When a person has sex, they’re not just having it with that partner, they’re having it with everybody that partner has had it with for the past ten years.
—OTIS RAY BROWN
Every once in a while a new disease will emerge and knock the socks off the human population. It happened with HIV/AIDS, and it can happen again.
—DR. ANTHONY FAUCI
In the fall of 1980 a very sick gay man entered the UCLA Medical Center. He was pale, very thin, had a mouth full of “cottage cheese,” and coughed painfully and uncontrollably.
Examination revealed he had a fungal infection of the mouth called thrush and an exceedingly rare illness—Pneumocystis carinii pneumonia (PCP)—caused by a protozoan. Doctors were baffled. Perhaps most puzzling was the complete absence of a certain population of white cells, called T4 cells, in the man’s blood.
Pentamidine is a drug used to treat PCP, but PCP is so rare that hospital pharmacies do not stock it. Such “orphan drugs” that treat rare diseases and are considered experimental can be obtained only from the Centers for Disease Control and Prevention. UCLA doctors contacted the CDC and ordered the experimental drug for their dying patient. The CDC, in fact, had received five orders for pentamidine between September 1980 and May 1981—five orders in an eight-month period when they had received only two requests between 1967 and 1979. Clearly, something was amiss.
In July 1981 a man with purplish blue splotches covering his body entered San Francisco’s General Hospital. He was a homosexual prostitute, and he had an extremely rare cancer. Called Kaposi’s sarcoma (KS), it affected the blood vessels of the skin, causing out-of-control growth. At about the same time a cluster of KS cases occurred in New York City and Los Angeles as well.
By the end of August 1981 the CDC would report 107 cases of either KS or PCP or a combination of the two. AIDS had emerged, and in the ensuing two decades would create an unprecedented public health crisis. Medicine would marshal its forces as never before to wage war against a disease that has threatened to become the worst plague ever to afflict humankind. Through the 1990s, it was the leading killer of Americans aged twenty-three to forty-four. In 2000 it newly infected more than three million people. Year 2001 saw more people diagnosed with HIV infection in England than ever before, and Britain’s Public Health Laboratory Service expects the number of cases to reach thirty-four thousand by 2005, a jump of 47 percent from 2000. As 2001 drew to a close, about twenty-two million people had died of AIDS worldwide, with another forty million infected, most asymptomatically. Nearly one million of them reside in the United States. The devastation it has wrought in Africa, where 70 percent of cases occur, is not to be believed. AIDS kills three times as many people in Africa as the next most common cause of death. In eleven million people it has also teamed up with the bacillus of tuberculosis, making TB many times more deadly (see Chapter 4, “The White Death”). The life expectancy of a child in Botswana has dropped from seventy years to under forty as a result of AIDS.
The AIDS virus presently kills three million people annually, and by the year 2020 will have caused more fatalities than any other disease outbreak in history, including the Black Death of the fourteenth century, which mowed down one-third of Europe’s population. Some extrapolations put the figure of those infected at one billion by the year 2050. That’s one out of every six people.
Thankfully, however, onset of the disease is no longer a death sentence. At one time AIDS, along with rabies, was considered the most lethal of all viral infections, with mortality figures at or very near 100 percent. Advances, however, in treatment of both AIDS and its many opportunistic infections have greatly extended life expectancies.
In the beginning AIDS was primarily, if not solely, found among homosexual males. And its most distinguishing feature was a total collapse of the immune system. Hence the disease’s original designation, gay-related immune deficiency—GRID.
Annihilation of the immune system in AIDS is accomplished by selective destruction of one key class of white blood cells—the helper Ts or T4 cells. As discussed earlier (see Chapter 6, “The Virus”), T4 cells are a central player in any immune response. Simply put, they release chemicals called interleukins that turn on every other component of the immune system. Destroy them, and the entire immune response goes kaput.
With no system to fight invading microorganisms, the body is prey to any number of weird and highly unusual diseases. Known as opportunistic infections, they are caused by germs that exploit a decimated immune system to gain biological advantage within our bodies. The two most common and devastating opportunistic infections are Pneumocystis carinii pneumonia and Kaposi’s sarcoma. Together they have become the signature of AIDS. But many other odd ailments make the life of an AIDS sufferer a veritable hell. Diseases formerly found only in sheep, cats, or birds suddenly appear in AIDS patients. Laurie Garrett, in her tome The Coming Plague, lists the more important ones:
Thrush caused by Candida fungal infections; pronounced herpes simplex-II throughout the body; blood contamination of active cytomegalovirus with unknown effect; mononucleosis due to Epstein-Barr virus; marked lymph node swelling; radical infections of the stomach and gastrointestinal tract with Entamoeba histolytica; diarrhea and gastric problems caused by the Cryptosporidium parasite; similar symptoms caused by, of all things, Mycobacterium avium, a tuberculosis bacteria usually found in chickens; galloping infections in many organs of the Cryptococcus fungus; out of control bacterial infections with common organisms such as Staphylococcus aureus, Escherichia coli and Klebsiella.
What on earth could so devastate an immune system? Was it a deadly chemical? Was it an infectious agent? Was it the wrath of God descending on homosexual sinners, as certain evangelists claimed? A frantic search for the cause of AIDS began.
