There are four nucleobases in the DNA molecule: adenine (A), guanine (G), thymine (T), and cytosine (C). Each nucleobase is attached to part of the “backbone” of the DNA molecule, a sugar-phosphate group. The resulting structure is called a nucleotide. The four nucleotides are also referred to as A, G, T, and C, according to which nucleobase is present. Because these pieces are the building blocks of DNA, biologists talk about nucleotides more than they do nucleobases.
There is a direct connection between the A, G, C, and T nucleotides of DNA and the 21 amino acids of proteins. The nucleotides exist in sets of three—called triplet codons. With three slots and four possible nucleotides per slot, altogether there are 64 combinations (4 x 4 x 4)—more than enough to code for the 21 amino acids and to include administrative tasks, such as noting the start and end of a gene. Codon ATG means the start of a gene, while TAT represents the amino acid tyrosine.
The DNA molecule is a double helix, like a twisted rope ladder. Two strands twist around each other, zipped together by bonds between the nucleobases along the length of each strand. The two strands are complementary: A always bonds with T, and C always bonds with G. So a section of DNA that reads ATCGTA on one strand will be joined to the sequence TAGCAT on the complementary strand. Only one strand holds the actual code, but the other strand is not redundant; it plays a very important role, as we will see.
The length of a section of DNA is normally given as a number of base pairs—each base pair being a single rung on the twisted rope ladder. That same measurement could just as well be bases or nucleotides. Genes are generally a few thousand base pairs (kbp) long. The gene for a protein called dystrophin, found in muscle, is the longest known; it is 2.4 million base pairs long. The entire length of DNA of an organism, copied in each cell (well, most of them), is that organism’s genome. Generally—although not always—the more complex an organism, the more base pairs will make up its genome. Bacterial genomes can range from around 150 thousand to about 12 million base pairs in length. In eukaryotes the number is much greater; for example, the human genome is about three billion base pairs long. A good deal of a genome’s DNA has no (or no known) function.
Loops, beads, and shoelaces
A single length of DNA, end to end, is called a chromosome, and it contains many genes along its length. The prokaryotic cells of Bacteria and Archaea have a single chromosome, arranged as a closed loop that floats free in the cytoplasm. Most eukaryotic cells have between several and many chromosomes, which are held inside the nucleus (see here). For most organisms, the genome is a huge length of DNA—in humans this amounts to a total of about 6 feet (2 meters)—in each cell. In order to fit all that in the nucleus, the DNA has to be very tightly packed.
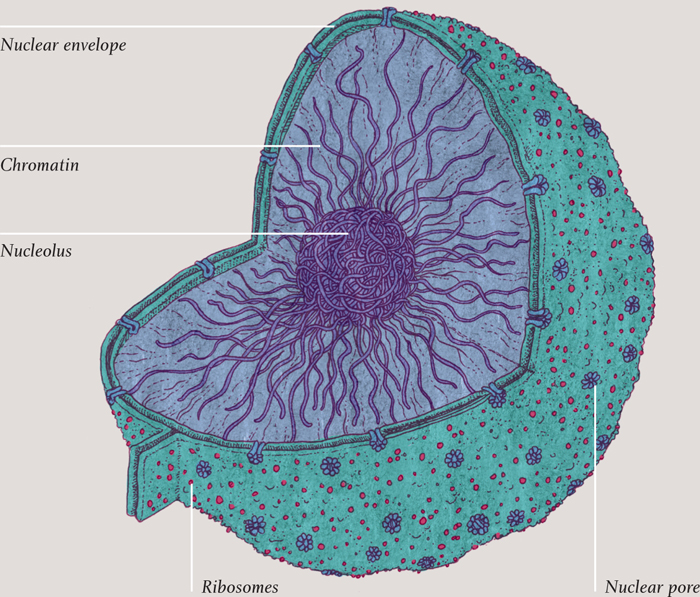
THE NUCLEUS
The function of the nucleus (found only in eukaryotic cells) is simply to keep the huge expanse of DNA in one place. The nucleus has a phospholipid bilayer membrane of its own, just like the cell’s main membrane. And, of course, it lets things in and out, just as the cell’s membrane does. There are two thousand or so pores in the nuclear membrane, each one a collection of proteins with a centered hole in it, embedded in the membrane. Various compounds pass in and out of the nucleus through these membranes, including the ingredients for making RNA, DNA, and proteins.
Around certain sections of DNA inside the nucleus is a dense crowding of molecules that constantly produce new ribosomes, the molecular machines that actually build the proteins. This densely packed region is called the nucleolus.
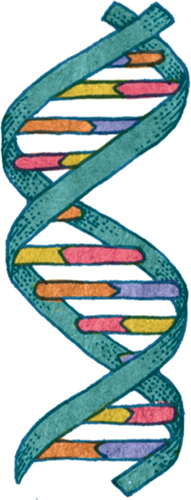
Schematic diagram of a section of DNA. The green twisting section represents the sugar-phosphate backbone, and the rungs are the nucleobases. A (purple) always bonds with T (orange), and C (yellow) with G (pink).
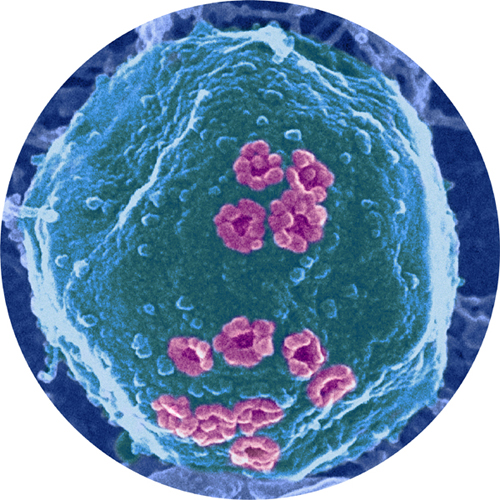
Colored scanning electron micrograph of a cell nucleus. The nucleus is colored pale blue, and the nuclear pore proteins are colored pink.
