6 Cells and Energy Transformation
I love this must know. It’s a simple statement (welcome back, first law of thermodynamics), yet it perfectly shapes how we learn about energy transformation in cells. This entire chapter—whether we’re learning about cellular respiration or photosynthesis—is based on the cell’s need to convert energy from one form to another.
ATP
This book is about life, and one of the characteristics of all living things is the need for energy. The idea of energy is an amorphous understanding that it has something to do with your ability to move, to do things, to live. But what, exactly, is energy? This question is a hefty one, but for our sakes, I will address it from a cell’s point of view: energy is a chemical called adenosine triphosphate (ATP). More accurately, ATP is the chemical form of energy all cells use to power their cellular work. ATP is an organic molecule composed of an adenine, a ribose, and three phosphate groups.
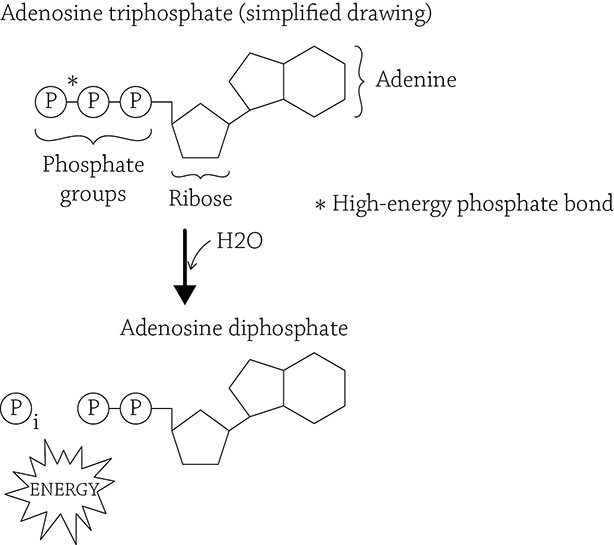
Adenosine triphosphate (ATP) and its low-energy-form, ADP
This is a cell’s chemical energy currency. The covalent bond between the second and third (outermost) phosphate groups hold a large amount of potential energy. When a cell needs to power an activity (such as building molecules via anabolic reactions) or when a multicellular organism needs to do something (such as move muscles), then the third phosphate group in ATP is cleaved off, releasing the stored energy for the cell to use. This leaves adenosine diphosphate (ADP) and a free inorganic phosphate group.
In order to recharge the battery (and reform ATP), there needs to be an input of energy in order to re-create ATP … keep this in mind as we discuss the process of cellular respiration in a little bit. The thing to focus on now is the idea that this chemical form of ATP needed to be “created” by the cell. Yet remember from Chapter 1 that there are these two ironclad rules of thermodynamics:
The most common question from my students at this point is: what is that small subscript “i” next to the free phosphate group supposed to mean? It stands for “inorganic,” which means the phosphate group is no longer part of a larger, carbon-based (organic) molecule. No big deal.
1. Energy cannot be created, nor can it be destroyed. It can, however, be converted from one form to another (our must know).
2. Any time energy is converted from one form to another (meaning it is being used by the critters of Earth), some of the potential energy is lost as heat. It is a thermodynamic rule that when energy is transformed, the process is never, ever 100% efficient (and most of the time, the lost energy escapes as heat).
Therefore, how can the cell create this chemical energy out of thin air? The answer is: it doesn’t (Rule 1: energy can’t be created from nothing!). Instead, the cell is following the second part of Rule 1: it is converting energy from one chemical form (glucose) into another (ATP). This glorious process is called cellular respiration.
Cellular Respiration
The process by which cells convert the chemical glucose into the chemical ATP is called cellular respiration. That is not to be confused with respiration, or breathing. The two are linked, however, because cellular respiration requires oxygen and generates carbon dioxide, and these gases are transported to (and from) the body’s cells by breathing.
As we focused on earlier, energy can be converted from one form to another. Cellular respiration takes the sugar glucose (C6H12O6) and, in the presence of oxygen, breaks up the sugar until it is nothing but carbon dioxide and water. By doing so, the energy stored in the sugar glucose is converted into another form of energy, ATP:
C6H12O6 + 6O2 → 6CO2 + 6H2O + ATP
This equation is a neat, concise overview of the entire process which is much more complicated … we will delve into the details later. Right now, let’s look at the idea of catabolic versus anabolic reactions, because it may help you understand.
Catabolic and anabolic reactions play a big role in this chapter. It will also be useful to understand the terms reduction and oxidation. In short, catabolism breaks things down, and anabolism builds things up. Now, when you think of breaking down and building up molecules, you must consider that you are either breaking or building covalent bonds. You need energy to create new bonds; you release energy when you break them. Therefore:
Oxidation refers to chemical reactions that involve an input of oxygen and a removal of hydrogens and electrons; reduction means that hydrogens and electrons are added. As we will see, when glucose is broken down in the catabolic (energy-releasing) reactions of cellular respiration, the glucose molecule is oxidized as many hydrogens and electrons are stripped away throughout the process. Furthermore, the “burning” of glucose requires an input of oxygen (once again, the definition of oxidation). On the flip side, photosynthesis is a reduction reaction because a bunch of hydrogens and electrons are added to CO2 in order to create C6H12O6; oxygen is also produced in the process. Therefore:
So, once again consider the overall equation for cellular respiration, but this time, look at the structural formulas for the carbon compounds glucose (a reactant) and carbon dioxide (a product):
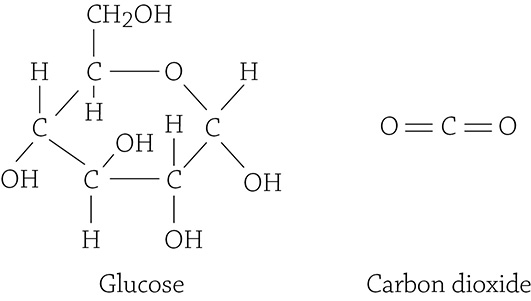
Glucose is a single molecule with six carbons, six oxygens, and twelve hydrogens. After the reaction commences, all the carbons locked into a molecule of glucose have been cleaved loose as six single carbon dioxide molecules (each with a single carbon). Was this an anabolic or a catabolic reaction? It was a catabolic reaction because one large molecule (glucose) was broken up into six small molecules (carbon dioxides). Was energy used or released in this reaction? It was released (which is the point of cellular respiration!).