It was apparent from the outset that AIDS was preferentially attacking gay men. What was it, epidemiologists wanted to know, that singled them out? One common factor among all the initial cases of 1980 and early 1981 was a promiscuous lifestyle. Ever since the 1969 Stonewall riots in Greenwich Village—when police tried to shut down a gay bar called Stonewall and arrest its patrons—the gay community had “come out of the closet.” Homosexuals began demonstrating, becoming vocal and politically active in their demands for equal rights. Gay bathhouses opened up that were nothing short of orgy centers. This sudden sense of freedom caused a small but significant group of gay men to overindulge in casual sex. For some men that meant as many as several hundred partners a year!
It was predominantly this population that was contracting AIDS. With promiscuity as a common thread, researchers came up with various and sundry theories to account for its transmission. Ideas ranged widely. One leading theory proposed that sperm of a sexual partner was finding its way into a victim’s bloodstream. His immune system then made antibodies to the sperm that cross-reacted with his own T cells. What was, in effect, happening was an autoimmune response triggered by foreign sperm. But why would sperm suddenly start turning deadly?
Another popular misconception was that amyl nitrite, known as “poppers,” was causing the symptoms. Used to intensify orgasms during sexual encounters, amyl nitrite also suppresses the immune system. There was no reason, however, to ascribe sustained and continued immune system collapse to poppers taken months earlier. More and more, GRID was exhibiting the transmission characteristics of an infectious, sexually transmitted disease. But what infectious agent was being transmitted?
At first the notion of “antigen overload” was promulgated by some researchers. They suggested that rather than a single germ causing GRID, a host of sexually transmitted viruses and bacteria were battering the immune system over the years, seriously overworking and depleting the T-cell population. However plausible and seductive the overload theory appeared to some investigators, it could not explain why, in the summer of 1982, hemophiliacs began developing GRID. Couple that with the appearance of GRID among intravenous drug addicts and people receiving blood transfusions, and it became clear that the disease was blood-borne—spread by some mysterious infectious agent in the blood. No longer a strictly homosexual affliction, the disease was renamed AIDS—acquired immune deficiency syndrome—in late 1982. Could it be that the wrath of God was now bearing down on innocent heterosexuals?
AIDS was an infectious, blood-borne disease that could also be transmitted sexually. Soon the mad pursuit began, with labs around the globe racing one another to isolate the bug that gobbled up T cells. Its discovery would certainly mean fame, fortune, and a Nobel Prize.
One scientist whose curiosity was piqued by the notion of an infectious agent, probably a virus because it was so hard to detect, was Robert Gallo. It was the involvement of T cells that most intrigued him. A few years earlier he had shocked the scientific establishment by announcing the discovery of a new and highly unique human virus that infected T cells, turning them cancerous. The virus was dubbed HTLV, named after the disease it caused—human T-cell leukemia. HTLV was the first human retrovirus ever isolated.
A retrovirus is a very special entity. Like many other viruses, it has RNA as its genetic material. But unlike other viruses, the RNA does not directly synthesize protein. First it goes in reverse, making DNA. The DNA then makes another RNA strand that finally goes about manufacturing protein (see Chapter 6, “The Virus”).
Why such needless nucleic acid synthesis? It is analogous to your taking a perfectly fine loaf of bread back to the store and exchanging it for a new loaf before making a sandwich. What a monumental waste of time and energy for both you and the virus. Or is it? Rarely do living systems evolve mechanisms that do not in some way benefit the organism, and retroviruses are no exception. The apparent senseless production of unnecessary DNA is of great benefit to the virus, for it allows it to incorporate itself into the host cell’s DNA. In a process called integration the viral DNA splices itself right into the host’s genome.
Integration, a process essential and unique to retroviral replication, affords the virus several distinct advantages. First, the virus, now part of the host genome, is well hidden from detection and destruction by the evervigilant immune system. Second, with each cell division the virus—or, more precisely, the viral DNA—replicates and goes into a new cell. Silently the virus spreads throughout the organism. And if infection happens to occur in gamete-producing cells of a testis or ovary, sperms and eggs might even carry the internalized virus to the host’s offspring.
Although many if not most retroviruses probably travel within host cells causing little or no harm, sometimes integration alters the cell in such a manner as to turn it malignant. This is the type of retrovirus—HTLV—that Robert Gallo discovered in 1979. In 1982 his lab isolated a second, closely related T-cell leukemia virus, which he named HTLV-2 (the first became HTLV-1).
Could AIDS, Gallo speculated, also be caused by a retrovirus? And if so, was it one of the HTLVs, or was it a totally new virus? Both HTLV and the AIDS virus infected T cells and suppressed the immune system. Both were transmitted through blood and sex. This was compelling evidence.
By mid-1982 Gallo’s lab initiated investigations to find the AIDS virus. Step one was to determine whether or not it was a retrovirus. This could be accomplished by growing the virus in cell culture and testing for retroviral activity. All retroviruses have a unique enzyme that allows them to churn out DNA from an RNA template. The enzyme is found only in retroviruses—their footprint, if you will—and demonstration of its presence in cell cultures is clear proof of retroviral infection.
The only problem, in early investigations, was the near impossibility of getting enough virus to grow in culture. The reason was simple. Unlike HTLV, which caused T4 cells to grow and multiply indefinitely (as cancerous cells are wont to do), the AIDS virus seemed to destroy the T cells they replicated within. Not until late 1983 was a T-cell line found that could maintain the suspected AIDS virus without itself being destroyed. Now the AIDS virus could be grown in abundance.
