Disease is not of the body, but of the place.
—SENECA
EPIDEMIOLOGISTS ARE BIOLOGICAL sleuths called in when epidemics strike to try to ascertain how and why they occurred. Robin Marantz Henig makes the interesting observation in her book A Dancing Matrix that, were a seasoned African epidemiologist to be asked, “What constitutes an epidemic worth looking into?” he would most likely answer, “The death of one white person.”
Life is cheaper in third-world countries than in developed nations. Preventable diseases that incapacitate and kill hundreds of millions of people throughout Africa, Asia, and Central and South America are allowed to run amok. The World Health Organization (WHO) estimated that in 1998 two billion children suffered acute respiratory infections and more than four million died—the vast majority in emerging countries. Eighty percent of these deaths were due to bacterial lung infections, largely preventable or curable, such as pneumonia. Much of the world’s population still suffers and dies from diseases contracted through unclean water. These include bacterial and viral diarrheas as well as diseases caused by parasites. Every year 1.7 billion people suffer from infections acquired through water contaminated by parasites or their animal hosts.
Strictly speaking, a parasite is an organism that lives on or in another organism upon which it feeds. By definition, then, bacteria and viruses are parasites, and the diseases they cause are parasitic diseases. Traditionally, however, biologists have used the term parasite in a more limited sense, to refer to larger, more complex living things, such as protozoa and worms. And tradition is hard to break. Even today the designation parasite is usually meant to exclude viruses and bacteria.
Once believed to be tiny animals because they are motile and not green, protozoa now belong to their own distinct kingdom. They are the simplest of the parasites. Although bacteria and protozoa are both one-celled organisms, the cell of a protozoan is larger and vastly more complex. Anyone who has taken biology in high school has seen protozoa under a microscope. They include the often-studied amoeba and paramecium. Protozoa are ubiquitous, being found in virtually any watery environment—ponds, rivers, lakes, fish tanks.
Most protozoa are free-living—they have no hosts—and cause people and other animals no harm. Unfortunately several are parasitic, and a few present very serious health problems to infected individuals. Way back in 1875 a protozoan was first shown to be a pathogen. It was Entamoeba histolytica, the dysentery amoeba familiar to anyone foolish enough to drink unbottled water in countries lacking proper sewage disposal. Two hundred years before that, the great microscopist Antonie van Leeuwenhoek noticed a protozoan flitting about in his feces. It was a “wee animalcule” of the Giardia genus. Since Leeuwenhoek’s discovery, Giardia lamblia and another protozoan, Cryptosporidium, have been implicated in many outbreaks of painful intestinal infections and severe diarrhea worldwide. They are found routinely in drinking water that has been polluted by human or animal feces. In April 1993, four hundred thousand Milwaukee residents came down with cryptosporidiosis; forty-four hundred were hospitalized. It seems that the protozoan is resistant to standard chlorination procedures used for drinking water and can, in fact, survive in full-strength Clorox. Chalk one up for the microbes.
Intestinal parasites aside, it is the bite of tropical insects that spreads the worst protozoan diseases—those that continue to plague third-world countries.
Sleeping sickness is one such deadly disease, caused by a small fishlike protozoan called a trypanosome, which swims in the blood by lashing a whiplike tail or flagellum. The illness is transmitted through the bite of a tsetse fly and eventually leads to loss of consciousness and death when the organisms invade the spinal cord and brain. Sleeping sickness is also a disease of livestock such as cattle, and in many areas where the disease is endemic it can literally wipe out the homegrown meat supply. Some experts feel that global warming can greatly expand the habitats of tsetse flies, prevalent throughout Africa, and hence the distribution of the disease they carry. In general global warming is threatening to expand the territories of many insect vectors.
In the year 2000 there were twelve million cases of leishmaniasis worldwide. It is a debilitating and often fatal affliction caused by the leishmania protozoan—an organism “no weightier than an eyelash,” to quote noted parasitologist Robert Desowitz. And the insect that brings this deadly scourge to humanity, a tiny blood-sucking sand fly, is not much weightier than an eyelash. But the two team up to produce epidemics that kill hundreds of thousands of people in India, China, northern Africa, and Brazil.
The most deadly form of the disease is visceral leishmaniasis, also called kala-azar. It has wiped out two-thirds of some hard-hit villages. The symptoms of kala-azar are unremitting fever, skin that turns dark gray, severe anemia, and a spleen and liver that are grossly enlarged, distending the abdomen. If left untreated the disease has a mortality rate approaching 100 percent. Leishmania protozoa actually live inside white blood cells that should swallow up and kill the protozoa. They do swallow them up but are unable to finish the job. The leishmania protozoa, in fact, thrive inside white blood cells. Over time they destroy these cells and, much like HIV, wreak havoc on the immune system. A depressed immune system allows other deadly bacterial infections such as pneumonia or dysentery to overcome the body’s defenses. Speaking of HIV, this deadly virus has been found increasingly coinfecting people along with the leishmania protozoan. Although most people bitten by leishmania-carrying sand flies do not develop disease, coinfection with HIV quickly evolves into severe leishmaniasis.
The standard treatment for leishmaniasis is an intravenous therapy using highly poisonous antimony compounds. If the disease doesn’t kill the patient, the cure will. Recently another, safer drug—miltefosine—administered orally has proven effective in animal tests. A possible vaccine also looms on the horizon. Then again, the mere possession of a successful vaccine means very little as diseases such as measles dramatically demonstrate.
