1 Chemical Bonds and Reactions
Biochemical reactions, in the simplest terms, are based on changing one thing into another. In order to do that, the covalent bonds within the starting molecule(s) must be broken and new bonds formed to create the new molecule(s).
The rearrangement of bonds is significant, because it is related to whether the reaction is spontaneous or not, or if energy is needed or released. From a biological point of view, this aspect of chemistry is really important because cellular metabolism is based on breaking and rearranging bonds in order to build molecules and release energy.
Yes, I am oversimplifying. But in regards to what I want my biology students to know, this is what we should focus on. It is, simply speaking, the must know concept!
Covalent, Ionic, and Intermolecular Bonds
Think back to chemistry class and you may remember the different types of bonds that occur within and between molecules: covalent, ionic, and intermolecular. Our must know concept will focus on the rearrangement of covalent bonds, but all three of these bond types are important.
An ion is a charged atom, and an ionic bond occurs between a positive and a negative ion. Whether an atom is an ion is based on the number of electrons it has. Normally, the number of electrons in an atom equals the number of protons; since each electron has a negative charge, and each proton has a positive charge, the overall charges will balance each other out and the atom will be neutral.
The atoms depicted on a periodic table are the neutral variety, with equal numbers of electrons and protons. An element’s atomic number equals the number of protons; the atomic number, therefore, also indicates the numbers of electrons. An element’s atomic mass is the sum of the number of protons and the number of neutrons. All the weight (mass) of the atom is in the nucleus, where the protons and neutrons reside. So, you add them all up!
If a neutral atom gains an extra electron, it becomes a negatively charged ion (anion). If a neutral atom loses an electron, it becomes a positively charged atom (cation). The saying “opposites attract” hold true here, because that negative anion and the positive cation seek out and hold on to one another. Table salt—NaCl—is created through an ionic bond formed between the Na+ and the Cl-. A covalent bond, unlike the ionic bond, is formed between atoms that are sharing electrons. A single covalent bond is created when two electrons are shared between two atoms; a double bond involves four shared electrons. Both ionic bonds and covalent bonds are called intramolecular bonds (intra = within) because they occur within a single molecule. An intermolecular bond (inter = between) is different because it occurs between two separate molecules. We will talk about two types of intermolecular forces in this book: van der Waals and hydrogen bonding. Hydrogen bonding occurs in molecules that contain a hydrogen atom bonded to an oxygen, nitrogen, or fluorine atom. We will talk more about hydrogen bonding once we get to the water section of this chapter.
Intermolecular Forces: van der Waals and Hydrogen Bonding
Entire molecules can have positively and negatively charged regions. That doesn’t mean they are ionic (having lost or gained an electron). It is simply that electrons like to hang out in some regions of a molecule more than other regions, creating an unequal charge distribution. Because one area has a slightly more positive charge (because the electrons are not hanging out there) and one area has a slightly more negative charge, separate molecules will stick to one another, just like magnets. These sorts of attractions are called van der Waals interactions, and they only occur if the molecules are really, really close to one another. I have a totally cool example of van der Waals forces in nature. You know what a gecko is? Geckos are those little lizards that live in all sorts of habitats, and they particularly like to hang out on rocks and the sides of trees.