ATP is indeed the energy currency of all cells. There’s a problem, however—ATP is very volatile and cannot be stored for long amounts of time. Once a cell makes some ATP, it needs to be used right away. That is why glucose is so important: it is how the body stores long-term energy. And to take that one step farther, how does our body store glucose for the long haul? It links all the glucose together into one lovely polymer called glycogen and stores it in our muscles and liver (think back to Part One!). So, when your cells need energy, it first releases individual glucose monomers from the stored glycogen, and then burns up the glucose to create ATP!
Glycolysis
The truth is more complex (and interesting) than the simple equation for cellular respiration: C6H12O6 + 6O2 → 6CO2 + 6H2O + ATP. The entire process of cellular respiration takes place in three big steps: glycolysis, Krebs cycle, and the electron transport chain. Glycolysis (“glyco” = sugar; “lysis” = split) is a 10-step process where glucose is split into two molecules of pyruvate. The breaking and rearrangement of covalent bonds releases energy that is immediately transformed into two molecules of ATP per glucose. Furthermore, along this pathway hydrogens and electrons are pulled from the carbon molecules (which used to be glucose) and are carried over to the electron transport chain for later use (we’ll get to that in a bit). The hydrogens and electrons are “held” by a molecule called NAD+; once NAD+ picks up its passengers (H+ and electrons, it becomes NADH. Once the passengers are dropped off at the electron transport chain, the NAD+ travels back to glycolysis to pick up more hydrogen ions and electrons.
In organic chemistry, the ending of some molecules’ names can end with either “-ate” or “-ic acid.”
This is pyruvic acid: CH3COCOOH− ← It’s a weak acid because of this hydrogen
This is pyruvate: CH3COCOO− ← It lost the hydrogen and lost its “acid” designation
Even though it’s better for us to say pyruvate, it’s forgivable if you refer to it as pyruvic acid.
Here is the simplified overview for the process of glycolysis:
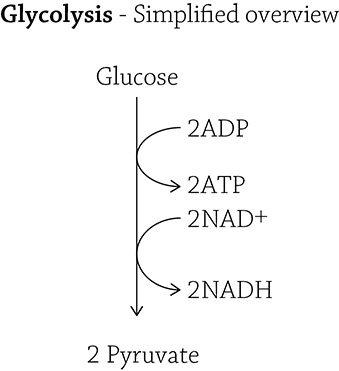
Glucose is broken up into two pyruvate molecules, and along the way, some ATP is made (using the energy released by breaking covalent bonds) and some of those NAD+ molecules pick up their passengers (electrons and hydrogens). I am including what I refer to in class as The Truth (see below): the full 10 steps of the glycolytic pathway.
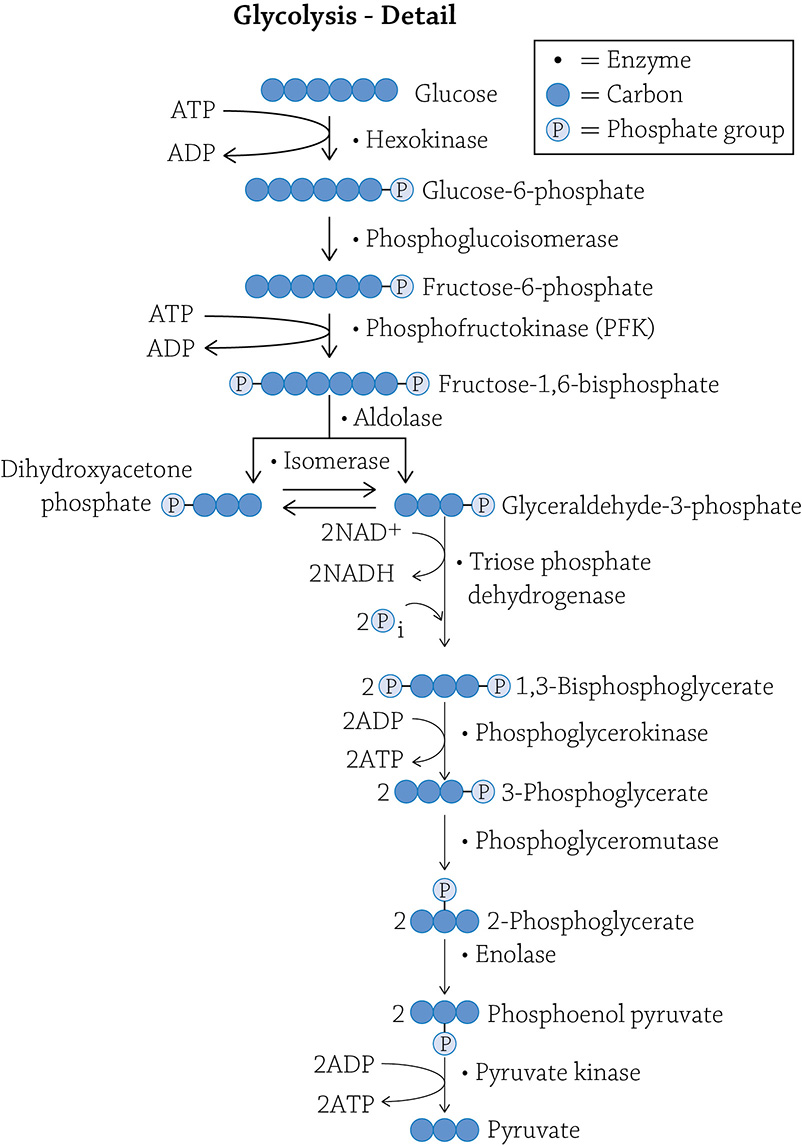
Each step shows you the carbon-skeleton structure of the molecule, and what enzyme is responsible for the reaction. There is a very good chance you do not need to know this much detail; if you do not, feel free to skip that part. For some people, however, it actually helps to see the detail.
The process of glycolysis doesn’t occur in any organelle. Instead, the enzymes that catalyze the ten steps reside in the cytoplasm. This is significant because prokaryotic cells do not have organelles. Since glycolysis doesn’t require organelles, it is found in all cell types! Now, consider this: what is the first cell type to evolve on Earth 3.5 billion years ago? Yup, a prokaryotic cell. Therefore, a logical deduction is that glycolysis was the first energy-producing biochemical pathway to evolve because it is found in simple, organelle-free prokaryotic cells.
Furthermore, glycolysis is an anaerobic process (it doesn’t require oxygen to function). If this is the first biochemical pathway to evolve on Earth, back when cells first appeared on the scene, is it logical that it would not need oxygen to function? Considering that atmospheric oxygen didn’t begin to accumulate until 2.7 billion years ago, it makes sense that glycolysis is an anaerobic process.
