Information for Healthcare Professionals
Current Interim Guidance
• Interim Guidance for Public Health Personnel Evaluating Persons Under Investigation (PUIs) and Asymptomatic Close Contacts of Confirmed Cases at Their Home or Non-Home Residential Settings
• Interim Guidance for Collection and Submission of Postmortem Specimens from Deceased Persons Under Investigation (PUI) for COVID-19, February 2020
• Evaluating and Reporting Persons Under Investigation (PUI)
• Healthcare Infection Control Guidance
• Clinical Care Guidance
• Home Care Guidance
• Guidance for EMS
• Healthcare Personnel with Potential Exposure Guidance
• Inpatient Obstetric Healthcare Guidance
Persons Under Investigation (PUI)

• Interim Guidance for Public Health Personnel Evaluating Persons Under Investigation (PUIs) and Asymptomatic Close Contacts of Confirmed Cases at Their Home or Non-Home Residential Settings
• Evaluating and Reporting PUI Guidance
• Reporting a PUI or Laboratory-Confirmed Case for COVID-19

• Clinical Care Guidance
• Disposition of Hospitalized Patients with COVID-2019
• Inpatient Obstetric Healthcare Guidance
Supply of Personal Protective Equipment
• Implementing Home Care of People Not Requiring Hospitalization
• Preventing COVID-19 from Spreading in Homes and Communities Disposition of Non-Hospitalized Patients with COVID-19
Webinar for Healthcare Professionals

Strategies for Healthcare Systems Preparedness and Optimizing N95 Supplies.
Find more information on supplies of personal protective equipment
View presentation slides
Evaluating and Testing Persons for Coronavirus Disease 2019 (COVID-19)
Summary of Recent Changes
Revisions were made on March 9, 2020, to reflect the following:
• Reorganized the Criteria to Guide Evaluation and Laboratory Testing for COVID-19 section
Revisions were made on March 4, 2020, to reflect the following:
• Criteria for evaluation of persons for testing for COVID-19 were expanded to include a wider group of symptomatic patients.
Limited information is available to characterize the spectrum of clinical illness associated with coronavirus disease 2019 (COVID-19). No vaccine or specific treatment for COVID-19 is available; care is supportive.
The CDC clinical criteria for considering testing for COVID-19 have been developed based on what is known about COVID-19 and are subject to change as additional information becomes available.

This is an official CDC Health Update
Update and Interim Guidance on Outbreak of Coronavirus Disease 2019 (COVID-19)
CDC continues to closely monitor an outbreak of respiratory illness caused by COVID-19 that was initially detected in Wuhan City, Hubei Province, China. This HAN Update provides a situational update and guidance to state and local health departments and health care providers.

Contact your local or state health department
Healthcare providers should immediately notify their local or state health department in the event of the identification of a PUI for COVID-19. When working with your local or state health department check their available hours.
Criteria to Guide Evaluation and Laboratory Testing for COVID-19
Clinicians should continue to work with their local and state health departments to coordinate testing through public health laboratories. In addition, COVID-19 diagnostic testing, authorized by the Food and Drug Administration under an Emergency Use Authorization (EUA), is becoming available in clinical laboratories. This additional testing capacity will allow clinicians to consider COVID-19 testing for a wider group of symptomatic patients.
Clinicians should use their judgment to determine if a patient has signs and symptoms compatible with COVID-19 and whether the patient should be tested. Most patients with confirmed COVID-19 have developed fever1 and/or symptoms of acute respiratory illness (e.g., cough, difficulty breathing). Priorities for testing may include:
1. Hospitalized patients who have signs and symptoms compatible with COVID-19 in order to inform decisions related to infection control.
2. Other symptomatic individuals such as, older adults and individuals with chronic medical conditions and/or an immunocompromised state that may put them at higher risk for poor outcomes (e.g., diabetes, heart disease, receiving immunosuppressive medications, chronic lung disease, chronic kidney disease).
3. Any persons including healthcare personnel2, who within 14 days of symptom onset had close contact3 with a suspect or laboratory-confirmed4 COVID-19 patient, or who have a history of travel from affected geographic areas5 (see below) within 14 days of their symptom onset.
There are epidemiologic factors that may also help guide decisions about COVID-19 testing. Documented COVID-19 infections in a jurisdiction and known community transmission may contribute to an epidemiologic risk assessment to inform testing decisions. Clinicians are strongly encouraged to test for other causes of respiratory illness (e.g., influenza).
Mildly ill patients should be encouraged to stay home and contact their healthcare provider by phone for guidance about clinical management. Patients who have severe symptoms, such as difficulty breathing, should seek care immediately. Older patients and individuals who have underlying medical conditions or are immunocompromised should contact their physician early in the course of even mild illness.
Recommendations for Reporting, Testing, and Specimen Collection
Clini1cians should immediately implement recommended infection prevention and control practices if a patient is suspected of having COVID-19. They should also notify infection control personnel at their healthcare facility and their state or local health department if a patient is classified as a PUI for COVID-19. State health departments that have identified a PUI or a laboratory-confirmed case should complete a PUI and Case Report form through the processes identified on CDC’s Coronavirus Disease 2019 website. State and local health departments can contact CDC’s Emergency Operations Center (EOC) at 770-488-7100 for assistance with obtaining, storing, and shipping appropriate specimens to CDC for testing, including after hours or on weekends or holidays.
For initial diagnostic testing for COVID-19, CDC recommends collecting and testing upper respiratory tract specimens (nasopharyngeal AND oropharyngeal swabs). CDC also recommends testing lower respiratory tract specimens, if available. For patients who develop a productive cough, sputum should be collected and tested for COVID-19. The induction of sputum is not recommended. For patients for whom it is clinically indicated (e.g., those receiving invasive mechanical ventilation), a lower respiratory tract aspirate or bronchoalveolar lavage sample should be collected and tested as a lower respiratory tract specimen. Specimens should be collected as soon as possible once a PUI is identified, regardless of the time of symptom onset. See Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Patients Under Investigation (PUIs) for COVID-19 and Biosafety FAQs for handling and processing specimens from suspected cases and PUIs.
Interim Guidance for Collection and Submission of Postmortem Specimens from Deceased Persons Under Investigation (PUI) for COVID-19, February 2020
State and local health departments who have identified a Persons Under Investigation (PUI) should immediately notify CDC’s Emergency Operations Center (EOC) at 770-488-7100 to report a deceased PUI and determine whether testing for SARS-CoV-2, the virus that causes COVID-19, at CDC is indicated. The EOC will assist local/state health departments to collect, store, and ship specimens appropriately to CDC, including during afterhours or on weekends/holidays.
CDC is available for urgent consultation in the event that an autopsy on a COVID-19 PUI is being considered. CDC can be reached for urgent consultation by calling the EOC at 770-488-7100.
This interim guidance is based on what is currently known about COVID-19. The Centers for Disease Control and Prevention (CDC) will update this interim guidance as needed and as additional information becomes available.
The CDC is closely monitoring an outbreak of respiratory illness caused by a novel (new) coronavirus (named SARS-CoV-2); this illness is now called coronavirus disease 2019 or COVID-19. This virus was first identified in Wuhan, Hubei Province, China and it continues to spread. CDC is working across the Department of Health and Human Services and other parts of the U.S. government in the public health response to COVID-19.
Much is unknown about COVID-19. Current knowledge is largely based on what is known about similar coronaviruses. Coronaviruses are a large family of viruses that are common in many different species of animals, including camels, cattle, cats, and bats. Rarely, animal coronaviruses can infect people and then spread between people such as with MERS-CoV, SARS-CoV, and now with SARS-CoV-2, the virus that causes COVID-19. Most often, spread from a living person happens with close contact (i.e., within about 6 feet) via respiratory droplets produced when an infected person coughs or sneezes, similar to how influenza and other respiratory pathogens spread. This route of transmission is not a concern when handling human remains or performing postmortem procedures. Postmortem activities should be conducted with a focus on avoiding aerosol generating procedures, and ensuring that if aerosol generation is likely (e.g., when using an oscillating saw) that appropriate engineering controls and personal protective equipment (PPE) are used. These precautions and the use of Standard Precautions should ensure that appropriate work practices are used to prevent direct contact with infectious material, percutaneous injury, and hazards related to moving heavy remains and handling embalming chemicals.
This document provides specific guidance for the collection and submission of postmortem specimens from deceased persons under investigation (PUI) for COVID-19. This document also provides recommendations for biosafety and infection control practices during specimen collection and handling, including during autopsy procedures. The guidance can be utilized by medical examiners, coroners, pathologists, other workers involved in the postmortem care of deceased PUI, and local and state health departments.
The following factors should be considered when determining if an autopsy will be performed for a deceased PUI: medicolegal jurisdiction, facility environmental controls, availability of recommended personal protective equipment (PPE), and family and cultural wishes.
If an autopsy is performed, collection of the following postmortem specimens is recommended:
• Postmortem clinical specimens for testing for SARS-CoV-2, the virus that causes COVID-19:
○ Upper respiratory tract swabs: Nasopharyngeal Swab AND Oropharyngeal Swab (NP swab and OP swab)
○ Lower respiratory tract swab: Lung swab from each lung
• Separate clinical specimens for testing of other respiratory pathogens and other postmortem testing as indicated
• Formalin-fixed autopsy tissues from lung, upper airway, and other major organs
If an autopsy is NOT performed, collection of the following postmortem specimens is recommended:
• Postmortem clinical specimens for testing for SARS-CoV-2, the virus that causes COVID-19, to include only upper respiratory tract swabs: Nasopharyngeal Swab AND Oropharyngeal Swab (NP swab and OP swab)
• Separate NP swab and OP swab specimens for testing of other respiratory pathogens
Detailed guidance for postmortem specimen collection can be found in the section: Collection of Postmortem Clinical and Pathologic Specimens.
In addition to postmortem specimens, submission of any remaining clinical specimens (e.g., NP swab, OP swab, sputum, serum, stool) that may have been collected prior to death is recommended. Please refer to Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons Under Investigation (PUIs) for Coronavirus Disease 2019 (COVID-19) for more information.
Recommended Biosafety and Infection Control Practices
Collection of Postmortem Upper Respiratory Tract Swab Specimens
Individuals in the room during the procedure should be limited to healthcare personnel (HCP) obtaining the specimen. If HCP are not performing an autopsy or conducting aerosol generating procedures (AGPs), follow Standard Precautions.
Engineering Control Recommendations:
Since collection of nasopharyngeal and oropharyngeal swab specimens from deceased persons will not induce coughing or sneezing, a negative pressure room is not required. Personnel should adhere to Standard Precautions as described above.
PPE Recommendations:
The following PPE should be worn at a minimum:
• Wear nonsterile, nitrile gloves when handling potentially infectious materials.
• If there is a risk of cuts, puncture wounds, or other injuries that break the skin, wear heavy-duty gloves over the nitrile gloves.
• Wear a clean, long-sleeved fluid-resistant or impermeable gown to protect skin and clothing.
• Use a plastic face shield or a face mask and goggles to protect the face, eyes, nose, and mouth from splashes of potentially infectious bodily fluids.
Autopsy Procedures
Standard Precautions, Contact Precautions, and Airborne Precautions with eye protection (e.g., goggles or a face shield) should be followed during autopsy. Many of the following procedures are consistent with existing guidelines for safe work practices in the autopsy setting; see Guidelines for Safe Work Practices in Human and Animal Medical Diagnostic Laboratories.
• AGPs such as use of an oscillating bone saw should be avoided for confirmed or suspected cases of COVID-19. Consider using hand shears as an alternative cutting tool. If an oscillating saw is used, attach a vacuum shroud to contain aerosols.
• Allow only one person to cut at a given time.
• Limit the number of personnel working in the autopsy suite at any given time to the minimum number of people necessary to safely conduct the autopsy.
• Limit the number of personnel working on the human body at any given time.
• Use a biosafety cabinet for the handling and examination of smaller specimens and other containment equipment whenever possible.
• Use caution when handling needles or other sharps, and dispose of contaminated sharps in puncture-proof, labeled, closable sharps containers.
• A logbook including names, dates, and activities of all workers participating in the postmortem and cleaning of the autopsy suite should be kept to assist in future follow up, if necessary. Include custodian staff entering after hours or during the day.
Engineering Control Recommendations
Autopsies on decedents with known or suspected COVID-19 should be conducted in Airborne Infection Isolation Rooms (AIIRs). These rooms are at negative pressure to surrounding areas, have a minimum of 6 air changes per hour (ACH) for existing structures and 12 ACH for renovated or new structures, and have air exhausted directly outside or through a HEPA filter. Doors to the room should be kept closed except during entry and egress. If an AIIR is not available, ensure the room is negative pressure with no air recirculation to adjacent spaces. A portable HEPA recirculation unit could be placed in the room to provide further reduction in aerosols. Local airflow control (i.e., laminar flow systems) can be used to direct aerosols away from personnel. If use of an AIIR or HEPA unit is not possible, the procedure should be performed in the most protective environment possible. Air should never be returned to the building interior, but should be exhausted outdoors, away from areas of human traffic or gathering spaces and away from other air intake systems.
PPE Recommendations:
The following PPE should be worn during autopsy procedures:
• Double surgical gloves interposed with a layer of cut-proof synthetic mesh gloves
• Fluid-resistant or impermeable gown
• Waterproof apron
• Goggles or face shield
• NIOSH-certified disposable N-95 respirator or higher
○ Powered, air-purifying respirators (PAPRs) with HEPA filters may provide increased worker comfort during extended autopsy procedures.
○ When respirators are necessary to protect workers, employers must implement a comprehensive respiratory protection program in accordance with the OSHA Respiratory Protection standard (29 CFR 1910.134) that includes medical exams, fit-testing, and training.
Surgical scrubs, shoe covers, and surgical cap should be used per routine protocols. Doff (take off) PPE carefully to avoid contaminating yourself and before leaving the autopsy suite or adjacent anteroom (https://www.cdc.gov/hai/pdfs/ppe/ppe-sequence.pdf).
After removing PPE, discard the PPE in the appropriate laundry or waste receptacle. Reusable PPE (e.g., goggles, face shields, and PAPRs) must be cleaned and disinfected according to the manufacturer’s recommendations before reuse. Immediately after doffing PPE, wash hands with soap and water for 20 seconds. If hands are not visibly dirty and soap and water are not available, an alcohol-based hand sanitizer that contains 60%-95% alcohol may be used. However, if hands are visibly dirty, always wash hands with soap and water before using alcohol-based hand sanitizer. Avoid touching the face with gloved or unwashed hands. Ensure that hand hygiene facilities are readily available at the point of use (e.g., at or adjacent to the PPE doffing area).
Additional safety and health guidance is available for workers handling deceased persons under investigation (PUI) for COVID-19 at the Occupational Safety and Health Administration (OSHA), COVID-19 website.
Collection of Postmortem Clinical and Pathologic Specimens
Implementing proper biosafety and infection control practices is critical when collecting specimens. Please refer to Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Coronavirus Disease 2019 (COVID-19) for additional information.
Collection of Postmortem Clinical Specimens for SARS-CoV-2 Testing
CDC recommends collecting and testing postmortem upper respiratory specimens (nasopharyngeal and oropharyngeal swabs) and, if an autopsy is performed, lower respiratory specimens (lung swabs).
Use only synthetic fiber swabs with plastic shafts. Do not use calcium alginate swabs or swabs with wooden shafts, as they may contain substances that inactivate some viruses and inhibit PCR testing. Place swabs immediately into sterile tubes containing 2-3 ml of viral transport media. NP, OP, and lung swab specimens should be kept in separate vials. Refrigerate specimen at 2-8°C and ship overnight to CDC on ice pack.
Upper Respiratory Tract Specimen Collection: Nasopharyngeal Swab AND Oropharyngeal Swabs (NP swab, OP swab)
• Nasopharyngeal swab: Insert a swab into the nostril parallel to the palate. Leave the swab in place for a few seconds to absorb secretions. Swab both nasopharyngeal areas with the same swab.
• Oropharyngeal swab (e.g., throat swab): Swab the posterior pharynx, avoiding the tongue.
Lower respiratory tract: Lung swabs
• Collect one swab from each lung.
Collection of Postmortem Clinical Specimens for Other Routine Diagnostic Testing
Separate clinical specimens (e.g., NP swab, OP swab, lung swabs) should be collected for routine testing of respiratory pathogens at either clinical or public health labs. Note that clinical laboratories should NOT attempt viral isolation from specimens collected from COVID-19 PUIs.
Other postmortem specimen collection and evaluations should be directed by the decedent’s clinical and exposure history, scene investigation, and gross autopsy findings, and may include routine bacterial cultures, toxicology, and other studies as indicated.
Collection of Fixed Autopsy Tissue Specimens
The preferred specimens would be a minimum of eight blocks and fixed tissue specimens representing samples from the respiratory sites listed below in addition to specimens from major organs (including liver, spleen, kidney, heart, GI tract) and any other tissues showing significant gross pathology.
The recommended respiratory sites include:
1. Trachea (proximal and distal)
2. Central (hilar) lung with segmental bronchi, right and left primary bronchi
3. Representative pulmonary parenchyma from right and left lung
Viral antigens and nucleic acid may be focal or sparsely distributed in patients with respiratory viral infections and are most frequently detected in respiratory epithelium of large airways. For example, larger airways (particularly primary and segmental bronchi) have the highest yield for detection of respiratory viruses by molecular testing and immunohistochemistry (IHC) staining. Performance of specific immunohistochemical, molecular, or other assays will be determined using clinical and epidemiologic information provided by the submitter and the histopathologic features identified in the submitted tissue specimens.
Collection of tissue samples roughly 4-5 mm in thickness (i.e., sample would fit in a tissue cassette) is recommended for optimal fixation. The volume of formalin used to fix tissues should be 10x the volume of tissue. Place tissue in 10% buffered formalin for three days (72 hours) for optimal fixation.
Safely Preparing the Specimens for Shipment
After collecting and properly securing and labeling specimens in primary containers with the appropriate media/solution, they must be transferred from the autopsy suite in a safe manner to laboratory staff who can process them for shipping.
1. Within the autopsy suite, primary containers should be placed into a larger secondary container.
2. If possible, the secondary container should then be placed into a resealable plastic bag that was not in the autopsy suite when the specimens were collected.
3. The resealable plastic bag should then be placed into a biological specimen bag with absorbent material; and then can be transferred outside of the autopsy suite.
a. Workers receiving the biological specimen bag outside the autopsy suite or anteroom should wear disposable nitrile gloves.
Submission of Specimens to CDC
State and local health departments who have identified a PUI should immediately notify CDC’s Emergency Operations Center (EOC) at 770-488-7100 to report a deceased PUI and determine whether SARS-CoV-2, the virus that causes COVID-19, testing at CDC is indicated. The EOC will assist local/state health departments to collect, store, and ship specimens appropriately to CDC, including during afterhours or on weekends/holidays.
Submission of Postmortem Clinical Specimens for SARS-CoV-2 Testing
This section applies to submission of postmortem NP swab, OP swab, and lung swabs
• Store specimens at 2-8°C and ship overnight to CDC on ice pack.
• Label each specimen container with the patient’s ID number (e.g., medical record number), unique specimen ID (e.g., laboratory requisition number), specimen type (e.g., tissue), and the date the sample was collected.
• Complete a CDC Form 50.34 for each specimen submitted.
• In the upper left box of the form provide the following: (1) for test requested select “Respiratory virus molecular detection (non-influenza) CDC-10401” and (2) for At CDC, bring to the attention of enter “Stephen Lindstrom: 2019-nCoV PUI – Autopsy specimens”.
Clinical specimens from COVD-19 PUIs must be packaged, shipped, and transported according to the current edition of the International Air Transport Association (IATA) Dangerous Goods Regulations. Store specimens at 2-8°C and ship overnight to CDC on ice pack. If a specimen is frozen at -70°C ship overnight to CDC on dry ice. Additional useful and detailed information on packing, shipping, and transporting specimens can be found at Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Coronavirus 2019 (COVID-19).
Submission of Fixed Autopsy Tissue Specimens
CDC’s Infectious Diseases Pathology Branch will perform histopathologic evaluation; testing for SARS-CoV-2, as well as other respiratory viral pathogens (e.g., influenza); and bacterial and other infections, as indicated.
Paraffin-embedded tissue blocks
In general, this is the preferred specimen and is especially important to submit in cases where tissues have been in formalin for a significant time. Prolonged fixation (>2 weeks) may interfere with some immunohistochemical and molecular diagnostic assays.
Wet tissue
If available, we highly recommend that unprocessed tissues in 10% neutral buffered formalin be submitted in addition to paraffin blocks.
Requirements for submitting fixed tissues to CDC
A. Contact CDC’s Infectious Diseases Pathology Branch at pathology@cdc.gov who will provide a pre-populated CDC Form 50.34 for your convenience. Include in the email:
1. A brief clinical history
2. A description of gross or histopathologic findings in the tissues to be submitted
B. After you receive email approval from pathology@cdc.gov:
1. Electronically fill, save, and print both pages of the CDC Form 50.34.
2. In the upper left box of the form, Select Test Order Code CDC-10365 (“Pathologic Evaluation of Tissues for Possible Infectious Etiologies”)
3. Enter “COVID-19 PUI” and provide any applicable CDC and State Case ID numbers in the Comments section on Page 2 of the CDC 50.34 form.
4. In addition to the CDC 50.34 form, enclose the following in the specimen submission package:
1. Surgical pathology, autopsy report (preliminary is acceptable), or both
2. Relevant clinical notes, including admission History and Physical (H&P), discharge summary, if applicable
C. Mailing/Contact Info:
1. Formalin-fixed wet tissues and/or formalin-fixed paraffin-embedded tissue blocks should be shipped in suitable packaging at ambient temperature. Do not freeze fixed tissues.
2. Ship to: Dr. Sherif Zaki, CDC, IDPB, 1600 Clifton Rd NE, MS: H18-SB, Atlanta, GA 30329-4027
3. Send tracking number to pathology@cdc.gov
4. Tel: 404-639-3132, Fax: 404-639-3043, Email: pathology@cdc.gov
Cleaning and Waste Disposal Recommendations
The following are general guidelines for cleaning and waste disposal following an autopsy of a decedent with confirmed or suspected COVID-19. The surface persistence of SARS-CoV-2 is uncertain at this time. Other coronaviruses such as those that cause MERS and SARS can persist on nonporous surfaces for 24 hours or more.
Routine cleaning and disinfection procedures (e.g., using cleaners and water to pre-clean surfaces prior to applying an Environmental Protection Agency (EPA)-registered, hospital-grade disinfectant for appropriate contact times as indicated on the product’s label) are appropriate for COVID-19 in these settings.
After an autopsy of a decedent with confirmed or suspected COVID-19, the following recommendations apply for the autopsy room (and anteroom if applicable):
• Keep ventilation systems active while cleaning is conducted.
• Wear disposable gloves recommended by the manufacturer of the cleaner or disinfectant while cleaning and when handling cleaning or disinfecting solutions.
