No field of science has cast more light on both the past and the future of our species than evolutionary biology. Recently, the pace of new discoveries about how we have evolved has increased (Culotta and Pennisi, 2005).
It is now clear that we are less unique than we used to think. Genetic and palaeontological evidence is now accumulating that hominids with a high level of intelligence, tool-making ability, and probably communication skills have evolved independently more than once. They evolved in Africa (our own ancestors), in Europe (the ancestors of the Neanderthals) and in Southeast Asia (the remarkable ‘hobbits’, who may be miniaturized and highly acculturated Homo erectus).
It is also becoming clear that the genes that contribute to the characteristics of our species can be found and that the histories of these genes can be understood. Comparisons of entire genomes have shown that genes involved in brain function have evolved more quickly in hominids than in more distantly related primates.
The genetic differences among human groups can now be investigated. Characters that we tend to think of as extremely important markers enabling us to distinguish among different human groups now turn out to be understandable at the genetic level, and their genetic history can be traced. Recently a single allelic difference between Europeans and Africans has been found (Lamason et al., 2005). This functional allelic difference accounts for about a third of the differences in skin pigmentation in these groups. Skin colour differences, in spite of the great importance they have assumed in human societies, are the result of natural selection acting on a small number of genes that are likely to have no effects beyond their influence on skin colour itself.
How do these and other recent findings from fields ranging from palaeontology to molecular biology fit into present-day evolution theory, and what light do they cast on how our species is likely to evolve in the future?
I will introduce this question by examining briefly how evolutionary change takes place. I will then turn to the role of environmental changes that have resulted in evolutionary changes in the past and extrapolate from those past changes to the changes that we can expect in the short-term and long-term future. These changes will be placed in the context of what we currently know about the evolution of our species. I will group these changes into physical changes and changes that stem from alterations of our own intellectual abilities. I will show that the latter have played and will continue to play a large role in our evolution and in the evolution of other animal and plant species with which we interact. Finally, I will turn to a specific examination of the probable course of future evolution of our species and of the other species on which we depend.
Evolutionary changes in populations, of humans and all other organisms, depend on five factors.
The first and perhaps the most essential is mutation. Evolution depends on the fact that genetic material does not replicate precisely, and that errors are inevitably introduced as genes are passed from one generation to the next. In the absence of mutation, evolutionary change would slow and eventually stop.
The effects of mutations are not necessarily correlated with the sizes of the mutational changes themselves. Single changes in the base sequence of DNA can have no effect or profound effects on the phenotype – the allelic differences that affect skin colour, as discussed in Section 3.1, can be traced to a single alteration in a base from G to A, changing one amino acid in the protein from an alanine to a threonine. At the other end of the spectrum, entire doublings of chromosome number, which take place commonly in plants and less often in animals, can disturb development dramatically – human babies who have twice the normal number of chromosomes die soon after birth. But such doubling can sometimes have little effect on the organism.
A fascinating source of mutation-like changes has recently been discovered. Viruses and other pieces of DNA can transfer genes from one animal, plant, or bacterial species to another, a process known as horizontal gene transfer. Such transfers appear to have played little part in our own recent history, but they have been involved in the acquisition of important, new capabilities in the past: the origin of our adaptive immune system is one remarkable example (Agrawal et al., 1998).
The most important mechanism that decides which of these mutational changes are preserved and which are lost is natural selection. We normally think of natural selection as taking place when the environment changes. But environmental change is not essential to evolution. Darwin realized that natural selection is taking place all the time. In each generation, even if the environment is unchanged, the fittest organisms are the most likely to survive and produce offspring. New mutations will continue to arise, a few of which will enable their carriers to take greater advantage of their environment even if it is not changing.
It is now realized that natural selection often acts to preserve genetic variation in populations. This type of selection, called balancing selection, results from a balance of selective pressures acting on genetic variation. It comes in many forms (Garrigan and Hedrick, 2003). Heterozygote advantage preserves the harmful sickle cell allele in human populations because people who are heterozygous for the allele are better able to resist the effects of malaria. A more prevalent type of balancing selection is frequency-dependent selection, in which a mutant allele may be beneficial when it is rare but loses that benefit as it rises in frequency. Such selection has the capability of maintaining many alleles at a genetic locus in a population. It also has the intriguing property that as alleles move to their internal equilibrium frequencies, the cost of maintaining the polymorphism goes down. This evolutionary “freebie” means that many frequency-dependent polymorphisms can be maintained in a population simultaneously.
Three other factors play important but usually subordinate roles in evolutionary change: genetic recombination, the chance effects caused by genetic drift, and gene flow between populations.
Arguments have been made that these evolutionary processes are having little effect on our species at the present time (Jones, 1991). If so, this is simply because our species is experiencing a rare halcyon period in its history. During the evolutionary eye blink of the last 10,000 years, since the invention of agriculture and the rise of technology, our population has expanded dramatically. The result has been that large numbers of individuals who would otherwise have died have been able to survive and reproduce. I have argued elsewhere (Wills, 1998) and will explore later in this chapter the thesis that even this halcyon period may be largely an illusion. Powerful psychological pressures and new environmental factors (Spira and Multigner, 1998) are currently playing a major role in determining who among us reproduces.
It seems likely that this halcyon period (if it really qualifies as one) will soon come to an end. This book examines many possible scenarios for such resurgence in the strength of natural selection, and in this chapter I will examine how these scenarios might affect our future evolution.
As I pointed out earlier, evolutionary change can continue even in the absence of environmental change, but its pace is likely to be slow because it primarily ‘fine-tunes’ the adaptation of organisms that are already well adapted to their environment. When environmental changes occur, they can spur the pace of evolutionary change and can also provide advantages to new adaptations that would not have been selected for in an unchanging environment. What will be the evolutionary effects of environmental change that we can expect in the future? To gauge the likelihood of such effects, we must begin by examining the evolutionary consequences of changes that took place in the past. Let us look first at completed evolutionary changes, in which the evolutionary consequences of an environmental change have been fully realized, and then examine ongoing evolutionary changes in which the evolutionary changes that result from environmental change have only begun to take place.
Throughout the history of life environmental changes, and the evolutionary changes that result, have sometimes been so extreme as to cause massive extinctions. Nonetheless, given enough time, our planet’s biosphere can recover and regain its former diversity. Consider the disaster that hit the Earth 65 million years ago. A brief description of what happened can hardly begin to convey its severity.
One day approximately 65 ± 1 million years ago, without warning, a 10 km wide asteroid plunged into the atmosphere above the Yucatan peninsula at a steep angle from the southeast. It traversed the distance from upper stratosphere to the shallow sea in 20 seconds, heating the air ahead of it to a blue-hot plasma. The asteroid hit the ocean and penetrated through the sea bottom, first into the Earth’s crust and then into the molten mantle beneath the crust. As it did so, it heated up and exploded. The energy released, equivalent to 100 million megatons of TNT, was at least a million times as great as the largest hydrogen bomb that we humans have ever exploded. The atmospheric shock wave moved at several times the speed of sound across North America, incinerating all the forests in its path. Crust and mantle material erupted towards the sky, cooling and forming an immense choking cloud as it spread outwards. Shock waves raced through the crust, triggering force-10 earthquakes around the planet, and a 300 m high tsunami spread further destruction across a wide swath of all the Earth’s coastal regions. Volcanoes erupted along the planet’s great fault lines, adding their own noxious gases and dust to the witches’ brew that was accumulating in the atmosphere.