In eukaryotic cells (and in archaea), the DNA is wrapped every so often along its length around blobs of a protein called histones; the result looks like beads (histones) on a string (DNA). The beaded string is further wrapped around itself, so that it becomes a much fatter string—although still just 30 nanometers (0.00003 millimeters) in diameter. So each chromosome exists as a 30-nanometer fiber, chaotically jumbled with the other chromosomes, like so many shoelaces in a bag. Order is imposed on chromosomes during cell division, however, when the chromosomes organize themselves into discrete packages, ready for duplication. This is a process that is developed in chapter three.
When a protein is to be made, enzymes unpack and unwind the section of the DNA containing the relevant gene, so that the code becomes exposed. It marks the beginning of an amazing process of molecular recipe-copying, protein assembly, modification, and delivery.
DNA PACKAGING
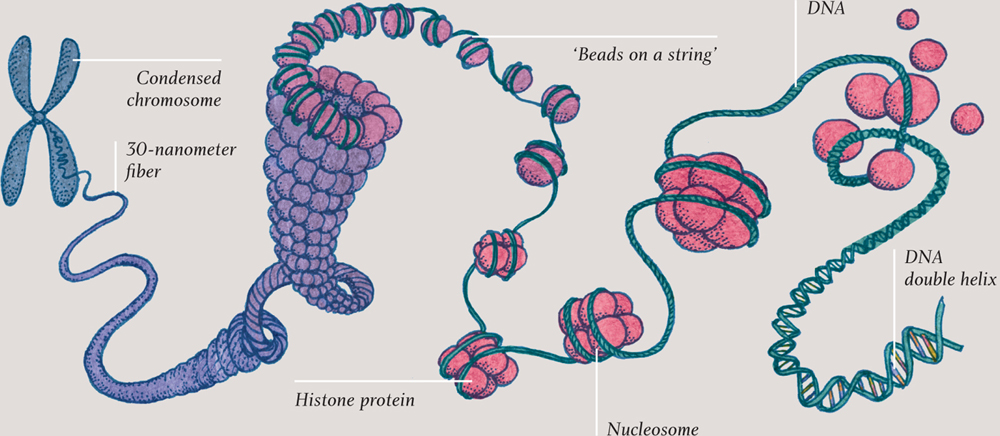
DNA packing, around histone proteins, into a 30-nanometer (0.00003-mm) fiber and into a condensed chromosome.
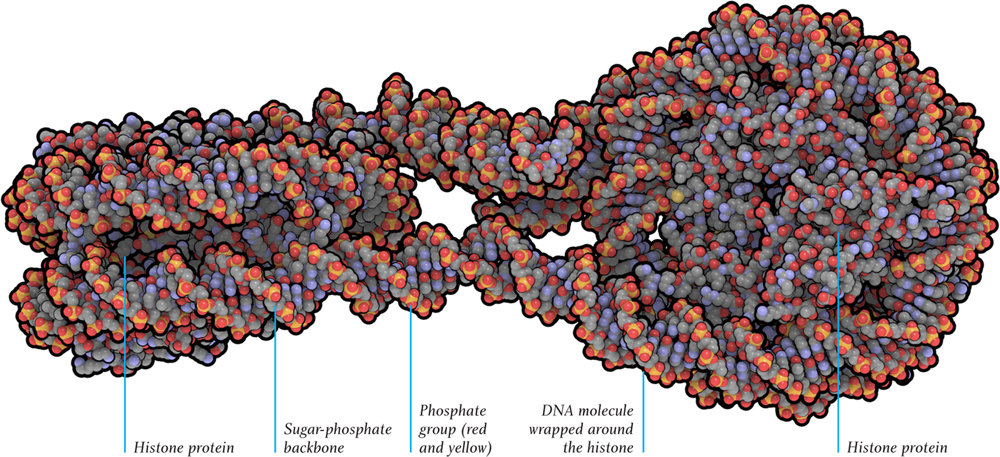
Molecular model of a length of DNA wrapped around histone protein molecules. Each end, with eight histone molecules at its core, is called a nucleosome.
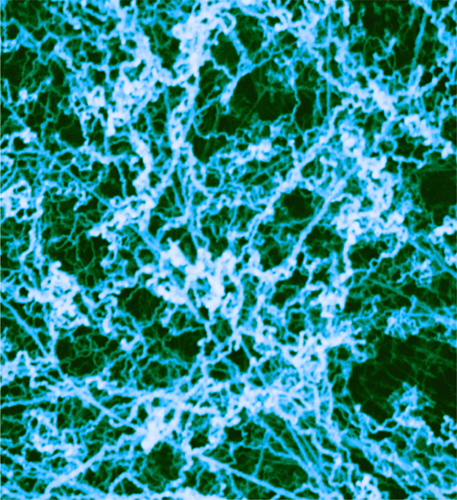
Transmission electron micrograph of chromatin, which is comprised of DNA wrapped around histone proteins. Here, in the cell nucleus, most of the chromatin is coiled around itself, forming 30-nanometer (0.00003-mm) fibers.