If a gecko happens to wander into somebody’s house, they are just as happy clinging to the walls as a rock. A gecko can hang upside down on a ceiling, even if it’s made of glass! These remarkable little lizards’ climbing abilities are so stunning, they have even been the subject of Aristotle’s praise. For years, scientists have studied this phenomenon to figure out how, exactly, this lizard can seemingly defy gravity and cling to almost any surface. Was it exuding some sort of sticky glue-like substance from its feet? Were there tiny little barbs that physically clung to the surface like Velcro? Maybe miniscule suction cups? Nope; it’s because of van der Waals forces between their toes and the wall! And there needs to be a ton of individual van der Waals interactions in order to support the weight of an entire gecko. This is possible because the gecko’s feet are flat and splayed out, and the entire surface is covered by a dizzying amount of tiny, microscopic folds and hairs. This creates a super-high surface area, thus increasing the potential number of van der Waals interactions. Behold! The amazing gecko and his glorious feet:

The common house gecko (Hemidactylus frenatus)
Author: Firos AK. https://commons.wikimedia.org/wiki/File:Asian_House_Gecko_close_up_from_bangalore.jpg
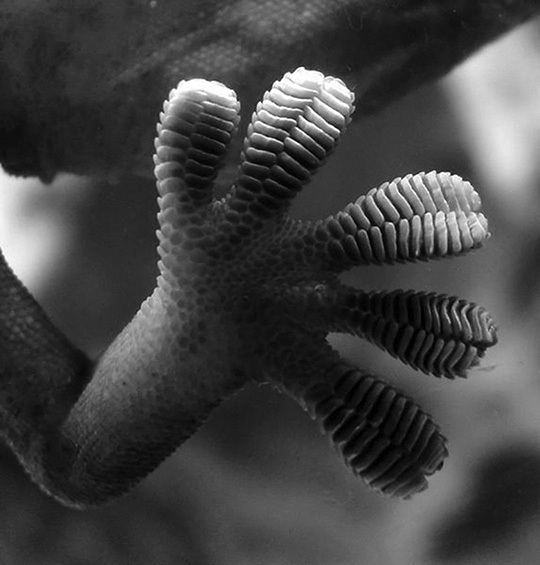
Close-up of the underside of a gecko’s foot as it walks on a glass wall
Author: Bjørn Christian Tørrissen. https://commons.wikimedia.org/wiki/File:Gecko_foot_on_glass.JPG
Gecko toes (and intermolecular interactions in general) are perfect examples of our must know concept that interactions between molecules are an important aspect of biology.
Energy Transformation (and How It Must Follow the Rules of the Universe)
Thermodynamics is the study of energy transformations, and it’s a perfect application of our must know concept. There are different forms of energy (e.g., chemical, thermal, mechanical) and transformation refers to switching one form to another, a very helpful ability for cells. On a small scale, this transformation occurs because the atoms and bonds of chemicals are broken up and rearranged in new ways. On a big scale, energy is transformed and transferred between us and the environment because organisms are referred to as “open systems” (a system in which mass or energy can be lost to or gained from the environment). For example: you eat food and it is broken down and transformed into the chemical form of energy called ATP (thank you, cellular respiration). During the process, some of the energy is lost as heat, radiating from our bodies into the surroundings. Two laws of thermodynamics explain the scenario above.
 First Law of Thermodynamics: Energy cannot be created or destroyed. It’s impossible to create energy from scratch; it can only be transformed from one form to another. This transformation is due to our must know concept of breaking bonds and rearranging atoms to create new things. Does your electric company create the energy that flows throughout your house? Nope. It converts it from one form (oil, coal, solar) into the electricity that travels through the power lines. In an upcoming chapter, we will cover two very important metabolic processes that transform energy from one form to another: photosynthesis and cellular respiration. Photosynthesis grabs photons of light from the sun (solar energy) and transforms it into chemical energy (glucose). Cellular respiration takes the chemical energy of glucose and converts it into another chemical form, ATP. The first law of thermodynamics aligns perfectly with our must know concept. Chemical reactions involve the breaking, reforming, and rearranging of bonds; we rely on breaking, reforming, and rearranging bonds in order to convert energy from one form to another.
First Law of Thermodynamics: Energy cannot be created or destroyed. It’s impossible to create energy from scratch; it can only be transformed from one form to another. This transformation is due to our must know concept of breaking bonds and rearranging atoms to create new things. Does your electric company create the energy that flows throughout your house? Nope. It converts it from one form (oil, coal, solar) into the electricity that travels through the power lines. In an upcoming chapter, we will cover two very important metabolic processes that transform energy from one form to another: photosynthesis and cellular respiration. Photosynthesis grabs photons of light from the sun (solar energy) and transforms it into chemical energy (glucose). Cellular respiration takes the chemical energy of glucose and converts it into another chemical form, ATP. The first law of thermodynamics aligns perfectly with our must know concept. Chemical reactions involve the breaking, reforming, and rearranging of bonds; we rely on breaking, reforming, and rearranging bonds in order to convert energy from one form to another.