Most of the animals and plants that lived in southern North America and the northern part of South America were killed by the direct effects of the impact. As the great cloud of dust blanketed the Earth over the next six months, blocking out the sun, many more animals and plants succumbed. There was no safe place on land or sea. Carbon dioxide released from the bolide impact caused a spike in temperatures worldwide (Beerling et al., 2002). All the dinosaurs perished, along with all the large flying and ocean-going reptiles and all the abundant nautilus-like ammonites that had swum in the oceans; of the mammals and birds, only a few survived.
When the dust eventually began to clear the landscape was ghastly and moonlike, with only a few timid ferns poking out of cracks in the seared rocks and soil, and a few tiny mammals and tattered birds surviving on the last of their stores of seeds. It took the better part of a million years for the planet to recover its former verdant exuberance. And it took another 4 million years before new species of mammals filled all the ecological niches that had been vacated by the ruling reptiles.
Asteroid impacts as large as the one that drove the dinosaurs to extinction are rare and have probably happened no more than two or three times during the last half billion years. But at least seven smaller impacts, each sufficiently severe to result in a wave of extinction, have occurred during that period. Each was followed by a recovery period, ranging up to a few million years (though many of the recovery times may have been less than that (Alroy et al., 2001)). During these recovery periods further extinctions took place and new clades of animals and plants appeared.
Asteroids are not the only source of environmental devastation. Massive volcanic eruptions that took place some 251 million years ago were the probable cause of the most massive wave of extinctions our planet has ever seen, the Permian-Triassic extinction (e.g., Benton, 2003). As befits the violence of that extinction event, the resulting alterations in the biosphere were profound. The event set in motion a wave of evolutionary change leading to mammal-like therapsid reptiles (although therapsids with some mammalian characteristics had already appeared before the extinction event). It also gave the ancestors of the dinosaurs an opportunity to expand into vacant ecological niches, though the earliest dinosaurs of which we have records did not appear in the fossil record until 20 million years after the extinction event (Flynn et al., 1999).
Completed catastrophic events are characterized by both mass extinctions and sufficient time for recovery to take place. In general, the more severe the extinction event, more are the differences found to separate the pre-event world from the recovered world. The characteristics of the recovered world are largely shaped by the types of organisms that survived the catastrophe, but complex and unexpected subsequent interactions can take place. Therapsids with some mammalian characteristics survived the Permian-Triassic extinction, and the descendants of these surviving therapsids dominated the world for much of the Triassic period. Nonetheless, halfway through the Triassic, the therapsids began to lose ground. Dinosaurs began to dominate the niches for large land animals, with the result that the mammalian lineages that survived this conflict were primarily small herbivores and insectivores. However, some mammals were able to occupy specialized niches and grow quite large (Ji et al., 2006), and others were sufficiently big and fierce that they were able to prey on small dinosaurs (Hu et al., 2005). The later Cretaceous-Tertiary extinction provided the mammals with the opportunity to take over from the dinosaurs once more.
A massive extinction event such as the Cretaceous-Tertiary event has a low likelihood of occurrence during any given short time period, and that probability has fluctuated only moderately over the course of the history of Earth, at least before the advent of humanity. But even though such major events are unlikely in the near future, less dramatic environmental changes, many driven by our own activities, are taking place at the present time. Glaciers have advanced and retreated at least 11 times during the last 2.4 million years. The diversity of vertebrates before the onset of this series of ice ages was far greater than the diversity of vertebrates of the present time (Barnosky et al., 2004; Zink and Slowinski, 1995). More recently, human hunting resulted in a wave of large mammal and bird extinctions in the late Pleistocene (Surovell et al., 2005).
There has been a relatively mild decrease in the number of species of mammal, compared with their great abundance in the early Ploicene. This decrease has resulted from Pliocene and Pleistocene climate change and from the human Pleistocene overkill. Now, the decrease is likely to become substantially steeper. It is clear that the relatively mild decrease in the number of species resulting from Pliocene and Pleistocene climate change and from the human Pleistocene overkill is likely to become substantially steeper. Some of the often discussed worst-case scenarios for the future range from the onset of an ice age of such severity that the planet freezes from poles to equator, to a series of nuclear wars or volcanic eruptions that irreversibly poison the atmosphere and oceans. If such terrifying scenarios do not transpire, however, we have a good chance of coming to terms with our environment and slowing the rate of extinction.
As we have seen, alterations in the environment can open up opportunities for evolutionary change as well as close them off through extinction. Even small environmental changes can sometimes have dramatic evolutionary consequences over short spans of time. Three species of diploid flowering plant (Tragopogon, Asteraceae) were introduced into western Washington State, North America, from Europe about 100 years ago. Two tetraploid species, arising from different combinations of these diploids, arose without human intervention soon afterwards and have thrived in this area (though such tetraploids have not appeared in their native Europe). DNA studies have shown that a variety of genetic modifications have occurred in these two tetraploids over a few decades (Cook et al., 1998). This report and many similar such stories of rapid recent evolution in both animals and plants indicate that evolutionary changes can take place within the span of a human lifetime.
New analyses of the fossil record suggest that recovery to former diversity levels from even severe environmental disasters may be more rapid than had previously been thought (Alroy et al., 2001). Present-day ecosystem diversity may also be regained rapidly after minor disturbances. We have recently shown that the diversity levels of tropical forest ecosystems are resilient, and that while these forests may not recover easily from severe environmental disasters there is an advantage to diversity that can lead to rapid recovery after limited damage (Wills et al., 2006).
Nothing illustrates the potential for rapid evolutionary response to environmental change in our own species more vividly than the discovery in 2004 of a previously unknown group of hominids, the ‘hobbits’. These tiny people, one metre tall, lived on the island of Flores and probably on other islands of what is now Indonesia, as recently as 12,000 years ago (Brown et al., 2004). They have been given the formal name Homo floresiensis, but I suspect that it is the name hobbit that will stick. Sophisticated tools found near their remains provide strong evidence that these people, who had brains no larger than those of chimpanzees, were nonetheless expert tool users and hunters. Stone points and blades, including small blades that showed signs of being hafted, were found in the same stratum as the skeletal remains (Brown et al., 2004). Using these tools the hobbits might have been able to kill (and perhaps even help to drive extinct!) the pygmy mastodons with which they shared the islands.