○ Dispose of gloves if they become damaged or soiled and when cleaning is completed, as described below. Never wash or reuse gloves.
• Use eye protection, such as a faceshield or goggles, if splashing of water, cleaner/disinfectant, or other fluids, is expected.
• Use respiratory protection if required on cleaner or disinfectant label.
• Ensure workers are trained on OSHA’s Hazard Communication standard, 29 CFR 1910.1200, to communicate with workers about the hazardous chemicals used in the workplace.
• Wear a clean, long-sleeved fluid-resistant gown to protect skin and clothing.
• Use disinfectants with EPA-approved products with label claims against human coronaviruses. All products should be used according to label instructions.
○ Clean the surface first, and then apply the disinfectant as instructed on the disinfectant manufacturer’s label. Ensure adequate contact time for effective disinfection.
○ Adhere to any safety precautions or other label recommendations as directed (e.g., allowing adequate ventilation in confined areas and proper disposal of unused product or used containers).
○ Avoid using product application methods that cause splashing or generate aerosols.
○ Cleaning activities should be supervised and inspected periodically to ensure correct procedures are followed.
• Do not use compressed air and/or water under pressure for cleaning, or any other methods that can cause splashing or might re-aerosolize infectious material.
• Gross contamination and liquids should be collected with absorbent materials, such as towels, by staff conducting the autopsy wearing designated PPE. Gross contamination and liquids should then be disposed of as described below:
○ Use of tongs and other utensils can minimize the need for personal contact with soiled absorbent materials.
○ Large areas contaminated with body fluids should be treated with disinfectant following removal of the fluid with absorbent material. The area should then be cleaned and given a final disinfection.
○ Small amounts of liquid waste (e.g., body fluids) can be flushed or washed down ordinary sanitary drains without special procedures.
○ Hard, nonporous surfaces may then be cleaned and disinfected as described above.
• Follow standard operating procedures for the containment and disposal of used PPE and regulated medical waste. SARS-CoV-2 is not considered a Category A infectious substance. State and local governments should be consulted for appropriate disposal decisions.
• Dispose of human tissues according to routine procedures for pathological waste.
• Clean and disinfect or autoclave non-disposable instruments using routine procedures, taking appropriate precautions with sharp objects.
• Materials or clothing that will be laundered can be removed from the autopsy suite (or anteroom, if applicable) in a sturdy, leak-proof biohazard bag that is tied shut and not reopened. These materials should then be sent for laundering according to routine procedures.
• Wash reusable, non-launderable items (e.g., aprons) with detergent solution, decontaminate using disinfectant, rinse with water, and allow items to dry before next use.
• Keep camera, telephones, computer keyboards, and other items that remain in the autopsy suite (or anteroom, if applicable) as clean as possible, but treat as if they are contaminated and handle with gloves. Wipe the items with appropriate disinfectant after use. If being removed from the autopsy suite, ensure complete decontamination with appropriate disinfectant according to the manufacturer’s recommendations prior to removal and reuse.
• When cleaning is complete and PPE has been removed, wash hands immediately with soap and water for 20 seconds. If hands are not visibly dirty and soap and water are not available, an alcohol-based hand sanitizer that contains 60%-95% alcohol may be used. However, if hands are visibly dirty, always wash hands with soap and water before using alcohol-based hand sanitizer. Avoid touching the face with gloved or unwashed hands. Ensure that hand hygiene facilities are readily available at the point of use (e.g., at or adjacent to the PPE doffing area).
Transportation of Human Remains
Follow standard routine procedures when transporting the body after specimens have been collected and the body has been bagged. Disinfect the outside of the bag with an EPA-registered hospital disinfectant applied according to the manufacturer’s recommendations. Wear disposable nitrile gloves when handling the body bag.
Interim Guidance for Emergency Medical Services (EMS) Systems and 911 Public Safety Answering Points (PSAPs) for COVID-19 in the United States
This guidance applies to all first responders, including law enforcement, fire services, emergency medical services, and emergency management officials, who anticipate close contact with persons with confirmed or possible COVID-19 in the course of their work.
Summary of Key Changes for the EMS Guidance:
• Updated PPE recommendations for the care of patients with known or suspected COVID-19:
○ Facemasks are an acceptable alternative until the supply chain is restored. Respirators should be prioritized for procedures that are likely to generate respiratory aerosols, which would pose the highest exposure risk to HCP.
○ Eye protection, gown, and gloves continue to be recommended.
■ If there are shortages of gowns, they should be prioritized for aerosol-generating procedures, care activities where splashes and sprays are anticipated, and high-contact patient care activities that provide opportunities for transfer of pathogens to the hands and clothing of HCP.
○ When the supply chain is restored, fit-tested EMS clinicians should return to use of respirators for patients with known or suspected COVID-19.
• Updated guidance about recommended EPA-registered disinfectants to include reference to a list now posted on the EPA website.
Background
Emergency medical services (EMS) play a vital role in responding to requests for assistance, triaging patients, and providing emergency medical treatment and transport for ill persons. However, unlike patient care in the controlled environment of a healthcare facility, care and transports by EMS present unique challenges because of the nature of the setting, enclosed space during transport, frequent need for rapid medical decision-making, interventions with limited information, and a varying range of patient acuity and jurisdictional healthcare resources.
When preparing for and responding to patients with confirmed or possible coronavirus disease 2019 (COVID-19), close coordination and effective communications are important among 911 Public Safety Answering Points (PSAPs)— commonly known as 911 call centers, the EMS system, healthcare facilities, and the public health system. Each PSAP and EMS system should seek the involvement of an EMS medical director to provide appropriate medical oversight. For the purposes of this guidance, “EMS clinician” means prehospital EMS and medical first responders. When COVID-19 is suspected in a patient needing emergency transport, prehospital care providers and healthcare facilities should be notified in advance that they may be caring for, transporting, or receiving a patient who may have COVID-19 infection.
Updated information about COVID-19 may be accessed at https://www.cdc.gov/coronavirus/2019-ncov/index.html. Infection prevention and control recommendations can be found here: https://www.cdc.gov/coronavirus/2019-nCoV/hcp/infection-control.html. Additional information for healthcare personnel can be found at https://www.cdc.gov/coronavirus/2019-nCoV/guidance-hcp.html.
Case Definition for COVID-19
CDC’s most current case definition for a person under investigation (PUI) for COVID-19 may be accessed at https://www.cdc.gov/coronavirus/2019-nCoV/clinical-criteria.html.
Recommendations for 911 PSAPs
Municipalities and local EMS authorities should coordinate with state and local public health, PSAPs, and other emergency call centers to determine need for modified caller queries about COVID-19, outlined below.
Development of these modified caller queries should be closely coordinated with an EMS medical director and informed by local, state, and federal public health authorities, including the city or county health department(s), state health department(s), and CDC.
Modified Caller Queries
PSAPs or Emergency Medical Dispatch (EMD) centers (as appropriate) should question callers and determine the possibility that this call concerns a person who may have signs or symptoms and risk factors for COVID-19. The query process should never supersede the provision of pre-arrival instructions to the caller when immediate lifesaving interventions (e.g., CPR or the Heimlich maneuver) are indicated. Patients in the United States who meet the appropriate criteria should be evaluated and transported as a PUI. Information on COVID-19 will be updated as the public health response proceeds. PSAPs and medical directors can access CDC’s PUI definitions here.
Information on a possible PUI should be communicated immediately to EMS clinicians before arrival on scene in order to allow use of appropriate personal protective equipment (PPE). PSAPs should utilize medical dispatch procedures that are coordinated with their EMS medical director and with the local or state public health department.
PSAPs and EMS units that respond to ill travelers at US international airports or other ports of entry to the United States (maritime ports or border crossings) should be in contact with the CDC quarantine station of jurisdiction for the port of entry (see: CDC Quarantine Station Contact List) for planning guidance. They should notify the quarantine station when responding to that location if a communicable disease is suspected in a traveler. CDC has provided job aids for this purpose to EMS units operating routinely at US ports of entry. The PSAP or EMS unit can also call CDC’s Emergency Operations Center at (770) 488-7100 to be connected with the appropriate CDC quarantine station.
Recommendations for EMS Clinicians and Medical First Responders
EMS clinician practices should be based on the most up-to-date COVID-19 clinical recommendations and information from appropriate public health authorities and EMS medical direction.
State and local EMS authorities may direct EMS clinicians to modify their practices as described below.
Patient assessment
• If PSAP call takers advise that the patient is suspected of having COVID-19, EMS clinicians should put on appropriate PPE before entering the scene. EMS clinicians should consider the signs, symptoms, and risk factors of COVID-19 (https://www.cdc.gov/coronavirus/2019-nCoV/clinical-criteria.html).
• If information about potential for COVID-19 has not been provided by the PSAP, EMS clinicians should exercise appropriate precautions when responding to any patient with signs or symptoms of a respiratory infection. Initial assessment should begin from a distance of at least 6 feet from the patient, if possible. Patient contact should be minimized to the extent possible until a facemask is on the patient. If COVID-19 is suspected, all PPE as described below should be used. If COVID-19 is not suspected, EMS clinicians should follow standard procedures and use appropriate PPE for evaluating a patient with a potential respiratory infection.
• A facemask should be worn by the patient for source control. If a nasal cannula is in place, a facemask should be worn over the nasal cannula. Alternatively, an oxygen mask can be used if clinically indicated. If the patient requires intubation, see below for additional precautions for aerosol-generating procedures.
• During transport, limit the number of providers in the patient compartment to essential personnel to minimize possible exposures.
Recommended Personal Protective Equipment (PPE)
• EMS clinicians who will directly care for a patient with possible COVID-19 infection or who will be in the compartment with the patient should follow Standard, Precautions and use the PPE as described below. Recommended PPE includes:
○ N-95 or higher-level respirator or facemask (if a respirator is not available),
■ N95 respirators or respirators that offer a higher level of protection should be used instead of a facemask when performing or present for an aerosol-generating procedure
○ Eye protection (i.e., goggles or disposable face shield that fully covers the front and sides of the face). Personal eyeglasses and contact lenses are NOT considered adequate eye protection.
○ A single pair of disposable patient examination gloves. Change gloves if they become torn or heavily contaminated, and isolation gown.,
■ If there are shortages of gowns, they should be prioritized for aerosol-generating procedures, care activities where splashes and sprays are anticipated, and high-contact patient care activities that provide opportunities for transfer of pathogens to the hands and clothing of EMS clinicians (e.g., moving patient onto a stretcher).
• When the supply chain is restored, fit-tested EMS clinicians should return to use of respirators for patients with known or suspected COVID-19.
• Drivers, if they provide direct patient care (e.g., moving patients onto stretchers), should wear all recommended PPE. After completing patient care and before entering an isolated driver’s compartment, the driver should remove and dispose of PPE and perform hand hygiene to avoid soiling the compartment.
○ If the transport vehicle does not have an isolated driver’s compartment, the driver should remove the face shield or goggles, gown and gloves and perform hand hygiene. A respirator or facemask should continue to be used during transport.
• All personnel should avoid touching their face while working.
• On arrival, after the patient is released to the facility, EMS clinicians should remove and discard PPE and perform hand hygiene. Used PPE should be discarded in accordance with routine procedures.
• Other required aspects of Standard Precautions (e.g., injection safety, hand hygiene) are not emphasized in this document but can be found in the guideline titled Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings.
Precautions for Aerosol-Generating Procedures
• If possible, consult with medical control before performing aerosol-generating procedures for specific guidance.
• An N-95 or higher-level respirator, instead of a facemask, should be worn in addition to the other PPE described above, for EMS clinicians present for or performing aerosol-generating procedures.,
• EMS clinicians should exercise caution if an aerosol-generating procedure (e.g., bag valve mask (BVM) ventilation, oropharyngeal suctioning, endotracheal intubation, nebulizer treatment, continuous positive airway pressure (CPAP), bi-phasic positive airway pressure (biPAP), or resuscitation involving emergency intubation or cardiopulmonary resuscitation (CPR)) is necessary.
○ BVMs, and other ventilatory equipment, should be equipped with HEPA filtration to filter expired air.
○ EMS organizations should consult their ventilator equipment manufacturer to confirm appropriate filtration capability and the effect of filtration on positive-pressure ventilation.
• If possible, the rear doors of the transport vehicle should be opened and the HVAC system should be activated during aerosol-generating procedures. This should be done away from pedestrian traffic.
EMS Transport of a PUI or Patient with Confirmed COVID-19 to a Healthcare Facility (including interfacility transport)
If a patient with an exposure history and signs and symptoms suggestive of COVID-19 requires transport to a healthcare facility for further evaluation and management (subject to EMS medical direction), the following actions should occur during transport:
• EMS clinicians should notify the receiving healthcare facility that the patient has an exposure history and signs and symptoms suggestive of COVID-19 so that appropriate infection control precautions may be taken prior to patient arrival.
• Keep the patient separated from other people as much as possible.
• Family members and other contacts of patients with possible COVID-19 should not ride in the transport vehicle, if possible. If riding in the transport vehicle, they should wear a facemask.
• Isolate the ambulance driver from the patient compartment and keep pass-through doors and windows tightly shut.
• When possible, use vehicles that have isolated driver and patient compartments that can provide separate ventilation to each area.
○ Close the door/window between these compartments before bringing the patient on board.
○ During transport, vehicle ventilation in both compartments should be on non-recirculated mode to maximize air changes that reduce potentially infectious particles in the vehicle.
○ If the vehicle has a rear exhaust fan, use it to draw air away from the cab, toward the patient-care area, and out the back end of the vehicle.
○ Some vehicles are equipped with a supplemental recirculating ventilation unit that passes air through HEPA filters before returning it to the vehicle. Such a unit can be used to increase the number of air changes per hour (ACH) (https://www.cdc.gov/niosh/hhe/reports/pdfs/1995-0031-2601.pdf).
• If a vehicle without an isolated driver compartment and ventilation must be used, open the outside air vents in the driver area and turn on the rear exhaust ventilation fans to the highest setting. This will create a negative pressure gradient in the patient area.
• Follow routine procedures for a transfer of the patient to the receiving healthcare facility (e.g., wheel the patient directly into an examination room).
Documentation of patient care
• Documentation of patient care should be done after EMS clinicians have completed transport, removed their PPE, and performed hand hygiene.
○ Any written documentation should match the verbal communication given to the emergency department providers at the time patient care was transferred.
• EMS documentation should include a listing of EMS clinicians and public safety providers involved in the response and level of contact with the patient (for example, no contact with patient, provided direct patient care). This documentation may need to be shared with local public health authorities.
Cleaning EMS Transport Vehicles after Transporting a PUI or Patient with Confirmed COVID-19
The following are general guidelines for cleaning or maintaining EMS transport vehicles and equipment after transporting a PUI:
• After transporting the patient, leave the rear doors of the transport vehicle open to allow for sufficient air changes to remove potentially infectious particles.
○ The time to complete transfer of the patient to the receiving facility and complete all documentation should provide sufficient air changes.
• When cleaning the vehicle, EMS clinicians should wear a disposable gown and gloves. A face shield or facemask and goggles should also be worn if splashes or sprays during cleaning are anticipated.
• Ensure that environmental cleaning and disinfection procedures are followed consistently and correctly, to include the provision of adequate ventilation when chemicals are in use. Doors should remain open when cleaning the vehicle.
• Routine cleaning and disinfection procedures (e.g., using cleaners and water to pre-clean surfaces prior to applying an EPA-registered, hospital-grade disinfectant to frequently touched surfaces or objects for appropriate contact times as indicated on the product’s label) are appropriate for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in healthcare settings, including those patient-care areas in which aerosol-generating procedures are performed.
• Products with EPA-approved emerging viral pathogens claims are recommended for use against SARS-CoV-2. Refer to List N on the EPA website for EPA-registered disinfectants that have qualified under EPA’s emerging viral pathogens program for use against SARS-CoV-2.
• Clean and disinfect the vehicle in accordance with standard operating procedures. All surfaces that may have come in contact with the patient or materials contaminated during patient care (e.g., stretcher, rails, control panels, floors, walls, work surfaces) should be thoroughly cleaned and disinfected using an EPA-registered hospital grade disinfectant in accordance with the product label.
• Clean and disinfect reusable patient-care equipment before use on another patient, according to manufacturer’s instructions.
• Follow standard operating procedures for the containment and disposal of used PPE and regulated medical waste.
• Follow standard operating procedures for containing and laundering used linen. Avoid shaking the linen.
Follow-up and/or Reporting Measures by EMS Clinicians After Caring for a PUI or Patient with Confirmed COVID-19
EMS clinicians should be aware of the follow-up and/or reporting measures they should take after caring for a PUI or patient with confirmed COVID-19:
• State or local public health authorities should be notified about the patient so appropriate follow-up monitoring can occur.
• EMS agencies should develop policies for assessing exposure risk and management of EMS personnel potentially exposed to SARS-CoV-2 in coordination with state or local public health authorities. Decisions for monitoring, excluding from work, or other public health actions for HCP with potential exposure to SARS-CoV-2 should be made in consultation with state or local public health authorities. Refer to the Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease 2019 (COVID-19) for additional information.
• EMS agencies should develop sick-leave policies for EMS personnel that are nonpunitive, flexible, and consistent with public health guidance. Ensure all EMS personnel, including staff who are not directly employed by the healthcare facility but provide essential daily services, are aware of the sick-leave policies.
• EMS personnel who have been exposed to a patient with suspected or confirmed COVID-19 should notify their chain of command to ensure appropriate follow-up.
○ Any unprotected exposure (e.g., not wearing recommended PPE) should be reported to occupational health services, a supervisor, or a designated infection control officer for evaluation.
○ EMS clinicians should be alert for fever or respiratory symptoms (e.g., cough, shortness of breath, sore throat). If symptoms develop, they should self-isolate and notify occupational health services and/or their public health authority to arrange for appropriate evaluation.
EMS Employer Responsibilities
The responsibilities described in this section are not specific for the care and transport of PUIs or patients with confirmed COVID-19. However, this interim guidance presents an opportunity to assess current practices and verify that training and procedures are up-to-date.
• EMS units should have infection control policies and procedures in place, including describing a recommended sequence for safely donning and doffing PPE.
• Provide all EMS clinicians with job- or task-specific education and training on preventing transmission of infectious agents, including refresher training.
• Ensure that EMS clinicians are educated, trained, and have practiced the appropriate use of PPE prior to caring for a patient, including attention to correct use of PPE and prevention of contamination of clothing, skin, and environment during the process of removing such equipment.
• Ensure EMS clinicians are medically cleared, trained, and fit tested for respiratory protection device use (e.g., N95 filtering facepiece respirators), or medically cleared and trained in the use of an alternative respiratory protection device (e.g., Powered Air-Purifying Respirator, PAPR) whenever respirators are required. OSHA has a number of respiratory training videos.
• EMS units should have an adequate supply of PPE.
• Ensure an adequate supply of or access to EPA-registered hospital grade disinfectants (see above for more information) for adequate decontamination of EMS transport vehicles and their contents.
• Ensure that EMS clinicians and biohazard cleaners contracted by the EMS employer tasked to the decontamination process are educated, trained, and have practiced the process according to the manufacturer’s recommendations or the EMS agency’s standard operating procedures.
Infection Control
• Interim Infection Prevention and Control Recommendations for Patients with Confirmed Coronavirus Disease 2019 (COVID-19) or Persons Under Investigation for COVID-19 in Healthcare Settings
• Interim Guidance for Collection and Submission of Postmortem Specimens from Deceased Persons Under Investigation (PUI) for COVID-19 (Recommended Biosafety and Infection Control Practices)
Frequently Asked Questions: Healthcare Infection Prevention and Control
Frequently Asked Questions about Personal Protective Equipment
Personal Protective Equipment (PPE) Supply Resources
• Strategies for Optimizing Supply of N95 Respirators
• Healthcare Supply of Personal Protective Equipment
• Checklist for Healthcare Facilities: Strategies for Optimizing the Supply of N95 Respirators
• Release of Stockpiled N95 Filtering Facepiece Respirators Beyond the Manufacturer-Designated Shelf Life
Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings
Summary of Changes to the Guidance:
• Updated PPE recommendations for the care of patients with known or suspected COVID-19:
○ Based on local and regional situational analysis of PPE supplies, facemasks are an acceptable alternative when the supply chain of respirators cannot meet the demand. During this time, available respirators should be prioritized for procedures that are likely to generate respiratory aerosols, which would pose the highest exposure risk to HCP.
■ Facemasks protect the wearer from splashes and sprays.
■ Respirators, which filter inspired air, offer respiratory protection.
○ When the supply chain is restored, facilities with a respiratory protection program should return to use of respirators for patients with known or suspected COVID-19. Facilities that do not currently have a respiratory protection program, but care for patients infected with pathogens for which a respirator is recommended, should implement a respiratory protection program.
○ Eye protection, gown, and gloves continue to be recommended.
■ If there are shortages of gowns, they should be prioritized for aerosol-generating procedures, care activities where splashes and sprays are anticipated, and high-contact patient care activities that provide opportunities for transfer of pathogens to the hands and clothing of HCP.
• Included are considerations for designating entire units within the facility, with dedicated HCP, to care for known or suspected COVID-19 patients and options for extended use of respirators, facemasks, and eye protection on such units. Updated recommendations regarding need for an airborne infection isolation room (AIIR).
○ Patients with known or suspected COVID-19 should be cared for in a single-person room with the door closed. Airborne Infection Isolation Rooms (AIIRs) (See definition of AIIR in appendix) should be reserved for patients undergoing aerosol-generating procedures (See Aerosol-Generating Procedures Section)
• Updated information in the background is based on currently available information about COVID-19 and the current situation in the United States, which includes reports of cases of community transmission, infections identified in healthcare personnel (HCP), and shortages of facemasks, N95 filtering facepiece respirators (FFRs) (commonly known as N95 respirators), and gowns.
○ Increased emphasis on early identification and implementation of source control (i.e., putting a face mask on patients presenting with symptoms of respiratory infection).
Healthcare Personnel (HCP)
For the purposes of this document, HCP refers to all paid and unpaid persons serving in healthcare settings who have the potential for direct or indirect exposure to patients or infectious materials, including:
• body substances
• contaminated medical supplies, devices, and equipment
• contaminated environmental surfaces
• contaminated air
Frequently Asked Questions: Healthcare Infection Prevention and Control
Background
This interim guidance has been updated based on currently available information about COVID-19 and the current situation in the United States, which includes reports of cases of community transmission, infections identified in healthcare personnel (HCP), and shortages of facemasks, N95 filtering facepiece respirators (FFRs) (commonly known as N95 respirators), and gowns. Here is what is currently known:
This guidance is applicable to all U.S. healthcare settings. This guidance is not intended for non-healthcare settings (e.g., schools) OR for persons outside of healthcare settings. For recommendations regarding clinical management, air or ground medical transport, or laboratory settings, refer to the main CDC COVID-19 website.