 Second Law of Thermodynamics: Energy transformations increase the disorder of the universe. If you think your life is always tending toward chaos, then congratulations, you’re fully aligned with the second law of thermodynamics: things tend toward disorder. Specifically, every energy transformation will increase the disorder of the universe. The term entropy is used as a measure of disorder and randomness. When energy is transformed, some of the potentially usable energy is lost as heat. For example, when your cells break and rearrange the bonds of glucose in order to transform it into the chemical energy of ATP, not all of the potential energy in glucose is used; some is lost as heat. This heat is radiating off your body, into the universe, increasing the overall disorder. Never realized you had such a huge impact on the universe, did you?
Second Law of Thermodynamics: Energy transformations increase the disorder of the universe. If you think your life is always tending toward chaos, then congratulations, you’re fully aligned with the second law of thermodynamics: things tend toward disorder. Specifically, every energy transformation will increase the disorder of the universe. The term entropy is used as a measure of disorder and randomness. When energy is transformed, some of the potentially usable energy is lost as heat. For example, when your cells break and rearrange the bonds of glucose in order to transform it into the chemical energy of ATP, not all of the potential energy in glucose is used; some is lost as heat. This heat is radiating off your body, into the universe, increasing the overall disorder. Never realized you had such a huge impact on the universe, did you?

IRL
Pseudoscience alert: There cannot be a perpetual motion machine. The two laws of thermodynamics are ironclad, and if a machine is converting one form of energy into motion, there needs to be a net input of energy because as per the second law, every transformation will result in a loss of some energy into the surroundings. A machine that is 100% efficient and no energy is ever lost? Nope, can’t be done. If you hear of someone claiming to invent such a machine, they have either made a grievous error in their results, or they are trying to scam someone.
Catabolic and Anabolic Reactions
Our must know focus is that chemical reactions involve the breaking and rearrangement of covalent bonds within a molecule. When creating a new bond and two atoms are glued together, energy is required. When that bond is broken, it releases the energy stored within it.
A catabolic reaction breaks larger, more complex molecules into smaller, simpler molecules. Covalent bonds are cleaved apart and energy is released. This is an example of an exergonic reaction: the products end up with less energy than the starting reactants and energy is released over the course of the reaction. Any reaction that has net release of energy has a negative ΔG.
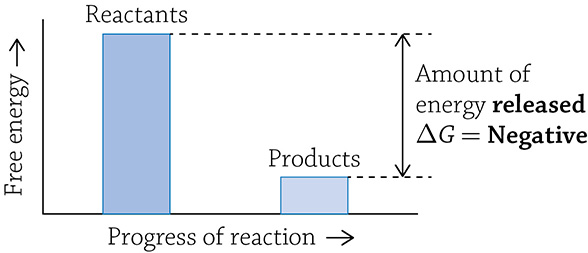
Graph of a catabolic reaction
An anabolic reaction is a “building-up” reaction (to help remember this, think about “anabolic steroids” and how they dangerously build up muscle mass). In order to form a new, complex molecule from smaller parts, new covalent bonds are created and energy is used; this is called an endergonic reaction. This means that the final product of an anabolic reaction stores more energy than the reactants it was made from. Any reaction that absorbs free energy has a positive ΔG.
