1.1 Introduction
For centuries, substantial extent of the world population (80%) has mainly relied on traditional medicinal practices involving herbal medicine for their routine healthcare needs. Herbal medicines are also referred as botanicals or phytomedicines. These medicines refer to herbs, herbal materials, herbal preparations, botanical formulations, and finished herbal products. The active ingredients of the herbal medicines are made of different plant parts, such as seeds, berries, roots, leaves, bark, or flowers (Sharma and Chaudhary 2015). Herbal medicines are exponentially gaining attention for its use in preventing and treating broad spectrum of health-related disorders (Kumar et al. 2012). Majority of the medical prescriptions used today for the treatment of innumerable chronic disorders are plant-based formulations or botanical-derived synthetic analogues. In the eastern part of the world, medicinal herbs have been used prophylactically and therapeutically for eras. The herbal medicines are presently practiced as a primary source of medicine for prevention and treatment of various health disorders. However, the herbal medicine practices are actively pursued in the rural areas mostly as compared to the urban places. Eastern countries have the unique distinction of having various medicinal practices associated with herbal medicine. With regard to the Indian herbal medicines, Ayurveda, Siddha, and Unani are well-known ancient medicinal practices in the healthcare system (Pandey et al. 2013).
Ayurveda is considered as a unique and distinct medicinal practice, which takes into consideration the physical, psychological, philosophical, ethical, and spiritual well-being of mankind. Ayurveda literally means “science of life” and has been used since folklore times for both preventive and curative measures. Around 25,000 effective plant-based formulations are used in ethnopharmacological approach and folklore Ayurvedic medicine in India (Alvari et al. 2012). This medicinal system has a holistic approach and thus has different approach in its therapeutic treatment as compared to the modern drug therapy. Instead of adopting the organ-oriented anatomy and physiology theory of the conventional medical science, Ayurveda has its own science which is known as the Panchamahabhuta. This is based on the five rudimentary elements: akasha (sky), vayu (air), agni (fire), prithvi (earth), and jala (water). Ayurveda, through reverse pharmacology, has made a pivotal contribution to the drug discovery processes along with new ways of identifying active compounds, reduction of drug-induced adverse reactions, and development costs. Plant components can act alone or along with other constituents from the same plant that may boost the activity of compounds or counter the toxic effects of compounds. Interestingly, an herbal medicine might have an additive, potentiating, synergistic, or antagonistic effect. This is the main path of action of Ayurvedic medicine where a combination of herbs is often prescribed. A significant number of traditional Ayurvedic medicine has been used to enhance cognitive functions and to ease symptoms that are correlated with cognitive disorders (Howes et al. 2003). The major Ayurvedic botanicals that have been evaluated for their potential to treat cognitive disorders are as follows: Withania somnifera (ashwagandha), Bacopa monnieri (brahmi), Curcuma longa (Indian ginseng), Convolvulus pluricaulis (shankapushpi), Clitoria ternatea (butterfly pea), Centella asiatica (Gotu kola), Celastrus paniculatus (jyotishmati), Terminalia chebula (yellow myrobalan or chebulic myrobalan), and Nardostachys jatamansi (jatamansi) (Ven Murthy et al. 2010; Solanki et al. 2015). The seeds and the seed oil of Celastrus paniculatus (Celastraceae) have been used for “stimulating intellectual ability and sharpening the memory” (Warrier et al. 1995; Howes and Houghton 2003). The roots of Clitoria ternatea (Leguminosae) have been shown to promote intelligence and enhance memory retention by affecting the cholinergic activity (Warrier et al. 1995; Misra 1998). Terminalia chebula’s (Combretaceae) ripe fruit is regarded as a brain power booster and memory promoter (Misra 1998; Manyam 1999; Naik et al. 2002). In the current article, we describe the cognitive neurodegenerative disorders and the therapeutic potential of Centella asiatica in relation to its neuroprotective properties.
1.2 Cognitive Disorders
Cognitive disorders are a group of mental disorders that can affect mood, functional activities, and behavior of an individual by impairing perception, memory, communication, reasoning, and judgment. Among cognitive impairments, dementia has attracted global attention for the past few decades. A slow and progressive neuronal dysfunction in the central nervous system (CNS) is the root cause of dementia, which ultimately leads to sensory dysfunction (Solanki et al. 2015). According to the definition given by the physician in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), dementia has symptoms such as decline of memory along with at least loss of one of the cognitive abilities such as coherent speech/language, perception, judgment, execution of motor functions, and/or reasoning. A definitive diagnosis of the loss of the cognitive abilities must be present for at least 6 months. There are various types of dementia such as mild cognitive impairment, vascular dementia, mixed dementia, Lewy body dementia, Alzheimer’s disease, Parkinson’s disease, frontotemporal dementia, Creutzfeldt-Jakob disease, normal pressure hydrocephalus, Huntington’s disease, and Wernicke-Korsakoff syndrome (Mori et al. 2012). Dementia is an age-related disorder and affects 10–15% of adults aged above 65 years, and its occurrence increases by 35–50% in adults of ages more than 85 years (Mori et al. 2012). It was estimated that in 2010, about 36 million people were suffering from some form of dementia worldwide. If left unchecked, it is expected to rise to 66 million by 2030 and 115 million by 2050 (Label 2009). Various types of dementias are as given in Table 1.1.
Table 1.1
Characteristic features of different forms of dementia
Types of dementia | Characteristic features |
|---|---|
Mild cognitive impairment | Memory loss, language impairment, other mental malfunction |
Vascular dementia | Elevated cholesterol levels, high blood pressure, hardening of the blood vessels (arterial walls), hyperglycemia, disrupted blood flow to the brain |
Mixed dementia | Vascular dementia and Alzheimer’s disease occur simultaneously |
Lewy body dementia | Alpha-synuclein composed of Lewy body deposition, progressive cognitive decline, memory impairment deterioration in visuospatial ability, visual hallucinations, rapid eye movement, sleep behavior disorder, severe neuroleptic sensitivity |
Parkinson’s disease | Bradykinesia, rigidity, postural instability, tremor or shaking of limbs, speech impairment, muscle stiffness, loss of autonomic movements. Lewy body deposition, drooling, seborrhea, constipation, sexual dysfunction, olfactory problem |
Frontotemporal dementia | Tremor, rigidity, memory impairment, loss of speech |
Creutzfeldt-Jakob disease | Progresses rapidly, depression, deposition of prion protein, memory deficit, movement disorders, mood swings |
Normal pressure hydrocephalus | Accumulation of cerebrospinal fluid in the ventricles of the brain and spinal cord, seizures, memory deficit, impaired vision |
Huntington’s disease | Degeneration of striatal neurons, movement, cognitive and psychiatric dysfunction, inherited changes in a single gene |
Wernicke-Korsakoff syndrome | Thiamine deficiency, ataxia, memory loss, alcoholism |
Other cognitive impairments
| |
Down syndrome | Due to genetic impairment chromosome 21 |
Prenatal alcohol/nicotine exposure | Excessive alcohol intake or nicotine exposure |
Homocysteinemia | Increased levels of homocysteine |
Alzheimer’s disease is a chronic progressive neurodegenerative cognitive disorder and is the most common type of age-related dementia (Hage et al. 2010). Alzheimer’s disease mainly affects the elderly people. Since the life expectancy in the United States (from 55 years to over 75 years of age) and around the world has increased, a strong correlation with a widespread of age-related cognitive disorders has been established (Gray et al. 2016). In the United States, Alzheimer’s disease is one of the most predominant irreversible neurodegenerative disorders, which gradually leads to the overall attenuation of higher cognitive abilities (Alzheimer’s Disease International 2009). Amnesic cognitive impairment (mild memory loss) is the early sign of Alzheimer’s disease (Howes et al. 2003). Mild memory loss usually leads to the decline in other cognitive abilities such as word finding, vision/spatial issues, and impaired reasoning. Based on time of the onset of disease, Alzheimer’s disease can be classified into two categories: familial Alzheimer’s disease (FAD) and sporadic Alzheimer’s disease (SAD). FAD occurs due to the uncontrolled genetic cause such as genetic mutations in the amyloid β precursor protein (APP) and presenilin (PS) genes which accounts for almost 3% of the cases and usually affects patients at an early age of less than 60 years (Mori et al. 2012). However, SAD is caused by the unknown pathological factors and represents 97% of the cases. SAD affects people of age 65 years or above and accounts for 50–60% of dementia cases (Howes et al. 2003; Label 2009). Irrespective of the types, both FAD and SAD have severe loss of memory, language, visuospatial skills, and emotion followed by inability to walk and swallow. Alzheimer’s disease patients with early onset tend to have more brain irregularities as compared to the late-onset disease. As this neurodegenerative disease progresses, it is further distinguished and classified depending on the severity of symptoms, such as mild Alzheimer’s disease, moderate Alzheimer’s disease, and severe Alzheimer’s disease. The stages of Alzheimer’s disease and their symptoms have been summarized in Table 1.2. However, it is not just the genetic and symptomatic features that are vital, but the prevention, therapy, drug interaction, patient care, and economic aspects also play a critical role in Alzheimer’s disease “care.” Hence, in the next section, we shall look into the prevalence and economic impacts of Alzheimer’s disease.
Table 1.2
Different stages and symptoms of Alzheimer’s disease
Stages of Alzheimer’s disease | Symptoms |
|---|---|
Mild | Mild coordination issues |
Mood swings | |
Recurrent recent memory loss | |
Repeating questions | |
Moderate | Confusions, misapprehensions, aggression, and paranoia |
Difficultly in recognizing family and friends | |
Disturbances in sleep | |
Feeling withdrawn and forgetfulness | |
Perpetual memory loss | |
Tremors and rigidity | |
Severe | Completely dependent on support giver for day-to-day activity |
Confusion between past and present | |
Immobility | |
Problems with swallowing and incontinence | |
Severe impairment of speech |
1.2.1 Prevalence of Alzheimer’s Disease
Universally, nearly 44 million people have been diagnosed with Alzheimer’s disease or a related dementia. Alzheimer’s disease is most common in Western Europe (North America is close behind) and is least prevalent in sub-Saharan Africa (Alzheimer’s Disease International). Alzheimer’s disease is the sixth leading cause of mortality in the United States. Based on Alzheimer’s Association statistics in 2015, an estimated 5.3 million Americans of all ages had Alzheimer’s disease and other dementia (Alzheimer’s Association 2015). These numbers are projected to rise to 15 million by the year 2050 (Jadidi-Niaragh et al. 2012). About 35.6 million people were diagnosed with dementia in 2010 worldwide (World Alzheimer’s report 2009). It has been predicted that the number will double every 20 years and rise to 65.7 million in 2030 and 115.4 million in 2050. Men have fewer incidences of Alzheimer’s disease and other dementia than women. It is postulated that almost two-thirds of Alzheimer’s diagnosed American population are women. It is estimated that in the United States, one in eight people of 65 years and older and nearly half of people of 85 years and older are Alzheimer’s patients. About 7.7 million new cases of dementia have been reported each year, thereby implying the prevalence of new case every 4 s in the world.
1.2.2 Etiology and Pathophysiology of Alzheimer’s Disease
Even though Alzheimer’s disease is an old disease, the specific etiological causes of this disease are still to be precisely elucidated and thus lead to the subject of continuous research. From the various studies carried on so far, it can be concluded that majority of the pathological features observed in the CNS are senile plaques (SPs), neurofibrillary tangles, oxidative stress, apoptosis, inflammation, excitotoxicity, and neurotransmitter disturbances (Howes et al. 2003). Based on the morphology and histology, the Alzheimer’s disease occurrence was hypothesized as cerebrovascular accumulation of amyloid beta (Aβ), predominant formation of neurofibrillary tangles in the cortex and hippocampus, followed by neuronal and synaptic loss, brain atrophy, and enlargement of cerebral ventricles (Khachaturian 1985; Cummings et al. 1998; Imbimbo et al. 2005). Diminutive neuronal loss or reactive gliosis is related with diffused Aβ plaques, whereas the Congo red and thioflavin S-positive plaques made up of fibrillary Aβ are related with neuronal loss, dystrophic neurites, and reactive astrocytes (Rozemuller et al. 1989; Itagaki et al. 1989). The neurofibrillary tangles (NFTs) are intracellular lesions consisting of twisted filaments of a cytoskeleton protein called tau protein (Castellani et al. 2010). The neuritic plaques, also known as senile plaques, are extracellular lesions composed of the 40–42 amino acid-long peptide Aβ fragments derived from amyloid precursor protein (APP) (Gomez-Isla et al. 1996; Selkoe 1998). Biochemically, the SPs are amyloid protein deposits mainly composed of beta-convoluted sheet of amyloid peptide of length 1–40 and 1–42 (Aβ1–40 and Aβ1–42) that gets cleaved from APP found in the neuronal cell membranes. NFTs are hyperphosphorylated tau proteins in the neuronal cells. Postmortem brain autopsy of Alzheimer’s disease expresses SP, NFTs, and atrophy of the cortical and temporal lobes of the brain. Such pathogenic hallmarks also co-localize with activated microglia, astrocytes, inflammatory cytokines, and proteins surrounding the dystrophic neuritis (Akiyama et al. 2000). Most of the cases of genetically inherited Alzheimer’s disease (FAD) are due to the mutations in one of the gene that encodes for the APP or in genes encoding APP (presenilin1-PSEN1 and presenilin 2-PSEN2) processing compound cellular machinery (Imbimbo et al. 2005; Piaceri et al. 2013). Conversely, SAD has been associated with several genetic and environmental factors. Despite numerous researches being carried out for almost a century, the etiology of SAD still remains vague (Piaceri et al. 2013). Out of several existing theories that have been put forth for understanding the pathogenesis of Alzheimer’s disease, Aβ and tau hypothesis are the widely accepted scientific theories currently. Nevertheless, the certainty of these theories still needs to be further elucidated.
1.2.2.1 Amyloid Beta (Aβ)-Induced Neurotoxicity in Alzheimer’s Disease
The main basis of amyloid cascade hypothesis is the characteristic extracellular senile plaques, one of the histological hallmarks of Alzheimer’s disease (Imbimbo et al. 2005). According to the Aβ toxicity or amyloid hypothesis, the primary causative agent for Alzheimer’s disease is Aβ (Aβ40 and Aβ42) peptides deposition (Cummings et al. 1998; Farlow 1998; Imbimbo et al. 2005; Karantzoulis and Galvin 2011; Piaceri et al. 2013). The Aβ peptides are derived by proteolysis of a type I transmembrane glycoprotein produced in many cells called the APP. In short, APP is a type I transmembrane protein processed by two competing catabolic pathways, non-amyloidogenic and amyloidogenic pathway (Hage et al. 2010). In non-amyloidogenic pathway, the initial proteolysis with α-secretase enzyme results in the generation of sAPPα and carboxyl-terminal 83-aa fragment (C83 or carboxyl-terminal fragments, CTFα), anchored at the membrane, followed by further proteolysis of C83 fragment with γ-secretase subsequently results in formation of N-terminal peptides, p3, and APP intracellular domain (AICD). Conversely, in the amyloidogenic pathway, the initial proteolysis of APP is performed by APP-cleaving enzyme, β-secretase that results in the formation of sAPPβ and carboxyl-terminal 99-aa fragment (C99 or β-CTF). The γ-secretase further processes C99 fragment to secrete Aβ peptides and APP intracellular domain (Hage et al. 2010). According to amyloid Alzheimer’s disease theory, these Aβ peptides of varying lengths oligomerize to form soluble oligomers and accumulate in the brain (Suh and Checler 2002). This is the significant initiating event in the development of Alzheimer’s disease. However, the specific species of Aβ that results in neurotoxicity is one of the many uncertainties that remain to be determined.
Beta-site APP-cleaving enzyme (BACE) is a single protein that is associated with β-secretase. β-site amyloid precursor protein-cleaving enzyme 1 (BACE-1) is a type 1 transmembrane aspartic protease related to the pepsin and retroviral aspartic protease families. It is mainly present in the acidic intracellular compartments (e.g., endosomes, trans-Golgi) with its active site in the lumen of the vesicles. The maximum activity and amount of BACE are found in the CNS (neurons), which is similar to β-secretase. BACE transfection (cDNA) and its antisense oligonucleotide exposure increased or decreased Aβ levels and β-secretase-cleaved APP fragments in APP-overexpressing cells. BACE2 is expressed in neurons (low levels), and it does not have the same cleavage activity on APP as β-secretase. Reformation of γ-secretase activity in yeast has discovered the presence of four components: presenilin, nicastrin, anterior pharynx-defective 1, and presenilin 2 (Gotz et al. 2004). FAD patients with mutations at APP, PSEN-1, and BACE protein confirmed the genetic framework to this theory, in turn confirming the pathological hallmarks of Aβ deposition in the brain of such patients less than 65 years (Citron et al. 1992; Piaceri et al. 2013). Increased BACE activity and linkage to the apolipoprotein E polymorphism found in sporadic Alzheimer’s disease patients have further confirmed that these are the key risk factors for developing late-onset Alzheimer’s disease (Bales et al. 1997; Farlow 1998). Various other biochemical studies also strengthen the Aβ theory associated with neuronal insult in cholinergic neurodegeneration. Aβ deposition prior to the formation of NFTs, tau fibril formation in the presence of Aβ, neuronal loss, and clinical symptoms of Alzheimer’s disease are the notable biochemical processes seen in the early stages of Alzheimer’s disease (Lewis et al. 2001; Mayeux et al. 2003; Hurtado et al. 2010; Chiu et al. 2012).
