5.
SIRT1, the “Rescue” Gene
Based on all the research and the study findings described so far, you should be convinced, as I am, of the efficacy of alternate-day dieting. You may, however, be wondering what it is about this plan that creates such benefits.
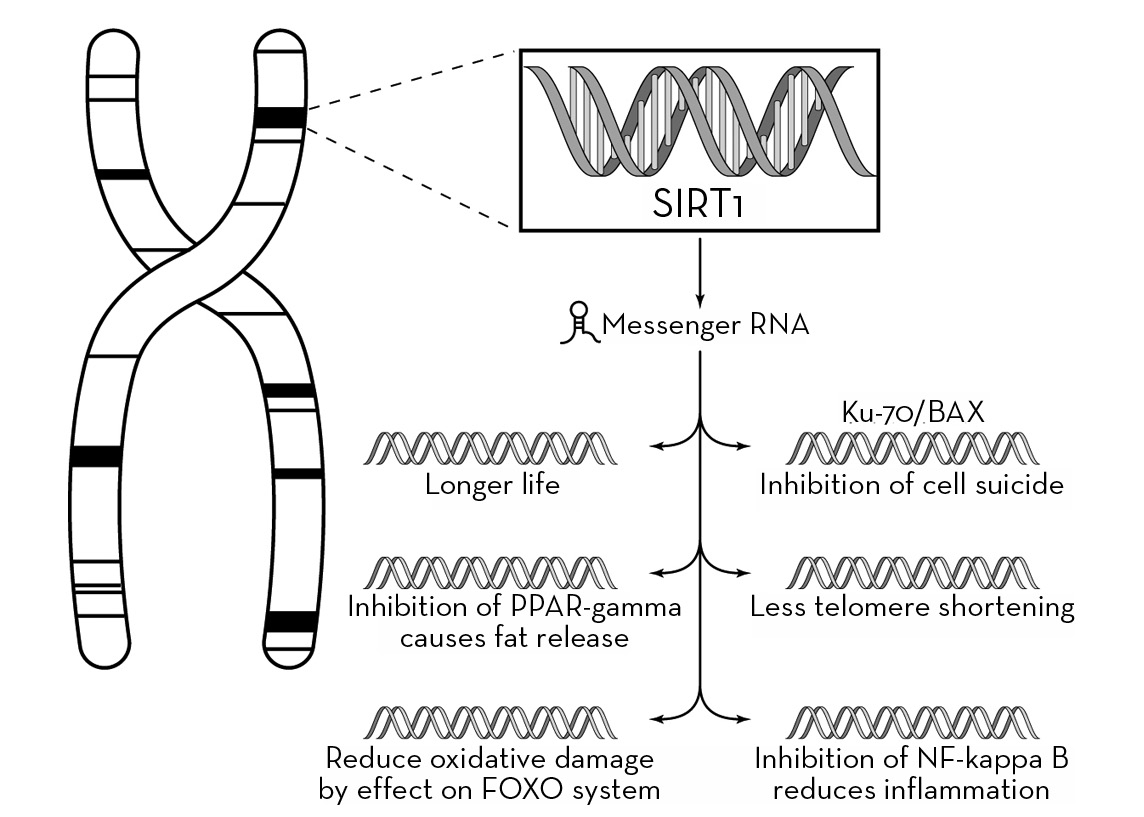
In large part, the answer appears to lie with a gene called SIRT1, which functions in mammals much the same way that a similar gene, Sir2, does in lower life forms such as yeast, worms, and fruit flies. Our bodies are made up of many different kinds of genes that perform myriad different functions. Some are responsible for determining eye, hair, and skin color, others may put us more or less at risk for particular diseases, and some work by activating or deactivating other genes. Sir2 and SIRT1 fall into this last category. These and other, similar genes are called “silent information regulators” because they appear to sense the levels of particular substances in the body and turn on downstream chemical reactions that regulate the manner in which we respond to these substances on a cellular level.
In 1999, researchers working in the lab of Leonard Guarente at MIT reported that brewer’s yeast lived longer when it contained higher levels of a gene called Sir2. With input from other researchers, it was established that reducing the food supply (instituting calorie restriction) to the yeast prolonged its lifespan through activation of Sir2. Since then, it has been demonstrated that the equivalent gene (called SIRT1) is activated in calorie-restricted mammals, including rats, mice, and humans.