Control of Glycolysis
It is important for a cell to regulate the speed at which metabolic pathways produce products; it’s wasteful to create a substance when it’s not needed! Glycolysis has one important regulatory step that serves as a good review of an earlier concept: allosteric enzymes.
Recall that an allosteric enzyme has two “forms”: a very-good-at-catalyzing-a-reaction (active) form, and a not-very-good-at-catalyzing-a-reaction (inactive) form. The enzyme can be “locked” into either of the forms, depending on the needs of the cell. Allosteric enzymes have the usual active site (into which a substrate binds), plus a second location called the allosteric site. If an activator binds at the allosteric site, the enzyme will take the active form; if an inhibitor binds at the active site, the enzyme will take on the inactive form.
Look at the detailed figure of glycolysis: the third step is under control of an enzyme called phosphofructokinase (PFK). If PFK is functioning properly, the glycolytic pathway chugs along and produces ATP by oxidizing (breaking apart) glucose. But imagine that you’re relaxing on your couch, playing a video game while snacking on cheesy puffs. Do you really need to produce a lot of energy at that moment? Probably not. Your cells respond properly by switching PFK to the inactive form, and glycolysis (and by association, ATP formation) slows down. Now here’s a question: what would be a logical inhibitor molecule to bind to the allosteric site to tell PFK to power down for a bit? Hint: Think negative feedback! If you want to stop a metabolic pathway that does this,
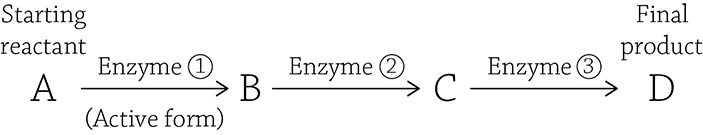
then the logical thing to bind to Enzyme 1 and tell it to stop making product D is a molecule of product D itself!
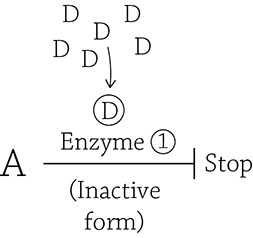
A high concentration of the end product ensures that a molecule of D (the inhibitor) will bind to the allosteric site and stabilize the inactive form of Enzyme 1. Therefore, the perfect choice for an inhibitor molecule to bind to PFK is a molecule of ATP! If you aren’t actively burning up your ATP (and there’s plenty floating around), an ATP molecule will bind to PFK’s allosteric site and stabilize the inactive form. Now, imagine that while you are relaxing on the couch, a giant radioactive ant breaks through your front door, inspiring you to quickly get off the couch and run out your back door. This uses up all your ATP, including that one molecule that glommed onto PFK, thus removing the inhibition (which is good, because you need a lot of energy to run away from a giant radioactive ant). Furthermore, a different allosteric molecule (an activator) will take its place and lock it in the active conformation. Here is another thought question: what is a good choice of activator? It once again has to do with the whole point of glycolysis: to make ATP. You need to think a bit deeper, though, about what happens when you use up ATP. Does it just … *poof* … go away? No. When you use ATP (adenosine triphosphate), you release its energy by breaking free the last phosphate group:
ATP → ADP + P
Once ATP is used, you’re left with ADP (adenosine diphosphate). Therefore, a good signal to PFK that it needs to speed up and make more ATP is … ADP (the “dead battery” form of ATP)! ADP is indeed one (of a few) activators for PFK. I just love the logic.
Krebs Cycle
At the end of glycolysis, the glucose molecule has been broken up and stripped of some electrons and hydrogens. What you have left are two molecules of pyruvate. These two molecules contain three carbons each and still have a significant amount of energy stored in their carbon-carbon bonds. Before the two molecules move on to the second stage of cellular respiration (the Krebs cycle), each must first be “tweaked” a bit: one carbon is removed, a hydrogen and electron is stripped from it, and the remaining two-carbon molecule (called acetate) is energized by a molecule called “coenzyme-A” (see figure on next page):
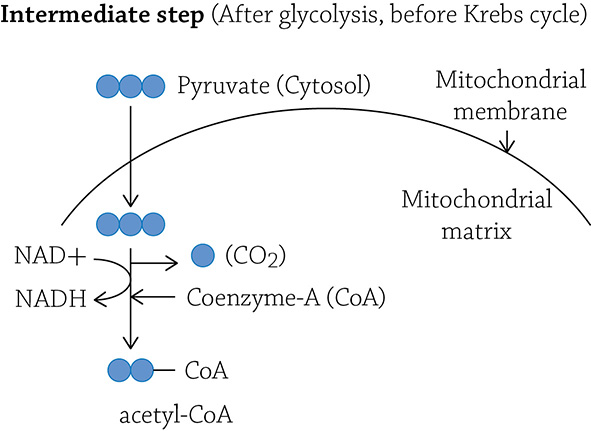
This intermediate step occurs in the mitochondrion and is necessary in order to get the carbon fragment ready for the next series of reactions in the Krebs cycle. The Krebs cycle (also called the Citric Acid cycle) is an eight-step cyclical series of reactions that occurs in the matrix of the mitochondrion. We refer to it as a cycle, because in these eight enzymatically driven steps, you cleverly end up back where you started.
Now the Krebs cycle will take over oxidation of the carbon molecule. After the tweaking step, you no longer have the three-carbon molecule called pyruvate, but instead, you have a two-carbon fragment called acetate. In order to be ferried over to the first step of Krebs, it must be held onto by a big moose of a molecule called coenzyme-A (the entire thing is called acetyl-CoA). The coenzyme-A (CoA) will drop off the acetate and head back to pick up another. (Why another? Recall at the end of glycolysis, we had split glucose into TWO molecules of pyruvate; therefore, there will be TWO molecules of acetate to drag into the Krebs cycle.)