With this new batch of T cells and AIDS-infected tissues, Gallo and his team of virologists went about testing for retroviral activity. When they found it, they tested for the presence of HTLV. This was done by mixing the infected samples with antibodies to HTLV. A reaction meant that the virus was most likely present in the tissues. At this point an even more conclusive test for HTLV presence was performed in which DNA probes for HTLV were used. Bonding of the probe with DNA in the AIDS tissues would indicate the presence of HTLV.
By April or May of 1984, after many months of growing and testing and learning how to handle the cultures, success was realized. A new virus had been found. It demonstrated the enzymatic activity of a retrovirus but did not react with antibodies to HTLV-1 or HTLV-2. Most important, it was found in a substantial number of AIDS patients and people at risk for AIDS. No evidence of the virus was found in 115 healthy heterosexuals. Robert Gallo named the virus HTLV-3 and declared it to be the infectious agent that produced AIDS.
As it turned out, Gallo was correct. HTLV-3 was the cause of AIDS. He was not, however, the first to discover it. That honor went to Luc Montagnier of the Pasteur Institute in France. A year earlier he had isolated a retrovirus from a section of swollen lymph node excised from an early-stage AIDS patient. But when he published his findings, claiming it to be the AIDS virus, the scientific community did not feel he had proven his case.
What ensued was a bitter and ugly battle between Gallo’s National Cancer Institute (part of the National Institutes of Health [NIH]) and Montagnier’s Pasteur Institute. Both demanded credit for discovery of the virus. Things really heated up when the DNA of each virus was sequenced and the two were compared. The match (nearly 99 percent) was so perfect between the two that they could only have come from the same patient.
The implications were enormous. Both labs, during the course of their investigations, had exchanged tissue samples as well as other reagents—antibodies, cell culture growth factors, and the like. Had Gallo taken Montagnier’s virus and deliberately tried to pass it off as his own independently isolated one? Had Montagnier’s virus mistakenly contaminated Gallo’s cultures, making its isolation an unfortunate but innocent laboratory error? No one would ever know for certain, but in March 1987 the governments of France and the United States intervened to call an armistice between the feuding labs. An agreement was announced officially recognizing the Montagnier and Gallo groups as codiscoverers of the AIDS virus, which was now being called human immunodeficiency virus—HIV.
The March 1987 accord also settled another long-standing dispute concerning patent rights to an AIDS blood test that had been developed in 1984. Gallo’s group first developed the test, but Montagnier claimed that he did so using the Pasteur Institute virus. At first the U.S. Patent and Trademark Office awarded patent rights to Gallo, only to reverse its decision later on. The awarding of these rights was by no means academic since it would generate huge sums of money as blood tests were sold and used worldwide.
Financial gain notwithstanding, President Ronald Reagan of the United States and Prime Minister Jacques Chirac of France agreed to include the names of both Gallo and Montagnier on any and all blood test patents. Any royalties that accrued would go toward funding future AIDS research. Cooler heads had finally prevailed.
By mid-1984 Gallo had finally found his AIDS virus. That was also an election year for President Reagan—a president who had been accused by many of being indifferent to the plight of AIDS sufferers. The last thing Reagan wanted to deal with was a tainted blood supply. So in June of 1984 five pharmaceutical companies were given twenty-five liters of T4 white cells infected with Gallo’s HTLV-3 AIDS virus. Each was instructed to develop a reliable, economical blood test for HIV infection that could be performed quickly and easily.
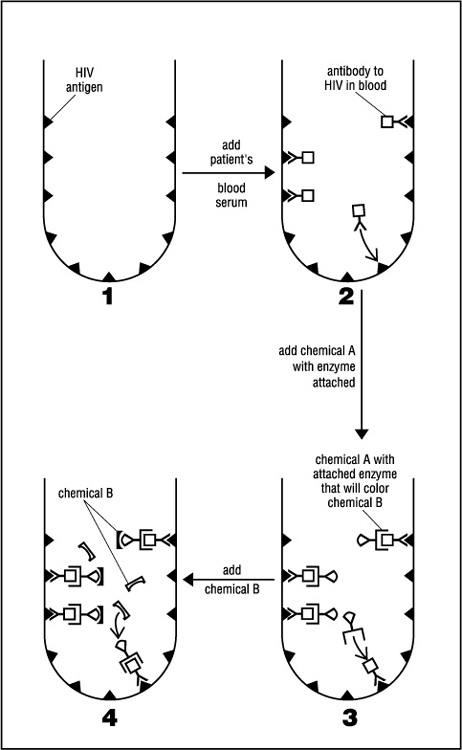
Right from the outset all five companies realized that a practical, easily performed test must search not for the hard-to-detect virus but for antibodies to the virus circulating in the blood. A technique was employed called the enzyme-linked immunosorbent assay, or ELISA for short. Today ELISA is still the most commonly used AIDS test for initial blood screening. It works something like this:
Small containers are provided by the pharmaceutical company. The walls of each container are impregnated with HIV antigens—the viral proteins that evoke strong antibody production during infection. Blood being tested is placed in the container and then washed out. Any antibodies to HIV present in the blood will cling to the antigens on the wall. Antibodies, however, are not visible. To determine whether any have stuck to the antigen layer, another chemical—chemical A—which binds to HIV antibodies, must be added. If HIV antibodies are present, chemical A forms a third layer that will not be washed off. Chemical A also has an enzyme attached to it that will make yet another chemical—chemical B—turn color. So a color change when chemical B is finally added indicates the presence of HIV antibodies and a positive test result for HIV infection. The procedure is summarized in Figure 2.