Mode of transmission: Early reports suggest person-to-person transmission most commonly happens during close exposure to a person infected with COVID-19, primarily via respiratory droplets produced when the infected person coughs or sneezes. Droplets can land in the mouths, noses, or eyes of people who are nearby or possibly be inhaled into the lungs of those within close proximity. The contribution of small respirable particles, sometimes called aerosols or droplet nuclei, to close proximity transmission is currently uncertain. However, airborne transmission from person-to-person over long distances is unlikely.
Shortage of personal protective equipment: Controlling exposures to occupational infections is a fundamental method of protecting HCP. Traditionally, a hierarchy of controls has been used as a means of determining how to implement feasible and effective control solutions. The hierarchy ranks controls according to their reliability and effectiveness and includes such controls as engineering controls, administrative controls, and ends with personal protective equipment (PPE). PPE is the least effective control because it involves a high level of worker involvement and is highly dependent on proper fit and correct, consistent use.
Major distributors in the United States have reported shortages of PPE, specifically N95 respirators, facemasks, and gowns. Healthcare facilities are responsible for protecting their HCP from exposure to pathogens, including by providing appropriate PPE.
In times of shortages, alternatives to N95s should be considered, including other classes of FFRs, elastomeric half-mask and full facepiece air purifying respirators, and powered air purifying respirators (PAPRs) where feasible. Special care should be taken to ensure that respirators are reserved for situations where respiratory protection is most important, such as performance of aerosol-generating procedures on suspected or confirmed COVID-19 patients or provision of care to patients with other infections for which respiratory protection is strongly indicated (e.g., tuberculosis, measles, varicella).
The anticipated timeline for return to routine levels of PPE is not yet known. Information about strategies to optimize the current supply of N95 respirators, including the use of devices that provide higher levels of respiratory protection (e.g., powered air purifying respirators [PAPRs]) when N95s are in limited supply and a companion checklist to help healthcare facilities prioritize the implementation of the strategies, is available.
Capacity across the healthcare continuum: Use of N95 or higher-level respirators are recommended for HCP who have been medically cleared, trained, and fit-tested, in the context of a facility’s respiratory protection program. The majority of nursing homes and outpatient clinics, including hemodialysis facilities, do not have respiratory protection programs nor have they fit-tested HCP, hampering implementation of recommendations in the previous version of this guidance. This can lead to unnecessary transfer of patients with known or suspected COVID-19 to another facility (e.g., acute care hospital) for evaluation and care. In areas with community transmission, acute care facilities will be quickly overwhelmed by transfers of patients who have only mild illness and do not require hospitalization.
Many of the recommendations described in this guidance (e.g., triage procedures, source control) should already be part of an infection control program designed to prevent transmission of seasonal respiratory infections. As it will be challenging to distinguish COVID-19 from other respiratory infections, interventions will need to be applied broadly and not limited to patients with confirmed COVID-19.
This guidance is applicable to all U.S. healthcare settings. This guidance is not intended for non-healthcare settings (e.g., schools) OR for persons outside of healthcare settings. For recommendations regarding clinical management, air or ground medical transport, or laboratory settings, refer to the main CDC COVID-19 website.
Definition of Healthcare Personnel (HCP) –For the purposes of this document, HCP refers to all paid and unpaid persons serving in healthcare settings who have the potential for direct or indirect exposure to patients or infectious materials, including body substances; contaminated medical supplies, devices, and equipment; contaminated environmental surfaces; or contaminated air.
Recommendations
1. Minimize Chance for Exposures
Ensure facility policies and practices are in place to minimize exposures to respiratory pathogens including SARS-CoV-2, the virus that causes COVID-19. Measures should be implemented before patient arrival, upon arrival, throughout the duration of the patient’s visit, and until the patient’s room is cleaned and disinfected. It is particularly important to protect individuals at increased risk for adverse outcomes from COVID-19 (e.g. older individuals with comorbid conditions), including HCP who are in a recognized risk category.
• Before Arrival
○ When scheduling appointments for routine medical care (e.g., annual physical, elective surgery), instruct patients to call ahead and discuss the need to reschedule their appointment if they develop symptoms of a respiratory infection (e.g., cough, sore throat, fever1) on the day they are scheduled to be seen.
○ When scheduling appointments for patients requesting evaluation for a respiratory infection, use nurse-directed triage protocols to determine if an appointment is necessary or if the patient can be managed from home.
■ If the patient must come in for an appointment, instruct them to call beforehand to inform triage personnel that they have symptoms of a respiratory infection (e.g., cough, sore throat, fever1) and to take appropriate preventive actions (e.g., follow triage procedures, wear a facemask upon entry and throughout their visit or, if a facemask cannot be tolerated, use a tissue to contain respiratory secretions).
○ If a patient is arriving via transport by emergency medical services (EMS), EMS personnel should contact the receiving emergency department (ED) or healthcare facility and follow previously agreed upon local or regional transport protocols. This will allow the healthcare facility to prepare for receipt of the patient.
• Upon Arrival and During the Visit
○ Consider limiting points of entry to the facility.
○ Take steps to ensure all persons with symptoms of COVID-19 or other respiratory infection (e.g., fever, cough) adhere to respiratory hygiene and cough etiquette (see appendix), hand hygiene, and triage procedures throughout the duration of the visit.
■ Post visual alerts (e.g., signs, posters) at the entrance and in strategic places (e.g., waiting areas, elevators, cafeterias) to provide patients and HCP with instructions (in appropriate languages) about hand hygiene, respiratory hygiene, and cough etiquette. Instructions should include how to use tissues to cover nose and mouth when coughing or sneezing, to dispose of tissues and contaminated items in waste receptacles, and how and when to perform hand hygiene.
■ Provide supplies for respiratory hygiene and cough etiquette, including alcohol-based hand rub (ABHR) with 60-95% alcohol, tissues, and no-touch receptacles for disposal, at healthcare facility entrances, waiting rooms, and patient check-ins.
■ Install physical barriers (e.g., glass or plastic windows) at reception areas to limit close contact between triage personnel and potentially infectious patients.
■ Consider establishing triage stations outside the facility to screen patients before they enter.
○ Ensure rapid safe triage and isolation of patients with symptoms of suspected COVID-19 or other respiratory infection (e.g., fever, cough).
■ Prioritize triage of patients with respiratory symptoms.
■ Triage personnel should have a supply of facemasks and tissues for patients with symptoms of respiratory infection. These should be provided to patients with symptoms of respiratory infection at check-in. Source control (putting a facemask over the mouth and nose of a symptomatic patient) can help to prevent transmission to others.
■ Ensure that, at the time of patient check-in, all patients are asked about the presence of symptoms of a respiratory infection and history of travel to areas experiencing transmission of COVID-19 or contact with possible COVID-19 patients.
■ Isolate the patient in an examination room with the door closed. If an examination room is not readily available ensure the patient is not allowed to wait among other patients seeking care.
■ Identify a separate, well-ventilated space that allows waiting patients to be separated by 6 or more feet, with easy access to respiratory hygiene supplies.
■ In some settings, patients might opt to wait in a personal vehicle or outside the healthcare facility where they can be contacted by mobile phone when it is their turn to be evaluated.
○ Incorporate questions about new onset of respiratory symptoms into daily assessments of all admitted patients. Monitor for and evaluate all new fevers and respiratory illnesses among patients. Place any patient with unexplained fever or respiratory symptoms on appropriate Transmission-Based Precautions and evaluate.
Additional considerations during periods of community transmission:
○ Explore alternatives to face-to-face triage and visits.
○ Learn more about how healthcare facilities can Prepare for Community Transmission
○ Designate an area at the facility (e.g., an ancillary building or temporary structure) or identify a location in the area to be a “respiratory virus evaluation center” where patients with fever or respiratory symptoms can seek evaluation and care.
○ Cancel group healthcare activities (e.g., group therapy, recreational activities).
○ Postpone elective procedures, surgeries, and non-urgent outpatient visits.
2. Adhere to Standard and Transmission-Based Precautions
Standard Precautions assume that every person is potentially infected or colonized with a pathogen that could be transmitted in the healthcare setting. Elements of Standard Precautions that apply to patients with respiratory infections, including COVID-19, are summarized below. Attention should be paid to training and proper donning (putting on), doffing (taking off), and disposal of any PPE. This document does not emphasize all aspects of Standard Precautions (e.g., injection safety) that are required for all patient care; the full description is provided in the Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings.
HCP (see Section 5 for measures for non-HCP visitors) who enter the room of a patient with known or suspected COVID-19 should adhere to Standard Precautions and use a respirator or facemask, gown, gloves, and eye protection. When available, respirators (instead of facemasks) are preferred; they should be prioritized for situations where respiratory protection is most important and the care of patients with pathogens requiring Airborne Precautions (e.g., tuberculosis, measles, varicella). Information about the recommended duration of Transmission-Based Precautions is available in the Interim Guidance for Discontinuation of Transmission-Based Precautions and Disposition of Hospitalized Patients with COVID-19
• Hand Hygiene
○ HCP should perform hand hygiene before and after all patient contact, contact with potentially infectious material, and before putting on and after removing PPE, including gloves. Hand hygiene after removing PPE is particularly important to remove any pathogens that might have been transferred to bare hands during the removal process.
○ HCP should perform hand hygiene by using ABHR with 60-95% alcohol or washing hands with soap and water for at least 20 seconds. If hands are visibly soiled, use soap and water before returning to ABHR.
○ Healthcare facilities should ensure that hand hygiene supplies are readily available to all personnel in every care location.
• Personal Protective Equipment
• Employers should select appropriate PPE and provide it to HCP in accordance with OSHA PPE standards (29 CFR 1910 Subpart I). HCP must receive training on and demonstrate an understanding of:
○ when to use PPE
○ what PPE is necessary
○ how to properly don, use, and doff PPE in a manner to prevent self-contamination
○ how to properly dispose of or disinfect and maintain PPE
○ the limitations of PPE.
Any reusable PPE must be properly cleaned, decontaminated, and maintained after and between uses. Facilities should have policies and procedures describing a recommended sequence for safely donning and doffing PPE. The PPE recommended when caring for a patient with known or suspected COVID-19 includes:
○ Respirator or Facemask
■ Put on a respirator or facemask (if a respirator is not available) before entry into the patient room or care area.
■ N95 respirators or respirators that offer a higher level of protection should be used instead of a facemask when performing or present for an aerosol-generating procedure (See Section 4). See appendix for respirator definition. Disposable respirators and facemasks should be removed and discarded after exiting the patient’s room or care area and closing the door. Perform hand hygiene after discarding the respirator or facemask. For guidance on extended use of respirators, refer to Strategies to Optimize the Current Supply of N95 Respirators
■ If reusable respirators (e.g., powered air purifying respirators [PAPRs]) are used, they must be cleaned and disinfected according to manufacturer’s reprocessing instructions prior to re-use.
■ When the supply chain is restored, facilities with a respiratory protection program should return to use of respirators for patients with known or suspected COVID-19. Those that do not currently have a respiratory protection program, but care for patients with pathogens for which a respirator is recommended, should implement a respiratory protection program.
• Eye Protection
○ Put on eye protection (i.e., goggles or a disposable face shield that covers the front and sides of the face) upon entry to the patient room or care area. Personal eyeglasses and contact lenses are NOT considered adequate eye protection.
○ Remove eye protection before leaving the patient room or care area.
○ Reusable eye protection (e.g., goggles) must be cleaned and disinfected according to manufacturer’s reprocessing instructions prior to re-use. Disposable eye protection should be discarded after use.
• Gloves
○ Put on clean, non-sterile gloves upon entry into the patient room or care area.
■ Change gloves if they become torn or heavily contaminated.
○ Remove and discard gloves when leaving the patient room or care area, and immediately perform hand hygiene.
• Gowns
○ Put on a clean isolation gown upon entry into the patient room or area. Change the gown if it becomes soiled. Remove and discard the gown in a dedicated container for waste or linen before leaving the patient room or care area. Disposable gowns should be discarded after use. Cloth gowns should be laundered after each use.
○ If there are shortages of gowns, they should be prioritized for:
■ aerosol-generating procedures
■ care activities where splashes and sprays are anticipated
■ high-contact patient care activities that provide opportunities for transfer of pathogens to the hands and clothing of HCP. Examples include:
■ dressing
■ bathing/showering
■ transferring
■ providing hygiene
■ changing linens
■ changing briefs or assisting with toileting
■ device care or use
■ wound care
3. Patient Placement
• For patients with COVID-19 or other respiratory infections, evaluate need for hospitalization. If hospitalization is not medically necessary, home care is preferable if the individual’s situation allows.
• If admitted, place a patient with known or suspected COVID-19 in a single-person room with the door closed. The patient should have a dedicated bathroom.
○ Airborne Infection Isolation Rooms (AIIRs) (See definition of AIIR in appendix) should be reserved for patients who will be undergoing aerosol-generating procedures (See Aerosol-Generating Procedures Section)
• As a measure to limit HCP exposure and conserve PPE, facilities could consider designating entire units within the facility, with dedicated HCP, to care for known or suspected COVID-19 patients. Dedicated means that HCP are assigned to care only for these patients during their shift.
○ Determine how staffing needs will be met as the number of patients with known or suspected COVID-19 increases and HCP become ill and are excluded from work.
○ It might not be possible to distinguish patients who have COVID-19 from patients with other respiratory viruses. As such, patients with different respiratory pathogens will likely be housed on the same unit. However, only patients with the same respiratory pathogen may be housed in the same room. For example, a patient with COVID-19 should not be housed in the same room as a patient with an undiagnosed respiratory infection.
○ During times of limited access to respirators or facemasks, facilities could consider having HCP remove only gloves and gowns (if used) and perform hand hygiene between patients with the same diagnosis (e.g., confirmed COVID-19) while continuing to wear the same eye protection and respirator or facemask (i.e., extended use). Risk of transmission from eye protection and facemasks during extended use is expected to be very low.
■ HCP must take care not to touch their eye protection and respirator or facemask.
■ Eye protection and the respirator or facemask should be removed, and hand hygiene performed if they become damaged or soiled and when leaving the unit.
○ HCP should strictly follow basic infection control practices between patients (e.g., hand hygiene, cleaning and disinfecting shared equipment).
• Limit transport and movement of the patient outside of the room to medically essential purposes.
○ Consider providing portable x-ray equipment in patient cohort areas to reduce the need for patient transport.
• To the extent possible, patients with known or suspected COVID-19 should be housed in the same room for the duration of their stay in the facility (e.g., minimize room transfers).
• Patients should wear a facemask to contain secretions during transport. If patients cannot tolerate a facemask or one is not available, they should use tissues to cover their mouth and nose.
• Personnel entering the room should use PPE as described above.
• To the extent possible, patients with known or suspected COVID-19 should be housed in the same room for the duration of their stay in the facility (e.g., minimize room transfers).
• To the extent possible, patients with known or suspected COVID-19 should be housed in the same room for the duration of their stay in the facility (e.g., minimize room transfers).
• Whenever possible, perform procedures/tests in the patient’s room.
• Once the patient has been discharged or transferred, HCP, including environmental services personnel, should refrain from entering the vacated room until sufficient time has elapsed for enough air changes to remove potentially infectious particles (more information on clearance rates under differing ventilation conditions is available). After this time has elapsed, the room should undergo appropriate cleaning and surface disinfection before it is returned to routine use (See Section 10).
4. Take Precautions When Performing Aerosol-Generating Procedures (AGPs)
• Some procedures performed on patient with known or suspected COVID-19 could generate infectious aerosols. In particular, procedures that are likely to induce coughing (e.g., sputum induction, open suctioning of airways) should be performed cautiously and avoided if possible.
• If performed, the following should occur:
○ HCP in the room should wear an N95 or higher-level respirator, eye protection, gloves, and a gown.
○ The number of HCP present during the procedure should be limited to only those essential for patient care and procedure support. Visitors should not be present for the procedure.
○ AGPs should ideally take place in an AIIR.
○ Clean and disinfect procedure room surfaces promptly as described in the section on environmental infection control below.
5. Collection of Diagnostic Respiratory Specimens
• When collecting diagnostic respiratory specimens (e.g., nasopharyngeal swab) from a possible COVID-19 patient, the following should occur:
○ HCP in the room should wear an N-95 or higher-level respirator (or facemask if a respirator is not available), eye protection, gloves, and a gown.
○ The number of HCP present during the procedure should be limited to only those essential for patient care and procedure support. Visitors should not be present for specimen collection.
○ Specimen collection should be performed in a normal examination room with the door closed.
○ Clean and disinfect procedure room surfaces promptly as described in the section on environmental infection control below.
6. Manage Visitor Access and Movement Within the Facility
• Establish procedures for monitoring, managing and training all visitors, which should include:
○ All visitors should perform frequent hand hygiene and follow respiratory hygiene and cough etiquette precautions while in the facility, especially common areas.
○ Passively screen visitors for symptoms of acute respiratory illness before entering the healthcare facility
■ Post visual alerts (e.g., signs, posters) at the entrance and in strategic places (e.g., waiting areas, elevators, cafeterias) advising visitors not to enter the facility when ill.
○ Informing visitors about appropriate PPE use according to current facility visitor policy
○ Visitors to the most vulnerable patients (e.g., oncology and transplant wards) should be limited; visitors should be screened for symptoms prior to entry to the unit.
• Limit visitors to patients with known or suspected COVID-19. Encourage use of alternative mechanisms for patient and visitor interactions such as video-call applications on cell phones or tablets. If visitation must occur, visits should be scheduled and controlled to allow for the following:
○ Facilities should evaluate risk to the health of the visitor (e.g., visitor might have underlying illness putting them at higher risk for COVID-19) and ability to comply with precautions.
○ Facilities should provide instruction, before visitors enter patients’ rooms, on hand hygiene, limiting surfaces touched, and use of PPE according to current facility policy while in the patient’s room.
○ Visitors should not be present during AGPs or other specimen collection procedures.
○ Visitors should be instructed to only visit the patient room. They should not go to other locations in the facility.
Additional considerations during periods of community transmission:
• All visitors should be actively assessed for fever and respiratory symptoms upon entry to the facility. If fever or respiratory symptoms are present, visitor should not be allowed entry into the facility.
• Determine the threshold at which screening of persons entering the facility will be initiated and at what point screening will escalate from passive (e.g., signs at the entrance) to active (e.g., direct questioning) to restricting all visitors to the facility.
• If restriction of all visitors is implemented, facilities can consider exceptions based on end-of-life situations or when a visitor is essential for the patient’s emotional well-being and care.
• Limit points of entry to the facility.
7. Implement Engineering Controls
• Design and install engineering controls to reduce or eliminate exposures by shielding HCP and other patients from infected individuals. Examples of engineering controls include:
○ physical barriers or partitions to guide patients through triage areas
○ curtains between patients in shared areas
○ air-handling systems (with appropriate directionality, filtration, exchange rate, etc.) that are installed and properly maintained
8. Monitor and Manage Ill and Exposed Healthcare Personnel
• Facilities and organizations providing healthcare should implement sick leave policies for HCP that are non-punitive, flexible, and consistent with public health guidance.
• Movement and monitoring decisions for HCP with exposure to COVID-19 should be made in consultation with public health authorities. Refer to the Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease 2019 (COVID-19) for additional information.
9. Train and Educate Healthcare Personnel
• Provide HCP with job- or task-specific education and training on preventing transmission of infectious agents, including refresher training.
• Ensure that HCP are educated, trained, and have practiced the appropriate use of PPE prior to caring for a patient, including attention to correct use of PPE and prevention of contamination of clothing, skin, and environment during the process of removing such equipment.
10. Implement Environmental Infection Control
• Dedicated medical equipment should be used when caring for patients with known or suspected COVID-19.
○ All non-dedicated, non-disposable medical equipment used for patient care should be cleaned and disinfected according to manufacturer’s instructions and facility policies.
• Ensure that environmental cleaning and disinfection procedures are followed consistently and correctly.
• Routine cleaning and disinfection procedures (e.g., using cleaners and water to pre-clean surfaces prior to applying an EPA-registered, hospital-grade disinfectant to frequently touched surfaces or objects for appropriate contact times as indicated on the product’s label) are appropriate for SARS-CoV-2 in healthcare settings, including those patient-care areas in which aerosol-generating procedures are performed.
○ Refer to List Ne on the EPA website for EPA-registered disinfectants that have qualified under EPA’s emerging viral pathogens program for use against SARS-CoV-2.
• Management of laundry, food service utensils, and medical waste should also be performed in accordance with routine procedures.
• Additional information about recommended practices for terminal cleaning of rooms and PPE to be worn by environmental services personnel is available in the Healthcare Infection Prevention and Control FAQs for COVID-19
11. Establish Reporting within and between Healthcare Facilities and to Public Health Authorities
• Implement mechanisms and policies that promote situational awareness for facility staff including infection control, healthcare epidemiology, facility leadership, occupational health, clinical laboratory, and frontline staff about known or suspected COVID-19 patients and facility plans for response.
• Communicate and collaborate with public health authorities.
○ Facilities should designate specific persons within the healthcare facility who are responsible for communication with public health officials and dissemination of information to HCP.
• Communicate information about known or suspected COVID-19 patients to appropriate personnel before transferring them to other departments in the facility (e.g., radiology) and to other healthcare facilities.
Appendix: Additional Information about Airborne Infection Isolation Rooms, Respirators and Facemasks
Information about Airborne Infection Isolation Rooms (AIIRs):
• AIIRs are single-patient rooms at negative pressure relative to the surrounding areas, and with a minimum of 6 air changes per hour (12 air changes per hour are recommended for new construction or renovation).
• Air from these rooms should be exhausted directly to the outside or be filtered through a high-efficiency particulate air (HEPA) filter directly before recirculation.
• Room doors should be kept closed except when entering or leaving the room, and entry and exit should be minimized.
• Facilities should monitor and document the proper negative-pressure function of these rooms.
Information about Respirators:
• A respirator is a personal protective device that is worn on the face, covers at least the nose and mouth, and is used to reduce the wearer’s risk of inhaling hazardous airborne particles (including dust particles and infectious agents), gases, or vapors. Respirators are certified by the CDC/NIOSH, including those intended for use in healthcare.
• Respirator use must be in the context of a complete respiratory protection program in accordance with OSHA Respiratory Protection standard (29 CFR 1910.134). HCP should be medically cleared and fit-tested if using respirators with tight-fitting facepieces (e.g., a NIOSH-approved N95 respirator) and trained in the proper use of respirators, safe removal and disposal, and medical contraindications to respirator use.
• NIOSH information about respirators
• OSHA Respiratory Protection eTool
• Strategies for Optimizing the Supply of N-95 Respirators
Filtering Facepiece Respirators (FFR) including N95 Respirators
• A commonly used respirator in healthcare settings is a filtering facepiece respirator (commonly referred to as an N95). FFRs are disposable half facepiece respirators that filter out particles.
• To work properly, FFRs must be worn throughout the period of exposure and be specially fitted for each person who wears one. This is called “fit-testing” and is usually done in a workplace where respirators are used.
• Three key factors for an N95 respirator to be effective
• FFR users should also perform a user seal check to ensure proper fit each time an FFR is used.