Writing out the recipe: transcription
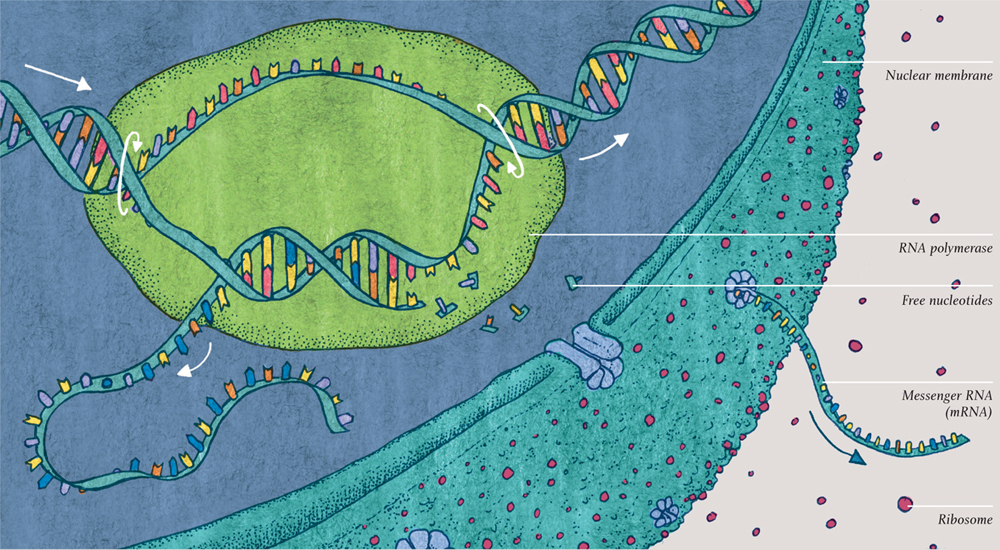
The DNA holds the key for protein building, but it doesn’t do the building, nor is the building done inside the nucleus. The first two stages in the process of protein building are copying out the recipe and taking it outside the nucleus. The copy is written as a length of RNA (ribonucleic acid), a compound very similar to DNA (deoxyribonucleic acid) but single-stranded. The RNA is copied from the complementary strand of DNA, not the coding strand. In this way, it is a copy of the coding strand—and that is why the second, complementary strand of DNA was so vital.
The “scribe,” which does the copying, is an enzyme—RNA polymerase. Before its job of transcription begins, a cluster of other molecules attaches to the DNA and prepares it for copying. The RNA polymerase then unzips the DNA double helix and makes a complementary copy of the complementary DNA strand from a multitude of nucleotides floating in the nucleus. The RNA is not exactly the same as the coding DNA of which it is a copy: all the T nucleotides have become U (the nucleobase called uracil), but the code still reads just as it did.
The RNA polymerase zips the DNA back up behind itself. As it moves swiftly along the DNA, a growing ribbon of RNA emerges from the side. When it reaches the end of the gene, the RNA polymerase molecule detaches, ready to read another gene, and the freshly made ribbon of RNA is let loose, ready for the next stage. This precious piece of genetic code is called messenger RNA (or mRNA), for it will carry the copied recipe to the manufacturing stage. Gatekeeper proteins embedded in the nucleus’s membrane encourage the mRNA out of the nucleus.
Do remember that this fundamental and intricate process is self-organized, not under any kind of central control. It is just a set of complex chemical reactions.
TRANSCRIPTION
TRANSLATION
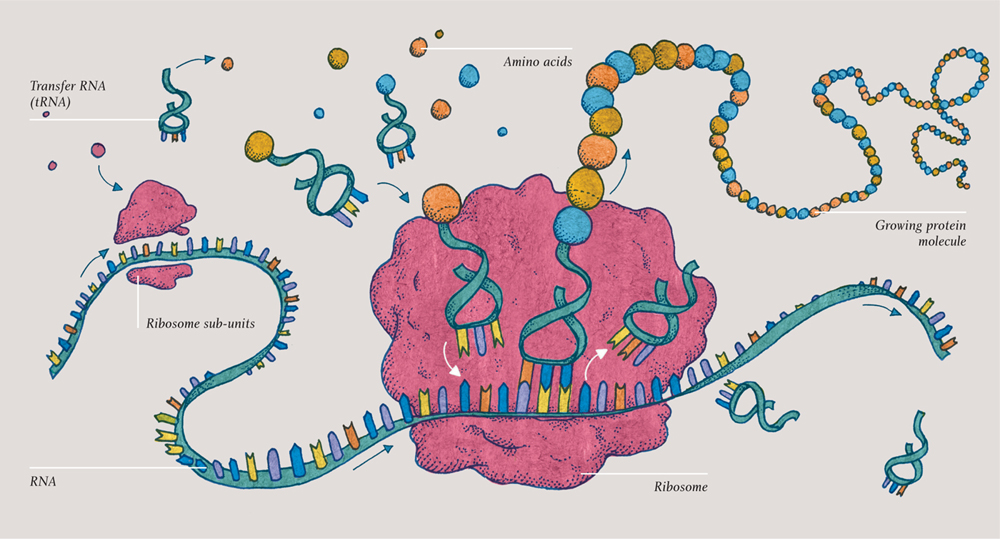
Reading the recipe: translation
So, a piece of messenger-RNA has just left the nucleus. This copy of the DNA’s recipe for a protein must now be translated into amino acid language. The protein has to be built up as a specific sequence of amino acids. Welcome to the remarkable process of protein translation.
A self-assembling molecular machine called a ribosome clamps onto the new ribbon of mRNA. The mRNA code passes codon-by-codon (three nucleotides at a time) through the heart of the ribosome—and smaller molecules now dock there, bringing one amino acid each. These new molecules are small lengths of transfer RNA (tRNA), so-called because they transfer the amino acids from the cell at large into the ribosome to assemble the protein. Each tRNA is just three nucleotides long, so that it corresponds to a particular codon. The amino acid it carries at its head corresponds to the codon it represents at its foot. Again, it is worth remembering that this is just a chemical reaction.
The three-toed foot of the tRNA molecule docks with the relevant codon on the mRNA at the heart of the ribosome … and then the next one sidles up next to it and does the same. The two amino acids join, and the ribosome moves to the next codon. The tRNA, now emptied of its load, is released and will soon be endowed with another identical amino acid, ready for its next transfer.
And so, just as a ribbon of mRNA emerged from the RNA polymerase as it moved along, the DNA inside the nucleus, now a growing chain of amino acids—a protein molecule—emerges from the ribosome as it makes its way along the mRNA. This amazing process is similar in prokaryotic cells, but the ribosomes and the step-by-step details are slightly different than those in eukaryotic cells.