ELISA is a very reliable test, but it is not foolproof. Sometimes it does produce false positives. To make certain the positives are genuine, a backup test is needed. The most common confirmatory procedure is the Western Blot.
Like ELISA, the Western Blot tests for HIV antibodies in the blood. But it uses a wider range of viral proteins, derived from mashed-up viruses that have been separated from one another and fixed onto a solid strip of nitrocellulose. The result is greater sensitivity and accuracy in picking up HIV antibodies when the strip is washed with infected blood. One other minor difference between the two procedures is use of a radioactive chemical in the Western Blot to recognize HIV antibodies adhering to the strip. The radioactivity is detected by placing the strip onto a photographic plate.
Sometimes the roles of antigen and antibody are reversed, and the initial “solid” layer is of HIV antibodies and not viral antigens. When washed with blood, viruses themselves will adhere to the test strip or container. The great advantage of this test is that it detects infection very early, before the person begins developing antibodies.
Early detection of HIV infection is critical. It is for this reason that researchers adopted a state-of-the-art viral identification procedure called PCR analysis from the forensic lab. PCR (polymerase chain reaction) made headlines during the 1995 murder trial of O. J. Simpson. The test was used to compare DNA from blood at the crime scene with that of Mr. Simpson. That is what PCR does. It takes minuscule amounts of DNA and, using special enzymes, makes millions of exact copies until there is enough to study and test. In the case of HIV the procedure is able to hunt down the tiniest amounts of virus present within infected cells long before symptoms of the disease or even detectable antibodies appear.
Through the use of PCR researchers made a startling discovery. People can be infected with HIV for years before their blood tests positive for HIV antibodies—years in which they are unwittingly contagious. When it comes to viral infection, it seems that HIV breaks all the rules. The more we learn about the virus, the more it confounds us. And we have learned an awful lot in a relatively short period of time.
Without doubt HIV has become the most intensively studied virus in history. Scientists have dissected its proteins and mapped its genes. They have studied its every move as it performs its most dastardly intracellular deeds. What have we learned?
In the spring of 1985, U.S. Secretary of Health and Human Services Margaret Heckler announced the discovery of a new retrovirus as the cause of AIDS. According to Ann Giudici Fettner in her book The Science of Viruses, an immunology researcher she was sitting with at the time blanched and groaned. “Oh God,” he said, “we’re in big trouble.”
His words were prophetic to say the least. Although Ms. Heckler predicted a vaccine in five years, it is now seventeen years since her speech, and no vaccine is in sight. Some researchers wonder if an effective prophylactic vaccine can ever be created given the nature of HIV. It is like no other virus.
The outer, fatty envelope of HIV is actually a snippet of cell membrane that it steals and cloaks itself with as it exits a cell. Protruding from the fatty or lipid membrane, like so many warts on a toad’s back, are knoblike projections of a sugary protein called glycoprotein 120 (gp 120). A second type of glycoprotein—gp 41—is embedded in the viral envelope and anchors the gp 120. These substances have been the subject of much research since they are the structures that first recognize and bind the virus to its target cells.
Beneath the lipid layer is a matrix layer of protein that surrounds the viral core. The core is the guts of the virus, where the nucleic acid resides in the form of two single RNA strands. These RNA strands house nine genes that code for fourteen different proteins. Joining the RNA within the core are several important enzymes that are needed to initiate infection.
By viral standards HIV is not particularly large or complex (although it is more complex than most other retroviruses). The smallpox virus is many times larger and contains enough DNA to control production of two hundred to three hundred proteins. Compare that to HIV’s paltry nine genes coding for fourteen viral proteins. Yet finding a vaccine against smallpox was child’s play compared to HIV. As Mark Caldwell so aptly put it in his August 1993 Discover article on AIDS, “Smallpox stumbles into the immune system like some dim-witted thug, setting off alarms everywhere.” In 1796 Edward Jenner, not even aware of the existence of viruses, was able to scare up a smallpox vaccine from the pus of a milkmaid’s cowpox sores. Why, by contrast, is HIV so impossibly difficult to battle?
In a word, stealth. HIV is no dim-witted thug, but rather a highly practiced and skilled cat burglar. Action begins when viruses enter the body through tainted blood or, as is the most common route today, through unprotected sex. AIDS is sexually transmitted by virtue of the fact that the semen of an infected male is loaded with T4 white blood cells, which are, in turn, teeming with HIV.
Once introduced into the vagina or anus of a sexual partner, HIV and HIV-infected cells make their way into the bloodstream via tiny cracks in the mucous membrane. Here the virus encounters T4 cells, which are the primary targets of HIV. Sitting on the surface of T4 cells are proteins called CD4 markers. It is, in fact, the CD4 surface protein that gives the helper T its name—T4. These CD4s fit hand-in-glove with the gp 120 viral projections. Using its gp 120s, the virus docks on the cell surface. Then, through a mechanism employing the other surface glycoprotein, gp 41, fusion of HIV’s lipid coat with the cell membrane takes place. This is how HIV gains entry into the T4 cell, opening up and spilling its guts into the cell’s interior.
