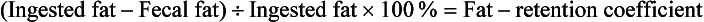Stool Tests
Overview
Reasons for Performing Stool Studies
Stool represents the waste products of digested food. It also includes bile, mucus, shed epithelial cells, bacteria, and other inorganic salts. Normally food is passed through the stomach, into the duodenum, and into the small bowel. There, most of the nutrient and electrolyte absorption occurs. The liquid stool is then passed into the colon, where most of the water is reabsorbed.
Stool studies are used to evaluate the function and integrity of the bowel. These studies are performed to evaluate patients with intestinal bleeding, infections, infestations, inflammation, malabsorption, and diarrhea.
In this chapter, we have listed some stool studies that are commonly performed. Other testing can be done on the stool but is more often performed on other specimens. In those situations, the study is listed in the appropriate chapter for the specimen that is more commonly used.
Although these specimens may be unpleasant to obtain, handle, and examine, the information obtained from stool studies is invaluable to proper care of the patient with gastrointestinal (GI) diseases.
Procedural Care for Stool Studies
Before
![]() Explain the method of stool collection to the patient. Be matter-of-fact to avoid any embarrassment to the patient.
Explain the method of stool collection to the patient. Be matter-of-fact to avoid any embarrassment to the patient.
![]() Instruct the patient not to mix urine or toilet paper with the stool specimen.
Instruct the patient not to mix urine or toilet paper with the stool specimen.
![]() Instruct the patient to use an appropriate collection container.
Instruct the patient to use an appropriate collection container.
• Determine if female patient is menstruating because vaginal blood may contaminate the specimen.
• Observe Standard Precautions when handling stool specimens.
During
• Ask the patient to defecate into a designated container.
• Place a small amount of stool in a sterile collection container.
• If a rectal swab is needed, wear gloves and insert the cotton-tipped swab at least 1 inch into the anal canal. Then rotate the swab for 30 seconds and place it in the clean container.
After
• Handle the stool specimen carefully, as though it were capable of causing infection. Wear gloves when obtaining and handling the specimen.
• Promptly send the stool specimen to the laboratory. Delays in transfer of the specimen may affect test results. If there will be a delay in laboratory handling of the stool specimen, follow laboratory procedures or guidelines concerning storage. Stools for ova and parasites should be kept warm. Stools for enteric pathogens and Clostridium difficile should be refrigerated. Another option is to add a preservative to the stool. For example, for stool culture, a buffered glycerol-saline solution may be combined with the stool as a preservative.
Apt Test (Downey Test, Qualitative Fetal Hemoglobin Stool Test, Stool for Swallowed Blood)
Indications
This is a screening test to indicate if blood present in the stool, emesis, or amniotic fluid of a newborn is fetal blood (possible intestinal bleeding) or swallowed maternal blood.
Test Explanation
Blood in the stool or emesis of a newborn must be rapidly evaluated. Although an adult can lose hundreds of milliliters of blood, that volume may represent the entire blood volume of a newborn child. Newborns may have a serious disease causing the blood in the intestinal tract or may simply be defecating maternal blood that was swallowed during birth or breastfeeding. It is important to rapidly tell the difference. The Apt test is performed on the stool specimen to differentiate the source of the blood. Fetal hemoglobin is resistant to alkali denaturization; adult hemoglobin (hemoglobin A) is not. When sodium hydroxide is added to the blood, maternal blood will dissolve, leaving only a brown hematin stain. Newborn blood (containing hydroxide-resistant hemoglobin) will not dissolve, and red blood will remain in the specimen. This test can be performed on stool, a stool-stained diaper, amniotic fluid, or vomitus.
Test Results
Maternal blood: A newborn usually defecates maternal blood in the first 3 to 5 days of life. If maternal nipple disease exists, the blood in the stool of a newborn can persist.
Fetal blood: This is an indication of disease within the gastrointestinal tract of the newborn and must be evaluated immediately.
Related Test
Stool for Occult Blood (p. 857). This is a method of identifying occult adult blood or substantiating the presence of adult blood in the stool.
Clostridium difficile Testing (C. diff., Clostridial Toxin Assay)
Indications
This test is indicated in patients with diarrhea who have been taking antibiotics for more than 5 days. It can also be performed on immunosuppressed patients with diarrhea even though they are not receiving antibiotics.