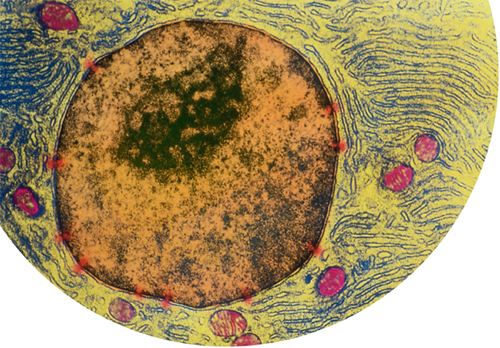
Colored transmission electron micrograph of the nucleus of a cell from a human pancreas. The dark area in the nucleus is the nucleolus, a region active in making mRNA. Nuclear pores, highlighted in red, let mRNA molecules out. The endoplasmic reticulum, studded with ribosomes, is colored dark blue. (The pink-colored structures are mitochondria.)
Taking the rough with the smooth
The membrane of the nucleus is a phospholipid bilayer, just like the cell membrane. Attached to the nucleus’s envelope, and extending from it, is yet another bilayer membrane structure, called the rough endoplasmic reticulum (RER). It is rough because it has ribosomes embedded in it, and it is endoplasmic because it is in the inside (endo-) of the living mixture (plasm) of the cell. And reticulum is just a fancy word for net. The RER looks a little like a net, although it is actually more like a maze that might be included in a puzzle book.
Ribosomes become embedded in the RER if a particular code is present in the mRNA protein recipe. Otherwise they do their protein manufacturing floating free in the cytoplasm. Proteins manufactured on the surface of the RER may have sugar molecules added, or may be better folded into the correct shape when made here. Most are destined for release from the cell inside a secretory vesicle, one of those champagne bubbles that will burst out of the cell membrane (see here).
Farther out but also connected to the nuclear envelope is another folded membranous structure: the smooth endoplasmic reticulum (SER). As its description suggests, this is not studded with ribosomes—so, unlike the RER, it has no role in protein building. Instead, lipids, phospholipids, and cholesterol and other steroids are made here. So cells that make steroid hormones, such as testosterone and estrogen, have more SER than most other cells. It is also involved in breaking down toxins, so the detoxifying cells in the liver also have more SER than most other cells.
VESICLES
The champagne bubble vesicles that deliver secretory proteins to the cell membrane are not the only kind of vesicle present inside cells. Plant cells have huge, membrane-bound spaces called vacuoles, which are really just large vesicles. The main function of a vacuole is to store useful—and potentially toxic—compounds. A vacuole also stores water; it can swell to occupy 95 percent of the cell’s volume. It stores pigments and also poisons that can protect the plant if the cell is attacked. For example, vacuoles inside the cells of a garlic bulb hold allicin, the sulfurous compound that gives garlic its flavor. A plant cell’s waste products are also processed inside the vacuole.
Plant cells have vacuoles, but the equivalent vesicles inside animal cells are called lysosomes. Inside the membrane of a lysosome, obsolete and toxic molecules are broken down into their constituent parts, which are then released for recycling if they are useful. It is in lysosomes that proteins are broken down into their constituent amino acids, for example, and released ready for ribosomes to use them to make new proteins.
Delivering the goods
Proteins that were manufactured by ribosomes embedded in the RER are now dispatched to another membranous structure: the Golgi apparatus. They travel in style, enclosed in vesicles. The Golgi apparatus is similar to the ER; it is made up of stacked layers of membranes. As a protein passes from layer to layer on its way up toward the cell’s outer membrane, it is altered in any of a number of ways. Its sugar molecules might be changed; its shape may change again; in some cases, part of the protein may be cut off, transforming it from an inactive to an active state. This last action, for example, is what happens to insulin as it passes through the Golgi apparatus.
Once through the layers of the Golgi apparatus, the secretory protein arrives at the cell membrane, ready to be released in a graceful bursting of the bubble. Often, however, proteins will be stored in the Golgi apparatus so that they can be released quickly. This is particularly true of neurotransmitters in neurone cells. When the cell receives a particular signal, the neurotransmitter proteins are released from vesicles that rapidly burst at the cell’s surface, ready to help make a new memory, play a part in perception, or cause a muscle to contract.
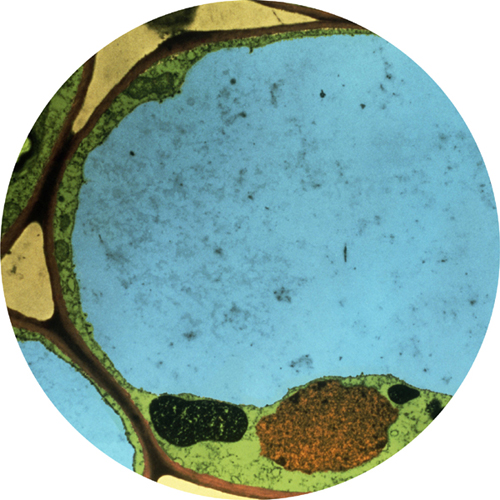
Colored transmission electron micrograph of yeast cells. The cell wall is colored green, and in the center is a kind of vesicle called a vacuole (pale blue). All fungal cells have vacuoles, whose main purpose is to hold water with either waste products or important compounds in solution.
Frames and motors
The vesicles that act as delivery vehicles inside eukaryotic cells don’t just float around randomly. Instead, proteins walk them along rigid tubular tracks inside the cell. These tracks are part of an extensive network of protein fibers known as the cytoskeleton.
The power of three
The cytoskeleton is found only in eukaryotic cells. It has several crucial roles beyond providing delivery tracks. It makes cells move; it supports organelles; it drags chromosomes around in the dance of cell division (the music for which begins in chapter three); and in cells with no cell wall, it determines the overall shape of the cell. The cytoskeleton is made of three different types of protein fiber, which can be considered as small, medium, and large. The names (or at least the first two) help: microfilaments, intermediate filaments, and microtubules. The smallest and largest—the microfilaments and microtubules respectively—are dynamic structures, constantly assembling and disassembling from smaller pieces called subunits. The intermediate filaments are less dynamic.