Neuronal toxicity is induced by Aβ via various mechanisms such as oxidative stress (Butterfield et al. 2001), disruption of mitochondrial activity (Schapira and Reichmann 1995), energy imbalance (Carvalho et al. 2012), stimulation of neuroinflammation (Verri et al. 2012), disturbance in calcium (Ca2+) homeostasis (Resende et al. 2007), perturbation of axonal transport (Decker et al. 2010), and activation of apoptotic signaling (Imaizumi et al. 1999; Kudo et al. 2012). These mechanisms eventually lead to the disturbance in neuronal cell integrity and CNS function, thereby resulting in accelerated neuronal cell death and Alzheimer’s disease pathogenesis. Excitotoxicity also plays a crucial role in neuronal cell death. This is distinctly evident in several chronic progressive neurodegenerative disorders (Thellung et al. 2013). Glutamate can act on various types of receptors (AMPA, NMDA, and kainate). Glutamate-like acetylcholine is also involved in cognitive functions such as learning and memory in the brain. The form of synaptic plasticity known as long-term potentiation takes place at glutamatergic synapses in the hippocampus, neocortex, and other parts of the brain. Excitotoxicity is mainly caused by the imbalance of Ca2+ homeostasis mediated by slow N-methyl-D-aspartate receptors ion channels resulting in the stimulation of various oxidative stress pathways. One of the theories is that the Aβ peptides induce synaptic discrepancies such as inhibition of long-term potentiation (LTP) in the hippocampal perforant pathway (Rowan et al. 2004; Parameshwaran et al. 2008; Ferreira et al. 2012). Aβ peptides also decrease surface expression and function of 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propionic acid receptors (AMPARs) and NMDARs, thereby impairing glutamatergic transmission (Parameshwaran et al. 2008). This Aβ deposition through induction of these harmful effects may lead to neurotoxicity, thereby resulting in the progression of Alzheimer’s disease.
1.2.2.2 Oxidative Stress: A Pathogenic Factor
Oxidative stress is a vital pathogenic factor underlying various neurodegenerative disorders (Karpińska and Gromadzka 2013). Oxidative stress is one of the well-studied neurotoxic mechanisms for the study of Alzheimer’s disease and has been investigated both as secondary and primary incident in Alzheimer’s disease pathogenesis (Markesbery 1997; Butterfield et al. 2001; Moreira et al. 2008). It is also contemplated that free radical generation and depletion of antioxidants may play a pivotal role in the pathogenesis of Alzheimer’s disease, similar to the oxidative stress theory of aging (Choi et al. 2012). In support of this theory, there are direct evidences which suggests that there is an increase in the level of metals (Al3+ and Mn2+) in the brains that are capable of stimulating free radical generation (Leskovjan et al. 2011). Remarkable amount and variety of reactive oxygen species (ROS) are generated in the early stages which decline with the disease progression (Nunomura et al. 2001). Studies have also found a significant increase in the lipid peroxidation and its product 4-hydroxynonenal in the ventricular fluids of Alzheimer’s disease patients and also in transgenic animals (Siegel et al. 2007). Lipid peroxidation is a product of the direct effect of free radicals on lipids. Lipids are highly vulnerable to oxidative stress. As stated by the amyloid theory, deposition of Aβ is the key initiating factor resulting in the ROS generation via several mechanisms such as interaction with metals. This results in the release of hydrogen peroxide that further induces the lipid peroxidation and consequent production of extremely reactive neurotoxic oxygen species, aldehyde, 4-hydroxynonenal (Abdul et al. 2008). Increased ROS leads to an altered activity of key enzyme complexes involved in energy metabolism and electron transport chain, thereby resulting in superoxide radical (O2−) formation. Such reactive species oxidize other molecules like nitric oxide (NO), leading to the formation of highly reactive peroxynitrite radical, a cytotoxic agent that induces peroxidation of lipid membranes, and other hydroxyl radical/free radicals causing neurotoxicity (Subathra et al. 2005; Chen et al. 2013). Furthermore, the oxidative stress causes an increase in the levels of damaged proteins and DNA in the brains of Alzheimer’s disease patients (Markesbery 1997; Moreira et al. 2008). Additionally, mitochondrial dysfunction associated with disrupted energy metabolism, notable Aβ release, and its deposition leads to programmed cell death (Siegel et al. 2007; Abdul et al. 2008; Chami and Checler 2012; Verri et al. 2012). Free radicals also attack DNA, resulting in DNA cleavage and damage, thereby leading to apoptotic changes in neurons (Kumar and Gupta 2002; Boland and Campbell 2004; Tabner et al. 2005) and, consequently, cell death (Sultana et al. 2006; Moreira et al. 2008; Abdul et al. 2008; Verri et al. 2012). It is known that an elevated ROS production in mitochondria can inhibit adenosine triphosphate (ATP) synthesis, release cytochrome c, and induce mitochondrial permeability transition (Ichas and Mazat 1998; Rizzuto et al. 2000; Tewari et al. 2016). Dysfunction in the powerhouse of the cell, mitochondria, can result in the release of pro-inflammatory and proapoptotic factors which initiate, intensify, and implement various signals resulting in apoptotic cell death (Kroemer and Reed 2000). Moreover, mitochondrial dysfunction and associated bioenergetic breakdown leads to aberrant cellular ion homeostasis, which can result in cellular disruption and swelling of the cells, ultimately causing necrotic cell death (Nieminen 2003). In conclusion, oxidative stress is known to be the earliest, most detrimental event and could be one of the targets for therapeutic interventions for Alzheimer’s disease.
1.2.2.3 Neurochemical Alterations
Noticeable change in the levels of several neurotransmitters has been observed in Alzheimer’s disease patients, which closely correlates with its different cognitive and noncognitive symptoms (Reinikainen et al. 1990). Various studies have associated the neuropathology resulting in memory loss due to the substantial deficits in cholinergic neurotransmission, one of the severities seen in Alzheimer’s disease (Bowen et al. 1976; Bierer et al. 1995; Francis et al. 1999; Gottwald and Rozanski 1999; Howes et al. 2003). Initially, various histological and pathological studies have discovered substantial degeneration of cholinergic nucleus basalis of Meynert in neocortical and hippocampal regions of the brains of Alzheimer’s disease patients. Drugs that stimulate cholinergic receptors prolong the availability of acetylcholine (Ach) by inhibiting the hydrolysis of Ach by acetylcholinesterase (AChE), and increased Ach release into the synaptic cleft can increase cholinergic neurotransmission. Cholinesterase inhibitors significantly improved cognitive functions, thereby supporting the pathogenic role of cholinergic deficiency (Gottwald and Rozanski 1999; Howes et al. 2003; Atri 2011; Hong-Qi et al. 2012). However, such treatments did not show permanent improvement in Alzheimer’s disease patients, because of neurodegenerative nature. The cortical regions of the brain of patients suffering from Alzheimer’s disease also showed variations in norepinephrine (NE) and serotonin (5-HT) levels, thereby resulting in secondary symptoms, such as language deficit, depression, and behavioral problems like agitation, disturbance in mood, aggression, and psychosis (Engelborghs and De Deyn 1997). Likewise, levels of the most abundant excitatory and inhibitory neurotransmitters of the brain, glutamate and gamma-aminobutyric acid (GABA), respectively, were also altered in patients suffering from Alzheimer’s disease. However, the glutamatergic system was significantly affected more than the GABAergic system (Hyman et al. 1987; Reinikainen et al. 1990; Engelborghs and De Deyn 1997; Butterfield and Pocernich 2003; Schwab et al. 2013). As mentioned earlier, the surface expression and function of NMDA and AMPA receptors play a major role in glutamatergic mode of neurotransmission. In neurodegeneration, observation of the synaptic levels of glutamate is imperative for neuronal physiology and survival. Varied levels of glutamate have been seen in different regions of the brains of Alzheimer’s disease patients. Glutamatergic transmission in neocortical and hippocampal regions is known to be severely affected in Alzheimer’s disease (Hyman et al. 1987; Butterfield and Pocernich 2003). Significantly high level of glutamate is also observed in the occipital lobe, and remarkable decreased content is seen in frontal and temporal lobes (Ernst et al. 1997; Fayed et al. 2011). Additionally, various APP transgenic mice model studies have confirmed the noticeable increase in the level of extracellular glutamate levels (Schallier et al. 2011). It has been hypothesized that the glutamate cytotoxicity is mainly mediated by NMDA subtypes of glutamate receptors. As mentioned earlier, NMDA receptors also mediate glutamate-induced processing of APP, which in turn results in enhanced Aβ secretion. Further increase in the level of Aβ in the brain results in oxidative stress leading to the oxidation of various lipids, sugars, and protein molecules in the neuronal cells. Glutamine synthetase is an enzyme involved in the conversion of glutamate to glutamine. In Alzheimer’s disease patients, reduced brain activity was noticed because of oxidized glutamine synthetase (Bowen et al. 1976). Similarly, both clinical and experimental cases of Alzheimer’s disease have observed reduction in activity and protein expression of glial glutamate transporter (GLT-1) (Sheldon and Robinson 2007). Due to the significant reduction in the glutamine synthetase activity and decreased GLT-1 levels in Alzheimer’s disease patients, there is a remarkable decrease in synaptic clearance of glutamate, which in turn causes NMDA-mediated neuronal injury. Additionally, NO production in response to the excitatory NMDA receptors activation causes extensive damage to cerebral neurocytes (Wang et al. 2010). Glutamate decarboxylase enzyme (GAD) converts glutamate to GABA. Recent studies show that 50% increase in the mRNA of GAD in the brains of Alzheimer’s disease patients indicates an increase in the GABAergic stimulation in the dorsal striatum (Reinikainen et al. 1990; Schwab et al. 2013). Increase in the level of GABA via presynaptic GABAA receptors results in prolonged inhibition of CNS neurons, which eventually leads to neuronal dystrophy, deafferentation, and increased neuronal degeneration (Schwab et al. 2013). All the above studies suggest that alteration in glutamate and GABA levels may play critical role in the pathogenesis of Alzheimer’s disease. Nevertheless, due to their narrow pathogenic potential and analgesic therapeutic effects, these neurotransmitter alterations are considered to be the indicators of collateral damage occurring as a result of severe neuronal degeneration in Alzheimer’s disease (Robichaud 2006). Changes in the cholinergic and glutamatergic system are considered to be the key element in the current treatment of Alzheimer’s disease.
1.2.2.4 Synaptic Deficits and Cognitive Dysfunction
Based on the electron microscopy and immunohistochemical staining in the cortical and hippocampal areas of the brains of Alzheimer’s disease patients, a significant decrease in synaptic density and loss of presynaptic and postsynaptic markers were observed (Masliah et al. 2001; Reddy et al. 2005). The loss of synapses is an early and consistent characteristic feature of Alzheimer’s disease that strongly associates with the severity of cognitive impairment (Masliah et al. 2001; Scheff et al. 2007). Various studies have proposed that synaptic dysfunction and neuronal loss are caused by Aβ peptides and hyperphosphorylated tau, respectively (Frautschy and Cole 2010). Soluble non-fibrillar Aβ assemblies [also known as Aβ-derived diffusible ligands (ADDLs)], oligomers, paranuclei, and protofibrils emerge long before the Aβ deposits and are thought to be the main culprit. Aβ-induced synaptic deficits are caused by three basic mechanisms: (1) toxic gain of function (due to novel interactions by new conformations of Aβ), (2) loss of usual physiological function, and (3) precipitation of physiological dysfunction by excessive Aβ. Under normal conditions, Aβ peptides play a vital role in homoeostatic plasticity by altering the excitatory transmission through AMPA and NMDA subtypes of glutamatergic receptors. Moreover, Aβ can also hinder LTP in the hippocampal perforant pathway (Chen et al. 2000; Wang et al. 2002; Rowan et al. 2004). It also decreases AMPAR and NMDAR surface expression and function. Dysfunctions caused by Aβ are internalization of NMDA receptors through calcineurin-dependent pathway (Dewachter et al. 2009) and weakening of synaptic transmission and plasticity via activation of group I metabotropic glutamate receptors (mGluRs) with p38 mitogen-activated protein kinase (MAPK) and calcineurin as downstream effectors (Hsieh et al. 2006; Li et al. 2009). Excitotoxicity and the resultant cell death caused by Aβ are primarily because of its interaction with NMDA receptors and disruption of the Ca2+ balance inside the cell. In several in vivo and in vitro studies, it has been observed that ADDLs readily bind to the dendritic arbors of a specific type of cultured neurons (Lacor et al. 2004; Laurén et al. 2009). In such studies, it has also been observed that ADDLs facilitate dendritic spine loss (Lacor et al. 2007), increase ROS generation, and disrupt excitatory and inhibitory neurotransmission balance, which in turn leads to epileptic behavior in transgenic mice. In various transgenic animal studies, synaptic deficits are the common disorders that are introduced for observing various pathophysiological aspects of Alzheimer’s disease. Therefore, damage to the synaptic connections and impairment of synaptic plasticity are elementary in pathogenesis of memory impairment in Alzheimer’s disease. Thus, further research in elucidating the underlying mechanisms of synaptic toxicity may help in the development of novel therapeutic medications.
1.2.2.5 Inflammation and Neuronal Cell Death
Inflammation also plays a pivotal role in various neurodegenerative and neurological disorders such as multiple sclerosis, Parkinson’s disease, Huntington’s disease, Alzheimer’s disease, and traumatic brain injury (Blasko et al. 2004). According to the inflammatory hypothesis of Alzheimer’s disease, chronic inflammation developing independently (due to several unknown factors) or as a result of Aβ pathology, plays a pivotal role in neurodegeneration. Along with oxidative stress, Aβ plaques, and NFTs, brain inflammation is an early pathological indication of Alzheimer’s disease (McGeer et al. 1989; Eikelenboom et al. 2010). Neuroinflammation is a chronic and self-sustaining pathogenic process which is specific to Alzheimer’s disease and is extremely capable of neuronal injury and neurodegeneration. Various direct and indirect evidences confirm the pathophysiological significance of neuroinflammation in Alzheimer’s disease (Akiyama et al. 2000). One of the confirmations is the co-localization of activated microglia, astroglia, and monocytes around the Aβ plaques and dystrophic neurites, specifically in the frontal neocortex and limbic cortex; however, no signs of inflammation are observed in the cerebellum (Rogers et al. 1988; Dickson et al. 1988). Furthermore, prudent but remarkable inflammation is observed in patients with low Braak scores for Alzheimer’s disease pathology (i.e., patients without any history of dementia but ample amount of Aβ and neurofibrillary tangles at autopsy). There is an upregulation of the pro-inflammatory genes (gene encoding for complement factors; major histocompatibility complex proteins II, cell adhesion molecules) and pro-inflammatory enzymes resulting in the enhanced synthesis of prostaglandin and various other pro-inflammatory cytokines (Lue et al. 1996; Blalock et al. 2004; Parachikova et al. 2007). Likewise, in numerous in vitro and in vivo studies, Aβ has demonstrated to activate complement cascade and stimulate secretion of pro-inflammatory cytokines, chemokines, and other inflammatory markers from the activated monocytes (O’Barr and Cooper 2000), microglia (Del Bo et al. 1995; Chong 1997), astrocytes (Hu et al. 1998), endothelial cells (Suo et al. 1998), and neurons (Del Bo et al. 1995; Du Yan et al. 1997). Nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) and myeloperoxidase pathway are induced by Aβ, resulting in the ROS and cytokines generation via stimulated monocytes, microglia, and neurons (Bianca et al. 1999).
Recent studies have demonstrated that the pro-inflammatory mediators such as interleukins 1 (IL-1) and 6 IL-6 and interferon-γ (IFN-γ) induce APP secretion and processing and also annihilate the production of soluble APP (sAPP; a well-known neuroprotectant) leading to Aβ deposition and neurotoxicity (Blasko et al. 1999). Patients suffering from Alzheimer’s disease have shown increased levels of gliosis, pro-inflammatory markers like IL-1, and conspicuous complement activation in postmortem brain (Rozemuller et al. 2005). Due to pathogenic role, anti-inflammatory therapies are known to be effective in delaying the onset of inflammation in Alzheimer’s disease or slowing the progression of Alzheimer’s disease (Jaturapatporn et al. 2012). Clinical trials have shown that the use of nonsteroidal anti-inflammatory drugs (NSAIDs) can decrease the risk of developing Alzheimer’s disease. This hypothesis is supported further when rheumatoid arthritis patients, who frequently use NSAIDs, have a lower incidence of Alzheimer’s disease (Breitner and Welsh 1995; Breitner 1996; McGeer et al. 1996; Howes et al. 2003). In addition to this, it is found that passive immunization therapy with Aβ antibodies have been proven beneficial to the Alzheimer’s disease suffering individuals (Aisen and Vellas 2013). All the above indications establish the pathogenic significance of chronic neuro-inflammatory processes in pathophysiology and progression of Alzheimer’s disease.