It has even been suggested that leishmaniasis did in the dinosaurs. Although not overly compelling, the evidence cannot be dismissed summarily. Remember Jurassic Park, a clever bit of science fiction in which dinosaurs were cloned using preserved insects that had sucked the blood of dinosaurs some seventy or eighty million years earlier? Dinosaurs, of course, cannot be cloned, but the fossil record reveals that they were indeed parasitized by insects, several species of which were sand flies. And DNA comparison of different leishmania species (some of which infect lizards) indicates that they also have been evolving for about eighty million years. It is therefore not unreasonable to speculate that prehistoric sand flies harbored leishmania protozoa that they injected into dinosaurs. If this is true, and it contributed to dinosaur extinction, then we owe quite a lot to the sand fly and leishmania protozoan, for it was extinction of the dinosaurs that allowed primitive mammals and eventually humans to evolve.
The word malaria means “bad air,” air that, according to the ancient Greeks, made people deathly ill with intermittent fever. But no one at the time could identify the precise cause of illness. Hippocrates believed miasmas—deadly mists—were at play, somehow upsetting the balance of the body’s four humors: blood, phlegm, black bile, and yellow bile. Sound silly? Well, the ancient Chinese attributed paroxysmal fever—malaria—to an imbalance or disharmony between the yin and yang, two opposing life forces in the body. And the great taxonomist Carolus Linnaeus, to whom we owe the genus-species method of scientifically naming organisms, hypothesized that tiny suspended particles of clay in drinking water clogged blood vessels, thereby bringing on the disease. Not until 1898 would the true cause of malaria be identified—the bite of a mosquito infected with a deadly protozoan.
That protozoan is Plasmodium falciparum, and it might well kill more humans than any other pathogen … save perhaps the bacillus of tuberculosis or the AIDS virus. Malaria is endemic throughout most of the world, but because so many deaths occur in third-world countries, annual fatality figures are rough estimates at best. Even so, the loss of life is staggering, somewhere between one and two million people, probably closer to the latter. Perhaps three hundred million people are diagnosed each year with clinical malaria. In severely malarious areas people are bitten several hundred times a year by Plasmodium-infected mosquitoes. Contracting the disease is almost inevitable. Robert Desowitz, in his book The Malaria Capers, offers us a vivid description of a young pregnant woman in the throes of a malarial “rigor”:
The attack came with surprising ferocity. In a moment the nausea yielded to a chill that made Amporn feel her body was encased in a shroud of ice. Under the blazing tropical sun she shook uncontrollably. During this “freezing” rigor, Amporn’s temperature had risen to 104°F. After an hour of tooth-chattering shakes the rigor abated and for a few moments in the eye of this parasitic storm Amporn thought she might yet live. The brief respite was followed by a feverishness that was as intense as the sensation of cold she had experienced during the rigor. Amporn’s temperature was now 106°F. Her senses reeled; consciousness blurred. She crawled into her house and collapsed upon the cool dirt floor, her sarong sodden with the sweat pouring from her burning body.
Ironically, it was the burning fever, so horrible a symptom of the disease, that convinced Viennese neurology professor Julius Wagner von Jauregg to inject people with the malaria protozoan. The year was 1917, and the seemingly insane idea was a new and revolutionary therapy for the treatment of late-stage syphilis. It was believed that the corkscrew-shaped heat-sensitive bacteria of syphilis would be killed by malaria-induced fevers. And it worked. A relatively harmless strain of malaria protozoan was injected into late-stage syphilis victims, those already showing signs of neurological damage. When the fever struck, it stopped the progression of the disease right in its tracks. People were not necessarily cured, but they got no worse. In this way tens of thousands of people were spared a certain, agonizing death, and in 1928 von Jauregg received a Nobel Prize for his insanity. Several years ago malaria therapy was even suggested for patients suffering Lyme disease, since it is caused by a spirochete very similar to the one responsible for syphilis. (For more on Lyme disease, see Chapter 5, “New Kids on the Block.”)
The rigor, or shakes, so symptomatic of malaria is a consequence of the way the insidious malarial protozoan lives its life. It is a very complex life that involves both man and insect. It is, however, a life we must understand in intimate detail if we are to conquer this “mother of fevers,” as the ancient Chinese called malaria.
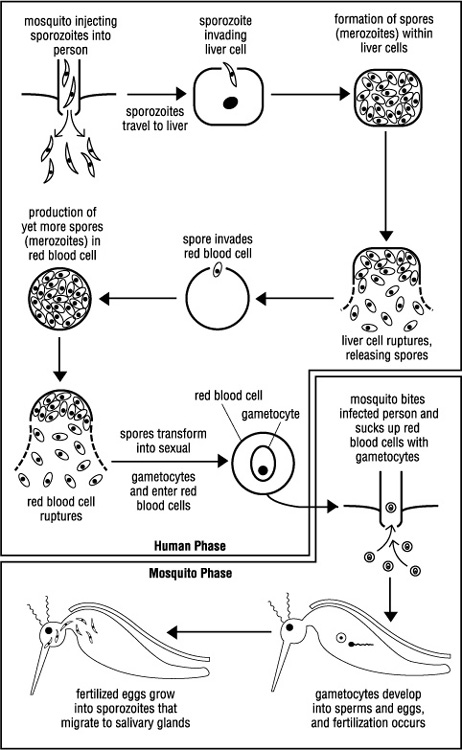
Life cycle of a killer. To be precise, there are four species of the Plasmodium protozoan that infect humans and cause malaria, but one, P. falciparum, is particularly virulent and lethal. It accounts for over 95 percent of all malarial deaths. All four, however, have a similar life cycle.
Human infection begins with a bite from a female anopheles mosquito. Only anopheles mosquitoes are vectors of human malaria, and only females bite. The male, to quote Dr. Desowitz, “flies about in a lifelong pursuit of sex and nectar.” (In many ways it is not unlike the human male.) With her bite the infected mosquito injects thousands of threadlike plasmodia called sporozoites into her victim. These sporozoites head straight for the liver, where each one infects a liver cell. In the liver cell the sporozoite rounds up and divides repeatedly, producing up to forty thousand spores. Spore production takes two weeks, during which time there are no signs of illness. But the seeds of malaria are being sown.