Microfilaments are extremely slender protein threads with a diameter of about 6 nanometers (0.000006 millimeters)—about one-thousandth the diameter of a typical human hair. Microfilaments have tensile strength; they are strong under tension, like the guy ropes of a tent. By pulling, they can change the shape of a cell—for example, extend an amoeba’s “foot.” In the cells that line our intestines, the microfilaments extend into tiny bristles called microvilli, which dramatically increase the surface area of the cells, enabling them to absorb more nutrients. Microfilaments also play a central role in the contraction of muscle cells, which will feature in chapter seven.
The intermediate filaments are medium in diameter and also in rigidity. They are like thick twisted ropes rather than thin fibers or rigid tubes. There are several kinds, each made of different proteins. Overall, they are responsible for maintaining a cell’s shape, including keeping the other two types of fiber in place.
One kind of intermediate filament, made of the protein lamin, forms a grid that gives shape to the membrane around the cell nucleus. The toughness of skin and hair is due to tangled, cross-linked intermediate fibers made of keratin. Yet another kind of intermediate fiber forms structures on the cell membrane that connect to similar structures in neighboring cells. These structures are called desmosomes, and in animals they hold cells together to form tissues, such as muscle and mucous membranes.

Light micrograph of a fibroblast cell, showing the actin filaments of the cytoskeleton, stained purple.

Light micrograph of microtubule filaments, made of the protein tubulin, which has taken up a red fluorescent stain (rhodamine).
MICROFILAMENT ASSEMBLY
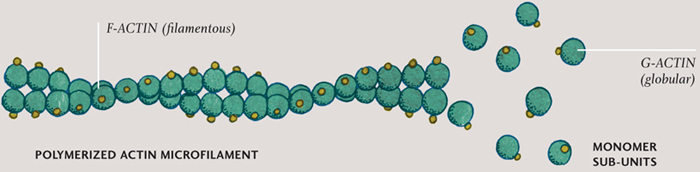
Individual molecules (monomers) connect together to form a repeating pattern (polymer). The G-actin molecules (green) are bound to ATP (yellow), and the resulting long, filamentous polymer resists tension.
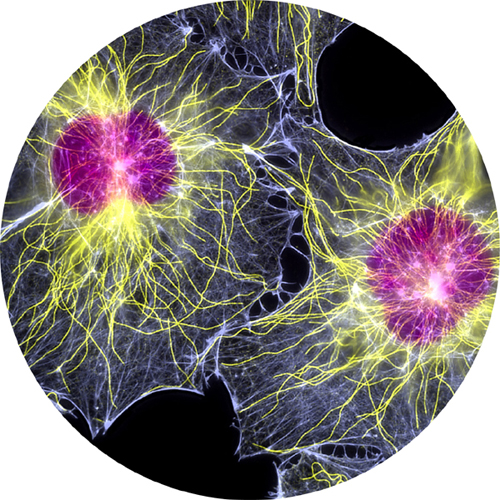
Fluorescent light micrograph of two fibroblast cells. Nuclei are purple, microtubules yellow, and actin microfilaments pale blue.
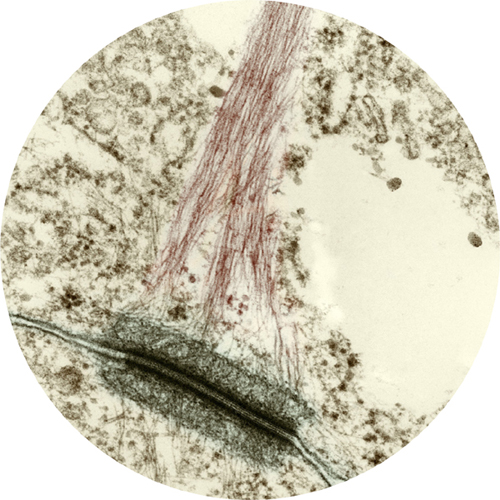
Colored transmission electron micrograph of a desmosome (dark green) attached to an intermediate filament called a tonofibril, which anchors the desmosome to the cytoskeleton.
Delivering the goods
It is the largest fibers, the microtubules, that form the remarkable network of tracks enabling vesicles to be delivered around the cell. Microtubules are rigid, hollow rods made of a protein called tubulin, which can disassemble as quickly as they can assemble. Small tubulin subunit molecules are everywhere, dissolved in the cytoplasm, ready to be used in a kind of cellular construction kit. The motor proteins that walk along the tracks are called kinesins and dyneins. Often the track is made just in front of the approaching kinesin, like laying down railroad tracks in front of a moving train.
The kinesins always walk towards the cell membrane, the dyneins away from it. The gait of these mobile molecules is a little awkward, but they race along, taking up to one hundred steps every second. One hundred motor protein steps move the proteins about 800 nanometers—which works out as a few millimeters per hour. The cargo, securely attached to the head of these incredible walking proteins, is not restricted to vesicles: it can be RNA, proteins, or even entire organelles. To move a particularly large organelle, two or more “walkers” team up—especially if there is some resistance to the organelle’s movement.
Whips and eyelashes
Microtubules extend into long, thin, hairlike extensions called cilia (the name is Latin for “eyelashes”). Many cilia carry the sensing part of sensory cells in the nervous system; this is true of the cells that give us our sense of smell (olfactory neurons) and the light-sensitive cells in the retinas of our eyes, which will feature in the final chapter. Kinesins and dyneins carry nutrients and other essentials to and fro inside each cilium, along its length. They play an even more dynamic role in some cilia, making them move back and forth. In the lining of our lungs and windpipe, for example, cilia are flapping back and forth between 10 and 20 times every second, pushing mucus-soaked dust particles and bacteria up to the top of the windpipe, where they can be swallowed. And the human egg is teased along the Fallopian tubes by flapping cilia. Inside these motile cilia, the kinesin and dynein grab hold of a centered column and walk up and down, so that the whole column of tubulin bends.