Even though T-helper cells’ role in Alzheimer’s disease is controversial, recent studies have shown that the T-cell-regulated inflammatory response plays a role in either pathophysiology, progression, or both in the most common dementia-related neurodegenerative disorder (Togo et al. 2002; Panossian et al. 2003; Magnus et al. 2005). It has also been observed that the blood-brain barrier (BBB) is permeable to activated T cells, which is intensely increased during systemic infection or other neurodegenerative diseases (Ransohoff et al. 2003). However, the number of T cells affecting the brains of Alzheimer’s disease patients is far less as compared to other neurodegenerative disorders like multiple sclerosis. A study by Itagaki et al. (1988) showed that the CD4 (T-helper-inducer) and CD8 (T-cytotoxic-suppressor) T-cell trafficking was altered in significantly large amounts in the hippocampus and temporal cortex of the brains of Alzheimer’s disease patients when compared to the normal brain tissues. Recent investigations are indicative of the involvement of dendritic and microglia cells in orchestrating the T-cell response in the brains of Alzheimer’s disease patients (Rogers et al. 1988; Magnus et al. 2005; Fisher et al. 2011). Microglia protects and supports the neurons as well as act as an immunoprotectant in the CNS. It is able to express major histocompatibility complex II, release cytokines, complement proteins, and in an activated states also possess scavenger and phagocytic properties (Moore and O’Banion 2002). Activated microglia expresses co-stimulatory molecules like CD80, CD86, and CD40 during CNS inflammation in neurodegenerative disorders. These molecules interact with T cells and modulate T-cell-mediated response, thereby promoting either their proliferation, T-effector functions (cytokine secretion), or both (Aloisi 2001; Magnus et al. 2005; Wirenfeldt et al. 2011). Microglia activation and T-cell-induced cytotoxic inflammatory response is mediated by the release of pro-inflammatory cytokines. Cytokines, IL-1, IL-2, IL-17a, and other secreted cellular products also play a vital role in the neuronal immune response by regulating the neuronal cell viability or BBB permeability (Huppert et al. 2010). These cytokines also play a role in the development and differentiation of T cell in a given disease state. In case of multiple sclerosis, an autoimmune disorder, the integrity of BBB is impaired by the IL-17 producing T-helper cells, which leads to the increase in its permeability to other lymphocytes. Increase in the tumor necrosis factors (TNF)-α protein expression is directly related with inflammatory response in Alzheimer’s disease (Fillit et al. 1991; Blasko et al. 1999; Perry et al. 2001). Tumor necrosis factor-alpha (TNF-alpha) is a potent central regulator of inflammation. The expression of TNF-α and IFN-γ helps Aβ peptides production, which leads to the deposition of Aβ. This leads to the disruption of homeostatic balance and plaque formation (Blasko et al. 1999). Additionally, production and release of prostaglandins are also stimulated by these cytokines, which may result in neurotoxic effects associated with Alzheimer’s disease pathology (Prasad et al. 1998).
1.3 Therapeutic Efficacy of Centella asiatica
Centella asiatica is a slender creeping perennial herb belonging to Umbelliferae (also known as Apiaceae) family. This plant has delicately scalloped hairy leaves with barely visible flowers. It is commonly found in tropical swampy areas. Centella species are widespread throughout tropical and subtropical countries worldwide (Awang 1998; Wattanathorn et al. 2008; Vasavi et al. 2014). Centella asiatica has numerous common names depending on the geographical location of origin. It is known as Gotu kola in Sinhala, mandukaparni in Sanskrit, kodakan in Malayalam, pegaga in Malayasia, Indian pennywort and Indian water navelwort in India, tsubokusa in Japan, and tungchian or luei gong gen in China. Botanically it is known as Hydrocotyle asiatica Linn and Trisanthus cochinchinensis Lour (Kalshetty et al. 2012). As described earlier, Centella asiatica is an ancient medical herbal drug and has been traditionally used for its plethora of therapeutic purposes in Indian Ayurvedic medicinal practices for eras. According to the Charaka Chikitsa I, an ancient Indian literature, the juice of Centella asiatica promotes longevity and cures a variety of diseases. Centella asiatica is an active component of “Medhya Rasayana” (Bhavna and Jyoti 2011) and considered as crucial herb for the treatment of various health-related disorders (Thomas et al. 2010; Ermertcan et al. 2008; Somboonwong et al. 2012; Subathra et al. 2005; Bian et al. 2013; Di Tomo et al. 2015; Abas et al. 2015; Jayathirtha and Mishra 2004; Belcaro et al. 2011; Shukla et al. 1999; Cesarone et al. 2001a, b; De Sanctis et al. 2001; Incandela et al. 2001; Paocharoen 2010).
Therapeutically, the whole aerial part of Centella asiatica is useful as it is very rich in numerous bioactive compounds (Kumar and Gupta 2002). Various in vitro and in vivo (animals and human clinical) studies have been undertaken to evaluate its bioactive components and the medicinal value of Centella asiatica. The basic molecular mechanisms related to the therapeutic efficacy are being investigated. Centella asiatica has been used extensively for the dermatological pathologies. It has been widely used in the treatment of wounds, burns, and ulcerous skin ailments and for the prevention of keloid and hypertrophic scars (Belcaro et al. 2011; Brinkhaus et al. 2000; Gohil et al. 2010; Paocharoen 2010). This botanical is also used to treat second- and third-degree burns and topically to accelerate healing, mainly in cases of chronic postsurgical and post-trauma wounds (Belcaro et al. 2011). Details of medicinal applications of Centella asiatica are summarized in Table 1.3.
Table 1.3
Medicinal use of Centella asiatica in cognitive disorders
Plant part used | Medicinal use | References |
|---|---|---|
Whole plant | Enhances learning and memory | Rao et al. (2005) |
Whole plant | Effective against MPTP-induced Parkinsonism | Ittiyavirah and Hameed (2014) |
Whole plant | Enhances cognitive functions | Gray et al. (2016) |
Whole plant | Significantly prevents the cognitive impairments | Gupta et al. (2003) |
Whole plant | Memory enhancer in Alzheimer’s disease | Hage et al. (2010) |
Whole plant | Prevents Alzheimer’s disease development | Chen et al. (2015) |
Whole plant | Enhances cognitive function | Kumar and Gupta (2002) |
Improves learning and memory | ||
Whole plant | Neuroprotective and beneficial in Alzheimer’s disease | Soumyanath et al. (2012) |
Whole plant | Prevents memory deficits | Kumar et al. (2011) |
Improves the memory impairment | ||
Whole plant | Significant neuroprotective effects | Subathra et al. (2005) |
Protects brain against age-related diseases | ||
Whole plant | Enhances the cognitive function | Omar et al. (2011) |
Neuroprotective actions | ||
Whole plant | Anti-Parkinson’s effect | Khotimah et al. (2015) |
Improves learning and memory | ||
Protects the brain from age-related oxidative damage | ||
Whole plant | Neuroprotective properties | Shinomol et al. (2011) |
Improves memory | ||
Prevents cognitive impairment | ||
Whole plant | Restores memory | Howes and Houghton (2012) |
Prevents dementia | ||
Improves cognitive function | ||
Whole plant | Cognitive enhancement | Iqbal et al. (2011) |
Whole plant | Increase memory | Krishna (2013) |
Whole plant | Enhances learning | Mohd Salim et al. (2013) |
Whole plant | Neuroprotective effect against Parkinson’s disease and Alzheimer’s disease | Orhan (2012) |
Whole plant | Memory-enhancing effects | Singh et al. (2008) |
Whole plant | Improves memory retention | Kumar and Gupta (2003) |
Prevents cognitive impairment | ||
Whole plant | Enhances cognitive performances | Xu et al. (2008) |
Whole plant | Possesses neuroprotective properties against Alzheimer’s disease | Dhanasekaran et al. (2009) |
Whole plant | Improves cognitive function Neuroprotective effects | Gray et al. (2015) |
Whole plant | Improves both speed and accuracy of working memory | Wattanathorn et al. (2008) |
Increases accuracy of word recognition | ||
Whole plant | Improves symptoms of Parkinson’s disease | Barbosa et al. (2008) |
Enhances memory | ||
Leaves | Improves memory and cognitive function | Howes and Houghton (2003) |
Prevent dementia | ||
Leaves | Alternatives for the treatment of Alzheimer’s disease | Akagi et al. (2015) |
Leaves | Increases memory power in children | Sarkar et al. (2015) |
Leaves | Memory-enhancing properties | Seevaratnam (2012) |
Protection against age-related changes in brain | ||
Leaves | Augments memory power | Tiwari et al. 2016) |
Leaves | Potent in reducing the Parkinsonian symptoms | Siddique et al. (2014) |
Leaves | Enhances memory | Mohandas Rao et al. (2012) |
Leaves | Neuroprotective properties | Kasture et al. (2014) |
Leaves | Protects against age-related changes in brain | Sugunabai and Karpagam (2015) |
Leaves | Memory improvement and improves symptoms of Alzheimer’s disease | Patel et al. (2014) |
Leaves | Neuroprotective effect | Bhavna and Jyoti (2011) |
Leaves | Improves memory against dementia and aging | Stafford et al. (2008) |
Leaves | Prophylactic neuroprotective action | |
Brain stimulant | ||
Leaves | Neuroprotective effects against neurodegenerative disorders including Alzheimer’s disease and Parkinson’s disease | Zhang et al. (2012) |
Leaves | Improves memory | Ashalatha and Shenoy (2015) |
Brain tonic | ||
Leaves | Brain booster for improving memory | Wanakhachornkrai et al. (2013) |
Leaves | Enhances memory retention | Rasoanaivo (2011) |
Leaves and stem | Improves symptoms of Alzheimer’s disease | Khatun et al. (2011) |
Neuroprotective effect in Parkinsonism | ||
Pure compound (asiatic acid) | Neuroprotective effects | Xu et al. (2012) |
Improves cognitive function against glutamate-induced dementia | ||
Pure compound (asiatic acid) | Enhances working memory | Sirichoat et al. (2015) |
Pure compound (asiaticoside) | Improves memory | Chen et al. (2014) |
Delays the onset and progression of neurological disorders | ||
Pure compound (madecassoside) | Improves cognitive function in Alzheimer’s disease | Du et al. (2014) |
1.3.1 Active Components of Centella asiatica
The major active chemical components of Centella asiatica are identified as triterpenes, flavonoids, polyphenols, and other compounds (Bhattachryya and Lythgoe 1949; Singh and Rastogi 1969; Zainol et al. 2003; Mangas et al. 2006; Zheng and Qin 2007; Shinomol and Muralidhara 2008a; Zhang et al. 2008; James and Dubery 2009; Thomas et al. 2010; Gohil et al. 2010; Kalshetty et al. 2012; Long et al. 2012; Tiwari et al. 2013). The triterpenoids exist in the forms of saponins and glycosides, such as asiaticoside and madecassoside with its respective ursane-type sapogenins (Fig. 1.1). Other compounds reported in Centella asiatica with therapeutic efficacy are amino acids (alanine, serine, aminobutyrate, aspartate, glutamate, histidine, lysine, and threonine), fatty acids (linoleic acids, linolenic acid, lignocene, oleic acid, palmitic acid, and stearic acid), phytosterols (campesterol, sitosterol, and stigmasterol), resin, tannin, and various other terpenoids (beta-caryophyllene, trans-beta-farnesene and germacrene D, alpha-pinene, and beta-pinene) (George and Gnanarethinam 1975). Madecassoside, a pentacyclic triterpenoid derivative saponin, is reported to possess anti-inflammatory (Li et al. 2009; Liu et al. 2008; Wu et al. 2012), antioxidant (Luo et al. 2014), and cardioprotective (anti-myocardial infraction) activities (Bian et al. 2008). Main compounds responsible for the antioxidant activity of Centella asiatica are reported to be flavonoids and phenolic compounds (Hussin et al. 2005).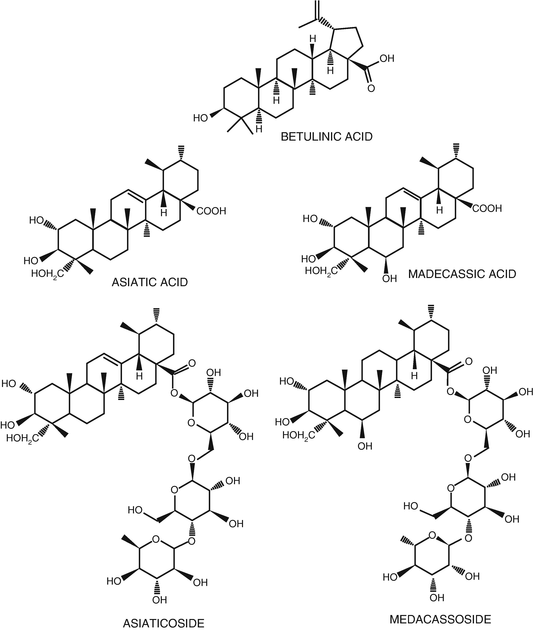

Fig. 1.1
Bioactive triterpenoids isolated from Centella asiatica
1.3.2 Learning and Memory-Enhancing Capability in Alzheimer’s Disease
Centella asiatica extract was well enumerated and may have pro-cognitive effects in humans and rodents (Dhanasekaran et al. 2009; Kumar and Gupta 2002; Wattanathorn et al. 2008; Gohil et al. 2010; Shinomol et al. 2011; Xu et al. 2012b). Pharmacological studies have established the cognitive enhancing capability of Centella asiatica extract in a series of behavioral tests and memory retention tasks such as active and passive avoidance, object recognition, and water maze in rodents (Nalini et al. 1992; Kumar and Gupta 2002; Gupta and Pansari 2003). In a clinical study, Centella asiatica extract positively modulated cognition and mood-enhancing quality of life in healthy elderly volunteer (Wattanathorn et al. 2008; Mato et al. 2011; Nasir et al. 2011). Additionally, a few human clinical studies have reported that Centella asiatica extract substantially improved cognitive ability of mentally retarded children (Appa Rao et al. 1973).
The activation of cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB) leads to the increase in BDNF which in turn modulates the NMDA receptor activity and its expression on the pre- and post- synaptic sites (Madara and Levine 2008). It is also observed that the synaptic localization of pre- and postsynaptic proteins such as synaptobrevin is enhanced by BDNF through activation of TrkB receptor. Synaptobrevin plays an important role in maintaining synaptic plasticity (Minichiello et al. 2002; Huang and Reichardt 2003). Several pathways such as protein kinase A (PKA), NO signaling and CaMKII activation lead to the phosphorylation of CREB. As mentioned in previous sections, Aβ initiates numerous cytotoxic pathways in the CNS which leads to neurodegeneration and progression of Alzheimer’s disease. Besides neuronal plaques formation, Aβ also stimulates the immune system of the CNS, which leads to the inflammatory-mediated response (Lue et al. 1996; Gupta and Pansari 2003). Additionally, it has been proven that the inflammatory mediators increase the APP processing in the neuronal cells, thereby accelerating and augmenting the secretion and deposition of Aβ (Blasko et al. 1999). It can thus be concluded that chronic inflammation may indirectly act as the main pathogenic agent in the formation of Aβ plaques in the brains of Alzheimer’s disease patients. Therefore, studies were conducted with Centella asiatica to evaluate its neuroprotective properties against Aβ toxicity in association with BDNF and inflammation (Xu et al. 2008).
Centella asiatica plays a key role in neuronal regeneration by stimulating the dendrites of rat brains, neurite elongations in human SH-SY5Y cells, and increasing axonal regeneration in rats. Synaptic strengthening and cognitive enhancement activities of Centella are by increasing CREB phosphorylation and thereby aiding the neuronal dendritic arborization and axonal regeneration in rats (Rao et al. 2005; Soumyanath et al. 2005). One of the physiological processes of memory formation is generally induced by CREB (a transcription factor) during LTP. The important early genes like c-fos and proteins such as brain-derived neurotrophic factor (BDNF) responsible for neuronal survival and protein synthesis are regulated by CREB (Malinow et al. 2000). Phosphorylation of CREB leads to the activation of factors such as several protein kinases, MAPK, calcium-/calmodulin-dependent protein kinase II (CaMKII), protein kinase C, and signaling factors like Wnt, which play an important role in neuronal survival and arborization (Wayman et al. 2006). Numerous studies have demonstrated BDNF to robustly stimulate the dendrite outgrowth by triggering TrKB receptors, resulting in the stimulation of various proteins including the activation of CAMKII (Minichiello et al. 2002; Huang and Reichardt 2003). Overall, these studies have concluded that one of the mechanisms mediating cognitive enhancement activity of Centella asiatica can be via increasing CREB signaling (Xu et al. 2008, Fig. 1.2).

Fig. 1.2
Mechanisms of action
1.3.3 Neuroprotective Activity in Alzheimer’s Disease
Numerous experiments have established that Centella asiatica has neuroprotective mechanisms of action significant to the therapeutic treatment of Alzheimer’s disease. As pointed out earlier, excessive levels of the neurotransmitter, glutamate, is found in the brains of Alzheimer’s disease patients. It has been demonstrated that Centella asiatica may have a neuroprotective effect when cultured neurons are exposed to glutamate (Lee et al. 2000; Ramanathan et al. 2007). Some studies have proven that Centella asiatica extract influences neurotransmitter systems such as dopamine and serotonin (5-HT) that regulate the production of Aβ peptide (Sakina and Dandiya 1990; Nalini et al. 1992; Nitsch et al. 1996), which consequently may serve to limit the production of Aβ peptide.