The first clinical attack—sweating and high fever—occurs when the liver cells burst, releasing their myriad spores into the bloodstream. Each spore invades a red blood cell within which it lives for a while, feeding on the cell’s hemoglobin and growing large. Finally there is a shattering of the spore into smaller fragments—an asexual means of producing yet more spores (scientific name merozoites). When the red blood cell bursts, the merozoites are released and invade new blood cells. Repeated cycles of red blood cell infection and rupture ultimately lead to a chronic malarial condition or death.
There is an amazing synchrony to the invasion and bursting of red blood cells. For reasons not fully understood, it seems that millions of infected blood cells burst simultaneously, releasing their merozoites. It is this timed, en-masse slaughter of red blood cells that brings on a bout of the shakes.
After several rounds of blood cell infection and reinfection some of the merozoites get down and dirty, transforming into male or female reproductive cells—the gametocytes. This is where the human phase of the life cycle ends. When an anopheles mosquito sucks up malaria-infected blood, she also sucks up millions of gametocytes. In her stomach the gametocytes develop into eggs and sperms, and fertilization occurs.
In a final act of brilliance, the fertilized eggs grow into threadlike sporozoites and make their way to the salivary glands, completing the cycle (see Figure 1, Plasmodium Life Cycle). The mosquito is now a deadly vector of malaria.
Figure 1 Plasmodium Life Cycle

Interestingly, the disease sickle-cell anemia and its milder form, sicklecell trait, offer sufferers protection against malaria. They do this by deforming the red blood cells, thereby preventing the protozoa from flourishing within them. Small wonder sickle-cell is so common an inherited disease in highly malarious parts of the world.
Malaria kills more than a million African children each year. Perhaps another million die in South America and Asia. It is in these wet, tropical continents that the mosquitoes of malaria thrive. Surprisingly, however, throughout much of recorded history malaria was very much a European disease. Spreading northward from Africa, it invaded Greece and Italy in A.D. 79, devastating the very susceptible populations of these nations. The Roman Empire, so formidable in its day, was crippled by malaria. Roman soldiers carried the disease as far north as England and Denmark. For two millennia it plagued much of Europe, until its eradication in the late 1940s.
Malaria was a relative newcomer to the New World, not arriving there until Columbus and other European explorers brought their parasites with them to the Americas. But it did not take long for malaria to establish itself and become a serious endemic disease from southern Chile to Montreal. President George Washington suffered from malaria. So did Abraham Lincoln. During the Civil War half of the white troops and four-fifths of the African-American soldiers in the Union Army contracted malaria annually. Altogether at least a million soldiers suffered from malaria during the Civil War. In the 1930s, six hundred thousand Americans contracted malaria domestically. Atlanta, Georgia, was a hotbed of malaria, which was very much an American disease until about 1950, when eradication efforts wiped it out. From a unit created during World War II to control malaria, the CDC was born.
Prevention. How does one proceed to wipe out malaria? The most successful methods attacked not the protozoa but the mosquito. Eliminate their breeding sites, the standing water in which their young grow and develop, and you’ve eliminated the problem. Easier said than done. To quote a Tanzanian medical director, “These little creatures can breed thousands of offspring in a puddle the size of a hippo’s foot.”
Yet eliminate their breeding sites is exactly what Benito Mussolini did in the 1930s. Through elaborate canals he drained a region known as the Campagna, the wet, fertile area extending westward from Rome to the sea. It was, however, a monumental effort achieved at great expense. Such antimalarial endeavors were not even feasible for the rest of Italy, let alone the rest of the world. Obviously other methods of pest control were necessary and were employed with varying degrees of success.
A tried-and-true course of action for killing anopheles larvae (known as wrigglers because they wriggled in the water) was to lay down a film of oil on the breeding pools. The oil clogged larval breathing tubes, and the larvae died. Unfortunately, oiling had to be repeated quite often or mosquitoes returned.
Then it was noticed that young larvae pretty much swept any floating particle of the proper size into their mouths. Why not dust the water with a chemical that, upon ingestion, would kill the young anophelines? By about 1920 a suitable larvicide was found—Paris green. Cheap, effective, and noninjurious to other animals, it could even be broadcast over large, swampy areas by airplane.
At about the same time Paris green was being deployed in Italy and other European nations, a tiny fish of the genus Gambusia was generating excitement among malariologists. Native to North America, it demonstrated a voracious appetite for mosquito larvae. Why not ship gambusia to highly malarious countries, where it could be used to stock anophelesinfested waters?
Attacking anopheles at its water-dependent larval stage was not the only strategy employed against these malaria vectors. Before the turn of the nineteenth century, homes were often fumigated with smoke to kill adult mosquitoes. It was not easily done, however, and ruined much of the household furnishings. In 1910 an aqueous solution of pyrethrum, a powder derived from chrysanthemum flowers, was put into a spray bottle and became the first widely used insecticide. It worked very well in killing adult anopheles mosquitoes as well as other disease-carrying insects. In fact a 1944 epidemic of typhus, transmitted by a louse, was quelled by dusting the inhabitants of wartorn Naples, Italy, with pyrethrum. Regrettably, pyrethrum suffered the same shortcomings as those that had preceded it; namely, the insecticide had to be applied to surfaces at least once a week to be effective.