It is probable that the hobbits had physically larger ancestors and that the hobbits themselves were selected for reduced stature when their ancestors reached islands such as Flores, where food was limited. It was this relatively minor change in the physical environment, one that nonetheless had a substantial effect on survival, that selected for the hobbits’ reduction in stature. At the same time, new hunting opportunities and additional selective pressures must have driven their ability to fashion sophisticated weapons.
The ancestor of the hobbits may have been Homo erectus, a hominid lineage that has remained distinct from ours for approximately 2 million years. But, puzzlingly, features of the hobbits’ skeletons indicate that they had retained a mix of different morphologies, some dating back to a period 3 million years ago – long before the evolution of the morphologically different genus Homo. The history of the hobbits is likely to be longer and more complex than we currently imagine (Dennell and Roebroeks, 2005).
Determining whether the hobbits are descendants of Homo erectus, or of earlier lineages such as Homo habilis, requires DNA evidence. No DNA has yet been isolated from the hobbit bones that have been found so far, because the bones are water-soaked and poorly preserved. In the absence of such evidence it is not possible to do more than speculate how long it took the hobbits to evolve from larger ancestors. But, when better-preserved hobbit remains are discovered and DNA sequences are obtained from them, much light will be cast on the details of this case of rapid and continuing evolvability of some of our closest relatives.
The evolution of the hobbits was strongly influenced by the colonization of islands by their ancestors. Such colonizations are common sources of evolutionary changes in both animals and plants. Is it possible that similar colonization events in the future could bring about a similar diversification of human types?
The answer to this question depends on the extent and effect of gene flow among the members of our species. At the moment the differences among human groups are being reduced because of gene flow that has been made possible by rapid and easy travel. Thus it is extremely unlikely that different human groups will diverge genetically because they will not be isolated. But widespread gene flow may not continue in the future. Consider one possible scenario described in the next paragraph.
If global warming results in a planet with a warm pole-to-pole climate, a pattern that was typical of the Miocene, there will be a rapid rise in sea level by 80 metres as all the world’s glaciers melt (Williams and Ferrigno, 1999). Depending on the rapidity of the melt, the sea level rise will be accompanied by repeated tsunamis as pieces of the Antarctic ice cap that are currently resting on land slide into the sea. There will also be massive disturbance of the ocean’s circulation pattern, probably including the diversion or loss of the Gulf Stream. Such changes could easily reduce the world’s arable land substantially – for example, all of California’s Central Valley and much of the southeastern United States would be under water. If the changes occur swiftly, the accompanying social upheavals would be substantial, possibly leading to warfare over the remaining resources. Almost certainly there would be substantial loss of human life, made far worse if atomic war breaks out. If the changes occur sufficiently slowly it is possible that alterations in our behaviour and the introduction of new agricultural technologies, may soften their impact.
What will be the evolutionary consequences to our species of such changes? Because of the wide current dispersal and portability of human technology, the ability to travel and communicate over long distances is unlikely to be lost completely as a result of such disasters unless the environmental disruption is extreme. But if a rapid decrease in population size were accompanied by societal breakdown and the loss of technology, the result could be geographic fragmentation of our species. There would then be a resumption of the genetic divergence among different human groups that had been taking place before the Age of Exploration (one extreme example of which is the evolution of the hobbits). Only under extremely adverse conditions, however, would the fragmentation persist long enough for distinctly different combinations of genes to become fixed in the different isolated groups. In all but the most extreme scenarios, technology and communication would become re-established over a span of a few generations, and gene flow between human groups would resume.
Both large and small changes in the physical environment can bring about evolutionary change. But even in the absence of such changes, cultural selective pressures that have acted on our species have had a large effect on our evolution and will continue to do so. To understand these pressures, we must put them into the context of hominid history. As we do so, we will see that cultural change has been a strong driving force of human evolution, and has also affected many other species with which we are associated.
About 6 million years ago, in Africa, our evolutionary lineage separated from the lineage that led to chimpanzees and bonobos. The recent discovery in Kenya of chimpanzee teeth that are half a million years old (McBrearty and Jablonski, 2005) shows that the chimpanzee lineage has remained distinct for most of that time from our own lineage, though the process of actual separation of the gene pools may have been a complicated one (Patterson et al., 2006). There is much evidence that our remote ancestors were morphologically closer to chimpanzees and bonobos than to modern humankind ourselves. The early hominid Ardipithecus ramidus, living in East Africa 4.4 million years ago, had skeletal features and a brain size resembling those of chimpanzees (White et al., 1994). Its skeleton differed from those of chimpanzees in only two crucial respects: a slightly more anterior position of the foramen magnum, which is the opening at the bottom of the skull through which the spinal cord passes, and molars with flat crowns like those of modern humans rather than the highly cusped molars of chimpanzees. If we could resurrect an A. ramidus it would probably look very much like a chimpanzee to us – though there is no doubt that A. ramidus and present-day chimpanzees would not recognize each other as members of the same species.
Evolutionary changes in the hominid line include a gradual movement in the direction of upright posture. The changes required a number of coordinated alterations in all parts of the skeleton but in the skull and the pelvis in particular. Perhaps the most striking feature of this movement towards upright posture is how gradual it has been. We can trace the change in posture through the gradual anterior movement of the skull’s foramen magnum that can be seen to have taken place from the oldest hominid fossils down to the most recent.
A second morphological change is of great interest. Hominid brains have undergone a substantial increase in size, with the result that modern human brains have more than three times the volume of a chimpanzee brain. Most of these increases have taken place during the last 2.5 million years of our history. The increases took place not only in our own immediate lineage but also in at least one other extinct lineage that branched off about a million years ago – the lineage of Europe and the Middle East that included the pre-Neanderthals and Neanderthals. It is worth emphasizing, however, that this overall evolutionary ‘trend’ may have counterexamples, in particular the apparent substantial reduction in both brain and body size in the H. floresiensis lineage. The remarkable abilities of the hobbits make it clear that brain size is not the only determiner of hominid success.
Our ability to manipulate objects and thus alter our environment has also undergone changes during the same period. A number of changes in the structure of hominid hands, such as the increase in brain size beginning at least 2.5 million years ago, have made them more flexible and sensitive. And our ability to communicate, too, has had a long evolutionary history, reflected in both physical and behavioural characteristics. The Neanderthals had a voice box indistinguishable from our own, suggesting that they were capable of speech (Arensburg et al., 1989). The ability of human children to learn a complex language quickly (and their enthusiasm for doing so) has only limited counterparts in other primates. Although some chimpanzees, bonobos and gorillas have shown remarkable understanding of human spoken language, their ability to produce language and to teach language to others is severely limited (Tagliatela et al., 2003).
Many of the changes in the hominid lineage have taken place since the beginning of the current series of glaciations 2.5 million years ago. There has been an accelerating increase in the number of animal and plant extinctions worldwide during this period. These include extinctions in our own lineage, such as those of H. habilis, H. erectus and H. ergaster, and more recently the Neanderthals and H. floresiensis. These extinctions have been accompanied by a rapid rate of evolution in our own lineage.