Many researchers are now investigating the complex nature of SIRT1 and how it affects our bodies—particularly how it is activated and how it conveys resistance to stress—and new information is constantly forthcoming. The interesting question is why Sir2 and SIRT1 exist. Because these genes are found in the most ancient life forms and appear to be present in all species in various forms, the typical speculation is that they act to protect the organism in times of adversity, such as when there is an inadequate food supply, allowing the animal to survive until food conditions improve. Whatever the original purpose of these genes, however, the recent scientific discoveries that have allowed us to understand (and eventually control) how they act are nothing short of miraculous. Among the most prominent of the scientists investigating these mechanisms is David Sinclair at the Harvard Medical School.
SIRT1 TO THE RESCUE
When a cell is exposed to fatal stress, such as extreme heat or starvation, it reacts one of two ways: It either necroses and dies immediately or it begins a process called apoptosis, which leads to a slower but inevitable programmed cell death.
Sinclair and his colleagues have shown that SIRT1 prevents this cell suicide by interfering with the action of a protein called BAX, which initiates the apoptosis process. This gives the cell time to repair the damage done by the stressor and continue to function normally.
What Exactly Is a “Stress Response”?
The most widely accepted theory of why calorie restriction prevents disease and/or delays the onset of age-related diseases is called “hormesis,” which means that a harmful stress—one that might be fatal in large quantities—is beneficial in small amounts. Thus, if an animal is starved, it dies, but if its daily calorie intake is reduced to 60 percent of normal, it lives longer in very good health. (If calories are reduced to less than 60 percent, the average lifespan is shortened.) The physiologic events that occur in response to a nonfatal stimulus constitute the stress response. At the level of gene expression, the stress response is believed to be initiated by SIRT1 activation. The downstream effects of SIRT1 activation include reduced oxidative stress and inflammation and reduced fat storage and anti-apoptosis.
In humans, as in animals, the stress response is probably “dose-related.” That is, zero calorie intake every other day activates the mechanism more intensely than daily calorie restriction. But, as I’ve said, and as Eric Ravussin also determined in his three-week study of volunteers who were employees in his lab (see WHAT IF YOU DON'T GORGE?), most humans would not willingly adhere to an every-other-day eating pattern on a long-term basis. That said, the Alternate-Day Diet will activate the stress response to the metabolic and oxidative insults to which we are constantly subjected.
SIRT1 is “turned on” to do its work in the body through a shift in the relative levels of the coenzymes nicotinamide and NAD+. When NAD+ is present, SIRT1 is activated. When, through a biochemical reaction, NAD+ is turned into nicotinamide (which is NAD+ minus one electron), SIRT1 is inhibited.
ALTERNATE-DAY TURNS ON SIRT1
You may be wondering what all this has to do with the Alternate-Day Diet. On the simplest level, the answer is that when energy (i.e., serum glucose) supplied to the cell is as low as it is in calorie restriction, the level of NAD+ rises, turning on SIRT1. David Sinclair has described the series of events that occur on the cellular level in response to reduced calorie intake as a metaphor for making a 911 call in an emergency. SIRT1 answers the call and dispatches the “rescuers” that prevent the stressed cells from dying. Some organs, such as the liver, are capable of regenerating themselves after cells are lost, but others, most notably the heart and the brain, are not. Preventing loss of critical cells due to apoptosis in organs that do not regenerate may, therefore, be one way SIRT1 promotes longevity.
Anthony E. Civitarese, Eric Ravussin, and their colleagues published a study that looked at three groups of overweight but not obese people for a period of six months. A control group ate 100 percent of their calorie requirements daily; a second group received 25 percent less; and a third group had their calorie intake reduced by 12.5 percent while also increasing their calorie expenditure through exercise by 12.5 percent. After six months, the researchers found, by doing biopsies of the subjects’ thigh muscle, that both those who were restricted 25 percent and those who combined exercise with a 12.5 percent calorie restriction had more mitochondria and less free-radical damage to their DNA, and had activated SIRT1. These results confirmed those of a previous study by the same team that showed SIRT1 activation after three weeks of eating every other day. Based on their findings, the researchers stated that eating fewer calories “can improve whole body metabolism in conjunction with an increase in SIRT1 gene expression in skeletal muscle. These results raise the possibility that SIRT1 may contribute to more efficient metabolism, less oxidative stress, and increased longevity in humans as it does in lower organisms.”
