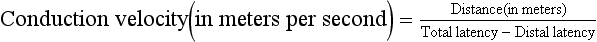Electrodiagnostic Tests
Reasons for Performing Electrodiagnostic Studies
Procedural Care for Electrodiagnostic Studies
Overview
Reasons for Performing Electrodiagnostic Studies
Most electrodiagnostic studies use electrical activity and electronic devices to evaluate disease or injury to a specified area of the body. The electrical impulses can be generated spontaneously or can be stimulated. For example, electrocardiography records spontaneous electrical impulses generated by the heart during the cardiac cycle. In electromyography, the electrical impulses are stimulated by an electrical shock applied to the body. For the caloric study, nystagmus is induced by irrigating the ear canal with water. The electrical activity is usually detected by electrodes placed on the body. The electrodes are attached to instruments for receiving and recording electrical impulses. Table 3-1 lists the various areas of the body that can be evaluated by electrodiagnostic studies.
TABLE 3-1
Body Areas Evaluated by Electrodiagnostic Studies
| Name of Test | Evaluation |
| Caloric study | Cranial nerve VIII |
| Cardiac stress | Cardiac muscle |
| Electrocardiography | Cardiac muscle and conduction system |
| Electroencephalography | Brain |
| Electromyography | Neuromuscular system |
| Electroneurography | Peripheral nerves |
| Electronystagmography | Oculovestibular reflex pathway |
| Electrophysiologic studies | Cardiac conduction system |
| Evoked potential studies | Sensory pathways of the eyes, ears, and peripheral nerves |
| Contraction stress (fetal) | Fetal viability |
| Nonstress (fetal) | Fetal viability |
| Holter monitoring | Cardiac rhythm |
| Pelvic floor sphincter electromyography | Urinary or fecal continence |
Procedural Care for Electrodiagnostic Studies
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Obtain baseline values for comparison during and after the test.
![]() Explain food restrictions, if indicated. For example, caloric studies require fasting to reduce the possibility of nausea and vomiting. On the other hand, fasting would affect electroencephalography results by causing hypoglycemia.
Explain food restrictions, if indicated. For example, caloric studies require fasting to reduce the possibility of nausea and vomiting. On the other hand, fasting would affect electroencephalography results by causing hypoglycemia.
• Determine if there are any drug restrictions. Sedatives may adversely affect most test results.
• Because of its stimulating effect, caffeine is restricted before most studies.
• Most of these studies are considered noninvasive and do not require a consent form.
During
• For most tests, some type of electrode is applied to the patient to record electrical activity.
• Some tests (such as electromyography) require some type of stimulation. Slight discomfort may be felt if electrical stimulation is applied.
• The patient needs to remain still during testing. Any movement can alter test results.
Caloric Study (Oculovestibular Reflex Study)
Indications
This test is used to evaluate the function of cranial nerve VIII. It also can indicate disease in the temporal portion of the cerebrum.
Test Explanation
Caloric studies are used to evaluate the vestibular portion of the eighth cranial nerve (CN VIII) by irrigating the external auditory canal with hot or cold water. This is usually part of a complete neurologic examination. Stimulation with cold water normally causes rotary nystagmus (involuntary rapid eye movement) away from the ear being irrigated; hot water induces nystagmus toward the side of the ear being irrigated. If the labyrinth is diseased or CN VIII is not functioning (e.g., from tumor compression), no nystagmus is induced. This study aids in the differential diagnosis of abnormalities that may occur in the vestibular system, brainstem, or cerebellum. When results are inconclusive, electronystagmography (p. 557) may be performed.
Procedure and Patient Care
During
• Although the exact procedures for caloric studies vary, note the following steps in a typical test:
1. Before the test, the patient is examined for the presence of nystagmus, postural deviation (Romberg sign), and past-pointing. This examination provides the baseline values for comparison during the test.
2. The ear canal should be examined and cleaned before testing to ensure that the water will flow freely to the middle ear area.
3. The ear on the suspected side is irrigated first because the patient's response may be minimal.
4. After an emesis basin is placed under the ear, the irrigation solution is directed into the external auditory canal until the patient complains of nausea and dizziness, or nystagmus is observed. This usually occurs in 20 to 30 seconds.
5. If after 3 minutes no symptoms occur, the irrigation is stopped.
6. The patient is tested again for nystagmus, past-pointing, and Romberg sign.
7. After approximately 5 minutes, the procedure is repeated on the other side.
• Note that this procedure is usually performed by a physician or technician in approximately 15 minutes.
![]() Tell the patient that he or she will probably experience nausea and dizziness during the test. If the patient has a decreased level of consciousness, position safely to avoid potential aspiration from vomiting.
Tell the patient that he or she will probably experience nausea and dizziness during the test. If the patient has a decreased level of consciousness, position safely to avoid potential aspiration from vomiting.
Test Results and Clinical Significance
Related Test
Electronystagmography (p. 557). During this test, nystagmus is stimulated in a manner similar to that described for caloric studies. The direction, velocity, and amplitude of the nystagmus are recorded through the use of electrodes.
Cardiac Stress Testing (Stress Testing, Exercise Testing, Electrocardiograph [EKG] Stress Testing, Nuclear Stress Testing, Echo Stress Testing)
Indications
Stress testing is used in the following situations:
1. To evaluate chest pain in a patient with suspected coronary disease (Occasionally a person may have significant coronary stenosis that is not apparent during normal physical activity. If, however, the pain can be reproduced with exercise, coronary occlusion may be present.)
2. To determine the limits of safe exercise during a cardiac rehabilitation program or to assist patients with cardiac disease in maintaining good physical fitness
3. To detect labile or exercise-related hypertension
4. To detect intermittent claudication in patients with suspected vascular occlusive disease in the extremities (In this situation, the patient may experience leg muscle cramping while performing the exercise.)
5. To evaluate the effectiveness of treatment in patients who take antianginal or antiarrhythmic medications
6. To evaluate the effectiveness of cardiac intervention (such as bypass grafting or angioplasty)
Test Explanation
Stress testing is a noninvasive study that provides information about the patient's cardiac function. In stress testing the heart is stressed in some way. The heart is then evaluated during the stress. Changes indicating ischemia point to coronary occlusive disease. By far the most commonly used method of stress is exercise (usually treadmill). Chemical stress methods are becoming more common because of their safety and increased accuracy. A third method, less frequently used, is pacer stress (Box 3-1).
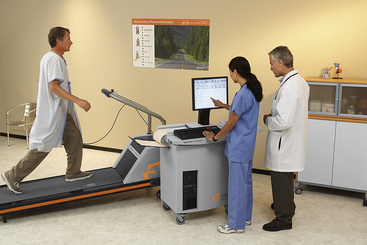
During exercise stress testing, the EKG, heart rate, and blood pressure are monitored while the patient engages in some type of physical activity (stress). Two methods of stress testing are pedaling a stationary bike and walking on a treadmill. With the stationary bicycle the pedaling tension is slowly increased to increase the heart rate. With the treadmill test the speed and grade of incline are increased. The treadmill test is the most frequently used because it is the most easily standardized and reproducible (Figure 3-1). The various grades of exercise are determined by the cardiologist in attendance based on estimation of cardiac function capabilities.
The usual goal of the exercise stress testing is to increase the heart rate to just below maximal levels or to the “target heart rate.” This target heart rate is usually 80% to 90% of the maximal heart rate. The test is usually discontinued if the patient reaches that target heart rate or develops any symptoms or EKG changes. The maximal heart rate is determined by a chart that takes into account the patient's age (about 220 minus the patient's age) and gender. The normal maximal heart rate for adults varies from 150 to 200 beats/min; patients taking calcium channel blockers and sympathetic blockers have a lower-than-expected maximal heart rate.
Exercise stress testing is based on the principle that occluded arteries will be unable to meet the heart's increased demand for blood during the testing. This may become obvious with symptoms (e.g., chest pain, fatigue, dyspnea, tachycardia, cardiac arrhythmias [dysrhythmias], fall in blood pressure) or EKG changes (e.g., ST-segment variance >1 mm, increasing premature ventricular contractions, other rhythm disturbances). An advantage of stress testing is that these symptoms can be stimulated and identified in a safe environment. Besides the electrodiagnostic method of cardiac evaluation, the stressed heart also can be evaluated by nuclear scanning or echocardiography, which are more sensitive and accurate. Findings of ischemia are discussed in “Test Results and Clinical Significance.”
When exercise testing is not advisable or the patient is unable to exercise to a level adequate to stress the heart (patients with an orthopedic, arthritic, neurologic, or pulmonary limitation), chemical stress testing is recommended. Chemical stress testing is being increasingly used because of its accuracy and ease of performance. Although chemical stress testing is less physiologic than exercise testing, it is safer and more controllable. Dipyridamole (Persantine) is a coronary vasodilator. If one coronary artery is significantly occluded, the coronary blood flow is diverted to the opened vessels. This causes a “steal syndrome” away from the stenotic or occluded coronary vessel. That is, the dipyridamole-induced vascular dilation “steals” the blood from the ischemic areas and diverts it to the open, dilated coronary vessels. Caution must be taken, however, because this can precipitate angina or myocardial infarction (MI). This test should be performed only with a cardiologist in attendance. Intravenous (IV) aminophylline can reverse the effect of dipyridamole. Adenosine works similarly to dipyridamole.