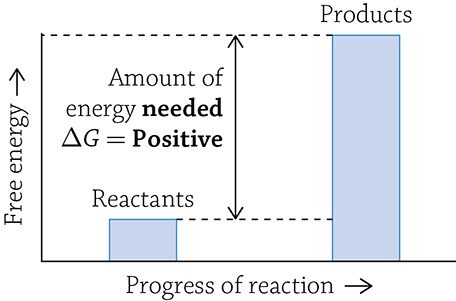
Graph of an anabolic reaction
What is this strange “delta gee” (ΔG) I speak of? The letter G stands for Gibbs free energy (or just free energy), and it means the amount of a reaction’s energy that can be used to do some sort of “work.” When you use ΔG to describe a reaction, you are talking about the change in free energy from the start of the reaction (the amount of energy in the reactants) to the end of the energy (the amount of energy in the products). If you have more energy stored at the start (in the reactants), then energy will be released once the products are formed; this indicates a -ΔG. Reactions with a -ΔG are spontaneous, meaning it is energetically favorable for the reaction to occur … it just wants to happen! If instead energy needs to be added in order to create the products, the reaction is said to have a +ΔG; this reaction is not spontaneous and needs a push (energy added) in order for it to occur.
A perfect example of energy-releasing (-ΔG) catabolic reactions and energy-storing (ΔG) anabolic reactions relates to something we will learn more about later: photosynthesis and cellular respiration.
This is the summary equation for the process of photosynthesis:
6CO2 + 6H2O (+ sun energy) → C6H12O6 + 6O2
And this is the summary equation for cellular respiration:
C6H12O6 + 6O2 → 6CO2 + 6H2O (+ chemical energy)
Even though each of these equations is actually the composite of many, many individual reactions, they summarize the biochemical pathways quite nicely. Photosynthesis is the process of combining six carbon dioxide molecules with six water molecules and, using the energy of the sun, rearranging the bonds to create a high-energy molecule of glucose and six molecules of diatomic oxygen (O2). The sugar molecule contains more energy in its bonds than found in the reactants (carbon dioxide and water) and the energy input of the sun was needed to make the final product (+ΔG); this is an anabolic reaction. The glucose molecule stores the energy of the sun within it! When this sugar molecule is subjected to the catabolic pathways of cellular respiration, it is broken apart and the stored energy is released (-ΔG). The small resulting molecules of carbon dioxide float away into the atmosphere, and the only way they will once again reform a molecule of glucose is if there is again an input of sun energy to once again rearrange the atoms of six carbon dioxides and six water molecules into a single energy-storing glucose molecule.
Enzyme Function
Your metabolism consists of trillions of reactions occurring in your body, in every cell, all the time. Some of these reactions, if left to their own devices, would occur so slowly as to not help the cell in its pursuit of a happy and metabolically productive life. Luckily, there are enzymes to facilitate the process. Enzymes help us understand both must know concepts: the interactions they participate in (with their substrate) are pivotal to their function (breaking and rearranging bonds).
Enzymes are biological catalysts that speed up chemical reactions but are themselves not used up in the reaction (they are reusable). The term biological catalyst is a fancy way of saying they are made by cells. Most enzymes are made of proteins, though there are RNA-based enzymes (called ribozymes). Since enzymes come from cells, it makes sense that they tend to prefer conditions that remind them of home: body temperature, neutral pH. There are definitely exceptions to this rule, and they’re cool to consider. You have enzymes in your stomach, for example, and conditions there are harshly acidic (a pH of 2!). There are certain species of archaebacteria (a type of prokaryotic cell) that enjoy living in extreme environments where no other form of life would have a chance of surviving. These single-celled critters’ enzymes have evolved to thrive in such punishing environments as 100°C hot springs, 0°C arctic waters, super-salty bodies of water, and solutions with a pH ranging from 2 to 11. These crazy “extremozymes” are, obviously, the exception to the rule.
Enzymes are very specific and will catalyze only one reaction. Generally speaking, a reaction can either be a building (anabolic) reaction or a breaking (catabolic) reaction. The molecule(s) the enzyme grabs onto is called the substrate, and it fits in a specific location called the enzyme’s active site. Once the enzyme grabs onto its target, it forms the enzyme-substrate complex, a temporary pairing of the substrate before the reaction actually occurs. If this is a breaking-apart reaction, the enzyme will squeeze a bit, putting stress on the covalent bonds within the substrate. The enzyme is making the substrate easier to break apart! Once it does, the enzyme releases the products and will happily await another substrate molecule.
