6
BROWNING
Guided Inquiry Activities (Web): 5, Amino Acids and Proteins; 7, Carbohydrate; 17, Browning; 23, Meat Cooking
6.1 INTRODUCTION
We will describe chemistry/molecular changes that take place in cheese browning, chemistry of Maillard browning of proteins, chemistry of caramelization browning of sugars, and enzymatic browning including some basic chemistry and biochemistry of enzymes.
Brown food can be both incredibly appetizing and unattractive. Grilling produces a delicious flavor that cannot be matched by poaching, while a browned apple slice is something we try to avoid eating (Fig. 6.1)! The chemical reactions that bring us the goodness and the off-flavors of browning are everywhere. Chocolate cocoa is bitter and astringent until reactions leading to brown color and flavor are created. Coffee and beer are both better for the browning that occurs during their making. Plant enzymes catalyze a different set of browning reactions, in this case to provide first aid for an injured plant and to detract animals from eating and further damaging the plant. There are thousands of flavors and aromas produced by these reactions; a good scientist and cook will have an appreciation and understanding of the process by which these compounds are formed.

Figure 6.1 Browning is better. The impact of browning reactions on the taste and attractiveness of our food. Both are cooked to a safe temperature but one is much more tasty.
This process that creates an amazing diversity of new molecules is often called browning. However, this term is not quite accurate as many of the chemical products are not brown but yellow or even colorless. Moreover, browning reactions can be organized in a number of different ways: caramelization, Maillard reactions, ascorbic acid browning, and fruit browning. A closer look reveals that each type of browning is a chemical process—bonds are broken and formed to make new molecules. In some instances, the browning occurs with the aid of enzymes and other browning reactions occur without a catalyst, also called nonenzymatic browning. To better understand the difference between an enzyme- and nonenzyme-catalyzed reaction, it will help to consider how a chemical reaction proceeds. A chemical reaction is the process by which one or more molecules (chemical substances) will change by the breaking and/or making of new bonds to form one or more new molecules (Fig. 6.2). The chemical equation for a reaction shows one or more starting substances called reactants forming new molecules called products. How fast the reaction happens is measured as the rate of the reaction.
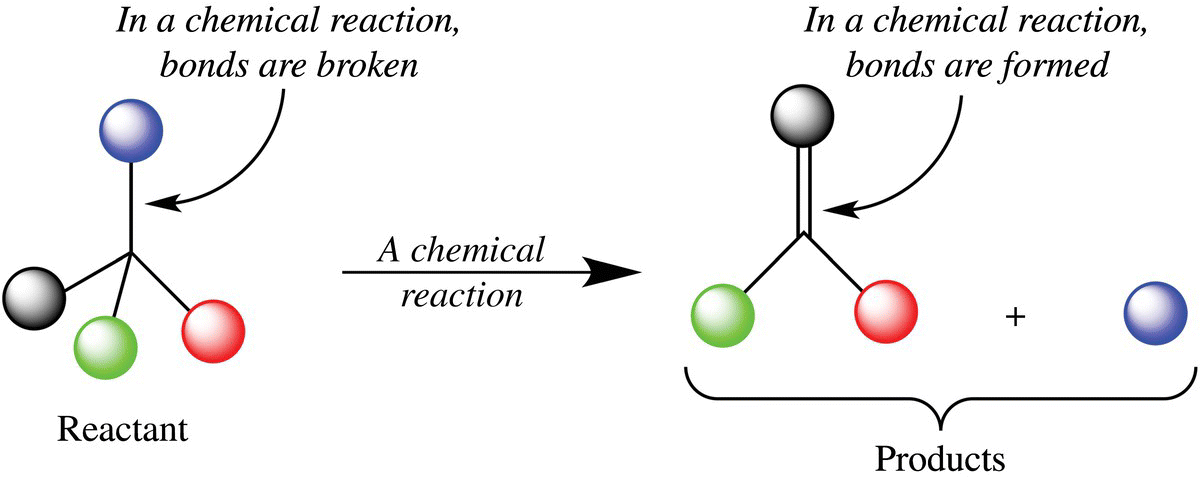
Figure 6.2 The anatomy of a chemical reaction. A chemical reaction (chemical change) takes place when a chemical bond is broken or made.
6.2 CHEMICAL REACTION KINETICS
When a chemical reaction involves the collision of two reactants, the reacting atoms or molecules must collide with enough force and correct geometry to change the bonds. When a chemical reaction involves the breakdown of one molecule into two or more, the reactant must vibrate with enough thermal energy to break the bonds. How fast the reactants collide or vibrate to form products depends on a number of parameters. Heating increases the energy of the molecules involved in a reaction. Increasing the energy of a system makes molecules vibrate more and move around more rapidly. When a reaction involves the collision of two molecules, increasing the temperature increases the number of collisions over time. Compared with reactions that take place at room temperature, every 50°F/10°C increase in temperature nearly doubles the rate of a reaction. Increasing the concentration of reactants also helps to increase the number of collisions of reacting molecules over time.
One class of browning reactions, the Maillard reaction, can take place at room temperature, albeit very slowly. Heating food increases the number and successful collisions between sugar and protein molecules that make up the Maillard reaction and thus browning takes place more effectively at higher temperatures. To better understand this process, it is helpful to investigate a concept called activation energy (Fig. 6.3). Activation energy is the amount of energy needed to start and continue a spontaneous reaction, that is a reaction where there is less total energy in the products than the starting reactants.
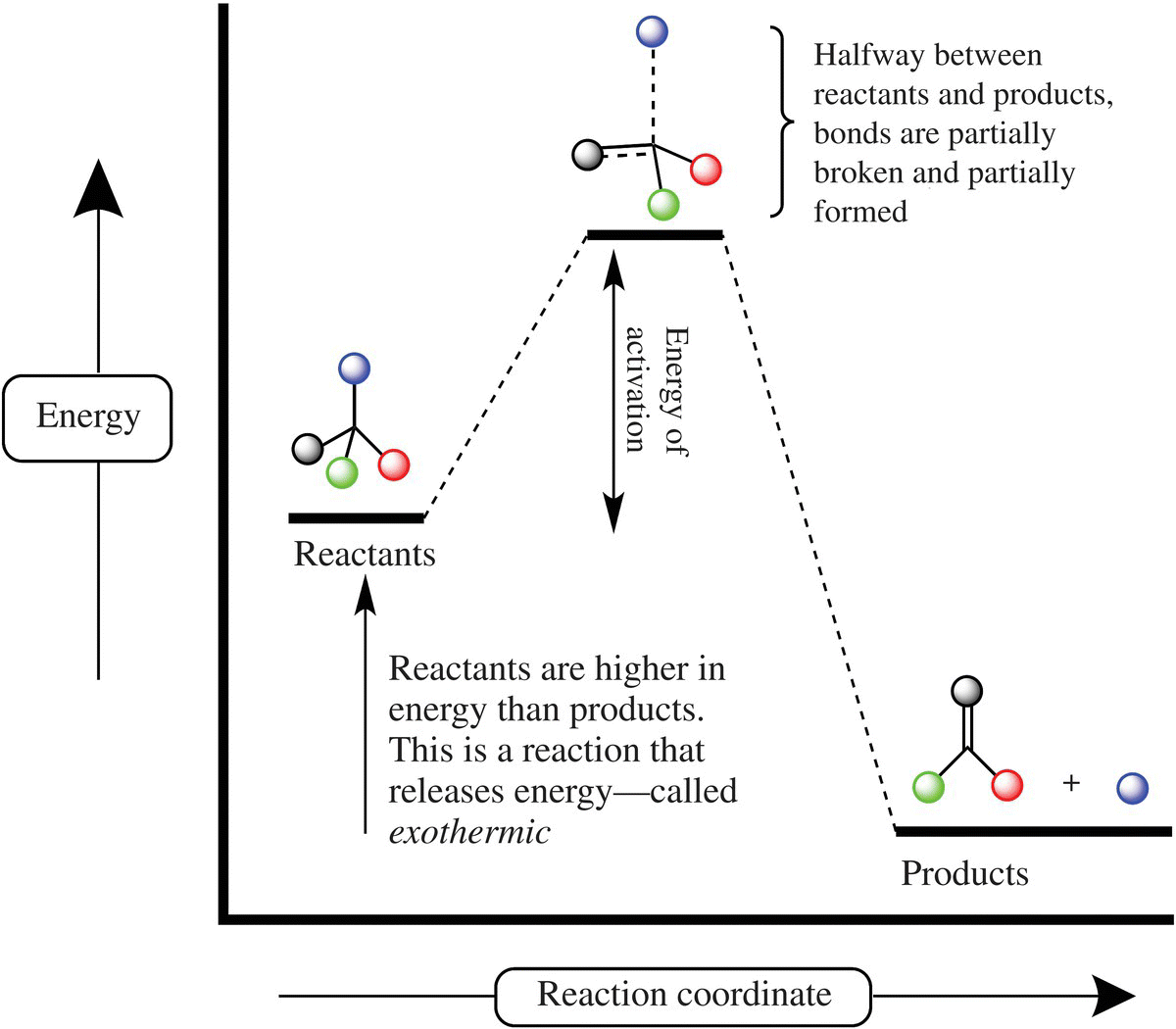
Figure 6.3 Energy diagram for a chemical reaction. The progress of a chemical reaction is shown on the horizontal axis (reaction coordinate) and the free energy of the substances involved in the reaction is shown on the vertical axis. The energy required for the reaction to reach the intermediate (also called a transition state) of the reaction is the energy of activation. A spontaneous reaction will end with less free energy than the starting compounds possessed.
To start a reaction the reacting molecules absorb energy from the surroundings and collide. Appropriately oriented collisions between reaction molecules may not result in a chemical reaction unless there is enough energy in the collision. The minimum energy of the collision is called the activation energy of a reaction. This is often shown by the energy diagram in Figure 6.3. The inherent energy of a substance (for our purpose a molecule from food) is shown on the vertical or Y-axis, while the horizontal or X-axis describes the process of a reaction, also called a reaction coordinate.
Let’s consider the reaction between sugar and oxygen producing CO2 and H2O. The inherent energy of the starting reactants is higher than the energy of the products. Thus overall, energy is given off, and the reaction is considered exothermic or spontaneous. However, the bag of sugar in your pantry has yet to react with oxygen in the air! That is a good thing; there is a lot of potential chemical energy in a bag of sucrose if all of the molecules were to react at one time! What stops the reaction? From the energy diagram you can see there is an intermediate or activated complex of the reaction. To produce the activated complex requires additional energy—called the energy barrier, an amount of energy that is given off as the reaction proceeds. The activated complex (also called a transition state) is short lived, and the overall energy of activation is released as the activated complex forms products. The energy barrier requirements prevent sugar and oxygen from reacting without the addition of heat. That is why to get the combustion of sugar, one has to add heat to overcome the energy barrier and produce a nice brown crust on a crème brûlée (Fig. 6.4).

Figure 6.4 Heat increasing the rate of a reaction—crème brûlée. Heat from the flame provides the energy needed for oxygen to react with sucrose sprinkled on the top of a desert.
Heating food helps to bring about reactions by increasing the energy of the reacting molecules in vibrations and collisions that overcome the energy barrier to products. Heating food increases the number of molecules that have the required energy to react. At room temperature, only a few molecules have enough energy for sugars and proteins to collide and form brown compounds. Thus, browning happens at a low temperature but very slowly. Increasing the temperature increases the number of molecules that have productive collisions and react. This is one of the reasons why we heat our food, to create more brown molecules!
Enzymes are protein catalysts. Catalysts reduce the activation energy for a reaction (Fig. 6.5). Thus, in a catalyzed reaction, it takes less energy to overcome the activation complex/energy barrier and more product molecules can be formed at a lower temperature. However, it should be noted that a catalyst does not change the total energy of the reaction. Heat can also speed up an enzyme-catalyzed reaction. The additional energy added to a system when cooking, as in a noncatalyzed reaction, results in more molecules with enough energy to overcome the activation energy barrier. However, remember that enzymes are proteins. Heat can also impact the structure of the protein. Many proteins will denature around 45–65°C (113–149°F). Therefore, heating an enzyme-catalyzed reaction will increase the rate until the protein is heated above its denaturation point, at which time the reaction will slow down with increased temperature.