What are the cultural pressures that have contributed to this rapid evolution? I have argued elsewhere (Wills, 1993) that a feedback loop involving our brains, our bodies, our genes, and our rapidly changing cultural environment has been an important contributor to morphological and behavioural changes. Feedback loops are common in evolution and have led to many extreme results of sexual selection in animals and of interactions with pollinators among flowering plants. A ‘runaway brain’ feedback can explain why rapid changes have taken place in the hominid lineage.
The entire human and chimpanzee genomes are now available for comparison, opening up an astounding new world of possibilities for scientific investigation (Chimpanzee Sequencing and Analysis Consortium, 2005). Overall comparisons of the sequences show that some 10 million genetic changes separate us from chimpanzees. We have hardly begun to understand which of these changes have played the most essential role in our evolution. Even at such early stages in these genome-wide investigations, however, we can measure the relative rate of change of different classes of genes as they have diverged in the two lineages leading to humans and chimpanzees. It is now possible to examine the evolution of genes that are involved in brain function in the hominid lineage and to compare these changes with the evolution of the equivalent (homologous) genes in other primates.
The first such intergenomic comparisons have now been made between genes that are known to be involved in brain growth and metabolism and genes that affect development and metabolic processes in other tissues of the body. Two types of information have emerged, both of which demonstrate the rapid evolution of the hominid lineage.
First, the genes that are expressed in brain tissue have undergone more regulatory change in the human lineage than they have in other primate lineages. Gene regulation determines whether and when a particular gene is expressed in a particular tissue. Such regulation, which can involve many different interactions between regulatory proteins and stretches of DNA, has a strong influence on how we develop from embryo to adult. As we begin to understand some of these regulatory mechanisms, it is becoming clear that they have played a key role in many evolutionary changes, including major changes in morphology and behaviour. We can examine the rate of evolution of these regulatory changes by comparing the ways in which members of this class of genes are expressed in the brains of ourselves and of our close relatives (Enard et al., 2002). One pattern that often emerges is that a given gene may be expressed at the same level (say high or low) in both chimpanzees and rhesus monkeys, but at a different level (say intermediate) in humans. Numerous genes show similar patterns, indicating that their regulation has undergone significantly more alterations in our lineage than in those of other primates. Unlike the genes involved in brain function, regulatory changes have not occurred preferentially in the hominid lineage in genes expressed in the blood and liver.
Second, genes that are implicated in brain function have undergone more meaningful changes in the human lineage than in other lineages. Genes that code for proteins undergo two types of changes: non-synonymous changes that alter the proteins that the genes code for, possibly changing their function, and synonymous changes that change the genes but have no effect on the proteins. When genes that are involved in brain function are compared among different mammalian lineages, significantly more potentially functional changes have occurred in the hominid lineage than in the other lineages (Clark et al., 2003). This finding shows clearly that in the hominid lineage strong natural selection has changed genes involved in brain function more rapidly than the changes that have taken place in other lineages.
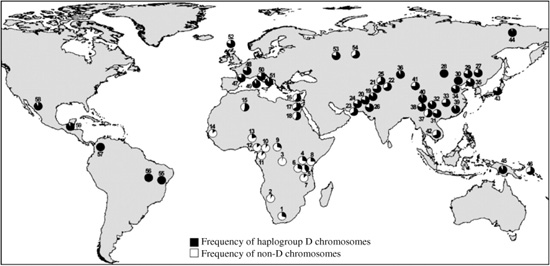
Specific changes in genes that are involved in brain function can now be followed in detail. Evidence is accumulating that six microcephalin genes are involved in the proliferation of neuroblasts during early brain development. One of these genes, MCPH1, has been found to carry a specific haplotype at high frequency throughout human populations (Evans et al., 2005), and it has reached highest frequency in Asia (Fig. 3.1). The haplotype has undergone some further mutations and recombinations since it first arose about 37,000 years ago, and these show strong linkage disequilibrium. Other alleles at this locus do not show such a pattern of disequilibrium. Because disequilibrium breaks down with time, it is clear that this recent haplotype has spread as a result of strong natural selection.
Fig. 3.1 Global frequencies of microcephalin haplogroup D chromosomes (defined as having the derived C allele at the G37995C diagnostic SNP) in a panel of 1184 individuals.
Fig. 3.2 Worldwide frequencies of ASPM haplogroup D chromosomes (defined as having the derived G allele at the A44871G diagnostic polymorphism), based on a panel of 1186 individuals.
Another gene also associated with microcephaly, abnormal spindle-like microcephaly-associated (ASPM), shows even more recent evidence of extremely strong selection (Mekel-Bobrov et al., 2005). An allele found chiefly in Europe and the Middle East, but at much lower frequencies in Asia (Fig. 3.2), appears to have arisen as recently as 5800 years ago. The spread of this allele has been so rapid that it must confer a selective advantage of several percent on its carriers.
Although both these alleles carry non-synonymous base changes, the allelic differences that are being selected may not be these changes but may be in linked regulatory regions. Further, a direct effect of these alleles on brain function has not been demonstrated. Nonetheless, the geographic patterns seen in these alleles indicate that natural selection is continuing to act powerfully on our species.
Hawks and coworkers (Hawks et al. 2007) have recently shown that the pattern of strong recent selection detectable by linkage disequilibrium extends to more than 2,000 regions of the human genome. They calculate that over the last 40,000 years our species has evolved at a rate 100 times as fast as our previous evolution. Such a rapid rate may require that many of the newly selected genes are maintained by frequency-dependent selection, to reduce the selective burden on our population.
All these pieces of evidence from our past history show that humans have retained the ability to evolve rapidly. But are we continuing to evolve at the present time? Without a doubt! Although the evolutionary pressures that are acting on us are often different from those that acted on our ancestors a million years ago or even 10,000 years ago, we are still exposed to many of the same pressures. Selection for resistance to infectious disease continues. We have conquered, at least temporarily, many human diseases, but many others – such as tuberculosis, AIDS, malaria, influenza, and many diarrhoeal diseases-continue to be killers on a massive scale (see Chapter 14, this volume). Selection for resistance to these diseases is a continuing process because the disease organisms themselves are also evolving.
Although the selective effects of psychological pressures are difficult to measure, they must also play a role. Michael Marmot and his colleagues have shown that an individual’s position in a work hierarchy has an impact on his or her health (Singh-Manoux et al., 2005). The members at the top level of the hierarchy live healthier lives than those at the bottom level – or even those who occupy positions slightly below the top level!
Reproduction also applies psychological pressures. The introduction of effective means of birth control has provided personal choice in reproduction for more people than at any time in the past. We can only surmise how selection for genes that influence reproductive decision-making will affect our future evolution. There is some evidence from twin studies, however, that heritability for reproductive traits, especially for age at first reproduction, is substantial (Kirk et al., 2001). Thus, rapid changes in population growth rates such as those being experienced in Europe and the former Soviet Union are likely to have evolutionary consequences.