An anabolic enzyme will work a bit differently, because it needs to bring together two reactants and create a larger molecule. The enzyme will help this process along by grabbing the reactants and making sure they line up perfectly for them to bond together.
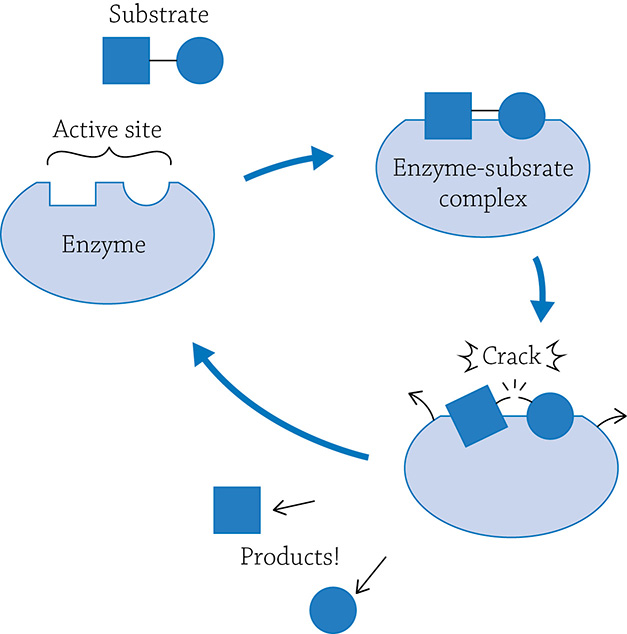
Example of breaking-apart reaction
Enzyme Inhibition
Enzymes are good at their job, but sometimes the cell needs to put on the metabolic brakes. The presence of inhibitors in a cell can help regulate enzyme activity. There are two different categories of enzyme inhibition, depending on where, exactly, this little inhibitor binds. As you know, the substrate must fit into the enzyme’s active site in order for catalysis to occur. If, for some reason, the substrate cannot get into that active site, then the reaction cannot happen! A competitive inhibitor does just what its name implies: it competes for the active site and blocks the actual substrate. It is a rude sort of molecule, shouldering its way into the space that is supposed to be for the substrate. This pairing, however, is not permanent (luckily, otherwise that particular enzyme molecule would be kaput). The competitive enzyme hangs out for a bit, then is eventually let go. The enzyme will then grab another molecule, whether it be the actual substrate or another competitive inhibitor. If there was a relatively small concentration of inhibitor, the chances of the next molecule being a substrate molecule are relatively high; if there was a high concentration of inhibitor, the enzyme might once again grab the wrong molecule.
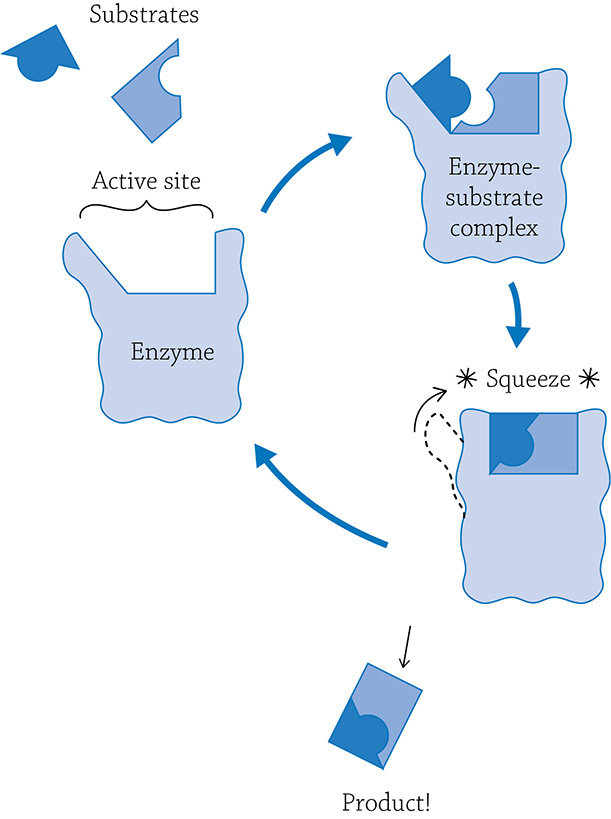
Example of building reaction
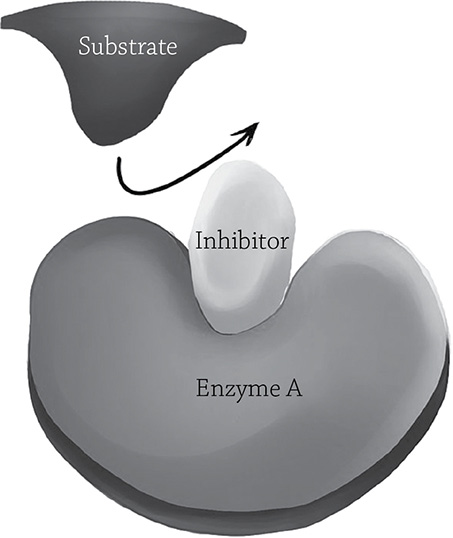
Competitive enzyme inhibition. If the competitive inhibitor binds the active site, it blocks the substrate from entering.