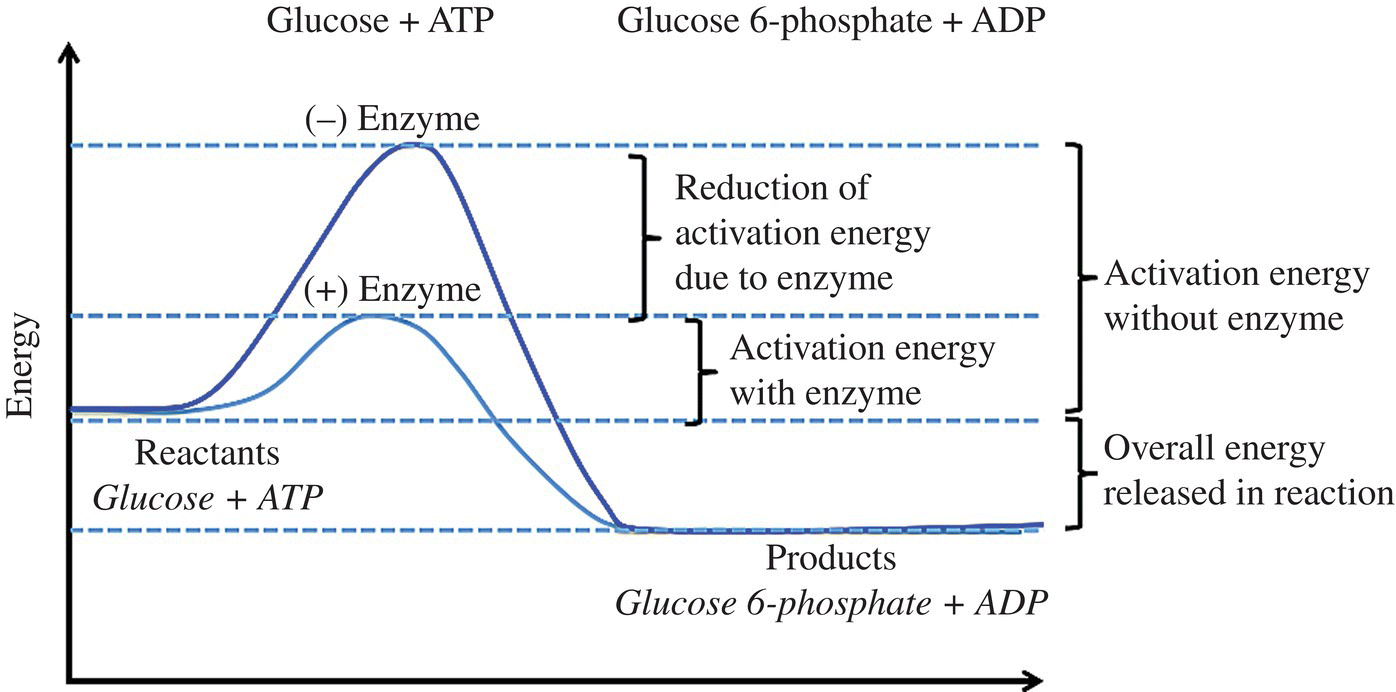
Figure 6.5 Effect of an enzyme catalyst on energy of a reaction.
So we catalogue browning reactions in two categories: enzyme-catalyzed reactions (vegetable and fruit browning) and nonenzyme-catalyzed reactions. These reactions include caramelization, the Maillard reactions, lipid oxidation, and ascorbic acid browning.
6.3 THE MAILLARD REACTION
The Maillard reaction is a complex series of reactions between some sugars and the amino groups of amino acids and proteins (Fig. 6.6). This reaction creates the pleasant odor and flavor of baked bread, cooked meat, and buttered popcorn flavor, among hundreds of others. The reaction is also responsible for the brown color of beer, roasted meat, coffee, and chocolate. The reaction was first described and eventually named after the French physician/scientist Louis Camille Maillard. In modern days, he would be considered a biochemist. At the age of 16, Maillard entered the University of Nancy in Lorraine, France, where he earned a master’s degree in science at the age of 19, became a physician, and began research on metabolism in 1903. There he studied metabolism of biological molecules, particularly the synthesis and breakdown of proteins. At the age of 34, he published the first article describing reactions between sugars and amino acids, starting with glycine and glucose [1]. Maillard established the formation of a class of dark-colored compounds he named “melanoidins.” Eventually he studied and found the order of reactivity for the kinds of sugars that react with different amino acids, defining the reactivity for each. While he eventually published 14 papers on reactions between sugars and amino acids, specifics on how the reactions were taking place remained elusive until the early 1950s when chemist John Hodge from the US Department of Agriculture (USDA) defined the chemical steps in the early stages of the browning process. Interestingly, Maillard referred to the reactions that create brown flavorful molecules as “my reaction.” However, the convention of calling these complex reactions the “Maillard reaction” had appeared in the literature (published in a review by General Foods and several chemical publications) by the early 1950s (Box 6.1). Thus, the large set of nonenzyme-catalyzed reactions between reducing sugars and amino groups are now called the Maillard reaction.

Figure 6.6 The Maillard browning reaction. Maillard browning is a reaction between select sugars (reducing) and nitrogen atoms of many amino acids.
The initial steps of the Maillard reaction are the simplest and, therefore, the easiest place to begin. For those interested in cooking, this is where the magic happens. Heat, sugars, and proteins or amino acids result in the brown tasty and aroma-filled goodness of cooked food. A closer look at the chemistry shows that the reaction takes place at the C O bond (a carbonyl group) of a simple sugar and the nitrogen of an amino group found on amino acids and proteins. Under higher temperatures (to surmount the activation energy barrier!) around, 100–140°C/38–60°F, we begin to observe the formation of significant amounts of browning products (Fig. 6.6).
O bond (a carbonyl group) of a simple sugar and the nitrogen of an amino group found on amino acids and proteins. Under higher temperatures (to surmount the activation energy barrier!) around, 100–140°C/38–60°F, we begin to observe the formation of significant amounts of browning products (Fig. 6.6).
Sugar molecules can take on two different structures or shapes: a straight or open chain and ring or closed form of the molecule. In sugars such as glucose, the ring-opening process produces a C O or carbonyl at the anomeric carbon. See Figure 6.7 for examples of several reducing sugars and the anomeric carbon. When dissolved in the water of our cells and our food, these sugars can be found in both the ring-opened and closed conformations. The carbonyl group formed upon ring opening is what participates in the Maillard reaction, and sugars that can “ring open” to form a C
O or carbonyl at the anomeric carbon. See Figure 6.7 for examples of several reducing sugars and the anomeric carbon. When dissolved in the water of our cells and our food, these sugars can be found in both the ring-opened and closed conformations. The carbonyl group formed upon ring opening is what participates in the Maillard reaction, and sugars that can “ring open” to form a C O group are called “reducing sugars.” However, in other sugars—such as fructose or sucrose—ring opening is not possible, and these sugars do not participate in the Maillard reaction. Examples of monosaccharides that can open and close to form carbonyl groups are glucose, ribose, galactose, and ribulose (Fig. 6.7). Disaccharides can be reducing sugars if at least one of the anomeric carbons is able to convert to the open chain format. The disaccharide found in milk, lactose, is composed of galactose bonded to glucose and has two anomeric carbons. The anomeric carbon of galactose is tied up in two C
O group are called “reducing sugars.” However, in other sugars—such as fructose or sucrose—ring opening is not possible, and these sugars do not participate in the Maillard reaction. Examples of monosaccharides that can open and close to form carbonyl groups are glucose, ribose, galactose, and ribulose (Fig. 6.7). Disaccharides can be reducing sugars if at least one of the anomeric carbons is able to convert to the open chain format. The disaccharide found in milk, lactose, is composed of galactose bonded to glucose and has two anomeric carbons. The anomeric carbon of galactose is tied up in two C O bonds and unable to convert to reducing sugar form. But the anomeric carbon of the glucose portion of lactose is able to open and close and thus can react to chemically reduce other compounds. Maltose (formed from the breakdown of starch found in sweet potato and cereals and used to make candies, syrups, and ketchup) is another example of a disaccharide that is a reducing sugar. Foods with milk and potatoes brown for this reason. However, don’t try to use table sugar (sucrose) to brown your food. Sucrose is a disaccharide of glucose and fructose has both of the anomeric carbons bonded to each other and is not a reducing sugar (Fig. 6.7).
O bonds and unable to convert to reducing sugar form. But the anomeric carbon of the glucose portion of lactose is able to open and close and thus can react to chemically reduce other compounds. Maltose (formed from the breakdown of starch found in sweet potato and cereals and used to make candies, syrups, and ketchup) is another example of a disaccharide that is a reducing sugar. Foods with milk and potatoes brown for this reason. However, don’t try to use table sugar (sucrose) to brown your food. Sucrose is a disaccharide of glucose and fructose has both of the anomeric carbons bonded to each other and is not a reducing sugar (Fig. 6.7).

Figure 6.7 Anomeric carbons and reducing sugars. Some sugars have ring-opened forms in which the anomeric carbon becomes a carbonyl group (C O), while in other sugars the anomeric carbon is trapped by two C
O), while in other sugars the anomeric carbon is trapped by two C O bonds and cannot ring open.
O bonds and cannot ring open.
Proteins and amino acids found in food are the second reactants in the Maillard reaction. The atom involved is the nitrogen of amino groups within amino acids, small proteins (peptides), or the amino groups of proteins themselves. In the reaction, the amino nitrogen bonds to the anomeric carbon of a reducing sugar. The combining of the reducing sugar (at the carbonyl carbon) and the nitrogen of amino groups is called a dehydration reaction and takes place with the loss of a water molecule. Very quickly the dehydration product rearranges to form a new compound of both the sugar and amino acid called an Amadori compound (Fig. 6.8).
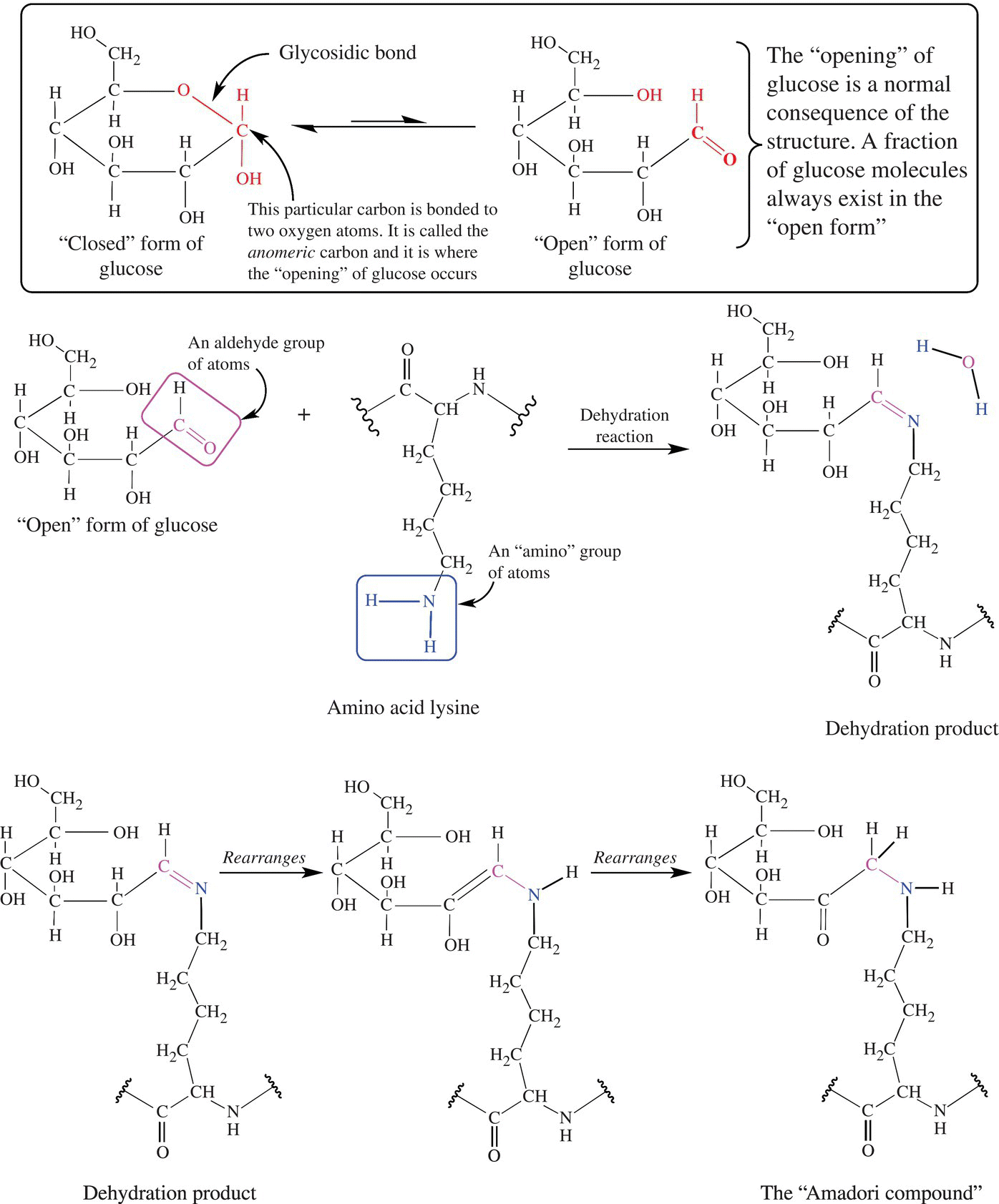
Figure 6.8 The Maillard reaction. The Maillard browning reaction requires a reducing “open” form of a sugar and the amino group of an amino acid. After the dehydration (also called a condensation) reaction, the compound will rearraign to form the intermediate “Amadori compound.”