In their desperation to achieve some degree of success in combating malaria, scientists became remarkably inventive. One epidemiologist set up a ring of twenty pigsties around a malarious village, believing that the mosquito vectors would choose swine to feed on in lieu of humans. He even boasted some success, but like Paris green, oiling, and pyrethrum, it just didn’t get the job done.
Then, in 1941, Paul Müller registered a new insecticide with the Swiss patent office—the office where Albert Einstein had worked while formulating his greatest theories. From this historic building would emerge patent no. 226150, the ultimate weapon against malaria—DDT. Dichlorodiphenyl-trichloroethane was everything anyone could ask for in an insecticide. It was inexpensive to manufacture. It appeared harmless to other animals, including humans. But most important, it killed all manner of insects and kept on killing. DDT was sprayed on walls, floors, and ceilings. Like pyrethrum, it was also dusted on people to kill the lice that spread typhus. But unlike pyrethrum, it had tremendous staying power. A DDT-treated surface would kill for up to six months. Needless to say, malariologists were ecstatic.
Early trials with DDT proved so successful that in 1947 the U.S. Congress appropriated $7 million for malaria eradication within its borders. By the early 1950s not one case of malaria could be found in America.
At long last eliminating malaria worldwide seemed more than just a dream. Malariologists predicted that massive and continued spraying in countries where malaria was endemic (most of the world) would completely stamp out the mosquito vector. Malaria would go the way of smallpox.
With such unbridled optimism WHO, backed mainly by U.S. dollars, embarked on a very ambitious five-year campaign to wipe malaria off the face of the planet. It began in 1958, and it almost worked. Malaria-ridden Sri Lanka, for example, went from one million cases in 1955 to just eighteen in 1963. Incidence of the disease was reduced dramatically in other countries as well.
The principal reason for DDT’s failure to completely eradicate malaria was the anopheles mosquito’s ability to develop resistance to the insecticide. Malariologists were experiencing what bacteriologists had encountered after the discovery of antibiotics. In time DDT-resistant populations of mosquitoes developed.
Growth of such resistant populations should have surprised no one. Individuals within a large population show varying degrees of DDT resistance. A select few might even be totally unaffected by the insecticide. Routine spraying would kill the vast majority of susceptible insects, leaving resistant survivors to go forth and multiply. To further complicate the issue, environmentalists began complaining that DDT was harming fish and fowl, demanding that its use be curtailed. Unfortunately, as soon as rigorous use of DDT was discontinued, the mosquitoes returned with a vengeance. Mortality due to malaria in 1993 was at a historic all-time high in Africa, and it is currently the only major disease other than AIDS and tuberculosis that is spreading steadily. A whopping 40 percent of the world’s population is at risk for contracting malaria. And the returning plague seemed to be more lethal than ever. A new wrinkle had been added. A cerebral form of malaria had developed and was attacking the brain, killing people with alarming suddenness. What, if anything, could be done?
Treatment. Scientists did have one other weapon in their antimalarial arsenal—drugs with which to treat patients. Robert Koch, the brilliant microbiologist of tuberculosis fame, often said of malaria, “Treat the patient, not the mosquito.” Unfortunately, in his day (late nineteenth century) there was only one drug—quinine.
Quinine, a preparation from the bark of the cinchona tree, had been used as an antimalarial for centuries by natives of South America. It was and still is an excellent antimalarial drug. Regrettably, however, it was and still is fairly toxic, often causing deafness when used in dosages necessary to kill plasmodium. Nonetheless, it was used universally and extensively until the 1930s, when other, less harmful derivatives were synthesized. Chief among them was chloroquine.
Shortly after its development chloroquine became the drug of choice for treating malaria. It would sit on the dinner table in stately homes alongside the salt and pepper shakers. Hospitals and the military handed out chloroquine as if it were candy. Undoubtedly chloroquine saved countless millions of lives through the 1940s and ’50s, but its widespread, indiscriminate use would also be its undoing. By the early 1960s physicians around the world were beginning to report cases of malaria that would not respond to chloroquine. Plasmodium falciparum was evolving, adapting, becoming resistant to the drug. Soon drug-resistant malaria—not only to chloroquine but to other pharmaceuticals as well—became the rule rather than the exception. The magic bullet had lost its magic.
Today the search continues for drugs that will effectively halt Plasmodium. In July 2000 the FDA approved Glaxo Wellcome’s Malarone for prevention and treatment of malaria. A synthetic drug called pyronaridine, developed in China, shows great promise. And there are others. Meanwhile, chloroquine has been largely replaced by mefloquine as the standard treatment for malaria. It is even recommended prophylactically for American travelers to Africa.
In retrospect the international effort to eradicate malaria might have been better served if conducted differently. Had all endemic areas been drenched with massive and repeated doses of DDT, had the entire tropical world been saturated simultaneously with chloroquine, the insect and/or its protozoan parasite might not have survived the onslaught. But that did not happen, and as soon as eradication efforts abated, the mosquito rebounded triumphantly.
Vaccination. By 1965 it became obvious to all concerned that the tag team of DDT and chloroquine was not going to snuff out malaria. WHO, looking for yet another magic bullet, turned its energies and financial resources to developing a vaccine. It had worked for Jenner and smallpox as well as a dozen other diseases. Why not malaria?
Why not? Because the malaria protozoan is vastly more complex than the viruses and bacteria that succumbed to vaccination. To begin with, plasmodium’s life cycle involves at least six antigenically distinct types of organisms. Against which one should a vaccine be generated? And, unlike bacteria or viruses, scientists could not successfully grow and study plasmodium in a culture dish until 1977.