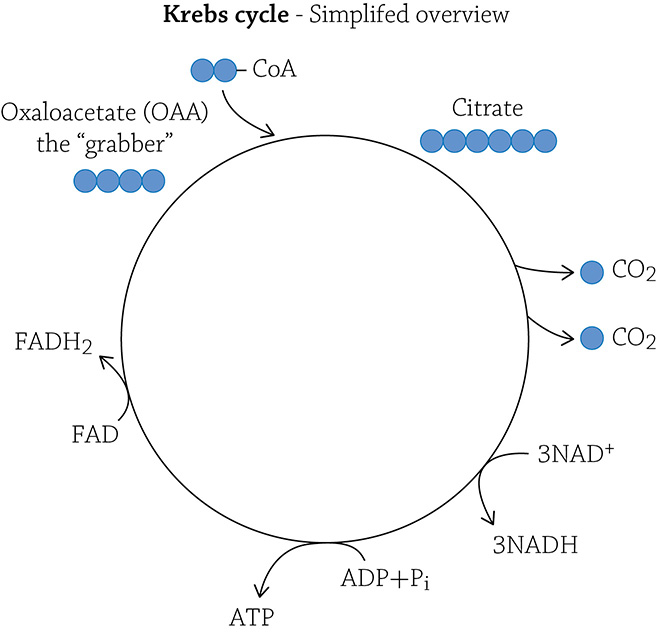
There is energy still stored in the covalent bonds of acetate, and to release the energy and convert it into ATP, your mitochondria need to break those remaining carbon-carbon bonds. The two-carbon fragment is first “grabbed” by a molecule waiting in the wings; it’s called oxaloacetate (or OAA). Oxaloacetate is a four-carbon molecule. You don’t need to stress out about its actual structural formula, but it is important to focus on the numbers of carbons. If OAA has four carbons, and it picks up (and covalently bonds with) an incoming acetate molecule, a new molecule called citric acid is produced. How many carbons must be in citric acid? Yes! Six! (The four carbons of OAA + the two carbons of acetate = a six-carbon compound called citric acid.)
Since this is a cycle, we need to regenerate what we started with: a molecule of OAA (which will then happily snag another incoming acetate). We are, therefore, allowed to cleave off *two* carbons (the amount added by an incoming acetate), both of which are released as molecules of carbon dioxide. By doing so, we are breaking covalent bonds and converting that energy into molecules of ATP! In truth, there is a fair amount of rearrangement that needs to accompany this ATP production and carbon-dioxide release, but don’t get lost in the details. Instead, keep in mind that the energy released from breaking and rearranging the covalent bonds in this remaining carbon compound (that used to be glucose) is being converted into the chemical form ATP (our must know concept!). In fact, each turn of the Krebs cycle yields one molecule of ATP. Here’s a question: How many total molecules of ATP are made in the Krebs cycle, per glucose? The answer is two (the Krebs will “spin” twice per glucose molecule, because there were two molecules of acetate to deal with).
Now, as in glycolysis, these eight steps are accompanied by molecules of NAD+ (and one molecule of its chemical cousin FAD+) swooping in, grabbing some hydrogens and electrons, and shuttling them over to the electron transport chain.
At this point, we have seen the last of our molecule of glucose. All the carbons have been cut free and “exhausted off” as carbon dioxide. And it is actual exhaust, by the way. When you exhale, carbon dioxide is released … all that carbon dioxide came from the lowly CO2 generated by the Krebs cycle (and the intermediate step). The helpful hydrogens and electrons have been stripped free and shuttled over to the electron transport chain.
Electron Transport Chain
Up to this point, we have made a paltry amount of ATP. For a single glucose molecule, we produced two molecules of ATP during glycolysis and a total of two after the Krebs cycle (one per spin). That is not a lot, considering the amount of potential energy locked up in sugar. Luckily, the spirit of glucose lives on in the electrons and hydrogen ions that have been plucked from its carbon skeleton throughout glycolysis and the Krebs cycle. The NADH and FADH2 molecules have traveled vast distances (on a cellular level) in order to arrive at their destination: the folded inner membrane of the mitochondrion.
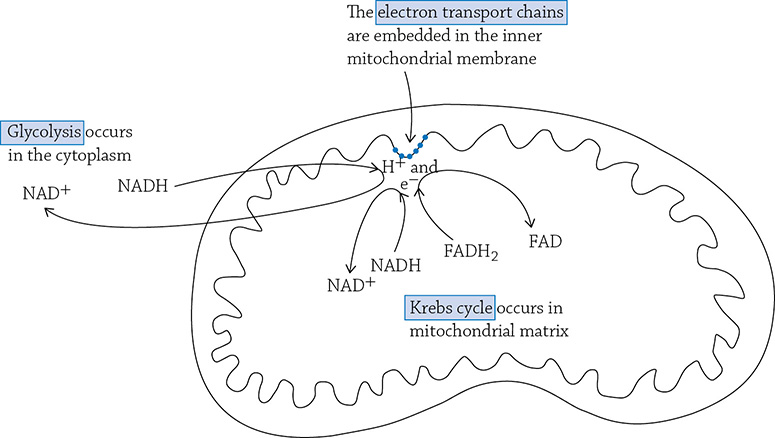
The locations of the three stages of cellular respiration. Notice that both glycolysis and Krebs send “shuttle buses” (NADH and FADH2) over to the electron transport chain in order to drop off their passengers (electrons and hydrogen ions).
Now is the time you get a huge payoff from those H+ and electrons, and it is through a process called chemiosmosis:
chemi—chemical
osmosis—referring to diffusion
Chemiosmosis is the diffusion of some sort of chemical, in this case, H+. And in order for something to want to diffuse, it needs to first be at a high concentration somewhere. And in order to concentrate something in the first place, you need to use energy … that’s where the electrons come in. These high-energy electrons use their energy to create a concentration gradient of hydrogen ions (they are stored in the inner membrane space of the mitochondrion). When the hydrogen ions diffuse back into the matrix, the only path they can take is through an enzyme called ATP synthase. This flow of hydrogen ions powers up the enzyme so it can make ATP. Consider this analogy: chemiosmosis is like a hydroelectric dam. In a hydroelectric dam, water is stored behind the dam wall (A). It can only pass through a small opening (B) before passing through a turbine (C), a machine that spins when the water passes through. The spinning of this machine creates energy. Eventually, the water reaches the region of lower water potential (D).