Once inside the cell, HIV, being a retrovirus, uses its two single strands of RNA and the enzyme attached to them—reverse transcriptase—to make double-stranded DNA. It then integrates that DNA into the host’s genome.
Although T4 cells are the primary targets of HIV, they are not the only ones infected. There is evidence that certain cells of the mucous membrane are also cloaked with CD4 markers. So cells susceptible to HIV infection probably line the vagina and anus. What more could a sexually transmitted virus ask for?
What more? How about the ability to infect macrophages? These, you may remember, are the large white blood cells that roam the body, swallowing up invading germs. Once inside macrophages, HIV get a free ride anywhere they want to go. It is believed that macrophages carry the viruses across the blood-brain barrier to the brain, where they bring about the dementia so common in advanced AIDS cases.
Many doctors and researchers feel that once integration occurs, there is little anyone can do to wipe the infection completely from the body. The immune system will not recognize and destroy cells that have HIV nestled snugly and safely within their nuclei. In effect, the body does not fight these viruses because these viruses have become part of the body.
Please do not, however, misunderstand what takes place during HIV infection. The virus does not remain quiescent for years as was originally thought. From the time a person first acquires HIV, many T4 cells begin actively replicating viral particles, and there is a vigorous initial immune defense. Large numbers of viruses are present in the bloodstream during this acute phase, and the patient experiences flulike symptoms for several weeks. Soon B cells start churning out antibodies to neutralize viruses not within cells, and activated killer Ts (see Chapter 6, “The Virus”) destroy those cells already infected.
This brings the infection under control, but a fierce struggle between immune system and virus persists throughout the asymptomatic period of the disease—which typically lasts eight or nine years. Hundreds of millions of HIV are killed daily. At the same time the immune system can lose up to a billion T cells a day. During this period of stalemate between the virus and the immune system, the victim is said to be HIV-positive but does not yet suffer from AIDS. Slowly, however, over the years, the battle turns in favor of the virus. The invading hordes of HIV eventually overpower the huge and efficient armies of killer T cells and antibodies. Success is primarily a function of the virus’s extreme mutability. Of all known viruses HIV has, by far, the highest rate of mutation. The same virus, over the course of infection in a single individual, can change its genetic makeup by as much as 30 percent. This is incredible variability—much more so even than the highly mutable influenza virus.
The reason for such genetic instability is the imprecise way in which reverse transcriptase makes DNA from viral RNA. In short, it makes mistakes, many of them, during transcription. And the high rate of viral replication, perhaps a billion new viral particles a day, only gives HIV more opportunity to mutate. Although most mutations hurt the virus and make it nonfunctional, every so often a mutant particle might arise with a slightly different surface protein—one that the immune system cannot recognize. Now a new population of viruses can run amok until the immune system catches up. Eventually so many mutants are generated that the immune army starts chasing its own tail, and the battle is lost.
Interestingly, much of the head-to-head combat between virus and T cell goes unnoticed because it occurs in the dozens of lymph nodes found throughout the body, not in circulating T4s. Lymph nodes are a part of our immune system, a filtering system of sorts that catches and destroys invading microbes. It now seems that T4 cells residing in lymph nodes are sitting ducks for HIV. During much of the asymptomatic period of AIDS infection, the viral load continues to build in these nodes. Meanwhile the T4 count in the bloodstream does not diminish appreciably. Finally the nodes “burn out,” and virus floods into the blood, signaling the onset of full-blown AIDS. It is at this point that doctors, monitoring the blood, begin to notice a significant drop in levels of circulating T4 cells.
Nasty little critters, these HIVs. Can anything be done to stop them or even slow them down? Yes, but the task is a daunting one.
Most experts agree that the global AIDS pandemic can be stopped only through an effective vaccine. The principle behind vaccination is remarkably simple. Present a harmless version of the germ to the immune system, and it will be duped into a state of readiness. Should the genuine article come along, it will be dealt with swiftly and harshly.
Such approaches have worked well with other viral diseases such as smallpox, polio, measles, and influenza. Either attenuated or killed viruses may be used. Attenuated virus seems to elicit better immune responses and is the vaccine of choice when feasible.
Concerning HIV, however, scientists are very reluctant to inject healthy people with weakened yet live virus. There is too great a danger that HIV will mutate to a not-so-weak form. Researchers dare not use even a killed vaccine for fear that some viral particles may have survived the killing process. One solitary surviving particle can invade a cell and initiate a cycle of infection.
Even if an attenuated HIV does not revert to virulence, there is always the possibility that it can reacquire the ability to integrate into the host genome. No one knows what long-term effect integrated HIV might have on a cell. With other human retroviruses it often leads to cancer. The last thing researchers want is a vaccine that will prevent AIDS only to produce a malignancy ten or fifteen years down the road.
For this reason there are no candidate live-attenuated HIV or whole-killed HIV vaccines in any human clinical trials. So where does that leave researchers? The only option is the creation of vaccines from harmless pieces, or subunits, of the virus. Up until recently, the focus has been on the envelope glycoproteins gp 120 and gp 41. They were the logical choice because they initiate infection, are easily accessible to antibodies, and do seem to elicit strong antibody production. Early vaccination attempts offered only short-lived and limited immunity, as mutability of the virus, once again, reared its ugly head. It is hard to find a magic bullet when the target keeps zigzagging every which way. A subunit vaccine can induce antibody production against only one type of subunit. If an HIV changes its subunit sufficiently, antibodies generated against the vaccine will not recognize it, and there will be no immunity.