Nonetheless, grants were awarded liberally to any scientist with a laboratory, some anopheles mosquitoes, and a desire for a Nobel Prize. Soon vaccines began to appear. Some were made from infected red blood cells, ruptured and treated with formalin to attenuate the protozoa. Mosquitoes carrying plasmodium were irradiated with x-rays in hopes of beating their protozoa into a suitable vaccine. Plasmodium itself was grown in culture, and different concoctions of whole and fragmented protozoa were melded into vaccines. Yet by 1990 all attempts to provide effective immunity through vaccination had largely failed.
Then the big guns were brought in. The 1980s had seen major advances in molecular biology—advances such as DNA analysis, genetic engineering, improved methods of protein synthesis, and antibody production. With these powerful and sophisticated tools researchers prodded and poked poor plasmodium. By 1992 the first glimmer of hope appeared. Dr. Manuel Patarroyo of Colombia created a vaccine from proteins he had manufactured in the lab, proteins that were identical to those of the protozoan. Now, in effect, he had a synthetic parasite, or at least part of one, that could induce an immune response without causing disease. Trial tests conducted first in South America and later in Africa initially proved effective, reducing by nearly 40 percent the rate of infection in vaccinated subjects. In subsequent trials, however, the vaccine gave almost no protection against malaria to inoculated children. Back to the drawing board. Today we have anti-sporozoite vaccines and anti-asexual blood stage vaccines and transmission-blocking vaccines under investigation.
Meanwhile other scientists are picking apart the genes of anopheles and plasmodium, learning which ones produced resistance to DDT and chloroquine. Hopefully this knowledge can be used to make the deadly disease vulnerable once again to drugs and insecticides.
Is there yet hope for humanity in its quest to make the world malariafree? Yes—but it won’t come soon, it won’t come easily, and it won’t come cheaply. For the foreseeable future, the smart money is on the ingenuity and evolutionary prowess of P. falciparum.
Protozoa are not the only parasites that find humans particularly hospitable. Ticks, lice, and mites are other examples of parasites—external parasites, which feed on people without entering their bodies. Most important, however, as far as human disease and death are concerned, are two groups of internally parasitic worms. They are the flatworms and the roundworms, simple animals that have given up free-living for freeloading.
Flatworms are the reason I do not eat steak tartare, for lurking in raw beef may be small, immature tapeworms. Fish and pigs are also carriers of tapeworm, so eating improperly cooked fish or pork is ill advised as well. Many Jewish people used to get tapeworm after eating a delicacy known as gefilte fish, prepared by boiling ground pike, carp, whiting, or other fish that has been seasoned and molded into balls. When gefilte fish is cooked insufficiently, tapeworm larvae can survive and find their way to the diner’s digestive tract. There is even danger in preparing gefilte fish, for cooks have a tendency to taste-test the partially cooked fish as it simmers.
Tapeworms derive their name from the fact that the adult worm is long and ribbonlike—sometimes very long. That baby worm you accidentally ingest, once it attaches to your small-intestine wall (by means of hooks or suckers on its head), may grow to be sixty feet long (more than eighteen meters). And the worm is a very interesting creature in the way it conducts its life—the ultimate parasite, if you will. It has no mouth and no trace of a digestive system. They have been lost over time as the animal adapted to a way of life in which it merely soaks up digested nutrients like a sponge. What the tapeworm does possess is a highly developed reproductive system, for perpetuation of the species is the name of the game. It is hermaphroditic, and each individual worm has many testes and ovaries. Eggs are self-fertilized and then released in huge numbers with the feces. Some of these eggs will find a cow or pig to parasitize, and the life cycle of infection continues. Fish tapeworms follow a somewhat more circuitous route, first infecting tiny copepods, which are then eaten by the fish.
As with most parasites of the digestive system, proper sanitation and sewage disposal are essential to eradication of the disease. And, of course, thorough cooking of meat and fish will kill the larvae and prevent infection.
Tapeworms may make you weak, undernourished, and anemic. They can cause diarrhea and digestive system problems. In some instances the worm travels to other organs, leading to serious complications. When doctors autopsied one woman who had died of epileptic convulsions, they found her brain riddled with small encysted tapeworms. Usually, however, infection with these worms is not life-threatening or even particularly debilitating. Many times one becomes aware of tapeworm infestation only when part of the worm breaks off and is seen in the stool. Elimination of the worm involves taking drugs orally that literally put the worm to sleep. Only then will the head release its grip on the intestine wall and allow itself to be flushed out with a purgative. If the head is not removed, the worm simply grows back.
Tapeworms are certainly a nuisance and a potential danger. As agents of suffering and death, however, they pale in comparison to another class of parasitic flatworms, the flukes.
Adult flukes are much smaller than tapeworms, but the devastation they cause is far greater. One type of fluke, the blood fluke or schistosome, is particularly nasty, causing a level of global debilitation by parasite that is second only to malaria. An estimated two hundred million people in seventy Asian, African, and South American countries harbor schistosomes. Three-quarters of a million will die this year. No one really knows, with any degree of accuracy, how many people suffer from schistosomiasis because the range of symptoms is so enormous. The worm weakens and kills in many unpleasant ways. Heart disease, epilepsy, kidney failure, cirrhosis of the liver, lung degeneration, and even cancer can result from infection with the blood fluke. It is an excruciating and debilitating disease affecting mostly school-age children.
Often the first sign of schistosomiasis occurs at puberty. A strange and scary thing happens—urine suddenly and inexplicably turns red with blood. So common is the event, signaling the onset of the disease, that among boys it has come to be considered a sort of male menstruation throughout much of Africa.