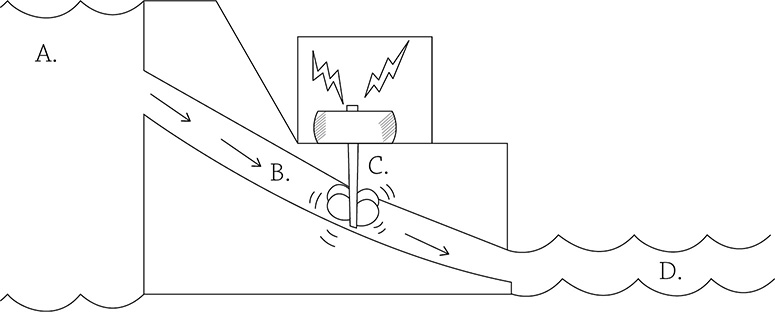
Chemiosmosis in a mitochondrion works the same way:
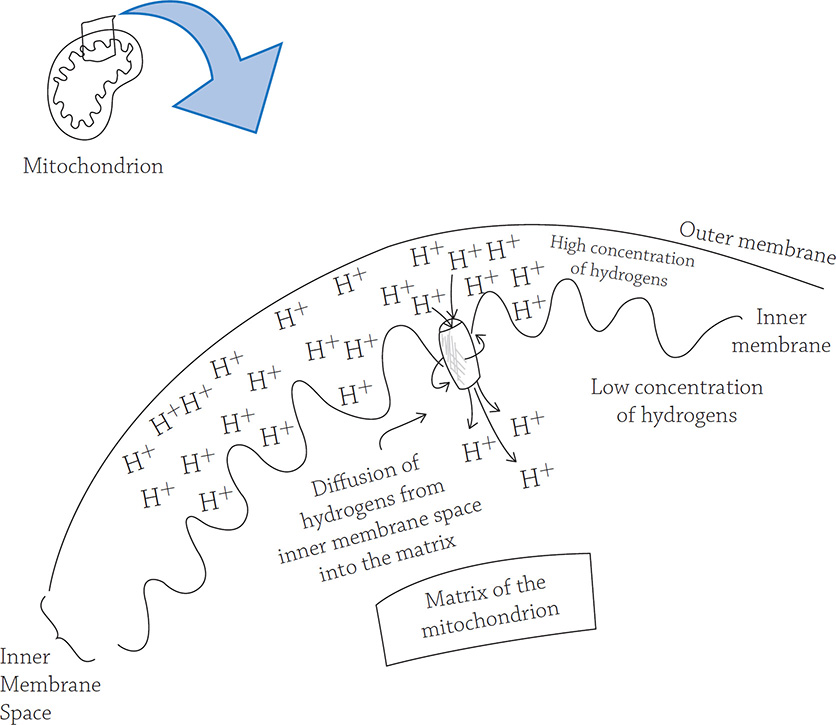
The “water” in a mitochondrion is the flow of hydrogen ions. A high concentration of hydrogen ions is stored behind the inner membrane “wall,” and they can only diffuse through a small tunnel: the enzyme called ATP synthase. As the hydrogens flow through, ATP synthase spins (like a turbine) and generates energy (just like the turbine)!
Here is the complete story of how the electron transport chain makes a ton of ATP:
 The electron and H+ carriers (NADH and FADH2) bring over their passengers from glycolysis (in the cytoplasm) and the Krebs cycle (right there in the matrix).
The electron and H+ carriers (NADH and FADH2) bring over their passengers from glycolysis (in the cytoplasm) and the Krebs cycle (right there in the matrix).
 The electrons are dropped off at the first protein in a series of proteins, all of which want to grab on to an electron. The first protein in the chain holds on to the electron at its highest energy level.
The electrons are dropped off at the first protein in a series of proteins, all of which want to grab on to an electron. The first protein in the chain holds on to the electron at its highest energy level.
 There’s an oxygen molecule at the end of the electron transport chain, and because of its high affinity for electrons, it’s “drawing” the electron toward it, down the chain of proteins.
There’s an oxygen molecule at the end of the electron transport chain, and because of its high affinity for electrons, it’s “drawing” the electron toward it, down the chain of proteins.
 The next electron transport protein in line is willing to take the electron from the first protein, but only if the e– “cools off” a bit and loses some energy. This process continues, allowing this electron to slowly, controllably, lose its energy.
The next electron transport protein in line is willing to take the electron from the first protein, but only if the e– “cools off” a bit and loses some energy. This process continues, allowing this electron to slowly, controllably, lose its energy.
 This energy is used to power tiny hydrogen pumps (often called “proton pumps”) embedded in the inner membrane. These pumps actively transport hydrogens that are hanging out in the matrix (remember: they were also dropped off by NADH and FADH2) and move them into the inner membrane space.
This energy is used to power tiny hydrogen pumps (often called “proton pumps”) embedded in the inner membrane. These pumps actively transport hydrogens that are hanging out in the matrix (remember: they were also dropped off by NADH and FADH2) and move them into the inner membrane space.
 This creates a high concentration of hydrogen ions in a small space. These H+ want to diffuse back into the matrix, but the only pathway available is through an enzyme called ATP synthase.
This creates a high concentration of hydrogen ions in a small space. These H+ want to diffuse back into the matrix, but the only pathway available is through an enzyme called ATP synthase.
 The flow of hydrogen ions through ATP synthase causes the enzyme to spin and catalyze the reaction ADP + P → ATP.
The flow of hydrogen ions through ATP synthase causes the enzyme to spin and catalyze the reaction ADP + P → ATP.
 Meanwhile, the electron gets to the bottom of the chain where it is grabbed by an awaiting O2 and combines with a couple of hydrogens, thus creating water.
Meanwhile, the electron gets to the bottom of the chain where it is grabbed by an awaiting O2 and combines with a couple of hydrogens, thus creating water.

IRL
Each day, you produce about 230 milliliters (8 ounces) of water generated from cellular respiration. That is a LOT of molecules of H2O produced at the end of many electron transport chains.
 This glorious, oxygen-driven process creates anywhere between 26–28 ATP per glucose!
This glorious, oxygen-driven process creates anywhere between 26–28 ATP per glucose!

IRL
As you know, cyanide is bad. What you may not know, cyanide kills because the chemical grabs hold of a certain protein of the electron transport chain and serves as a road block, stopping the flow of electrons down the chain. Without electrons to actively create a hydrogen ion gradient, there can be no ATP production … and a cell without ATP is dead (as are you, if you ingest enough cyanide). Interestingly enough, a major source of cyanide is the cassava root from South America. If you eat raw cassava, you will most likely die due to cyanide poisoning. If, however, you dry and boil the cassava root long enough, you will inactivate the poison from the plant tissue and turn the root into … tapioca!
NADH versus FADH2–Why One Is Better Than the Other
The oxidation of glucose is a slow, controlled process. The energy is released slowly so it can be harnessed to do cellular work; otherwise, it would literally be tiny little explosions of burning glucose! Heat and light and *poof*! That wouldn’t do anyone any good.
At key steps in glycolysis and the Krebs cycle, electrons and hydrogens are stripped off of the glucose molecule to be used in the electron transport chain. The electrons and hydrogens, however, need a “shuttle bus” to drive them over to the inner mitochondrial membrane. The molecular shuttle buses are the molecules NAD+ and FAD. Once these buses pick up their passengers, they are reduced into NADH and FADH2. When electrons are transferred from glucose to NAD+, they retain almost all of their stored energy (remember: glucose is a sugar made with the energy of the sun, so any electrons stripped from this molecule are super energized and chock full o’ energy).