• Learn more about how to perform a user seal check
• For more information on how to perform a user seal check: https://www.cdc.gov/niosh/docs/2018-130/pdfs/2018-130.pdf?id=10.26616/NIOSHPUB2018130
NIOSH-approved N95 respirators list.
• PAPRs have a battery-powered blower that pulls air through attached filters, canisters, or cartridges. They provide protection against gases, vapors, or particles, when equipped with the appropriate cartridge, canister, or filter.
• Loose-fitting PAPRs do not require fit testing and can be used with facial hair.
• A list of NIOSH-approved PAPRs is located on the NIOSH Certified Equipment List.
Information about Facemasks:
• If worn properly, a facemask helps block respiratory secretions produced by the wearer from contaminating other persons and surfaces (often called source control).
• Facemasks are cleared by the U.S. Food and Drug Administration (FDA) for use as medical devices. Facemasks should be used once and then thrown away in the trash.
Interim Guidance for Implementing Home Care of People Not Requiring Hospitalization for COVID-19
CDC has developed interim guidance for staff at local and state health departments, infection prevention and control professionals, healthcare providers, and healthcare workers who are coordinating the home care and isolation of people who are confirmed to have, or being evaluated for (COVID-19 (see Criteria to Guide Evaluation of Patients Under Investigation (PUI) for COVID-19).
Interim Guidance for Implementing Home Care of People Not Requiring Hospitalization forCOVID-19)
Important Links
• Respirator Trusted-Source Information
• Respirator Fact Sheet
Healthcare Infection Prevention and Control FAQs for COVID-19
Page Summary
This page was updated on March 10, 2020 to align with the revised Interim Infection Prevention and Control Recommendations for Patients with Confirmed Coronavirus Disease 2019 (COVID-19) or Persons Under Investigation for COVID-19 in Healthcare Settings.
Who is this for: Healthcare personnel who may care for patients who are confirmed with or under investigation for COVID-19.
What is it for: This creates FAQs to support the existing Healthcare Infection Prevention and Control Guidance for COVID-19.
How is it used: To assist healthcare facilities in preventing transmission of COVID-19 in healthcare settings.
1. What personal protective equipment (PPE) should be worn by individuals transporting patients who are confirmed with or under investigation for COVID-19 within a healthcare facility? For example, what PPE should be worn when transporting a patient to radiology for imaging that cannot be performed in the patient room?
In general, transport and movement of the patient outside of their room should be limited to medically essential purposes. If being transported outside of the room, such as to radiology, healthcare personnel (HCP) in the receiving area should be notified in advance of transporting the patient. For transport, the patient should wear a facemask to contain secretions and be covered with a clean sheet.
If transport personnel must prepare the patient for transport (e.g., transfer them to the wheelchair or gurney), transport personnel should wear all recommended PPE (gloves, a gown, respiratory protection that is at least as protective as a fit-tested NIOSH-certified disposable N95 filtering facepiece respirator or facemask—if a respirator is not available—and eye protection [i.e., goggles or disposable face shield that covers the front and sides of the face]). This recommendation is needed because these interactions typically involve close, often face-to-face, contact with the patient in an enclosed space (e.g., patient room). Once the patient has been transferred to the wheelchair or gurney (and prior to exiting the room), transporters should remove their gown, gloves, and eye protection and perform hand hygiene.
If the patient is wearing a facemask, no recommendation for PPE is made typically for HCP transporting patients with a respiratory infection from the patient’s room to the destination. However, given current limitations in knowledge regarding COVID-19 and following the currently cautious approach for risk stratification and monitoring of healthcare personnel caring for patients with COVID-19, use of a facemask by the transporter is recommended for anything more than brief encounters with COVID-19 patients. Additional PPE should not be required unless there is an anticipated need to provide medical assistance during transport (e.g., helping the patient replace a dislodged facemask).
After arrival at their destination, receiving personnel (e.g., in radiology) and the transporter (if assisting with transfer) should perform hand hygiene and wear all recommended PPE. If still wearing their original respirator or facemask, the transporter should take care to avoid self-contamination when donning the remainder of the recommended PPE. This cautious approach will be refined and updated as more information becomes available and as response needs change in the United States.
Interim guidance for EMS personnel transporting patients with confirmed or suspected COVID-19 is available here. EMS personnel should wear all recommended PPE because they are providing direct medical care and in close contact with the patient for longer periods of time.
2. What PPE should be worn by HCP providing care to asymptomatic patients with a history of exposure to COVID-19 who are being evaluated for a non-infectious complaint (e.g., hypertension or hyperglycemia)?
Standard Precautions should be followed when caring for any patient, regardless of suspected or confirmed COVID-19. If the patient is afebrile (temperature is less than 100.0oF) and otherwise without even mild symptoms* that might be consistent with COVID-19 (e.g., cough, sore throat, shortness of breath), then precautions specific to COVID-19 are not required. However, until the patient is determined to be without such symptoms, HCP should wear all recommended PPE for the patient encounter. If the primary evaluation confirms the patient is without symptoms, management and need for any Transmission-Based Precautions should be based with the condition for which they are being evaluated (e.g., patient colonized with a drug-resistant organism), rather than potential exposure to COVID-19.
This public health response is an important opportunity to reinforce the importance of strict adherence to Standard Precautions during all patient encounters. Standard Precautions are based on the principles that all blood, body fluids, secretions, excretions except sweat, nonintact skin, and mucous membranes may contain transmissible infectious agents. The application of Standard Precautions is determined by the nature of the HCP-patient interaction and the extent of anticipated blood, body fluids, and pathogen exposure. For example, a facemask and eye protection should be worn during the care of any patient if splashes, sprays, or coughs could occur during the patient encounter. Similarly, gloves should be worn if contact with body fluids, mucous membranes, or nonintact skin are anticipated.
3. What personal protective equipment (PPE) should be worn by environmental services (EVS) personnel who clean and disinfect rooms of hospitalized patients with COVID-19?
In general, only essential personnel should enter the room of patients with COVID-19. Healthcare facilities should consider assigning daily cleaning and disinfection of high-touch surfaces to nursing personnel who will already be in the room providing care to the patient. If this responsibility is assigned to EVS personnel, they should wear all recommended PPE when in the room. PPE should be removed upon leaving the room, immediately followed by performance of hand hygiene.
After discharge, terminal cleaning may be performed by EVS personnel. They should delay entry into the room until a sufficient time has elapsed for enough air changes to remove potentially infectious particles. We do not yet know how long SARS-CoV-2 remains infectious in the air. Regardless, EVS personnel should refrain from entering the vacated room until sufficient time has elapsed for enough air changes to remove potentially infectious particles (more information on clearance rates under differing ventilation conditions is available). After this time has elapsed, EVS personnel may enter the room and should wear a gown and gloves when performing terminal cleaning. A facemask and eye protection should be added if splashes or sprays during cleaning and disinfection activities are anticipated or otherwise required based on the selected cleaning products. Shoe covers are not recommended at this time for personnel caring for patients with COVID-19.
Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19)
Summary of Recent Changes
Revisions were made on March 7, 2020, to reflect the following:
• Characteristics of patients with confirmed COVID-19 based on recent epidemiologic data from China, including characteristics of patients with COVID-19 admitted to the intensive care unit and data on pediatric cases
• Data regarding SARS-CoV-2 viral shedding among asymptomatic persons, and data from a recent report of viable SARS-CoV-2 isolation from stool
• Accessibility of investigational drug therapies for COVID-19 treatment through clinical trial enrollment in the United States
• Recently published pediatric surviving sepsis guidance
This interim guidance is for clinicians caring for patients with confirmed infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease (COVID-19).
This update includes additional information from recent reports including:
• Characteristics of patients with confirmed COVID-19 based on recent epidemiologic data from China, including characteristics of patients with COVID-19 admitted to the intensive care unit and data on pediatric cases
• Data regarding SARS-CoV-2 viral shedding among asymptomatic persons, and data from a recent report of viable SARS-CoV-2 isolation from stool
• Accessibility of investigational drug therapies for COVID-19 treatment through clinical trial enrollment in the United States
• Recently published pediatric surviving sepsis guidance
CDC will update this interim guidance as more information becomes available.
Clinical Presentation
Among reports that describe the clinical presentation of patients with confirmed COVID-19, most are limited to hospitalized patients with pneumonia. The incubation period is estimated at 4 days (interquartile range: 2 to 7 days). [1] Some studies have estimated a wider range for the incubation period; data for human infection with other coronaviruses (e.g. MERS-CoV, SARS-CoV) suggest that the incubation period may range from 2-14 days. Frequently reported signs and symptoms of patients admitted to the hospital include fever (77–98%), cough (46%–82%), myalgia or fatigue (11–52%), and shortness of breath (3-31%) at illness onset. [2–5] Among 1,099 hospitalized COVID-19 patients, fever was present in 44% at hospital admission, and developed in 89% during hospitalization. [6] Other less commonly reported respiratory symptoms include sore throat, headache, cough with sputum production and/or hemoptysis. Some patients have experienced gastrointestinal symptoms such as diarrhea and nausea prior to developing fever and lower respiratory tract signs and symptoms. The fever course among patients with COVID-19 is not fully understood; it may be prolonged and intermittent. A limited number of reports describe identification of asymptomatic or subclinical infection on the basis of detection of SARS-CoV-2 RNA or live virus from throat swab specimens of contacts of confirmed patients. [7–8]
Risk factors for severe illness are not yet clear, although older patients and those with chronic medical conditions may be at higher risk for severe illness. Among more than 44,000 confirmed cases of COVID-19 in China as of Feb 11, 2020, most occurred among patients aged 30–69 years (77.8%), and approximately 19% were severely or critically ill [9]. Case-fatality proportion among cases aged ≥60 years was: 60-69 years: 3.6%; 70-79 years: 8%; ≥80 years: 14.8%. Patients who reported no underlying medical conditions had an overall case fatality of 0.9%, but case fatality was higher for patients with comorbidities: 10.5% for those with cardiovascular disease, 7% for diabetes, and 6% each for chronic respiratory disease, hypertension, and cancer. Case fatality for patients who developed respiratory failure, septic shock, or multiple organ dysfunction was 49%. [9]
Limited information is available about the clinical presentation, clinical course, and risk factors for severe COVID-19 in children. Of confirmed COVID-19 patients in China as of Feb 11, 2020, only 2.1% were aged >20 years, and no deaths were reported among those >10 years of age [9]. From limited published reports, signs and symptoms among children with COVID-19 may be more mild than adults, with most pediatric patients presenting with fever, cough, congestion, and rhinorrhea [10, 12–13], and one report of primarily gastrointestinal symptoms (vomiting and diarrhea) [13]. Prolonged detection of SARS-CoV RNA has been reported in respiratory specimens (up to 22 days after illness onset) and stool specimens (at least 30 days after illness onset) [10–11]. Severe complications of acute respiratory distress syndrome and septic shock were reported in a 13-month old with COVID-19 in China [13].
Clinical Course
Clinical presentation among reported cases of COVID-19 varies in severity from asymptomatic infection to mild illness to severe or fatal illness. Some reports suggest the potential for clinical deterioration during the second week of illness.[2,5] In one report, among patients with confirmed COVID-19 and pneumonia, just over half of patients developed dyspnea a median of 8 days after illness onset (range: 5–13 days). [2] In another report, the mean time from illness onset to hospital admission with pneumonia was 9 days.[1] Acute respiratory distress syndrome (ARDS) developed in 17–29% of hospitalized patients, and secondary infection developed in 10%. [2,4] In one report, the median time from symptom onset to ARDS was 8 days.[3]
Approximately 20-30% of hospitalized patients with COVID-19 and pneumonia have required intensive care for respiratory support.[2–3] Compared to patients not admitted to an intensive care unit, critically ill patients were older (median age 66 years versus 51 years), and were more likely to have underlying co-morbid conditions (72% versus 37%). [3] Among critically ill patients admitted to an intensive care unit, 11–64% received high-flow oxygen therapy and 47-71% received mechanical ventilation; some hospitalized patients have required advanced organ support with endotracheal intubation and mechanical ventilation (4–42%).[3–4,9] A small proportion have also been supported with extracorporeal membrane oxygenation (ECMO, 3–12%).[3–4,9] Other reported complications include cardiac injury, arrhythmia, septic shock, liver dysfunction, acute kidney injury, and multi-organ failure. Post-mortem biopsies in one patient who died of ARDS reported pulmonary findings of diffuse alveolar damage. [14]
An overall case fatality proportion of 2.3% has been reported among confirmed cases of COVID-19 in China. [9] However, the majority of these cases were among hospitalized patients and therefore this estimate of mortality is likely biased upward. Among hospitalized patients with pneumonia, the case fatality proportion has been reported as 4–15%.[2–4] Among critically ill COVID-19 patients in China, the reported case fatality proportion was 49%. In a report from one hospital, 61.5% of critically ill patients with COVID-19 had died by day 28 of ICU admission. [9,15]
Diagnostic Testing
Information on specimen collection, handling, and storage is available at: Real-Time RT-PCR Panel for Detection 2019-Novel Coronavirus. After initial confirmation of COVID-10, additional testing of clinical specimens can help inform clinical management, including discharge planning.
Laboratory and Radiographic Findings
The most common laboratory abnormalities reported among hospitalized patients with pneumonia on admission included leukopenia (9–25%), leukocytosis (24–30%), lymphopenia (63%), and elevated alanine aminotransferase and aspartate aminotransferase levels (37%). [2,4] Among 1,099 COVID-19 patients, lymphocytopenia was present in 83%; 36% had thrombocytopenia, and 34% had leukopenia. [6] Most patients had normal serum levels of procalcitonin on admission. Chest CT images have shown bilateral involvement in most patients. Multiple areas of consolidation and ground glass opacities are typical findings reported to date. [2–4, 16–24] However, one study that evaluated the time from symptom onset to initial CT scan found that 56% of patients who presented within 2 days had a normal CT. [20]
Limited data are available about the detection of SARS-CoV-2 RNA and infectious virus in clinical specimens. SARS-CoV-2 RNA has been detected from upper and lower respiratory tract specimens, and the virus has been isolated in cell culture from upper respiratory tract specimens and bronchoalveolar lavage fluid. In one case series SARS-CoV-2 viral RNA levels in the first 3 days after symptom onset were higher in specimens collected from the nose than from the throat (as demonstrated by lower cycle threshold values in the nose). [25] A similar time course and pattern of viral RNA detection was reported in one asymptomatic patient after exposure to a patient with confirmed COVID-19. [25]
SARS-CoV-2 RNA has been detected in blood and stool specimens and SARS-CoV-2 virus has been isolated in cell culture from the stool of a patient with pneumonia 15 days after symptom onset. [26–29]. The duration of SARS-CoV-2 RNA detection in the upper and lower respiratory tracts and in extrapulmonary specimens is not yet known. It is possible that RNA could be detected for weeks, which has occurred in some cases of MERS-CoV or SARS-CoV infection. [30–37] Viable SARS-CoV has been isolated from respiratory, blood, urine, and stool specimens. In contrast, viable MERS-CoV has been isolated only from respiratory tract specimens. [37–39]
Clinical Management and Treatment
For information regarding infection prevention and control recommendations, please see Interim Infection Prevention and Control Recommendations for Patients with Confirmed Coronavirus Disease 2019 (COVID-19) or Persons Under Investigation for COVID-19 in Healthcare Settings.
Patients with a mild clinical presentation may not initially require hospitalization. However, clinical signs and symptoms may worsen with progression to lower respiratory tract disease in the second week of illness; all patients should be monitored closely. Possible risk factors for progressing to severe illness may include, but are not limited to, older age, and underlying chronic medical conditions such as lung disease, cancer, heart failure, cerebrovascular disease, renal disease, liver disease, diabetes, immunocompromising conditions, and pregnancy.
The decision to monitor a patient in the inpatient or outpatient setting should be made on a case-by-case basis. This decision will depend not only on the clinical presentation, but also on the patient’s ability to engage in monitoring, home isolation, and the risk of transmission in the patient’s home environment. For more information, see Evaluating and Reporting Persons Under Investigation (PUI)
No specific treatment for COVID-19 is currently available. Clinical management includes prompt implementation of recommended infection prevention and control measures and supportive management of complications, including advanced organ support if indicated.
Corticosteroids should be avoided, because of the potential for prolonging viral replication as observed in MERS-CoV patients, unless indicated for other reasons. [31, 40–42] For example, for a chronic obstructive pulmonary disease exacerbation or for septic shock per Surviving Sepsis guidelines for adults and children.
For more information, see: WHO interim guidance on clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected, Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America, and Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children
Investigational Therapeutics
There are currently no antiviral drugs licensed by the U.S. Food and Drug Administration (FDA) to treat patients with COVID-19. In the United States, the National Institutes of Health (NIH) and collaborators are working on development of candidate vaccines and therapeutics for COVID-19. Some in-vitro or in-vivo studies suggest potential therapeutic activity of compounds against related coronaviruses, but there are no available data from randomized controlled trials in humans to support recommending any investigational therapeutics for patients with confirmed or suspected COVID-19 at this time.
Remdesivir is an investigational antiviral drug that was reported to have in-vitro activity against SARS-CoV-2. [43] Some patients with COVID-19 have received intravenous remdesivir for compassionate use outside of a clinical trial setting. In China, multiple clinical trials of investigational therapeutics have been implemented, including two clinical trials of remdesivir. An NIH adaptive randomized controlled clinical trial of investigational therapeutics for hospitalized COVID-19 patients in the United States was approved by the Food and Drug Administration; the first investigational therapeutic to be studied is remdesivir. Other remdesivir trials for COVID-19 patients in the U.S. are available (participants with severe and moderate coronavirus disease). Some COVID-19 patients have received uncontrolled treatment with other investigational antivirals. [4, 28, 44] For information on specific clinical trials underway for treatment of patients with COVID-19, see clinicaltrials.gov, and www.chictr.org.cn
In the absence of an approved vaccine, community mitigation measures are the primary way to reduce SARS-CoV-2 transmission among persons in the community, and adherence to recommended infection prevention and control measures can reduce the risk of SARS-CoV-2 spread in healthcare facilities. In the absence of an approved therapeutic with demonstrated safety and efficacy in patients with COVID-19, clinical management of COVID-19 patients includes avoidance of corticosteroids, and supportive care of complications, including advanced organ support.
Discontinuation of Transmission-Based Precautions or Home Isolation
For recommendations on discontinuation of transmission-based precautions or home isolation for patients who have recovered from COVID-19 illness, please see: Interim Guidance for Discontinuation of Transmission-Based Precautions and Disposition of Hospitalized Patients with COVID-19 and Interim Guidance for Discontinuation of In-Home Isolation for Patients with COVID-19
Additional resources:
• Evaluating and Reporting Persons Under Investigation (PUI)
• Resources for Hospitals and Healthcare Professionals Preparing for Patients with Suspected or Confirmed COVID-19
• Interim Infection Prevention and Control Recommendations for Patients with Confirmed Coronavirus Disease 2019 (COVID-19) or Persons Under Investigation for COVID-19 in Healthcare Settings World Health Organization. Interim Guidance on Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected
• American Thoracic Society and Infectious Diseases Society of America Clinical Practice Guidelines. Diagnosis and treatment of adults with community-acquired pneumonia
• Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016
• Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children
• Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza
References
1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMoa2002032. [Epub ahead of print]
2. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020 Jan 24.
3. Wang D, Hu B, Hu C, Zhu F, Liu X et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan. Published online February 7, 2020.
4. Chen N, Zhou M, Dong X, Qu J, Gong F. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 Jan 30. [Epub ahead of print]
5. Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020 Jan 31. doi: 10.1056/NEJMoa2001191. [Epub ahead of print]Huang C, Wang Y, Li X, Ren L, Zhao J, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Jan 24. [Epub ahead of print]
6. Li Q, Guan X, Wu P, Wang X, Zhou L, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020 Jan 29.
7. Chan JF, Yuan S, Kok K, To KK, Chu H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020 Jan 24. [Epub ahead of print]
8. Hoehl S, Berger A, Kortenbusch M, Cinatl J, Bojkova D, Rabenau H, Behrens P, Böddinghaus B, Götsch U, Naujoks F, Neumann P. Evidence of SARS-CoV-2 Infection in Returning Travelers from Wuhan, China. New England Journal of Medicine. 2020 Feb 18.
9. Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. [The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. DOI:10.3760/cma.j.issn.0254-6450.2020.02.003.
10. Kam KQ, Yung CF, Cui L, Lin Tzer Pin R, Mak TM, Maiwald M, Li J, Chong CY, Nadua K, Tan NWH, Thoon KC. A Well Infant with Coronavirus Disease 2019 (COVID-19) with High Viral Load. Clin Infect Dis. 2020 Feb 28. pii: ciaa201. doi: 10.1093/cid/ciaa201. [Epub ahead of print]
11. Cai J, Xu J, Lin D, Yang Z, Xu L, Qu Z, Zhang Y, Zhang H, Jia R, Liu P, Wang X, Ge Y, Xia A, Tian H, Chang H, Wang C, Li J, Wang J, Zeng M. A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020 Feb 28. pii: ciaa198. doi: 10.1093/cid/ciaa198. [Epub ahead of print]
12. Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel Coronavirus Infection in Hospitalized Infants Under 1 Year of Age in China. JAMA. 2020 Feb 14. doi: 10.1001/jama.2020.2131. [Epub ahead of print]
13. Chen F, Liu ZS, Zhang FR, Xiong RH, Chen Y, Cheng XF, Wang WY, Ren J. [First case of severe childhood novel coronavirus pneumonia in China].Zhonghua Er Ke Za Zhi. 2020 Feb 11;58(0):E005. doi: 10.3760/cma.j.issn.0578-1310.2020.0005. [Epub ahead of print] Chinese.
14. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Feb 18. pii: S2213-2600(20)30076-X. doi: 10.1016/S2213-2600(20)30076-X. [Epub ahead of print]
15. Yang X, Yu Y, Xu J, Shu H, Xia J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020. Published Online February 21, 2020 https://doi.org/10.1016/S2213-2600(20)30079-5
16. Chang D, Minggui L, Wei L, Lixin X, Guangfa Z et al. Epidemiologic and Clinical Characteristics of Novel Coronavirus Infections Involving 13 Patients Outside Wuhan China. Published online February 7, 2020.
17. Zhu N, Zhang D, Wang W, Li X, Yang B, et al; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020 Jan 24. [Epub ahead of print]
18. Phan LT, Nguyen TV, Luong QC, Nguyen TV, Nguyen HT et al. Importation and Human-to-Human Transmission of a Novel Coronavirus in Vietnam. N Engl J Med. 2020 Jan 28. doi: 10.1056/NEJMc2001272. [Epub ahead of print]
19. Lei J, Li J, Li X, Qi X. CT Imaging of the 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology. 2020 Jan 31:200236. doi: 10.1148/radiol.2020200236. [Epub ahead of print]
20. Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, Diao K, Lin B, Zhu X, Li K, Li S. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology. 2020 Feb 20:200463.