Dobutamine is another chemical that can stress the heart. Dobutamine stimulates heart muscle function. This entails administration of progressively greater amounts of dobutamine over 3-minute intervals. The normal heart muscle increases its contractility (wall motion). Ischemic muscle has no augmentation. In fact, in time the ischemic area becomes hypokinetic. Infarcted tissue is akinetic. In chemical stress testing the stressed heart is evaluated by nuclear scanning or echocardiography.
Pacing is another method of stress testing. In patients with permanent pacemakers, the rate of capture can be increased to a rate that would be considered a cardiac stress. The heart is then evaluated electrodiagnostically or with nuclear scanning or echocardiography.
As indicated in Box 3-2, the methods of evaluating the heart are nuclear scanning, echocardiography, and electrophysiologic parameters. Echocardiography is fast becoming the method of choice for urgent and elective cardiac evaluation with or without stress testing.
Stress testing is discontinued with any of the criteria noted in Box 3-3.
Contraindications
• Patients with unstable angina, because stress may induce an infarction.
• Patients with severe aortic valvular heart disease (especially stenotic lesions), because their stress tolerance is easily reached and is quite low.
• Patients who cannot participate in an exercise program because of their impaired lung or motor function. However, they can be stressed chemically.
• Patients who have recently had a myocardial infarction (MI). However, limited stress testing may be done.
• Patients with severe congestive heart failure.
• Patients who have severe claudication and cannot walk adequately to stress their hearts. However, they can be stressed chemically.
• Patients with known severe left main coronary artery disease.
Interfering Factors
• Heavy meals before testing can divert blood to the gastrointestinal tract.
• Nicotine from smoking can cause coronary artery spasm.
• Caffeine blocks the effect of dipyridamole.
• Medical problems such as hypertension, valvular heart disease (especially of the aortic valve), severe anemia, hypoxemia, and chronic pulmonary disease can affect results.
• Left ventricular hypertrophy may affect test results.
• The EKG is not a reliable indicator of ischemia in patients with left bundle branch block.
![]() Drugs that can affect test results include beta blockers (e.g., propranolol [Inderal]), calcium channel blockers, digoxin, and nitroglycerin.
Drugs that can affect test results include beta blockers (e.g., propranolol [Inderal]), calcium channel blockers, digoxin, and nitroglycerin.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Instruct the patient to abstain from eating, drinking, and smoking for 4 hours.
Instruct the patient to abstain from eating, drinking, and smoking for 4 hours.
![]() Inform the patient about the risks of the test and obtain informed consent.
Inform the patient about the risks of the test and obtain informed consent.
![]() Instruct the patient to bring comfortable clothing and athletic shoes for exercise. Slippers are not acceptable.
Instruct the patient to bring comfortable clothing and athletic shoes for exercise. Slippers are not acceptable.
![]() Inform the patient if any medications should be discontinued before testing.
Inform the patient if any medications should be discontinued before testing.
• Record the patient's vital signs for baseline values. Monitor the blood pressure during the testing.
During
• Note that a physician usually is present during stress testing.
• After the patient begins to exercise, adjust the treadmill machine settings to apply increasing levels of stress at specific intervals. Encourage and support the patient at each level of increased stress.
![]() Encourage patients to verbalize any symptoms.
Encourage patients to verbalize any symptoms.
• Note that during the test the EKG tracing and vital signs are monitored continuously.
• Terminate the test if the patient complains of chest pain, exhaustion, dyspnea, fatigue, or dizziness.
• Note that testing usually takes approximately 45 minutes.
![]() Inform the patient that the physician in attendance usually interprets the results and explains them to the patient.
Inform the patient that the physician in attendance usually interprets the results and explains them to the patient.
Test Results and Clinical Significance
Coronary artery occlusive disease: Subclinical coronary artery occlusive disease often becomes evident with stress testing.
Exercise-related hypertension or hypotension: The blood pressure is higher or lower than what is considered normal for the level of exercise.
Intermittent claudication: As with the coronary system, peripheral vascular stenosis or occlusion may not become evident until the legs are stressed as in an exercise stress test.
Abnormal cardiac rhythms such as ventricular tachycardia or supraventricular tachycardia: Ectopy may not occur or become symptomatic until the person is stressed.
Electrocardiography (Electrocardiogram [ECG, EKG])
Indications
This electrodiagnostic test records the electrical impulses that stimulate the heart to contract. It is used to evaluate arrhythmias, conduction defects, myocardial injury and damage, hypertrophy—both left and right, and pericardial diseases. It is also used to assist in the diagnosis of other noncardiac conditions such as electrolyte abnormalities, drug level abnormalities, and pulmonary diseases.
Test Explanation
The EKG is a graphic representation of the electrical impulses that the heart generates during the cardiac cycle. These electrical impulses are conducted to the body's surface, where they are detected by electrodes placed on the patient's limbs and chest. The monitoring electrodes detect the electrical activity of the heart from a variety of spatial perspectives. The EKG lead system is composed of several electrodes that are placed on each of the four extremities and at varying sites on the chest. Each combination of electrodes is called a lead.
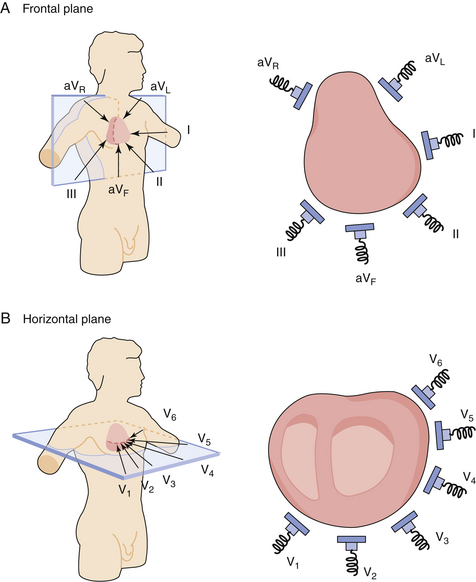
A 12-lead EKG provides a comprehensive view of the flow of the heart's electrical currents in two different planes. There are six limb leads (combination of electrodes on the extremities) and six chest leads (corresponding to six sites on the chest).
The limb leads provide a frontal plane view that bisects the body, separating it front to back. The chest leads provide a horizontal plane view that bisects the body, separating it top to bottom (Figure 3-2). Leads I, II, and III are considered the standard limb leads. Lead I records the difference in electrical potential between the left arm (LA) and the right arm (RA). Lead II records the electrical potential between the RA and the left leg (LL). Lead III reflects the difference between the LA and the LL. The right leg (RL) electrode is an inactive ground in all leads. There are three augmented limb leads: aVR, aVL, and aVF (a, augmented; V, vector [unipolar]; R, right arm; L, left arm; F, left foot or leg). The augmented leads measure the electrical potential between the center of the heart and the right arm (aVR), the left arm (aVL), and the left leg (aVF). The six standard chest, or precordial, leads (V1, V2, V3, V4, V5, V6) are recorded by placing electrodes at six different positions on the chest, surrounding the heart. (The exact locations of the leads are indicated in “Procedure and Patient Care.”)
In general, leads II, III, and aVF look at the inferior portion of the heart. Leads aVL and I look at the lateral portion of the heart. Leads V2 to V4 look at the anterior portion of the heart.
The EKG is recorded on special paper with a graphic background of horizontal and vertical lines for rapid measurement of time intervals (X coordinate) and voltages (Y coordinate). Time duration is measured by vertical lines 1 mm apart, each representing 0.04 second. Voltage is measured by horizontal lines 1 mm apart. Five 1-mm squares equal 0.5 mV.
The normal EKG pattern is composed of waves arbitrarily designated by the letters P, Q, R, S, and T. The Q, R, and S waves are grouped together and described as the QRS complex. The significance of the waves and the time intervals are as follows (Figure 3-3):
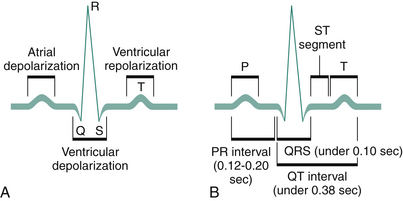
Figure 3-3 A, Normal EKG deflections during depolarization and repolarization of the atria and ventricles. B, Principal EKG intervals between P, QRS, and T waves.
• P wave. This represents atrial electrical depolarization associated with atrial contraction. It represents electrical activity associated with the spread of the original impulse from the sinoatrial (SA) node through the atria. If the P waves are absent or altered, the cardiac impulse originates outside the SA node.
• PR interval. This represents the time required for the impulse to travel from the SA node to the atrioventricular node. If this interval is prolonged, a conduction delay exists in the atrioventricular node (e.g., a first-degree heart block). If the PR interval is shortened, the impulse must have reached the ventricle through a “shortcut” (as in Wolff-Parkinson-White syndrome).
• QRS complex. This represents ventricular electrical depolarization associated with ventricular contraction. This complex consists of an initial downward (negative) deflection (Q wave), a large upward (positive) deflection (R wave), and a small downward deflection (S wave). A widened QRS complex indicates abnormal or prolonged ventricular depolarization time (as in a bundle branch block).
• ST segment. This represents the period between the completion of depolarization and the beginning of repolarization of the ventricular muscle. This segment may be elevated or depressed in transient muscle ischemia (e.g., angina) or in muscle injury (as in the early stages of myocardial infarction [MI]).