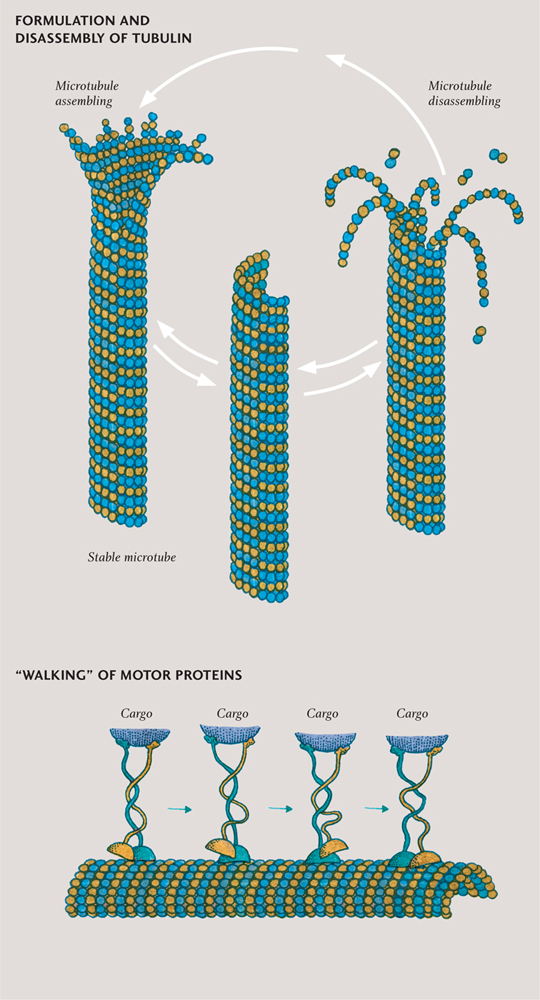
Microtubules (top) are built from units each consisting of two proteins, alpha- and beta-tubulin. They are dynamic structures, with even apparently stable tubes constantly being assembled and disassembled. Motor proteins (bottom) move along the surface of microtubules in a walking action.
While only eukaryotic cells have a cytoskeleton and cilia, there is a related structure that is found in some bacterial and archaeal cells as well as eukaryotic ones: the flagellum, named after the Latin for “whip.” Flagella are much longer and fewer in number than cilia, but they work in a similar way. Eukaryotic flagella have a framework of tubulin and work just as motile cilia do. There is a large flagellum located on the back of each sperm cell, pushing it along like a motorboat. The framework inside a bacterial flagellum is made of tubes of a similar protein, called flagellin. Rather than waving to and fro, this flagellum is made to rotate, at a rate of several hundred revolutions per minute, by a molecular motor at its root. These motors are very effective: a waterborne bacterium can move up to 50 times its own length every second—the equivalent of a person swimming at about 100 mph (160 km/h).
The energy required to build the cytoskeleton, to power the motor proteins that walk along the microtubules, and to drive flagella comes from cellular respiration. The energy is delivered in little packages of a compound called adenosine triphosphate (ATP). The “walking” proteins kinesin and dynein require one ATP for each step, up to 100 per second.
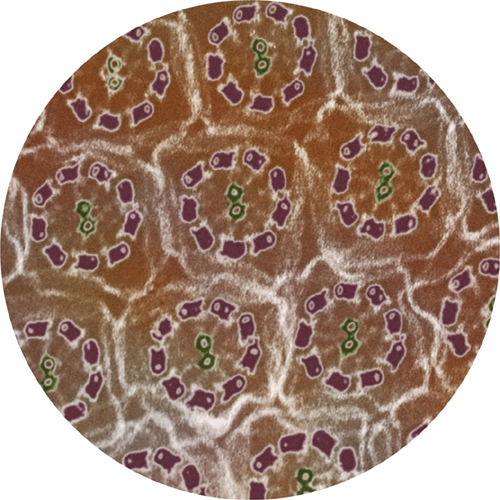
Inside flagella and cilia microtubules are arranged in neat geometric bundles.
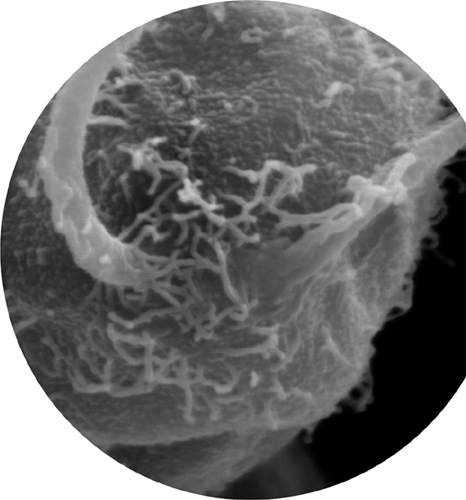
Scanning electron micrograph showing the root of the two flagella of Chlamydomonas reinhardtii, a single-celled green alga (a eukaryote).
Feeding the fire
All cells obtain the energy they need to survive, grow, and reproduce from energy-rich chemical compounds, such as glucose. Some cells are able to manufacture their own glucose, using the energy of sunlight.
Invest to spend
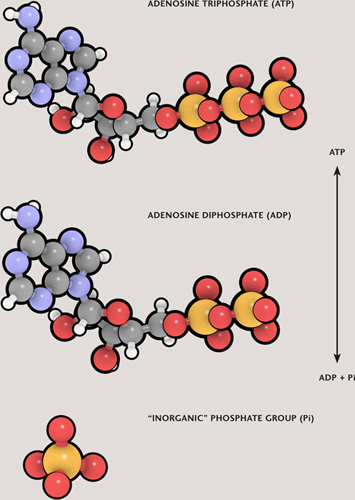
The two main processes by which energy is released from cellular fuels, such as glucose, are respiration and fermentation. There are several different variations on these two themes, but in every case the energy released by processing cellular fuels ends up being stored in cells’ universal currency—molecules of adenosine triphosphate, ATP.
The ATP molecules are built up from lower-energy adenosine diphosphate (ADP) by adding a phosphate group, an operation that requires energy. It may help to think of ADP as an empty wallet and ATP a full one, with the extra phosphate as the cash that fills that second wallet. ATP molecules at large throughout the cell spend their energy powering a multitude of different processes. In the most common and most efficient form of respiration, called aerobic respiration, each glucose molecule ultimately yields about 36 ATP molecules.