This notwithstanding, the AIDS vaccine furthest along in the clinical testing process is of the subunit class. Called AIDSVAX, it is the first vaccine to enter Phase III trials—the final stage of testing—in which the effectiveness of the product is evaluated in large numbers of humans. Phase I and Phase II clinical testing deals primarily with safety issues.
A more recent vaccination strategy, the “naked DNA” vaccine, seeks to confer protection by using the actual genes of HIV. Once introduced into the skin or muscle, the genetic material is taken up by cells of the body, which then produce HIV proteins that stimulate the immune system. Experimental vaccines using SIV (simian immunodeficiency virus) genes to protect monkeys against their form of AIDS have proved successful and Phase I trials with HIV genes are underway now in humans.
Genes of HIV need not, of course, be injected as naked bits of DNA. They can be inserted into a harmless virus or bacterium and then introduced into the body via this microbial vector. Once in the body, HIV genes will begin to express themselves and produce HIV proteins to which the immune system will respond. The concept is not new. Bacteria molecularly engineered to contain human insulin or growth hormone genes have been pumping out these hormones for more than twenty years.
In the eighteenth century, Edward Jenner discovered that immunization with cowpox protected people against variola virus, the cause of smallpox in humans (see Chapter 2, “Germs and Disease: A Brief History”). This concept of “Jennerian” vaccines, based on the use of similar but not identical viruses to those that cause disease, is being considered for HIV. Although not in clinical trials yet, possibilities include the simian equivalent of HIV (SIV) and a weaker strain of HIV called HIV-2.
Because of the extreme difficulty in trying to mount an immune assault against the wily, ever-metamorphosing HIV, efforts are being made to create a vaccine that directs immune responses against the human host cell receptors that serve as HIV’s port of entry into cells. By blocking HIV receptors such as CD4, the virus will be unable to attach to the cell surface, preventing infection. It is a clever and novel approach and one that still awaits clinical testing.
With so many irons in the fire, one would hope that an AIDS vaccine is close at hand. But vaccine research is painstakingly slow. Clinical trials seem to take forever and often fail to produce the desired results. What appears so promising in a test tube or an experimental animal can fail miserably in human testing. The bottom line is that prospects for an effective, reliable prophylactic vaccine in the near future remain dim. Researchers feel that we’ll be lucky to have even a crude one on the market by 2007. At current rates of infection, an additional fifty to one hundred million people will have contracted the virus by then, and millions will have died. In light of this, virologists and immunologists are working feverishly to find antiviral drugs that can stop or at least slow HIV infection. Since 1987 the drug of choice has been AZT.
AZT, also known as azidothymidine, zidovudine, and retrovir, is one of a class of compounds dubbed nucleoside analogs, or nukes. Others include abacavir, 3TC, ddC, ddI, and D4T (the longer scientific names are not important). All are molecules that very much resemble one of the subunits—nucleosides—that are strung together by reverse transcriptase to make retroviral DNA. They are, in fact, DNA building blocks. What AZT and the other nukes do is fool the transcriptase into using them instead of the proper nucleoside. Proper nucleosides have a chemical hook on either end. One hook attaches to the growing DNA strand, while the other allows additional nucleosides to be attached. With nukes there is only one hook. Once the nuke is in place along the DNA chain, growth of the chain terminates and viral replication is halted.
There is another class of drugs that also cripple the functioning of reverse transcriptase. They are not, however, nucleoside analogs, hence the term non-nucleoside reverse transcriptase inhibitors (NNRTIs), and their mode of action is entirely different from the nukes.
Unfortunately, in a matter of months variants of reverse transcriptase appear in HIV that can produce viral DNA in the presence of either variety of inhibitor. Add to this the high toxicity level of nukes—up to 40 percent of the people on AZT are unable to tolerate it for more than six weeks due to the severe anemia it causes—and they are far from the ideal drugs.
The thought then crossed the minds of AIDS researchers to use two or even three nukes simultaneously, forcing the virus to undergo many more mutations to become resistant to the combination. Such double and triple drug therapies have indeed proved more effective than AZT alone and should buy more time for the AIDS sufferer. They are not, however, the knockout punch scientists are looking for. HIV will not succumb to one or even a combination of drugs aimed at a single step in its life cycle.
Which brings us to protease inhibitors (PIs)—another important weapon in our battle with HIV. Protease is one of the enzymes that HIV carries in its core. During the latter stages of viral infection protease is called on to perform a necessary step in synthesis of intact viral particles. It acts as a molecular scissors, snipping long protein molecules into smaller functional units. If this is not done, the virus cannot be assembled properly.
Scientists, of course, would like nothing more than to keep HIV from assembling properly. Toward this end they have come up with drugs that interfere with protease action—gum up the scissors, if you will. They are the protease inhibitors.