The electron transport chain consists of a series of proteins embedded in the inner mitochondrial membrane. The chain starts at a high-energy end and finishes at a low energy end; with each step “down” the chain, the electron releases a bit of its stored energy. Oxygen is impatiently waiting at the very end of the chain, and it has such a love for electrons it “pulls” the electrons toward it. Once oxygen gets a hold of the electrons, it snags a couple of hydrogen ions and turns into H2O.
The total amount of energy released in this drop—starting at the highest end of the electron transport chain and ending at the oxygen—is used to make ATP. NADH is very good at its job and drops off its passengers at the top of the chain, yielding 2.5 ATP per molecule of NADH. Now, if NADH is a bus and the electron transport chain is a hill, the bus drives to the top of the hill to drop off the electrons. FADH2 is also a bus, but it’s not quite able to make it to the top of the hill. Instead, it drops off its passengers a bit lower. Because of its failure to make it to the top, the electrons don’t have as far to fall, and they don’t release as much energy as their NADH-riding brethren. Therefore, each FADH2 will yield only 1.5 ATP.
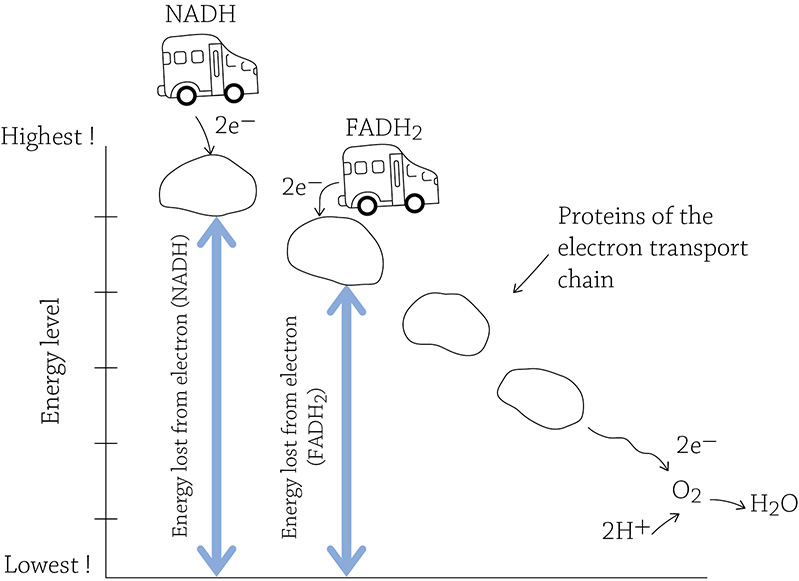
Relative amounts of energy released in the electron transport chain (NADH versus FADH2)
My students always ask this excellent question: if the electron carrier NADH yields more ATP, then why did natural selection foster the formation of FADH2? Why isn’t there only the better shuttle bus, NADH? It has to do with the molecules from which the electrons and hydrogens are torn. For some reason, NAD+ couldn’t do the job, but FAD was able to. It’s better to get in there and grab any unused electrons, even if they are not as full of energy as others.
The Role of Oxygen (and what to do if there isn’t any)
Let’s refer back to the overall equation of cellular respiration:
C6H12O6 + 6O2 → 6CO2 + 6H2O + ATP
Now we can see where each of these molecules played a role:
The process of cellular respiration is an aerobic process, meaning it requires oxygen to run. This seems strange, considering that oxygen’s role is seemingly small, right at the end of the end of the electron transport chain. Yet if there is no oxygen to grab the electrons as they hit the bottom of the chain, a subatomic particle traffic jam will form. A backup of the electron transport chain will also cause the Krebs cycle to come to a screeching halt; all that’s left running is glycolysis. This process—glycolysis providing ATP in the absence of oxygen—is fermentation.
Fermentation
Fermentation is an anaerobic process, meaning it occurs in the absence of oxygen. When there’s no oxygen, two major pathways of cellular respiration come to a screeching halt (Krebs cycle and electron transport chain); that leaves only glycolysis to run. Some cells, such as yeast, some bacteria, and our muscle cells, can create energy using only glycolysis when there’s no oxygen around. Considering that glycolysis made two molecules of ATP per glucose (compared to the 34–36 ATP when the entire process of cellular respiration is running), it isn’t as efficient. Yet, some ATP is better than none.
In order for glycolysis to occur, each of the 10 steps needs to happen. A couple of the steps requires that the carbon compound is oxidized—electrons and hydrogens are removed and transferred to NAD+, creating NADH. If NAD+ is not available to swoop in and grab its passengers (electrons and hydrogens), then glycolysis cannot proceed.
Consider our little passenger bus NADH. It’s carrying its passengers: electrons and H+. Where does NADH drop off its passengers? Yup, the electron transport chain. Now, if the chain is clogged because there’s no oxygen sweeping away the low-energy electrons, then NADH can’t swing by to unload its cargo. If NADH can’t do that, it isn’t able to turn back into its oxidized form of NAD+ and play its important role for glycolysis. If glycolysis stops … well, that’s bad. Luckily, many cells can switch to a fermentation pathway in order to keep glycolysis running, even in the absence of oxygen.
Fermentation is the energy-converting process that relies solely on glycolysis. The covalent bonds of glucose will be broken and rearranged, yielding a net gain of two precious molecules of ATP. The big difference, however, is an “add-on” is necessary to oxidize NADH back into NAD+. Instead of NADH dumping its e– and H+ onto the electron transport chain, it uses a carbon molecule that’s readily available (considering that it cannot continue to be broken up in the Krebs cycle): pyruvate! Once pyruvate is reduced—and depending on the cell type in which fermentation occurs—it will turn into either ethanol or lactic acid.
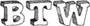
It’s easy to make the mistake of thinking the purpose of fermentation is the production of lactic acid or ethanol. The creation of those products is, actually, inconsequential. The purpose of the fermentation pathways is to regenerate NAD+ in order to keep glycolysis running in the absence of oxygen!
Ethanol Fermentation
Ethanol fermentation occurs in yeast cells and some bacteria. This is a two-step process that requires that pyruvate is first turned into a two-carbon molecule called acetaldehyde. The acetaldehyde then picks up the e– and H+ from NADH, turning into ethanol. Keep in mind, the point of this process is not to produce ethanol; the purpose is to regenerate NAD+ in order to keep glycolysis running. The alcohol and carbon dioxide are both by-products, and not particularly wanted by the cells creating it. Humans, however, have harnessed yeast cells to do their bidding as long ago as 7000 BC; fermenting foods and brewing alcoholic beverages have been in our culture for a long time.