• T wave. This represents ventricular repolarization (i.e., return to neutral electrical activity).
• QT interval. This represents the time between the onset of ventricular depolarization and the end of ventricular depolarization. This interval varies with age, sex, heart rate, and medications.
• U wave. This deflection follows the T wave and is usually quite small. It represents repolarization of the Purkinje fibers within the ventricles.
Through the analysis of these wave forms and time intervals, valuable information about the heart may be obtained. The EKG is used primarily to identify abnormal heart rhythms (arrhythmias, or dysrhythmias) and to diagnose acute MI, conduction defects, and ventricular hypertrophy. It is important to note that the EKG may be normal, even in the presence of heart disease, if the heart disorder does not affect the electrical activity of the heart.
For some patients at high risk for malignant ventricular dysrhythmias, a signal-averaged EKG (SAEKG) can be performed. This test averages several hundred QRS waveforms to detect late potentials that are likely to lead to ventricular dysrhythmias. SAEKG has been a useful precursor to electrophysiologic studies (EPS) (p. 559) because it can identify ventricular tachycardia in patients with unexplained syncope. The SAEKG can be performed at the bedside in 15 to 20 minutes and must be ordered separately from a standard EKG.
Microvolt T-wave alternans (MTWA) detects T-wave alternans (variations in the vector and amplitude of the T waves) on EKG signals as small as one-millionth of a volt. Microvolt T-wave alternans is defined as an alternation in the morphology of the T-wave in an every-other-beat pattern. It has long been associated with ventricular arrhythmias and sudden death. T-wave alternans is linked to the rapid onset of ventricular tachyarrhythmias.
MTWA is significant in the clinical context because it acts as a risk stratifier between patients who need implantable cardiac defibrillators (ICDs) and those who do not. Patients who test negative for MTWA have a very low risk for sudden cardiac death and are less likely to require implantable cardiac defibrillators than those who test positive.
In this test, high-fidelity EKG leads are placed on the patient's chest during an exercise test. The goal is to get the patient walking fast enough to get the heart rate in the range of 105 to 110 beats/min, but no higher. Minute changes in T waves are measured and recorded via computer analysis.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Tell the patient that no food or fluid restriction is necessary.
Tell the patient that no food or fluid restriction is necessary.
![]() Assure the patient that the flow of electric current is from the patient. He or she will feel nothing during this procedure.
Assure the patient that the flow of electric current is from the patient. He or she will feel nothing during this procedure.
• Expose only the patient's chest, arms, and lower legs. Keep the abdomen and thighs adequately covered.
During
• Note the following procedural steps:
1. The skin areas designated for electrode placement are prepared by using alcohol swabs or sandpaper to remove skin oil or debris. Sometimes the skin is shaved if the patient has a large amount of hair.
2. Prelubricated leads are applied to ensure electrical conduction between the skin and the electrodes.
3. The four limb leads are usually held in place by clamps that can easily be opened and applied to the extremity.
4. Many cardiologists recommend that arm electrodes be placed on the upper arm, because fewer muscle tremors are detected there.
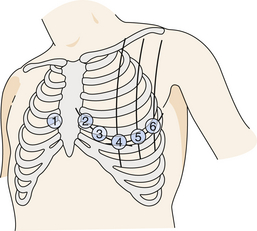
5. The chest leads are applied one at a time, three at a time, or six at a time, depending on the type of EKG machine used. These leads are positioned (Figure 3-4) as follows:
• Note that cardiac technicians, nurses, or physicians perform this procedure in less than 5 minutes at the bedside or in the cardiology clinic.
![]() Tell the patient that although this procedure causes no discomfort, he or she must lie still in the supine position without talking while the EKG is recorded.
Tell the patient that although this procedure causes no discomfort, he or she must lie still in the supine position without talking while the EKG is recorded.
Test Results and Clinical Significance
Arrhythmia (dysrhythmia): Arrhythmias can start in the atrium or the ventricle. They can cause the heart to speed up (tachyarrhythmias) or to slow down (bradyarrhythmias). With serious arrhythmias, cardiac output can fall significantly, causing the patient to lose consciousness (syncope). Often the patient may experience palpitations during some arrhythmias. Most arrhythmias are asymptomatic, however.
Acute myocardial muscle damage is often seen as elevations in the ST segment or as inverted T waves. Old MIs (or areas of dead muscle tissue) appear as deep Q waves on the EKG. The EKG should be one of the first tests to be performed on an adult patient who complains of chest pain.
Wolff-Parkinson-White syndrome:
The number and type of conduction defects are too great to discuss within the scope of this book. Some conduction defects slow the normal conduction of electrical voltage through the heart (e.g., bundle branch block). Some (e.g., Wolff-Parkinson-White syndrome) speed up the electrical conduction.
Ventricular hypertrophy: As a result of prolonged strain on the left ventricle (e.g., aortic stenosis), the thickened myocardium produces large R waves in V5 and V6 and large S waves in V1.
The right heart strain associated with acute pulmonary diseases (e.g., embolism) is called acute cor pulmonale. The classic EKG findings are “S1 Q3 T3,” which means the presence of an S wave in lead I, a Q wave in lead III, and T wave inversion in lead III. Many times, however, there may be no changes other than tachycardia associated with pulmonary emboli.
Electrolyte imbalance: Each electrolyte abnormality is associated with different EKG changes (Table 3-2).
TABLE 3-2
Electrolyte Abnormalities and Associated EKG Abnormalities
| Electrolyte Abnormality | EKG Abnormality |
| Increased calcium | Prolonged PR interval |
| Shortened QT interval | |
| Decreased calcium | Prolonged QT interval |
| Increased potassium | Narrowed, elevated T waves |
| AV conduction changes | |
| Widened QRS complex | |
| Decreased potassium | Prolonged U wave |
| Prolonged QT interval |
Pericarditis: The EKG findings of pericarditis are classic for that disease. There are widespread elevations of the ST segments involving most of the leads (except aVR). The QRS complexes are normal. When effusion is associated with the pericarditis, the voltages are diminished throughout.
Electroencephalography (Electroencephalogram [EEG])
Indications
This electrodiagnostic test is performed to identify and evaluate patients with seizures. Pathologic conditions involving the brain cortex (such as tumors, infarction) can also be detected. The EEG is also a confirmatory test for determination of brain death.
Test Explanation
The EEG is a graphic recording of the electrical activity of the brain. EEG electrodes are placed on the scalp overlying multiple areas of the brain to detect and record electrical impulses within the brain. This study is invaluable in the investigation of epileptic states, in which the focus of seizure activity is characterized by rapid, spiking waves seen on the graph. Patients with cerebral lesions (e.g., tumors, infarctions) will have abnormally slow EEG waves, depending on the size and location of the lesion. Because this study determines the overall electrical activity of the brain, it can be used to evaluate trauma and drug intoxication and also to determine cerebral death in comatose patients.
The EEG also can be used to monitor the electrophysiologic effects of cerebral blood flow during surgical procedures. For example, during carotid endarterectomy, the carotid vessel must be temporarily occluded. When this surgery is performed with the patient under general anesthesia, the EEG can be used for the early detection of cerebral tissue ischemia, which would indicate that continued carotid occlusion will result in a cerebrovascular accident (stroke) syndrome. Temporary shunting of the blood during the surgery is then required.
Electrocorticography (ECoG) is a form of EEG performed during craniotomy in which electrodes are placed directly on the exposed surface of the brain to record electrical activity from the cerebral cortex. ECoG is currently considered to be the “gold standard” for defining epileptogenic zones before attempts at surgical interruption are carried out. This procedure is invasive. The same information can be obtained by a noninvasive brain imaging technique called magnetoencephalography (MEG).
MEG measures the magnetic fields produced by electrical activity in the brain with an extremely sensitive device called a superconducting quantum interference device (SQID). The data obtained by MEG are commonly used to assist neurosurgeons in localizing pathology or defining sites of origin for epileptic seizures. MEG is also used in localizing important adjacent cortical areas for surgical planning in patients with brain tumors or intractable epilepsy. This allows the surgeon to identify and avoid injury of important nearby cortical tissue that, if injured, would cause grave neurologic defects (such as blindness, aphasia, or loss of sensation).
Interfering Factors
• Fasting may cause hypoglycemia, which could modify the EEG pattern.
• Drinks containing caffeine (e.g., coffee, tea, cocoa, cola) interfere with the test results.
• Body and eye movements during the test can cause changes in the brain wave patterns.
• Lights (especially bright or flashing) can alter test results.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Assure the patient that this test cannot “read the mind” or detect senility.
Assure the patient that this test cannot “read the mind” or detect senility.
![]() Assure the patient that the flow of electrical activity is from the patient. He or she will not feel anything during the test.
Assure the patient that the flow of electrical activity is from the patient. He or she will not feel anything during the test.
![]() Instruct the patient to wash his or her hair the night before the test. No oils, sprays, or lotion should be used.
Instruct the patient to wash his or her hair the night before the test. No oils, sprays, or lotion should be used.
• Check if the physician wants the patient to discontinue any medications before the study. (Anticonvulsants should be taken unless contraindicated by the physician.)
![]() Instruct the patient if sleeping time should be shortened the night before the test. Adults may not be allowed to sleep more than 4 or 5 hours, and children not more than 5 to 7 hours, if a sleep EEG will be attempted at the time of testing.