Centella asiatica alters fabrication of extracellular matrix molecules (Maquart et al. 1999). Deposition of fibrillar Aβ is known to promote extracellular matrix molecule, perlecan, in rodent brain (Snow et al. 1994; Holcomb et al. 2000). Whereas, laminin, an extracellular matrix molecule, has recently been demonstrated to hinder Aβ fibril formation (Castillo et al. 2000). Centella asiatica may function as an anti-fibrillogenic agent, limiting the formation of amyloid deposits through alterations in the production of key extracellular matrix proteins. Centella asiatica has shown to exhibit preventive effects on cognitive deficits in streptozotocin-treated animal model of memory deficit (Kumar and Gupta 2003). Furthermore, reduction in the protein carbonyl production was seen in the brains of aged rats when treated with Centella asiatica (Subathra et al. 2005). Overall, these studies have demonstrated that Centella asiatica may reduce neuropathologies of Alzheimer’s disease.
Researchers have found that prior to the increase of Aβ 1–40 and 1–42 levels or noticeable Aβ deposition in the Alzheimer’s disease mouse model, augmented lipid peroxidation takes place (Praticò et al. 2001). Studies conducted in our research laboratory (Dhanasekaran et al. 2009) have found that Centella asiatica could block lipid peroxidation, thereby suggesting that the primary action could be hindering the hydroxyl radical-generated membrane damage. This study further documented that Centella asiatica may also help in reducing Aβ deposition and elicited protection to proteins, sugars, and nucleic acids from oxidative and Aβ damage.
Asiatic acid and asiaticoside present in Centella asiatica provoke the acceleration of learning and memory performance (Kumar and Gupta 2003; Gupta et al. 2003; Rao et al. 2005). Asiatic acid from Centella also exhibits neuroprotective properties and has shown to protect cortical neurons from glutamate-induced excitotoxicity in vitro (Mook-Jung et al. 1999; Lee et al. 2000; Xiong et al. 2009; Krishnamurthy et al. 2009).
Ceramides derived from sphingosine are known to cause mitochondrial dysfunction which results in neuronal cell death (Arboleda et al. 2009; Zhang et al. 2012b). Several researchers have found that ceramides may play a role in various neurological disorders such as Alzheimer’s disease (Cutler et al. 2004; Jana et al. 2009), Parkinson’s disease (France-Lanord et al. 1997; Bras et al. 2008), and cerebral ischemia (Novgorodov and Gudz 2009). Specifically, C2-ceramide (short chain active analog of ceramide) causes mitochondrial disruptions and apoptosis. Zhang et al. (2012) demonstrated that Centella asiatica exhibited protective activity against C2-ceramide-induced mitochondria-dependent apoptosis may be by regulating the ERK1/2 signaling pathway (Movsesyan et al. 2002; Stoica et al. 2005).
Apoptosis is seen in peripheral chronic disorders and in various neurological disorders mainly in Alzheimer’s disease and Parkinson’s disease. Neurodegeneration is mainly caused by apoptosis that can be triggered by various pathways (LeBlanc 2005). In a recent study, it has been hypothesized that early induction of caspase-6 leads to the activation of downstream apoptotic mediators such as the caspase-3. Induction of caspase-6 results in the disruption of the cytoskeleton of neurites and also leads to the damage of proper trafficking of proteins and organelles, thereby resulting in neurodegeneration and synaptic loss in Alzheimer’s disease (LeBlanc 2005). Prakash and Kumar (2012) showed that Centella asiatica exhibited anti-apoptotic activity by effectively inhibiting the caspase activity induced by D-galactose neurotoxicity in rats.
In the double transgenic mice model of Alzheimer’s disease, Aβ plaque accumulation occurs in the frontal cortex and has DNA fragmentation (Feng et al. 2004; Wang et al. 2005; Migliore et al. 2005). A single-strand break in DNA may lead to the double-strand breaks, which may result in apoptosis, or may inactivate vital genes, or may also cause chromosomal aberrations (Barzilai and Yamamoto 2004). Disturbance of the supercoiled form of the plasmid DNA caused by exposure to hydrogen peroxide and UV light was inhibited by Centella asiatica, thereby preventing the single-strand or double-strand breaks formation of DNA.
In addition to improving cognition, Centella asiatica may also be used to treat the motor agitation and mood changes of people suffering from Alzheimer’s disease. It is postulated that Centella also helps in promoting a deep state of relaxation and mental calmness during meditation practices (Brown 1995). Formulations of Centella asiatica in combination with other herbs are used to alleviate depression and anxiety (Bradwejn et al. 2000). The antidepressant and anxiolytic activities of Centella asiatica are thought to be mediated through the hypothalamic-pituitary-adrenocortical axis, which relieves stress and alters the components of the monoaminergic neurotransmitters (Chen et al. 2005; Bobade et al. 2015 Fig. 1.3). In addition, Centella asiatica has been shown to possess antioxidant activity in the hippocampus and corpus striatum (Ittiyavirah and Hameed 2014).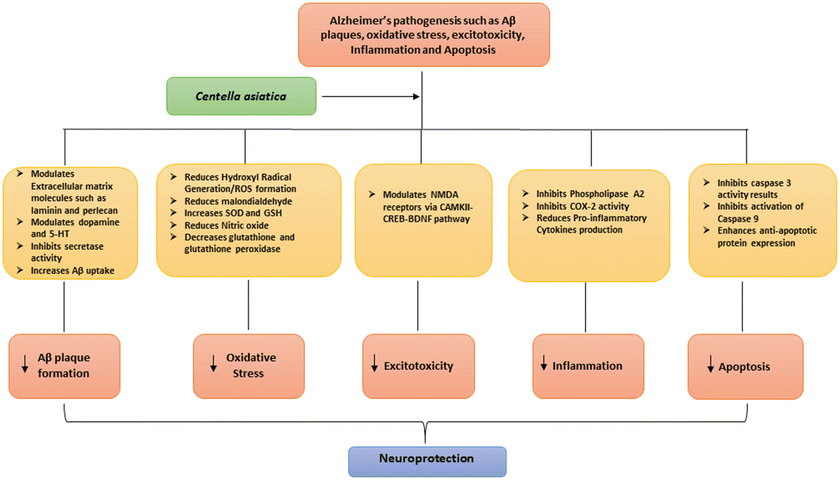

Fig. 1.3
Mode of action of Centella asiatica in the pathogenesis of Alzheimer’s disease
1.3.4 Antioxidant and Anti-inflammatory Activities in Alzheimer’s Disease
Phenolic compounds exhibit potent antioxidant activity and also act as mitochondrial energizers. Free radicals are responsible to initiate cell injury (Spiteller 1993; Maxwell 1995; Howes et al. 2003). Thus, the use of antioxidants has been considered to slow the neuronal degeneration and progression of Alzheimer’s disease (Howes et al. 2003). Centella asiatica is known to have an effective antioxidant and anti-inflammatory capabilities since it has shown to attenuate cyclooxygenase, lipoxygenase, and phospholipase expression. A study by Shinomol and Muralishara demonstrated that Centella asiatica reduces endogenous oxidative markers, enhances antioxidant defenses in cytosol and other brain regions of prepubertal mice (Shinomol and Muralidhara 2008a), and has neuroprotective properties (Shinomol and Muralidhara 2008b). Centella inhibits phospholipase 2, thereby highlighting its significant role in anti-inflammatory activities (Defillipo et al. 2012). The two major components of arachidonic pathway are COX-2 and 15-lipoxygenase (15-LOX) enzymes. The arachidonic pathway leads to the formation of pro-inflammatory prostaglandins, leukotrienes, and other mediators, which are associated with numerous chronic neurological disorders (Hoozemans et al. 2004). Centella asiatica significantly inhibits the activity of Ca2+-independent phospholipase A2 (iPLA2) and cytosolic phospholipase A2 (PLA2) in the rat brain. Drugs affecting either of the phospholipase will hinder the profound inflammatory reaction and reduce neurodegeneration (Kolko et al. 2002, Burnett et al. 2011, Fig. 1.3).
1.3.5 Study in Double Transgenic Alzheimer’s Disease Mice Model
Based on the extensive scientific literature, it is clear that Centella asiatica has been used for memory enhancement in Ayurvedic medicine for years. In our laboratory, we had undertaken a chronic study to evaluate the neuroprotective effects of C. asiatica extract on amyloid pathology in the PSAPP mouse model of Alzheimer’s disease (Dhanasekaran et al. 2009). PSAPP mice expressing the “Swedish” amyloid precursor protein and the M146L presenilin 1 mutations are a well-characterized model for spontaneous Aβ plaque formation. PSAPP doubly transgenic mice were obtained by crossbreeding Tg2576 mouse line with “Swedish” APP mutation (APPK670NM671L) with mutant presenilin 1 mouse line (PS- 1M146L6.2). The resultant breed was due to these two mutations that caused and elevated the production of Aβ peptide with a measureable deposition of plaques as early as 3–4 months of age (Holcomb et al. 1998, 1999; McGowan et al. 1999; Emilien et al. 2000). In a chronic therapeutic approach (8 month treatment), we had demonstrated that C. asiatica extracts decline the Aβ 1–40 and 1–42 levels in the hippocampus and cortex. Extracts of C. asiatica decreased the amyloid load due to fibrillar Aβ. Dense-core plaque formation has recently been shown to occur predominantly and is associated with the vasculature in PSAPP mice, suggesting a malfunction of the clearance of Aβ through the neurovascular system that may impact amyloid deposition (Kumar-Singh et al. 2005). It has been reported that C. asiatica effects in regulating the Aβ clearance or deposition as noticeable plaques are due to the alteration of extracellular matrix composition, perlecan or fibril formation subdued by other extracellular matrix molecule, laminin (Holcomb et al. 2000; Castillo et al. 2000). Asiaticoside upregulates biglycan, another heparin sulfate proteoglycan in other amyloidosis, which is also known to bind Aβ (Snow et al. 1994). Centella asiatica extract-based treatment thus may cause the regional alterations of extracellular matrix molecules and influence the fibrillar amyloid load detected in PSAPP mouse brain. In Tg2576 mouse, one of the PSAPP parental lines, previous researches have concluded that there is a significant elevation in lipid peroxidation before the increase of Aβ 1–40 and 1–42 levels or noticeable amyloid deposition (Praticò et al. 2001). Centella asiatica blocked lipid peroxidation, which in turn infers that the basic mechanism is by inhibiting the membrane damage caused by the hydroxyl free radical, thereby reducing the oxidative stress. It can thereby be concluded that the continuous and chronic treatment with C. asiatica may alter the Aβ pathology in the PSAPP Alzheimer’s disease mouse model. Based on our research data, it is clear that the antioxidant and anti-inflammatory properties of C. asiatica may prevent neurodegeneration (Table 1.4).
Table 1.4
Various studies on Centella asiatica to validate its efficacy
Model used | Toxin/task | Mechanism of action (MOA) | References |
|---|---|---|---|
B103 cells | H2O2
| Inhibition of Aβ and free radical-induced cell death | Mook-Jung et al. (1999) |
Staurosporine | |||
Mice | Passive avoidance task | Modulate dopamine, 5-HT and noradrenaline systems | |
Albino rats | |||
Male Wistar rats | H2O2
| Antioxidant, reduce the oxidative stress by decreasing the lipid peroxidation | Kumar and Gupta (2002) |
Rat cortical neurons cells | Glutamate | Increase the levels of glutathione and glutathione peroxidase and reduced the overproduction of NO | Lee et al. (2000) |
Mice | Nootropic, radial arm maze | Increase release of acetylcholine in the hippocampus | Rao et al. (2005) |
Increase dendritic arborization of hippocampal CA3 neurons | |||
Neonatal mice in vivo | Glutamate | Restores lipid peroxidation and glutathione content | Xu et al. (2012b) |
Reinstates superoxide dismutase activity in the hippocampus and cortex | |||
Human neuroblastoma SH-SY5Y cells in vitro | |||
Regulates NMDA receptors | |||
Reduced reactive oxygen species | |||
Stabilizes the mitochondrial membrane potential | |||
Promotes the expression of PGC-1α and Sirt1 | |||
Male Sprague-Dawley rats | Aβ | Increasephosphorylation of cyclic AMP response element-binding protein and thereby aiding the neuronal dendritic arborization and axonal regeneration | Soumyanath et al. (2005) |
Human SH-SY5Y cells | |||
Neonatal Wistar rat pups | Aβ | Increase phosphorylation of cyclic AMP response element-binding protein and thereby aiding the neuronal dendritic arborization and axonal regeneration | Mohandas Rao et al. (2006) |
N2a neuroblastoma cells expressing Aβ1–42 | Aβ | Increase phosphorylation of cyclic AMP response element-binding protein that leads to the increase in BDNF | Xu et al. (2008) |
H2O2
| |||
Rat embryonic cortical cells | |||
Modulates the NMDA receptor | |||
Wistar rats | FeCl2
| Antioxidant action | Subathra et al. (2005) |
Reduces lipid peroxidation and protein carbonyl production | |||
PSAPP mice | Aβ | Decreases amyloid beta deposition | Dhanasekaran et al. (2009) |
H2O2
| |||
Antioxidant effect | |||
Scavenged free radicals, reduced lipid peroxidation, and protected against DNA damage | |||
Rat embryonic cortical cells | C2-ceramide | Inhibits caspase 3 and the dephosphorylation of ERK1/2 | Zhang et al. (2012a) |
Rat embryonic cortical cells | Arachidonic acid | Inhibits cytosolic phospholipase A2 and secretory phospholipases A2 | Defillipo et al. (2012) |
Swiss albino mice | D-galactose | Anti-apoptotic activity by effectively controlling the caspase-3 activity | Prakash and Kumar (2012) |
Prepubertal male CFT-Swiss mice | 3-Nitropropionic acid (3-NPA) | Antioxidant activity | Shinomol and Muralidhara (2008a) |
Enhances GSH and thiols levels | |||
RAW 264.7 murine macrophage cells | Lipopolysaccharide | Inhibits inducible nitric oxide, COX-2, tumor necrosis factor-alpha, interleukin-1 beta, and IL-6 via the downregulation of nuclear factor-kappaB activation which in turn suppresses neuronal apoptosis | Won et al. (2010) |
Male Wistar rats | Pentylenetetrazol | Inhibits oxidative stress | Gupta et al. (2003) |
Reduce malondialdehyde levels and increase in the glutathione levels | |||
Adult male Wistar rats | Arachidonic acid | Inhibits the activity of Ca2+-independent phospholipase A2 and cytosolic phospholipase A2 | Barbosa et al. (2008) |
Female Sprague-Dawley rats | Glutamate | Antioxidant effects | Ramanathan et al. (2007) |
Reduces catalase, superoxide dismutase, and lipid peroxides | |||
C57Bl/6 mice | Glutamate | Modulates antioxidant and mitochondrial pathways | Gray et al. (2016) |
Increased the expression of synaptic genes | |||
MC65 | Tetracycline | Modulates antioxidant and mitochondrial pathway | Gray et al. (2015) |
SH-SY5Y neuroblastoma cells | |||
Human neuroblastoma SH-SY5Y cells | Buthionine sulfoximine (BSO) | Inhibits the activation of caspase-9 pathway | Omar et al. (2011) |
Male Sprague-Dawley rats | Ischemia reperfusion injury | Reduce the levels of malondialdehyde, nitric oxide, pro-inflammatory cytokines and nuclear factor kappa-light-chain-enhancer of activated B cells | Luo et al. (2014) |
Mouse NG108-15 neural cell line | Aβ25–35 | Blocks the conversion of light chain3-I to light chain3-II (protects microtubule-associated proteins) | Du et al. (2014) |
Human neuroblastoma cell SH-SY5Y | |||
Reduce Beclin-1 | |||
Increase anti-apoptotic protein Bcl-2 level | |||
Adult zebra fish | Rotenone | Decreases reactive oxygen species probability of radical attack to a-synuclein protein | Khotimah et al. (2015) |
Increase dopamine level | |||
Rat PC12 pheochromocytoma cells | Aβ1–40 | Modulates the activities of superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase and alters the levels of glutathione and glutathione disulfide | Chen et al. (2015) |
Human IMR32 neuroblastoma cells | |||
Male Wistar rats | Colchicine | Reduces AChE activity | Kumar et al. (2009) |
IMR-32 human neuroblastoma cells | Aβ | Upregulates the level of activated ERK1/2 and protein kinase B (Akt) | Wanakhachornkrai et al. (2013) |
Tg2576 mouse | Glutamate | Modulates the toxic effects of Aβ by inhibiting Aβ-induced nitric oxide (NO) | Soumyanath et al. (2012) |
SH-SY5Y cells and MC65 human neuroblastoma cells | Aβ | ||
PD model Drosophila flies | Transgenic Drosophila fly lines that expresses wild-type human synuclein | Reduces lipid peroxidation, protein carbonyl, GST, and MDA content | Siddique et al. (2014) |
Increase GSH content | |||
Male Laca mice | D-Galactose | Upregulates NADH dehydrogenase, succinate dehydrogenase activity, | Kumar et al. (2011) |
Increased neuronal viability | |||
Reduces acetylcholine esterase | |||
Adult Wistar rats | Silver nitrate | BDNF-like action | Mohandas Rao et al. (2012) |
Activate the expression of the early genes such as activity-regulated cytoskeleton-associated protein (arc) leading to enhancement of the dendritic arborization | |||
In vitro | Markers of oxidative stress | Antioxidants prevent chain initiation, binding of transition metal ion, decomposition of peroxides in addition to free radical scavenging | Sugunabai and Karpagam (2015) |
Male Wistar rats | Streptozotocin | Decreases MDA and increase in glutathione and catalase levels | Kumar and Gupta (2003) |
Improves acetylcholine synthesis | |||
Male Sprague-Dawley rats | Behavioral cognitive | Increase doublecortin (DCX) and Notch1 protein levels in the hippocampus | Sirichoat et al. (2015) |
Male ICR mice | Transient cerebral ischemia-reperfusion | Reduced the microglial overactivation and the phosphorylation of p38 mitogen-activated protein kinases | Chen et al. (2014) |
Adult male Wistar rats | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) | Antioxidant maintain the metabolic balance of dopamine and increasing ratio of apoptosis regulator Bcl-2/apoptosis regulator Bax | Xu et al. (2012a) |
1.3.6 Other Neurological Disorders
3-Nitropropionic acid (3-NPA) is an irreversible complex II inhibitor of the electron transport chain and Krebs cycle in the mouse brain (Shinomol and Muralidhara 2008b). 3-NPA is a fungal neurotoxin known to induce selective striatal pathology that is encountered in Huntington’s disease. It was observed that upon administration of 3-NPA, there was a significant increase in the level of ROS and malondialdehyde (MDA) indicating the oxidative stress in cytoplasmic and mitochondrial fractions of striatum and other brain regions of prepubertal mice (Binienda et al. 1998; Hussin et al. 2005). Upon treatment, it has been demonstrated that the prophylactic activity of C. asiatica resulted in the protection against the glutathione depletion, protein oxidation damage, and diminishing of Na+, K+ ATPase activity caused by 3-NPA (Shinomol and Muralidhara 2008b). The study thereby concluded that Centella asiatica helps in the protection of oxidative stress and mitochondrial dysfunction which is usually observed in the neurodegenerative disorders (Shinomol and Muralidhara 2008b).