At this point, you can begin to see the diversity of new molecules that can be formed by the Maillard browning reaction. There are many reducing sugars found in meat, vegetables, and fruits. Each of the 20 amino acids has an amino group, and one amino acid, lysine, has a side chain that contains an additional amino group. Some food scientists report that arginine can also form the Amadori compound of a Maillard reaction. Considering that 18 of the amino acids can react once and two of the amino acids can react with three different outcomes (the amino group, side chain, or both), the number of Amadori compounds possible with cooking is significant! If we limit the reducing sugars to eight of the more common reducing sugars, we can predict the possible number of initial products of the Maillard reaction. Looking at just the 18 amino acids that can only react with one of the eight sugars, there are 18 × 8 = 144. If two of the amino acids have three different combinations of sugar reactions, there are an additional 2 × 3 × 8 = 48 possible products. Therefore in our simple calculation that ignores the many other sugars, peptides, and proteins that also react to form flavor molecules, there are 192 Amadori compounds that can be made from these reducing sugars and amino acids. When you then consider that there is a minimum of three different paths that each Amadori product can take to create the final compound, there are an additional 192 × 3 = 576 different compounds from eight different sugars and 20 amino acids. That is a lot of flavor, color, and aroma compounds! When you factor in the many additional reactions that follow the initial Amadori step, one can begin to comprehend the thousands of possible new products and the complexity of food color, flavor, and aroma.
There is an order of reactivity for both sugars and amino acids for the Maillard reaction. The most reactive reducing sugars are the smallest sugars. Five-carbon sugars such as ribose react faster than six-carbon sugars such as glucose, galactose, and fructose. Disaccharides are much slower to react than monosaccharides. Sorbitol and other sugar alcohols used as low-calorie substitutes do not react and if used in baking or cooking will not brown. Free amino acids react faster than peptides or amino acid residues within proteins. Lysine is the most reactive amino acid and browns very well with foods rich in ribose. Foods made with wheat such as bread are rich in lysine-containing proteins and produce a rich flavor and dark color when toasted. The whey protein in milk is also rich in lysine and is often used as a browning agent to increase browning in foods. Cheese and aged meat whose proteins have been degraded to peptides and amino acids and have a high concentration of sugars are particularly quick to brown. Ribose in meat is created by the breakdown of ATP and nucleotides making up DNA and RNA molecules. In baking, egg washes contribute to the flavor and browned color of baked good by providing amino acids, including lysine. The amino acid cysteine reacts well with ribose, creating a strong meaty flavor and aroma. In fact, through the Maillard reactions, over 200 different volatile aroma compounds from the reaction between cysteine and reducing sugars have been found in cooked meat. See Figure 6.9 for an example of potatoes cooked in different sugar–amino acid compounds (Table 6.1).

Figure 6.9 Browning potatoes. Potatoes dipped in a dilute solution of sugar (ribose) and an amino acid was lightly fried for 3 min and 45 s. (a) Water control—no sugar or amino acid, (b) ribose–leucine, (c) ribose–lysine, and (d) ribose–glycine.
Table 6.1 Intensity of Maillard Browning of Common Amino Acids and Sugars.
| Absorbance at 420 nm | ||||
| Amino Compound | +D-Glucose | +D-Fructose | +D-Ribose | +α-Lactose |
| Lvs | 0.947 | 1.04 | 1.22 | 1.23 |
| Glv | 0.942 | 1.07 | 1.34 | 1.49 |
| Trp | 0.826 | 0.853 | 0.972 | 1.32 |
| Tvr | 0.809 | 0.857 | 0.951 | 1.06 |
| Pro | 0.770 | 0.783 | 0.792 | 0.876 |
| Leu | 0.764 | 0.747 | 0.895 | 1.11 |
| Ile | 0.746 | 0.797 | 0.870 | 0.986 |
| Ala | 0.739 | 0.792 | 0.945 | 1 .06 |
| Phe | 0.703 | 0.751 | 0.800 | 0.941 |
| Met | 0.668 | 0.669 | 0.828 | 0.888 |
| Val | 0.663 | 0.800 | 0.772 | 0.900 |
| Gln | 0.602 | 0.644 | 0.633 | 0.639 |
| Ser | 0.600 | 0.646 | 0.679 | 0.751 |
| Asn | 0.560 | 0.578 | 0.565 | 0.560 |
| His | 0.535 | 0.573 | 0.529 | 0.609 |
| Thr | 0.509 | 0.601 | 0.590 | 0.600 |
| Asp | 0.353 | 0.426 | 0.378 | 0.336 |
| Arg | 0.335 | 0.331 | 0.370 | 0.312 |
| Glu | 0.294 | 0.338 | 0.341 | 0.320 |
| Cvs | 0.144 | 0.202 | 0.150 | 0.273 |
Amino acids and sugars were mixed at a 1 : 1 ratio with a final concentration of 5 mM dissolved in 40 mM carbonate buffer pH 9.0. Each solution was autoclaved at 121°C for 10 min. The absorbance after each sample was treated was taken at 420 nm [2].
6.4 FACTORS THAT IMPACT MAILLARD REACTION BROWNING: pH, TEMPERATURE, AND TIME
There are a number of interesting factors that impact the rate and completeness of the Maillard browning reaction, the most impressive of which is pH. The acid level of a food can dramatically alter the rate and ability of a food to brown. To create their characteristic dark brown color, old-fashioned pretzels were dipped in lye and then cooked. Lye is a strongly basic/alkaline solution of sodium or potassium hydroxide (NaOH or KOH) and is used in a number of dishes including lutefisk and some types of noodles. In the case of the pretzels, the lye created a basic/alkaline pH on the outside of the pretzel dough, which increases the rate and quantity of Maillard browning reactions. Rates of browning reactions can be increased severalfold when the pH is above 7. Conversely, lower, more acidic pHs inhibit the Maillard reaction (Fig. 6.10). The explanation for this becomes clear if one considers the chemistry for the first stage of Maillard browning. The open/closed formation of reducing sugars is dependent on pH. In acidic solutions, reducing sugars are mostly in the ring conformation and unable to react. In more alkaline conditions, more sugar molecules are in the ring-opened form, which allows the anomeric carbon to react with the amino group. In addition, when the pH is low, the amino group becomes a protonated ammonium ion, which is very unreactive toward the reducing sugar. When the pH is raised, the proton is removed from the amino group and the reaction can occur. This explains why most marinades, which include an acid to break down proteins in tougher cuts of meat, take longer times and higher temperatures to brown. Figure 6.11 shows the acid/base chemistry impacting both the sugar and amino acid in the Maillard reaction.
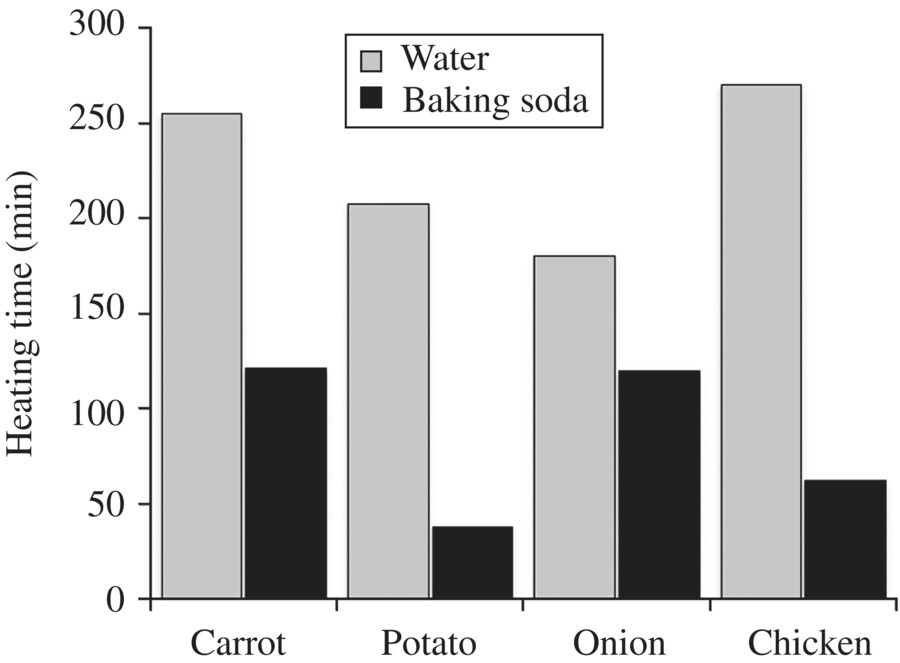
Figure 6.10 Alkali conditions speed browning time. 2.5 cm cubed sections of foods were dipped in water or a dilute solution of baking soda (sodium bicarbonate) and lightly fried for the indicated time. Note that decreasing the pH with baking soda decreased the time needed to brown each food item.
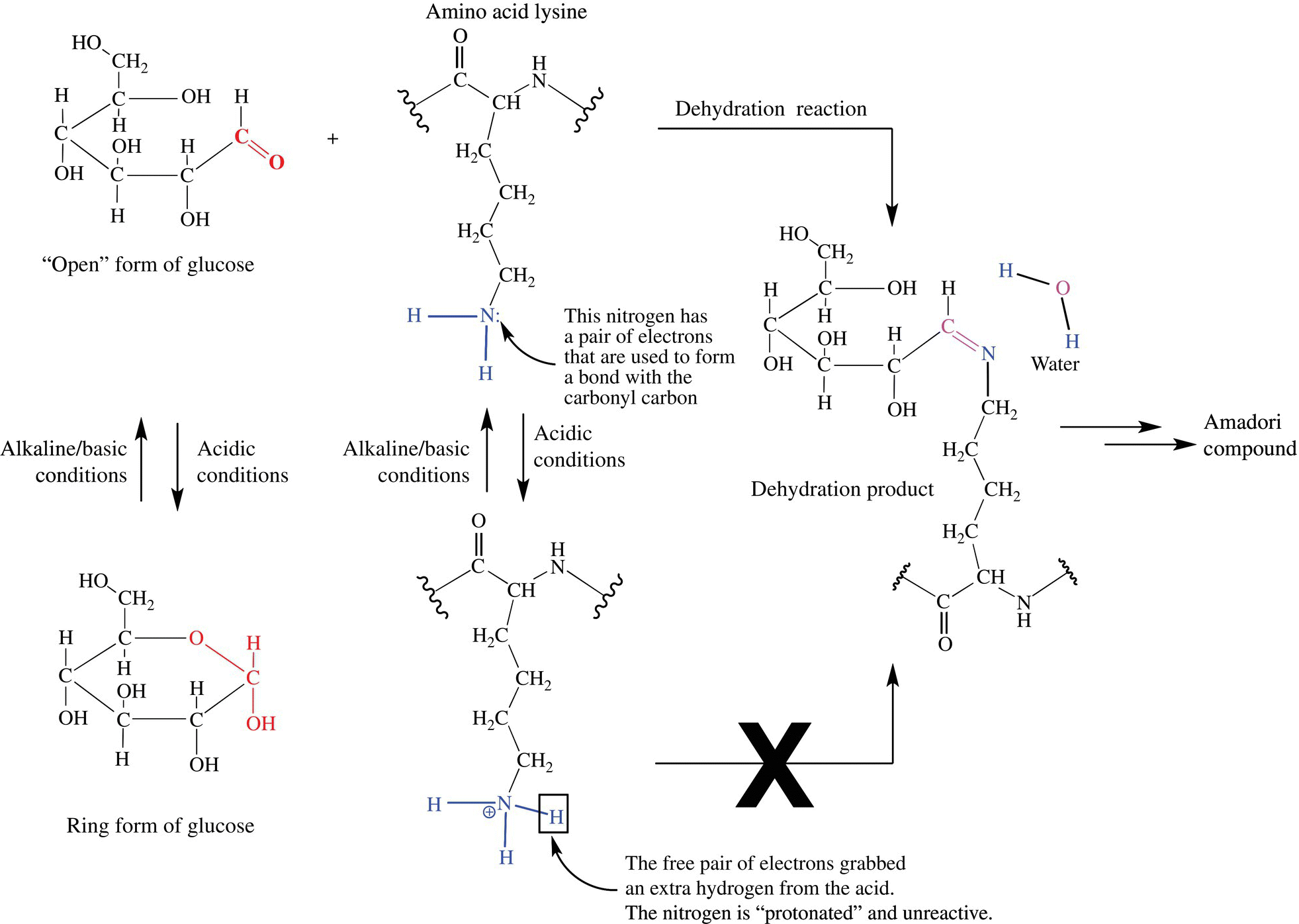
Figure 6.11 Acid/base chemistry of the initial phase of the Maillard reaction.
Temperature also impacts the rate of the reaction. As presented earlier in this chapter, increasing the temperature increases the number of collisions and the number of molecules with enough energy to overcome the energy barrier to reaction, allowing a spontaneous reaction to proceed. However, most foods will have some browning at lower temperatures, just not at an appreciable rate. Most Maillard reactions begin above 212°F/100°C and will continue quickly if heated above 284°F/140°C. Aged champagne, which is not allowed to warm significantly, still develops a yellow color due to the proteins and sugars found in the grapes undergoing Maillard reactions. But because the temperature is cool, the rates of the reactions producing the yellow Maillard compounds are very slow.
Another way in which heating food increases the rate of Maillard reaction is by evaporating water. Water content slows the reaction. In part, this is due to the energy that water absorbs during heating. While there remains a significant amount of water in the food, the temperature of the food will stay at the boiling point of water, 210°F/100°C. As the water content at the surface of the food decreases with heating (i.e., the water evaporates), the temperature of the food can then rise to the temperatures needed for Maillard browning to occur. Fried or grilled meat can have a thin layer of browned tissue where it touches the heat source, but the reaction is limited to this semidried area of the food. Similarly, toasted or baked bread is dried and brown on the surface but moist and unreacted in the middle. This is one of the reasons why oil is used to fry food. Oil efficiently transfers heat from the pan or heating element to the surface of the food.