Instruct the patient if sleeping time should be shortened the night before the test. Adults may not be allowed to sleep more than 4 or 5 hours, and children not more than 5 to 7 hours, if a sleep EEG will be attempted at the time of testing.
• Do not administer any sedatives or hypnotics before the test because they will cause abnormal waves on the EEG.
![]() Tell the patient not to fast before the study. Fasting may cause hypoglycemia, which could alter test results.
Tell the patient not to fast before the study. Fasting may cause hypoglycemia, which could alter test results.
![]() Instruct the patient not to drink any coffee, tea, cocoa, or cola on the morning of the test because of their stimulating effect.
Instruct the patient not to drink any coffee, tea, cocoa, or cola on the morning of the test because of their stimulating effect.
![]() Tell the patient that he or she needs to remain still during the test. Any movement, including opening the eyes, will create interference and alter the EEG recording.
Tell the patient that he or she needs to remain still during the test. Any movement, including opening the eyes, will create interference and alter the EEG recording.
During
• Note the following procedural steps:
1. The EEG is usually performed in a specially constructed room that is shielded from outside disturbances.
2. The patient is placed in a supine position on a bed or reclining on a chair.
3. Sixteen or more electrodes are applied to the scalp with electrode paste in a specified pattern (as determined by the 10-20 system) over both sides of the head, covering the prefrontal, frontal, temporal, parietal, and occipital areas (Figures 3-5 and 3-6). In some laboratories the electrodes are tiny needles superficially placed in the skin of the scalp.
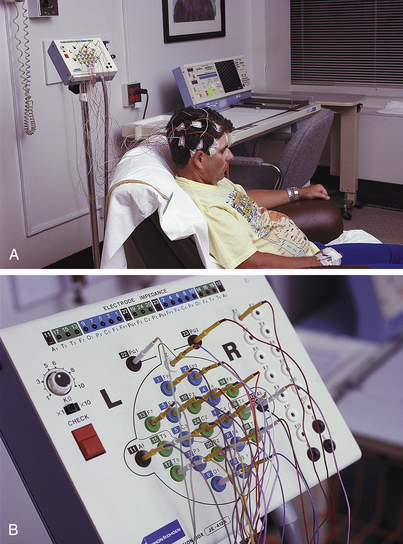
Figure 3-5 Electroencephalography (EEG). A routine EEG takes approximately 1¼ hours. The actual test lasts approximately 30 minutes. Electrodes are attached to the patient's head (A) with the wires leading to corresponding areas on the equipment (B) for recording brain wave activity.
4. One electrode may be applied to each earlobe for grounding.
5. After the electrodes are applied, the patient is instructed to lie still with his or her eyes closed.
6. The technician continuously observes the patient during the EEG recording for any movements that could alter results.
7. Approximately every 5 minutes the recording is interrupted to permit the patient to move if desired.
• In addition to the resting EEG, note that the following activating procedures can be performed:
1. The patient is hyperventilated (asked to breathe deeply 20 times a minute for 3 minutes) to induce alkalosis and cerebral vasoconstriction, which can activate otherwise hidden abnormalities.
2. Photostimulation is performed by flashing a light at variable speeds over the patient's face with the eyes opened or closed. Photostimulated seizure activity may be seen on the EEG.
3. A sleep EEG may be performed to aid in the detection of some abnormal brain waves that are seen only if the patient is sleeping (e.g., frontal lobe epilepsy). The sleep EEG is performed after orally administering a sedative or hypnotic. A recording is performed while the patient is falling asleep, while the patient is asleep, and while the patient is waking.
• Note that this study is performed by an EEG technician in approximately 45 minutes to 2 hours.
![]() Tell the patient that no discomfort is associated with this study, other than possibly missing sleep.
Tell the patient that no discomfort is associated with this study, other than possibly missing sleep.
After
• Help the patient to remove the electrode paste. The paste may be removed with acetone or witch hazel.
![]() Instruct the patient to shampoo the hair.
Instruct the patient to shampoo the hair.
• Ensure safety precautions until the effects of any sedatives have worn off. Keep the bed's side rails up.
![]() Tell the patient who has had a sleep EEG not to drive home alone.
Tell the patient who has had a sleep EEG not to drive home alone.
Test Results and Clinical Significance
Seizure disorders (e.g., epilepsy): Major, minor, and focal motor seizures can be detected by the EEG only when they are occurring. Between seizures the EEG may be normal.
Cerebral death: Cerebral death is total cessation of brain blood flow and function while the patient is being ventilated. The EEG is flat, that is, there is no electrical activity. Box 3-4 lists the criteria for brain death.
Encephalitis: Diffuse global slowing of the EEG waves may be noted.
Narcolepsy: Sleep waves are noted during what are normally waking hours.
Metabolic encephalopathy: This may be drug induced or may occur with hypoxia (e.g., after a cardiac arrest), hypoglycemia, etc. The EEG usually shows diffuse slowing of electrical activity.
Related Test
Evoked Potential Studies (p. 562). These tests are used to evaluate specific areas of the cortex that receive incoming stimulus from the eyes, ears, and lower- or upper-extremity sensory nerves.
Electromyography (EMG)
Indications
This test is used in the evaluation of patients with diffuse or localized muscle weakness/atrophy. Combined with electroneurography, EMG can identify primary muscle diseases and differentiate them from primary neurologic pathologic conditions.
This test is also used to evaluate the peripheral nervous system in patients with paresthesias and neurogenic pain.
Test Explanation
By placing a recording electrode into a skeletal muscle, one can monitor the electrical activity of a skeletal muscle in a way very similar to electrocardiography. The electrical activity is displayed on an oscilloscope as an electrical waveform. An audio electrical amplifier can be added to the system so that both the appearance and sound of the electrical potentials can be analyzed and compared simultaneously. EMG is used to detect primary muscular disorders as well as muscular abnormalities caused by other system diseases (e.g., nerve dysfunction, sarcoidosis, paraneoplastic syndrome).
Spontaneous muscle movement, such as fibrillation and fasciculation, can be detected during EMG. When evident, these waveforms indicate injury or disease of the nerve or muscle being evaluated. A decrease in the number of muscle fibers able to contract is typically observed with peripheral nerve damage. This study is usually done in conjunction with nerve conduction studies (p. 574) and also may be called electromyoneurography.
The EMG is performed by a physiatrist, musculoskeletal physician, or neurologist in approximately 30 to 60 minutes. The small needle size helps reduce discomfort.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient. Allay any fears and allow the patient to express concerns.
Explain the procedure to the patient. Allay any fears and allow the patient to express concerns.
• Obtain informed consent if required by the institution.
![]() Tell the patient that fasting is not usually required; however, some facilities may restrict stimulants (coffee, tea, cocoa, cola, cigarettes) for 2 to 3 hours before the test.
Tell the patient that fasting is not usually required; however, some facilities may restrict stimulants (coffee, tea, cocoa, cola, cigarettes) for 2 to 3 hours before the test.
• If serum enzyme tests (e.g., aspartate aminotransferase [AST], creatine phosphokinase [CPK], lactic dehydrogenase [LDH]) are ordered, the specimen should be drawn before EMG or 5 to 10 days afterward because the penetration of the muscle by the electrodes may cause misleading elevations of these enzymes, which can be produced by the muscle tissue.
• Premedication or sedation is usually avoided because of the need for patient cooperation. The small needle size makes the test nearly painless.
During
• Note the following procedural steps:
1. This study is usually done in an EMG laboratory. This may be specially designed (with copper-lined walls) to minimize extraneous electrical activity.
2. The patient's position and the position of the electrode depend on the muscle being studied.
3. A tiny needle that acts as a reference electrode is inserted into the muscle being examined (Figure 3-7) or overlying the nerve itself. In most circumstances, however, that reference electrode is in the needle itself.
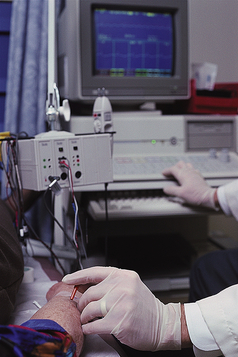
Figure 3-7 Patient having electromyogram (EMG) of forearm. Tiny needle size makes procedure nearly painless.
4. A reference electrode is placed nearby on the skin surface.
5. The patient is asked to keep the muscle at rest.
6. The oscilloscope display is viewed for any evidence of spontaneous electrical activity, such as fasciculation or fibrillation.
7. The patient is asked to contract the muscle slowly and progressively.
8. The electrical waves produced are examined for their number, form, and amplitude. This evaluates the muscular component of the test.
9. Next, a nerve innervating a particular muscle group is stimulated, and the resulting muscle contraction is evaluated as described if nerve conduction studies are performed concomitantly.
Test Results And Clinical Significance
Polymyositis: This disease is evidenced by early to recruit, small, spontaneous waveforms (myotonia), caused by hyperirritability of the muscle membrane.
These primary muscle diseases are denoted by decreased electrical activity and amplitude. Even with nerve stimulation, little or no activity is seen. This indicates weakened muscle tissue.
These endocrine diseases are marked by decreased electrical activity in both amplitude and frequency. This indicates weakened muscle tissue.
Paraneoplastic syndrome (e.g., lung cancer),
These two diseases can be associated with ectopic production of adrenocorticotropic hormone. As in hyperadrenalism, decreased electrical activity in both amplitude and frequency are noted. This indicates weakened muscle tissue.