Concerns over dysgenic pressures on the human population resulting from the build-up of harmful genes (Muller, 1950) have abated. It is now realized that even in the absence of selection, harmful mutant alleles will accumulate only slowly in our gene pool and that many human characteristics have a complex genetic component so that it is impossible to predict the effects of selection on them. Alleles that are clearly harmful – sickle cell, Tay-Sachs, muscular dystrophy and others – will soon be amenable to replacement by functional alleles through precise gene surgery performed on germ line cells. Such surgery, even though it will be of enormous benefit to individuals, is unlikely to have much of an effect on our enormous gene pool unless it becomes extremely inexpensive.
One intriguing direction for current and future human evolution that has received little or no attention is selection for intellectual diversity. The measurement of human intellectual capabilities, and their heritability, is at a primitive stage. The heritability of IQ has received a great deal of attention, but a recent meta-analysis estimates broad heritability of IQ to be 0.5 and narrow heritability (the component of heritability that measures selectable phenotypic variation) to be as low as 0.34 (Devlin et al., 1997). But IQ is only one aspect of human intelligence, and other aspects of intelligence need investigation. Daniel Goleman has proposed that social intelligence, the ability to interact with others, is at least as important as IQ (Goleman, 1995), and Howard Gardner has explored multiple intelligences ranging from artistic and musical through political to mechanical (Gardner, 1993). All of us have a different mix of such intelligences. To the extent that genes contribute to them, these genes are likely to be polymorphic – that is, to have a number of alleles, each at appreciable frequency, in the human population.
This hypothesis of polymorphic genes involved in behaviour leads to two predictions. First, loci influencing brain function in humans should have more alleles than the same loci in chimpanzees. Note, however, that most of the functional polymorphic differences are likely to be found, not in structural genes, but in the regulatory regions that influence how these genes are expressed. Because of the difficulty of determining which genetic differences in the polymorphisms are responsible for the phenotypic effects, it may be some time before this prediction can be tested.
The second prediction is that some type of balancing selection, probably with a frequency-dependent component, is likely to be maintaining these alleles in the human population.
When an allele has an advantage if it is rare but loses that advantage if it is common, it will tend to be maintained at the frequency at which there is neither an advantage nor a disadvantage. If we suppose that alleles influencing many behaviours or skills in the human population provide an advantage when they are rare, but lose that advantage when they are common, there will be a tendency for the population to accumulate these alleles at such intermediate frequencies. And, as noted earlier, if these genes are maintained by frequency dependence, the cost to the population of maintaining this diversity can be low.
Numerous examples of frequency-dependent balancing selection have been found in populations. One that influences behaviours has been found in Drosophila melanogaster. Natural populations of this fly are polymorphic for two alleles at a locus (for, standing for forager) that codes for a protein kinase. A recessive allele at this locus, sitter, causes larvae to sit in the same spot while feeding. The dominant allele, rover, causes its carriers to move about while feeding. Neither allele can take over (reach fixation) in the population. Rover has an advantage when food is scarce, because rover larvae can find more food and grow more quickly than sitter larvae. Sitter has an advantage when food is plentiful. If they are surrounded by abundance, sitter larvae that do not waste time and effort moving about can mature more quickly than rovers (Sokolowski et al., 1997).
It will be fascinating to see whether behaviour-influencing polymorphisms such as those at the for locus are common in human populations. If so, one type of evolutionary change may be the addition of new alleles at these loci as our culture and technology become more complex and opportunities for new types of behaviours arise. It is striking that the common MCPH1 allele has not reached fixation in any human population, even though it has been under positive selection since before modern humans spread to Europe. It may be that this allele is advantageous when it is rare but loses that advantage when it becomes common. There appear to be no behavioral effects associated with this allele (Mekel-Bobrov et al., 2007), but detailed studies may reveal small differences. Natural selection can work on small phenotypic differences as well as large, and much of our recent evolution may have resulted from selection for genes with small effects.
There has been much speculation about the effects of genetic engineering on the future of our species, including the possibility that a ‘genetic elite’ may emerge that would benefit from such engineering to the exclusion of other human groups (e.g., Silver, 1998). Two strong counterarguments to this viewpoint can be made.
First, the number of genes that can potentially be modified in our species is immense. Assuming 50,000 genes per diploid human genome and 6 billion individuals, the number of genes in our gene pool is 3 × 1014. The task of changing even a tiny fraction of these genes would be enormous, especially since each such change could lead to dangerous and unexpected side effects. It is far more likely that our growing understanding of gene function will enable us to design specific drugs and other compounds that can produce desirable changes in our phenotypes and that these changes will be sufficiently easy and inexpensive that they will not be confined to a genetic elite (Wills, 1998). Even such milder phenotypic manipulations are fraught with danger, however, as we have seen from the negative effects that steroid and growth hormone treatments have had on athletes.
Second, the likelihood that a ‘genetic elite’ will become established seems remote. The modest narrow (selectable) heritability of IQ mentioned earlier shows the difficulty of establishing a genetic elite through selection. Heritabilities that are even lower are likely to be the rule for other physical or behavioural characteristics that we currently look upon as desirable.
Attempts to establish groups of clones of people with supposedly desirable characters would also have unexpected and unpredictable effects, in this case because of the environment. Clones of Bill Gates or Mother Teresa, growing up at a different time and in a different place, would turn into people who reflected the influences of their unique upbringing, just as the originals of such hypothetical clones did. And, luckily, environmental effects can work to defang evil dysgenic schemes as well as utopian eugenic ones. ‘The Boys from Brazil’ notwithstanding, it seems likely that if clones of Adolf Hitler were to be adopted into well-adjusted families in healthy societies they would grow up to be nice, well-adjusted young men.
Discussions of human evolution have tended to ignore the fact that we have greatly influenced the evolution of other species of animals and plants. These species have in turn influenced our own evolution. The abundant cereals that made the agricultural revolution possible were produced by unsung generations of primitive agriculturalists who carried out a process of long-continued artificial selection. Some results of such extremely effective selection are seen in Indian corn, which is an almost unrecognizable descendent of the wild grass teosinte, and domesticated wheat, which is an allohexaploid with genetic contributions from three different wild grasses. The current immense human population depends absolutely on these plants and also on other plants and animals that are the products of thousands of generations of artificial selection.
One consequence of climate change such as global warming is that the agriculture of the future will have to undergo rapid adaptations (see also Chapter 13, this volume). Southern corn leaf blight, a fungus that severely damaged corn production in the Southeast United States during the 1970s, was controlled by the introduction of resistant strains, but only after severe losses. If the climate warms, similar outbreaks of blight and other diseases that are prevalent in tropical and subtropical regions will become a growing threat to the world’s vast agricultural areas.