Cerebral ischemia which can result in cerebral infarction or stroke is the leading cause of disability and mortality in the world (Lo et al. 2003) and can only be cured by the restoration of adequate blood flow in brain. However, the reperfusion can cause tissue damage that is well past the ischemia, via is excitotoxicity, oxidative stress, inflammation, and apoptosis (Luo et al. 2014). Madecassic acid from Centella asiatica is reported to reduce oxidative stress by diminishing 3,4-methylenedioxyamphetamine level, increasing glutathione and superoxide dismutase activity, and reducing COX-2 expression and production of prostaglandin E2. It also reduces the plasma levels of TNF-α and IL-6 (Won et al. 2010). Generally, oxidative stress regulates anti-inflammatory activity by triggering toll-like receptors and NF-κB. Activation of NF-κB is known to mediate inflammation and apoptosis and augment the expression of various inflammation markers (TNF-α, IL-1β, COX-2), inducible nitric oxide synthase, and additional mediators such as adhesion molecules (Tak and Firestein 2001). Madacasic acid reduces activation of NF-κB, thereby suppressing the IL-1β, IL-6, and TNF-α levels, which in turn suppresses neuronal apoptosis. Madacasic acid found in Centella asiatica is protected against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity.
It is postulated that Aβ peptide increases vasoactivity in a single transgenic mouse model of Alzheimer’s disease (Paris et al. 2000). C. asiatica is also known to exert a positive effect in the treatment of venous insufficiency (Cataldi et al. 2001), wound healing by stimulating type 1 collagen synthesis in fibroblast cells, and has been shown to help normalize the vasculature (Widgerow et al. 2000; Brinkhaus et al. 2000; Lee et al. 2006).
1.4 Conclusions
An old Sinhalese proverb righteously depicts about the therapeutic effect of Centella asiatica, “Two leaves (of Centella asiatica) a day keeps old age away.” True to the above, Centella asiatica has shown to possess significant neuroprotective phytochemicals and nutrients. These neuroprotectants exhibit multiple pharmacological effects which can prevent cell injury, attenuate toxic insults, and protect against endogenous and exogenous lethality. Centella asiatica has significantly shown to protect against dermal and CNS disorders. Without any uncertainties, it is definite to conclude that Centella asiatica possesses significant memory-enhancing effects with very minimal adverse effects. Hence, Centella asiatica and its active components can be a potent botanical with memory-enhancing properties, prevent neurodegeneration, and additionally have therapeutic value in preventing cognitive impairment.
References
Abas R, Othman F, Thent ZC (2015) Effect of Momordica charantia fruit extract on vascular complication in type 1 diabetic rats. EXCLI J 14:179–189. doi:10.17179/excli2014-539
PubMedPubMedCentral
Abdul HM, Sultana R, St Clair DK, Markesbery WR, Butterfield DA (2008) Oxidative damage in brain from human mutant APP/PS-1 double knock-in mice as a function of age. Free Radic Biol Med 45:1420–1425. doi:10.1016/j.freeradbiomed.2008.08.012
PubMedPubMedCentral
Acosta D, Wortmann M (2009) World Alzheimer Report
Aisen PS, Vellas B (2013) Passive immunotherapy for Alzheimer’s disease: what have we learned, and where are we headed? J Nutr Health Aging 17:49–50. doi:10.1007/s12603-013-0001-3
PubMed
Akagi M, Matsui N, Akae H, Hirashima N, Fukuishi N, Fukuyama Y, Akagi R (2015) Nonpeptide neurotrophic agents useful in the treatment of neurodegenerative diseases such as Alzheimer’s disease. J Pharmacol Sci 127:155–163. doi:10.1016/j.jphs.2014.12.015
PubMed
Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T (2000) Inflammation and Alzheimer’s disease. Neurobiol Aging 21:383–421PubMedPubMedCentral
Aloisi F (2001) Immune function of microglia. Glia 36:165–179PubMed
Alvari A, Mehrnaz SOR, Ahmad FJ, Abdin MZ (2012) Contemporary overview on clinical trials and future prospects of hepato-protective herbal medicines. Rev Recent Clin Trials 7:214–223PubMed
Alzheimer’s Association (2015) Alzheimer’s disease facts and figures. Alzheimers Dement 11:332–84
Alzheimer’s disease International (2009) Alzheimer’s Disease International Report 2009. London
Appa Rao MV, Srinivasan K, Rao KT (1973) Effect of Man-dookaparni (Centella asiatica) on the general mental ability (Medhya) of mentally retarded children. J Res Indian Med 8:9–16
Arboleda G, Morales LC, Benítez B, Arboleda H (2009) Regulation of ceramide-induced neuronal death: cell metabolism meets neurodegeneration. Brain Res Rev 59:333–346. doi:10.1016/j.brainresrev.2008.10.001
PubMed
Ashalatha M, Shenoy LN (2015) Preliminary pharmacognostic study of Brahmi. Internat Ayurvedic Med J 3:2465–2469
Atri A (2011) Effective pharmacological management of Alzheimer’s disease. Am J Manag Care 17(Suppl 1):S346–S355PubMed
Awang D (1998) Gotu kola. Can Pharm J 7:42–46
Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, Piccardo P, Ghetti B, Paul SM (1997) Lack of apolipoprotein E dramatically reduces amyloid beta-peptide deposition. Nat Genet 17:263–264PubMed
Barbosa NR, Pittella F, Gattaz WF (2008) Centella asiatica water extract inhibits iPLA2 and cPLA2 activities in rat cerebellum. Phytomedicine 15:896–900. doi:10.1016/j.phymed.2008.02.007
PubMed
Barzilai A, Yamamoto K-I (2004) DNA damage responses to oxidative stress. DNA Repair (Amst) 3:1109–1115. doi:10.1016/j.dnarep.2004.03.002
Belcaro G, Maquart F-X, Scoccianti M, Dugall M, Hosoi M, Cesarone MR, Luzzi R, Cornelli U, Ledda A, Feragalli B (2011) TECA (Titrated Extract of Centella Asiatica): new microcirculatory, biomolecular, and vascular application in preventive and clinical medicine. A status paper. Panminerva Med 53:105–118PubMed
Bhattachryya SC, Lythgoe B (1949) Triterpene acids. Nature 163:259
Bhavna D, Jyoti K (2011) Centella asiatica: the elixir of life. IJRAP 2:431–438
Bian G-X, Li G-G, Yang Y, Liu R-T, Ren J-P, Wen L-Q, Guo S-M, Lu Q-J (2008) Madecassoside reduces ischemia-reperfusion injury on regional ischemia induced heart infarction in rat. Biol Pharm Bull 31:458–463PubMed
Bian D, Zhang J, Wu X, Dou Y, Yang Y, Tan Q, Xia Y, Gong Z, Dai Y (2013) Asiatic acid isolated from Centella asiatica inhibits TGF-β1-induced collagen expression in human keloid fibroblasts via PPAR-γ activation. Int J Biol Sci 9:1032–1042. doi:10.7150/ijbs.7273
PubMedPubMedCentral
Bianca VD, Dusi S, Bianchini E, Dal Prà I, Rossi F (1999) beta-amyloid activates the O-2 forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer’s disease. J Biol Chem 274:15493–15499PubMed
Bierer LM, Haroutunian V, Gabriel S, Knott PJ, Carlin LS, Purohit DP, Perl DP, Schmeidler J, Kanof P, Davis KL (1995) Neurochemical correlates of dementia severity in Alzheimer’s disease: relative importance of the cholinergic deficits. J Neurochem 64:749–760PubMed
Binienda Z, Simmons C, Hussain S, Slikker W, Ali SF (1998) Effect of acute exposure to 3-nitropropionic acid on activities of endogenous antioxidants in the rat brain. Neurosci Lett 251:173–176PubMed
Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW (2004) Incipient Alzheimer’s disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci U S A 101:2173–2178. doi:10.1073/pnas.0308512100
PubMedPubMedCentral
Blasko I, Marx F, Steiner E, Hartmann T, Grubeck-Loebenstein B (1999) TNFalpha plus IFNgamma induce the production of Alzheimer beta-amyloid peptides and decrease the secretion of APPs. FASEB J 13:63–68PubMed
Blasko I, Stampfer-Kountchev M, Robatscher P, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B (2004) How chronic inflammation can affect the brain and support the development of Alzheimer’s disease in old age: the role of microglia and astrocytes. Aging Cell 3:169–176. doi:10.1111/j.1474-9728.2004.00101.x
PubMed
Bobade V, Bodhankar SL, Aswar U, Mohan V, Thakurdesai P (2015) Prophylactic effects of asiaticoside-based standardized extract of Centella asiatica ( L.) urban leaves on experimental migraine: involvement of 5HT1A/1B receptors. Chin J Nat Med 13:274–282. doi:10.1016/S1875-5364(15)30014-5
PubMed
Boland B, Campbell V (2004) Abeta-mediated activation of the apoptotic cascade in cultured cortical neurones: a role for cathepsin-L. Neurobiol Aging 25:83–91
Bowen DM, Smith CB, White P, Davison AN (1976) Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain 99:459–496PubMed
Bradwejn J, Zhou Y, Koszycki D, Shlik J (2000) A double-blind, placebo-controlled study on the effects of Gotu Kola (Centella asiatica) on acoustic startle response in healthy subjects. J Clin Psychopharmacol 20:680–684PubMed
Bras J, Singleton A, Cookson MR, Hardy J (2008) Emerging pathways in genetic Parkinson’s disease: potential role of ceramide metabolism in Lewy body disease. FEBS J 275:5767–5773. doi:10.1111/j.1742-4658.2008.06709.x
PubMedPubMedCentral
Breitner JC (1996) APOE genotyping and Alzheimer’s disease. Lancet (London, England) 347:1184–1185
Breitner JC, Welsh KA (1995) Diagnosis and management of memory loss and cognitive disorders among elderly persons. Psychiatr Serv 46:29–35. doi:10.1176/ps.46.1.29
PubMed
Brinkhaus B, Lindner M, Schuppan D, Hahn EG (2000) Chemical, pharmacological and clinical profile of the East Asian medical plant Centella asiatica. Phytomedicine 7:427–448PubMed
Brown D (1995) Encyclopedia of herbs and their uses. Doring Kindersley, Boston
Burnett BP, Bitto A, Altavilla D, Squadrito F, Levy RM, Pillai L (2011) Flavocoxid inhibits phospholipase A2, peroxidase moieties of the cyclooxygenases (COX), and 5-lipoxygenase, modifies COX-2 gene expression, and acts as an antioxidant. Mediat Inflamm 2011:385780. doi:10.1155/2011/385780
Butterfield DA, Pocernich CB (2003) The glutamatergic system and Alzheimer’s disease: therapeutic implications. CNS Drugs 17:641–652PubMed
Butterfield DA, Drake J, Pocernich C, Castegna A (2001) Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol Med 7:548–554PubMed
Carvalho C, Cardoso S, Correia SC, Santos RX, Santos MS, Baldeiras I, Oliveira CR, Moreira PI (2012) Metabolic alterations induced by sucrose intake and Alzheimer’s disease promote similar brain mitochondrial abnormalities. Diabetes 61:1234–1242. doi:10.2337/db11-1186
PubMedPubMedCentral
Castellani RJ, Rolston RK, Smith MA (2010) Alzheimer disease. Dis Mon 56:484–546. doi:10.1016/j.disamonth.2010.06.001
PubMedPubMedCentral
Castillo GM, Lukito W, Peskind E, Raskind M, Kirschner DA, Yee AG, Snow AD (2000) Laminin inhibition of beta-amyloid protein (Abeta) fibrillogenesis and identification of an Abeta binding site localized to the globular domain repeats on the laminin a chain. J Neurosci Res 62:451–462PubMed
Cataldi A, Gasbarro V, Viaggi R, Soverini R, Gresta E, Mascoli F (2001) Effectiveness of the combination of alpha tocopherol, rutin, melilotus, and Centella asiatica in the treatment of patients with chronic venous insufficiency. Minerva Cardioangiol 49:159–163PubMed
Cesarone MR, Incandela L, De Sanctis MT, Belcaro G, Bavera P, Bucci M, Ippolito E (2001a) Evaluation of treatment of diabetic microangiopathy with total triterpenic fraction of Centella asiatica: a clinical prospective randomized trial with a microcirculatory model. Angiology 52(Suppl 2):S49–S54
Cesarone MR, Incandela L, De Sanctis MT, Belcaro G, Geroulakos G, Griffin M, Lennox A, Di Renzo AD, Cacchio M, Bucci M (2001b) Flight microangiopathy in medium- to long-distance flights: prevention of edema and microcirculation alterations with total triterpenic fraction of Centella asiatica. Angiology 52(Suppl 2):S33–S37PubMed
Chami L, Checler F (2012) BACE1 is at the crossroad of a toxic vicious cycle involving cellular stress and beta-amyloid production in Alzheimer’s disease. Mol Neurodegener 7:52. doi:10.1186/1750-1326-7-52
Chen QS, Kagan BL, Hirakura Y, Xie CW (2000) Impairment of hippocampal long-term potentiation by Alzheimer amyloid beta-peptides. J Neurosci Res 60:65–72PubMed
Chen Y, Han T, Rui Y, Yin M, Qin L, Zheng H (2005) Effects of total triterpenes of Centella asiatica on the corticosterone levels in serum and contents of monoamine in depression rat brain. Zhong Yao Cai 28:492–496PubMed
Chen J, Wang X, Yi X, Wang Y, Liu Q, Ge R (2013) Induction of KLF4 contributes to the neurotoxicity of MPP + in M17 cells: a new implication in Parkinson’s disease. J Mol Neurosci 51:109–117. doi:10.1007/s12031-013-9961-3
PubMed
Chen S, Yin Z, Jiang C, Ma Z, Fu Q, Qu R, Ma S (2014) Pharmacology, biochemistry and behavior Asiaticoside attenuates memory impairment induced by transient cerebral ischemia – reperfusion in mice through anti-inflammatory mechanism. Pharmacol Biochem Behav 122:7–15. doi:10.1016/j.pbb.2014.03.004
PubMed
Chen CL, Tsai WH, Chen CJ, Pan TM (2015) Centella asiatica extract protects against amyloid??<inf>1–40</inf>−induced neurotoxicity in neuronal cells by activating the antioxidative defence system. J Tradit Complement Med 1–8. doi: 10.1016/j.jtcme.2015.07.002
Chiu MJ, Yang SY, Chen TF, Chieh JJ, Huang TZ, Yip PK, Yang HC, Cheng TW, Chen YF, Hua MS, Horng HE (2012) New assay for old markers-plasma beta amyloid of mild cognitive impairment and Alzheimer’s disease. Curr Alzheimer Res 9:1142–1148PubMed
Choi YJ, Lee JY, Chung CP, Park YJ (2012) Cell-penetrating superoxide dismutase attenuates oxidative stress-induced senescence by regulating the p53-p21(Cip1) pathway and restores osteoblastic differentiation in human dental pulp stem cells. Int J Nanomedicine 7:5091–5106. doi:10.2147/IJN.S31723
PubMedPubMedCentral
Chong Y (1997) Effect of a carboxy-terminal fragment of the Alzheimer’s amyloid precursor protein on expression of proinflammatory cytokines in rat glial cells. Life Sci 61:2323–2333PubMed
Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ (1992) Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature 360:672–674. doi:10.1038/360672a0
PubMed
Cummings JL, Vinters HV, Cole GM, Khachaturian ZS (1998) Alzheimer’s disease: etiologies, pathophysiology, cognitive reserve, and treatment opportunities. Neurology 51:S2–17. discussion S65–7PubMed
Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP (2004) Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci U S A 101:2070–2075. doi:10.1073/pnas.0305799101
PubMedPubMedCentral
De Sanctis MT, Belcaro G, Incandela L, Cesarone MR, Griffin M, Ippolito E, Cacchio M (2001) Treatment of edema and increased capillary filtration in venous hypertension with total triterpenic fraction of Centella asiatica: a clinical, prospective, placebo-controlled, randomized, dose-ranging trial. Angiology 52(Suppl 2):S55–S59PubMed
Decker H, Lo KY, Unger SM, Ferreira ST, Silverman MA (2010) Amyloid-beta peptide oligomers disrupt axonal transport through an NMDA receptor-dependent mechanism that is mediated by glycogen synthase kinase 3beta in primary cultured hippocampal neurons. J Neurosci 30:9166–9171. doi:10.1523/JNEUROSCI.1074-10.2010