Thus, one of the challenges when browning food is how long and how high does one heat the food in order to achieve the Maillard reaction, but not dry out the food. No one wants to eat a dried piece of brown bread or meat. While boiling, poaching, or sous vide cooking can produce moist, cooked food, food cooked with these methods will not brown, nor will it have the flavor bouquet of the Maillard reaction. Simply dropping the food onto a hot pan may not be the right fix, as the moisture at the surface of the food will inhibit the browning until the high temperatures sufficiently dry the tissue. While it might be enticing to dip food into lye, the flavor impact of the strong base can be problematic and unsavory, and use of strongly basic solutions can be dangerous. One way to use our understanding of science and cooking is to use a dilute solution of a weak base such as baking soda (aka sodium bicarbonate) to raise the pH and add a wash of lysine-containing food and a bit of ribose (purchased from health stores) to the surface of a food item for a quick browning. This is why tempura batters include baking soda—for quick and tasty browning during cooking!
6.5 MAILLARD IS COMPLICATED
The Maillard browning process is actually a very intricate series of reactions that start with the formation of the Amadori complex. In the early 1950s, USDA scientist John Hodge created a three-phase scheme describing the possible pathways by which the Maillard reactions could form many final products [2]. Three possible pathways combined with many different sugars and amino acids available to react in most foods give us an insight to the many possible products and flavors of browning (Fig. 6.12). See Table 6.2 for examples of some of these products [3].
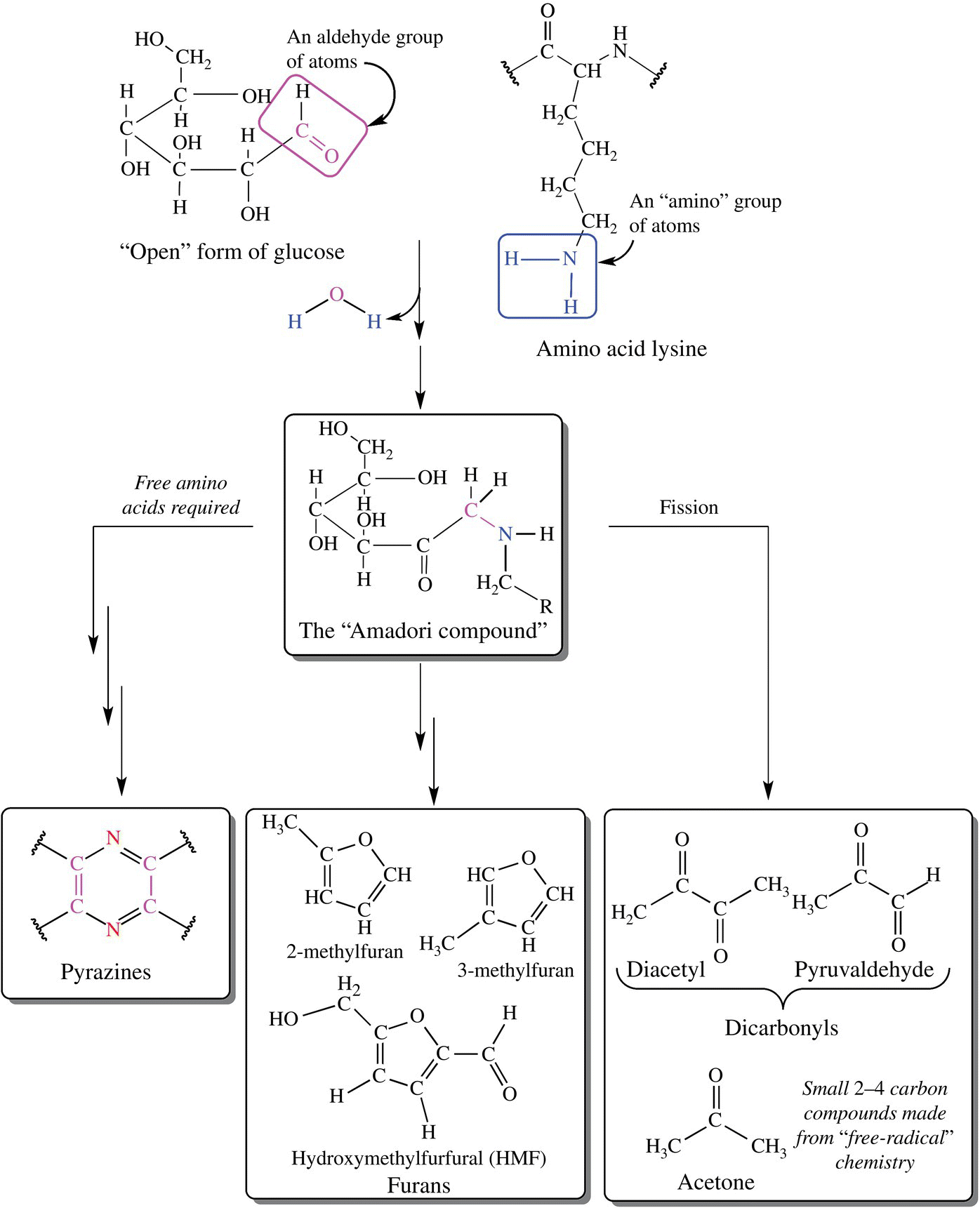
Figure 6.12 The pathways of the Maillard reaction. A simplified version of the reactions described by Hodge and others [2].
Table 6.2 Select Maillard Products.
|
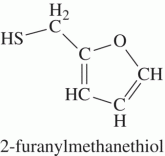
This is one of the browning compounds responsible for the aroma of fresh roasted coffee |
|
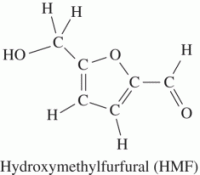
HMF is an intermediate of the Maillard reaction and is found in corn syrup |
|
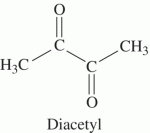
Diacetyl is the compound produced from fats in butter and gives the strong buttery and butterscotch flavor. This compound is also a common product of browning through the fission Maillard reaction |
|
Methyl furans (some containing sulfurs—not shown here) are responsible for meaty flavors and are often the result of heating onion and garlic-like flavors. This is different from the caramelization flavor and aroma also found with onions |
Shorter chain products are formed by the fission pathway. This pathway involves single electron compounds called free radicals. While complex in nature, this pathway can result in shorter 2- and 3-carbon compounds. These compounds can react with other sugars or amino acids, giving rise to interesting compounds including diacetyl (the smell of butter) and acetone (a fruity smell).
The dehydration pathway involves the loss of a water and nitrogen (amine) group. This pathway can produce ring structures including the furan-based molecules, which give roasted coffee smells, flavors of meat, and if sulfur is present a burnt bread odor and flavor.
A third pathway providing a large number of aromatic and brown-colored compounds especially in roasting is the Strecker degradation. This reaction can occur among the products of the Maillard reaction but can also occur apart from Maillard chemistry. In this reaction, dicarbonyl species (two C O bonds) produced from breakdown of the Amadori product or elsewhere react with free amino acids to create aminoketones. These aminoketones condense and after oxidation (typically from oxygen in the air), pyrazines are formed. Pyrazines have strong flavors that add to the complexity of the Maillard bouquet.
O bonds) produced from breakdown of the Amadori product or elsewhere react with free amino acids to create aminoketones. These aminoketones condense and after oxidation (typically from oxygen in the air), pyrazines are formed. Pyrazines have strong flavors that add to the complexity of the Maillard bouquet.
In addition to their fabulous aromas and flavors, many of the final products from any one of these pathways can further react in polymerization reactions to create brown, high molecular weight pigments called “melanoidins.” These pigments provide the dark color for beer and coffee as well as the brown color associated with browned meats and breads (Box 6.2).
6.6 CARAMELIZATION: BROWNING BEYOND THE MAILLARD
Caramelization involves another complex set of nonenzymatic browning reactions. While both Maillard reactions and caramelization create brown flavorful compounds, caramelization differs from the Maillard reaction in several important ways. Firstly, the reaction takes place between sugar molecules; no proteins or amino acids are involved in this reaction. Secondly, the required temperature needed to induce the reaction is higher for caramelization (starting at 320–356°F/160–180°C) than the Maillard reaction (typically 12–284°F/100–140°C). Finally, the reaction for caramelization is an oxidation reaction leading to long polymers of sugars with some shorter volatile compounds.
With the exception of most candies, which are primarily sugar and fats, most cooked dishes have Maillard and caramelization reactions happening concurrently, and both contribute to flavor. Braised beef, beer, and chocolate are just a few foods and drinks that owe much of their taste to a combination of Maillard reactions and caramelization. Caramels made solely from sugars are used in puddings or desserts including nougats, caramel brittles, and custards, while the process of caramelizing sugars in protein-containing foods contributes to the flavors one associates with browned onions, carrots, and coffee/cocoa beans. Browning describes both flavoring processes, but it is important for a careful cook to know the difference. Once both processes are understood, there is a world of opportunity to create interesting flavors and foods.
Caramelization is the process of degrading mono- and disaccharide sugars to form large polymers and volatile flavor molecules. These reactions happen when sugar is heated at or above its melting point. Like the Maillard reaction, caramelization requires heat to drive the complex, multistep reaction. The first part of the reaction is to melt the solid sugar crystals into liquid form. Then the sugars are further heated where they begin to lose OH groups and C atoms as smaller, often volatile compounds. Further heating will induce the collisions necessary to join many of the degraded sugars into large, viscous, dark-colored polymers. It is possible to heat the sugar too much; extended heating will create a burned, carbon (charcoal-like) residue. Complete oxidation (burning) of sugars will convert all the carbon, hydrogen, and oxygen atoms to CO2 and H2O—leaving nothing behind.
For solid, crystalline sugars—in contrast to liquid sugars like honey or syrups—there are two common techniques to make caramel, the wet and the dry methods. The dry method is simple and begins with a slow heating of solid sugar, such as sucrose (i.e., table sugar), until the solid is turned to a liquid. As a solid is heated, eventually the noncovalent intermolecular interactions holding the molecules in the crystal lattice are defeated, and the sugar molecules melt and begin to move more freely. This melting occurs as the sugar molecules acquire enough energy to overcome the intermolecular forces binding them into an orderly crystalline lattice. Melting does not occur instantaneously, because molecules bound to each other in a lattice must absorb the energy and then physically break the intermolecular forces. Typically, the outside of a crystal will melt faster than the inside, as it takes time for the heat to penetrate.
For many years, the conventional thinking was that addition of heat first melted sucrose into a liquid, and then the liquefied sugar broke down via chemical changes to a caramel product. However, Dr. Shelly Schmidt, a food chemist at the University of Illinois, published interesting research findings in a series of papers [4–7]. This work demonstrates that when heated slowly, some of the sugar molecules begin to chemically change into caramel products while still in a solid, crystal lattice form. This chemical change seems to take place before the molecules separate from solid into liquid form and not after melting takes place, as is the case for most solids. Dr. Schmidt showed that at lower temperatures and with a slow rate of heating, the sugar molecules began to chemically change and liquefy at 145°C/290°F while still solid, instead of at the expected temperature (190°C/380°F) and with higher rates of heating used to make the liquid sugar. The caramelized sugars made at this lower temperature did not contain the undesirable compounds made by caramelization at higher temperatures. Some caramelization products are flavorful and have a pleasing odor, while others impart a bitter and burnt taste to food. Anyone who has caramelized sucrose too quickly at high temperatures understands the poor tasting, burned result. While still being investigated this is a shift in the traditional understanding of how solids make the transition from a solid to melted form, although how this all happens (what scientists call “mechanism”) has yet to be determine. Dr. Schmidt’s work impacts how food is processed to control the kinds of caramelization products formed. Applying this new understanding of how to make one set of compounds over the other by the careful application of heat and time yields more predictable results (Box 6.3).
The first step of caramelization chemistry requires reducing sugars. The reaction proceeds as reducing sugars lose a water molecule (dehydration) and rearrange into new compounds. This reaction requires the presence of the carbonyl (C O) of a reducing sugar. Sucrose, as you may recall, is made of glucose and fructose and is not a reducing sugar. Therefore sucrose must first be converted to its individual components: glucose and fructose. This can happen in a few ways. In solution, sucrose will very slowly break down into glucose and fructose monosaccharides. This process is increased by heat (thus the melting of sucrose, or dissolving sucrose prior to heating) and accelerated by acids including tartaric and lemon juice. Two enzymes—invertase from plant cells and sucrase from animal cells—can also break the glycosidic bond of sucrose into glucose and fructose. The process of converting sucrose to its individual units is called inversion and will produce what is commonly called “inverted sugar.” An examination of the temperatures required to start the caramelization reaction reflects the need to “invert” sucrose. Table 6.3 also explains why foods cooked and baked with fructose (including honey) brown faster and darker than those with sucrose or glucose.