21. Xu X, Yu C, Qu J, Zhang L, Jiang S, Huang D, Chen B, Zhang Z, Guan W, Ling Z, Jiang R, Hu T, Ding Y, Lin L, Gan Q, Luo L, Tang X, Liu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020 Feb 28. doi: 10.1007/s00259-020-04735-9. [Epub ahead of print]
22. Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, Dai J, Sun Q, Zhao F, Qu J, Yan F. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):A multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020 Feb 26. pii: S0163-4453(20)30099-2. doi: 10.1016/j.jinf.2020.02.016. [Epub ahead of print]
23. Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020 Feb 26:200642. doi: 10.1148/radiol.2020200642. [Epub ahead of print]
24. Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020 Feb 24. pii: S1473-3099(20)30086-4. doi: 10.1016/S1473-3099(20)30086-4. [Epub ahead of print]
25. Zou L, Ruan F, Huang M, Liang L, Huang H, et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med. 2020 Feb 19. doi: 10.1056/NEJMc2001737. [Epub ahead of print]
26. Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, Wang YY, Xiao GF, Yan B, Shi ZL, Zhou P. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerging Microbes & Infections. 2020 Jan 1;9(1):386-9.
27. Chen W, Lan Y, Yuan X, Deng X, Li Y, Cai X, Li L, He R, Tan Y, Deng X, Gao M, Tang G, Zhao L, Wang J, Fan Q, Wen C, Tong Y, Tang Y, Hu F, Li F, Tang X. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect. 2020 Dec;9(1):469-473.
28. Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, Ng OT, Marimuthu K, Ang LW, Mak TM, Lau SK, Anderson DE, Chan KS, Tan TY, Ng TY, Cui L, Said Z, Kurupatham L, Chen MI, Chan M, Vasoo S, Wang LF, Tan BH, Lin RTP, Lee VJM, Leo YS, Lye DC; Singapore 2019 Novel Coronavirus Outbreak Research Team. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA. 2020 Mar 3. doi: 10.1001/jama.2020.3204. [Epub ahead of print]
29. Zhang Y, Chen C, Zhu S, Shu C, Wang D, Song J, et al. Isolation of 2019-nCoV from a Stool Specimen of a Laboratory-Confirmed Case of the Coronavirus Disease 2019 (COVID-19)[J]. China CDC Weekly, 2020, 2(8): 123-124
30. Memish ZA, Assiri AM, Al-Tawfiq JA. Middle East respiratory syndrome coronavirus (MERS-CoV) viral shedding in the respiratory tract: an observational analysis with infection control implications. Int J Infect Dis. 2014 Dec;29:307-8.
31. Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015 Sep 5;386(9997):995-1007. doi: 10.1016/S0140-6736(15)60454-8. Epub 2015 Jun 3. Review.
32. Chan KH, Poon LL, Cheng VC, Guan Y, Hung IF et al. Detection of SARS coronavirus in patients with suspected SARS. Emerg Infect Dis. 2004 Feb;10(2):294-9.
33. Cheng PK, Wong DA, Tong LK, Ip SM, Lo AC et al. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004 May 22;363(9422):1699-700.
34. Hung IF, Cheng VC, Wu AK, Tang BS, Chan KH et al. Viral loads in clinical specimens and SARS manifestations. Emerg Infect Dis. 2004 Sep;10(9):1550-7.
35. Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, et al; HKU/UCH SARS Study Group. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003 May 24;361(9371):1767-72.
36. Liu W, Tang F, Fontanet A, Zhan L, Zhao QM et al. Long-term SARS coronavirus excretion from patient cohort, China. Emerg Infect Dis. 2004 Oct;10(10):1841-3.
37. Corman VM, Albarrak AM, Omrani AS, Albarrak MM, Farah ME, et al. Viral Shedding and Antibody Response in 37 Patients With Middle East Respiratory Syndrome Coronavirus Infection. Clin Infect Dis. 2016 Feb 15;62(4):477-483.
38. Al-Abdely HM, Midgley CM, Alkhamis AM, Abedi GR, Lu X, et al. Middle East respiratory syndrome coronavirus infection dynamics and antibody responses among clinically diverse patients, Saudi Arabia. Emerg Infect Dis. 2019 Apr;25(4):753-766.
39. Al-Abdely HM, Midgley CM, Alkhamis AM, Abedi GR, Tamin A et al. Infectious MERS-CoV Isolated From a Mildly Ill Patient, Saudi Arabia. Open Forum Infect Dis. 2018 May 15;5(6):ofy111.
40. Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, et al; Saudi Critical Care Trial Group. Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am J Respir Crit Care Med. 2018 Mar 15;197(6):757-767.
41. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020 Feb 6; S0140-6736(20)30305-6.
42. Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-e67.
43. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 Feb 4. doi: 1038/s41422-020-0282-0. [Epub ahead of print] PubMed PMID: 32020029.
44. Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, Li SB, Wang HY, Zhang S, Gao HN, Sheng JF, Cai HL, Qiu YQ, Li LJ. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020 Feb 19;368:m606. doi:10.1136/bmj.m606.
Information for Pediatric Healthcare Providers
Who this is for: Pediatric Healthcare Providers
What this is for: To inform pediatric healthcare providers of information available on children with COVID-19.
How to use: Refer to this information when managing pediatric patients with confirmed or suspected COVID-19.
Burden of COVID-19 and epidemiologic risk factors in children
Relatively few cases of COVID-19 caused by SARS-CoV-2 infection have been reported in children compared with the total number of cases in the general population. As of Feb. 20, 2020, 2.4% of the 75,465 cases (confirmed and suspected) in China had occurred among persons younger than 19 years old. An analysis from one large city in southern China suggests that, among all cases, the proportion of children younger than 15 years old may have increased from 2% to 13% from early to later in the outbreak.
Of the cases reported in China to date, most children had exposure to household members with confirmed COVID-19. In one case, a three-month-old child visited a healthcare setting before COVID-19 was confirmed and was thought to be the first case in a family cluster—the source of infection (healthcare or community) was not determined. At least one child who primarily had gastrointestinal symptoms sought care at multiple outpatient healthcare centers before becoming a confirmed case.
During previous outbreaks caused by related zoonotic beta coronaviruses, Severe Acute Respiratory Syndrome (SARS) and Middle Eastern Respiratory Syndrome (MERS), the majority of confirmed cases occurred among adults. During the 2002-2003 SARS epidemic, less than 5% of cases were diagnosed in patients younger than 18. The majority of SARS cases in patients younger than 18 were thought to have occurred through household transmission, though some cases were hospital-acquired. The majority of MERS-CoV cases in children were also thought to be due to household transmission.
Children may play a role in the spread of SARS-CoV-2 in the community. In one report examining 10 infected children in China, SARS-CoV-2 ribonucleic acid (RNA) was detected in respiratory specimens up to 22 days after symptoms began and in stool up to 30 days after symptoms began. A case report of a 6-month-old infant describes detection of SARS-CoV-2 RNA in blood, stool, and multiple nasopharyngeal swab samples, even though the infant’s only documented manifestation of illness was one recorded temperature of 38.5° C (101.3° F). Viral culture was not performed on specimens in these reports; therefore, it is uncertain whether persistent or asymptomatic RNA detection represented potentially transmissible virus.
Currently, it is unknown if differences in reported incidence of confirmed COVID-19 among children versus adults in China is because of difference in exposures (e.g., children are less likely to care for sick contacts), disease severity, testing, or surveillance (e.g., symptoms at presentation differ from case definitions for surveillance or diagnosis).
Clinical presentation in children
Symptoms in Pediatric Patients
Illness among pediatric cases appear to be mild, with most cases presenting with:
• symptoms of respiratory infection
• cough
• nasal congestion
• rhinorrhea
• sore throat
The predominant signs and symptoms of COVID-19 reported to date among all patients are similar to other viral respiratory infections. These include fever, cough, and difficulty breathing. Gastrointestinal symptoms, including abdominal pain, diarrhea, nausea, and vomiting, were reported in a minority of adult patients.
In a report of nine hospitalized infants in China with confirmed COVID-19, only half presented with fever. At least one child to date had primarily gastrointestinal symptoms of vomiting, diarrhea, and anorexia at initial presentation. There have been multiple reports to date of children with asymptomatic SARS-CoV-2 infection.
Data from pediatric cases of SARS and MERS also show milder symptoms among children compared with adults, and adolescents with SARS had more severe symptoms compared with younger children. Co-detection of other respiratory pathogens (influenza, respiratory syncytial virus, Mycoplasma pneumoniae) have been described in children with COVID-19.
Signs and symptoms of COVID-19 in children may be similar to those for common viral respiratory infections or other childhood illnesses. It is important for pediatric providers to have an appropriate suspicion of COVID-19, but also to continue to consider and test for other diagnoses, such as influenza (see CDC’s Flu Information for Healthcare Professionals for more information).
Clinical course and complications in children
Complications of COVID-19 appear to be milder among children compared with adults based on limited reports from China. Severe complications (e.g., acute respiratory distress syndrome, septic shock) were reported in one case of a 13-month old with confirmed COVID-19. Other reports describe a mild disease course, including in infants. As of February 20, 2020, just one of the 2,114 deaths among 55,924 confirmed COVID-19 cases in China occurred among children younger than 20 years old. No further details were provided about this patient.
Chest X-rays of children with COVID-19 show patchy infiltrates consistent with viral pneumonia, and chest CT scans have shown nodular ground glass opacities.
During the 2003-2004 SARS outbreak, patients younger than 12 years old had milder and shorter illnesses than adults, and no deaths were reported. Death was rare among children with MERS. One pediatric death from MERS was reported in a child with cystic fibrosis who had respiratory specimens also positive for influenza A(H1N1)pdm09 and multidrug-resistant Pseudomonas.
Though symptoms and disease course for COVID-19 may be milder in children than adults, it is unknown if children with underlying medical conditions are at increased risk of severe disease.
Treatment and prevention
Currently, there are no antiviral drugs recommended or licensed by the U.S. Food and Drug Administration (FDA) for COVID-19. Clinical management includes promptly using recommended infection prevention and control measures (e.g., a respirator or facemask, gloves, gown, eye protection) in healthcare settings and supportive management of complications.
Lopinavir/ritonavir and interferon-alpha have been used for treatment of children with COVID-19 in China but safety and efficacy of these drugs have not been determined. Remdesivir is an investigational antiviral drug that has been reported to have in-vitro activity against SARS-CoV-2. Some adult patients with COVID-19 have received intravenous remdesivir through clinical trials or compassionate use, although remdesivir has not been used for treatment of children with COVID-19. For additional information on investigational therapeutics, please see Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19).
Additional information
For general information on caring for patients with confirmed or possible COVID-19 please see: What Healthcare Personnel Should Know about Caring for Patients with Confirmed or Possible COVID-19 Infection
For CDC’s latest clinical guidance for management of patients with COVID-10 please see: Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease 2019 (COVID-19)
For information on infection prevention and control recommendations, please see: Interim Infection Prevention and Control Recommendations for Patients with Confirmed Coronavirus Disease 2019 (COVID-19) or Persons Under Investigation for COVID-19 in Healthcare Settings
For information on COVID-19 and pregnancy, please see: Frequently Asked Questions and Answers: Coronavirus Disease 2019 (COVID-19) and Pregnancy
For information on COVID-19 and breastfeeding, please see: Interim Guidance on Breastfeeding for a Mother Confirmed or Under Investigation For COVID-19
For information for healthcare facilities on how to prepare for the COVID-19 outbreak: Steps Healthcare Facilities Can Take Now to Prepare for Coronavirus Disease 2019 (COVID-19)
References
1. Al-Tawfiq, J.A., R.F. Kattan, and Z.A. Memish, Middle East respiratory syndrome coronavirus disease is rare in children: An update from Saudi Arabia. World J Clin Pediatr, 2016. 5(4): p. 391-396.
2. Bartenfeld, M., et al., Middle East Respiratory Syndrome Coronavirus and Children. Clin Pediatr (Phila), 2017. 56(2): p. 187-189.
3. Cai, J., et al., A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis, 2020.
4. Centers for Disease Control and Prevention. Coronavirus. 2020; Available from: https://www.cdc.gov/coronavirus/index.html; accessed 6 Mar 2020.
5. Chan, J.F., et al., A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet, 2020. 24: p. 24.
6. Chen, F., et al., [First case of severe childhood novel coronavirus pneumonia in China]. Zhonghua Erke Zazhi, 2020. 58(0): p. E005.
7. China Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi, 2020. 41(2): p. 145-151.
8. Dowell, S.F. and M.S. Ho, Seasonality of infectious diseases and severe acute respiratory syndrome-what we don’t know can hurt us. Lancet Infect Dis, 2004. 4(11): p. 704-8.
9.• Feng, K., et al., [Analysis of CT features of 15 Children with 2019 novel coronavirus infection]. Zhonghua Er Ke Za Zhi, 2020. 58(0): p. E007.
10. Hon, K.L., et al., Clinical presentations and outcome of severe acute respiratory syndrome in children. Lancet, 2003. 361(9370): p. 1701-3.
11. Liu, J., et al., Community Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, Shenzhen, China, 2020. Emerging Infectious Diseases, 2020. 26(6). https://doi.org/10.3201/eid2606.200239
12. Memish, Z.A., et al., Middle East respiratory syndrome coronavirus disease in children. Pediatr Infect Dis J, 2014. 33(9): p. 904-6.
13. Monto, A.S., J.S. Koopman, and I.M. Longini, Jr., Tecumseh study of illness. XIII. Influenza infection and disease, 1976-1981. Am J Epidemiol, 1985. 121(6): p. 811-22.
14. Pan, X., et al., Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis, 2020.
15. Shen, K., et al., Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts’ consensus statement. World J Pediatr, 2020.
16. Stockman, L.J., et al., Severe acute respiratory syndrome in children. Pediatr Infect Dis J, 2007. 26(1): p. 68-74.
17. Wang, D., et al., Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA, 2020.
18. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 Feb 4. doi: 1038/s41422-020-0282-0. [Epub ahead of print]
19. Wei, M., et al., Novel Coronavirus Infection in Hospitalized Infants Under 1 Year of Age in China. Jama, 2020.
20. Worby, C.J., et al., On the relative role of different age groups in influenza epidemics. Epidemics, 2015. 13: p. 10-16.
21. World Health Organization – People’s Republic of China Joint Mission. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Available at: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf; accessed 6 Mar 2020.
22. Xia W., et al., Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatric Pulmonology. 2020;1-6. https://doi.org/10.1002/ppul.24718.
23. Zhang, Y.H., et al., [2019-novel coronavirus infection in a three-month-old baby]. Zhonghua Er Ke Za Zhi, 2020. 58(0): p. E006.
Interim Guidance for Discontinuation of Transmission-Based Precautions and Disposition of Hospitalized Patients with COVID-19
Summary Page
Who this is for: Healthcare providers and public health officials managing patients with coronavirus disease (COVID-19).
What this is for: To help prevent the spread of COVID-19 in healthcare facilities.
How to use: Reference to guide healthcare staff and public health officials regarding discontinuing transmission-based precautions and discharging hospitalized patients with COVID-19.
Summary of Recent Changes
Revisions were made on March10, 2020, to reflect the updated Interim Infection Prevention and Control Recommendations for Patients with Confirmed Coronavirus Disease 2019 (COVID-19) or Persons Under Investigation for COVID-19 in Healthcare Settings.
Limited information is available to characterize the spectrum of clinical illness, transmission efficiency, and the duration of viral shedding for patients with COVID-19. Interim guidance for discontinuation of Transmission-Based Precautions and disposition of hospitalized patients has been developed based on available information about COVID-19 and what is known about similar diseases caused by related coronaviruses (MERS-CoV and SARS-CoV). This guidance is subject to change as additional information becomes available.
For non-hospitalized patients, see (Interim Guidance for Discontinuation of In-Home Isolation for Patients with COVID-19).
Limited information is available to characterize the spectrum of clinical illness, transmission efficiency, and the duration of viral shedding for patients with COVID-19. Interim guidance for discontinuation of Transmission-Based Precautions and disposition of hospitalized patients has been developed based on available information about COVID-19 and what is known about similar diseases caused by related coronaviruses (MERS-CoV and SARS-CoV). This guidance is subject to change as additional information becomes available.
For non-hospitalized patients, see (Interim Guidance for Discontinuation of In-Home Isolation for Patients with COVID-19).
For Hospitalized Patients with COVID-19 Under Transmission-Based Precautions:
• The decision to discontinue Transmission-Based Precautions for hospitalized patients with COVID-19 should be made on a case-by-case basis in consultation with clinicians, infection prevention and control specialists, and public health officials. This decision should consider disease severity, illness signs and symptoms, and results of laboratory testing for COVID-19 in respiratory specimens. Guidance for discontinuation of in-home isolation precautions is the same as that to discontinue Transmission-Based Precautions for hospitalized patients with COVID-19. Considerations to discontinue Transmission-Based Precautions include all of the following:
○ Resolution of fever, without use of antipyretic medication
○ Improvement in illness signs and symptoms
○ Negative results of an FDA Emergency Use Authorized molecular assay for COVID-19 from at least two consecutive sets of paired nasopharyngeal and throat swabs specimens collected ≥24 hours apart* (total of four negative specimens—two nasopharyngeal and two throat). See Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Patients Under Investigation (PUIs) for 2019 Novel Coronavirus (2019-nCoV) for specimen collection guidance.
Footnote
* Initial guidance is based upon limited information and is subject to change as more information becomes available. In persons with a persistent productive cough, SARS-CoV-2-RNA might be detected for longer periods in sputum specimens than in upper respiratory tract (nasopharyngeal swab and throat swab) specimens.
Disposition of Hospitalized Patients with COVID-19:
• Patients can be discharged from the healthcare facility whenever clinically indicated.
• Isolation should be maintained at home if the patient returns home before the decision is made to discontinue Transmission-Based Precautions. The decision to send the patient home should be made in consultation with the patient’s clinical care team and local or state public health departments and should include considerations of the home’s suitability for and patient’s ability to adhere to home isolation recommendations, and potential risk of secondary transmission to household members with immunocompromising conditions. See CDC Interim Guidance for Home Care of patients with confirmed COVID-19 and persons under investigation for COVID-19, Interim Guidance for Preventing 2019-nCoV from Spreading to Others in Homes and Communities and Interim Guidance for Discontinuation of In-Home Isolation for Patients with COVID-19.
References
• Al-Abdely HM, Midgley CM, Alkhamis AM, et al. Middle East respiratory syndrome coronavirus infection dynamics and antibody responses among clinically diverse patients, Saudi Arabia. Emerg Infect Dis. 2019 Apr;25(4):753–66.
• Al-Abdely HM, Midgley CM, Alkhamis AM, et al. Infectious MERS-CoV isolated from a mildly ill patient, Saudi Arabia. Open Forum Infect Dis. 2018 May 15;5(6):ofy111.
• Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020 Jan 24; 395(10223);514–23. doi: 10.1016/S0140-6736(20)30154-9.
• Chan KH, Poon LL, Cheng VC, et al. Detection of SARS coronavirus in patients with suspected SARS. Emerg Infect Dis. 2004 Feb;10(2):294–9.
• Cheng PK, Wong DA, Tong LK, et al. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004 May 22;363(9422):1699–700.
• Corman VM, Albarrak AM, Omrani AS, et al. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis. 2016 Feb 15;62(4):477–83.
• Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020 Jan 31. doi: 10.1056/NEJMoa2001191. [Epub ahead of print]
• Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Jan 24;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5
• Hung IF, Cheng VC, Wu AK, et al. Viral loads in clinical specimens and SARS manifestations. Emerg Infect Dis. 2004 Sep;10(9):1550–7.
• Liu W, Tang F, Fontanet A, et al. Long-term SARS coronavirus excretion from patient cohort, China. Emerg Infect Dis. 2004 Oct;10(10):1841–3.
• Memish ZA, Assiri AM, Al-Tawfiq JA. Middle East respiratory syndrome coronavirus (MERS-CoV) viral shedding in the respiratory tract: an observational analysis with infection control implications. Int J Infect Dis. 2014 Dec;29:307–8.
• Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 Jan 24. doi: 10.1056/NEJMoa2001017 [Epub ahead of print]
Interim Considerations for Infection Prevention and Control of Coronavirus Disease 2019 (COVID-19) in Inpatient Obstetric Healthcare Settings
These infection prevention and control considerations are for healthcare facilities providing obstetric care for pregnant patients with confirmed coronavirus disease (COVID-19) or pregnant persons under investigation (PUI) in inpatient obstetric healthcare settings including obstetrical triage, labor and delivery, recovery and inpatient postpartum settings.
This information is intended to aid hospitals and clinicians in applying broader CDC interim guidance on infection control (Interim Infection Prevention and Control Recommendations for Patients with Confirmed Coronavirus Disease 2019 (COVID-19) or Persons Under Investigation for COVID-19 in Healthcare Settings).
Since maternity and newborn care units vary in physical configuration, each facility should consider their appropriate space and staffing needs to prevent transmission of the virus that causes COVID-19. These considerations include appropriate isolation of pregnant patients who have confirmed COVID-19 or are PUIs; basic and refresher training for all healthcare personnel on those units to include correct adherence to infection control practices and personal protective equipment (PPE) use and handling; sufficient and appropriate PPE supplies positioned at all points of care; and processes to protect newborns from risk of COVID-19.
These considerations are based upon the limited evidence available to date about transmission of the virus that causes COVID-19, and knowledge of other viruses that cause severe respiratory illness including influenza, severe acute respiratory syndrome coronavirus (SARS-CoV), and Middle East Respiratory Syndrome coronavirus (MERS-CoV). The approaches outlined below are intentionally cautious until additional data become available to refine recommendations for prevention of person-to-person transmission in inpatient obstetric care settings.
Prehospital Considerations
• Pregnant patients who have confirmed COVID-19 or who are PUIs should notify the obstetric unit prior to arrival so the facility can make appropriate infection control preparations (e.g., identifying the most appropriate room for labor and delivery, ensuring infection prevention and control supplies and PPE are correctly positioned, informing all healthcare personnel who will be involved in the patient’s care of infection control expectations) before the patient’s arrival.
• If a pregnant patient who has confirmed COVID-19 or is a PUI is arriving via transport by emergency medical services, the driver should contact the receiving emergency department or healthcare facility and follow previously agreed-upon local or regional transport protocols. For more information refer to the Interim Guidance for Emergency Medical Services (EMS) Systems and 911 Public Safety Answering Points (PSAPs) for COVID-19 in the United States.
• Healthcare providers should promptly notify infection control personnel at their facility of the anticipated arrival of a pregnant patient who has confirmed COVID-19 or is a PUI.
During Hospitalization
• Healthcare facilities should ensure recommended infection control practices for hospitalized pregnant patients who have confirmed COVID-19 or are PUIs are consistent with Interim Infection Prevention and Control Recommendations for Patients with Confirmed Coronavirus Disease 2019 (COVID-19) or Persons Under Investigation for COVID-19 in Healthcare Settings.