Bloody urine is a manifestation of the way in which the parasite conducts itself within its human host. Infection begins when a person, often a young child bathing or a farmer wading through his rice paddy, enters water polluted with tiny schistosome larvae. These tadpolelike critters, the size of pinheads, penetrate the skin, causing an initial and transient rash. Disappearance of the rash, however, does not indicate the end of infection. After a brief respite the worm enters the bloodstream and travels first to the liver, where it matures, and then to a vein. Here the schistosome takes up residence. The vein it chooses is a function of the schistosome species. One species heads for veins of the lower intestine. Another settles in the upper intestine. Yet a third kind makes its home in the veins surrounding the urinary bladder.
Male schistosomes are of a decent size, stout and about three-quarters of an inch long. The worm attaches to the vein’s inner wall with two suckers on its head. Females, narrower and shorter, nestle themselves into grooves that run down the length of the male. Here they remain in monogamous conjugal bliss for the rest of their lives, which may be as long as thirty years. For most of those thirty years females will crank out an enormous number of eggs—about thirty-five hundred daily.
It is these eggs that are the chief source of pathogenicity in people. They do not remain harmlessly in the venous home of their parents but begin to burrow through the blood vessel. The eggs must make it to the interior of the bladder or intestine, where they will be discharged with the urine or feces. Only then can their life cycle continue, a life cycle that requires that they next parasitize a water snail. If there are no proper snails—and they have only a day to find one—the flukes cannot develop into the forms that will infect humans. The schistosomes will perish, and the chain of transmission will be broken. Unfortunately there are lots of snails in the feces-contaminated waters of the third-world tropics.
Many if not most of the eggs that leave the vein of their birth do not make it to the lumen of the intestine or bladder. Caught in the tissues of various organs, they bring about a cascade of immune system responses and overresponses. There is inflammation and swelling around the eggs. Abnormal tissue masses develop, and ultimately the symptoms of schistosomiasis set in.
At first glance, eradication of schistosomiasis does not seem to be insurmountable. There is no counterpart to the omnipresent malaria mosquito, whose bite is impossible to avoid. The solution in the case of schistosomiasis should be quite simple: stay out of snail-infested waters. Easier said than done. Rice farming is everywhere in southern Asia and Africa, and in the watery rice paddies snails abound. Merely add untreated human waste, with its untold numbers of schistosomes, and, presto, you have pain and suffering on a grand scale.
One human endeavor has even expanded—and greatly so—the mantle of the schistosome. It is the construction of dams to provide hydroelectric power for developing nations. Two of the most ambitious and most disastrous of these water impoundment projects were the Volta River Dam in Ghana and the Aswan High Dam in Egypt. Both created huge lakes of standing water hundreds of miles across with thousands of miles of shoreline. And both lakes became breeding grounds for the snails of schistosomiasis. In areas where the disease had been virtually unknown, infection has soared to over 90 percent of the population. So much for progress.
Robert Desowitz makes the point in his book New Guinea Tapeworms and Jewish Grandmothers that “if schistosomiasis were present in Sweden or the United States it would not be tolerated.” The infection would be treated—we have drugs such as praziquantel that are effective against the fluke and not unduly toxic to humans. The vectors would be eliminated through molluscicides (snails are mollusks) and drainage programs. Proper treatment of sewage would destroy the fluke before it had a chance to infect any surviving snails. In short, measures would be taken to ensure the good health of the citizenry. Life is, indeed, cheaper in third-world countries.
When you buy a puppy or a kitten, standard veterinary procedure is to deworm the animal. A simple pill or two will do the trick. The parasites your young pet suffers from are roundworms, long threadlike creatures that can be seen wriggling about in the feces. In fact their scientific name, nematode, comes from the Greek word for “thread,” nema. They are called roundworms because their bodies are not flattened as are those of tapeworms and flukes.
Roundworms are the most common and widespread of all parasites. No animal is without its roundworms; they even parasitize plants. Fiftyodd species of roundworms live within humans, a dozen of which are common parasites causing disease, disfigurement, and death. One in every four people suffers some kind of roundworm infection. As biological opportunists seeking to benefit at the expense of other organisms, roundworms have no peers.
The guinea worm has long been one of the more serious discomforts of the tropics. Only a decade ago in some villages, a quarter of the population was periodically incapacitated with fits of vomiting, diarrhea, and dizziness—all the result of the female guinea. Two to four feet long (the male is only an inch), she wanders through the body for a while before settling down just under the skin. Her appearance is that of a coiled varicose vein. But veins do not create blisters that discharge a milky fluid when submerged in water. The fluid contains thousands of larvae. Upon release they go on to infect a tiny crustacean called cyclops. It is the swallowing of larvaeladen cyclops that brings about human infection.
The guinea worm—known worldwide as the “fiery serpent”—most commonly attacks the legs, often causing painful inflammation and crippling muscle damage. Legs, however, are by no means the only sites of infection. As Dr. Donald Hopkins, an expert in tropical parasites, points out in the October 30, 1995, issue of People magazine: “I can show you pictures of a worm emerging from the back of a child’s head. They come out of the chest and genitals. One once came out under a man’s tongue. The swelling was so painful he couldn’t swallow and he starved to death.”
Native medicine men and even medical doctors would remove the worm by slowly and painfully winding it out onto a stick, perhaps a turn or two a day. In endemic regions it is not uncommon to see people walking with sticks taped to their legs around which worms are coiled. Evidence of worm infestation has been found in three-thousand-year-old mummies, and some speculate that the caduceus—symbol of the healing arts—is a visual representation of this ancient medical practice. If the procedure is not done properly, bacterial infection sets in and loss of a limb or death can result.
Recently there has been a serious and successful effort to wipe out the guinea worm through hygiene and water purification, which may involve the use of simple nylon swatch filters. A disease that a decade and a half ago afflicted 3.5 million people now debilitates a mere 25,000. It is anticipated that soon the guinea worm will become the second disease to be eliminated from the world (after smallpox)—and without “magic bullet” vaccines or medications.