O) of a reducing sugar. Sucrose, as you may recall, is made of glucose and fructose and is not a reducing sugar. Therefore sucrose must first be converted to its individual components: glucose and fructose. This can happen in a few ways. In solution, sucrose will very slowly break down into glucose and fructose monosaccharides. This process is increased by heat (thus the melting of sucrose, or dissolving sucrose prior to heating) and accelerated by acids including tartaric and lemon juice. Two enzymes—invertase from plant cells and sucrase from animal cells—can also break the glycosidic bond of sucrose into glucose and fructose. The process of converting sucrose to its individual units is called inversion and will produce what is commonly called “inverted sugar.” An examination of the temperatures required to start the caramelization reaction reflects the need to “invert” sucrose. Table 6.3 also explains why foods cooked and baked with fructose (including honey) brown faster and darker than those with sucrose or glucose.
Table 6.3 Temperature Required for Caramelization of Sugars.
| Fructose | 110°C |
| Glucose | 160°C |
| Sucrose | 160–180°C |
| Maltose | 180°C |
Like the Maillard reaction, the diversity of products of the caramelization reaction is immense and depends on the sugars involved in the reaction and the chemical environment of the reaction. As we saw in Chapter 1, after the loss of water, individual sugar molecules can then react with the reducing C O of another sugar to form a sugar anhydride. This is a condensation reaction, also called a dehydration reaction (Fig. 6.13), where two molecules are joined in a covalent bond by the loss of a water molecule. The result is a larger molecule. Depending on the time, temperature, and process, this reaction will continue with some sections of the molecule breaking from the parent molecule (fragmentation) forming small aromatic molecules. Fructose will begin this reaction forming difructose anhydride (Fig. 6.14). As the sugar polymer continues to grow by dehydration condensation, there can be transformation of carbons, oxygen, and hydrogen molecules into different structures. Heating the sugars in acidic or basic conditions leads to a range of different products and colors where the protons and hydroxyl ions serve as catalysts for the chemical rearrangement of the intermediate caramelization compounds.
O of another sugar to form a sugar anhydride. This is a condensation reaction, also called a dehydration reaction (Fig. 6.13), where two molecules are joined in a covalent bond by the loss of a water molecule. The result is a larger molecule. Depending on the time, temperature, and process, this reaction will continue with some sections of the molecule breaking from the parent molecule (fragmentation) forming small aromatic molecules. Fructose will begin this reaction forming difructose anhydride (Fig. 6.14). As the sugar polymer continues to grow by dehydration condensation, there can be transformation of carbons, oxygen, and hydrogen molecules into different structures. Heating the sugars in acidic or basic conditions leads to a range of different products and colors where the protons and hydroxyl ions serve as catalysts for the chemical rearrangement of the intermediate caramelization compounds.

Figure 6.13 Polymerization reaction.
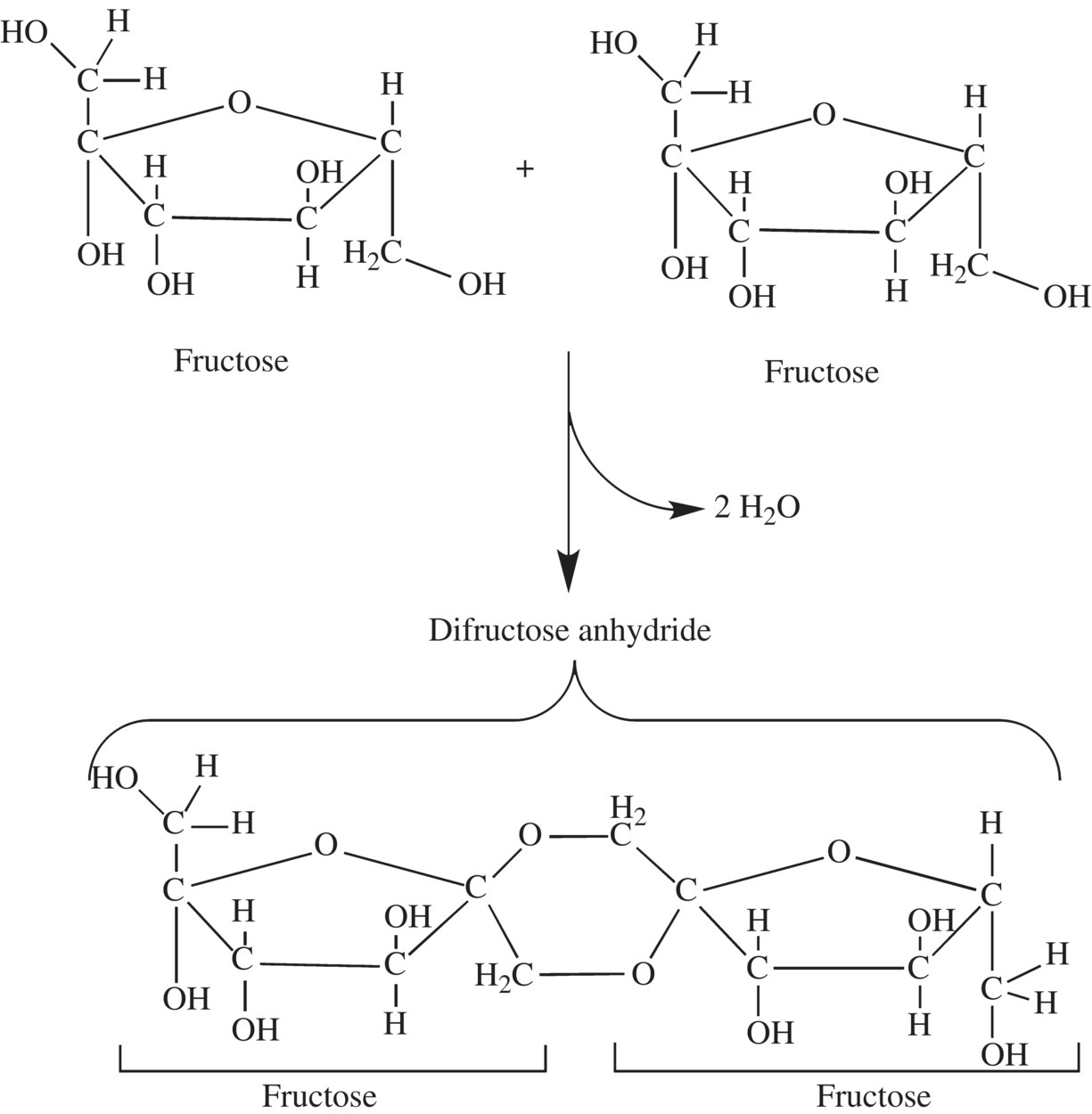
Figure 6.14 Dehydration reaction forming difructose anhydride.
Several organizations including the US Food and Drug Administration classify the colors and flavors of the process based on the chemical solutions used during caramelization. When a sugar solution is heated under different conditions, the resulting caramel colors are grouped into four classes for different products and uses.
The caramelization reaction will continue to form both small volatile compounds and very large polymers of the sugar monomers. Initial heating will decompose and dehydrate the sugar monomers, and a population of the compounds will also give rise to smaller volatile molecules. Fructose and, to a lesser extent, glucose can decompose to the nutty, buttery smelling 2-hydroxymethylfurfural (HMF). Diacetyl is another small volatile decomposition product responsible for the buttery flavor and smell of caramel. Heating fructose, glucose, or sucrose for 1–2 h causes a loss of one water molecule per carbohydrate monomer. Continued heating of the sugar leads to additional loss of water molecules and condensation of the dehydrated sugar monomers into larger polymers. The building blocks of these polymers arise from modification of fructose to difructose dianhydrides (DFA; Fig. 6.14).
Continued heating of sugars will create a complex mixture of small volatile molecules and larger nonvolatile polymers. Using a sensitive analytical technique called mass spectrometry, over 300 distinct major compounds and up to several thousand compounds have been identified. Often polymers of up to six fused glucose or fructose molecules are chemically altered to produce the large, nonvolatile, colored caramel products. While the complete chemistry of the reactions is not well understood, caramelization polymers are classified into three complex mixtures first described based on their size, color, and flavor characteristics (Fig. 6.15):
- Caramelans are a class of caramelization products with the formula C12H18O9 formed by the loss of 12 water molecules. Caramelans have a bitter taste and a nutty-brown color.
- Darker brown compounds, the caramelens (C36H50O25), are produced after an additional hour of cooking where each sugar loses about eight water molecules and condenses with other reactants.
- Longer heating of sugar will result in a darker and deeply flavored compound made of larger particles called caramelin (C125H188O80). This molecule is very dark and does not dissolve well in water.
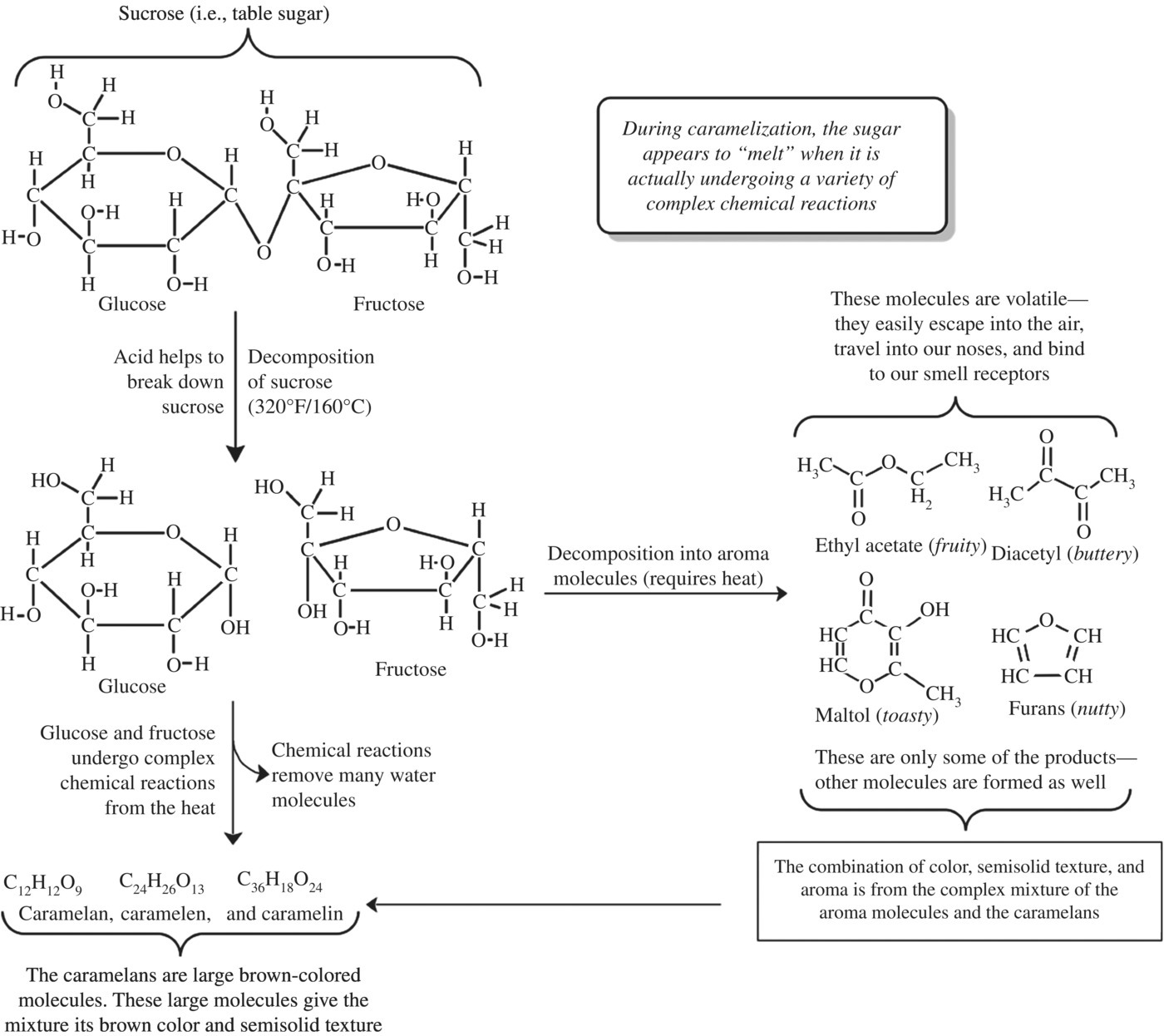
Figure 6.15 The many reactions of caramelization.
Caramel is one of the oldest and most widely used flavor compounds with over 50 metric tons of caramel produced and consumed in foods each year. As indicated in Table 6.4, the products of caramelization are used frequently in food and drink for many different properties including taste. For example, colas owe their color and some of their flavor to caramelization products. Some caramelization products can act as emulsifiers and help keep molecules in solution. Several of the caramels found in coffee and honey have antibacterial properties. One compound produced by both caramelization and via the Maillard reaction, 4-methylimidazole, or 4-MEI for short, is found in dark beers, coffee, and roasted meats and is part of the caramel coloring and flavor in some cola soft drinks. While a natural reaction during heating of these foods and drinks, some people have a concern about the dark flavored molecules. When mice were exposed to very high concentrations of 4-methylimidazole—levels that far exceed the average consumption of a human—a moderate but statistically significant increase in tumors was documented in the animals. This has led several to be concerned about drinking colas, coffee, or dark beer! While California now includes 4-MEI on its list of probable carcinogens, one would have to drink tens of thousands of cans of the soft drink a day to approach the level of concern for the compound!