Test Explanation
Clostridium difficile–associated diarrhea (CDAD) bacterial infections usually affects the intestine (colitis) and occur in patients who are immunocompromised or taking broad-spectrum antibiotics (e.g., clindamycin, ampicillin, and cephalosporins). The disease severity can range from mild nuisance diarrhea to severe pseudomembranous colitis and bowel perforation. The overwhelming predisposing factor is ongoing antibiotic therapy. Patient age, length of hospital stay, acuity of illness, and comorbidities are risk factors.
The infection possibly results from depression of the normal flora of the bowel caused by the administration of antibiotics. The clostridial bacterium produces two toxins (A and B) that cause inflammation and necrosis of the colonic epithelium. The standard for laboratory detection of Clostridium difficile toxins is the cytotoxicity assay in cell cultures. The specificity of the reaction is determined by the neutralization of the toxins with antisera directed to the toxin in the stool. However, the cytotoxin assay is labor intensive and may take up to 48 hours to obtain a result. Toxin detection by EIA is insensitive. C. difficile can also be diagnosed by obtaining colonic-rectal tissue for this toxin. Stool cultures (p. 855) for C. difficile can be performed but are also labor intensive and take longer to get results.
A PCR assay for the qualitative in vitro rapid detection of C. difficile toxin B gene (tcdB) in human liquid or soft stool specimens is available. This method rapidly provides a definitive diagnosis of C. difficile. Quickly reaching a definitive diagnosis allows CDAD patients to get the proper treatment without delay and reduce hospital stays for inpatients with CDAD. At the same time they can be placed in isolation sooner to reduce transmission and prevent outbreaks. Definitive results can reduce inappropriate antimicrobial use in negative patients.
A positive PCR result for the presence of the gene-regulating toxin production (tcdC) indicates the presence of Clostridium difficile and toxin A and/or B. A negative result indicates the absence of detectable Clostridium difficile tcdC DNA in the specimen, but does not rule out Clostridium difficile infection. False-negative results may occur because of inhibition of PCR, sequence variability underlying the primers and/or probes, or the presence of Clostridium difficile in quantities less than the limit of detection of the assay.
Treatment of CDAD typically involves withdrawal of the associated antimicrobial(s) and, if symptoms persist, orally administered and intraluminally active metronidazole, vancomycin, or fidaxomicin. Intravenous metronidazole may be used if an oral agent cannot be administered. In recent years, a more severe form of CDAD with increased morbidity and mortality has been recognized as being caused by an epidemic toxin-hyperproducing strain of Clostridium difficile (NAP1 strain). Many toxin-hyperproducing isolates also contain the binary toxin gene and are resistant quinolones.
Procedure and Patient Care
Before
![]() Explain the method of stool collection to the patient. Be matter of fact to avoid embarrassment to the patient.
Explain the method of stool collection to the patient. Be matter of fact to avoid embarrassment to the patient.
![]() Instruct the patient not to mix urine or toilet paper with the stool specimen.
Instruct the patient not to mix urine or toilet paper with the stool specimen.
• Handle the specimen carefully, as though it were capable of causing infection. If someone is assisting with the specimen collection, gloves should be worn.
During
![]() Instruct the patient to defecate into a clean container. A rectal swab cannot be used, because it collects inadequate amounts of stool. The stool cannot be retrieved from the toilet.
Instruct the patient to defecate into a clean container. A rectal swab cannot be used, because it collects inadequate amounts of stool. The stool cannot be retrieved from the toilet.
• Stool can be obtained from incontinence pads.
• A stool specimen also can be collected by proctoscopy or colonoscopy.
• Place the specimen in a closed container and then transport it to the laboratory to prevent deterioration of the toxin.
• If the specimen cannot be processed immediately, refrigerate it (depending on laboratory protocol).
• Submission of more than one specimen for testing is not recommended.
Fecal Fat (Fat Absorption, Quantitative Stool Fat Determination)
Indications
This test is performed to confirm the diagnosis of steatorrhea. Steatorrhea is suspected when the patient has large, greasy, and foul-smelling stools. Determining an abnormally high fecal fat content confirms the diagnosis.
Test Explanation
The fecal fat test measures the fat content in the stool. This qualitative or quantitative test is performed to confirm the diagnosis of steatorrhea. Steatorrhea occurs when fat content in the stool is high. Short-gut syndrome and any condition that may cause malabsorption (e.g., sprue, Crohn disease, Whipple disease) or maldigestion (e.g., bile duct obstruction, pancreatic duct obstruction secondary to tumor or gallstones) are also associated with increased fecal fat.