What do they call sushi in New Orleans? Bait. Well, in Japan they call this preparation of sliced raw fish (and sashimi, a similar dish) a gastronomic delight. It is loved by millions of people who indulge their appetites daily. Unfortunately, several hundred Japanese diners pay dearly each year for their sushi and sashimi fondness. They contract anisakiasis.
Although not a serious worldwide problem in terms of numbers, the disease has spread to other countries, including the United States. Any lover of raw fish should be aware of the possible dangers involved. Often only hours after eating the fish, excruciating abdominal pain and vomiting of blood may occur. The one-and-a-half-inch larva of anisakis has been ingested along with the food and has buried its head in the inner stomach lining. A look through a gastroscope (a tube stuck down the throat and into the stomach) reveals an angry-looking, bloody ulcer two inches in diameter. At the center of the inflamed, craterlike wound, the worm’s body, anchored by its head, undulates obscenely to and fro.
Sometimes the worm can be removed by pincers at the end of the gastroscope and no further medical intervention is required. At other times surgery of affected tissue is required. The worm larva may also attach to the small intestine wall farther down the digestive tract. When it does, symptoms of severe stabbing pain are not as immediate.
Thankfully, when one considers the sheer numbers of people that consume sushi and sashimi daily, infection with anisakis larvae is quite rare. This is not the case with hookworm, another intestinal roundworm parasite that is ubiquitous throughout much of the tropical and subtropical world.
The parasite is small—adults attain a length of about a half inch—and derives its name from the tiny hooks or plates within its mouth. These plates bite down onto the intestinal wall as the worm sucks in blood and tissue fluids. The outcome, if worms are numerous (several hundred or more), is severe, crippling anemia. It is the legacy of the disease. Infection as a child often results in physical and mental retardation.
As is the case with so many parasites, the worm is transmitted from one host to another through feces. Within the human waste are microscopic eggs that mature into tiny larvae when deposited into the soil. The hookworm larvae will then burrow through the skin of anyone walking barefoot. From there they enter the bloodstream and take an express trip to the intestine.
Interestingly, hookworm was a very serious problem in the United States until the latter half of the twentieth century. Wherever winters were mild and the soil did not freeze (which kills the worms) hookworm was endemic. Millions of people in the southern states were afflicted. The “barefoot boy with cheek of tan” often grew into the stereotypically lazy, shiftless, dull-witted southerner as a result of being bled from within by this insidious parasite. The scientific name, in fact, for one of the several common hookworm species is Nectarus americanus—“American killer.”
Better sanitation and public health awareness and the wearing of shoes have minimized the problem in the United States. Worldwide, however, tens—perhaps hundreds—of millions of people still suffer from hookworm. Because symptoms of the illness are so easily misdiagnosed, one cannot even hazard a guess as to exact numbers. Estimates run as high as 25 percent of the planet’s population, with sixty-five thousand dying each year. A 1980 World Bank study found that 85 percent of the residents of Java had hookworm. In these countries it continues to shorten life and greatly reduce its quality.
One cannot talk of crippling parasitic diseases without mention of the roundworms known as filaria. They are of great importance in western and central Africa, the Middle and Far East, and the New World from Mexico to Brazil. Of particular concern are two filarial worms that are close cousins. One causes river blindness and the other elephantiasis. Together they infect 140 million people.
Elephantiasis is the more dramatic of the two diseases. It derives its name from the immense swelling that is its most outstanding feature. Often the legs are involved, becoming so enlarged they resemble those of an elephant. Men’s genitalia, when affected, assume gargantuan dimensions. One male elephantiasis sufferer could not walk unless he placed his testicles—the size of watermelons—in a wheelbarrow and carted them before him. Women’s breasts may be similarly altered.
Such extreme swelling is the consequence of the female worm’s reproductive activity. She is three to four inches long and lies in a lymph gland or duct, looking like coiled string. Each day she pumps out thousands of progeny in the form of tiny threadlike larvae called microfilaria. Over time the microfilaria clog lymph ducts, causing lymphatic fluid to collect and swell affected body parts.
It is through the bite of a mosquito known as Culex that the worm is spread from one human to another. Culex, ubiquitous throughout the tropics and subtropics, is particularly fond of filthy, stagnant water such as raw sewage. Not surprisingly, growth of large cities without proper sanitation has greatly increased its numbers. The filaria of elephantiasis was even introduced into the southern United States with African slaves but was eradicated by the 1920s.
When Culex sinks its little proboscis into a human, it sucks up, along with its blood meal, the microfilaria of elephantiasis. Within the mosquito the larvae continue to develop. Soon the insect is ready to bite again and transmit its parasite. This transmission can be halted by treating infected individuals once a year over a four- to six-year period with a single-dose combination of oral medicines. It is one of about a half dozen infectious diseases in the world considered to be completely eradicable. So is its filariasis twin—river blindness.
River blindness—endemic primarily in Africa—is transmitted by the blackfly, an insect that lives and breeds in fast-flowing streams and rivers rather than stagnant pools. Its bite introduces into the human host a species of filaria that is happiest when infiltrating the eyes and outer layers of the skin. It is here that thousands of the snakelike microfilaria can be found. A common outcome of ocular infection is impaired vision or blindness. (In a related disease, Loa loa, worms can actually be seen moving across the front of the eyeball.) People with skin parasites fare no better. The microfilaria cause disfiguring lesions, leading to ostracism. Itching can become so intense and unrelenting that it has driven some sufferers to suicide.