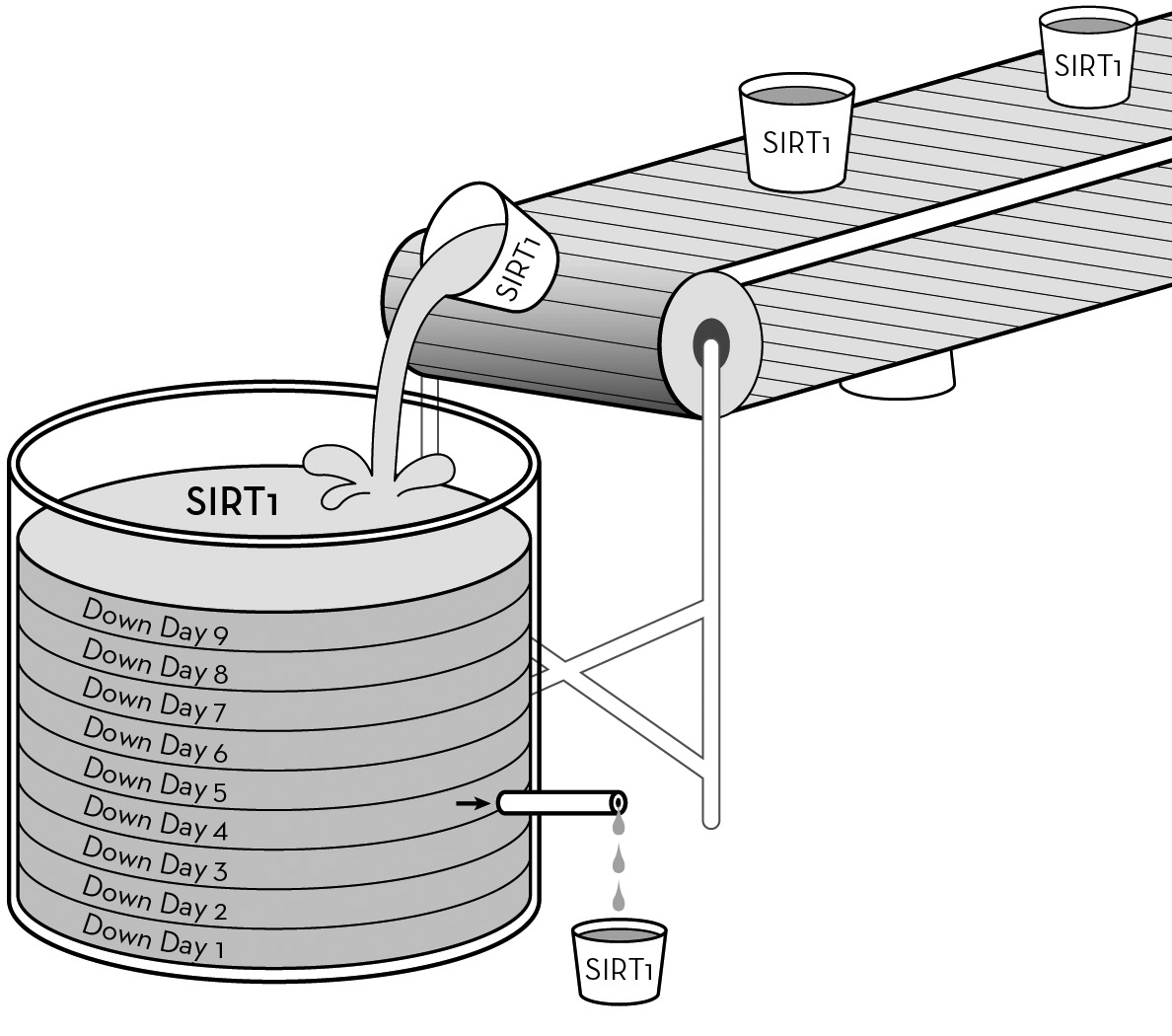
A single 36-hour period of low-energy supply (reduced calories) causes a rise in levels of the SIRT1 protein that diminishes over several days. Each down day causes a similar rise in SIRT1, and levels remain elevated even when eating normally every other day.
Genes are “expressed” in response to a variety of stimuli. In the Ravussin study, SIRT1 was expressed over the six-month duration of the study. But it probably only takes skipping a meal to increase our SIRT1 levels. In mice it has been shown that a 24-hour fast activates SIRT1 and that the increased activity is still measurable more than 24 hours after the end of the fast. This effect is not unlike that which my colleagues and I have observed in asthmatics. Not only were their markers for oxidative stress reduced after only one down day, indicating the rapid onset of SIRT1, but after following the Alternate-Day Diet for several weeks and then stopping, their symptoms remained improved for approximately ten to fourteen days, suggesting that SIRT1 was still active and its downstream effects were still operating. Formation of SIRT1 protein and its gradual decay over a period of days would explain this phenomenon.
Furthermore, based on our findings, we believe that alternate- day calorie restriction without actual fasting as provided by alternate-day dieting is sufficient to create the stress reaction that activates SIRT1 and also that its expression will continue to intensify and strengthen as long as this eating pattern is continued.