Peripheral nerve injury, entrapment, or compression,
Related Test
Nerve Conduction Studies (p. 574). This is similar to EMG except that it evaluates the integrity of the peripheral nerves. This test is often performed with EMG.
Electronystagmography (ENG, Electrooculography)
Indications
This electrodiagnostic test is used to evaluate patients with vertigo and to differentiate organic from psychogenic vertigo. With this test, central (cerebellum, brainstem, eighth cranial nerve) pathologic conditions can be differentiated from peripheral (vestibular-cochlear) pathologic conditions. If a known lesion exists, ENG can identify the site of the lesion. This test is also used to evaluate unilateral deafness.
Test Explanation
ENG is used to evaluate nystagmus (involuntary rapid eye movement) and the muscles controlling eye movement. By measuring changes in the electrical field around the eye, this study can make a permanent recording of eye movement at rest, with a change in head position, and in response to various stimuli. The test delineates the presence or absence of nystagmus, which is caused by the initiation of the oculovestibular reflex. Nystagmus should occur when initiated by positional, visual, or caloric (p. 557) stimuli. Unlike caloric studies, in which nystagmus is usually determined visually, with ENG, the direction, velocity, and degree of nystagmus can be recorded. If nystagmus does not occur with stimulation, the vestibular-cochlear apparatus, cerebral cortex (temporal lobe), auditory nerve, or brainstem is abnormal. Tumors, infection, ischemia, and degeneration can cause such abnormalities. The pattern of nystagmus when put together with the entire clinical picture helps in the differentiation between central and peripheral vertigo. This test is used in the differential diagnosis of lesions in the vestibular system, brainstem, and cerebellum.
It also may help evaluate unilateral hearing loss and vertigo. Unilateral hearing loss may be related to middle ear problems or nerve injury. If the patient experiences nystagmus with stimulation, the auditory nerve is working and hearing loss can be blamed on the middle ear.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Instruct the patient not to apply facial makeup before the test because electrodes will be taped to the skin around the eyes.
Instruct the patient not to apply facial makeup before the test because electrodes will be taped to the skin around the eyes.
![]() Instruct the patient not to eat solid food before the test to reduce the likelihood of vomiting.
Instruct the patient not to eat solid food before the test to reduce the likelihood of vomiting.
![]() Instruct the patient not to drink caffeine or alcoholic beverages for approximately 24 to 48 hours (as ordered) before the test.
Instruct the patient not to drink caffeine or alcoholic beverages for approximately 24 to 48 hours (as ordered) before the test.
• Check with the physician regarding withholding any medications that could interfere with the test results.
During
• Note the following procedural steps:
1. This procedure is usually performed in a darkened room with the patient seated or lying down on an examining table.
2. If there is any wax in the ear, it is removed.
3. Electrodes are taped to the skin around the eyes (Figure 3-8).
4. Various procedures are used to stimulate nystagmus, such as pendulum tracking, changing head position, changing gaze position, and caloric studies (p. 538).
5. Several recordings are made with the patient at rest and demonstrating patient response to various procedures (e.g., blowing air into the ear, irrigating the ear with water).
6. Nystagmus response is compared with the expected ranges, and the results are recorded as “normal,” “borderline,” or “abnormal.”
• Note that this procedure is performed by a physician or audiologist in approximately 1 hour.
![]() Tell the patient that nausea and vomiting may occur during the test.
Tell the patient that nausea and vomiting may occur during the test.
Test Results and Clinical Significance
Related Test
Caloric Study (p. 538). This is a test in which nystagmus is stimulated by warm or cold water (or air) and the presence or absence of nystagmus is observed.
Electrophysiologic Study (EPS, Cardiac Mapping)
Indications
EPS is a method of studying evoked potentials within the heart. It is used to evaluate patients with syncope, palpitations, or arrhythmias. It is used to identify the location of conduction defects that cause abnormal electroconduction and arrhythmias. It can also be used to monitor antiarrhythmic therapy. Through EPS the area known to induce arrhythmias can be obliterated by radiofrequency waves.
Test Explanation
In this invasive procedure, fluoroscopic guidance is used to place multiple-electrode catheters through a peripheral vein and into the right atrium and/or ventricle or, less often, through an artery into the left atrium and/or ventricle. With close cardiac monitoring the electrode catheters are used to pace the heart and potentially induce arrhythmias (dysrhythmias). Defects in the heart conduction system can then be identified; arrhythmias that are otherwise not apparent also can be induced, identified, and treated. The effectiveness of antiarrhythmic drugs (e.g., lidocaine, phenytoin, quinidine) can be assessed by determining the electrical threshold required to induce arrhythmias.
EPS can also be therapeutic. With the use of radiofrequency waves, sites with documented low thresholds for inducing arrhythmias can be obliterated to stop the arrhythmias.
Procedure and Patient Care
Before
![]() Instruct the patient to fast for 6 to 8 hours before the procedure. Fluids are usually permitted until 3 hours before the test.
Instruct the patient to fast for 6 to 8 hours before the procedure. Fluids are usually permitted until 3 hours before the test.
• Obtain an informed consent from the patient.
• Encourage the patient to verbalize any fears regarding this test.
• Prepare the catheter insertion site as directed.
• Collect a blood sample for potassium or drug levels, if indicated.
• Obtain peripheral intravenous (IV) access for the administration of drugs.
During
• Note the following procedural steps:
1. After being transported to the cardiac catheterization laboratory, the patient has electrocardiographic (EKG) leads attached.
2. The catheter insertion site, usually the femoral artery or vein, is prepared and draped in a sterile manner.
3. Under fluoroscopic guidance the catheter is passed to the atrium and ventricle.
4. Baseline surface intracardiac EKGs are recorded.
5. Various parts of the cardiac electroconduction system are stimulated by atrial or ventricular pacing.
6. Mapping of the electroconduction system and its defects is performed by measuring evoked potentials.
7. Arrhythmias (dysrhythmias) are identified.
8. Drugs may be administered to assess their efficacy in preventing EPS-induced arrhythmias.
9. Because dangerous arrhythmias can be prolonged, cardioversion must be immediately available.
10. Not only are vital signs and the heart monitored, but also the patient is constantly engaged in light conversation to assess mental status and consciousness.
• Note that this procedure is performed by a cardiologist within a darkened cardiac catheterization laboratory in approximately 1 to 4 hours.
![]() Tell the patient that he or she may experience palpitations, light-headedness, or dizziness when arrhythmias are induced. The patient should report these sensations to the physician. For most patients, this is an anxiety-producing experience.
Tell the patient that he or she may experience palpitations, light-headedness, or dizziness when arrhythmias are induced. The patient should report these sensations to the physician. For most patients, this is an anxiety-producing experience.
![]() Inform the patient that discomfort from catheter insertion is minimal.
Inform the patient that discomfort from catheter insertion is minimal.
After
• Keep the patient on bed rest for approximately 6 to 8 hours.
• Apply pressure to the catheter insertion site. Evaluate the venous access site for swelling and bleeding.
• Monitor the patient's vital signs for at least 2 to 4 hours for hypotension and arrhythmias (dysrhythmias). Additional monitoring is especially important for certain medications that the patient received during the test. For example, if the patient received quinidine, he or she should be monitored for hypotension and abdominal cramping.
• Continue cardiac monitoring to identify arrhythmias. Transfer arrangements to a monitored unit may be necessary.
• Cover the area with sterile dressings if the electrical catheter is left in place for subsequent studies.
Test Results and Clinical Significance
Sinoatrial node defects (e.g., sick sinus syndrome),
Atrioventricular node defects and heart blocks,
Inducible arrhythmias (e.g., ventricular tachycardia and Wolff-Parkinson-White):
Related Test
Electrocardiography (EKG) (p. 544). This is the only other mechanism available to identify and locate the source of arrhythmia.
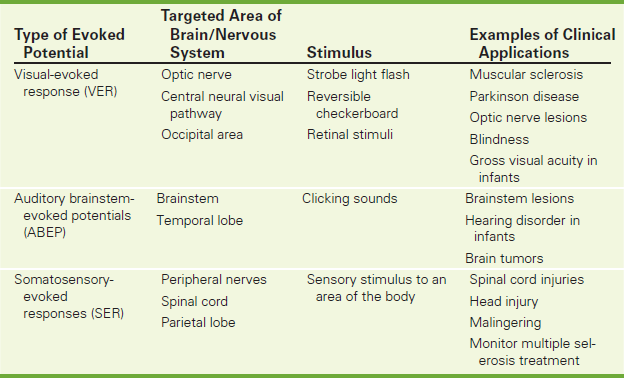
Evoked Potential Studies (EP Studies, Evoked Brain Potentials, Evoked Responses, Visual-Evoked Responses [VERs], Auditory Brainstem-Evoked Potentials [ABEPs], Somatosensory-Evoked Responses [SERs])
Indications
EP studies are indicated for patients who have a suspected sensory deficit but are unable to indicate or are unreliable in indicating recognition of a stimulus. These may include infants, comatose patients, or patients who are unable to communicate. These tests are used to evaluate specific areas of the cortex that receive incoming stimulus from the eyes, ears, and lower or upper extremities' sensory nerves. They are used to monitor natural progression or treatment of deteriorating neurologic diseases (e.g., multiple sclerosis). Finally, they are also used to identify histrionic or malingering patients who have sensory deficit complaints.