• All healthcare facilities that provide obstetric care must ensure that their personnel are correctly trained and capable of implementing recommended infection control interventions. Individual healthcare personnel should ensure they understand and can adhere to infection control requirements.
• Healthcare facilities should follow the above infection control guidance on managing visitor access, including essential support persons for women in labor (e.g., spouse, partner).
• Infants born to mothers with confirmed COVID-19 should be considered PUIs. As such, infants should be isolated according to the Infection Prevention and Control Guidance for PUIs.
Mother/Baby Contact
• It is unknown whether newborns with COVID-19 are at increased risk for severe complications. Transmission after birth via contact with infectious respiratory secretions is a concern. To reduce the risk of transmission of the virus that causes COVID-19 from the mother to the newborn, facilities should consider temporarily separating (e.g., separate rooms) the mother who has confirmed COVID-19 or is a PUI from her baby until the mother’s transmission-based precautions are discontinued, as described in the Interim Considerations for Disposition of Hospitalized Patients with COVID-19. See the considerations below for temporary separation:
○ The risks and benefits of temporary separation of the mother from her baby should be discussed with the mother by the healthcare team.
○ A separate isolation room should be available for the infant while they remain a PUI. Healthcare facilities should consider limiting visitors, with the exception of a healthy parent or caregiver. Visitors should be instructed to wear appropriate PPE, including gown, gloves, face mask, and eye protection. If another healthy family or staff member is present to provide care (e.g., diapering, bathing) and feeding for the newborn, they should use appropriate PPE. For healthy family members, appropriate PPE includes gown, gloves, face mask, and eye protection. For healthcare personnel, recommendations for appropriate PPE are outlined in the Infection Prevention and Control Recommendations.
○ The decision to discontinue temporary separation of the mother from her baby should be made on a case-by-case basis in consultation with clinicians, infection prevention and control specialists, and public health officials. The decision should take into account disease severity, illness signs and symptoms, and results of laboratory testing for the virus that causes COVID-19, SARS-CoV-2. Considerations to discontinue temporary separation are the same as those to discontinue transmission-based precautions for hospitalized patients with COVID-19. Please see Interim Considerations for Disposition of Hospitalized Patients with COVID-19.
○ If colocation (sometimes referred to as “rooming in”) of the newborn with his/her ill mother in the same hospital room occurs in accordance with the mother’s wishes or is unavoidable due to facility limitations, facilities should consider implementing measures to reduce exposure of the newborn to the virus that causes COVID-19.
■ Consider using engineering controls like physical barriers (e.g., a curtain between the mother and newborn) and keeping the newborn ≥6 feet away from the ill mother.
■ If no other healthy adult is present in the room to care for the newborn, a mother who has confirmed COVID-19 or is a PUI should put on a facemask and practice hand hygiene1 before each feeding or other close contact with her newborn. The facemask should remain in place during contact with the newborn. These practices should continue while the mother is on transmission-based precautions in a healthcare facility.
Breastfeeding
• During temporary separation, mothers who intend to breastfeed should be encouraged to express their breast milk to establish and maintain milk supply. If possible, a dedicated breast pump should be provided. Prior to expressing breast milk, mothers should practice hand hygiene.1 After each pumping session, all parts that come into contact with breast milk should be thoroughly washed and the entire pump should be appropriately disinfected per the manufacturer’s instructions. This expressed breast milk should be fed to the newborn by a healthy caregiver.
• If a mother and newborn do room-in and the mother wishes to feed at the breast, she should put on a facemask and practice hand hygiene before each feeding.
Hospital Discharge
• Discharge for postpartum women should follow recommendations described in the Interim Considerations for Disposition of Hospitalized Patients with COVID-19.
• For infants with pending testing results or who test negative for the virus that causes COVID-19 upon hospital discharge, caretakers should take steps to reduce the risk of transmission to the infant, including following the Interim Guidance for Preventing Spread of Coronavirus Disease 2019 (COVID-19) in Homes and Residential Communities.
Footnote:
1 Hand hygiene includes use of alcohol-based hand sanitizer that contains 60% to 95% alcohol before and after all patient contact, contact with potentially infectious material, and before putting on and upon removal of PPE, including gloves. Hand hygiene can also be performed by washing with soap and water for at least 20 seconds. If hands are visibly soiled, use soap and water before returning to alcohol-based hand sanitizer.
What Healthcare Personnel Should Know about Caring for Patients with Confirmed or Possible COVID-19 Infection
Healthcare personnel (HCP) are on the front lines of caring for patients with confirmed or possible infection with coronavirus disease 2019 (COVID-19) and therefore have an increased risk of exposure to this virus. HCPs can minimize their risk of exposure when caring for confirmed or possible COVID-19 patients by following Interim Infection Prevention and Control Recommendations for Patients with Confirmed Coronavirus Disease 2019 (COVID-19) or Persons Under Investigation for COVID-19 in Healthcare Settings.
How COVID-19 Spreads
There is much to learn about the newly emerged COVID-19, including how and how easily it spreads. Based on what is currently known about COVID-19 and what is known about other coronaviruses, spread is thought to occur mostly from person-to-person via respiratory droplets among close contacts.
Close contact can occur while caring for a patient, including:
• being within approximately 6 feet (2 meters) of a patient with COVID-19 for a prolonged period of time.
• having direct contact with infectious secretions from a patient with COVID-19. Infectious secretions may include sputum, serum, blood, and respiratory droplets.
If close contact occurs while not wearing all recommended PPE, healthcare personnel may be at risk of infection.
How You Can Protect Yourself
Healthcare personnel caring for patients with confirmed or possible COVID-19 should adhere to CDC recommendations for infection prevention and control (IPC):
• Assess and triage these patients with acute respiratory symptoms and risk factors for COVID-19 to minimize chances of exposure, including placing a facemask on the patient and placing them in an examination room with the door closed.
• Use Standard and Transmission-Based Precautions when caring for patients with confirmed or possible COVID-19.
• Perform hand hygiene with alcohol-based hand rub before and after all patient contact, contact with potentially infectious material, and before putting on and upon removal of PPE, including gloves. Use soap and water if hands are visibly soiled.
• Practice how to properly in a manner to prevent self-contamination.
• Perform aerosol-generating procedures, in an AIIR, while following appropriate IPC practices, including use of appropriate PPE.
Environmental Cleaning and Disinfection
Routine cleaning and disinfection procedures are appropriate for SARS-CoV-2 in healthcare settings, including those patient-care areas in which aerosol-generating procedures are performed. Products with EPA-approved emerging viral pathogens claims are recommended for use against SARS-CoV-2. Management of laundry, food service utensils, and medical waste should also be performed in accordance with routine procedures.
When to Contact Occupational Health Services
If you have an unprotected exposure (i.e., not wearing recommended PPE) to a confirmed or possible COVID-19 patient, contact your supervisor or occupational health immediately.
If you develop symptoms consistent with COVID-19 (fever, cough, or difficulty breathing), do not report to work. Contact your occupational health services.
For more information for healthcare personnel, visit: https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html
Strategies for Optimizing the Supply of N95 Respirators
Summary of Changes
• Clarification of introductory language
• Information added on Crisis/Alternative Strategies
• Information added to expand upon strategies, including two new resources:
○ Checklist for Healthcare Facilities: Strategies for Optimizing the Supply of N95 Respirators during the COVID-19 Response
○ Release of Stockpiled N95 Filtering Facepiece Respirators Beyond the Manufacturer-Designated Shelf Life: Considerations for the COVID-19 Response
Audience: These considerations are intended for use by federal, state, and local public health officials, respiratory protection program managers, occupational health service leaders, infection prevention and control program leaders, and other leaders in healthcare settings who are responsible for developing and implementing policies and procedures for preventing pathogen transmission in healthcare settings.
Purpose: This document offers a series of strategies or options to optimize supplies of disposable N95 filtering facepiece respirators (commonly called “N95 respirators”) in healthcare settings when there is limited supply. It does not address other aspects of pandemic planning; for those, healthcare settings can refer to existing influenza preparedness plans to address other aspects of preparing to respond to novel coronavirus disease 2019 (COVID-19). The strategies are also listed in order of priority and preference in the Checklist for Healthcare Facilities: Strategies for Optimizing the Supply of N95 Respirators during the COVID-19 Response in an easy-to-use format for healthcare facilities.
The following strategies are based upon these assumptions: 1) facilities understand their current N95 respirator inventory and supply chain, 2) facilities understand their N95 respirators utilization rate, and 3) facilities are in communication with state and local public health partners (e.g., public health emergency preparedness and response staff) and healthcare coalitions. While these strategies are targeted for optimizing the supply of N95 respirators, some of these strategies may be applicable to optimizing the supply of other personal protective equipment such as gowns, gloves, and eye protection.
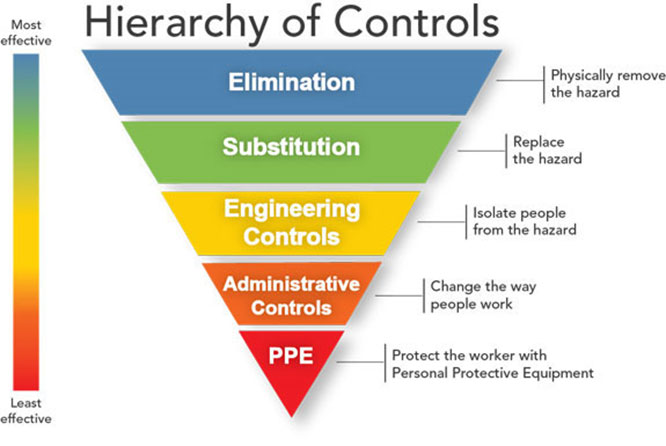
Controlling exposures to occupational hazards is a fundamental way to protect personnel. Conventionally, a hierarchy has been used to achieve feasible and effective controls. Multiple control strategies can be implemented concurrently and or sequentially. This hierarchy can be represented as follows:
• Elimination
• Substitution
• Engineering controls
• Administrative controls
• Personal protective equipment (PPE)
To prevent infectious disease transmission, elimination (physically removing the hazard) and substitution (replacing the hazard) are not typically options for the healthcare setting. However, exposures to transmissible respiratory pathogens in healthcare facilities can often be reduced or possibly avoided through engineering and administrative controls and PPE. Prompt detection and effective triage and isolation of potentially infectious patients are essential to prevent unnecessary exposures among patients, healthcare personnel (HCP), and visitors at the facility.
N95 respirators are the PPE most often used to control exposures to infections transmitted via the airborne route, though their effectiveness is highly dependent upon proper fit and use. The optimal way to prevent airborne transmission is to use a combination of interventions from across the hierarchy of controls, not just PPE alone. Applying a combination of controls can provide an additional degree of protection, even if one intervention fails or is not available.
Respirators, when required to protect HCP from airborne contaminants such as infectious agents, must be used in the context of a comprehensive, written respiratory protection program that meets the requirements of OSHA’s Respiratory Protection standard. The program should include medical evaluations, training, and fit testing.
Surge capacity refers to the ability to manage a sudden, unexpected increase in patient volume that would otherwise severely challenge or exceed the present capacity of a facility. While there are no commonly accepted measurements or triggers to distinguish surge capacity from daily patient care capacity, surge capacity is a useful framework to approach a decreased supply of N95 respirators during the COVID-19 response. Three general strata have been used to describe surge capacity and can be used to prioritize measures to conserve N95 respirator supplies along the continuum of care.1
• Conventional capacity: measures consist of providing patient care without any change in daily contemporary practices. This set of measures, consisting of engineering, administrative, and PPE controls should already be implemented in general infection prevention and control plans in healthcare settings.
• Contingency capacity: measures may change daily contemporary practices but may not have any significant impact on the care delivered to the patient or the safety of the HCP. These practices may be used temporarily when demands exceed resources.
• Crisis capacity: alternate strategies that are not commensurate with contemporary U.S. standards of care. These measures, or a combination of these measures, may need to be considered during periods of expected or known N95 respirator shortages.
Decisions to implement measures in contingency capacity and then crisis capacity should be based on:
• Consideration of all conventional capacity strategies first.
• The availability of N95 respirators and other types of respiratory protection.
• Consultation with entities that include some combination of: local healthcare coalitions, federal, state, or local public health officials, appropriate state agencies that are managing the overall emergency response related to COVID-19, and state crisis standards of care committees. Even when state/local coalitions or public health authorities can shift resources between health care facilities, these strategies may still be necessary.
References
1. Hick JL, Barbera JA, Kelen GD. Refining surge capacity: conventional, contingency, and crisis capacity. Disaster Med Public Health Prep. 2009;3(2 Suppl): S59-67.
Strategies for Optimizing the Supply of N95 Respirators: Conventional Capacity Strategies
Conventional Capacity Strategies
In the continuum of care, the following measures can be categorized as conventional capacity, which consists of providing patient care without any change in daily practices. This set of controls should already be implemented in general infection prevention and control plans in healthcare settings.
Engineering Controls
Engineering controls reduce exposures for HCP by placing a barrier between the hazard and the HCP. Engineering controls can be very effective as part of a suite of strategies to protect HCP without placing primary responsibility of implementation on them (i.e., they function without HCP having to take an action).
Isolation in airborne infection isolation room
Patients with known or suspected COVID-19 (i.e., person under investigation [PUI]) should be placed in an airborne infection isolation room (AIIR) that has been constructed and maintained in accordance with current guidelines, as recommended in the Interim Infection Prevention and Control Recommendations for Patients with Confirmed Coronavirus Disease 2019 (COVID-19) or Persons Under Investigation for COVID-19 in Healthcare Settings.
Use of physical barriers
Barriers such as glass/plastic windows can be an effective solution for reducing exposures among HCP to potentially infectious patients. This approach can be effective in reception areas (e.g., intake desk at emergency department, triage station, information booth, pharmacy drop-off/pick-up windows) where patients may first report upon arrival to a healthcare facility. Other examples include the use of curtains between patients in shared areas and closed suctioning systems for airway suctioning for intubated patients.
Properly maintained ventilation systems
Another cornerstone of engineering controls are ventilation systems that provide air movement from a clean (HCP workstation or area) to contaminated (sick patient) flow direction (along with appropriate filtration, exchange rate) that are installed and properly maintained.
Administrative Controls
Administrative controls are employer-dictated work practices and policies that reduce or prevent hazardous exposures. Their effectiveness depends on employer commitment and HCP acceptance and consistent use of the strategies. Regular training, monitoring and reinforcement are necessary to ensure that policies and procedures are followed consistently. Many of these strategies should already be incorporated into existing infection prevention and control policies in healthcare settings.
Limit number of patients going to hospital or outpatient settings
Consider developing mechanisms to screen patients for acute respiratory illness prior to their non-urgent care or elective visits or procedures, such as through the appointment reminder system. Postpone and reschedule those with signs and symptoms presenting for these non-acute visits.
Exclude all HCP not directly involved in patient care
Current CDC guidance recommends that, for COVID-19, only essential personnel enter the patient care area, and that facilities consider caring for these patients with dedicated HCP. Further limiting the numbers of healthcare personnel and patient contacts to those that are medically essential (e.g., excluding dietary personnel, environmental services) could limit the number of respirators used. The medically essential personnel would assume food delivery and environmental services.
Limit face-to-face HCP encounters with patient
Measures can be explored to limit face-to-face contact encounters between HCP and patients with confirmed or suspected COVID-19. HCP may consider bundling care activities to minimize room entries, and bundling may occur across HCP types (e.g., food trays are delivered by HCP performing other care). Alternative mechanisms for HCP and patient interactions include telephones, video monitoring, and video-call applications on cell phones or tablets.
Exclude visitors to patients with known or suspected COVID-19
Restrict visitors from entering the rooms of patients with known COVID-19 or suspected (PUI) COVID-19, as recommended in CDC’s guidance. Alternative mechanisms for patient and visitor interactions, such as video-call applications on cell phones or tablets should be explored. Facilities can consider exceptions based on end-of-life situations or when a visitor is essential for the patient’s emotional well-being and care. If visitors must enter the room of a known or suspected COVID-19 patient, facilities should provide instruction, before visitors enter patients’ rooms on use of PPE according to current facility policy while in the patient’s room.
Source control
Identify and assess patients who may be ill with or who may have been exposed to a person with known COVID-19. Patients with symptoms of suspected COVID-19 or other respiratory infection (e.g., fever, cough) presenting to care should use facemasks for source control until they can be placed in an airborne infection isolation room or a private room. Instructions should include how to use facemasks. Patients with these symptoms should not wear N95 respirators. If these patients need to leave their room for services in other areas of the hospital (e.g., radiology), they should also wear facemasks for source control.
Cohorting patients
Cohorting is the practice of grouping together patients who are infected with the same organism to confine their care to one area and prevent contact with other patients. Cohorts are created based on clinical diagnosis, microbiologic confirmation when available, epidemiology, and mode of transmission of the infectious agent. Cohorting has been used extensively for managing outbreaks of multidrug resistant organisms including MRSA, VRE, MDR-ESBLs, Pseudomonas aeruginosa; methicillin-susceptible Staphylococcus aureus, RSV, adenovirus keratoconjunctivitis, rotavirus, and SARS. When single patient rooms are not available, patients with confirmed COVID-19 may be placed in the same room. Cohorting patients could minimize respirator use when extended wear of respirators is implemented. For more information on cohorting of patients, refer to 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings.
Cohorting HCP
Assigning designated teams of HCP to provide care for all patients with suspected or confirmed COVID-19 could minimize respirator use when extended wear of RPDs is implemented. This strategy can also limit the number of exposed HCP who need to be fit tested.
Telemedicine
Nurse advice lines and telemedicine can screen and manage patients with suspected COVID-19 without the need for the HCP to use respiratory protection. Promoting the use of these technologies and referral networks can help triage persons to the appropriate level of care, potentially reducing the influx of patients to healthcare facilities seeking evaluation.
Training on indications for use of N95 respirators
It is important that HCP be trained on indications for use of N95 respirators. The OSHA Respiratory Protection standard requires employers to provide respirator training prior to requiring an employee to use a respirator in the workplace. For example, HCP should use N95 respirators when caring for patients under airborne precautions for infectious diseases including COVID-19, tuberculosis, measles, and varicella. HCP should generally not need to use N95 respirators when caring for patients under droplet precautions for infectious diseases except under certain circumstances (e.g., aerosol-generating procedures for influenza).
Training on use of N95 respirators
Training employees on the proper use of respirators, including putting on and removing them, limitations on their use, and maintenance is essential for effective use of respiratory protection. HCP should be thoroughly trained before they are fit tested to ensure they are comfortable donning the respirator and know how to conduct a user seal check. HCP should be trained on the respirator they are expecting to use at work.
Just in time fit testing
Just-in-time fit testing refers to the capacity of healthcare facilities to do larger scale evaluation, training, and fit testing of employees when necessary during a pandemic. Facilities may adopt a plan to use the “just-in-time” method for fit testing, which has been incorporated into pandemic plans for many facilities. For large facilities, it may not be feasible to fit test all employees, especially if their job does not typically place them at risk for exposure to airborne infectious diseases such as tuberculosis. If healthcare facilities are expecting to receive COVID-19 patients, they should begin training and start to plan for fit testing now. It is essential to have HCP trained and fit tested prior to receiving patients.
Limiting respirators during training
In order to conserve the supply of N95 respirators, healthcare facilities should understand which of their HCP do and do not need to be in a respiratory protection program and thus medically evaluated, trained, and fit tested. If training and fit testing are conducted during two separate steps, it is possible to allow limited re-use of N95 respirators used by individual HCP during both steps. Employees should be fit tested after they are comfortable donning the respirator and have passed a user seal check. Employees should be trained on the respirator they are expecting to use at work. The respirator can be saved and used for fit testing and patient care.
Qualitative fit testing
Respirator fit test methods are classified as either qualitative or quantitative, and there are multiple protocols of each classification that are NIOSH-recommended or meet the requirements of OSHA’s Respiratory Protection Standard. A qualitative fit test is a pass/fail test to assess the adequacy of respirator fit that relies on the individual’s sensory detection of a test agent. A quantitative fit test numerically measures the effectiveness of the respirator to seal with the wearer’s face, without relying on the wearer’s voluntary or involuntary response to a test agent. Quantitative fit tests involve adaptation of the respirator to the fit testing equipment, which can involve making holes in the respirator.
Many healthcare systems already use qualitative fit test methods for fit testing HCP. For those using quantitative fit test methods, considerations can be made to use qualitative fit test methods to minimize the destruction of an N95 respirator used in fit testing and allow for the re-use of the same N95 respirator by the HCP. Qualitative fit methods may also allow for rapid fit testing of larger numbers of HCP. Any switch in methods should be assessed to ensure proficiency of the fit testers in carrying out the test.
Personal Protective Equipment and Respiratory Protection
While engineering and administrative controls should be considered first when selecting controls, the use of personal protective equipment (PPE) should also be part of a suite of strategies used to protect personnel. Proper use of respiratory protection by HCP requires a comprehensive program (including medical clearance, training, and fit testing) that complies with OSHA’s Respiratory Protection Standard and a high level of HCP involvement and commitment. The program should also include provisions for the cleaning, disinfecting, inspection, repair, and storage of respirators used by workers on the job. Proper storage conditions can maximize shelf life of respirators. The following strategies in this section are traditionally used by some healthcare systems. If not already implemented, these strategies can be considered by healthcare settings in the face of a potential N95 respirator shortage before implementing the contingency strategies that are listed further below.
Surgical N95 respirators
Surgical N95 respirators (sometimes called medical respirators) are recommended only for use by HCP who need protection from both airborne and fluid hazards (e.g., splashes, sprays). These respirators are approved by NIOSH and regulated by the FDA and are not used or needed outside of healthcare settings. In times of shortage, only HCP who are working in a sterile field or who may be exposed to high velocity splashes, sprays, or splatters of blood or body fluids should be provided these respirators. Other HCP can use standard N95 respirators. If surgical N95 respirators are not available, and there is a risk that the worker may be exposed to high velocity splashes, sprays, or splatters of blood or body fluids, then a faceshield should be worn over the standard N95 respirator.
Use of alternatives to N95 respirators
Use alternatives to N95 respirators where feasible. These include other classes of filtering facepiece respirators, elastomeric half-mask and full facepiece air purifying respirators, powered air purifying respirators (PAPRs) where feasible. All of these alternatives will provide equivalent or higher protection than N95 respirators when properly worn. NIOSH maintains a searchable, online version of the certified equipment list identifying all NIOSH-approved respirators.
NIOSH approves other filtering facepiece respirators that are at least as protective as the N95. These include N99, N100, P95, P99, P100, R95, R99, and R100.
Elastomeric respirators are half-facepiece, tight-fitting respirators that are made of synthetic or rubber material permitting them to be repeatedly disinfected, cleaned, and reused. They are equipped with exchangeable filter cartridges. Similar to N95 respirators, elastomeric respirators require annual fit testing. Elastomeric respirators should not be used in surgical settings due to concerns that air coming out of the exhalation valve may contaminate the sterile field.