It is highly likely, therefore, that this ancient genetic mechanism functions in humans much the same way it does in mice.
SIRT1, NF-KAPPA B, AND INFLAMMATION
Another effect of SIRT1 is to inhibit a protein called NF-kappa B, which causes inflammation. Inflammation exists to defend against germs and tumors, but it can also act in a harmful way to cause cancer, arthritis, asthma, heart disease, and neurodegeneration. What this means is that activation of SIRT1 could be expected to reduce the incidence of these diseases caused by inflammation.
SIRT1 CAN ALSO MAKE YOU THIN
SIRT1 has a broad range of effects on metabolism. For example, limiting food intake increases SIRT1 activity in fat cells, causing fat to move into the bloodstream, where it is used as energy.
In the same way that SIRT1 acts upon BAX to prevent cellular apoptosis, it also works to inhibit fat storage by turning off another gene, called PPAR-gamma. Because PPAR-gamma is responsible for allowing the deposition of fat, fat storage is inhibited when it is deactivated.
In terms of weight loss this is good news not only because, obviously, the more fat you store the fatter you become, but also because reduced levels of body fat mean an increased ratio of muscle to fat. Also, because there are far more mitochondria in muscle tissue, it burns calories more efficiently than fat. More muscle means faster metabolism, which means that you will use the calories you eat more quickly.
In terms of health and increased longevity, however, the effect of SIRT1 on fat storage may have even greater significance because chemicals called inflammatory cytokines (such as tumor necrosis factor-alpha, previously discussed), which cause the chronic inflammation that contributes to the accumulation of damage to the organs we describe as aging, are produced by fat cells. What this means is that activating SIRT1 in fat cells could significantly slow the aging process and prevent specific diseases such as Type 2 diabetes, atherosclerosis, arthritis, osteoporosis, neurodegenerative disorders such as Alzheimer’s disease, and some types of cancer, which are very probably mediated by pro-inflammatory cytokines.
As Dr. Guarente has said, “The accumulation of WAT [white adipose tissue—i.e., fat] during ageing is associated with several adverse complications, such as insulin resistance, Type 2 diabetes and atherosclerosis. Given the impact of SIRT1 on PPAR-g activity and because PPAR-g activity helps determine age-related insulin resistance, SIRT1 may have an important role in metabolic diseases and link the effects of food consumption to body fat mass and diseases of ageing.”
BOOSTING YOUR LEVELS OF SIRT1
Based on the many apparent benefits of activating SIRT1, it would seem logical to make sure it’s working for you as much as possible. With the discovery that high levels of NAD+ activated SIRT1, the possibility that naturally occurring chemicals might “artificially” turn on this mechanism occurred to David Sinclair and workers at Biomol Laboratories. Testing a “library” of thousands of compounds, they found that a chemical named resveratrol and sixteen other plant-derived substances prolonged the lifespan of yeast by up to 70 percent.
An antioxidant known to be present in red wine, resveratrol had been targeted by Serge Renaud in the early 1990s as possibly responsible for what he termed the “French Paradox.” He suggested that drinking red wine might be a factor in the explanation of why the French, despite eating a diet high in saturated fat and cholesterol as compared with Americans, had a much lower risk of heart disease. In other words, drinking red wine might have been protecting them against heart disease.
According to the article “Therapeutic Potential of Resveratrol: The in vivo Evidence,” by David Sinclair and Joseph A. Baur, published in Nature Reviews (June 2006), “Since then, dozens of reports have shown that resveratrol can prevent or slow the progression of a wide variety of illnesses, including cancer, cardiovascular disease and ischaemic injuries [i.e., stroke], as well as enhance stress resistance and extend the lifespans of various organisms from yeast to vertebrates.”
The article cites numerous studies in which resveratrol has been shown to inhibit tumor growth in rodents, to reduce the markers of oxidative stress in hypertensive rats, to reduce inflammation, and to reduce brain damage following a stroke. In fact, Sinclair and Baur conclude, “It is becoming clear that resveratrol and more potent mimetics show great promise in the treatment of the leading causes of morbidity and mortality in the Western world. . . . Could resveratrol and similar molecules form the next class of wonder-drugs? Clinical trials are currently underway in several locations . . . and could soon answer this question.”
Alcohol: A Little Is Good
Drinking alcohol in moderation, up to two drinks per day for men and one drink a day for women, reduces heart attacks by 30 to 40 percent compared with nondrinkers. In people under age forty, however, there is no health benefit, since heart attack is rare in any case. Drinking it all on one or two days of the week doesn’t do it. And the kind of alcohol doesn’t matter. Red wine is probably no better.