Test Explanation
EP studies focus on changes and responses in brain waves that are evoked from stimulation of a sensory pathway. The study of EPs grew out of early work with the electroencephalogram (EEG) (p. 549). Although the EEG measures “spontaneous” brain electrical activity, the sensory EP study measures minute voltage changes produced in response to a specific stimulus, such as a light pattern, an audible click, or a shock. In contrast to the EEG, which records signals that reach amplitudes of up to 50 to 100 mV, EP signals are usually less than 5 mV. Because of this, they can be detected only with an averaging computer. The computer averages out (or cancels) unwanted random waves to sum the evoked response that occurs at a specific time after a given stimulus.
EP studies allow one to measure and assess the entire sensory pathway from the peripheral sensory organ all the way to the brain cortex (recognition of the stimulus). Clinical abnormalities are usually detected by an increase in latency, which refers to the delay between the stimulus and the wave response. Normal latency times are calculated depending on body size, position of the body where the stimulus is applied, conduction velocity of axons in the neural pathways, number of synapses in the system, location of nerve generators of EP components (brainstem or cortex), and presence of central nervous system (CNS) pathologic conditions. Conduction delays indicate damage or disease anywhere along the neural pathway from the sensory organ to the cortex.
Sensory stimuli used for the EP study can be visual, auditory, or somatosensory. The sensory stimulus chosen depends on what sensory system is suspected to be pathologic (e.g., questionable blindness, deafness, or numbness). Also, the sensory stimulus chosen may depend on the area of brain in which abnormality is suspected. (Auditory stimuli check the brainstem and temporal lobes of the brain; visual stimuli test the optic nerve, central neural visual pathway, and occipital portions of the brain; somatosensory stimuli check the peripheral nerves, spinal cord, and parietal lobe of the brain.) Increased latency (i.e., abnormally prolonged period from the time of stimulus to the time of brain EEG recognition) indicates a pathologic condition of the sensory organ or the specific neural pathway as described previously. See Table 3-3.
Visual-evoked responses (VERs) are usually stimulated by a strobe light flash, reversible checkerboard pattern, or retinal stimuli (Figure 3-9). A visual stimulus to the eye causes an electrical response in the occipital area that can be recorded with “EEG-like” electrodes placed on the scalp overlying the vertex and on the occipital lobes. Ninety percent of patients with multiple sclerosis show abnormal latencies in VERs, a phenomenon attributed to demyelination of nerve fibers. In addition, patients with other neurologic disorders (e.g., Parkinson disease) show an abnormal latency with VERs. The degree of latency seems to correlate with the disease severity. Abnormal results also may be seen in patients with lesions of the optic nerve, optic tract, visual center, and the eye itself. Absence of binocularity, which is a neurologic developmental disorder in infants, can be detected and evaluated by VERs. Eyesight problems or blindness can be detected in infants through VERs or electroretinography. This test also can be used during eye surgery to provide a warning of possible damage to the optic nerve. Infants' gross visual acuity can even be checked using VERs.

Figure 3-9 Patient undergoing test for visual-evoked responses. The patient is asked to concentrate on the yellow dot in the middle of the screen while the checkerboard pattern moves. Usually a patch is placed over one eye at a time. The room is darkened for the actual procedure.
Auditory brainstem-evoked potentials (ABEPs) are usually stimulated by clicking sounds to evaluate the central auditory pathways of the brainstem (Figure 3-10). Either ear can be evoked to detect lesions in the brainstem that involve the auditory pathway without affecting hearing. One of the most successful applications of ABEPs has been screening low-birth-weight (LBW) newborns and other infants for hearing disorders. Recognition of deafness enables infants to be fitted with corrective devices as early as possible. Use of these devices before affected children learn to speak helps prevent speech abnormalities. ABEPs also have great therapeutic implications in the early detection of posterior fossa brain tumors.
Somatosensory-evoked responses (SERs) are usually initiated by sensory stimulus to an area of the body. The time is then measured for the current of the stimulus to travel along the nerve to the cortex of the brain. SERs are used to evaluate patients with spinal cord injuries and to monitor spinal cord functioning during spinal surgery. They are also used to monitor treatment of diseases (e.g., multiple sclerosis), to evaluate the location and extent of areas of brain dysfunction after head injury, and to pinpoint tumors at an early stage. These tests can also be used to identify malingering or hysterical numbness. The latency is normal in these patients despite the fact that they indicated numbness.
One of the main benefits of EPs is their objectivity, because voluntary patient response is not needed. This makes EPs useful with nonverbal and uncooperative patients. This objectivity permits the distinction of organic from psychogenic problems. This is invaluable in settling lawsuits concerning workers' compensation insurance. The projected future of EPs is that they will aid in diagnosing and monitoring mental disorders and learning disabilities.
Procedure and Patient Care
During
• Note that the position of the electrode depends on the type of EP study to be done:
1. For VERs, electrodes are placed on the scalp along the vertex and the cortex lobes. Stimulation occurs by using a strobe light, checkerboard pattern, or retinal stimuli.
2. ABEPs are stimulated with clicking noises or tone bursts delivered via earphones. The responses are detected by scalp electrodes placed along the vertex and on each earlobe.
3. SERs are stimulated using electrical stimuli applied to nerves at the wrist (medial nerve) or the knee (peroneal nerve). The response is detected by electrodes placed over the sensory cortex of the opposite hemisphere on the scalp.
• Note that this study is performed by a physician or technician in less than 30 minutes.
![]() Tell the patient that little or no discomfort is associated with this study.
Tell the patient that little or no discomfort is associated with this study.
Test Results and Clinical Significance
Prolonged Latency for VERs
Demyelinating diseases (e.g., multiple sclerosis):
Optic nerve damage: In the absence of a functioning optic nerve, the stimulus cannot reach the cortex. VER latency will be prolonged or absent.
Without visual sensory functioning, the stimulus cannot reach the cortex, so stimulus recognition will not occur.
Prolonged Latency for ABEPs
Demyelinating diseases (e.g., multiple sclerosis): Demyelinating diseases destroy the function and integrity of the peripheral and central nervous system. Latency is prolonged.
Tumor—acoustic neuroma: These tumors grow where the eighth cranial nerve passes under the temporal lobe. Destruction by compression prolongs latency.
Infarctions of either portion of the brain will prolong ABEP latency. The brainstem is an important part of the reflex auditory mechanism.
Auditory nerve damage: If the auditory nerve is not functioning, the stimulus cannot reach the cortex. ABEP latency will be prolonged or absent.
Deafness: Without auditory sensory functioning, the stimulus cannot reach the cortex. Stimulus recognition will not occur. The test can be performed with vibratory stimuli, however. This bypasses the function of the inner ear.
Abnormal Latency for SERs
Spinal cord demyelinating diseases:
Because the spinal cord is the path by which the stimulus reaches the cortex, diseases affecting the spinal cord will prolong latency.
Peripheral nerve injury, transection, or disease: The somatic stimulus must travel by way of sensory peripheral nerves to the spinal cord. Diseases that affect the function of these nerves will prolong latency.
Parietal cortical tumor or CVA: Unilateral or bilateral latency may be noted in diseases that compress or destroy parietal cortical tissue.
Related Test
Electroencephalography (EEG) (p. 549). This electrodiagnostic test is used to detect large electrical waves generated by the cortical structures of the brain and to identify areas of seizure activity or wave slowing compatible with specific pathologic conditions.
Fetal Contraction Stress Test (CST, Oxytocin Challenge Test [OCT])
Indications
The fetal CST is a method to evaluate the viability of a fetus. It documents the ability of the placenta to provide an adequate blood supply to the fetus. The CST can be used to evaluate any high-risk pregnancy in which fetal well-being may be threatened. These pregnancies include those marked by diabetes, hypertensive disease of pregnancy (toxemia), intrauterine growth restriction, Rh-factor sensitization, history of stillbirth, postmaturity, or low estriol levels.
Test Explanation
The CST, frequently called the oxytocin challenge test (OCT), is a relatively noninvasive test of fetoplacental adequacy used in the assessment of high-risk pregnancy. (Other tests used to evaluate the fetoplacental unit are listed in Box 3-5.) For this study, a temporary stress in the form of uterine contractions is applied to the fetus after the intravenous (IV) administration of oxytocin. The reaction of the fetus to the contractions is assessed by an external fetal heart monitor. Uterine contractions cause transient impediment of placental blood flow. If the placental reserve is adequate, the maternal-fetal oxygen transfer is not significantly compromised during the contractions and the fetal heart rate (FHR) remains normal (a negative test). The fetoplacental unit can then be considered adequate for the next 7 days.
If the placental reserve is inadequate, the fetus does not receive enough oxygen during the contraction. This results in intrauterine hypoxia and late deceleration of the FHR. The test is considered to be positive if consistent, persistent, late decelerations of the FHR occur with two or more uterine contractions. False-positive results caused by uterine hyperstimulation can occur in 10% to 30% of patients. Thus, positive test results warrant a complete review of other studies (e.g., amniocentesis) before the pregnancy is terminated by delivery.
The test is considered to be unsatisfactory if the results cannot be interpreted (e.g., because of hyperstimulation of the uterus, excessive movement of the mother, or deceleration of unknown meaning [not associated with contractions]). In the case of unsatisfactory results, other means of evaluation should be considered (ultrasound or amniocentesis).