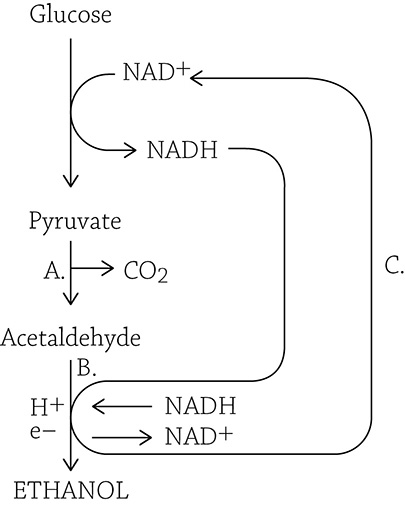
Alcohol fermentation
Let’s walk through the above figure. First, pyruvate is changed into acetaldehyde (A), releasing a carbon dioxide in the process. NADH deposits its hydrogens and electrons onto acetaldehyde (B), which is reduced to ethanol. Meanwhile, the oxidized NAD+ returns to glycolysis (C) to keep the pathway going.
The carbon dioxide that is released from pyruvate conveniently provides the carbonation found in beer and other fermented drinks. In the brewing process, we stick yeast in an airtight vat with plenty of sugar for them to use for cellular respiration. Eventually, however, the oxygen runs out and the little yeasty-beasties switch to fermentation. The alcohol and carbon dioxide build up in this sealed system and, eventually, the poor yeast cells die in their own toxic pool of alcohol. Interestingly, this is the exact same fermentation that occurs when baking bread, but unlike beer, you don’t need to be 21 years old to eat a dinner roll. When you allow bread to “rise” (ferment), the alcohol immediately evaporates as the carbon dioxide causes the dough to puff up.
Lactic Acid Fermentation
Lactic acid fermentation occurs in certain fungi, and you are now familiar with how we harness bacteria for the production of cheese and yogurt (the lactic acid produced is key to the production of these foods). In animals, muscle cells can switch from aerobic respiration to lactic acid fermentation when the need for ATP outpaces the supply of oxygen brought in by the blood. As in ethanol fermentation, this process serves as a means simply to regenerate NAD+. Once pyruvate is reduced by e– and H+, it turns into lactate, a weak acid. The lactic acid isn’t wasted; it is carried over to the liver where it is converted back into pyruvate. The liver has plenty of oxygen, so the liver cells can send the pyruvate into their mitochondria to complete the process of cellular respiration (Krebs and electron transport chain)!
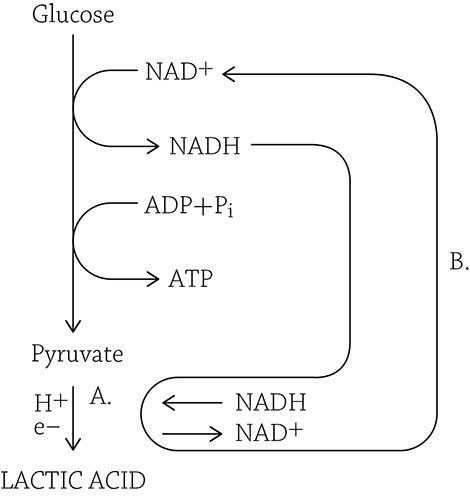
Lactic acid fermentation
In the figure above, we can see that NADH deposits its hydrogens and electrons onto pyruvate (A), which is reduced to lactic acid. Meanwhile, the oxidized NAD+ returns to glycolysis (B) to keep the pathway going.

IRL
You often hear people say their legs are burning because of the lactic acid generated after a hearty sprint or extended exercise session. Actually, this is not true. Yeah, acid burns, but the pain you feel is from tiny tears in the muscle tissue, not any fermentation by-product. Furthermore, lactate is not a bad thing; once oxygen is again available, it will be reconverted back into pyruvate and used in cellular respiration.
Photosynthesis: Light Reactions and the Calvin Cycle (AKA the dark reactions)
All eukaryotic cells undergo cellular respiration in order to convert the energy stored in glucose to the useable form of ATP. Where that glucose initially comes from, however, depends on the cell. If you are an animal cell, you must obtain the glucose from eating. If you are a photosynthetic cell, you make the glucose yourself through the process of photosynthesis.
Just like cellular respiration, the overall equation for photosynthesis sums up the process quite nicely: 6CO2 + 6H2O + sunlight → C6H12O6 + 6O2.
Notice that the equation for photosynthesis is the reverse of the equation for cellular respiration! This is significant, because if cellular respiration releases stored energy by breaking up glucose (a catabolic reaction), then photosynthesis needs an input of energy in order to create glucose (an anabolic reaction).
Photosynthesis can be broken up into two big halves: the light reactions and the Calvin cycle. The light reactions make ATP and NADPH; the Calvin cycle uses ATP and NADPH in order to create glucose from carbon dioxide. Think about it … if a cell needs to make glucose (C6H12O6) from carbon dioxide (CO2), it obviously needs some hydrogen atoms (there aren’t any on carbon dioxide!). Furthermore, in order to glue together six carbon dioxides into a single molecule of glucose—including all those new hydrogens—there needs to be an input of energy (energy is required to create covalent bonds). These two things—a source of hydrogens and a source of energy—are provided by the light reactions!
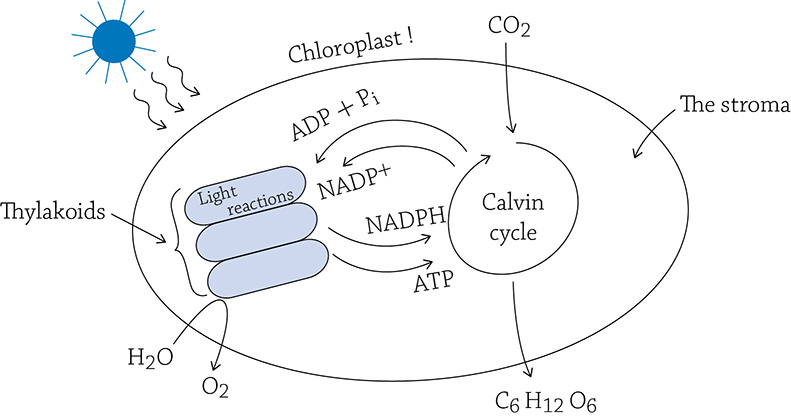
A single chloroplast. The light reactions are occurring on the thylakoid membrane, whereas the enzymes of the Calvin cycle reside in the stroma.