PubMed
Defillipo PP, Raposo AH, Fedoce AG, Ferreira AS, Polonini HC, Gattaz WF, Raposo NRB (2012) Inhibition of cPLA2 and sPLA2 activities in primary cultures of rat cortical neurons by Centella asiatica water extract. Nat Prod Commun 7:841–843PubMed
Del Bo R, Angeretti N, Lucca E, De Simoni MG, Forloni G (1995) Reciprocal control of inflammatory cytokines, IL-1 and IL-6, and beta-amyloid production in cultures. Neurosci Lett 188:70–74PubMed
Dewachter I, Filipkowski RK, Priller C, Ris L, Neyton J, Croes S, Terwel D, Gysemans M, Devijver H, Borghgraef P, Godaux E, Kaczmarek L, Herms J, Van Leuven F (2009) Deregulation of NMDA-receptor function and down-stream signaling in APP[V717I] transgenic mice. Neurobiol Aging 30:241–256. doi:10.1016/j.neurobiolaging.2007.06.011
PubMed
Dhanasekaran M, Holcomb LA, Hitt AR, Tharakan B, Porter JW, Young KA, Manyam BV (2009) Centella asiatica extract selectively decreases amyloid beta levels in hippocampus of Alzheimer’s disease animal model. Phytother Res 23:14–19. doi:10.1002/ptr.2405
PubMed
Di Tomo P, Di Silvestre S, Cordone VGP, Giardinelli A, Faricelli B, Pipino C, Lanuti P, Peng T, Formoso G, Yang D, Arduini A, Chiarelli F, Pandolfi A, Di Pietro N (2015) Centella asiatica and lipoic acid, or a combination thereof, inhibit monocyte adhesion to endothelial cells from umbilical cords of gestational diabetic women. Nutr Metab Cardiovasc Dis 25:659–666. doi:10.1016/j.numecd.2015.04.002
PubMed
Dickson DW, Farlo J, Davies P, Crystal H, Fuld P, Yen SH (1988) Alzheimer’s disease. A double-labeling immunohistochemical study of senile plaques. Am J Pathol 132:86–101PubMedPubMedCentral
Du Yan S, Zhu H, Fu J, Yan SF, Roher A, Tourtellotte WW, Rajavashisth T, Chen X, Godman GC, Stern D, Schmidt AM (1997) Amyloid-beta peptide-receptor for advanced glycation endproduct interaction elicits neuronal expression of macrophage-colony stimulating factor: a proinflammatory pathway in Alzheimer disease. Proc Natl Acad Sci U S A 94:5296–5301PubMedPubMedCentral
Du B, Zhang Z, Li N (2014) Madecassoside prevents Aβ25-35-induced inflammatory responses and autophagy in neuronal cells through the class III PI3K/Beclin-1/Bcl-2 pathway. Int Immunopharmacol 20:221–228. doi:10.1016/j.intimp.2014.02.036
PubMed
Eikelenboom P, van Exel E, Hoozemans JJM, Veerhuis R, Rozemuller AJM, van Gool WA (2010) Neuroinflammation – an early event in both the history and pathogenesis of Alzheimer’s disease. Neurodegener Dis 7:38–41. doi:10.1159/000283480
PubMed
Emilien G, Beyreuther K, Masters CL, Maloteaux JM (2000) Prospects for pharmacological intervention in Alzheimer disease. Arch Neurol 57:454–459PubMed
Engelborghs S, De Deyn PP (1997) The neurochemistry of Alzheimer’s disease. Acta Neurol Belg 97:67–84PubMed
Ermertcan AT, Inan S, Ozturkcan S, Bilac C, Cilaker S (2008) Comparison of the effects of collagenase and extract of Centella asiatica in an experimental model of wound healing: an immunohistochemical and histopathological study. Wound Repair Regen 16:674–681. doi:10.1111/j.1524-475X.2008.00417.x
PubMed
Ernst T, Chang L, Melchor R, Mehringer CM (1997) Frontotemporal dementia and early Alzheimer disease: differentiation with frontal lobe H-1 MR spectroscopy. Radiology 203:829–836. doi:10.1148/radiology.203.3.9169712
PubMed
Farlow MR (1998) Etiology and pathogenesis of Alzheimer’s disease. Am J Health Syst Pharm 55(Suppl 2):S5–10PubMed
Fayed N, Modrego PJ, Rojas-Salinas G, Aguilar K (2011) Brain glutamate levels are decreased in Alzheimer’s disease: a magnetic resonance spectroscopy study. Am J Alzheimers Dis Other Demen 26:450–456. doi:10.1177/1533317511421780
PubMed
Feng Z, Chang Y, Cheng Y, Zhang B, Qu Z, Qin C, Zhang J (2004) Melatonin alleviates behavioral deficits associated with apoptosis and cholinergic system dysfunction in the APP 695 transgenic mouse model of Alzheimer’s disease. J Pineal Res 37:129–136. doi:10.1111/j.1600-079X.2004.00144.x
PubMed
Ferreira IL, Bajouco LM, Mota SI, Auberson YP, Oliveira CR, Rego AC (2012) Amyloid beta peptide 1-42 disturbs intracellular calcium homeostasis through activation of GluN2B-containing N-methyl-d-aspartate receptors in cortical cultures. Cell Calcium 51:95–106. doi:10.1016/j.ceca.2011.11.008
PubMed
Fillit H, Ding WH, Buee L, Kalman J, Altstiel L, Lawlor B, Wolf-Klein G (1991) Elevated circulating tumor necrosis factor levels in Alzheimer’s disease. Neurosci Lett 129:318–320PubMed
Fisher Y, Nemirovsky A, Baron R, Monsonego A (2011) Dendritic cells regulate amyloid-β-specific T-cell entry into the brain: the role of perivascular amyloid-β. J Alzheimers Dis 27:99–111. doi:10.3233/JAD-2011-102034
PubMed
France-Lanord V, Brugg B, Michel PP, Agid Y, Ruberg M (1997) Mitochondrial free radical signal in ceramide-dependent apoptosis: a putative mechanism for neuronal death in Parkinson’s disease. J Neurochem 69:1612–1621PubMed
Francis PT, Palmer AM, Snape M, Wilcock GK (1999) The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry 66:137–147PubMedPubMedCentral
Frautschy SA, Cole GM (2010) Why pleiotropic interventions are needed for Alzheimer’s disease. Mol Neurobiol 41:392–409. doi:10.1007/s12035-010-8137-1
PubMedPubMedCentral
George V, Gnanarethinam J (1975) Free amino acids in Centella asiatica. Curr Sci 44:790
Gohil KJ, Patel JA, Gajjar AK (2010) Pharmacological review on Centella asiatica: a potential herbal cure-all. Indian J Pharm Sci 72:546–556. doi:10.4103/0250-474X.78519
PubMedPubMedCentral
Gomez-Isla T, Price JL, McKeel DWJ, Morris JC, Growdon JH, Hyman BT (1996) Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci 16:4491–4500PubMed
Gottwald MD, Rozanski RI (1999) Rivastigmine, a brain-region selective acetylcholinesterase inhibitor for treating Alzheimer’s disease: review and current status. Expert Opin Investig Drugs 8:1673–1682. doi:10.1517/13543784.8.10.1673
PubMed
Gotz J, Streffer JR, David D, Schild A, Hoerndli F, Pennanen L, Kurosinski P, Chen F (2004) Transgenic animal models of Alzheimer’s disease and related disorders: histopathology, behavior and therapy. Mol Psychiatry 9:664–683. doi:10.1038/sj.mp.4001508
PubMed
Gray NE, Sampath H, Zweig JA, Quinn JF, Soumyanath A (2015) Centella asiatica attenuates amyloid-β-induced oxidative stress and mitochondrial dysfunction. J Alzheimers Dis 45:933–946. doi:10.3233/JAD-142217.Centella
PubMedPubMedCentral
Gray NE, Harris CJ, Quinn JF, Soumyanath A (2016) Centella asiatica modulates antioxidant and mitochondrial pathways and improves cognitive function in mice. J Ethnopharmacol 180:78–86PubMedPubMedCentral
Gupta A, Pansari K (2003) Inflammation and Alzheimer’s disease. Int J Clin Pract 57:36–39PubMed
Gupta YK, Veerendra Kumar MH, Srivastava AK (2003) Effect of Centella asiatica on pentylenetetrazole-induced kindling, cognition and oxidative stress in rats. Pharmacol Biochem Behav 74:579–585PubMed
Hage S, Kienlen-campard P, Octave J, Quetin-leclercq J (2010) In vitro screening on β -amyloid peptide production of plants used in traditional medicine for cognitive disorders. J Ethnopharmacol 131:585–591. doi:10.1016/j.jep.2010.07.044
PubMed
Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O’Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K (1998) Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med 4:97–100PubMed
Holcomb LA, Gordon MN, Jantzen P, Hsiao K, Duff K, Morgan D (1999) Behavioral changes in transgenic mice expressing both amyloid precursor protein and presenilin-1 mutations: lack of association with amyloid deposits. Behav Genet 29:177–185PubMed
Holcomb LA, Gordon MN, Benkovic SA, Morgan DG (2000) A beta and perlecan in rat brain: glial activation, gradual clearance and limited neurotoxicity. Mech Ageing Dev 112:135–152PubMed
Hong-Qi Y, Zhi-Kun S, Sheng-Di C (2012) Current advances in the treatment of Alzheimer’s disease: focused on considerations targeting Aβ and tau. Transl Neurodegener 1:21. doi:10.1186/2047-9158-1-21
PubMedPubMedCentral
Hoozemans JJM, Veerhuis R, Rozemuller AJM, Arendt T, Eikelenboom P (2004) Neuronal COX-2 expression and phosphorylation of pRb precede p38 MAPK activation and neurofibrillary changes in AD temporal cortex. Neurobiol Dis 15:492–499. doi:10.1016/j.nbd.2003.11.028
PubMed
Howes MR, Houghton PJ (2003) Plants used in Chinese and Indian traditional medicine for improvement of memory and cognitive function. Pharmacol Biochem Behav 75:513–527. doi:10.1016/S0091-3057(03)00128-X
PubMed
Howes M-J R, Houghton PJ (2012) Ethnobotanical treatment strategies against Alzheimer’s disease. Curr Alzheimer Res 9:67–85. doi:10.2174/156720512799015046
PubMed
Howes M-JR, Perry NSL, Houghton PJ (2003) Plants with traditional uses and activities, relevant to the management of Alzheimer’s disease and other cognitive disorders. Phytother Res 17:1–18. doi:10.1002/ptr.1280
PubMed
Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R (2006) AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron 52:831–843. doi:10.1016/j.neuron.2006.10.035
PubMedPubMedCentral
Hu J, Akama KT, Krafft GA, Chromy BA, Van Eldik LJ (1998) Amyloid-beta peptide activates cultured astrocytes: morphological alterations, cytokine induction and nitric oxide release. Brain Res 785:195–206PubMed
Huang EJ, Reichardt LF (2003) Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72:609–642. doi:10.1146/annurev.biochem.72.121801.161629
PubMed
Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, Bechmann I, Becher B, Luhmann HJ, Waisman A, Kuhlmann CRW (2010) Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J 24:1023–1034. doi:10.1096/fj.09-141978
PubMed
Hurtado DE, Molina-Porcel L, Iba M, Aboagye AK, Paul SM, Trojanowski JQ, Lee VM-Y (2010) A{beta} accelerates the spatiotemporal progression of tau pathology and augments tau amyloidosis in an Alzheimer mouse model. Am J Pathol 177:1977–1988. doi:10.2353/ajpath.2010.100346
PubMedPubMedCentral
Hussin N, Jaafar J, Naing NN, Mat HA, Muhamad AH, Mamat MN (2005) A review of dengue fever incidence in Kota Bharu, Kelantan, Malaysia during the years 1998–2003. SE Asian J Trop Med Public Health 36:1179–1186
Hyman BT, Van Hoesen GW, Damasio AR (1987) Alzheimer’s disease: glutamate depletion in the hippocampal perforant pathway zone. Ann Neurol 22:37–40. doi:10.1002/ana.410220110
PubMed
Ichas F, Mazat JP (1998) From calcium signaling to cell death: two conformations for the mitochondrial permeability transition pore. Switching from low- to high-conductance state. Biochim Biophys Acta 1366:33–50PubMed
Imaizumi K, Morihara T, Mori Y, Katayama T, Tsuda M, Furuyama T, Wanaka A, Takeda M, Tohyama M (1999) The cell death-promoting gene DP5, which interacts with the BCL2 family, is induced during neuronal apoptosis following exposure to amyloid beta protein. J Biol Chem 274:7975–7981PubMed
Imbimbo BP, Lombard J, Pomara N (2005) Pathophysiology of Alzheimer’s disease. Neuroimaging Clin N Am 15:727–753., ix. doi:10.1016/j.nic.2005.09.009
PubMed
Incandela L, Belcaro G, Cesarone MR, De Sanctis MT, Nargi E, Patricelli P, Bucci M (2001) Treatment of diabetic microangiopathy and edema with total triterpenic fraction of Centella asiatica: a prospective, placebo-controlled randomized study. Angiology 52(Suppl 2):S27–S31PubMed
Iqbal H, Sher Z, Khan ZU (2011) Medicinal plants from salt range Pind Dadan Khan, district Jhelum, Punjab, Pakistan. J Med Plant Res 5:2157–2168
Itagaki S, McGeer PL, Akiyama H (1988) Presence of T-cytotoxic suppressor and leucocyte common antigen positive cells in Alzheimer’s disease brain tissue. Neurosci Lett 91:259–264PubMed
Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D (1989) Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol 24:173–182PubMed
Ittiyavirah SP, Hameed J (2014) Herbs treating Parkinson’s disease. Biomed Aging Pathol 4:369–376. doi:10.1016/j.biomag.2014.08.003
Jadidi-Niaragh F, Shegarfi H, Naddafi F, Mirshafiey A (2012) The role of natural killer cells in Alzheimer’s disease. Scand J Immunol 76:451–456. doi:10.1111/j.1365-3083.2012.02769.x
PubMed
James JT, Dubery IA (2009) Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L) Urban. Molecules 14:3922–3941. doi:10.3390/molecules14103922
PubMed
Jana A, Hogan EL, Pahan K (2009) Ceramide and neurodegeneration: susceptibility of neurons and oligodendrocytes to cell damage and death. J Neurol Sci 278:5–15. doi:10.1016/j.jns.2008.12.010
PubMedPubMedCentral
Jaturapatporn D, Isaac MGEKN, McCleery J, Tabet N (2012) Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer’s disease. Cochrane Database Syst Rev 2:CD006378. doi:10.1002/14651858.CD006378.pub2
Jayathirtha MG, Mishra SH (2004) Preliminary immunomodulatory activities of methanol extracts of Eclipta alba and Centella asiatica. Phytomedicine 11:361–365PubMed
Kalshetty P, Aswar U, Bodhankar S, Sinnathambi A, Mohan V (2012) Antidepressant effects of standardized extract of Centella asiatica L in olfactory bulbectomy model. Biomed Aging Pathol 2:48–53. doi:10.1016/j.biomag.2012.03.005
Karantzoulis S, Galvin JE (2011) Distinguishing Alzheimer’s disease from other major forms of dementia. Expert Rev Neurother 11:1579–1591. doi:10.1586/ern.11.155
PubMedPubMedCentral
Karpińska A, Gromadzka G (2013) Oxidative stress and natural antioxidant mechanisms: the role in neurodegeneration. From molecular mechanisms to therapeutic strategies. Postepy Hig Med Dosw (Online) 67:43–53
Kasture VS, Gosavi SA, Ajage RK, Deshpande SG (2014) Comparative study of Brahmi and Brhamanduki: a review. World J Pharm Pharm Sci 3:2217–2230
Khachaturian ZS (1985) Diagnosis of Alzheimer’s disease. Arch Neurol 42:1097–1105PubMed
Khatun MA, Harun-Or-Rashid M, Rahmatullah M (2011) Scientific validation of eight medicinal plants used in traditional medicinal systems of Malaysia: a review. Am J Sustain Agric 5:67–75
Khotimah H, Ali M, Sumitro SB, Widodo MA (2015) Decreasing α-synuclein aggregation by methanolic extract of Centella asiatica in zebrafish Parkinson’s model. Asian Pac J Trop Biomed 5:948–954. doi:10.1016/j.apjtb.2015.07.024
Kolko M, Nielsen M, Bazan NG, Diemer NH (2002) Secretory phospholipase A(2) induces delayed neuronal COX-2 expression compared with glutamate. J Neurosci Res 69:169–177. doi:10.1002/jnr.10288
PubMed
Krishna G (2013) Neurodegenerative disorders: combining novelty with an ancient science – a review. IAJPR 3(10):8480–8486
Krishnamurthy RG, Senut M-C, Zemke D, Min J, Frenkel MB, Greenberg EJ, Yu S-W, Ahn N, Goudreau J, Kassab M, Panickar KS, Majid A (2009) Asiatic acid, a pentacyclic triterpene from Centella asiatica, is neuroprotective in a mouse model of focal cerebral ischemia. J Neurosci Res 87:2541–2550. doi:10.1002/jnr.22071
PubMedPubMedCentral
Kroemer G, Reed JC (2000) Mitochondrial control of cell death. Nat Med 6:513–519. doi:10.1038/74994
PubMed