Although this test can be performed reliably at 32 weeks of gestation, it usually is done after 34 weeks. The CST can induce labor, and a fetus at 34 weeks is more likely to survive an unexpectedly induced delivery than a fetus at 32 weeks. The Fetal Nonstress Test (p. 569) is the preferred test in almost every instance and can be performed more safely at 32 weeks; it can then be followed 2 weeks later by the CST if necessary. The CST may be performed weekly until delivery terminates pregnancy.
Although rarely done, there is a noninvasive, alternative method of performing the CST called the breast stimulation or nipple stimulation technique. Stimulation of the nipple causes nerve impulses to the hypothalamus that trigger the release of oxytocin into the mother's bloodstream. This causes uterine contractions and may eliminate the need for IV administration of oxytocin. Uterine contractions are usually satisfactory after 15 minutes of nipple stimulation (gentle twisting of the nipples). Advantages of this technique include the ease of performing the test, shorter duration of the study, and elimination of the need to start, monitor, and stop IV infusions. If sufficient contractions do not result from nipple stimulation, the standard CST procedure is followed.
The CST is performed safely on an outpatient basis in the labor and delivery unit, where qualified nurses and necessary equipment are available. The test is performed by a nurse with a physician available. The duration of this study is approximately 2 hours. The discomfort associated with the CST may consist of mild labor contractions. Breathing exercises are usually sufficient to control any discomfort.
Contraindications
• Patients pregnant with multiple fetuses, because the myometrium is under greater tension and is more likely to be stimulated to premature labor
• Patients with a prematurely ruptured membrane, because labor may be stimulated by the CST
• Patients with placenta previa, because vaginal delivery may be induced
• Patients with abruptio placentae, because the placenta may separate from the uterus as a result of the oxytocin-induced uterine contractions
• Patients with a previous hysterotomy, because the strong uterine contractions may cause uterine rupture
• Patients with a previous vertical or classic cesarean section, because the strong uterine contractions may cause uterine rupture. (The test can be performed, however, if it is carefully monitored and controlled.)
• Patients with pregnancies of less than 32 weeks, because early delivery may be induced by the procedure
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
• Obtain informed consent for the procedure.
![]() Teach the patient breathing and relaxation techniques.
Teach the patient breathing and relaxation techniques.
• Record the patient's blood pressure and the FHR before the test as baseline values.
• If the CST is performed on an elective basis, the patient may be kept on nothing by mouth (NPO) status in case labor occurs.
During
• Note the following procedural steps:
1. After the patient empties her bladder, place her in a semi-Fowler's position and tilted slightly to one side to avoid vena caval compression by the enlarged uterus.
2. Check her blood pressure every 10 minutes to avoid hypotension, which may cause diminished placental blood flow and a false-positive test result.
3. Place an external fetal monitor over the patient's abdomen to record the fetal heart tones. Attach an external tocodynamometer to the abdomen at the fundal region to monitor uterine contractions.
4. Record the output of the fetal heart tones and uterine contractions on a two-channel strip recorder.
5. Monitor baseline FHR and uterine activity for 20 minutes.
6. If uterine contractions are detected during this pretest period, withhold oxytocin and monitor the response of the fetal heart tone to spontaneous uterine contractions.
7. If no spontaneous uterine contractions occur, administer oxytocin (Pitocin) by IV infusion pump.
8. Increase the rate of oxytocin infusion until the patient is having moderate contractions, then record the FHR pattern.
9. After the oxytocin infusion is discontinued, continue FHR monitoring for another 30 minutes until the uterine activity has returned to its preoxytocin state. The body metabolizes oxytocin in approximately 20 to 25 minutes.
Fetal Nonstress Test (NST, Fetal Activity Determination)
Indications
The NST is a method to evaluate the viability of a fetus. It documents the placenta's ability to provide an adequate blood supply to the fetus. The NST can be used to evaluate any high-risk pregnancy in which fetal well-being may be threatened. These pregnancies include those marked by diabetes, hypertensive disease of pregnancy (toxemia), intrauterine growth restriction, Rh-factor sensitization, history of stillbirth, postmaturity, or low estriol levels.
Test Explanation
The NST is a noninvasive study that monitors acceleration of the fetal heart rate (FHR) in response to fetal movement. This FHR acceleration reflects the integrity of the central nervous system (CNS) and fetal well-being. Fetal activity may be spontaneous, induced by uterine contraction, or induced by external manipulation. Oxytocin stimulation is not used. Fetal response is characterized as “reactive” or “nonreactive.” The NST indicates a reactive fetus when, with fetal movement, two or more FHR accelerations are detected, each of which must be at least 15 beats/min for 15 seconds or more within any 10-minute period. The test is 99% reliable in indicating fetal viability and negates the need for the fetal contraction stress test (CST, p. 566). If the test detects a nonreactive fetus (i.e., no FHR acceleration with fetal movement) within 40 minutes, the patient is a candidate for the CST. A 40-minute test period is used because this is the average duration of the sleep-wake cycle of the fetus. The cycle may vary considerably, however.
The NST is useful in screening high-risk pregnancies and in selecting those patients who may require the CST. The NST is how routinely performed before the CST to avoid the complications associated with oxytocin administration. No complications are associated with the NST.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Encourage verbalization of the patient's fears. The necessity for the study usually raises realistic fears in the expectant mother.
Encourage verbalization of the patient's fears. The necessity for the study usually raises realistic fears in the expectant mother.
![]() If the patient is hungry, instruct her to eat before the NST is begun. Fetal activity is enhanced with a high maternal serum glucose level.
If the patient is hungry, instruct her to eat before the NST is begun. Fetal activity is enhanced with a high maternal serum glucose level.
During
• After the patient empties her bladder, place her in the Sims' position.
• Place an external fetal monitor on the patient's abdomen to record the FHR. The mother can indicate fetal movement by pressing a button on the fetal monitor whenever she feels the fetus move. The FHR and fetal movement are concomitantly recorded on a two-channel strip graph.
• Observe the fetal monitor for FHR accelerations associated with fetal movement.
• If the fetus is quiet for 20 minutes, stimulate fetal activity by external methods, such as rubbing or compressing the mother's abdomen, ringing a bell near the abdomen, or placing a pan on the abdomen and hitting the pan.
• Note that a nurse performs the NST in approximately 20 to 40 minutes in the physician's office or a hospital unit.
![]() Tell the patient that no discomfort is associated with the NST.
Tell the patient that no discomfort is associated with the NST.
Test Results and Clinical Significance
Nonreactive fetus: This result alone does not indicate fetal distress, but when it is combined with other noninvasive tests such as CST, biophysical profile, alpha-fetoprotein, pregnanediol, and obstetric ultrasound, fetal health can be accurately determined.
Related Test
Fetal Contraction Stress Test (p. 566). This test is frequently called the oxytocin challenge test. It is a relatively noninvasive test of fetoplacental adequacy used in the assessment of high-risk pregnancy.
Holter Monitoring (Ambulatory Monitoring, Ambulatory Electrocardiography, Event Recorder)
Indications
Holter monitoring is used to record a patient's heart rate and rhythm for one or more days. It is indicated in patients who experience syncope, palpitations, atypical chest pains, or unexplained dyspnea.
Test Explanation
Holter monitoring is a continuous recording of the electrical activity of the heart. This can be performed for periods up to 72 hours. With this technique, an electrocardiogram (EKG) is recorded continuously on magnetic tape during unrestricted activity, rest, and sleep. The Holter monitor is equipped with a clock that permits accurate time monitoring on the EKG tape. The patient is asked to carry a diary and record daily activities, as well as any cardiac symptoms that may develop during the period of monitoring (Figure 3-11).
Most units are equipped with an “event marker.” This is a button the patient can push when symptoms such as chest pain, syncope, or palpitations are experienced. This type of monitor is referred to as an event recorder. Many recorders store the rhythm immediately preceding activation of the recorder. Stored information can be transmitted by telephone to a recording station.
The Holter monitor is used primarily to identify suspected cardiac rhythm disturbances and to correlate these disturbances with symptoms such as dizziness, syncope, palpitations, or chest pain. The monitor is also used to assess pacemaker function and the effectiveness of antiarrhythmic medications.
After completion of the determined time period, usually 24 to 72 hours, the Holter monitor is removed from the patient and the record tape is played back at high speed. The EKG tracing is usually interpreted by computer, which can detect any significant abnormal waveform patterns that occurred during the testing. Two different computer printouts can be generated. The first is generated from an “event recording,” in which representative tracings during noted events are printed out. Tracings demonstrating maximum and minimum heart rates are also printed. A report then can be generated regarding the frequency and severity of abnormal cardiac events, especially in relation to the patient's symptoms. The second type of report is generated from a “full disclosure recording,” in which all the beats are printed out and are scanned by a technologist, who looks for aberrant waveforms. These aberrations are then provided to the cardiologist for review.
Implantable loop recorders (ILRs) are used when long-term monitoring is required. These recorders are implanted subcutaneously via a small incision. They record electrocardiographic tracings continuously or only when purposefully activated by the patient. The recording device can be automatically activated by a predefined arrhythmia that will trigger device recording. If nothing irregular happens, the information is subsequently erased. But if an arrhythmia does occur, the device locks it in and saves it to memory. ILRs can provide a diagnosis in many patients with unexplained syncope or presyncope.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Instruct the patient about care of the Holter monitor (Figure 3-12).