Neutral fats include the monoglycerides, diglycerides, and triglycerides, whereas split fats are the free fatty acids that are liberated from them. Maldigestion (impaired synthesis or secretion of pancreatic enzymes or bile) may cause an increase in neutral fats, whereas an increase in split fats suggests malabsorption.
The total output of fecal fat can be tested on a random stool specimen but is more accurate when total 24-, 48-, or 72-hour collection is carried out. Abnormal results from a random specimen should be confirmed by submission of a timed collection. Test values for random fecal fat collections are reported in terms of percent fat.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient and/or the parent (if a child).
Explain the procedure to the patient and/or the parent (if a child).
![]() Instruct the patient to abstain from alcohol ingestion 3 days before testing.
Instruct the patient to abstain from alcohol ingestion 3 days before testing.
![]() Give the patient instructions regarding the appropriate diet (a diet diary may be requested by the laboratory):
Give the patient instructions regarding the appropriate diet (a diet diary may be requested by the laboratory):
1. For adults, usually 100 g of fat per day is suggested for 3 days before and throughout the collection period.
2. Children, and especially infants, cannot ingest 100 g of fat. Therefore a fat-retention coefficient is determined by measuring the difference between ingested fat and fecal fat and then expressing that difference (the amount of fat retained) as a percentage of the ingested fat:
• Note that the normal fat-retention coefficient is 95% or greater. A low value indicates steatorrhea.
![]() Instruct the patient to defecate into a dry, clean container. Occasionally a tongue blade is required to transfer the stool to the specimen container.
Instruct the patient to defecate into a dry, clean container. Occasionally a tongue blade is required to transfer the stool to the specimen container.
![]() Tell the patient not to urinate into the stool container.
Tell the patient not to urinate into the stool container.
![]() Inform the patient that even diarrheal stools should be collected.
Inform the patient that even diarrheal stools should be collected.
![]() Instruct the patient that toilet paper should not be placed in the stool container.
Instruct the patient that toilet paper should not be placed in the stool container.
![]() Tell the patient not to take any laxatives or enemas during this test because they will interfere with intestinal motility and alter test results.
Tell the patient not to take any laxatives or enemas during this test because they will interfere with intestinal motility and alter test results.
Test Results and Clinical Significance
 Increased Levels
Increased Levels
Cystic fibrosis: These patients experience maldigestion of fat because their pancreatic function is poor. They cannot absorb fat from the gut. As a result, they have steatorrhea.
Malabsorption secondary to sprue, celiac disease, Whipple disease, Crohn disease (regional enteritis), or radiation enteritis: The absorptive capability of the stool is markedly reduced. Transit time is markedly decreased. As a result of these changes, fat is not absorbed. Steatorrhea is the result.
Maldigestion secondary to obstruction of the pancreatobiliary tree (e.g., cancer, stricture, gallstones): Exocrine secretion of the pancreatobiliary tree is necessary for digestion of dietary fat. When disease affects these organs, steatorrhea results.
Short-gut syndrome secondary to surgical resection, surgical bypass, or congenital anomaly: The transit time in these patients is markedly diminished. The time available for digestion and absorption of fat is inadequate. Steatorrhea results.
Related Test
D-Xylose Absorption (p. 533). This test is used to evaluate the absorptive capability of the intestines. It is used in the evaluation of patients with suspected malabsorption.
Lactoferrin
Indications
Lactoferrin is used to diagnose inflammatory bowel diseases such as ulcerative colitis or Crohn disease. It is also used as a screening test to determine the possibility of bacterial colitis.
Test Explanation
Lactoferrin is a glycoprotein expressed by activated neutrophils. The detection of lactoferrin in a fecal sample therefore serves as a surrogate marker for inflammatory white blood cells (WBCs) in the intestinal tract. WBCs in the stool are not stable and may be easily destroyed by temperature changes, delays in testing, and toxins within the stool. As a result, WBCs may not be detected by common microscopic methods. Lactoferrin assay has allowed the identification of inflammatory cells in the stool without the use of microscopy.
Detection of fecal lactoferrin allows for the differentiation of inflammatory and noninflammatory intestinal disorders in patients with diarrhea. Usually the test is used as a diagnostic aid to help identify patients with active inflammatory bowel disease (such as Crohn disease or ulcerative colitis) and rule out those with active irritable bowel syndrome, which is noninflammatory. Lactoferrin is also present in patients with bacterial enteritis such as Shigella, Salmonella, Campylobacter jejuni, and Clostridium difficile. Diarrhea caused by viruses and most parasites is not associated with elevated lactoferrin levels. Lactoferrin testing is often used as a screening test for patients who may have bacterial enteritis. If the stool is negative for lactoferrin, it is unlikely that a stool culture will be positive.