Table 6.4 Classification of Caramel Used in Food and Beverage.
| Class | Classification | Preparation | Uses |
| I | Plain or spirit caramel | No ammonium or sulfur compounds can be used | Distilled high alcohol spirits such as whisky |
| II | Caustic sulfite caramel | High pH (NaOH) and sulfite (SO32−) used | Beer, malt bread, sherry, malt vinegars |
| III | Ammonia caramel | No sulfites but ammonium compounds can be used | Beer, sugar candies, soy sauce |
| IV | Sulfite ammonia caramel | Both sulfite and ammonium can be used | Widely used for soft drink and in acidic solutions |
Complicating our understanding of the process and products of caramelization is that these reactions take place side by side with the Maillard reaction. Many of the intermediates from both browning reactions can react to form new aromas, flavors, and colors. However, the distinction between the two processes remains simple. Maillard reactions take place at lower temperatures than caramelization, and the reaction is between a reducing sugar and amino group of an amino acid or protein. Caramelization proceeds at higher temperatures than the Maillard reaction and is the degradation and combination of two or more sugar monomers.
A review of grandmother’s caramel recipe shows how much kitchen chemistry she was conducting when making the soft chewy candies. Her basic recipe included sugar, milk or whipping cream, and butter. Her selection of sugar (corn syrup, honey, table sugar, or fructose) would provide different starting monosaccharides producing different possible flavors. Sometimes she would add vanilla extract for an additional flavor, but now we know she didn’t really need to as both browning reactions create plenty of flavors. At times, she might mix all the ingredients together before heating, producing a mixture of the two browning reactions. At other times her instructions would direct her to heat the sugar before adding the milk or cream, allowing the caramelization reaction to produce its compounds without competing with Maillard browning. Both methods will produce a fine candy, but the flavor profile of each as we now understand is very different. We now know why the directions also included heating the mixture to either 120°C/250°F for lighter colored candy or to 180°C/350°F for a darker more richly flavored candy. Addition of milk, evaporated milk, or cream provided fats for texture and proteins for Maillard browning. And the combination of caramelization and the Maillard reaction produces flavors including diacetyl, the odor, and flavoring of butter and butterscotch! Whew, what a scientist she was!
Finally, a discussion of caramelization would not be complete if one only considered table sugar! The cooking of many different foods produces caramelization reactions. Green coffee beans are roasted providing caramel color and flavors from the sugars in the coffee bean or seed. Roasting coffee beans first requires drying the bean at 190–220°C/375–425°F. Steam produced from this heating swells the seed and produces the “first crack” as the bean splits its shell. Roasting for longer and higher temperatures will produce a darker bean with strong flavors. Of course, the flavors are a combination of browning reactions. Seeds contain many complex and simple sugars as well as proteins and amino acids to react and form colorful flavors and odors. Braising tough cuts of meat is a way to slow cook beef, poultry, or pork for a more tender result. Braised meat is typically browned on the stovetop by Maillard reactions before placing it into a tight fitting pot or kettle with a small amount of liquid. Addition of sugars (corn syrup or brown sugar) and vegetables high in sugar content such as carrots and onions are often used to add flavor as the meat is simmered at or near the temperature of caramelization.
Carrots, corn, potatoes, and onions have high sugar content and are great vegetables for caramelization. Adding a small amount of fructose to low sugar vegetables like broccoli and cauliflower allows them to brown during roasting. Baking potatoes doesn’t get hot or dry enough to induce caramelization at 150°C/300°F. However, slices of potatoes brown nicely if warmed enough to release the steam (unpacking the complex starches) and then heated sufficiently to get the caramelization rolling at a higher temperature. Carrots and onions are particularly high in the reducing sugars fructose and glucose, which promotes browning reactions. Remember to get the reaction right; it takes time and high temperatures (up to 356°F/180°C) for the reactions to occur. Directions for onion and carrot caramelization stress the importance of heating the vegetable for nearly an hour. Of course, an impatient cook might use too much heat and burn the food instead of caramelizing it. Again, this is where you can use a little science of cooking to ease the process. Remember that caramelization takes place between reducing sugars that can form open or straight chains. The equilibrium between closed and open chain is shifted to the open form in basic or alkaline conditions. A sprinkle of baking soda helps to raise the pH of onions and carrots to the open chain, where the higher concentration of the open chain reducing sugar can dehydrate and polymerize into caramel goodness. Of course, the Maillard reaction is also taking place at a higher rate at alkaline pH, and more flavor is always better, isn’t it?
6.7 ASCORBIC ACID BROWNING
Left alone, fresh-squeezed citrus fruit juices will brown in under 3 days. Sugars and amino acids both play a key role in this browning; however, there is another reaction involving vitamin C, also called ascorbic acid. Ascorbic acid is a water-soluble weak acid, also known as vitamin C, naturally found in citrus fruits and many vegetables. Citric fruit juice is especially rich in ascorbic acid and the breakdown of ascorbic acid generates brown pigments and off-flavors over time. This browning, like caramelization and the Maillard reaction, is a nonenzymatic browning process.
Now that we’ve studied the complex chemistry of amino acid and sugar browning reactions, it will surprise no one to learn there are also many possible products for the breakdown of ascorbic acid, and they depend upon the conditions of the juice during degradation. Up to 17 different ascorbic acid breakdown products have been identified so far, but two of these products are present. There are two key degradation products of ascorbic acid, furfural and dehydroascorbic acid. In the presence of oxygen (aerobic conditions), ascorbic acid is converted to dehydroascorbic acid and then further degraded to brown compounds. Dehydroascorbic acid produced in ascorbic acid degradation then reacts in the Maillard pathway taking part in the Strecker reaction producing brown pigments. In acidic and low (anaerobic) conditions (that found in concentrated, canned, and fresh citrus juice), furfural is one of the main components of ascorbic acid breakdown. This compound is then further degraded to form brown pigments after reacting with amino acids in the fruit juice (Fig. 6.16).
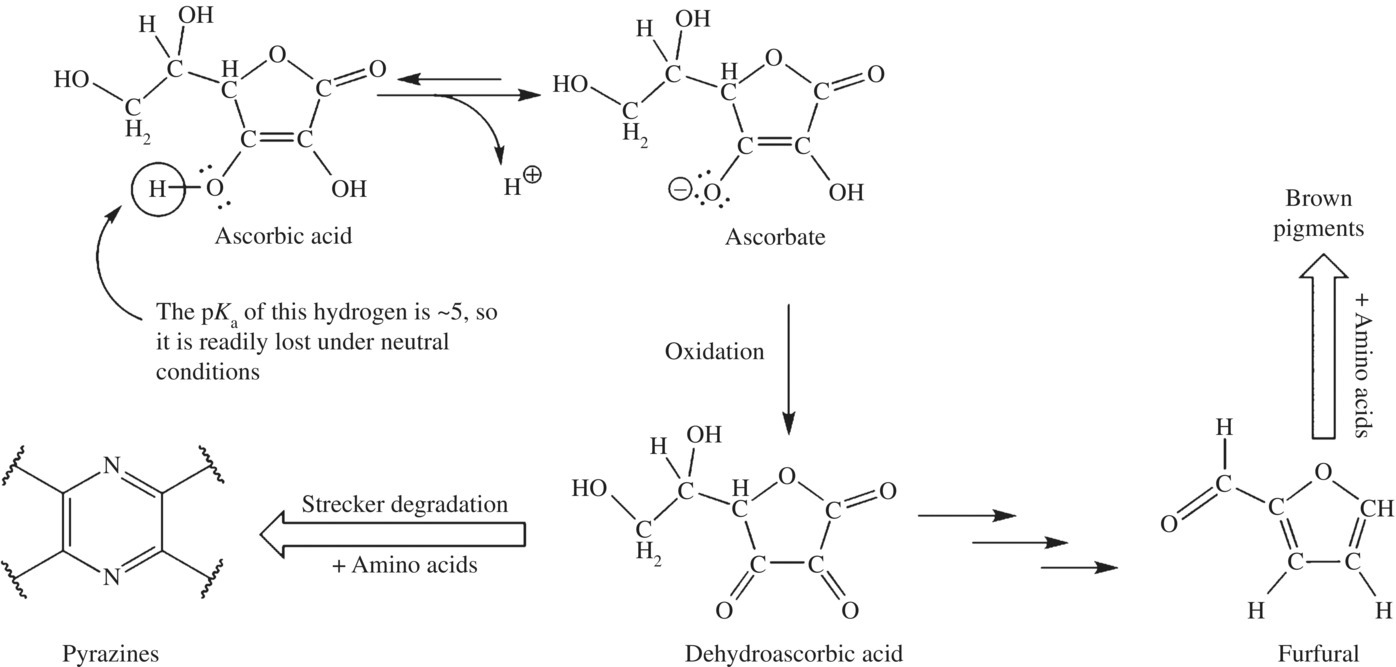
Figure 6.16 Ascorbic acid and browning reactions common to fruit juices.
Ascorbic acid degrades to dehydroascorbic acid and furfural, both of which, through very different pathways, react with amino acids to create bitter off-flavor brown pigments, which decrease the quality of the juice. Several researchers in the food industry have found that treating the juice to remove amino can stop the final few steps of each browning pathway. While the browning reaction can take place with or without oxygen, it proceeds more slowly in the absence of oxygen. Reducing head space (the gap above the juice in the container) or exchanging the air for nitrogen gas can limit exposure of the juice to oxygen. One of history’s oldest food additives, sulfites are used to compete with ascorbic degrading reactions, sparing the vitamin from breakdown. For example, sodium metabisulfite (Na2S2O4) is used in wine, beer, and other foods and reduces browning severalfold even when juices are heated to over 212°F/100°C. The sulfite inhibits the oxidation of ascorbic acid to dehydroascorbic acid, which prevents later reactions leading to the production of the brown pigments.
6.8 ENZYME-CATALYZED BROWNING
Cut into an apple or banana and leave it on the counter for an hour or so. The once enticing food is now brown and bitter tasting. Vegetables, fruits, and even some shellfish will all brown after being cut or damaged. Unlike caramelization or the Maillard reaction, this browning reaction is unpleasant and catalyzed by enzymes not heat. The browning occurs when plant compounds called phenols react with oxygen to form large, polyphenolic brown pigments.
Phenols are rings of carbon atoms with alternating single and double bonds and with at least one hydroxyl group ( OH) coming off the ring. For example, the amino acid tyrosine, found in almost every protein in nearly every organism, is a phenol. Polyphenols are compounds with several of these phenol rings bonded together (Fig. 6.17).
OH) coming off the ring. For example, the amino acid tyrosine, found in almost every protein in nearly every organism, is a phenol. Polyphenols are compounds with several of these phenol rings bonded together (Fig. 6.17).
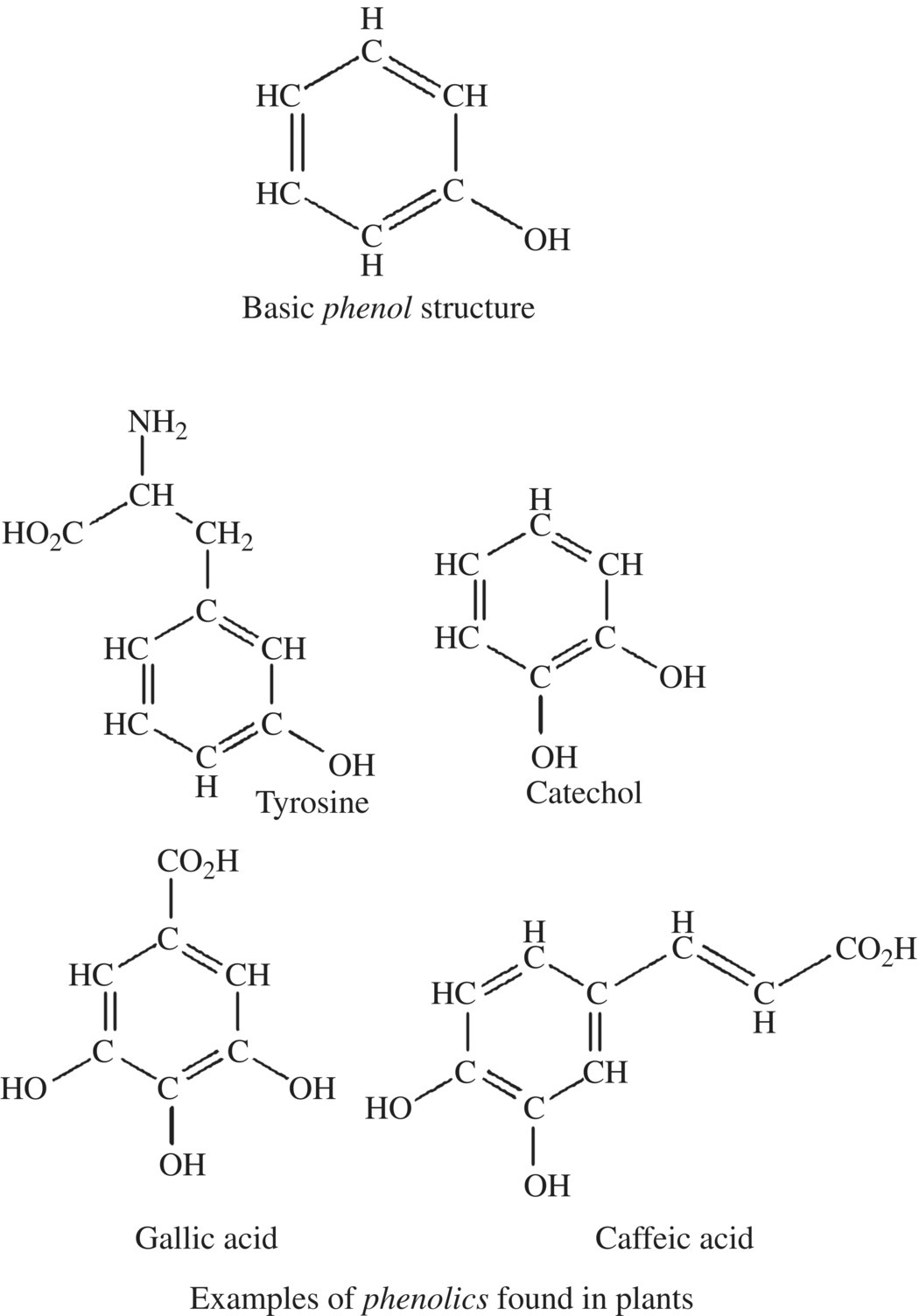
Figure 6.17 Phenols.