Author: California16. https://commons.wikimedia.org/wiki/File:Enzyme_inhibition.png
There is this other kind of inhibitor that is a bit sneakier. A non-competitive inhibitor binds at a site other than the active site, but once it binds, it makes the active site change shape so the substrate no longer fits. As was the case in the competitive inhibitor, the non-competitive inhibitor is not permanently bound to the enzyme. But if you increase the concentration of the actual substrate, would that increase the chance of the reaction occurring (as it did with the competitive inhibitor)? Nope. Since this particular passive-aggressive inhibitor is casually latching into a different location, it couldn’t care less how much substrate is around; it’s still going to screw up enzyme activity.
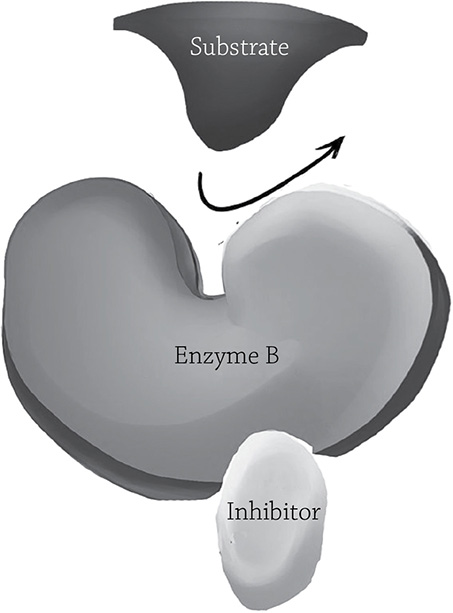
Non-competitive enzyme inhibition. The inhibitor binds a region other than the active site, so it is not in direct competition with the substrate.
Author: California16. https://commons.wikimedia.org/wiki/File:Competitive%26NonCompetitive_Enzyme_Inhibition.jpg
Allosteric Enzymes
Some enzymes have evolved clever means to finely control their activity. It’s important that enzymes aren’t running at full speed, all the time … that would be wasteful and inefficient. Enzymes that can tweak their reaction speeds are called allosteric enzymes, and they are regulated in a way very similar to the non-competitive inhibition example from earlier.
Allosteric enzymes usually are composed of multiple protein subunits, each with their own active site. An allosteric enzyme vacillates between two forms: active and inactive. There are also other binding sites to which a regulator protein can bind.