The lactoferrin analyte may be qualitatively detected by two distinct methods: (1) A latex agglutination procedure (the most commonly used) and (2) a microwell enzyme immunoassay procedure. The former method has been used primarily in the evaluation of patients with diagnoses of bacterial infectious gastroenteritis, while the latter method has been developed primarily as a diagnostic aid to distinguish between active inflammatory bowel disease and active noninflammatory irritable bowel syndrome.
Interfering Factors
• Delays in testing can interfere with test results: The stool specimen should be examined immediately. In some instances a specific stool preservative–enteric transport media (Cary-Blair) can be used.
• Breast feeding can affect test results: Because lactoferrin is a component of human breast milk, the test will be positive in breast-fed children and should not be used to evaluate neonates receiving breast milk. However, the test uses a human lactoferrin–specific antibody that does not cross react with lactoferrin in cow’s milk.
Stool Culture (Stool for Culture and Sensitivity [Stool C&S], Stool for Ova and Parasites [O&P])
Indications
Stool cultures are indicated in patients who have unrelenting diarrhea, fever, and abdominal bloating. One is especially suspicious if the patient has been drinking well water, has been receiving a prolonged course of antibiotics, or has traveled outside of the United States.
Test Explanation
Normally stool contains many bacteria and fungi. The more common bacteria include Enterococcus, Escherichia coli, Proteus, Pseudomonas, Staphylococcus aureus, Candida albicans, Bacteroides, and Clostridium. Bacteria are indigenous to the bowel; however, several bacteria act as pathogens within the bowel. These include Salmonella, Shigella, Campylobacter, Yersinia, pathogenic E. coli, Clostridium, and Staphylococcus.
Parasites also may affect the stool. Common parasites are Ascaris (hookworm), Strongyloides (tapeworm), and Giardia (protozoans), and Cryptosporidium (especially in acquired immunodeficiency syndrome [AIDS] patients). Identification of any of these pathogens in the stool incriminates that organism as the cause of the infectious enteritis.
Sometimes the normal stool flora can become pathogenic if overgrowth of the bacteria occurs as a result of antibiotics (e.g., C. difficile), immunosuppression, or overaggressive catharsis. Helicobacter pylori can be found in the stool but indicates an increased risk for peptic ulcer disease and gastritis. Usually, however, this is better cultured from the stomach or determined by a serologic test on the blood.
Infections of the bowel from bacteria, virus, or parasites usually present as acute diarrhea, excessive flatus, abdominal discomfort, and fever. This may progress to toxic megacolon.
Interfering Factors
• Urine may inhibit the growth of bacteria. Therefore urine should not be mixed with the feces during collection of a stool sample.
• Recent barium studies may obscure the detection of parasites.
![]() Drugs that may affect test results include antibiotics, bismuth, and mineral oil.
Drugs that may affect test results include antibiotics, bismuth, and mineral oil.
Procedure and Patient Care
During
![]() Instruct the patient to defecate into a designated clean container.
Instruct the patient to defecate into a designated clean container.
• Place a small amount of stool in a sterile collection container.
• Send mucus and blood streaks with the specimen.
• If a rectal swab is to be used, wear gloves and insert the cotton-tipped swab at least 1 inch into the anal canal. Then rotate the swab for 30 seconds and place it in the clean container.
Tape Test
• Use this test when pinworms (Enterobius) are suspected.
• Place a strip of clear tape in the patient’s perianal region. (This is especially helpful in children.)
• Because the female worm lays her eggs at night around the perianal area, apply the tape before bedtime and remove it in the morning before the patient gets out of bed.
• Press the sticky surface of the tape directly to a glass slide and examine microscopically for pinworm ova.
After
• Handle the stool specimen carefully, as though it were capable of causing infection.
• Promptly send the stool specimen to the laboratory. Delays in transfer of the specimen may affect viability of the organism. If long delays are necessary, obtain a buffered glycerol-saline solution to be combined with the stool and used as a preservative.
• Note that some enteric pathogens may take as long as 6 weeks to isolate.
• When pathogens are detected, maintain isolation of the patient’s stool until therapy is completed. Other people who have had close contact with the patient should be tested and treated to prevent spread of the infection.