PAPRs are reusable respirators that are typically loose-fitting hoods or helmets. These respirators are battery-powered with blower that pulls air through attached filters or cartridges. The filter is typically a high-efficiency particulate air (HEPA) filter. Loose-fitting PAPRs do not require fit-testing and can be worn by people with facial hair. However, PAPRs should not be used in surgical settings due to concerns that the blower exhaust and exhaled air may contaminate the sterile field.
Facilities using elastomeric respirators and PAPRs should have up to date cleaning/disinfection procedures, which are an essential part of use for protection against infectious agents.
Strategies for Optimizing the Supply of N95 Respirators: Contingency Capacity Strategies
Contingency Capacity Strategies
In the continuum of care, the following measures can be categorized as contingency capacity, which may change daily practices but may not have any significant impact on the care delivered to the patient or the safety of the HCP. The following measures may be considered in the setting of a potential impending shortage of N95 respirators. The decision to implement these practices should be made on a case by case basis taking into account known characteristics of the SARS-CoV-2 and local conditions (e.g., number of disposable N95 respirators available, current respirator usage rate, success of other respirator conservation strategies, etc.).
Administrative Controls
Decrease length of hospital stay for medically stable patients with COVID-19
Currently, CDC recommends discharge of patients with confirmed COVID-19 when they are medically stable and have an appropriate home environment to which to return. CDC lists considerations for care at home in: Interim Guidance for Implementing Home Care of People Not Requiring Hospitalization for Coronavirus Disease 2019 (COVID-19). If patients cannot be discharged to home for social rather than medical reasons, public health officials might need to identify alternative non-hospital housing where those patients can convalesce.
Personal Protective Equipment and Respiratory Protection
Use of N95 respirators beyond the manufacturer-designated shelf life for training and fit testing
In times of shortage, consideration can be made to use N95 respirators beyond the manufacturer-designated shelf life. However, expired respirators might not perform to the requirements for which they were certified. Over time, components such as the strap and material may degrade, which can affect the quality of the fit and seal. Because of this, use of expired respirators could be prioritized for situations where HCP are NOT exposed to pathogens, such as training and fit testing. As expired respirators can still serve an important purpose, healthcare facilities should retain all N95 respirators during the early phases of this outbreak.
Extended use of N95 respirators
Practices allowing extended use of N95 respirators, when acceptable, can also be considered. The decision to implement policies that permit extended use of N95 respirators should be made by the professionals who manage the institution’s respiratory protection program, in consultation with their occupational health and infection control departments with input from the state/local public health departments. CDC has recommended guidance on implementation of extended use of N95 respirators in healthcare settings. Extended use has been recommended and widely used as an option for conserving respirators during previous respiratory pathogen outbreaks and pandemics.
Extended use refers to the practice of wearing the same N95 respirator for repeated close contact encounters with several different patients, without removing the respirator between patient encounters. Extended use is well suited to situations wherein multiple patients with the same infectious disease diagnosis, whose care requires use of a respirator, are cohorted (e.g., housed on the same hospital unit). It can also be considered to be used for care of patients with tuberculosis, varicella, and measles.
Limited re-use of N95 respirators for tuberculosis
Re-use refers to the practice of using the same N95 respirator by one HCP for multiple encounters with different patients but removing it (i.e. doffing) after each encounter. This practice is often referred to as “limited reuse” because restrictions are in place to limit the number of times the same respirator is reused. It is important to consult with the respirator manufacturer regarding the maximum number of donnings or uses they recommend for the N95 respirator model. If no manufacturer guidance is available, data suggests limiting the number of reuses to no more than five uses per device to ensure an adequate safety margin.1 N95 and other disposable respirators should not be shared by multiple HCP. CDC has recommended guidance on implementation of limited re-use of N95 respirators in healthcare settings.
For pathogens for which contact transmission is not a concern, routine limited reuse of single-use disposable respirators has been practiced for decades. For example, for tuberculosis prevention, a respirator classified as disposable can be reused by the same provider as long as the respirator maintains its structural and functional integrity. To extend the supply of N95 respirators during an anticipated dwindling supply, HCP could be encouraged to reuse their N95 respirators when caring for patients with tuberculosis disease.
To maintain the integrity of the respirator, it is important for HCP to hang used respirators in a designated storage area or keep them in a clean, breathable container such as a paper bag between uses. It is not recommended to modify the N95 respirator by placing any material within the respirator or over the respirator. Modification may negatively affect the performance of the respirator and could void the NIOSH approval.
References
1. Hick JL, Barbera JA, Kelen GD. Refining surge capacity: conventional, contingency, and crisis capacity. Disaster Med Public Health Prep. 2009;3(2 Suppl): S59-67.
Strategies for Optimizing the Supply of N95 Respirators: Crisis/Alternate Strategies
Crisis/Alternate Strategies
These crisis capacity or alternate strategies accompany and build on the conventional and contingency capacity strategies. The following measures are not commensurate with current U.S. standards of care. However, individual measures or a combination of these measures may need to be considered during periods of expected or known N95 respirator shortages. It is important to consult with entities that include some combination of: local healthcare coalitions, federal, state, or local public health officials, appropriate state agencies that are managing the overall emergency response related to COVID-19, and state crisis standards of care committees. Even when state/local healthcare coalitions or public health authorities can shift resources between health care facilities, these strategies may still be necessary.
When N95 Supplies are Running Low
Personal Protective Equipment and Respiratory Protection
Use of respirators beyond the manufacturer-designated shelf life for healthcare delivery
Consideration can be made to use N95 respirators beyond the manufacturer-designated shelf life for care of patients with COVID-19, tuberculosis, measles, and varicella. However, respirators beyond the manufacturer-designated shelf life may not perform to the requirements for which they were certified. Over time, components such as the straps and nose bridge material may degrade, which can affect the quality of the fit and seal. Many models found in U.S. stockpiles and stockpiles of healthcare facilities have been found to continue to perform in accordance with NIOSH performance standards. However, fluid resistance and flammability were not assessed. Use of the N95 respirators recommended in Release of Stockpiled N95 Filtering Facepiece Respirators Beyond the Manufacturer-Designated Shelf Life: Considerations for the COVID-19 Response can be considered. It is optimal to use these respirators in the context of a respiratory protection program that includes medical evaluation, training, and fit testing. If used in healthcare delivery, it is particularly important that HCP perform the expected seal check, prior to entering a patient care area. CDC does not recommend using N95s beyond the manufacturer-designated shelf life in surgical settings.
Use of respirators approved under standards used in other countries that are similar to NIOSH-approved N95 respirators
Other countries approve respirators for occupational use and approve respirators to these standards. These products are evaluated using some methods similar to those used by NIOSH, and some methods that are different, but are expected to protect HCPs. These respirators are expected to provide protection to workers. Those with equivalent or similar protection to NIOSH-approved respirators may be available to provide respiratory protection to workers exposed to harmful airborne particulate matter. These devices are expected to be suitable alternatives to provide protection during the COVID-19 response when supplies are short. The country, conformity assessment standards, acceptable product classifications, standards and guidance documents, and protection factor determination are provided in alphabetical order. All of these respirators have protection factors of at least 10 in the countries listed below, as outlined in the standards and guidance documents specified.
|
Country
|
Performance Standard
|
Acceptable product classifications
|
Standards/Guidance Documents
|
Protection Factor ≥ 10
|
|
Australia
|
AS/NZS 1716:2012
|
P3
P2
|
AS/NZS 1715:2009
|
YES
|
|
Brazil
|
ABNT/NBR 13694:1996 and 13697:2010
|
P3
P2
|
Fundacentro CDU 614.894
|
YES
|
|
China
|
GB 2626-2006
|
KN 100 KP100
KN95 KP95
|
GB/T 18664—2002
|
YES
|
|
Europe
|
EN 149-2001
|
FFP3
FFP2
|
EN 529:2005
|
YES
|
|
Japan
|
JMHLW-2000
|
DS/DL3
DS/DL2
|
JIS T8150: 2006
|
YES
|
|
Korea
|
KMOEL-2017-64
|
Special
1st
|
KOSHA GUIDE H-82-2015
|
YES
|
|
Mexico
|
NOM-116-2009
|
N100, P100, R100
N99, P99, R99
N95, P95, R95
|
NOM-116
|
YES
|
|
US NIOSH Requirements
|
NIOSH approved
42 CFR 84
|
N100, P100, R100
N99, P99, R99
N95, P95, R95
|
OSHA 29CFR1910.134
|
YES
|
Limited re-use of N95 respirators for COVID-19 patients
Limited re-use of N95 respirators when caring for patients with COVID-19 might become necessary. However, it is unknown what the potential contribution of contact transmission is for SARS-CoV-2, and caution should be used. Re-use should be implemented according to CDC guidance. Re-use has been recommended as an option for conserving respirators during previous respiratory pathogen outbreaks and pandemics. It may also be necessary to re-use N95 respirators when caring for patients with varicella or measles, although contact transmission poses a risk to HCP who implement this practice.
Use of additional respirators beyond the manufacturer-designated shelf life for healthcare delivery
Use of additional N95 respirators beyond the manufacturer-designated shelf life for care of patients with COVID-19, tuberculosis, measles, and varicella can be considered. However, respirators beyond the manufacturer-designated shelf life may not perform to the requirements for which they were certified. Over time, components such as the straps and nose bridge material may degrade, which can affect the quality of the fit and seal. Some models have been found NOT to perform in accordance with NIOSH performances standards, and consideration may be given to use these respirators as identified in Release of Stockpiled N95 Filtering Facepiece Respirators Beyond the Manufacturer-Designated Shelf Life: Considerations for the COVID-19 Response. In addition, consideration can be given to use N95 respirators beyond the manufacturer-designated shelf life that have not been evaluated by NIOSH. It is optimal to use these respirators in the context of a respiratory protection program that includes medical evaluation, training, and fit testing. It is particularly important that HCP perform the expected seal check, prior to entering a patient care area.
Prioritize the use of N95 respirators and facemasks by activity type
The number of infectious particles required to cause an infection (infectious dose) is often uncertain or unknown for respiratory pathogens. Further, there is often uncertainty about the influence of factors such as exposure duration and nature of clinical symptoms on the likelihood of infection transmission from person-to-person. When facemasks must be used by HCP entering a patient care area, source control (i.e. masking of symptomatic patients) and maintaining distance from the patient are particularly important to reduce the risk of transmission.
This prioritization approach to conservation is intended to be used when N95 respirators are so limited that routinely practiced standards of care for all HCP wearing N95 respirators when caring for a COVID-19 patient are no longer possible. N95 respirators beyond their manufacture-designated shelf life, when available, are preferable to use of facemasks. The use of N95s or elastomeric respirators or PAPRs should be prioritized for HCP with the highest potential exposures including being present in the room during aerosol generating procedures performed on symptomatic persons.
Suggested facemask or respirator use, based upon distance from a patient with suspected or known COVID-19 and use of source control*
|
HCP planned proximity to the case patient during encounter
|
Facemask or respirator determination
|
|
Patient masked for entire encounter (i.e., with source control)
|
Unmasked patient or mask needs to be removed for any period of time during the patient encounter
|
|
HCP will remain at greater than 6 feet from symptomatic patient
|
HCP remaining at this distance from the patient should not need to enter the patient care area; if entry required: no facemask or respirator
|
HCP remaining at this distance from the patient should not need to enter the patient care area; if entry required: no facemask or respirator
|
|
HCP will be within 3 to 6 feet of symptomatic patient
|
HCP remaining at this distance from the patient should not need to enter the patient care area; if entry required: facemask
|
HCP remaining at this distance from the patient should not need to enter the patient care area; if entry required: facemask
|
|
HCP will be within 3 feet of symptomatic patient, including providing direct patient care
|
Facemask
|
N95 respirator/ elastomeric /PAPR, based on availability
|
|
HCP will be present in the room during aerosol generating procedures performed on symptomatic persons
|
N95 respirator/ elastomeric /PAPR, based on availability
|
N95 respirator/ elastomeric /PAPR, based on availability
|
|
* Based on availability, organizations may require and/or individuals may voluntarily choose to utilize higher levels of protection
|
When No Respirators are Left
Administrative Controls
Exclude HCP at higher risk for severe illness from COVID-19 from contact with known or suspected COVID-19 patients
During severe resource limitations, consider excluding HCP who may be at higher risk for severe illness from COVID-19, such as those of older age, those with chronic medical conditions, or those who may be pregnant, from caring for patients with confirmed or suspected COVID-19 infection.
Designate convalescent HCP for provision of care to known or suspected COVID-19 patients
It may be possible to designate HCP who have clinically recovered from COVID-19 to preferentially provide care for additional patients with COVID-19. Individuals who have recovered from COVID-19 infection may have developed some protective immunity, but this has not yet been confirmed.
Engineering Controls
Expedient patient isolation rooms for risk-reduction
Portable fan devices with high-efficiency particulate air (HEPA) filtration that are carefully placed can increase the effective air changes per hour of clean air to the patient room, reducing risk to individuals entering the room without respiratory protection. NIOSH has developed guidance for using portable HEPA filtration systems to create expedient patient isolation rooms. The expedient patient isolation room approach involves establishing a high-ventilation-rate, negative pressure, inner isolation zone that sits within a “clean” larger ventilated zone. In the absence of any remaining supply of N95 respirators, it may be possible to use this technology in conjunction with HCP wearing facemasks.
Ventilated Headboards
NIOSH has developed the ventilated headboard that draws exhaled air from a patient in bed into a HEPA filter, decreasing risk of HCP exposure to patient-generated aerosol. This technology consists of lightweight, sturdy, and adjustable aluminum framing with a retractable plastic canopy. The ventilated headboard can be deployed in combination with HEPA fan/filter units to provide surge isolation capacity within a variety of environments, from traditional patient rooms to triage stations, and emergency medical shelters. In the absence of any remaining supply of N95 respirators, it may be possible to use this technology in conjunction with HCP and/or patients wearing facemasks.
Personal Protective Equipment and Respiratory Protection
HCP use of non-NIOSH approved masks or homemade masks
In settings where N95 respirators are so limited that routinely practiced standards of care for wearing N95 respirators and equivalent or higher level of protection respirators are no longer possible, and surgical masks are not available, as a last resort, it may be necessary for HCP to use masks that have never been evaluated or approved by NIOSH or homemade masks. It may be considered to use these masks for care of patients with COVID-19, tuberculosis, measles, and varicella. However, caution should be exercised when considering this option.1,2
References
1. Dato, VM, Hostler, D, and Hahn, ME. Simple Respiratory Mask, Emerg Infect Dis. 2006; 12(6): 1033–1034.
2. Rengasamy S, Eimer B, and Shaffer R. Simple respiratory protection-evaluation of the filtration performance of cloth masks and common fabric materials against 20-1000 nm size particles, Ann Occup Hyg. 2010;54(7):789-98.
Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease (COVID-19)
Summary of Recent Changes
Update: This Interim Guidance was updated on March 7, 2020 to make the following changes:
○ Updating recommendations regarding HCP contact tracing, monitoring, and work restrictions in selected circumstances. These include allowances for asymptomatic HCP who have had an exposure to a COVID-19 patient to continue to work after options to improve staffing have been exhausted and in consultation with their occupational health program. (See Additional Considerations and Recommendations at the end of the document)
○ Removed requirement under “self monitoring with delegated supervision” for healthcare facilities to actively verify absence of fever and respiratory symptoms when healthcare personnel (HCP) report for work. This is now optional.
○ Simplified risk exposure categories based on most common scenarios with focus on presence/absence of source control measures; use of personal protective equipment (PPE) by HCP; and degree of contact with the patient (i.e., prolonged versus brief)
○ Added language advising HCP to inform their occupational health program if they have travel or community-associated exposures as defined in Interim US Guidance for Risk Assessment and Public Health Management of Persons with Potential Coronavirus Disease (COVID-19) Exposure in Travel-associated or Community Settings.
Background
Coronaviruses are a large family of viruses that are common in humans and in many different species of animals, including camels, cattle, cats, and bats. Rarely, animal coronaviruses can infect people and then spread between people such as with SARS-CoV, MERS-CoV, and now with SARS-CoV-2.
Published and early reports suggest spread from person-to-person most frequently happens during close exposure to a person infected with COVID-19. Person-to-person appears to occur similar to other respiratory viruses, mainly via respiratory droplets produced when an infected person coughs or sneezes. These droplets can land in the mouths, noses, or eyes of people who are nearby or possibly be inhaled into the lungs. Although not likely to be the predominant mode of transmission, it is not clear the extent to which touching a surface contaminated with the virus and then touching the mouth, nose, or eyes contributes to transmission.
Purpose
This interim guidance is intended to assist with assessment of risk, monitoring, and work restriction decisions for HCP with potential exposure to COVID-19. For guidance on assessment and management of exposure risk in non-healthcare settings, refer to the Interim US Guidance for Risk Assessment and Public Health Management of Persons with Potential Coronavirus Disease (COVID-19) Exposure in Travel-associated or Community Settings. The guidance for non-healthcare settings can also be used to identify the movement, public activity and travel restrictions that apply to the HCP included here.
Because of their often extensive and close contact with vulnerable individuals in healthcare settings, a conservative approach to HCP monitoring and restriction from work was taken to quickly identify early symptoms and prevent transmission from potentially contagious HCP to patients, HCP, and visitors. The signs and symptoms* described in this guidance are broader than those described when assessing exposures for individuals not working in healthcare. Healthcare facilities should have a low threshold for evaluating symptoms and testing symptomatic HCP, particularly those who fall into the high- and medium- risk categories described in this guidance.
This guidance is based on currently available data about COVID-19. Recommendations regarding which HCP are restricted from work may not anticipate every potential scenario and will change if indicated by new information.
Healthcare facilities, in consultation with public health authorities, should use clinical judgement as well as the principles outlined in this guidance to assign risk and determine need for work restrictions. CDC remains available for further consultation by calling the Emergency Operations Center at 770-488-7100. This cautious approach will be refined and updated as more information becomes available and as response needs change in the United States.
Other Resources
For guidance on risk assessment and public health management of persons not working in a U.S. healthcare setting refer to: Interim US Guidance for Risk Assessment and Public Health Management of Persons with Potential Coronavirus Disease (COVID-19) Exposure in Travel-associated or Community Settings.
For infection prevention and control guidance for healthcare settings caring for Persons with Known or Under Investigation (PUI) for Coronavirus Disease (COVID-19), refer to the Interim Infection Prevention and Control Recommendations for Patients with Known or Patients Under Investigation for Coronavirus Disease (COVID-19) in a Healthcare Setting.
I. Definitions Used in this Guidance
Self-monitoring means HCP should monitor themselves for fever by taking their temperature twice a day and remain alert for respiratory symptoms (e.g., cough, shortness of breath, sore throat)*. Anyone on self-monitoring should be provided a plan for whom to contact if they develop fever or respiratory symptoms during the self-monitoring period to determine whether medical evaluation is needed.
Active monitoring means that the state or local public health authority assumes responsibility for establishing regular communication with potentially exposed people to assess for the presence of fever or respiratory symptoms (e.g., cough, shortness of breath, sore throat)*. For HCP with high- or medium-risk exposures, CDC recommends this communication occurs at least once each day. The mode of communication can be determined by the state or local public health authority and may include telephone calls or any electronic or internet-based means of communication.
For HCP, active monitoring can be delegated by the health department to the HCP’s healthcare facility occupational health or infection control program, if both the health department and the facility are in agreement. Note, inter-jurisdictional coordination will be needed if HCP live in a different local health jurisdiction than where the healthcare facility is located.
Self-Monitoring with delegated supervision in a healthcare setting means HCP perform self-monitoring with oversight by their healthcare facility’s occupational health or infection control program in coordination with the health department of jurisdiction, if both the health department and the facility are in agreement. On days HCP are scheduled to work, healthcare facilities could consider measuring temperature and assessing symptoms prior to starting work. Alternatively, a facility may consider having HCP report temperature and absence of symptoms to occupational health prior to starting work. Modes of communication may include telephone calls or any electronic or internet-based means of communication.
Occupational health or infection control personnel should establish points of contact between the organization, the self-monitoring personnel, and the local or state health departments of authority in the location where self-monitoring personnel will be during the self-monitoring period. This communication should result in agreement on a plan for medical evaluation of personnel who develop fever or respiratory symptoms (e.g., cough, shortness of breath, sore throat)* during the self-monitoring period. The plan should include instructions for notifying occupational health and the local public health authority, and transportation arrangements to a designated hospital, if medically necessary, with advance notice if fever or respiratory symptoms occur. The supervising organization should remain in contact with HCP through the self-monitoring period to manage self-monitoring activities and provide timely and appropriate follow-up if symptoms occur in a HCP. Note, inter-jurisdictional coordination will be needed if HCP live in a different local health jurisdiction than where the healthcare facility is located.
Close contact for healthcare exposures is defined as follows: a) being within approximately 6 feet (2 meters), of a person with COVID-19 for a prolonged period of time (such as caring for or visiting the patient; or sitting within 6 feet of the patient in a healthcare waiting area or room); or b) having unprotected direct contact with infectious secretions or excretions of the patient (e.g., being coughed on, touching used tissues with a bare hand).
Data are limited for definitions of close contact. Factors for consideration include the duration of exposure (e.g., longer exposure time likely increases exposure risk), clinical symptoms of the patient (e.g., coughing likely increases exposure risk) and whether the patient was wearing a facemask (which can efficiently block respiratory secretions from contaminating others and the environment), PPE used by personnel, and whether aerosol-generating procedures were performed.
Data are insufficient to precisely define the duration of time that constitutes a prolonged exposure. However, until more is known about transmission risks, it is reasonable to consider an exposure greater than a few minutes as a prolonged exposure. Brief interactions are less likely to result in transmission; however, clinical symptoms of the patient and type of interaction (e.g., did the patient cough directly into the face of the HCP) remain important. Recommendations will be updated as more information becomes available.
Risk stratification can be made in consultation with public health authorities. Examples of brief interactions include: briefly entering the patient room without having direct contact with the patient or their secretions/excretions, brief conversation at a triage desk with a patient who was not wearing a facemask. See Table 1 for more detailed information.
Healthcare Personnel: For the purposes of this document HCP refers to all paid and unpaid persons serving in healthcare settings who have the potential for direct or indirect exposure to patients or infectious materials, including body substances; contaminated medical supplies, devices, and equipment; contaminated environmental surfaces; or contaminated air. For this document, HCP does not include clinical laboratory personnel.
II. Defining Exposure Risk Category
While body fluids other than respiratory secretions have not been clearly implicated in transmission of COVID-19, unprotected contact with other body fluids, including blood, stool, vomit, and urine, might put HCP at risk of COVID-19.
Table 1 describes possible scenarios that can be used to assist with risk assessment. These scenarios do not cover all potential exposure scenarios and should not replace an individual assessment of risk for the purpose of clinical decision making or individualized public health management. Any public health decisions that place restrictions on an individual’s or group’s movements or impose specific monitoring requirements should be based on an assessment of risk for the individual or group. Healthcare facilities, in consultation with public health authorities should use the concepts outlined in this guidance along with clinical judgement to assign risk and determine need for work restrictions.