Serious testing of protease inhibitors began in 1995, with much scientific fanfare and unbridled optimism. To begin with, PIs were much less toxic than AZT and other nukes. And initial studies showed that they brought about almost complete cessation of viral replication. But, as with AZT, the protease inhibitors must deal with HIV’s incredible ability to alter itself and its proteases so inhibitors will no longer inhibit. This explains the rationale behind combination drug therapy—the prescribing of one or more nukes in conjunction with protease inhibitors. To quote Dr. David Ho, head of New York’s Aaron Diamond AIDS Research Center: “With protease there’s been virtually a 98 to 99 percent inhibition of the virus. That’s pretty potent. Add another drug like that, or a third one, and you start to pile them up, and just think about the kind of pressure that you put on the virus” (New York Magazine, February 20, 1995).
These AIDS “cocktails”—formally known as highly active antiretroviral therapy (HAART)—are a grueling regimen of strictly scheduled pill popping. Add to that the antivirals and antibacterials needed to control opportunistic infections, and the number of capsules climbs to as many as two dozen a day. But they are strikingly effective, and by 1997 had substantially reduced the number of AIDS deaths in the United States and Europe. It is lamentable that, at upwards of fifteen thousand dollars a person per annum, the treatments were largely unavailable to the millions of afflicted in developing nations.
Nothing is simple when dealing with as crafty an adversary as HIV. Scientists are continually searching for its Achilles’ heel, and they think they may have found something in a class of compounds known as entry inhibitors. As the name suggests, these drugs attempt to thwart infection by preventing the virus from getting into our cells. The strategy is to confuse HIV by releasing “decoys” into the blood. One such decoy is the CD4 molecule, which, when on the surface of a T cell, acts as a docking site for the virus. It is reasoned that HIV will attach to these free CD4s instead of the CD4s on T cells, and infection will be averted.
One type of entry inhibitor furthest along in development is called T-20, a compound that mimics not the CD4 site of the T cell, but rather the gp 41 portion of the HIV protein envelope. It is gp 41 that facilitates fusion of HIV with the cell membrane, allowing admittance into the cell. What T-20 molecules do is bind with uninfected immune cells, thereby denying HIV the opportunity to infect.
Regardless of how they work, what all entry inhibitors share is an ability to thwart the virus outside the cell, before infection occurs. And that, some experts believe, may give them a better chance of dispatching HIV than the currently available antivirals, all of which work inside cells already infected. “Working outside the cell gives them, in theory, a major advantage,” says David Ho, the 1996 Time Man of the Year, “because cell membranes can present barriers to some drugs, and some have molecules that pump out drugs that manage to get inside.” These drugs are also effective in lower doses and safer for patients than the traditional drug cocktail therapies.
Buoyed by the success of these trials, researchers are busy investigating other compounds that would interrupt HIV’s reproductive cycle at critical points. One particularly attractive drug would block HIV from inserting its DNA into the host’s genome. And then there are the antisense drugs that target the actual genetic material, the RNA, of the virus. It works something like this:
The RNA of HIV is single stranded, unlike DNA, which has a complementary strand bonded to it (the famous double helix). One of the primary missions of this single strand of viral RNA is to churn out millions of molecules of a specific protein it was designed to manufacture. But what if biochemists synthesize a tiny stretch of RNA, called antisense RNA, that is complementary to a tiny stretch of HIV RNA? Such antisense snippets, when mixed with the virus, will hopefully stick to its RNA and effectively halt viral protein synthesis.
All of the sundry approaches to defeating HIV through pharmaceuticals underscore one central fact: the more we learn of HIV and how it operates the better and more varied our weapons against it can be. Not all drugs that show great promise in the test tube will pan out in clinical trials, for our knowledge of the interaction between HIV and our immune system is incomplete. Yet we have made great strides in slowing down viral replication, adding many years to the lives of those with full-blown AIDS. And until an effective vaccine is developed, antiretroviral drugs will remain the cornerstone of the anti-HIV effort.
The year 1981 will be remembered as a year of infamy, the year the AIDS pandemic struck—not only in America but in Europe and Africa as well. Actually the first AIDS case has been traced to an English sailor who died of the disease way back in 1959. His opportunistic infections so astounded doctors treating him that they froze and saved his blood and tissue samples. Sure enough, when tested decades later, they proved to be HIV-positive.
Before 1959 human blood—that available for testing—seems to have been free of HIV. So where did it come from, and why did it suddenly strike with such a vengeance?
Right from the beginning there has been no shortage of theories concerning the origin of AIDS. Many wild speculations abound. First there were conspiracy theories—either the Soviets or the Americans had genetically engineered a hybrid virus from a human and a sheep retrovirus. The fact that the nucleic acid of HIV was radically different from that of either virus it allegedly derived from did not seem to matter. Nor did the fact that in 1959 the technology for splicing new viruses together simply was not there. And why, pray tell, would any government purposely design a biological warfare germ that had an asymptomatic incubation period averaging nine years?
Another theory that seems to make more sense is that culturing of the poliovirus in the 1950s for the development of a vaccine introduced HIV into the human population. The reasoning goes like this: poliovirus is grown in live monkey cells—live monkey kidney cells, to be precise. Back then it seemed to be the only place they grew well. (Today poliovirus is grown in both monkey and human cells.) Unfortunately, monkeys sometimes harbor SIV, which is remarkably similar to HIV. Couldn’t contamination of a polio vaccine with SIV have occurred?