Related Test
Clostridial Toxin Assay (p. 849). This test allows one to make the diagnosis of pseudomembranous colitis based on the presence of the bacterium C. difficile.
Stool for Occult Blood (Stool for OB, Fecal Occult Blood Test [FOBT], Fecal Immunochemical Test [FIT], DNA Stool Sample)
Indications
This test is used for colorectal cancer screening of asymptomatic individuals. It can also detect occult blood from other causes (e.g., ulcers, hemorrhoids, diverticulosis).
Test Explanation
Normally only minimal quantities (2 to 2.5 mL) of blood are passed into the gastrointestinal (GI) tract. Usually this bleeding is not significant enough to cause a positive result in the stool for occult blood (OB) testing. This test can detect OB when as little as 5 mL of blood is lost per day.
Tumors of the intestine grow into the lumen and are subjected to repeated trauma by the fecal stream. Eventually the friable neovascular tumor ulcerates and bleeding occurs. Most often, bleeding is so slight that gross blood is not seen in the stool. The blood can be detected by chemical assay or by immunohistochemistry. Guaiac is the most commonly performed chemical assay. The peroxidase-like activity of hemoglobin catalyzes the reaction of peroxide and a chromogen called orthotolidine to form a blue-stained oxidized orthotolidine.
OB can also be detected by immunochemical methods that detect the human globin portion of hemoglobin using monoclonal antibodies. These tests are called fecal immunochemical test (FIT) or immunochemical fecal occult blood test (iFOBT). These methods are as sensitive as guaiac testing but are not affected by red meats or plant oxidizers as described below (see Interfering Factors). Immunochemical methods may fail to recognize occult blood from the upper GI tract because the globin is digested by the time it gets in the stool.
The DNA stool sample test is more sensitive than guaiac testing in the detection of significant colorectal precancerous, benign, and malignant tumors. Because most precancerous polyps do not bleed, they can be missed by FOBT. In contrast, all precancerous polyps shed cells that contain abnormal DNA. So, a stool-based DNA test designed to detect this DNA promises to be more accurate in the detection of precancerous polyps—which, when detected, can be removed before they turn into cancer.
Benign and malignant GI tumors, ulcers, inflammatory bowel disease, arteriovenous malformations, diverticulosis, and hematobilia (hemobilia) can all cause OB within the stool. Other more common abnormalities (e.g., hemorrhoids, swallowed blood from oral or nasopharyngeal bleeding) may also cause OB within the stool.
When OB testing is properly performed, a positive result obtained on multiple specimens collected on successive days warrants a thorough GI evaluation—usually EGD (see p. 608) and colonoscopy (see p. 591). Regular screening, beginning at age 50, can reduce the number of people who die from colorectal cancer by as much as 60%. There are several tests used for colorectal cancer screening (Table 9-1). Yet despite the availability of such screening tools, more than half of American adults have never undergone colorectal cancer screening. This fact highlights the need for more user friendly testing methods such as stool DNA testing. Several scientific organizations, including the U.S. Preventive Services Task Force (USPSTF) and other federal agencies, recommend regular screening for all adults aged 50 or older.
TABLE 9-1
Testing Options for Colorectal Cancer∗
| Test | Frequency |
| Fecal occult blood test (FOBT, or FIT) | Every year |
| Flexible sigmoidoscopy | Every 5 years |
| Double-contrast barium enema | Every 5 years |
| Colonoscopy† | Every 10 years |
| Virtual colonoscopy | Every 10 years |
∗(See Table 4-3, p. 593, colonoscopy, p. 591). People at higher risk of developing colorectal cancer should begin screening at a younger age, and may need to be tested more frequently.
†Colonoscopy can be used as a follow-up diagnostic tool when the results of another screening test are positive.
Reducing or oxidizing agents (such as iron, radish, cantaloupe or cauliflower, and vitamin C) can affect the results of guaiac or fecal immunochemical test (FIT). Furthermore neither FIT nor guaiac testing detects slow upper gastrointestinal (GI) bleeding because globin and heme are degraded during intestinal transit. To evaluate occult GI bleeding in these patients, a fluorometric method that will detect any hemoglobin or heme-derived porphyrins in the stool is very sensitive and provides quantitative results.
Interfering Factors
• Ingestion of red meat within 3 days before testing.
• Bleeding gums following a dental procedure or disease may affect results.