Burning up
Both respiration and fermentation involve oxidation. A classic, readily understood example of oxidation is combustion, or burning. When wood burns, for instance, oxygen from the air combines with carbon and hydrogen atoms from the wood, producing carbon dioxide, CO2, and dihydrogen oxide (better known as water, H20), and releasing energy in the form of heat. However, despite the name, oxidation doesn’t necessarily involve oxygen at all. The process is actually about the transfer of electrons: atoms or molecules become oxidized when they lose electrons.
CELLS’ ENERGY CURRENCY
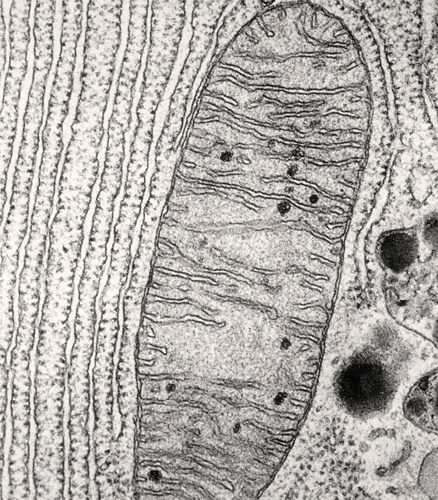
Transmission electron micrograph of a mitochondrion showing the double membrane. Visible to the left of the membrane is another membranous organelle—the rough endoplasmic reticulum.
The opposite of oxidation is reduction, which happens whenever elements and compounds gain electrons. Inside iron ore, for example, each iron atom is missing two or three electrons and is, therefore, a positively charged iron ion. Smelting the ore involves reducing the iron ions by reacting the ore with electron-donating elements, such as carbon. Once pure, the iron slowly reacts with air and water—both act as oxidizing agents, stealing electrons from the iron atoms. The result is that the pure iron becomes oxidized as rust (a mixture of iron oxides and iron hydroxides). Reducing an element or compound requires an input of energy, and oxidation releases that energy. The carbon atoms in fats and proteins and in carbohydrates, such as glucose, are all in a reduced state, having been made that way by metabolic reactions inside cells.
Something in the air
Aerobic respiration is a three-step process. It begins when glucose is split in two; this step (glycolysis) actually requires energy. The rewards soon start coming in, however: in the next step, a complex series of reactions taps much of the available energy. This step, called the Krebs cycle or citric acid cycle, produces an array of intermediate compounds. Ultimately it provides several molecules of ATP and a lot of molecules of another high-energy compound called nicotinamide adenine dinucleotide (reduced form)—thankfully known by its acronym, NADH. Most processes in the cell recognize only ATP, so the last step is a kind of currency conversion.
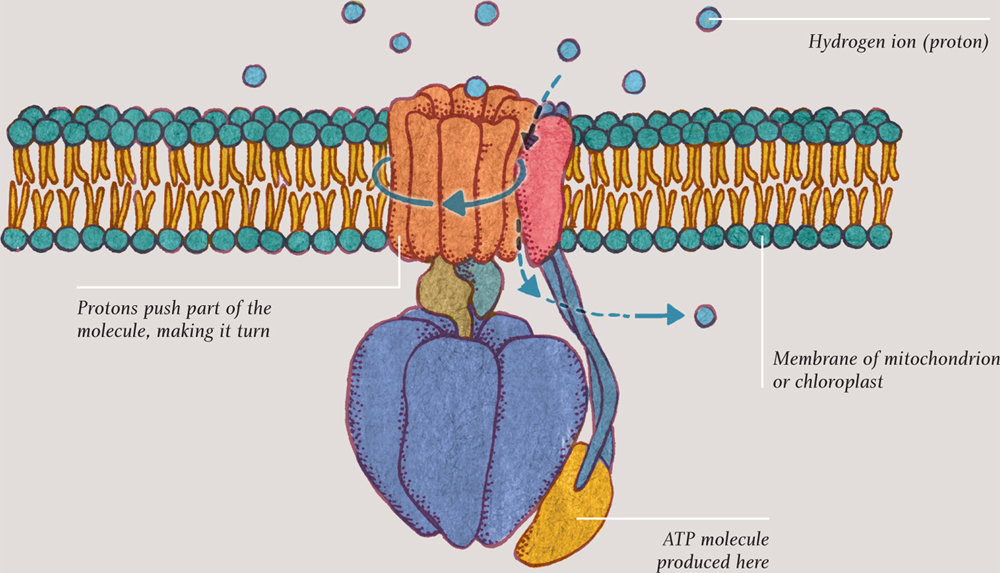
The ATP produced in the final step is constructed by a very special enzyme, ATP synthase, which sits in a membrane. This step is called the electron transport chain. Electrons from NADH are carried back and forth across the membrane, each time “pumping” hydrogen ions, aka protons, from one side of the membrane to the other. The resulting pressure pushes protons back across the membrane, through the ATP synthase molecule, making one part of it literally rotate. The energy made available in this way unites ADP molecules with spare phosphates to make molecules of ATP. This remarkable process is called oxidative phosphorylation.
ATP SYNTHASE
LIVING WITHOUT OXYGEN
Some cells get by without oxygen—either as their only form of respiration or as a way of carrying on respiration when no oxygen is available. Anaerobic respiration is very similar to aerobic respiration but uses alternative oxidizing agents, such as sulfur or nitrates. Nearly all anaerobes are bacteria or archaea.
The other main alternative is fermentation. This involves the breakdown of compounds produced by what is normally step one of cellular respiration. The products of fermentation are normally ethanol (alcohol) and carbon dioxide gas—putting the fermentation process at the heart of brewing and baking. Sometimes lactic acid is produced; an athlete’s overworked muscle cells produce lactic acid when oxygen is just not arriving fast enough, for example, and the buildup of this acid causes pain in hardworking muscles. Lactic acid produced by fermenting bacteria curdles milk, a vital step in making yogurt.