Amid all this human anguish are safe and effective antifilarial drugs. In the early 1980s the pharmaceutical giant Merck and Co. invented ivermectin, an antiparasite drug that works well against the filaria of river blindness, as well as elephantiasis. Several years later Merck donated the drug to needy nations (and continues to do so), where half a million people were already blind from the roundworms and a hundred million more were at risk. But the effort was a dismal failure. The health programs of these nations were so poorly run that ivermectin never made it to the people. Such are the difficulties in developing countries. In 1995 a second effort was initiated by the World Bank to treat the peoples of sixteen African nations where river blindness is most devastating. It met with limited success. Today philanthropic organizations such as the Carter Center have joined the endeavor, realizing that if 85 percent of the people in endemic regions are given a dose of ivermectin twice in one year the disease can be wiped out. It is a goal on which the Carter Center has put a 2007 target date.
Almost two billion people, a sizable fraction of Earth’s inhabitants, suffer parasite infections. They kill; they maim; they make life unbearable. So prevalent are parasitic diarrheas that they have simply become a way of life. A doctor, upon examining the watery stool of his patient, asked how long it had been like that. With some surprise, the patient replied, “It’s always been like that.”
It doesn’t have to be like that. Most of the serious parasitic diseases—malaria, kala-azar, schistosomiasis, filariasis—can be brought under control and in most cases eradicated. What it takes is determination and a serious commitment. Unfortunately in many cases neither the rich developed nations nor the governments of the affected nations themselves exhibit such commitment. And pharmaceutical companies will rarely do anything unless there is a profit. Where does that leave several billion people? Living with debilitating illness and dying young.
All life on this planet can be sorted into five kingdoms. The obvious ones are plants and animals. Flatworm and roundworm parasites discussed in this chapter belong to the animal kingdom.
With the invention and widespread use of the microscope, an unseen living world suddenly burst into view. It was the world of one-celled organisms. The larger unicellulars, those with a complex cell structure, were classified as protists. Protozoan pathogens are found in this kingdom. Bacteria, tiny, simple, and single-celled, comprise yet a fourth kingdom. They are life at its most fundamental. Viruses, inert particles of nucleic acid and protein, have no cellular structure and are not considered living entities. As such, they belong to no kingdom of living things.
There is, however, a fifth kingdom of life, one populated by organisms in which cells are uniquely fused into microscopic filaments and reproductive spores perpetuate the many different species. It is the kingdom fungi.
Most fungi are nonpathogenic, growing in the soil and feeding on its organic matter. Some derive sustenance from dead plant and animal remains. They are called saprophytes. A mushroom is a saprophytic fungus, as is the fuzzy green mold growing on your three-day-old strawberries. Some fungi, however, are parasitic, living on and feeding off the living. In the process they cause disease. We have all suffered at one time or another from a rash or infection of the skin as a result of a fungal invasion. Athlete’s foot, jock itch, and ringworm are familiar examples.
Breathing in spores released by certain fungi can also cause illness. Most of the time it is due to allergic reaction and not infection. Mold spores are, after all, second only to dust mites as the most common indoor allergen. But in some people, usually those with enervated immunity, fungal spores can establish lung and then systemic infections. AIDS sufferers are constantly plagued by normally harmless fungi.
Being the consummate opportunists, fungi exploit any and every weakness in an individual. Candida is a perfect example. Every year Candida, a single-celled fungus better known as yeast, gives rise to twenty million cases of vaginal infection, primarily in women who are on antibiotic therapy. The antibiotics kill normal flora (bacteria) in the vaginal tract that keep yeast growth in check. The yeast then overrun the area. In babies, Candida causes diaper rash and in the immunosuppressed, the mouth infection thrush.
Recently, another fungus has been implicated in a number of serious illnesses. It is Stachybotrys, a wet, greenish-black growth that the media have dubbed “killer mold.” Is this merely hyperbole, or does Stachybotrys deserve its menacing moniker? No one really knows. In 1993 and 1994 acute pulmonary hemorrhage struck forty-seven infants living in the Cleveland, Ohio, area after homes had been flooded. Sixteen died. Since then sporadic cases have been reported. A CDC investigation concluded that significant exposure to Stachybotrys growing on and in the walls of many of the homes played a significant role in the development of this severe and oftentimes fatal lung disease. But other more common molds—aspergillus, penicillium, cladosporium—were also present and might have contributed to the problem.
Even if it is not the mass murderer portrayed in the tabloids, Stachybotrys does cause myriad symptoms that have come to be known as “sick building syndrome.” These include coughing, wheezing, runny nose, eye irritation, sore throat, skin rash, diarrhea, headache, fatigue, and general malaise. Mycologist and Stachybotrys investigator Dr. Dorr Dearborn says, “it’s like having a bad cold that doesn’t go away.” As with other fungi, Stachybotrys thrives in dark, wet places. Basements, especially those that take in groundwater or become very humid during warm weather, provide a perfect environment for Stachybotrys. Well-insulated, airtight buildings that do not “breathe” can exacerbate the situation. Studies have found the killer mold in 1 percent to 3 percent of homes tested. And as Stachybotrys grows it produces powerful toxins that can be found in its spores. Inhaling these poisonous spores in high enough concentration produces the flulike symptoms of sick building syndrome or more serious lung disease.
Medical experts predict a dramatic rise in fungal infections as the population of weak and immunocompromised individuals in our society continues to increase. Nosocomials, those infections contracted in hospitals, will show the most striking upsurge with the old, sick, and weak being kept alive longer through the miracle of medicine. AIDS presents a frightening picture of how fungi can completely overwhelm the human body. Coccidiodes, a rare fungal lung infection, is found in 10 percent of AIDS sufferers. Thrush, the oral yeast infection with its patches of creamy white exudate overlaying a painful reddish inflammation, is one of the signature diseases of AIDS. But more on AIDS—acquired immunodeficiency syndrome—in the next chapter.