Kudo W, Lee H-P, Smith MA, Zhu X, Matsuyama S, Lee H-G (2012) Inhibition of Bax protects neuronal cells from oligomeric Abeta neurotoxicity. Cell Death Dis 3:e309. doi:10.1038/cddis.2012.43
PubMedPubMedCentral
Kumar MHV, Gupta YK (2002) Effect of different extracts of Centella asiatica on cognition and markers of oxidative stress in rats. J Ethnopharmacol 79:253–260
Kumar MH, Gupta YK (2003) Effect of Centella asiatica on cognition and oxidative stress in an intracerebroventricular streptozotocin model of Alzheimer’s disease in rats. Clin Exp Pharmacol Physiol 30:336–342
Kumar A, Dogra S, Prakash A (2009) Neuroprotective effects of Centella asiatica against intracerebroventricular colchicine-induced cognitive impairment and oxidative stress. Int J Alzheimers Dis. doi:10.4061/2009/972178
Kumar A, Prakash A, Dogra S (2011) Centella asiatica attenuates D-galactose-induced cognitive impairment, oxidative and mitochondrial dysfunction in mice. Int J Alzheimers Dis 2011:347569. doi:10.4061/2011/347569
PubMedPubMedCentral
Kumar H, More SV, Han S-D, Choi J-Y, Choi D-K (2012) Promising therapeutics with natural bioactive compounds for improving learning and memory – a review of randomized trials. Molecules 17:10503–10539. doi:10.3390/molecules170910503
PubMed
Kumar-Singh S, Pirici D, McGowan E, Serneels S, Ceuterick C, Hardy J, Duff K, Dickson D, Van Broeckhoven C (2005) Dense-core plaques in Tg2576 and PSAPP mouse models of Alzheimer’s disease are centered on vessel walls. Am J Pathol 167:527–543. doi:10.1016/S0002-9440(10)62995-1
PubMedPubMedCentral
Label L (2009) Dementia facts and statistics. Disabled world towards tomorrow. https://www.disabled-world.com/health/aging/dementia/statistics.php
Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL (2004) Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci 24:10191–10200. doi:10.1523/JNEUROSCI.3432-04.2004
PubMed
Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL (2007) Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci 27:796–807. doi:10.1523/JNEUROSCI.3501-06.2007
PubMed
Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM (2009) Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature 457:1128–1132. doi:10.1038/nature07761
PubMedPubMedCentral
LeBlanc AC (2005) The role of apoptotic pathways in Alzheimer’s disease neurodegeneration and cell death. Curr Alzheimer Res 2:389–402PubMed
Lee MK, Kim SR, Sung SH, Lim D, Kim H, Choi H, Park HK, Je S, Ki YC (2000) Asiatic acid derivatives protect cultured cortical neurons from glutamate-induced excitotoxicity. Res Commun Mol Pathol Pharmacol 108:75–86PubMed
Lee J, Jung E, Kim Y, Park J, Park J, Hong S, Kim J, Hyun C, Kim YS, Park D (2006) Asiaticoside induces human collagen I synthesis through TGFbeta receptor I kinase (TbetaRI kinase)-independent Smad signaling. Planta Med 72:324–328. doi:10.1055/s-2005-916227
PubMed
Leskovjan AC, Kretlow A, Lanzirotti A, Barrea R, Vogt S, Miller LM (2011) Increased brain iron coincides with early plaque formation in a mouse model of Alzheimer’s disease. NeuroImage 55:32–38. doi:10.1016/j.neuroimage.2010.11.073
PubMed
Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E (2001) Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293:1487–1491. doi:10.1126/science.1058189
PubMed
Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D (2009) Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 62:788–801. doi:10.1016/j.neuron.2009.05.012
PubMedPubMedCentral
Liu M, Dai Y, Yao X, Li Y, Luo Y, Xia Y, Gong Z (2008) Anti-rheumatoid arthritic effect of madecassoside on type II collagen-induced arthritis in mice. Int Immunopharmacol 8:1561–1566. doi:10.1016/j.intimp.2008.06.011
PubMed
Lo C-J, Lin J-G, Kuo J-S, Chiang S-Y, Chen S-C, Liao E-T, Hsieh C-L (2003) Effect of salvia miltiorrhiza bunge on cerebral infarct in ischemia-reperfusion injured rats. Am J Chin Med 31:191–200. doi:10.1142/S0192415X03000916
PubMed
Long HS, Stander MA, Van Wyk B (2012) Notes on the occurrence and significance of triterpenoids (asiaticoside and related compounds) and caffeoylquinic acids in Centella species. S Afr J Bot 82:53–59. doi:10.1016/j.sajb.2012.07.017
Lue LF, Brachova L, Civin WH, Rogers J (1996) Inflammation, A beta deposition, and neurofibrillary tangle formation as correlates of Alzheimer’s disease neurodegeneration. J Neuropathol Exp Neurol 55:1083–1088PubMed
Luo Y, Yang Y, Liu J, Li W, Yang J, Sui X, Yuan X, Nie Z, Liu Y, Chen D, Lin S, Wang Y (2014) Neuroprotective effects of madecassoside against focal cerebral ischemia reperfusion injury in rats. Brain Res 1565:37–47. doi:10.1016/j.brainres.2014.04.008
PubMed
Madara JC, Levine ES, 2008. Presynaptic and postsynaptic NMDA receptors mediate distinct effects of brain-derived neurotrophic factor on synaptic transmission. J Neurophysiol 100, 3175–3184. doi:10.1152/jn.90880.2008
Magnus T, Schreiner B, Korn T, Jack C, Guo H, Antel J, Ifergan I, Chen L, Bischof F, Bar-Or A, Wiendl H (2005) Microglial expression of the B7 family member B7 homolog 1 confers strong immune inhibition: implications for immune responses and autoimmunity in the CNS. J Neurosci 25:2537–2546. doi:10.1523/JNEUROSCI.4794-04.2005
PubMed
Malinow R, Mainen ZF, Hayashi Y (2000) LTP mechanisms: from silence to four-lane traffic. Curr Opin Neurobiol 10:352–357PubMed
Mangas S, Bonfill M, Osuna L, Moyano E, Tortoriello J, Cusido RM, Piñol MT, Palazón J (2006) The effect of methyl jasmonate on triterpene and sterol metabolisms of Centella asiatica, Ruscus aculeatus and Galphimia glauca cultured plants. Phytochemistry 67:2041–2049. doi:10.1016/j.phytochem.2006.06.025
PubMed
Manyam BV (1999) Dementia in Ayurveda. J Altern Complement Med 5:81–88. doi:10.1089/acm.1999.5.81
PubMed
Maquart FX, Chastang F, Simeon A, Birembaut P, Gillery P, Wegrowski Y (1999) Triterpenes from Centella asiatica stimulate extracellular matrix accumulation in rat experimental wounds. Eur J Dermatol 9:289–296PubMed
Markesbery WR (1997) Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med 23:134–147PubMed
Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Morris JC (2001) Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology 56:127–129PubMed
Mato L, Wattanathorn J, Muchimapura S, Tongun T, Piyawatkul N, Yimtae K, Thanawirattananit P, Sripanidkulchai B (2011) Centella asiatica improves physical performance and health-related quality of life in healthy elderly volunteer. Evid Based Complement Alternat Med 2011:579467. doi:10.1093/ecam/nep177
PubMedPubMedCentral
Maxwell SR (1995) Prospects for the use of antioxidant therapies. Drugs 49:345–361PubMed
Mayeux R, Honig LS, Tang M-X, Manly J, Stern Y, Schupf N, Mehta PD (2003) Plasma A[beta]40 and A[beta]42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology 61:1185–1190PubMed
McGeer PL, Akiyama H, Itagaki S, McGeer EG (1989) Activation of the classical complement pathway in brain tissue of Alzheimer patients. Neurosci Lett 107:341–346PubMed
McGeer PL, Schulzer M, McGeer EG (1996) Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology 47:425–432PubMed
McGowan E, Sanders S, Iwatsubo T, Takeuchi A, Saido T, Zehr C, Yu X, Uljon S, Wang R, Mann D, Dickson D, Duff K (1999) Amyloid phenotype characterization of transgenic mice overexpressing both mutant amyloid precursor protein and mutant presenilin 1 transgenes. Neurobiol Dis 6:231–244. doi:10.1006/nbdi.1999.0243
PubMed
Migliore L, Fontana I, Trippi F, Colognato R, Coppedè F, Tognoni G, Nucciarone B, Siciliano G (2005) Oxidative DNA damage in peripheral leukocytes of mild cognitive impairment and AD patients. Neurobiol Aging 26:567–573. doi:10.1016/j.neurobiolaging.2004.07.016
PubMed
Minichiello L, Calella AM, Medina DL, Bonhoeffer T, Klein R, Korte M (2002) Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron 36:121–137PubMed
Misra R (1998) Modern drug development from traditional medicinal plants using radioligand receptor-binding assays. Med Res Rev 18:383–402PubMed
Mohandas Rao KG, Muddanna Rao S, Gurumadhva Rao S (2006) Centella asiatica (L.) leaf extract treatment during the growth spurt period enhances hippocampal CA3 neuronal dendritic arborization in rats. Evid Based Complement Alternat Med 3:349–357. doi:10.1093/ecam/nel024
PubMedPubMedCentral
Mohandas Rao KG, Rao MS, Rao GS (2012) Evaluation of amygdaloid neuronal dendritic arborization enhancing effect of Centella asiatica (Linn) fresh leaf extract in adult rats. Chin J Integr Med 1–6. doi: 10.1007/s11655-012-1235-3
Mohd Salim RJ, Adenan MI, Amid A, Jauri MH, Sued AS (2013) Statistical analysis of metal chelating activity of Centella asiatica and Erythroxylum cuneatum using response surface methodology. Biotechnol Res Int 2013:137851. doi:10.1155/2013/137851
PubMedPubMedCentral
Mook-Jung I, Shin JE, Yun SH, Huh K, Koh JY, Park HK, Jew SS, Jung MW (1999) Protective effects of asiaticoside derivatives against beta-amyloid neurotoxicity. J Neurosci Res 58:417–425PubMed
Moore AH, O’Banion MK (2002) Neuroinflammation and anti-inflammatory therapy for Alzheimer’s disease. Adv Drug Deliv Rev 54:1627–1656PubMed
Moreira PI, Nunomura A, Nakamura M, Takeda A, Shenk JC, Aliev G, Smith MA, Perry G (2008) Nucleic acid oxidation in Alzheimer disease. Free Radic Biol Med 44:1493–1505. doi:10.1016/j.freeradbiomed.2008.01.002
PubMed
Mori T, Rezai-Zadeh K, Koyama N, Arendash GW, Yamaguchi H, Kakuda N, Horikoshi-Sakuraba Y, Tan J, Town T (2012) Tannic acid is a natural beta-secretase inhibitor that prevents cognitive impairment and mitigates Alzheimer-like pathology in transgenic mice. J Biol Chem 287:6912–6927. doi:10.1074/jbc.M111.294025
PubMedPubMedCentral
Movsesyan VA, Yakovlev AG, Dabaghyan EA, Stoica BA, Faden AI (2002) Ceramide induces neuronal apoptosis through the caspase-9/caspase-3 pathway. Biochem Biophys Res Commun 299:201–207PubMed
Naik MT, Chang YC, Huang T (2002) Folding kinetics of the lipoic acid-bearing domain of human mitochondrial branched chain alpha-ketoacid dehydrogenase complex. FEBS Lett 530:133–138PubMed
Nalini K, Aroor A, Rao A, Karanth K (1992) Effect of Centella asiatica fresh leaf aqueous extract on learning and memory and biogenic amine turnover in albino rats. Fitoterapia 63:231–238
Nasir MN, Habsah M, Zamzuri I, Rammes G, Hasnan J, Abdullah J, 2011. Effects of asiatic acid on passive and active avoidance task in male Spraque-Dawley rats. J Ethnopharmacol 134, 203–209. doi:10.1016/j.jep.2010.12.010
Nieminen AL (2003) Apoptosis and necrosis in health and disease: role of mitochondria. Int Rev Cytol 224:29–55PubMed
Nitsch RM, Wurtman RJ, Growdon JH (1996) Regulation of APP processing. Potential for the therapeutical reduction of brain amyloid burden. Ann N Y Acad Sci 777:175–182PubMed
Novgorodov SA, Gudz TI (2009) Ceramide and mitochondria in ischemia/reperfusion. J Cardiovasc Pharmacol 53:198–208. doi:10.1097/FJC.0b013e31819b52d5
PubMedPubMedCentral
Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA (2001) Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol 60:759–767PubMed
O’Barr S, Cooper NR (2000) The C5a complement activation peptide increases IL-1beta and IL-6 release from amyloid-beta primed human monocytes: implications for Alzheimer’s disease. J Neuroimmunol 109:87–94PubMed
Omar NS, Zakaria ZAC, Mian TS, Ngah WZW, Mazlam M (2011) Centella asiatica modulates neuron cell survival by altering caspase-9 pathway. J Med Plant Res 5:2201–2209
Orhan IE (2012) Centella asiatica (L.) urban: from traditional medicine to modern medicine with neuroprotective potential. Evid Based Complement Alternat Med. doi:10.1155/2012/946259
Pandey MM, Rastogi S, Rawat AKS (2013) Indian traditional ayurvedic system of medicine and nutritional supplementation. Evid Based Complement Alternat Med 2013:376327. doi:10.1155/2013/376327
PubMedPubMedCentral
Panossian LA, Porter VR, Valenzuela HF, Zhu X, Reback E, Masterman D, Cummings JL, Effros RB (2003) Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol Aging 24:77–84PubMed
Paocharoen V (2010) The efficacy and side effects of oral Centella asiatica extract for wound healing promotion in diabetic wound patients. J Med Assoc Thail 93(Suppl 7):S166–S170
Parachikova A, Agadjanyan MG, Cribbs DH, Blurton-Jones M, Perreau V, Rogers J, Beach TG, Cotman CW (2007) Inflammatory changes parallel the early stages of Alzheimer disease. Neurobiol Aging 28:1821–1833. doi:10.1016/j.neurobiolaging.2006.08.014
PubMed
Parameshwaran K, Dhanasekaran M, Suppiramaniam V (2008) Amyloid beta peptides and glutamatergic synaptic dysregulation. Exp Neurol 210:7–13. doi:10.1016/j.expneurol.2007.10.008
PubMed
Paris D, Town T, Mori T, Parker TA, Humphrey J, Mullan M (2000) Soluble beta-amyloid peptides mediate vasoactivity via activation of a pro-inflammatory pathway. Neurobiol Aging 21:183–197PubMed
Patel KC, Pramanik S, Patil VC (2014) Ayurvedic approach with a prospective to treat and prevent Alzheimer’s and other cognitive diseases: a review. World J Pharm Sci 3:234–252
Perry RT, Collins JS, Wiener H, Acton R, Go RC (2001) The role of TNF and its receptors in Alzheimer’s disease. Neurobiol Aging 22:873–883PubMed
Piaceri I, Nacmias B, Sorbi S (2013) Genetics of familial and sporadic Alzheimer’s disease. Front Biosci (Elite Ed) 5:167–177
Prakash AK, Kumar A (2012) Ameliorative effect of Centella asiatica on memory dysfunction in D-galactose-induced senescence mice. Alzheimers Dement 7:S124. doi:10.1016/j.jalz.2011.05.325
Prasad KN, Hovland AR, La Rosa FG, Hovland PG (1998) Prostaglandins as putative neurotoxins in Alzheimer’s disease. Proc Soc Exp Biol Med 219:120–125PubMed
Praticò D, Uryu K, Leight S, Trojanoswki JQ, Lee VM (2001) Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci 21:4183–4187PubMed
Ramanathan M, Sivakumar S, Anandvijayakumar PR, Saravanababu C, Pandian PR (2007) Neuroprotective evaluation of standardized extract of Centella asiatica in monosodium glutamate treated rats. Indian J Exp Biol 45:425–431PubMed
Ransohoff RM, Kivisäkk P, Kidd G (2003) Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol 3:569–581. doi:10.1038/nri1130
PubMed
Rao SB, Chetana M, Devi PU (2005) Centella asiatica treatment during postnatal period enhances learning and memory in mice. Physiol Behav 86:449–457. doi:10.1016/j.physbeh.2005.07.019
PubMed
Rasoanaivo P (2011) Drugs and phytomedicines in Indian ocean and Madagascar: issues in research, policy and public health. Asian Biotechnol Dev Rev 13:7–25. doi:10.13140/2.1.2631.6162
Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell WJ, Kaye J, Manczak M (2005) Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J Alzheimers Dis 7:103–180PubMed
Reinikainen KJ, Soininen H, Riekkinen PJ (1990) Neurotransmitter changes in Alzheimer’s disease: implications to diagnostics and therapy. J Neurosci Res 27:576–586. doi:10.1002/jnr.490270419
PubMed