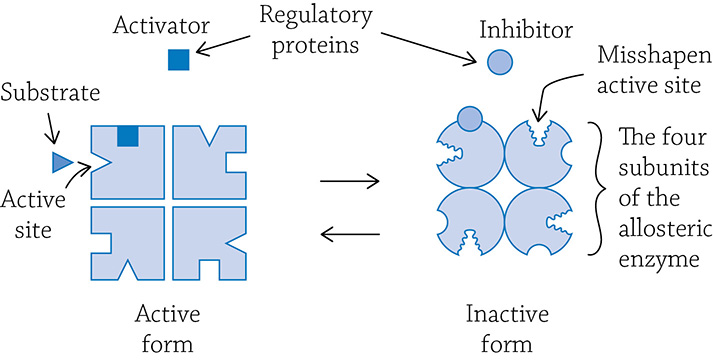
Allosteric enzyme oscillating between its active or inactive forms. An activator regulatory protein binds to an allosteric site and stabilizes the active form, allowing the substrate to fit into the active site.
There are two different kinds of regulator molecules: activators and inhibitors. If an activator binds to the regulatory site, the subunits are stabilized in the active shape. It is “active” because the shape of the active site perfectly fits the substrate. This speeds up the reaction. If, however, the inhibitor binds to the regulatory site, then all the subunits are stabilized in the inactive form. This allows the cell to fine-tune enzyme activity through the presence of activators and inhibitors. One important allosteric enzyme has a major role in adjusting your metabolic production of ATP to fit the current energy needs. The enzyme phosphofructokinase (PFK) is an allosteric enzyme that regulates the speed of glycolysis, and we’ll learn more about PFK in Chapter 6. Enzyme function is rooted in our must know concept of crucial interactions between molecules being a foundation of proper biological function.
REVIEW QUESTIONS
1. Molecules of water have an uneven distribution of electrons, resulting in one end of the molecule having a slightly negative charge. The slightly negative end of one water molecule will adhere to the slightly positive end of a second water molecule. What type of chemical bond is occurring between these two water molecules?
2. Cellular respiration is the biochemical pathway cells use to break up a molecule of glucose in order to convert the energy into the chemical form, ATP. This releases heat in the process, which provides body heat. Which law(s) of thermodynamics applies to this example?
3. Choose the right term from each of the follow pairs: A reaction that breaks bonds is called a(n) anabolic/catabolic reaction because energy is needed/released.
4. You run an experiment with an enzyme that catalyzes the reaction of turning A (reactant) into B (the product). Your friend plays a joke on you by adding a non-competitive inhibitor to the reaction, slowing the reaction down significantly.
a. Where on the enzyme does the inhibitor bind?
b. Can the reaction be sped up again by adding more of the enzyme’s substrate (A)?
5. Choose the correct answer: A(n) ionic/covalent/intermolecular bond involves sharing two electrons between two atoms.
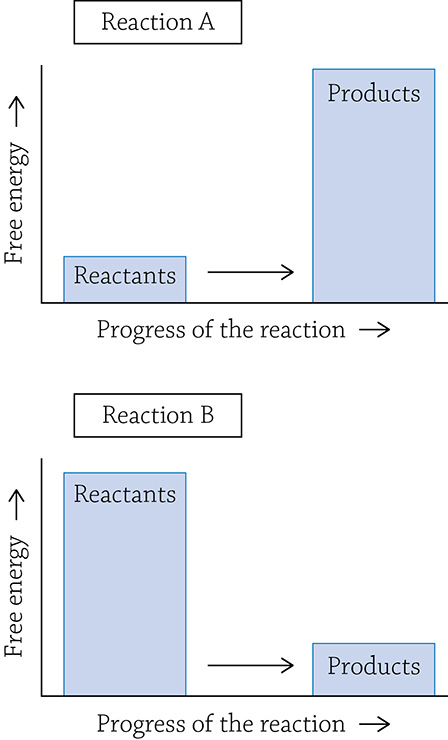
6. Referring to the two reaction diagrams below, indicate which of the two reactions: Requires an input of energy? Creates products that contain more energy than the reactants? Has a negative delta G?
7. Why are enzymes called biological catalysts?
8. How does an enzyme help a catabolic reaction occur?
9. How is an allosteric enzyme similar to a non-competitive inhibitor? How is it different?

 KNOW
KNOW Atoms are rearranged and bonds are broken and formed in chemical reactions.
Atoms are rearranged and bonds are broken and formed in chemical reactions.