As you can see in the above figure, the light reactions provide the Calvin cycle with ATP (a source of energy). In turn, after the Calvin cycle uses the energy of ATP, it sends the “dead battery” (ADP and the lone phosphate) back to the light reactions to get recharged. What would be a good source of energy to use when recharging the dead battery? Yes, the sun! Our must know concept in all its glory: the chloroplast is transforming the energy of the sun into the chemical form of ATP.
What about that source of hydrogens? You have probably deduced that NADPH, like the NADH of cellular respiration, is a shuttle bus for electrons and hydrogens. Sure enough, if you refer to the previous figure, you can see that the “empty bus” (NADP+), after having dropped off its passengers, goes back to the light reaction to pick up more.
You want an easy way to remember that NADPH is used in photosynthesis and NADH is used in cellular respiration? Imagine the “P” in NADPH stands for “photosynthesis”!
You probably know that chloroplasts are green. The green comes from pigment molecules called chlorophyll that are embedded in the thylakoid membrane. Pigments like to absorb light, which is why chlorophyll molecules are part of the light reactions. The pigments absorb so much light, in fact, an electron is energized to the point that it jumps out of the chlorophyll molecule. As in cellular respiration, this high-energy electron is passed down a series of proteins (an electron transport chain) that is embedded in the thylakoid membrane. And just as we learned in cellular respiration, the energy released by the electron is used to power tiny hydrogen pumps. These pumps move hydrogen ions from the stroma into the hollow thylakoid (called the thylakoid space). There is now a high concentration of H+ stuffed into the thylakoid space, and they want to diffuse out back into the stroma. The only pathway through which they can diffuse, however, is through our old friend, the enzyme ATP synthase. The flow of hydrogens (chemiosmosis) powers the enzyme, and it catalyzes the reaction of adding Pi to ADP, creating ATP!
The electron that jumped down the chain isn’t done, however. It is reenergized by another group of chlorophyll molecules and more sunlight. Once energized, it combines with NADP+ and a lone H+, creating NADPH.
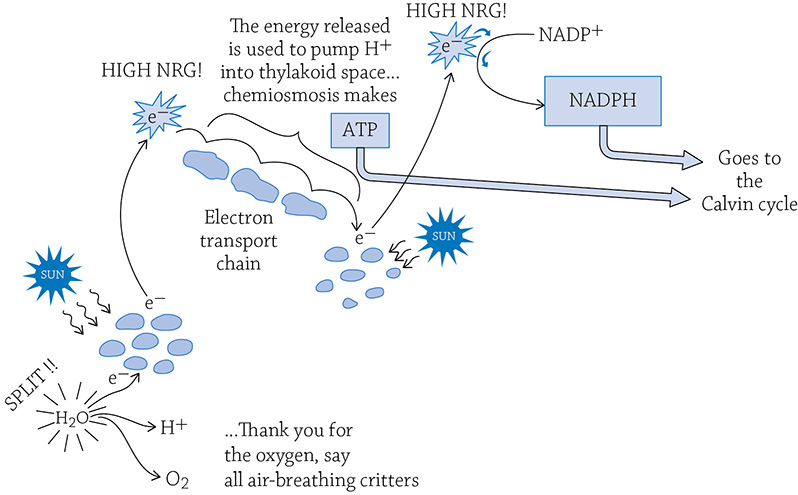
Overview of the light reactions. All of this is taking place on and in the thylakoid.
That little electron began its journey as a part of a chlorophyll molecule. It was energized by the sun, provided the power to create a hydrogen gradient inside the thylakoid, was reenergized by the sun a second time, and eventually ended up part of NADPH.
Do you see that molecule of water in the previous figure? It’s important. We are missing an electron (the same one that ended up on NADPH). We need to split apart water to get a new electron to fill in the hole. When you rip apart water, you also release some H+ (useful for chemiosmosis) and oxygen (useful for us!).
Want to blow your mind? What is the final-final resting place of that energized electron? It takes a ride on NADPH and goes over to the Calvin cycle, where it will become part of a brand-new molecule of sugar. When cellular respiration tears apart that glucose molecule and uses the electrons to power the mitochondrion’s electron transport chain, the electron doesn’t need sun to boost it up to a high energy level … why? Because it’s already energized because it just absorbed all this sun energy in the light reactions of a chloroplast when the glucose was made! Our mitochondria are harnessing the power of the sun, though it has been converted into different forms along the way!
REVIEW QUESTIONS
1. Choose the best answer: The energy in ATP is released by cleaving the bond between:
a. The first phosphate and the ribose
b. The adenine and the ribose
c. The third phosphate group and the second phosphate group
2. Cellular respiration is the process where cells convert the stored energy of _________________ into the useable form, _________________.
3. Cellular respiration is a(n) [choose one: anabolic/catabolic] reaction because energy is released as a molecule of glucose is broken down into smaller molecules of carbon dioxide.
4. What is the overall purpose of fermentation?
5. In photosynthesis, the light reactions create ________ (a source of energy) and ________ (a source of hydrogen).
6. Cellular respiration and photosynthesis both use chemiosmosis (the diffusion of hydrogen ions) in order to power ATP synthase and make ATP. Where is the high concentration of hydrogen ions stored in the mitochondrion? Where is it stored in the chloroplast?
7. If adenosine triphosphate is analogous to a battery, the fully charged form is [choose one: ATP/ADP] and the dead battery form is [choose one: ATP/ADP].
8. In glycolysis, two ADP are converted into two ________, two NAD+ are reduced to form two _______________, and glucose is oxidized into two molecules of _______________.
9. What is the role of oxygen in the mitochondrion’s electron transport chain? What molecule does oxygen end up in?
10. Choose the correct term from the pair: Photosynthesis is a(n) anabolic/catabolic reaction because energy is required in order to build a molecule of glucose from smaller molecules of carbon dioxide.
11. The Calvin cycle creates glucose from carbon dioxide. In order to do so, it needs a source of _______________ (provided by NADPH) and _______________ in order to create new covalent bonds (provided by ATP). Both NADPH and ATP are provided by the _______________ occurring on the thylakoid membranes.

 KNOW
KNOW Energy is neither created nor destroyed; a cell can only convert it from one form to another.
Energy is neither created nor destroyed; a cell can only convert it from one form to another.