The incidence of breast cancer in women and colon cancer in both men and women is higher among those who are moderate drinkers than among non-drinkers. If you do drink, taking a folic acid supplement appears to reduce the cancer risk.
In addition to being potentially addictive and causing a third of all traffic fatalities, excess drinking increases the risk of a variety of cancers as well as high blood pressure and liver disease. Moreover, you can get the same heart health benefit from increasing your exercise level, so don’t feel compelled to start drinking if you don’t already.
Although there is still much to be learned about exactly what mechanisms are responsible for these effects, it seems clear that at least one of the ways resveratrol works is to activate SIRT1.
IF RESVERATROL CAN TURN ON SIRT1, WHY NOT JUST SKIP THE DIET?
Wouldn’t it be great if we could forget dieting altogether and just pop a couple of resveratrol capsules along with our daily vitamins? Whether or not this might be a viable alternative probably depends on whether you are trying to get the potential health benefits of resveratrol or to lose weight. There is a big range in possible doses, but resveratrol and related chemicals may well have weight-loss effects in addition to significant health benefits.
Two landmark studies that appeared toward the end of 2006 showed for the first time that resveratrol had effects in mice similar to those seen in lower animals. In the first, David Sinclair, Rafael de Cabo, and associates showed that mice fed a high-calorie diet and 24 milligrams per kilogram of body weight of resveratrol survived 31 percent longer than mice fed a standard diet. Another group of mice fed 5 mg per kg of body weight showed many of the same health benefits as the higher dose. Among other findings, the resveratrol-fed mice did not develop atherosclerosis, had improved motor function, and showed an increased number of mitochondria, all in the absence of weight loss. The improved motor function in this study is an indication that the negative effects of a high-calorie diet on the brain were reversed by the resveratrol.
Does Fat Make You Dumb?
Another negative aspect of obesity that is not widely appreciated is the very significant effect it has on mental ability. Numerous studies document the fact that obesity and high-saturated-fat, high-calorie diets negatively affect cognitive function in animals and humans. Insulin resistance and Type 2 diabetes magnify this effect. Resveratrol has been shown to improve neuromuscular function in mice, indicating a positive effect on the central nervous system.
In addition, resveratrol has been shown to reverse severe cognitive deficits in an Alzheimer’s disease mouse model. This is the first potential agent that could actually improve cognitive function in neurodegenerative diseases such as Alzheimer’s.
The second study appeared in the journal Cell in December 2006. Here, scientists working with David Sinclair and Johan Auwerx in Illkirch, France, showed that mice fed 200 to 400 mg of resveratrol per kg of body weight mixed with their food became lean and were able to run twice as far. It is possible, then, that humans could lose weight if they were willing to take 15 or 20 grams of resveratrol per day. But this dose is extremely high, and its safety has not been established at that level.
The resveratrol-fed mice had much bigger and more numerous mitochondria in their muscle cells, and the authors concluded this was a direct effect of the resveratrol. In addition, the animals fed resveratrol had faster metabolisms (greater energy expenditure) and improved results on tests of the central nervous system, such as balance and strength. They also had less insulin resistance and improved glucose tolerance, both of which are abnormal in many overweight people.
Resveratrol Supplements and Drugs
At the moment, resveratrol is being sold by many vendors on the Internet, and it is possible that the Alternate-Day Diet might be even more effective if you were also to take resveratrol, but an effective dose would probably have to be quite high, and the risks of taking high doses over the long term have not yet been established.
David Sinclair, in partnership with Christoph Westphal, has formed a corporation, Sirtris, to develop drugs based on resveratrol as well as other SIRT1 activators. Reportedly, pharmaceutical giants such as Merck and Pfizer are also interested in developing SIRT1 activators.
Dysfunction of the mitochondria is seen in heart disease, neurodegenerative diseases (like Alzheimer’s), and metabolic diseases (like Type 2 diabetes), all of which could potentially be treated with resveratrol-type drugs. Also, both exercise and calorie reduction increase the number and function of the mitochondria and are the cornerstones of treatment of metabolic syndrome, a condition involving multiple risk factors for cardiovascular disease, including abdominal obesity, insulin resistance and glucose intolerance, elevated blood pressure, and a pro-inflammatory state. Thus SIRT1 activators like resveratrol might be very useful for treating this common condition.
Resveratrol was effective in reversing the cognitive loss in a mouse model of Alzheimer’s disease and in preventing the death of neurons associated with the process. This lends promise to the possibility of effective treatment of Alzheimer’s disease.