• Ingestion of peroxidase-rich vegetables and fruits (turnips, artichokes, mushrooms, radishes, broccoli, bean sprouts, cauliflower, oranges, bananas, cantaloupes, and grapes) and horseradish may affect results.
![]() Drugs that may cause GI bleeding include anticoagulants, aspirin, colchicine, iron preparations (large doses), nonsteroidal antiarthritics, and steroids. Although these drugs do not interfere with the performance of the test, they can cause GI bleeding not associated with pathology.
Drugs that may cause GI bleeding include anticoagulants, aspirin, colchicine, iron preparations (large doses), nonsteroidal antiarthritics, and steroids. Although these drugs do not interfere with the performance of the test, they can cause GI bleeding not associated with pathology.
![]() Drugs that may instigate the peroxidation reaction and cause false-positive results include boric acid, bromides, colchicine, iodine, iron, and rauwolfia derivatives.
Drugs that may instigate the peroxidation reaction and cause false-positive results include boric acid, bromides, colchicine, iodine, iron, and rauwolfia derivatives.
![]() Vitamin C may cause false-negative results by inhibiting the peroxidation reaction.
Vitamin C may cause false-negative results by inhibiting the peroxidation reaction.
Procedure and Patient Care
Before
![]() Explain the procedure to the patient.
Explain the procedure to the patient.
![]() Instruct the patient to refrain from eating any red meat for at least 3 days before the test.
Instruct the patient to refrain from eating any red meat for at least 3 days before the test.
![]() Instruct the patient to refrain from drugs known to interfere with OB testing.
Instruct the patient to refrain from drugs known to interfere with OB testing.
![]() Instruct the patient in the method of obtaining appropriate stool specimens. Many procedures are available (e.g., specimen cards, tissue wipes, test paper). Tests may be done at home with specimen cards (Hemoccult) and mailed to a local testing laboratory or doctor’s office when collected.
Instruct the patient in the method of obtaining appropriate stool specimens. Many procedures are available (e.g., specimen cards, tissue wipes, test paper). Tests may be done at home with specimen cards (Hemoccult) and mailed to a local testing laboratory or doctor’s office when collected.
![]() Instruct the patient not to mix urine with the stool specimen.
Instruct the patient not to mix urine with the stool specimen.
![]() Inform the patient about the need for multiple specimens obtained on separate days to increase the test’s accuracy.
Inform the patient about the need for multiple specimens obtained on separate days to increase the test’s accuracy.
• Note that in some centers a high-residue diet is recommended to increase the abrasive effect of the stool.
• Be gentle in obtaining stool by digital rectal examination. Traumatic digital examination can cause a false-positive result, especially in patients with prior anorectal disease such as hemorrhoids.
Test Results and Clinical Significance
GI tumor (cancers and polyps): The mucosa overlying neoplasm is friable. Bleeding occurs when stool passes by.
Peptic diseases (esophagitis, gastritis, and ulceration): In peptic disease, the mucosa becomes inflamed, thickened, and friable. Bleeding easily occurs. Ulcers can erode into blood vessels within the wall of the gut.
Varices: Caused by portal hypertension, these large venous complexes are covered by a thin lining of mucosa. With increased intraabdominal pressure, these can rupture and bleed.
Inflammatory bowel disease (ulcerative colitis, Crohn disease): The inflammatory reaction causes a thickened and friable mucosa, which causes bleeding.
Ischemic bowel disease: The mucosa of the bowel is the first layer to be affected by diminished blood supply. This mucosa easily sloughs, and minor bleeding can occur.
GI trauma: Penetrating or blunt trauma can cause bleeding into the gut.
Recent GI surgery: Small amounts of bleeding occur at the new GI anastomosis.
Hemorrhoids and other anorectal problems: An anorectal pathologic condition is the most common nonneoplastic cause of blood in the stool.
Related Tests
Colonoscopy (p. 591). This test allows endoscopic evaluation of the entire colon.
Esophagogastroduodenoscopy (p. 608). This endoscopic procedure visualizes the esophagus, stomach, and duodenum.
Barium Enema (p. 994). This test uses barium to provide x-ray visualization of the colon.
Upper GI Series (p. 1072). This test uses barium to provide x-ray visualization of the upper GI tract.
Small Bowel Series (p. 1064). This test uses barium to provide x-ray visualization of the small intestines.
Septin 9 DNA Methylation Assay (p. 460). This blood test is used to screen asymptomatic patients for colorectal cancer.