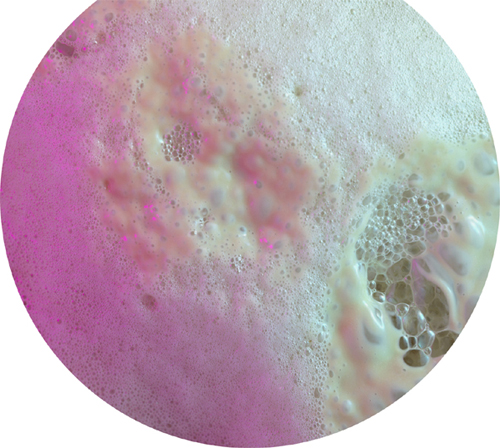
Bubbles of carbon dioxide gas produced by yeast accessing the energy in sugar via fermentation.
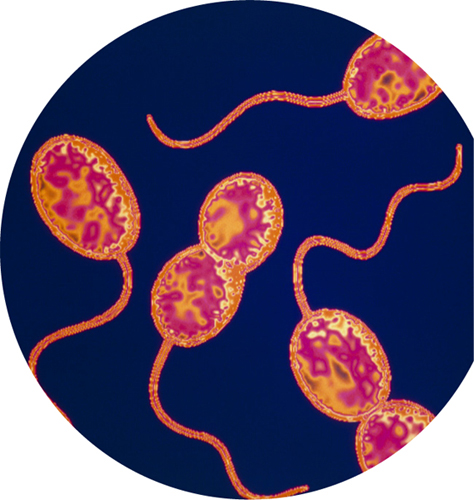
Colored transmission electron micrograph of the sulfur-eating bacteria Thiocystis. These bacteria live in sulfur-rich lakes with little or no oxygen.
The reaction needs a final step to mop up the now low-energy electrons that carried protons across the membrane. Something that mops up electrons is an oxidizing agent; in aerobic respiration, that agent is oxygen. It steals the electrons, which unite with used protons (hydrogen ions) that have come back through the membrane, via the ATP synthase molecule, to make molecules of water. There is a range of options available for cells that live in, or find themselves in, an environment with little or no oxygen (see here).
In the membrane
When aerobic respiration takes place inside prokaryotic cells, such as aerobic bacteria, ATP synthase sits in the actual cell membrane. This is part of the reason why bacteria have such a protective cell wall, or even a second membrane outside the main one. Eukaryotic cells, however, contain specialized, membrane-bound organelles called mitochondria. The second and third stages of cellular respiration both occur inside these power stations. Mitochondria have an inner and an outer membrane—and it is the inner membrane that is studded with ATP synthase molecules.
A mitochondrion is like a self-contained cell-within-a-cell. Its outer membrane acts in precisely the same way as the main cell membrane. Oxygen diffuses into the mitochondria, and the products of the first stage of cellular respiration (glycolysis) are taken in through selective membrane proteins. Similarly, carbon dioxide diffuses out, and the freshly produced ATP passes through membrane proteins. Mitochondria even contain their own DNA and their own ribosomes. These facts (and others) strongly suggest that mitochondria originated as free-living bacteria and that, millions of years ago, one of them entered into a beneficial relationship (symbiosis) inside (endo-) a host cell. This theory of endosymbiosis applies equally to another membrane-bound organelle found in plants and algae: the chloroplast.
Catching the sun
(Eukaryotic) plants and algae use energy in sunlight to manufacture glucose in the process called photosynthesis. They use the glucose as food—fuel for aerobic respiration—so they are self-feeders, or autotrophs. They also use glucose as the starting point for building energy-storage molecules, such as starch, and structural polymers, such as cellulose. Photosynthesis also takes place in some prokaryotic cells but, unlike plants and algae, they do not possess chloroplasts.
Inside, a chloroplast has its very own DNA and ribosomes, evidence of endosymbiosis, as it is in the case of mitochondria. Also like mitochondria, much of the action takes place on membranes that are studded with ATP synthase—and, once again, high-energy electrons pump protons across one side of the membrane to make ATP. But in a chloroplast the electrons come from water. Light energy captured by a pigment called chlorophyll splits water molecules, producing hydrogen ions (the protons) and high-energy electrons. The water’s oxygen is released. The other ingredient of photosynthesis is carbon dioxide, which has been “breathed in” from the air. In a series of reactions called the Calvin cycle, which involves the electrons and hydrogen ions from the first step, the carbon dioxide is reduced to form glucose, the final product of photosynthesis. To summarize a complex series of events into a simple statement, photosynthesis is water and carbon dioxide in, glucose out. Of course, there are variations on that theme, too: just as there are some organisms that do not use oxygen in respiration, there are some autotrophs that do not use water in photosynthesis. The equivalent to anaerobic respiration is “anoxygenic photosynthesis.”
All the remarkable happenings we have glimpsed in this chapter would be for nothing were it not for the things that we will glimpse in the next—for it is there we will look at how cells reproduce.
CHLOROPLAST
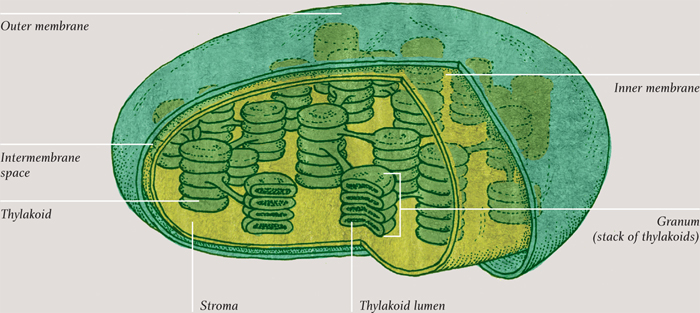

Colored transmission electron micrograph of two chloroplasts in a plant cell. The grana (stacks of membranes, yellow) hold the chlorophyll molecules that absorb light energy. The black areas are starch granules, made from the sugars produced by photosynthesis.










