Resende R, Pereira C, Agostinho P, Vieira AP, Malva JO, Oliveira CR (2007) Susceptibility of hippocampal neurons to Abeta peptide toxicity is associated with perturbation of Ca2+ homeostasis. Brain Res 1143:11–21. doi:10.1016/j.brainres.2007.01.071
PubMed
Rizzuto R, Bernardi P, Pozzan T (2000) Mitochondria as all-round players of the calcium game. J Physiol 529(Pt 1):37–47PubMedPubMedCentral
Robichaud AJ (2006) Approaches to palliative therapies for Alzheimer’s disease. Curr Top Med Chem 6:553–568PubMed
Rogers J, Luber-Narod J, Styren SD, Civin WH (1988) Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. Neurobiol Aging 9:339–349PubMed
Rowan MJ, Klyubin I, Wang Q, Anwyl R (2004) Mechanisms of the inhibitory effects of amyloid beta-protein on synaptic plasticity. Exp Gerontol 39:1661–1667. doi:10.1016/j.exger.2004.06.020
PubMed
Rozemuller JM, Eikelenboom P, Pals ST, Stam FC (1989) Microglial cells around amyloid plaques in Alzheimer’s disease express leucocyte adhesion molecules of the LFA-1 family. Neurosci Lett 101:288–292PubMed
Rozemuller AJM, van Gool WA, Eikelenboom P (2005) The neuroinflammatory response in plaques and amyloid angiopathy in Alzheimer’s disease: therapeutic implications. Curr Drug Targets CNS Neurol Disord 4:223–233PubMed
Sakina MR, Dandiya PC (1990) A psycho-neuropharmacological profile of Centella asiatica extract. Fitoterapia 61:291–296
Sarkar P, Lohith KDH, Dhumal C, Panigrahi SS, Choudhary R (2015) Traditional and ayurvedic foods of Indian origin. J Ethn Foods 2:97–109. doi:10.1016/j.jef.2015.08.003
Schallier A, Smolders I, Van Dam D, Loyens E, De Deyn PP, Michotte A, Michotte Y, Massie A (2011) Region- and age-specific changes in glutamate transport in the AbetaPP23 mouse model for Alzheimer’s disease. J Alzheimers Dis 24:287–300. doi:10.3233/JAD-2011-101005
PubMed
Schapira AH, Reichmann H (1995) Electron transport chain defects in Alzheimer’s disease. Neurology 45:599–600PubMed
Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ (2007) Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 68:1501–1508. doi:10.1212/01.wnl.0000260698.46517.8f
PubMed
Schwab C, Yu S, Wong W, McGeer EG, McGeer PL (2013) GAD65, GAD67, and GABAT immunostaining in human brain and apparent GAD65 loss in Alzheimer’s disease. J Alzheimers Dis 33:1073–1088. doi:10.3233/JAD-2012-121330
PubMed
Seevaratnam V (2012) Functional properties of Centella asiatica (L.): a review. Int J Pharm Pharm Sci 4:8–14
Selkoe DJ (1998) The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer’s disease. Trends Cell Biol 8:447–453PubMed
Sharma V, Chaudhary AK (2015) Ayurvedic pharmacology and herbal medicine. IJGP 9:192–198
Sheldon AL, Robinson MB (2007) The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int 51:333–355. doi:10.1016/j.neuint.2007.03.012
PubMedPubMedCentral
Shinomol GK, Muralidhara (2008a) Prophylactic neuroprotective property of Centella asiatica against 3-nitropropionic acid induced oxidative stress and mitochondrial dysfunctions in brain regions of prepubertal mice. Neurotoxicology 29:948–957. doi:10.1016/j.neuro.2008.09.009
PubMed
Shinomol GK, Muralidhara à (2008b) Effect of Centella asiatica leaf powder on oxidative markers in brain regions of prepubertal mice in vivo and its in vitro efficacy to ameliorate 3-NPA-induced oxidative stress in mitochondria. Phytomedicine 15:971–984. doi:10.1016/j.phymed.2008.04.010
PubMed
Shinomol GK, Muralidhara, MMS B (2011) Exploring the role of & Brahmi (Bocopa monnieri and Centella asiatica) in brain function and therapy. Recent Pat Endocr Metab Immune Drug Discov 5:33–49. doi:10.2174/187221411794351833
PubMed
Shukla A, Rasik AM, Jain GK, Shankar R, Kulshrestha DK, Dhawan BN (1999) In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica. J Ethnopharmacol 65:1–11PubMed
Siddique YH, Naz F, Jyoti S, Fatima A, Khanam S, Rahul AF, Mujtaba SF, Faisal M (2014) Effect of Centella asiatica leaf extract on the dietary supplementation in transgenic Drosophila model of Parkinson’s disease. Parkinsons Dis 2014:1–11. doi:10.1155/2014/262058
Siegel SJ, Bieschke J, Powers ET, Kelly JW (2007) The oxidative stress metabolite 4-hydroxynonenal promotes Alzheimer protofibril formation. Biochemistry 46:1503–1510. doi:10.1021/bi061853s
PubMedPubMedCentral
Singh B, Rastogi RP (1969) A reinvestigation of the triterpenes of Centella asiatica. Phytochemistry 8:917–921. doi:10.1016/S0031-9422(00)85884-7
Singh RH, Narsimhamurthy K, Singh G (2008) Neuronutrient impact of Ayurvedic Rasayana therapy in brain aging. Biogerontology 9:369–374. doi:10.1007/s10522-008-9185-z
PubMed
Sirichoat A, Chaijaroonkhanarak W, Prachaney P, Pannangrong W, Leksomboon R, Chaichun A, Wigmore P, Welbat J (2015) Effects of asiatic acid on spatial working memory and cell proliferation in the adult rat hippocampus. Forum Nutr 7:8413–8423. doi:10.3390/nu7105401
Snow AD, Sekiguchi R, Nochlin D, Fraser P, Kimata K, Mizutani A, Arai M, Schreier WA, Morgan DG (1994) An important role of heparan sulfate proteoglycan (Perlecan) in a model system for the deposition and persistence of fibrillar A beta-amyloid in rat brain. Neuron 12:219–234PubMed
Solanki I, Parihar P, Parihar MS (2015) Neurochemistry international neurodegenerative diseases: from available treatments to prospective herbal therapy. Neurochem Int 95:100–108. doi:10.1016/j.neuint.2015.11.001
PubMed
Somboonwong J, Kankaisre M, Tantisira B, Tantisira MH (2012) Wound healing activities of different extracts of Centella asiatica in incision and burn wound models: an experimental animal study. BMC Complement Altern Med 12:103. doi:10.1186/1472-6882-12-103
PubMedPubMedCentral
Soumyanath A, Zhong YP, Gold SA, Yu X, Koop DR, Bourdette D, Gold BG (2005) Centella asiatica accelerates nerve regeneration upon oral administration and contains multiple active fractions increasing neurite elongation in-vitro. J Pharm Pharmacol 57:1221–1229. doi:10.1211/jpp.57.9.0018
PubMed
Soumyanath A, Zhong YP, Henson E, Wadsworth T, Bishop J, Gold BG, Quinn JF (2012) Centella asiatica extract improves behavioral deficits in a mouse model of Alzheimer’s disease: investigation of a possible mechanism of action. Int J Alzheimers Dis 2012:381974. doi:10.1155/2012/381974
PubMedPubMedCentral
Spiteller G (1993) Review: on the chemistry of oxidative stress. J Lipid Mediat 7:199–221PubMed
Stafford GI, Pedersen ME, Van Staden J, Jäger AK (2008) Review on plants with CNS-effects used in traditional South African medicine against mental diseases. J Ethnopharmacol 119:513–537. doi:10.1016/j.jep.2008.08.010
PubMed
Stoica BA, Movsesyan VA, Knoblach SM, Faden AI (2005) Ceramide induces neuronal apoptosis through mitogen-activated protein kinases and causes release of multiple mitochondrial proteins. Mol Cell Neurosci 29:355–371. doi:10.1016/j.mcn.2005.02.009
PubMed
Subathra M, Shila S, Devi MA, Panneerselvam C (2005) Emerging role of Centella asiatica in improving age-related neurological antioxidant status. Exp Gerontol 40:707–715. doi:10.1016/j.exger.2005.06.001
PubMed
Sugunabai J, Karpagam T (2015) Analysis of functional compounds and antioxidant activity of Centella asiatica. WJPS 4:1982–1993
Suh YH, Checler F (2002) Amyloid precursor protein, presenilins, and alpha-synuclein: molecular pathogenesis and pharmacological applications in Alzheimer’s disease. Pharmacol Rev 54:469–525PubMed
Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, Markesbery WR, Zhou XZ, Lu KP, Butterfield DA (2006) Oxidative modification and down-regulation of Pin1 in Alzheimer’s disease hippocampus: a redox proteomics analysis. Neurobiol Aging 27:918–925. doi:10.1016/j.neurobiolaging.2005.05.005
PubMed
Suo Z, Tan J, Placzek A, Crawford F, Fang C, Mullan M (1998) Alzheimer’s beta-amyloid peptides induce inflammatory cascade in human vascular cells: the roles of cytokines and CD40. Brain Res 807:110–117PubMed
Tabner BJ, El-Agnaf OMA, Turnbull S, German MJ, Paleologou KE, Hayashi Y, Cooper LJ, Fullwood NJ, Allsop D (2005) Hydrogen peroxide is generated during the very early stages of aggregation of the amyloid peptides implicated in Alzheimer disease and familial British dementia. J Biol Chem 280:35789–35792. doi:10.1074/jbc.C500238200
PubMed
Tak PP, Firestein GS (2001) NF-kappaB: a key role in inflammatory diseases. J Clin Invest 107:7–11. doi:10.1172/JCI11830
PubMedPubMedCentral
Tewari D, Mukhopadhyay M, Nekkanti MS, Vallabhaneni S, Sahu G, Jetti SK, Preethidan DS, Bera AK (2016) Cytoprotective effect of Centella asiatica is mediated through the modulation of mitochondrial voltage-dependent anion channel (VDAC) and scavenging of free radicals. J Funct Foods 21:301–311. doi:10.1016/j.jff.2015.11.047
Thellung S, Gatta E, Pellistri F, Corsaro A, Villa V, Vassalli M, Robello M, Florio T (2013) Excitotoxicity through NMDA receptors mediates cerebellar granule neuron apoptosis induced by prion protein 90-231 fragment. Neurotox Res 23:301–314. doi:10.1007/s12640-012-9340-9
PubMed
Thomas M, Kurup R, John A, Purushothaman S, Jacob P, Dan M, Baby S (2010) Elite genotypes/chemotypes, with high contents of madecassoside and asiaticoside, from sixty accessions of Centella asiatica of south India and the Andaman Islands: for cultivation and utility in cosmetic and herbal drug applications. Ind Crop Prod 32:545–550. doi:10.1016/j.indcrop.2010.07.003
Tiwari C, Bakshi M, Vichitra A (2013) A rapid two step protocol of in vitro propagation of an important medicinal herb Centella asiatica Linn. Afr J Biotechnol 12:1084–1090. doi:10.5897/AJB2012.2945
Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, Oda T, Tsuchiya K, Kosaka K (2002) Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J Neuroimmunol 124:83–92PubMed
Vasavi HS, Arun AB, Rekha PD (2014) Anti-quorum sensing activity of flavonoid- rich fraction from Centella asiatica L. against Pseudomonas aeruginosa PAO1. J Microbiol Immunol Infect 49:8–15. doi:10.1016/j.jmii.2014.03.012
PubMed
Ven Murthy MR, Ranjekar PK, Ramassamy C, Deshpande M (2010) Scientific basis for the use of Indian ayurvedic medicinal plants in the treatment of neurodegenerative disorders: ashwagandha. Cent Nerv Syst Agents Med Chem 10:238–246PubMed
Verri M, Pastoris O, Dossena M, Aquilani R, Guerriero F, Cuzzoni G, Venturini L, Ricevuti G, Bongiorno AI (2012) Mitochondrial alterations, oxidative stress and neuroinflammation in Alzheimer’s disease. Int J Immunopathol Pharmacol 25:345–353PubMed
Wanakhachornkrai O, Pongrakhananon V, Chunhacha P, Wanasuntronwong A, Vattanajun A, Tantisira B, Chanvorachote P, Tantisira MH (2013) Neuritogenic effect of standardized extract of Centella asiatica ECa233 on human neuroblastoma cells. BMC Complement Altern Med 13:204. doi:10.1186/1472-6882-13-204
PubMedPubMedCentral
Wang Z, Song D, Berger TW (2002) Contribution of NMDA receptor channels to the expression of LTP in the hippocampal dentate gyrus. Hippocampus 12:680–688. doi:10.1002/hipo.10104
PubMed
Wang J, Ho L, Qin W, Rocher AB, Seror I, Humala N, Maniar K, Dolios G, Wang R, Hof PR, Pasinetti GM (2005) Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer’s disease. FASEB J 19:659–661. doi:10.1096/fj.04-3182fje
PubMed
Wang Y, Pei L, Liu C-Y, Pei Y-R, Jia X-M (2010) Effects of Tianbingtiaodu capsule on improvement of learning and memory abilities and expression of NMDAR1 of epileptic rats in the hippocampus. Zhong Yao Cai 33:947–951PubMed
Warrier P, Nambiar V, Ramankutty C (1995) Indian medicinal plants: a compendium of 500 species. Orient Longman Ltd, Madras
Wattanathorn J, Mator L, Muchimapura S (2008) Positive modulation of cognition and mood in the healthy elderly volunteer following the administration of Centella asiatica. J Ethnopharmacol 116:325–332. doi:10.1016/j.jep.2007.11.038
PubMed
Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR (2006) Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron 50:897–909. doi:10.1016/j.neuron.2006.05.008
PubMed
Widgerow AD, Chait LA, Stals R, Stals PJ (2000) New innovations in scar management. Aesthet Plast Surg 24:227–234
Wirenfeldt M, Babcock AA, Vinters HV (2011) Microglia – insights into immune system structure, function, and reactivity in the central nervous system. Histol Histopathol 26:519–530PubMed
Won JH, Shin JS, Park HJ, Jung H-J, Koh DJ, Jo BG, Lee JY, Yun K, Lee KT (2010) Anti-inflammatory effects of madecassic acid via the suppression of NF-kappaB pathway in LPS-induced RAW 264.7 macrophage cells. Planta Med 76:251–257. doi:10.1055/s-0029-1186142
PubMed
Wu F, Bian D, Xia Y, Gong Z, Tan Q, Chen J, Dai Y (2012) Identification of major active ingredients responsible for burn wound healing of Centella asiatica herbs. Evid Based Complement Alternat Med 2012:848093. doi:10.1155/2012/848093
PubMedPubMedCentral
Xiong Y, Ding H, Xu M, Gao J (2009) Protective effects of asiatic acid on rotenone- or H2O2-induced injury in SH-SY5Y cells. Neurochem Res 34:746–754. doi:10.1007/s11064-008-9844-0
PubMed
Xu Y, Cao Z, Khan I, Luo Y (2008) Gotu Kola (Centella Asiatica) extract enhances phosphorylation of cyclic AMP response element binding protein in neuroblastoma cells expressing amyloid beta peptide. J Alzheimers Dis 13:341–349PubMed
Xu CL, Wang QZ, Sun LM, Li XM, Deng JM, Li LF, Zhang J, Xu R, Ma SP (2012a) Asiaticoside: attenuation of neurotoxicity induced by MPTP in a rat model of Parkinsonism via maintaining redox balance and up-regulating the ratio of Bcl-2/Bax. Pharmacol Biochem Behav 100:413–418. doi:10.1016/j.pbb.2011.09.014
PubMed
Xu M, Xiong Y, Liu J, Qian J, Zhu L, Gao J (2012b) Asiatic acid, a pentacyclic triterpene in Centella asiatica, attenuates glutamate-induced cognitive deficits in mice and apoptosis in SH-SY5Y cells. Acta Pharmacol Sin 33:578–587. doi:10.1038/aps.2012.3
PubMedPubMedCentral
Zainol M, Abd-Hamid A, Yusof S, Muse R (2003) Antioxidative activity and total phenolic compounds of leaf, root and petiole of four accessions of Centella asiatica (L.) urban. Food Chem 81:575–581. doi:10.1016/S0308-8146(02)00498-3
Zhang F-L, Wei Y-J, Zhu J, Gong Z-N (2008) Simultaneous quantitation of three major triterpenoid glycosides in Centella asiatica extracts by high performance liquid chromatography with evaporative light scattering detection. Biomed Chromatogr 22:119–124. doi:10.1002/bmc.901
PubMed
Zhang W, Bai M, Xi Y, Hao J, Liu L, Mao N, Su C, Miao J, Li Z (2012a) Early memory deficits precede plaque deposition in APPswe/PS1dE9 mice: involvement of oxidative stress and cholinergic dysfunction. Free Radic Biol Med 52:1443–1452. doi:10.1016/j.freeradbiomed.2012.01.023
PubMed
Zhang X, Wu J, Dou Y, Xia B, Rong W, Rimbach G, Lou Y (2012b) Asiatic acid protects primary neurons against C 2 -ceramide-induced apoptosis. Eur J Pharmacol 679:51–59. doi:10.1016/j.ejphar.2012.01.006
PubMed
Zheng C, Qin L (2007) Chemical components of Centella asiatica and their bioactivities. Zhong Xi Yi Jie He Xue Bao 5:348–351PubMed