Phenols and polyphenols are found in nearly every living organism. They have interesting biological roles in a range of organisms including mold, bacteria, algae, and higher animals. There are over 8000 different phenolic compounds in plants with just as many diverse roles including the color and flavors of fruit, bark, and leaves. Tannins are a family of polyphenols found in plants; they provide color and function as pesticides for the plant, and some tannins are plant hormones regulating root development, fruit softening, and flowering. Tannins also have an astringent taste. Tannins are responsible for the dry pucker that accompanies a sip of strong black tea or red wine.
Common phenolic compounds found in food and browning reactions are grouped into four classes. The simple phenols include tyrosine and mono- and diphenols such as catechol, resorcinol, and dihydroquinone. The addition of a carboxylic acid group distinguishes another class called the phenolic acids. Gallic acid is found in tea, grapes, and apples and was used for dyes, possibly even as an invisible ink. One of the acid phenols is chlorogenic acid, a key substrate for polyphenol oxidase (PPO) in food browning especially potato, peach, and prunes. Chlorogenic acid is also present at relatively high concentration in coffee beans, and the metabolic products of the acid may be responsible for some of the heart health benefits of drinking coffee. Flavonoids are a diverse group of polyphenols providing color and the building blocks for plant hormones. Several of the polyphenols of the flavonoid family are targets for PPO and are responsible for the astringency of some fruits as well as color of dark teas.
Fruit and vegetable browning of phenol and polyphenol compounds is controlled by an enzyme that binds and starts the series of reactions that lead to bitter brown compounds (Fig. 6.18). The basic function of enzymes is to decrease the activation energy needed for a reaction to occur. Enzymes are proteins, which bind very specifically to their reactant (also called a substrate) producing a single product. The browning reaction in fruits and vegetables is complex, but like other browning reactions we’ve studied, the first step is key for the production of the final compound.
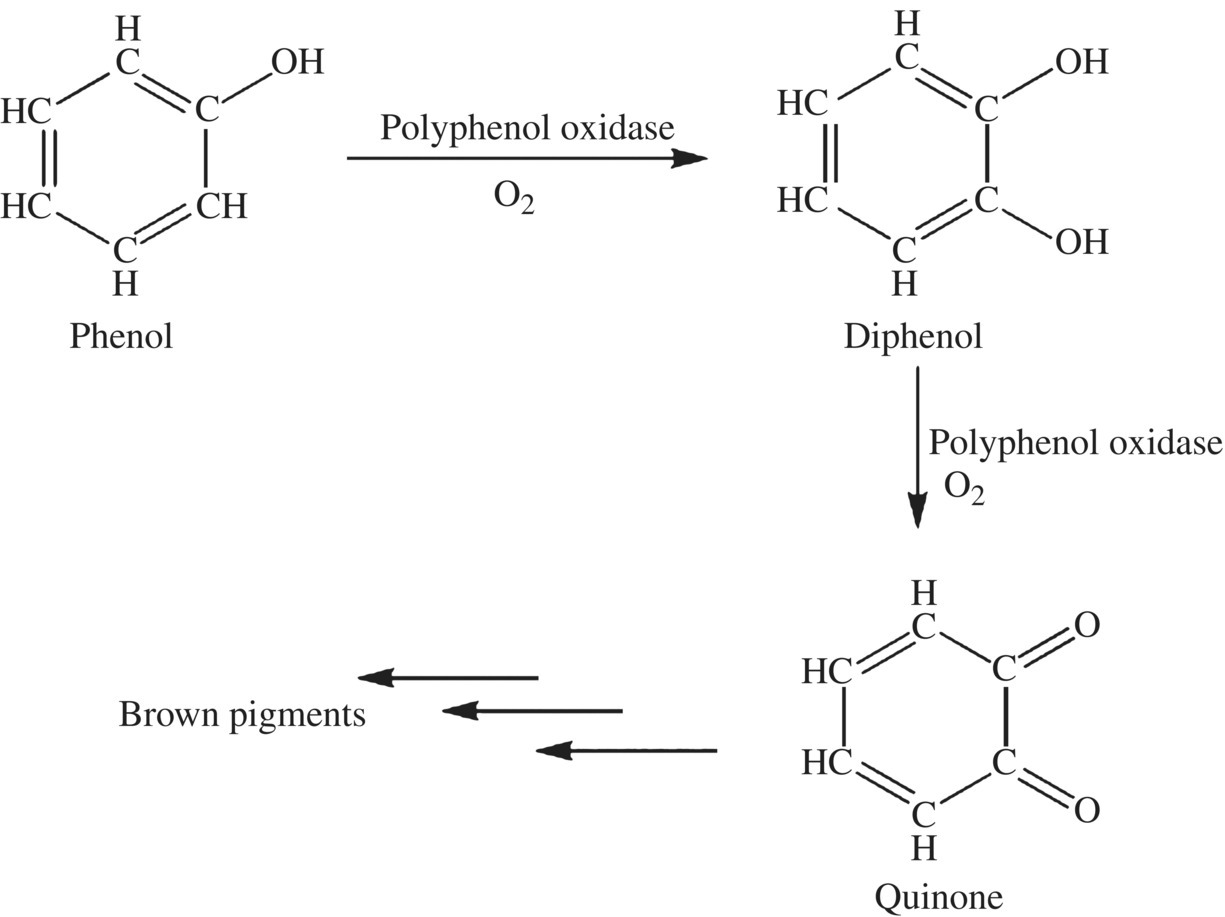
Figure 6.18 Enzyme-catalyzed browning. The actions of polyphenol oxidase on many of the phenols found in plants lead to the addition of oxygen atoms and a brown bitter tasting pigment.
The critical first step in enzyme-catalyzed browning starts with a phenolic compound, the enzyme PPO, and oxygen.
There are several common phenolic compounds found in food and browning reactions. The simple phenols include tyrosine and mono- and diphenols such as catechol, resorcinol, and dihydroquinone (Figs. 6.17 and 6.19). The addition of a carboxylic acid group to a phenol distinguishes the phenolic acids. Gallic acid is found in tea, grapes, and apples and was used for dyes, possibly even as an invisible ink. One of the acid phenols is chlorogenic acid, a key substrate for PPO in food browning especially potato, peach, and prunes. Chlorogenic acid is also present at relatively high concentration in coffee beans, and the metabolic products of the acid may be responsible for some of the heart health benefits of drinking coffee. Flavonoids are part of a diverse group of polyphenols providing color and the building blocks for plant hormones. Several of the polyphenols of the flavonoid family are targets for PPO and are responsible for the astringency of some fruits as well as color of dark teas.
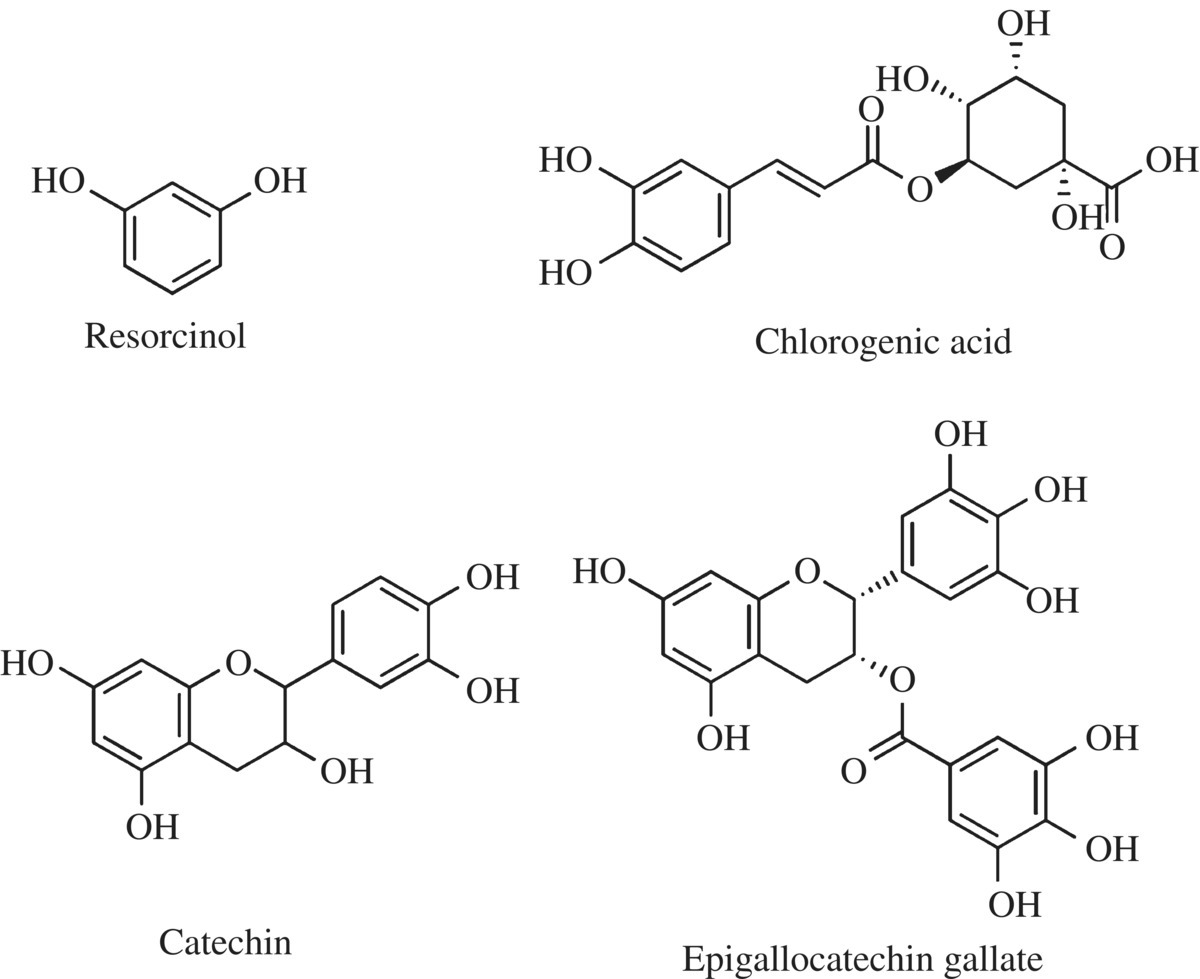
Figure 6.19 Examples of plant phenols.
PPOs are enzymes found in all plants and animals that catalyze the formation of quinones from mono- or diphenolic molecules. These enzymes are responsible for the first reactions in the browning process. There are three types of PPOs (tyrosinase, catechol oxidase, and laccase); each is a different protein made from different genes, but each protein binds and catalyzes the same basic reaction. Tyrosinase catalyzes the addition of an  OH group onto monophenols and then converts the product diphenol to quinones. Tyrosinase is also responsible for the synthesis of the neurochemical dopamine and melanin, the pigment in the skin, hair, and eyes. Laccase also converts diphenols to quinones. Catechol oxidase (aka diphenol oxidase) converts diphenols, and to a lesser extent monophenols, into quinone compounds. All three enzymes are called PPOs in the scientific literature and for the rest of this chapter. The bottom line is that these enzymes alter phenols to quinone compounds, which starts the browning reaction producing complex melanin products.
OH group onto monophenols and then converts the product diphenol to quinones. Tyrosinase is also responsible for the synthesis of the neurochemical dopamine and melanin, the pigment in the skin, hair, and eyes. Laccase also converts diphenols to quinones. Catechol oxidase (aka diphenol oxidase) converts diphenols, and to a lesser extent monophenols, into quinone compounds. All three enzymes are called PPOs in the scientific literature and for the rest of this chapter. The bottom line is that these enzymes alter phenols to quinone compounds, which starts the browning reaction producing complex melanin products.
Plant cells are filled with a variety of phenols and polyphenols and two or more PPO enzymes. Yet, vegetables and fruits don’t brown until cut or damaged. This is because the plant cell has done a brilliant job of separating the substrate from the enzyme, until a reaction is beneficial to the plant. PPOs are made as inactive longer proteins (called preproteins), and many of the phenols used in the browning reaction are sequestered in cellular compartments called vacuoles. Browning is slow to progress in intact vegetables and fruits, and only with aging does the reaction significantly occur. However, once cut or damaged, the PPOs are activated into shorter, mature enzymes, and the cellular destruction of the knife releases the phenols from the vacuoles allowing phenols to mix with enzyme and oxygen, beginning the process of browning (Fig. 6.20).