Our ability to construct new strains and varieties of animals and plants that are resistant to disease, drought, and other probable effects of climate change depends on the establishment and maintenance of stocks of wild ancestral species. Such stocks are difficult to maintain for long periods because governments and granting agencies tend to lose interest and because societal upheavals can sometimes destroy them. Some of the stocks of wild species related to domestic crops that were collected by Russian geneticist Nikolai Vavilov in the early part of the twentieth century have been lost, taking with them an unknown number of genes of great potential importance. It may be possible to avoid such losses in the future through the construction of multiple seed banks and gene banks to safeguard samples of the planet’s genetic diversity. The Norwegian government recently opened a bunker on Svalbard, an Arctic island, designed to hold around 2 million seeds, representing all known varieties of the world’s crops. Unfortunately, however, there are no plans to replicate this stock centre elsewhere.
Technology may aid us in adjusting to environmental change, provided that our technological capabilities remain intact during future periods of rapid environmental change. To cite one such example, a transgenic tomato strain capable of storing excess salt in its leaves while leaving its fruit relatively salt-free has been produced by overexpression of an Arabidopsis transporter gene. The salt-resistant plants can grow at levels of salt 50 times higher than those found in normal soil (Zhang and Blumwald, 2001). The ability to produce crop plants capable of growing under extreme environmental conditions may enable us to go on feeding our population even as we are confronted with shrinking areas of arable land.
Even a large global catastrophe such as a 10 km asteroidal/cometary impact would not spell doom for our species if we would manage to spread to other solar systems by the time the impactor arrives. We can, however, postulate a number of scenarios, short of extinction, that will test our ability to survive as a species. I will not discuss here scenarios involving intelligent machines or more radical forms of technology-enabled human transformation.
If climatic change either due to slow (e.g., anthropogenic) or due to sudden (e.g., supervolcanic) causative agents is severe and the survivors are few in number, there may be no time to adapt. The fate of the early medieval Norse colonists in Greenland, who died out when the climate changed because they could not shift from eating meat to eating fish (Berglund, 1986) stands as a vivid example. Jared Diamond (2005) has argued in a recent book that climate change has been an important factor in several cases of societal collapse in human history.
If the environmental change occurs over generations rather than years or decades, there may be time for us to alter our behaviours deliberately. These behavioural changes will not be evolutionary changes, at least at first, although as we will see the ability to make such changes depends on our evolutionary and cultural history. Rather they will consist of changes in memes (Dawkins, 1976), the non-genetic learned or imitated behaviours that form an essential part of human societies and technology. The change that will have the largest immediate effect will be population control, through voluntary or coerced means or both. Such changes are already having an effect. The one-child policy enforced by the Chinese government, imperfect though it is in practice, has accelerated that country’s demographic transition and helped it to gain a 20-fold increase in per capita income over the last quarter century.
Such demographic transitions are taking place with increasing rapidity in most parts of the world, even in the absence of government coercion. Predictions of a human population of 12 billion by 2050 were made by the United Nations in 1960. These frightening projections envisioned that our population would continue to increase rapidly even after 2050. These estimates have now been replaced by less extreme predictions that average nine billion by 2050, and some of these revised projections actually predict a slow decline in world population after mid-century. Demographic transitions in sub-Saharan Africa and South Asia will lag behind the rest of the planet, but there is no reason to suppose that these regions will not catch up during this century as education, particularly the education of women, continues to spread. These demographic transitions are unlikely to be reversed in the future, as long as education continues to spread. Recently the chief of a small village on the remote island of Rinca in Indonesia complained to me that all his six children wanted to go to medical school, and he could not imagine how he could send them all.
Accompanying the demographic transitions will be technological changes in how the planet is fed. If rising ocean levels cause the loss of immense amounts of arable land (including major parts of entire countries, such as low-lying Bangladesh), hordes of refugees will have to be fed and housed. Technology and alterations in our eating habits hold out some hope. Soy protein is similar in amino acid content to animal proteins, and its production has increased 400% in the past 30 years. This crop is already beginning to change our eating habits. And new agricultural infrastructure, such as intense hydroponic agriculture carried out under immense translucent geodesic domes with equipment for recycling water, will rapidly become adopted when the alternative is starvation.
Little will be done to confront these problems without a series of catastrophic events that make it clear even to the most reactionary societies and governments that drastic change is needed. These catastrophes are already beginning to throw into sharp relief current social behaviours that are inadequate for future challenges, such as a disproportionate use of the world’s limited resources by particular countries and restrictions on the free flow of information by dictatorial governments. Societal models based on national self-interest or on the preservation of power by a few will prove inadequate in the face of rapid and dramatic environmental changes. I will predict that – in spite of the widespread resistance to the idea – a global governmental organization with super-national powers, equivalent on a global scale to the European Union, will inevitably emerge. As we confront repeated catastrophes, we will see played out in the economic realm a strong selection against individual and societal behaviours that cannot be tolerated in a world of scarcity.
Discomfiting as such predictions may be to some, the accelerating rate of environmental change will make them inevitable. If we are to retain a substantial human population as the planet alters, our behaviours must alter as well. Otherwise, our societal upheavals may result in long-term ecological damage that can be reversed only after tens or hundreds of thousands of years.
Scream and argue and fight as we may about how to behave in the future, our past evolutionary history has provided most of us with the ability to change how we behave. This is our remarkable strength as a species.
A third future scenario, that of the colonization of other planets, is rapidly moving from the realm of science fiction to a real possibility. More than 200 extrasolar planets have been discovered in the last decade. These are mostly Jupiter-sized or larger, but it is safe to predict that within years or decades Earth-sized extrasolar planets, some of them showing evidence of life, will be found. The smallest extrasolar planet yet found is a recently discovered mere 5-Earth masses companion to Gliese 581 which lies in the habitable zone and is likely to possess surface water (Beaulieu et al., 2006). In view of the selection effects applicable to the surveys thus far, the discoveries of even smaller planets are inevitable.
This prediction is at variance with the argument presented by Ward and Brownlee (2000) that planets harbouring complex life are likely to be extremely rare in our galaxy. However, that argument was based on a biased interpretation of the available data and the most restrictive view on how planetary systems form (Kasting, 2001).
No more exciting moment of scientific discovery can be imagined than when we first obtain an image or spectrum of an Earth-sized planet with an oxygen-rich atmosphere circling a nearby star (soon to become possible with the advent of Darwin, Kepler, Gaia and several other terrestrial planet-seeking missions in the next several years). It is likely that the challenge of visiting and perhaps colonizing these planets is one that we as a species will be unable to resist.
The colonization of other planets will result in an explosive Darwinian adaptive radiation, involving both our species and the animals and plants that accompany us. Just as the mammals radiated into new ecological niches after the extinction of the dinosaurs, and the finches and land tortoises that Darwin encountered on the Galápagos Islands radiated adaptively as they spread to different islands, we will adapt in different ways to new planets that we explore and colonize.
The new planetary environments will be different indeed. How will we be able to colonize new planets peopled with indigenous life forms that have a different biochemistry and mechanism of inheritance from us? Could we, and the animals and plants that we bring with us, coexist with these indigenous and highly adapted life forms? Could we do so without damaging the ecology of the planets we will be occupying? And how will competition with these life forms change us? Will we be able to direct and accelerate these changes to ourselves by deliberately modifying the genes of these small populations of colonists?