Instruct the patient about care of the Holter monitor (Figure 3-12).
![]() Inform the patient about the necessity of ensuring good contact between the electrodes and the skin.
Inform the patient about the necessity of ensuring good contact between the electrodes and the skin.
![]() Teach the patient how to maintain an accurate diary. Stress the need to record activities and significant symptoms.
Teach the patient how to maintain an accurate diary. Stress the need to record activities and significant symptoms.
![]() Instruct the patient to note in the diary if any interruption in Holter monitoring occurs.
Instruct the patient to note in the diary if any interruption in Holter monitoring occurs.
![]() Assure the patient that the electrical flow is coming from the patient and that he or she will not experience any electrical stimulation from the machine.
Assure the patient that the electrical flow is coming from the patient and that he or she will not experience any electrical stimulation from the machine.
![]() Instruct the patient not to bathe during the period of cardiac monitoring.
Instruct the patient not to bathe during the period of cardiac monitoring.
![]() Tell the patient to minimize the use of electrical devices (e.g., electric toothbrushes, shavers), which may cause artificial changes in the EKG tracing.
Tell the patient to minimize the use of electrical devices (e.g., electric toothbrushes, shavers), which may cause artificial changes in the EKG tracing.
During
• Prepare the sites for electrode placement with alcohol (this is usually done in the cardiology department by a technologist). (See equipment in Figure 3-12.)
• Securely place the gel and electrodes at the appropriate sites. The chest and abdomen are usually the most appropriate locations for limb-lead electrode placement. The precordial leads also may be placed.
• Usually, do not use the extremities for electrode placement to minimize alterations in tracing that occur with normal physical activity.
![]() Encourage the patient to call if he or she has any difficulties.
Encourage the patient to call if he or she has any difficulties.
• Use a tight undershirt or netlike dressing to hold the leads in place.
Test Results and Clinical Significance
Cardiac arrhythmia (dysrhythmia): Tachycardia or bradycardia may be noted and may be a cause of syncope. Frequent premature beats may be identified.
Ischemic changes: If a patient experiences unusual pain symptoms during a particular exercise, a monitor can be applied and that particular exercise performed. If the pain occurs and associated EKG ischemic changes are noted on the monitor, the diagnosis of angina can be made even though the pain is atypical.
Related Test
Electrocardiography (EKG) (p. 544). This is an electrodiagnostic test of the heart taken at one point in time, whereas Holter monitoring continually records EKG data during the entire monitoring period.
Nerve Conduction Studies (NCS, Electroneurography)
Normal Findings
No evidence of peripheral nerve injury or disease (Conduction velocity is usually decreased in elderly people.)
Indications
This test is performed to identify peripheral nerve injury in patients with localized or diffuse weakness, muscle atrophy, dysesthesia, paresthesia, and neurogenic pain to differentiate primary peripheral nerve disease from muscular injury. NCS can document the severity of injury. It also is used to monitor the nerve injury and response to treatment.
Test Explanation
Nerve conduction studies evaluate the integrity of the nerves and allow the detection and location of peripheral nerve injury or disease. By initiating an electrical impulse at one site (proximal, when evaluating motor nerves or distal when evaluating sensory nerves) of a nerve and recording the time required for that impulse to travel to a second site (opposite above) of the same nerve, the conduction velocity of an impulse in that nerve can be determined. This study is usually done in conjunction with EMG (p. 554) and also may be called electromyoneurography.
The normal value for conduction velocity may only slightly vary. It is always best to compare the conduction velocity of the suspected side with the contralateral nerve conduction velocity. In general, a range of normal conduction velocity for the upper extremities will be approximately 50 to 60 m/sec. For the lower extremities, normal conduction velocity is 40 to 50 m/sec.
Trauma to or contusion of a nerve usually cause slowing of conduction velocity in the affected side compared with the normal side. Neuropathies, both local and generalized, also cause a slowing of conduction velocity. A velocity greater than normal does not indicate a pathologic condition. With complete nerve transection, no nerve conduction is noted.
Because conduction velocity may require contraction of a muscle as an indication of an impulse arriving at the recording electrode, significant primary muscular disorders may cause a falsely slow nerve conduction velocity. This “muscular” variable is eliminated if one evaluates the suspected pathologic muscle group before performing nerve conduction studies. This muscular factor is evaluated by measuring distal latency (i.e., the time required for stimulation of the nerve to cause muscular contraction). As the distal latency is calculated, the motor nerve conduction study is performed normally by stimulating the nerve bundle. Conduction velocity can then be determined by the following equation:
NCS can also indicate diseases affecting either the motor or sensory nerves. Diseases affecting the neuromuscular junction, nerve axon loss, and variations in nerve recovery time can be evaluated.
NCS takes about 15 minutes and is performed by a physiatrist, neurologist, or trained technologist. It may be uncomfortable because a mild shock is required for nerve impulse stimulation.
Procedure and Patient Care
During
• Note the following procedural steps:
1. This test can be performed in a nerve conduction laboratory, office setting, or at the patient's bedside.
2. The patient's position depends on the area of suspected peripheral nerve injury or disease.
3. A recording electrode is placed on the skin overlying a muscle innervated solely by the relevant nerve.
4. A reference electrode is placed nearby.
5. All skin-to-electrode connections are ensured by using electrical paste.
6. The nerve is stimulated by a shock-emitting device at an adjacent location.
7. For the evaluation of a motor nerve, the time between nerve impulse and muscular contraction (distal latency) is measured in milliseconds on an EMG machine.
8. The nerve is similarly stimulated at a location proximal to the area of suspected injury or disease.
9. The time required for the impulse to travel from the site of initiation to muscle contraction (total latency) is recorded in milliseconds.
10. The distance between the site of stimulation and the recording electrode is measured in centimeters.
11. Conduction velocity is converted to meters per second and is computed as in the previous equation.
• Note that this test takes approximately 40 minutes and is performed by a physiatrist, neurologist, or trained technologist.
• This test may be uncomfortable because a mild shock is required for nerve impulse stimulation.
Test Results and Clinical Significance
Related Test
Electromyography (EMG) (p. 554). This test is often performed simultaneously with electroneurography to determine distal latency (muscular contraction) and identify muscular disorder as a contributing cause of weakness.
Pelvic Floor Sphincter Electromyography (Pelvic Floor Sphincter EMG, Rectal EMG Procedure)
Indications
This test is used to document pelvic diaphragm muscle weakness or paralysis. It is performed most often in patients who have urinary or fecal incontinence. The pathologic condition causing the muscle weakness can be muscular or neurologic.
Test Explanation
This urodynamic test uses the placement of electrodes on or in the pelvic floor musculature to evaluate the neuromuscular function of the urinary or anal sphincter. The main benefit of this study is to evaluate the external sphincter (skeletal muscle) activity during voiding. This test is also used to evaluate the bulbocavernosus reflex and voluntary control of external sphincter or pelvic floor muscles. The pelvic floor sphincter EMG also aids in the investigation of “functional” or “psychologic” disturbances of voiding. Fecal incontinence caused by muscular dysfunction can also be identified by rectal sphincter EMG.
Three electrodes are used for this procedure. Recordings may be made from surface electrodes or needle electrodes within the muscle; surface electrodes are most often used. These electrodes allow for observation of and change in the muscle activity before and during voiding.
Patient cooperation is essential. If the patient does not cooperate, the interpretation of the test results will be difficult. This test is performed by a urologist, physiatrist, or neurologist in less than 30 minutes. This study is slightly more uncomfortable than urethral catheterization.
Procedure and Patient Care
During
• Note the following procedural steps:
1. Two electrodes are placed at the 2 o'clock and 10 o'clock positions on the perianal skin to monitor the pelvic floor musculature during voiding.
2. The third electrode is usually placed on the thigh and serves as a ground.
3. Electrical activity is recorded with the bladder empty and the patient relaxed.
4. Reflex activity is evaluated by asking the patient to cough and by stimulating the urethra and trigone by gently tugging on an inserted Foley catheter (bulbocavernosus reflex).
5. Voluntary activity is evaluated by asking the patient to contract and relax the sphincter muscle.
6. The bladder is filled with sterile water at room temperature at a rate of 100 mL/min.
7. The EMG responses to filling and detrusor hyperreflexia (if present) are recorded.
8. Finally, when the bladder is full and with the patient in a voiding position, the filling catheter is removed and the patient is asked to urinate. In the normal patient, the EMG signals build during bladder filling and cease promptly on voluntary micturition, remaining silent until the pelvic floor contracts at the end of voiding.
9. The electrical waves produced are examined for their number, amplitude, and form.
10. The patient may be asked when there is the first urge to void and when there is a strong urge to void. This is recorded.
Test Results and Clinical Significance
Neuromuscular dysfunction of the lower urinary sphincter,
Pelvic floor muscle dysfunction of the anal sphincter:
With overly relaxed pelvic musculature, the frequency and amplitude of the electrical waveform are diminished. This is most commonly seen in older adult women who have had significant muscle stretching during childbirth. It is also seen in patients who have neurologic injury to the nerves innervating the pelvic muscles. The resultant weakness can affect the posterior portion of the pelvic sling and cause anal incontinence. It can affect the anterior portion of the pelvic muscle and cause cystocele, uterine prolapse, and/or urinary incontinence.