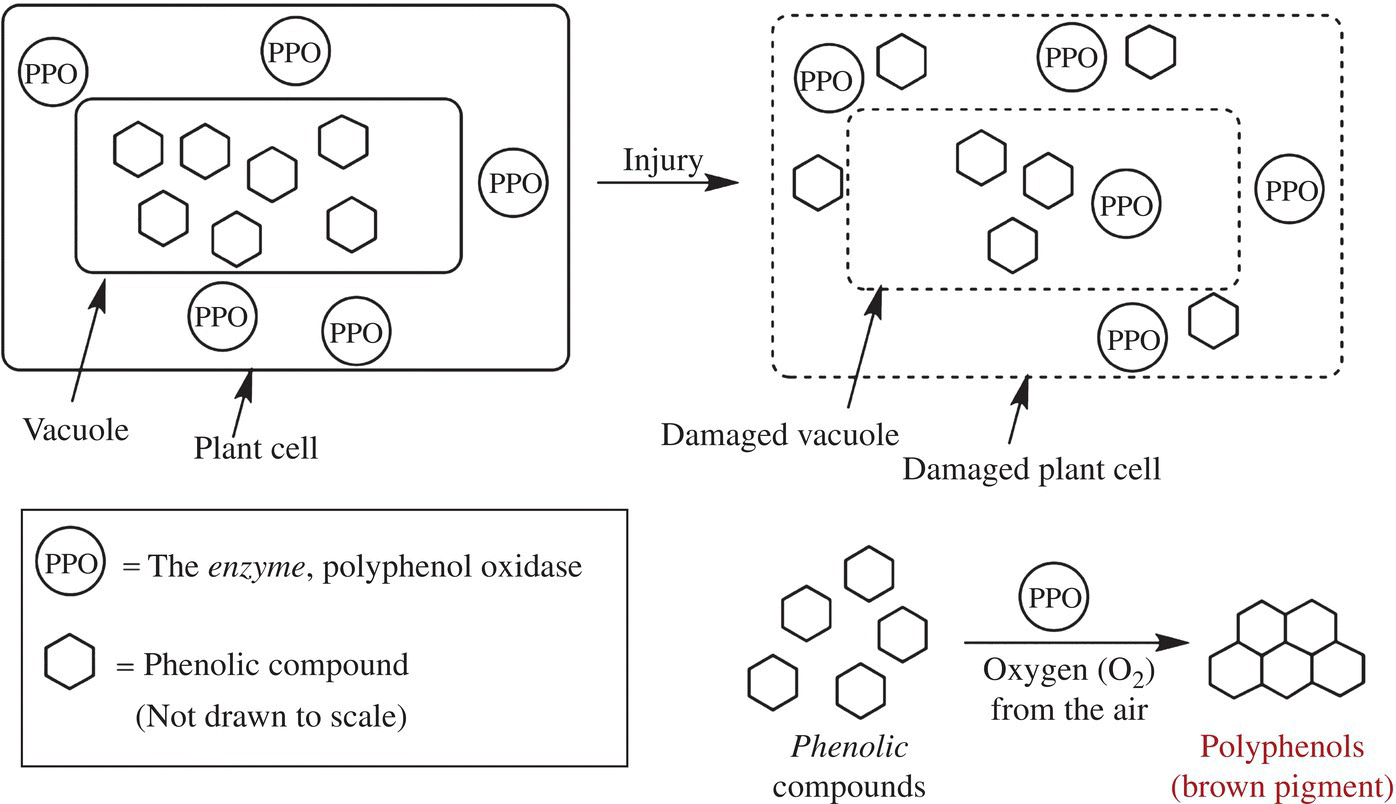
Figure 6.20 The browning of plants. Damage to plant cells (fruits or vegetables) causes the release of the enzyme polyphenol oxidase (PPO), which can then interact and react with its substrate—the phenolic compounds found on the plant cell wall.
Monophenols react with oxygen and PPOs to form diphenol compounds. The oxidase enzymes will then use a second molecule of oxygen to oxidize each alcohol of the diphenol to a carbonyl group (C O) of the product quinone. Once formed, the quinones react with other compounds including amino acids to form large complex brown polymers. In animals, the brown melanin pigments found in skin and hair are formed by similar reactions that start with tyrosine.
O) of the product quinone. Once formed, the quinones react with other compounds including amino acids to form large complex brown polymers. In animals, the brown melanin pigments found in skin and hair are formed by similar reactions that start with tyrosine.
The natural role for phenols and PPO enzymes depends on the plant in question. Some polyphenols are used by plants to generate plant hormones, and others are used to strengthen the cell walls, while other polyphenol products create color and UV light protection. Tea leaves, figs, and cocoa plants all utilize PPO-catalyzed synthesis of polyphenols to create a darker color and new flavors. There are several interesting scientific studies that suggest the brown compounds produced by PPO enzymes are beneficial to the plant. When insects bite or other physical damage occurs to the plant, the resulting wound provides an opportunity for microbial pathogens to infect the plant tissue. In this case, polyphenols are a defense mechanism, a kind of immune response for the plants. In tomato, tobacco, and other plants, wounding activates the PPO enzymes and the resulting enzyme-catalyzed browning produces polyphenol compounds that inhibit the parasitic growth of mold, fungus, and other microbes while promoting wound closure. The bitter taste of the brown compounds is thought to discourage further attacks on the plant by insects and herbivores.
In addition to being less attractive to insects, brown food is bitter and unattractive to humans. Nearly half of our vegetable and fruit crop each year is lost and wasted due to browning after the food is harvested and before it gets to our table. This represents a significant waste of farming resources and agriculture. One interesting approach to avoid such loss is the creation of genetically modified organisms (GMO)—in this case, a genetically modified plant that will not produce brown polyphenols. The Arctic Apple, a GMO product, is a nonbrowning apple where the eight genes for PPO enzymes have been silenced. The approach used to reduce the production of PPO in the apple uses a technique called RNA interference. This molecular therapy is being developed as a treatment for cancer, tissue transplantation, liver disease, as well as other human diseases.
Unless you can use RNA interference to stop browning food in your kitchen, the average person will have to use an understanding of enzymatic browning to block the reaction at home. To start, recall that inhibition of PPO will block the browning compounds from forming. Polyphenols, oxygen, and enzyme: stop any one of these three components and you’ve just created a way to keep food from browning. Limiting access to oxygen is easy but only mildly effective. Immersing vegetables and fruits in water prior to and during cutting reduces the availability of oxygen to bind to the enzyme. Potatoes, which quickly brown, are prepared this way for industrial production of French fries and potato chips. Some have experimented with packaging fruit in carbon dioxide gas to eliminate oxygen, but the results were limited in success. There are several compounds that bind and block the ability of PPO to react with polyphenols. As classic enzyme inhibitors, many of these compounds have nearly the same structure as the phenolic substrates of PPOs. These substrate-mimicking compounds compete with the natural enzyme substrate inhibiting the polyphenol reaction. Guaiacol, resorcinol, and phloroglucinol are all examples of such “competitive inhibitors.”
Blanching vegetables and some fruits is an effective way to inhibit the enzyme and soften the cell wall. Blanching is the short submersion of a vegetable in boiling water for a short period of time. Some, but not all, PPO enzymes are denatured and rendered inactive after short periods of 212°F/100°C heat of boiling water. Longer exposures are needed to kill several forms of PPO and would result in cooking the vegetable (Box 6.4).
Some acids are also effective inhibitors of PPO. The pH optimum for PPO activity lies between 6.0 and 6.5. If the pH ranges greater than 1 unit above or below these points, almost no activity remains. Phosphate buffers are sometimes used as they can buffer the pH at either end of this range and are considered food safe. Citric acid, malic acid, and ascorbic acids are effective inhibitors of the enzyme; less for their acidic properties, but instead for other properties. Both citric acid and malic acid can bind and strip copper away from PPO enzymes. Copper is absolutely required by the enzyme. However, each acid will only partially inhibit the production of brown compounds in apples and other fruits or vegetables (Fig. 6.21). While ascorbic acid is involved with browning in citric juices, the acid is an effective inhibitor of PPO function and does not impart taste to food like malic and citric (sour and tart). Ascorbic acid will react with quinone compounds, reversing the PPO reaction. As long as ascorbic acid is present in excess, the quinone products cannot continue the reaction to form brown pigments. However, once the ascorbic acid is consumed in the reaction, browning can occur.
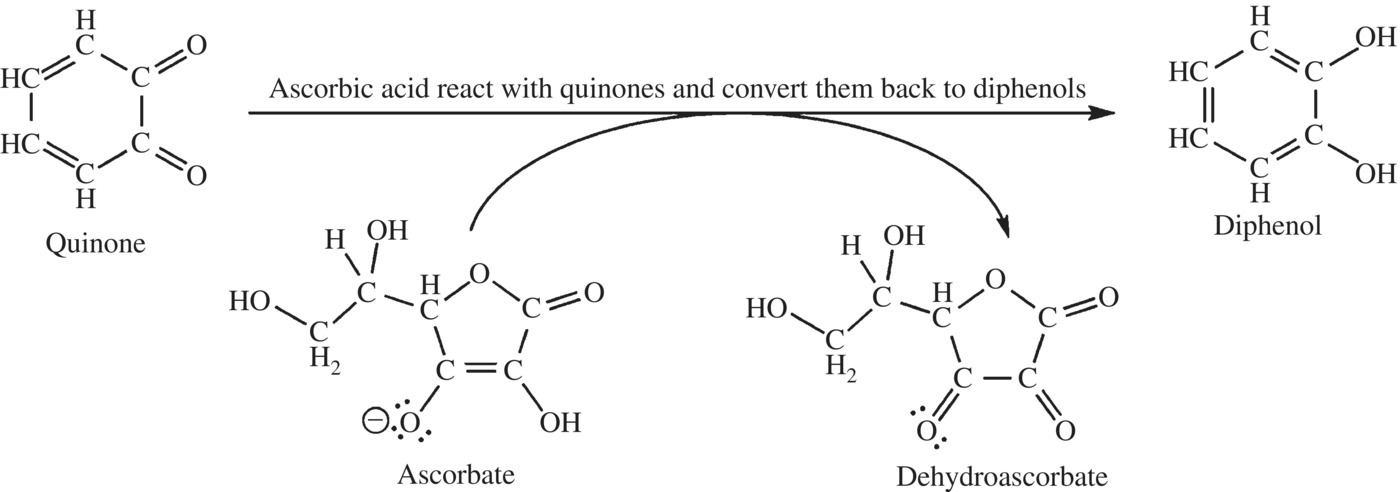
Figure 6.21 Ascorbic acid is an inhibitor of PPO. Ascorbic acid can reverse the production of the brown pigment forming quinones.
Calcium chloride is another modest inhibitor of PPO activity. When used alone, any one of these PPO inhibitors is moderately effective, but combinations of inhibitors are even better at preventing browning. Several combinations of the acids mentioned previously and calcium ions have a strong effect on PPO activity. NatureSeal is a company producing a range of products to inhibit browning in packaged salads, fruit, and other produce. NatureSeal is a mixture of calcium and ascorbic acid. Used as a dip, the combination can inhibit browning in apples for up to 14 days. Using this technology, one fast-food restaurant sold nearly $0.5 billion in just sliced apples in 2010 (Box 6.5).
REFERENCES
- [1] Hodge, J. (1953) Chemistry of browning reactions in model system. J. Agric. Food Chem., 1: 928–943.
- [2] Ashoor, S.H. and Zent, J.B. (1984) Maillard browning of common amino acids and sugars. J. Food Sci. 49: 1206–1207.
- [3] Somoza, V. and Fogliano, V. (2013) 100 years of the Maillard reaction: why our food turns brown. J. Agric. Food Chem. 61: 10197–10197.
- [4] Schmidt, S.J. (2012) Exploring the sucrose–water state diagram. Manuf. Confect. 92(1): 79–89.
- [5] Lee, J.W., Thomas, L.C. and Schmidt, S.J. (2011) Investigation of the heating rate dependency associated with the loss of crystalline structure in sucrose, glucose, and fructose using a thermal analysis approach (Part I). J. Agric. Food Chem. 59: 684–701.
- [6] Lee, J.W., Thomas, L.C., Schmidt, S.J. (2011) Investigation of thermal decomposition as the kinetic process that causes the loss of crystalline structure in sucrose using a chemical analysis approach (Part II). J. Agric. Food Chem. 59: 702–712.
- [7] Lee, J.W., Thomas, L.C., Schmidt, S.J. (2011) Can the thermodynamic melting temperature of sucrose, glucose, and fructose be measured using rapid-scanning differential scanning calorimetry (DSC)? J. Agric. Food Chem. 59(7): 3306–3310.
- [8] Roos, Y.H., Franks, F., Karel, M., Labuza, T.P., Levine, H., Mathlouthi, M., Reid, D, Shalaev, E., Slade, L. (2012) Comment on the melting and decomposition of sugars. J. Agric. Food Chem. 60(41): 10359–10362.
- [9] Lee, J.W., Thomas, L.C. and Schmidt, S.J. (2012) Response to comment on the melting and decomposition of sugars. J. Agric. Food Chem. 60(41): 10363–10371.