If we are able to adapt to these new environments, then 10,000 or 100,000 years from now our species will be spread over so wide a region that no single environment-caused disaster would be able to wipe us all out. But our continued existence would still be fraught with danger. Will we, collectively, still recognize each other as human? What new and needless prejudices will divide us, and what new misunderstandings will lead to pointless conflict?
Kareiva, P., Watts, S., McDonald, R., and Boucher, T. (2007). Domesticated nature: shaping landscapes and ecosystems for human welfare. Science, 316, 1866-1869. The likelihood that we will permanently alter the world’s ecosystems for our own benefit is explored in this paper.
Myers, N. and Knoll, A.H. (2001). The biotic crisis and the future of evolution. Proc. Natl. Acad. Sci. (USA), 98, 5389-5392. Discomfiting predictions about the course of future evolution can be found in this paper.
Palumbi, S.R. (2001). Humans as the world’s greatest evolutionary force. Science, 293, 1786-1790. The influence of humans on the evolution of other organisms is examined.
Unfortunately, none of these authors deals with the consequences of changes in evolutionary pressures on our own species.
Agrawal, A., Eastman, Q.M., and Schatz, D.G. (1998). Implications of transposition mediated by V(D) J-recombination proteins RAG1 and RAG2 for origins of antigen-specific immunity. Nature, 394, 744-751.
Alroy, J., Marshall, C.R., Bambach, R.K., Bezusko, K., Foote, M., Fursich, F.T., Hansen, T.A., Holland, S.M., Ivany, L.C., Jablonski, D., Jacobs, D.K., Jones, D.C., Kosnik, M.A., Lidgard, S., Low, S., Miller, A.I., Novack-Gottshall, P.M., Olszewski, T.D., Patzkowsky, M.E., Raup, D.M., Roy, K., Sepkoski, J.J., Jr, Ommers, M.G., Wagner, P.J., and Webber, A. (2001). Effects of sampling standardization on estimates of Phanerozoic marine diversification. Proc. Natl. Acad. Sci. (USA), 98, 6261–6266.
Arensburg, B., Tillier, A.M., Vandermeersch, B., Duday, H., Schepartz, L.A., and Rak, Y. (1989). A Middle Paleolithic hyoid bone. Nature, 338, 758–760.
Barnosky, A.D., Bell, C.J., Emslie, S.D., Goodwin, H.T., Mead, J.I., Repenning, C.A., Scott, E., and Shabel, A.B. (2004). Exceptional record of mid-Pleistocene vertebrates helps differentiate climatic from anthropogenic ecosystem perturbations. Proc. Natl. Acad. Sci. (USA), 101, 9297–9302.
Beaulieu, J.-P., Bennett, D.P., Fouque, P., Williams, A., Dominik, M., J0rgensen, U.G., Kubas, D., Cassan, A., Coutures, C., Greenhill, J., Hill, K., Menzies, J., Sackett, P.D., Albrow, M., Brillant, S., Caldwell, J.A.R., Calitz, J.J., Cook, K.H., Corrales, E., Desort, M., Dieters, S., Dominis, D., Donatowicz, J., Hoffman, M., Kane, S., Marquette, J.-B., Martin, R., Meintjes, P., Pollard, K., Sahu, K., Vinter, C., Wambsganss, J., Woller, K., Horne, K., Steele, I., Bramich, D.M., Burgdorf, M., Snodgrass, C., Bode, M., Udalski, A., Szymaski, M.K., Kubiak, M., Wickowski, T., Pietrzyski, G., Soszyski, I., Szewczyk, O., Wyrzykowski, L., Paczyski, B., Abe, F., Bond, I.A., Britton, T.R., Gilmore, A.C., Hearnshaw, J.B., Itow, Y., Kamiya, K., Kilmartin, P.M., Korpela, A.V., Masuda, K., Matsubara, Y., Motomura, M., Muraki, Y., Nakamura, S., Okada, C., Ohnishi, K., Rattenbury, N.J., Sako, T., Sato, S., Sasaki, M., Sekiguchi, T., Sullivan, D.J., Tristram, P.J., Yock, P.C.M., and Yoshioka, T. (2006). Discovery of a cool planet of 5.5 Earth masses through gravitational microlensing. Nature, 439, 437.
Beerling, D.J., Lomax, B.H., Royer, D.L., Upchurch, G.R., Jr, and Kump, L.R. (2002). An atmospheric pCO 2 reconstruction across the Cretaceous-Tertiary boundary from leafmegafossils. Proc. Natl. Acad. Sci. (USA), 99, 7836–7840.
Benton, M.J., and Twitchett, R.J. (2003) How to kill (almost) all life: the end-Permian extinction event, Trends Ecol. Evol. 18, 358–365.
Berglund, J. (1986). The decline of the Norse settlements in Greenland. Arc. Anthropol., 23, 109–136.
Brown, P., Sutikna, T., Morwood, M.J., Soejono, R.P., Jatmiko, Saptomo, E.W., and Due, R.A. (2004). A new small-bodied hominin from the Late Pleistocene of Flores, Indonesia. Nature, 431, 1055–1061.
Clark, A.G., Glanowski, S., Nielsen, R., Thomas, P.D., Kejariwal, A., Todd, M.A., Tanenbaum, D.M., Civello, D., Lu, F., Murphy, B., Ferriera, S., Wang, G., Zheng, X., White, T.J., Sninsky, J.J., Adams, M.D., and Cargill, M. (2003). Inferring nonneutral evolution from human-chimp-mouse orthologous gene trios. Science, 302, 1960 – 1963.
Chimpanzee Sequencing and Analysis Consortium, T.C.S. (2005). Initial sequence of the chimpanzee genome and comparison with the human genome. Nature, 437, 69–87.
Cook, L.M., Soltis, P.M., Brunsfeld, S.J., and Soltis, D.E. (1998). Multiple independent formations of Tragopogon tetraploids (Asteraceae): evidence from RAPD markers. Mol. Ecol., 7, 1293–1302.
Culotta, E. and Pennisi, E. (2005). Evolution in action. Science, 310, 1878–1879.
Dawkins, R. (1976). The Selfish Gene (New York, NY: Oxford University Press).
Dennell, R. and Roebroeks, W. (2005). An Asian perspective on early human dispersal from Africa. Nature, 438, 1099–1104.
Devlin, B., Daniels, M., and Roeder, K. (1997). The heritability of IQ. Nature, 388, 468–471.
Diamond, Jared (2005). Collapse: How Societies Choose to Fail or Succeed. Viking, New York.
Enard, W., Khaitovich, P., Klose, J., Zöllner, S., Heissig, F., Giavalisco, P., Nieselt-Struwe, K., Muchmore, E., Varki, A., Ravid, R., Doxiadis, G.M., Bontrop, R.E., and Pääbo, S. (2002). Intra- and interspecific variation in primate gene expression patterns. Science, 296, 340–343.