Certainly it could have happened, although that’s unlikely. To begin with, SIV is restricted to African monkeys, whereas the monkeys used in the manufacture of polio vaccine came almost exclusively from Asia. And there are other pieces of the puzzle that don’t quite fit, such as the time frame and the strain of SIV being implicated. Yet the theory is hard to dismiss outright, and it has gained wide popularity. In fact, several years ago a father of a girl who inexplicably contracted AIDS sued the provider of her polio vaccine.
Although laboratory-grown monkey cells are not the likely source of HIV, it most likely was an SIV from which the AIDS virus evolved. The evolutionary trail, however, is not an easy one to trace. For starters, there are two types of HIV. HIV-1 is the original AIDS virus. It is responsible for the pandemic the world is now experiencing. But in 1985 a second HIV was found and dubbed HIV-2. We now know that it is much less virulent than HIV-1, often causing no illness, and is found almost exclusively in Africa. Also, because it seems to be more benign, HIV-2 is probably an older virus that has existed in the human population for centuries. To complicate matters further, there are many different types of SIV, infecting a wide range of primates. African green monkeys, macaques, sooty mangabeys, cynomolgus monkeys, and chimpanzees all have their SIVs. Which if any are related to HIV-1 and HIV-2?
Interestingly, HIV-1 and HIV-2 are related only distantly, sharing a mere 43 percent of their DNA sequences. Compare that with the 75 percent homology between HIV-2 and SIV of the African green monkey. And HIV-2 is so closely related to the SIV of macaques that some scientists took to using a single designation for the two—HIV/SIVmac. What does all this mean? Zoonosis, or the cross-species transmission of virus from primates to humans, has most likely occurred and is probably still occurring. There is also little doubt that such genetic similarity indicates a common ancestry between HIV-2 and the SIVs.
But what about HIV-1—the AIDS virus—and its origins? The evolutionary trail becomes even more tortuous and difficult to follow. When a computer was enlisted to analyze all of the accumulated data, what emerged were six distinct groups or subtypes of HIV-1. Each group, labeled A through F, had its own unique geographic distribution, mode of transmission, and type of infectivity. Subtype B, for example, was the only one found in North America. It was spread easily through homosexual sex and often resulted in opportunistic infections such as PCP and Kaposi’s sarcoma. Subtype A, on the other hand, was the AIDS virus of central Africa and India. It was more readily transmitted through heterosexual sex than subtype B and was much more apt to cause chronic diarrhea and a wasting of the body.
Some researchers believe that HIV-1 has existed at a low level in human populations for hundreds of years, and human activity, not biological change, brought on its emergence. But the latest modeling, using the world’s fastest supercomputer, Nirvana, at Los Alamos National Laboratory, suggests otherwise. Based on the genetic dissimilarity of the various subtypes and known mutation rates of HIV-1, this virus most likely made the transition from chimpanzees circa 1930. It was then that the chimp SIV crossed over to a new genus and species, perhaps infecting humans when they killed and ate the animals. Whatever its evolutionary past, the present and future of HIV are what most concern virologists.
AIDS is a most insidious disease. It seeks out and destroys the very cells designed to protect and defend us. Trying to assess our progress in dealing with HIV is like trying to decide if the glass is half full or half empty. To most it would appear half empty. There is no cure for the disease. There is no prophylactic vaccine. To quote Mark Caldwell in his 1993 Discover article, “The truth is that nobody really understands what’s going on between these invading retroviruses and the host’s immune system.” As we approach the year 2003, much of what Caldwell said still rings true. Scientists, as yet, don’t have a clue as to how HIV kills all the T4 cells it does. A majority of these cells do not even have HIV replicating within them. Several theories have been promulgated. One is that cells, even uninfected ones, can have certain of their genes turned on, causing them, in effect, to commit suicide. The phenomenon, known as apoptosis, has its share of proponents. Another belief is that antibodies to viral glycoproteins attack and destroy healthy cells—the old autoimmune theme. In yet a third theory, scientists speculate that perhaps HIV kills the immature cells of the immune system that go on to produce T cells.
Perhaps, perhaps. Meanwhile people keep dying. But there is light at the end of the tunnel. The nations of the world seem finally to be of one mind in their resolve to defeat this scourge on humanity. In 2001 the United Nations General Assembly convened a session on HIV/AIDS, the first ever devoted to a public health crisis. A global action plan was drafted, one that will require seven to ten billion dollars a year to implement, coming mostly from developed nations. Private individuals and corporations are contributing their resources to fund groups such as the International AIDS Vaccine Initiative (IAVI), a New York-based agency presently financing five companies involved in vaccine research. Its goal is to have eight to twelve vaccines in development by 2007. Bill and Melinda Gates, of Microsoft fame, have created a foundation that has already pumped more than twenty-four billion dollars into promoting health care in the developing world. Much of the investment is aimed at defeating HIV. With AIDS champion Dr. Anthony Fauci at its helm, the National Institute for Allergy and Infectious Diseases (NIAID) created the HIV Vaccine Trials Network (HVTN), with twelve trial sites within the United States and thirteen outside the country. It is working hand-in-glove with Merck and Co. and other vaccine manufacturers.
Hope springs eternal. On the optimistic side, we appear to be on the threshold of discovering new drugs and drug combinations that will greatly slow down, if not halt, the virus in its tracks. We know its genes, and we know its proteins. We know a lot about how they go about their business. It is a multistep affair, and we are attempting to foil the virus at each and every step. Too many great minds and great technologies are at work worldwide for us not to succeed. Let’s hope it will not take another two decades.