Evans, P.D., Gilbert, S.L., Mekel-Bobrov, N., Vallender, E.J., Anderson, J.R., Vaez-Azizi, L.M., Tishkoff, S.A., Hudson, R.R., and Lahn, B.T. (2005). Microcephalin, a gene regulating brain size, continues to evolve adaptively in humans. Science, 309, 1717–1720.
Flynn, J.J., Parrish, J.M., Rakotosamimanana, B., Simpson, W.F., Whatley, R.L., and Wyss, A.R. (1999). A Triassic fauna from Madagascar, including early dinosaurs. Science, 286, 763–765.
Gardner, H. (1993). Multiple Intelligences: The Theory in Practice (New York: Basic Books).
Garrigan, D. and Hedrick, P.W. (2003). Perspective: detecting adaptive molecular polymorphism: lessons from the MHC. Evolution, 57, 1707–1722.
Goleman, D. (1995). Emotional Intelligence (New York: Bantam Books).
Hawks, J., Wang, E.T., Cochran, G.M., Harpending, H.C. and Moyzis, R.K. (2007) Recent acceleration of human adaptive evolution. Proceedings of the National Academy of Sciences (US), 104, 20753–20758.
Hu, Y., Meng, J., Wang, Y., and Li, C. (2005). Large Mesozoic mammals fed on young dinosaurs. Nature, 433, 149–152.
Ji, Q., Luo, Z.-X., Yuan, C.-X., and Tabrum, A.R. (2006). A swimming mammaliaform from the middle Jurassic and ecomorphological diversification of early mammals. Science, 311, 1123–1127.
Jones, J.S. (1991). Is evolution over? If we can be sure about anything, it’s that humanity won’t become superhuman. New York Times, p.E17.
Kasting, J.F. (2001). Peter Ward and Donald Brownlee’s ‘Rare Earth’. Persp. Biol. Med., 44, 117–131.
Kirk, K.M., Blomberg, S.P., Duffy, D.L., Heath, A.C., Owens, I.P.F., and Martin, N.G. (2001). Natural selection and quantitative genetics of life-history traits in Western women: a twin study. Evolution, 55, 423–435.
Lamason, R.L., Mohideen, M.-A.P.K., Mest, J.R., Wong, A.C., Norton, H.L., Aros, M.C., Jurynec, M.J., Mao, X., Humphreville, V.R., Humbert, J.E., Sinha, S., Moore, J.L., Jagadeeswaran, P., Zhao, W., Ning, G., Makalowska, I., McKeigue, P.M., O’Donnell, D., Kittles, R., Parra, J., Mangini, N.J., Grunwald, D.J., Shriver, M.D., Canfield, V.A., and Cheng, K.C. (2005). SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science, 310, 1782–1786.
McBrearty, S. and Jablonski, N.G. (2005). First fossil chimpanzee. Nature, 437, 105–108.
Mekel-Bobrov, N., Posthuma, D., Gilbert, S.L., Lind, P., Gosso, M.F., Luciano, M., Harris, S.E., Bates, T.C., Polderman, T.J.C., Whalley, L.J., Fox, H., Starr, J.M., Evans, P.D., Montgomery, G.W., Fernandes, C., Heutink, P., Martin, N.G., Boomsma, D.I., Deary, I.J., Wright, M.J., de Geus, E.J.C. and Lahn, B.T. (2007) The ongoing adaptive evolution of ASPM and Microcephalin is not explained by increased intelligence. Human Molecular Genetics 16, 600–608.
Mekel-Bobrov, N., Gilbert, S.L., Evans, P.D., Vallender, E.J., Anderson, J.R., Hudson, R.R., Tishkoff, S.A., and Lahn, B.T. (2005). Ongoing adaptive evolution of ASPM, a brain size determinant in Homo sapiens. Science, 309, 1720–1722.
Muller, H.J. (1950). Our load of mutations. Am. J. Human Genet., 2, 111–176.
Patterson, N., Richter, D.J., Gnerre, S., Lander, E.S., and Reich, D. (2006). Genetic evidence for complex speciation of humans and chimpanzees. Nature, 441, 1103–1108.
Silver, L. (1998). Remaking Eden (New York: Harper).
Singh-Manoux, A., Marmot, M.G., and Adler, N.E. (2005). Does subjective social status predict health and change in health status better than objective status? Psychosomatic Medicine, 67, 855–861.
Sokolowski, M.B., Pereira, H.S., and Hughes, K. (1997). Evolution of foraging behavior in Drosophila by density-dependent selection. Proc. Natl. Acad. Sci. (USA), 94, 7373–7377.
Spira, A. and Multigner, L. (1998). Environmental factors and male infertility. Human Reprod., 13, 2041 -2042.
Surovell, T., Waguespack, N., and Brantingham, P.J. (2005). Global archaeological evidence for proboscidean overkill. Proc. Natl. Acad. Sci. (USA), 102, 6231–6236.
Tagliatela, J.P., Savage-Rumbaugh, S., and Baker, L.A. (2003). Vocal production by a language-competent Pan paniscus. Int. J. Primatol., 24, 1 -17.
Ward, P. and Brownlee, D. (2000). Rare Earth: Why Complex Life Is Uncommon in the Universe (New York: Copernicus Books).
White, T.D., Suwa, G., and Asfaw, B. (1994). Australopithecus ramidus, a new species of early hominid from Aramis, Ethiopia. Nature, 371, 306–312.
Williams, R.S. and Ferrigno, J. (1999). Estimated present-day area and volume of glaciers and maximum sea level rise potential. pp. 1–10. Satellite Image Atlas of Glaciers of the World. Washington DC: US Geological Survey.
Wills, C. (1993). The Runaway Brain: The Evolution of Human Uniqueness (New York: Basic Books).
Wills, C. (1998). Children of Prometheus: The Accelerating Pace of Human Evolution (Reading, MA: Perseus Books [formerly Addison-Wesley]).
Wills, C., Harms, K.E., Condit, R., King, D., Thompson, J., He, F., Muller-Landau, H.C., Ashton, P., Losos, E., Comita, L., Hubbell, S., LaFrankie, J., Bunyavejchewin, S., Dattaraja, H.S., Davies, S., Esufali, S., Foster, R., Gunatilleke, N., Gunatilleke, S., Hall, P., Itoh, A., John, R., Kiratiprayoon, S., de Lao, S.L., Massa, M., Nath, C., Noor, M.N.S., Kassim, A.R., Sukumar, R., Suresh, H.S., Sun, I.-F., Tan, S., Yamakura, T., and Zimmerman, J. (2006). Nonrandom processes maintain diversity in tropical forests. Science, 311, 527–531.
Zhang, H.-X. and Blumwald, E. (2001). Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat. Biotechnol., 19, 765–768.
Zink, R.M. and Slowinski, J.B. (1995). Evidence from molecular systematics for decreased avian diversification in the Pleistocene epoch. Proc. Natl. Acad. Sci. (USA), 92, 5832–5835.