For this guidance high-risk exposures refer to HCP who have had prolonged close contact with patients with COVID-19 who were not wearing a facemask while HCP nose and mouth were exposed to material potentially infectious with the virus causing COVID-19. Being present in the room for procedures that generate aerosols or during which respiratory secretions are likely to be poorly controlled (e.g., cardiopulmonary resuscitation, intubation, extubation, bronchoscopy, nebulizer therapy, sputum induction) on patients with COVID-19 when the healthcare providers’ eyes, nose, or mouth were not protected, is also considered high-risk.
Medium-risk exposures generally include HCP who had prolonged close contact with patients with COVID-19 who were wearing a facemask while HCP nose and mouth were exposed to material potentially infectious with the virus causing COVID-19. Some low-risk exposures are considered medium-risk depending on the type of care activity performed. For example, HCP who were wearing a gown, gloves, eye protection and a facemask (instead of a respirator) during an aerosol-generating procedure would be considered to have a medium-risk exposure. If an aerosol-generating procedure had not been performed, they would have been considered low-risk. See Table 1 for additional examples.
Low-risk exposures generally refer to brief interactions with patients with COVID-19 or prolonged close contact with patients who were wearing a facemask for source control while HCP were wearing a facemask or respirator. Use of eye protection, in addition to a facemask or respirator would further lower the risk of exposure.
Proper adherence to currently recommended infection control practices, including all recommended PPE, should protect HCP having prolonged close contact with patients infected with COVID-19. However, to account for any inconsistencies in use or adherence that could result in unrecognized exposures HCP should still perform self-monitoring with delegated supervision.
HCP with no direct patient contact and no entry into active patient management areas who adhere to routine safety precautions do not have a risk of exposure to COVID-19 (i.e., they have no identifiable risk.)
Currently, this guidance applies to HCP with potential exposure in a healthcare setting to patients with confirmed COVID-19. However, HCP exposures could involve a PUI who is awaiting testing. Implementation of monitoring and work restrictions described in this guidance could be applied to HCP exposed to a PUI if test results for the PUI are not expected to return within 48 to 72 hours. A record of HCP exposed to a PUI should be maintained and HCP should be encouraged to perform self-monitoring while awaiting test results. If the results will be delayed more than 72 hours or the patient is positive for COVID-19, then the monitoring and work restrictions described in this document should be followed.
Table 1: Epidemiologic Risk Classification1 for Asymptomatic Healthcare Personnel Following Exposure to Patients with Coronavirus Disease (COVID-19) or their Secretions/Excretions in a Healthcare Setting, and their Associated Monitoring and Work Restriction Recommendations
Both high- and medium-risk exposures place HCP at more than low-risk for developing infection; therefore, the recommendations for active monitoring and work restrictions are the same for these exposures. However, these risk categories were created to align with risk categories described in the Interim US Guidance for Risk Assessment and Public Health Management of Persons with Potential Coronavirus Disease (COVID-19) Exposure in Travel-associated or Community Settings, which outlines criteria for quarantine and travel restrictions specific to high-risk exposures. Use that Interim Guidance for information about the movement, public activity, and travel restrictions that apply to the HCP included here.
The highest risk exposure category that applies to each person should be used to guide monitoring and work restrictions.
Note: While respirators confer a higher level of protection than facemasks, and are recommended when caring for patients with COVID-19, facemasks still confer some level of protection to HCP, which was factored into our assessment of risk.
|
Epidemiologic risk factors
|
Exposure category
|
Recommended Monitoring for COVID-19 (until 14 days after last potential exposure)
|
Work Restrictions for Asymptomatic HCP
|
|
Prolonged close contact with a COVID-19 patient who was wearing a facemask (i.e., source control)
|
|
HCP PPE: None
|
Medium
|
Active
|
Exclude from work for 14 days after last exposure
|
|
HCP PPE: Not wearing a facemask or respirator
|
Medium
|
Active
|
Exclude from work for 14 days after last exposure
|
|
HCP PPE: Not wearing eye protection
|
Low
|
Self with delegated supervision
|
None
|
|
HCP PPE: Not wearing gown or glovesa
|
Low
|
Self with delegated supervision
|
None
|
|
HCP PPE: Wearing all recommended PPE (except wearing a facemask instead of a respirator)
|
Low
|
Self with delegated supervision
|
None
|
|
Prolonged close contact with a COVID-19 patient who was not wearing a facemask (i.e., no source control)
|
|
HCP PPE: None
|
High
|
Active
|
Exclude from work for 14 days after last exposure
|
|
HCP PPE: Not wearing a facemask or respirator
|
High
|
Active
|
Exclude from work for 14 days after last exposure
|
|
HCP PPE: Not wearing eye protectionb
|
Medium
|
Active
|
Exclude from work for 14 days after last exposure
|
|
HCP PPE: Not wearing gown or glovesa,b
|
Low
|
Self with delegated supervision
|
None
|
|
HCP PPE: Wearing all recommended PPE (except wearing a facemask instead of a respirator)b
|
Low
|
Self with delegated supervision
|
None
|
HCP=healthcare personnel; PPE=personal protective equipment
a The risk category for these rows would be elevated by one level if HCP had extensive body contact with the patients (e.g., rolling the patient).
b The risk category for these rows would be elevated by one level if HCP performed or were present for a procedure likely to generate higher concentrations of respiratory secretions or aerosols (e.g., cardiopulmonary resuscitation, intubation, extubation, bronchoscopy, nebulizer therapy, sputum induction). For example, HCP who were wearing a gown, gloves, eye protection and a facemask (instead of a respirator) during an aerosol-generating procedure would be considered to have a medium-risk exposure.
Additional Scenarios:
• Refer to the footnotes above for scenarios that would elevate the risk level for exposed HCP. For example, HCP who were wearing a gown, gloves, eye protection and a facemask (instead of a respirator) during an aerosol-generating procedure would be considered to have a medium-risk exposure.
• Proper adherence to currently recommended infection control practices, including all recommended PPE, should protect HCP having prolonged close contact with patients infected with COVID-19. However, to account for any inconsistencies in use or adherence that could result in unrecognized exposures, HCP should still perform self-monitoring with delegated supervision.
• HCP not using all recommended PPE who have only brief interactions with a patient regardless of whether patient was wearing a facemask are considered low-risk. Examples of brief interactions include: brief conversation at a triage desk; briefly entering a patient room but not having direct contact with the patient or the patient’s secretions/excretions; entering the patient room immediately after the patient was discharged.
• HCP who walk by a patient or who have no direct contact with the patient or their secretions/excretions and no entry into the patient room are considered to have no identifiable risk.
III. Recommendations for Monitoring Based on COVID-19 Exposure Risk
HCP in any of the risk exposure categories who develop signs or symptoms compatible with COVID-19 must contact their established point of contact (public health authorities or their facility’s occupational health program) for medical evaluation prior to returning to work
1. High- and Medium-risk Exposure Category
HCP in the high- or medium-risk category should undergo active monitoring, including restriction from work in any healthcare setting until 14 days after their last exposure. If they develop any fever (measured temperature >100.0oF or subjective fever) OR respiratory symptoms consistent with COVID-19 (e.g., cough, shortness of breath, sore throat)* they should immediately self-isolate (separate themselves from others) and notify their local or state public health authority and healthcare facility promptly so that they can coordinate consultation and referral to a healthcare provider for further evaluation.
2. Low-risk Exposure Category
HCP in the low-risk category should perform self-monitoring with delegated supervision until 14 days after the last potential exposure. Asymptomatic HCP in this category are not restricted from work. They should check their temperature twice daily and remain alert for respiratory symptoms consistent with COVID-19 (e.g., cough, shortness of breath, sore throat)*. They should ensure they are afebrile and asymptomatic before leaving home and reporting for work. If they do not have fever or respiratory symptoms they may report to work. If they develop fever (measured temperature > 100.0 oF or subjective fever) OR respiratory symptoms they should immediately self-isolate (separate themselves from others) and notify their local or state public health authority or healthcare facility promptly so that they can coordinate consultation and referral to a healthcare provider for further evaluation. On days HCP are scheduled to work, healthcare facilities could consider measuring temperature and assessing symptoms prior to starting work. Alternatively, facilities could consider having HCP report temperature and symptoms to occupational health prior to starting work. Modes of communication may include telephone calls or any electronic or internet-based means of communication.
3. HCP who Adhere to All Recommended Infection Prevention and Control Practices
Proper adherence to currently recommended infection control practices, including all recommended PPE, should protect HCP having prolonged close contact with patients infected with COVID-19. However, to account for any inconsistencies in use or adherence that could result in unrecognized exposures, HCP should still perform self-monitoring with delegated supervision as described under the low-risk exposure category.
4. No Identifiable risk Exposure Category
HCP in the no identifiable risk category do not require monitoring or restriction from work.
5. Community or travel-associated exposures
HCP with potential exposures to COVID-19 in community settings, should have their exposure risk assessed according to CDC guidance. HCP should inform their facility’s occupational health program that they have had a community or travel-associated exposure. HCP who have a community or travel-associated exposure should undergo monitoring as defined by that guidance. Those who fall into the high- or medium- risk category described there should be excluded from work in a healthcare setting until 14 days after their exposure. HCP who develop signs or symptoms compatible with COVID-19 should contact their established point of contact (public health authorities or their facility’s occupational health program) for medical evaluation prior to returning to work.
Additional Considerations and Recommendations:
While contact tracing and risk assessment, with appropriate implementation of HCP work restrictions, of potentially exposed HCP remains the recommended strategy for identifying and reducing the risk of transmission of COVID-19 to HCP, patients, and others, it is not practical or achievable in all situations. Community transmission of COVID-19 in the United States has been reported in multiple areas. This development means some recommended actions (e.g., contact tracing and risk assessment of all potentially exposed HCP) are impractical for implementation by healthcare facilities. In the setting of community transmission, all HCP are at some risk for exposure to COVID-19, whether in the workplace or in the community. Devoting resources to contact tracing and retrospective risk assessment could divert resources from other important infection prevention and control activities. Facilities should shift emphasis to more routine practices, which include asking HCP to report recognized exposures, regularly monitor themselves for fever and symptoms of respiratory infection and not report to work when ill. Facilities should develop a plan for how they will screen for symptoms and evaluate ill HCP. This could include having HCP report absence of fever and symptoms prior to starting work each day.
Facilities could consider allowing asymptomatic HCP who have had an exposure to a COVID-19 patient to continue to work after options to improve staffing have been exhausted and in consultation with their occupational health program. These HCP should still report temperature and absence of symptoms each day prior to starting work. Facilities could have exposed HCP wear a facemask while at work for the 14 days after the exposure event if there is a sufficient supply of facemasks. If HCP develop even mild symptoms consistent with COVID-19, they must cease patient care activities, don a facemask (if not already wearing), and notify their supervisor or occupational health services prior to leaving work.
Resources for Hospitals and Healthcare Professionals Preparing for Patients with Suspected or Confirmed COVID-19
To aid healthcare professionals and hospitals, CDC has developed two checklists that identify key actions that can be taken now to enhance preparedness for potential or confirmed patients with coronavirus disease 2019 (COVID-19).
Healthcare Providers Preparedness Checklist
A checklist to aid healthcare providers in identifying key actions to enhance preparedness for patients potentially infected with SARS-CoV-2.
Hospital Preparedness Tool
A checklist to highlight important areas for hospitals to review in preparation for potential arrivals of COVID-19 patients.
Healthcare Professional Preparedness Checklist For Transport and Arrival of Patients With Confirmed or Possible COVID-19
Healthcare Personnel Preparedness Checklist for COVID-19[PDF]
Front-line healthcare personnel in the United States should be prepared to evaluate patients for coronavirus disease 2019 (COVID-19). The following checklist highlights key steps for healthcare personnel in preparation for transport and arrival of patients with confirmed or possible COVID-19.
□ Stay up to date on the latest information about signs and symptoms, diagnostic testing, and case definitions for coronavirus disease 2019.
□ Review your infection prevention and control policies and CDC infection control recommendations for COVID-19 for:
□ Assessment and triage of patients with acute respiratory symptoms
□ Patient placement
□ Implementation of Standard, Contact, and Airborne Precautions, including the use of eye protection
□ Visitor management and exclusion
□ Source control measures for patients (e.g., put facemask on suspect patients)
□ Requirements for performing aerosol generating procedures
□ Be alert for patients who meet the persons under investigation (PUI) definition
□ Know how to report a potential COVID-19 case or exposure to facility infection control leads and public health officials
□ Know who, when, and how to seek evaluation by occupational health following an unprotected exposure (i.e., not wearing recommended PPE) to a suspected or confirmed coronavirus disease 2019 patient
□ Remain at home, and notify occupational health services, if you are ill
□ Know how to contact and receive information from your state or local public health agency
Coronavirus Disease 2019 (COVID-19) Hospital Preparedness Assessment Tool
All U.S. hospitals should be prepared for the possible arrival of patients with Coronavirus Disease 2019 (COVID-19).
All hospitals should ensure their staff are trained, equipped and capable of practices needed to:
• Prevent the spread of respiratory diseases including COVID-19 within the facility
• Promptly identify and isolate patients with possible COVID-19 and inform the correct facility staff and public health authorities
• Care for a limited number of patients with confirmed or suspected COVID-19 as part of routine operations
• Potentially care for a larger number of patients in the context of an escalating outbreak
• Monitor and manage any healthcare personnel that might be exposed to COVID-19
• Communicate effectively within the facility and plan for appropriate external communication related to COVID-19
The following checklist does not describe mandatory requirements or standards; rather, it highlights important areas for hospitals to review in preparation for potential arrivals of COVID-19 patients.
Coronavirus Disease 2019 (COVID-19) Hospital Preparedness Tool

On February 11, 2020 the World Health Organization announced an official name for the disease that is causing the current outbreak of coronavirus disease, COVID-19. CDC will be updating our website and other CDC materials to reflect the updated name.
Interim Guidance for Implementing Home Care of People Not Requiring Hospitalization for Coronavirus Disease 2019 (COVID-19)
This interim guidance is for staff at local and state health departments, infection prevention and control professionals, and healthcare personnel who are coordinating the home care and isolation1 of people with confirmed or suspected COVID-19 infection, including persons under investigation (see Criteria to Guide Evaluation of Persons Under Investigation (PUI) for COVID-19). This includes patients evaluated in an outpatient setting who do not require hospitalization (i.e., patients who are medically stable and can receive care at home) or patients who are discharged home following a hospitalization with confirmed COVID-19 infection.
In general, people should adhere to home isolation until the risk of secondary transmission is thought to be low. Visit the Preventing the Spread of Coronavirus Disease 2019 in Homes and Residential Communities page for more information.

Preventing 2COVID-19 from Spreading in Homes and Communities: Interim guidance that may help prevent COVID-19 from spreading among people in homes and in communities.
This document does not apply to patients in healthcare settings. For interim healthcare infection prevention and control recommendations, see Interim Infection Prevention and Control Recommendations for Patients with Known or Persons Under Investigation forCoronavirus Disease 2019 (COVID-19) in a Healthcare Setting. CDC will update this interim guidance as needed and as more information becomes available.
Assess the Suitability of the Residential Setting for Home Care
In consultation with state or local health department staff, a healthcare professional should assess whether the residential setting is appropriate for home care. Considerations for care at home include whether:
• The patient is stable enough to receive care at home.
• Appropriate caregivers are available at home.
• There is a separate bedroom where the patient can recover without sharing immediate space with others.
• Resources for access to food and other necessities are available.
• The patient and other household members have access to appropriate, recommended personal protective equipment (at a minimum, gloves and facemask) and are capable of adhering to precautions recommended as part of home care or isolation (e.g., respiratory hygiene and cough etiquette, hand hygiene);
• There are household members who may be at increased risk of complications from COVID-19 infection (.e.g., people >65 years old, young children, pregnant women, people who are immunocompromised or who have chronic heart, lung, or kidney conditions).
Provide Guidance for Precautions to Implement during Home Care
A healthcare professional should
• Provide CDC’s Interim Guidance for Preventing Coronavirus Disease 2019 (COVID-19) from Spreading to Others in Homes and Communities to the patient, caregiver, and household members; and
• Contact their state or local health department to discuss criteria for discontinuing any such measures.
Preventing the Spread of Coronavirus Disease 2019 in Homes and Residential Communities
Interim Guidance
(This guidance provides clarification regarding evaluation for home isolation and a new section with information regarding preventative steps for household members, intimate partners, and caregivers in a nonhealthcare setting of a person with symptomatic, laboratory-confirmed COVID-19.)
This interim guidance is based on what is currently known about the epidemiology of COVID-19 and the transmission of other viral respiratory diseases. CDC will update this interim guidance as needed and as additional information becomes available.
Coronaviruses are a large family of viruses, some causing illness in people and others that circulate among animals, including camels, cats, and bats. Rarely, animal coronaviruses can infect people exposed to infected animals, and then spread among people, as has been seen with MERS-CoV and SARS-CoV, and likely now with SARS-CoV-2, the virus that causes COVID-19. This interim guidance may help prevent this virus from spreading among people in their homes and in other residential communities.
This interim guidance is intended for:
• People with confirmed or suspected COVID-19, including persons under investigation, who do not need to be hospitalized and who can receive care at home (see Interim Guidance for Implementing Home Care of People Not Requiring Hospitalization for Coronavirus Disease 2019 (COVID-19));
• People with confirmed COVID-19, who were hospitalized and then determined to be medically stable to go home (see Interim Guidance for Implementing Home Care of People Not Requiring Hospitalization for Coronavirus Disease 2019 (COVID-19));
• Household members, intimate partners, and caregivers in a nonhealthcare setting of a person with symptomatic, laboratory-confirmed COVID-19.
Prevention steps for
People with confirmed or suspected COVID-19 (including persons under investigation) who do not need to be hospitalized
and
People with confirmed COVID-19 who were hospitalized and determined to be medically stable to go home
Your healthcare provider and public health staff will evaluate whether you can be cared for at home. If it is determined that you do not need to be hospitalized and can be isolated at home, you will be monitored by staff from your local or state health department. You should follow the prevention steps below until a healthcare provider or local or state health department says you can return to your normal activities.
Stay home except to get medical care
People who are mildly ill with COVID-19 are able to isolate at home during their illness. You should restrict activities outside your home, except for getting medical care. Do not go to work, school, or public areas. Avoid using public transportation, ride-sharing, or taxis.
Separate yourself from other people and animals in your home
People: As much as possible, you should stay in a specific room and away from other people in your home. Also, you should use a separate bathroom, if available.
Animals: You should restrict contact with pets and other animals while you are sick with COVID-19, just like you would around other people. Although there have not been reports of pets or other animals becoming sick with COVID-19, it is still recommended that people sick with COVID-19 limit contact with animals until more information is known about the virus. When possible, have another member of your household care for your animals while you are sick. If you are sick with COVID-19, avoid contact with your pet, including petting, snuggling, being kissed or licked, and sharing food. If you must care for your pet or be around animals while you are sick, wash your hands before and after you interact with pets and wear a facemask. See COVID-19 and Animals for more information.
Call ahead before visiting your doctor
If you have a medical appointment, call the healthcare provider and tell them that you have or may have COVID-19. This will help the healthcare provider’s office take steps to keep other people from getting infected or exposed.
Wear a facemask
You should wear a facemask when you are around other people (e.g., sharing a room or vehicle) or pets and before you enter a healthcare provider’s office. If you are not able to wear a facemask (for example, because it causes trouble breathing), then people who live with you should not stay in the same room with you, or they should wear a facemask if they enter your room.
Cover your coughs and sneezes
Cover your mouth and nose with a tissue when you cough or sneeze. Throw used tissues in a lined trash can. Immediately wash your hands with soap and water for at least 20 seconds or, if soap and water are not available, clean your hands with an alcohol-based hand sanitizer that contains at least 60% alcohol.
Clean your hands often
Wash your hands often with soap and water for at least 20 seconds, especially after blowing your nose, coughing, or sneezing; going to the bathroom; and before eating or preparing food. If soap and water are not readily available, use an alcohol-based hand sanitizer with at least 60% alcohol, covering all surfaces of your hands and rubbing them together until they feel dry.
Soap and water are the best option if hands are visibly dirty. Avoid touching your eyes, nose, and mouth with unwashed hands.
Avoid sharing personal household items
You should not share dishes, drinking glasses, cups, eating utensils, towels, or bedding with other people or pets in your home. After using these items, they should be washed thoroughly with soap and water.
Clean all “high-touch” surfaces everyday
High touch surfaces include counters, tabletops, doorknobs, bathroom fixtures, toilets, phones, keyboards, tablets, and bedside tables. Also, clean any surfaces that may have blood, stool, or body fluids on them. Use a household cleaning spray or wipe, according to the label instructions. Labels contain instructions for safe and effective use of the cleaning product including precautions you should take when applying the product, such as wearing gloves and making sure you have good ventilation during use of the product.
Monitor your symptoms
Seek prompt medical attention if your illness is worsening (e.g., difficulty breathing). Before seeking care, call your healthcare provider and tell them that you have, or are being evaluated for, COVID-19. Put on a facemask before you enter the facility. These steps will help the healthcare provider’s office to keep other people in the office or waiting room from getting infected or exposed. Ask your healthcare provider to call the local or state health department. Persons who are placed under active monitoring or facilitated self-monitoring should follow instructions provided by their local health department or occupational health professionals, as appropriate.
If you have a medical emergency and need to call 911, notify the dispatch personnel that you have, or are being evaluated for COVID-19. If possible, put on a facemask before emergency medical services arrive.
Discontinuing home isolation
Patients with confirmed COVID-19 should remain under home isolation precautions until the risk of secondary transmission to others is thought to be low. The decision to discontinue home isolation precautions should be made on a case-by-case basis, in consultation with healthcare providers and state and local health departments.
Recommended precautions for household members, intimate partners, and caregivers in a nonhealthcare setting1 of
A patient with symptomatic laboratory-confirmed COVID-19
or
A patient under investigation
Household members, intimate partners, and caregivers in a nonhealthcare setting may have close contact2 with a person with symptomatic, laboratory-confirmed COVID-19 or a person under investigation. Close contacts should monitor their health; they should call their healthcare provider right away if they develop symptoms suggestive of COVID-19 (e.g., fever, cough, shortness of breath) (see Interim US Guidance for Risk Assessment and Public Health Management of Persons with Potential Coronavirus Disease 2019 (COVID-19) Exposure in Travel-associated or Community Settings.)
Close contacts should also follow these recommendations:
• Make sure that you understand and can help the patient follow their healthcare provider’s instructions for medication(s) and care. You should help the patient with basic needs in the home and provide support for getting groceries, prescriptions, and other personal needs.
• Monitor the patient’s symptoms. If the patient is getting sicker, call his or her healthcare provider and tell them that the patient has laboratory-confirmed COVID-19. This will help the healthcare provider’s office take steps to keep other people in the office or waiting room from getting infected. Ask the healthcare provider to call the local or state health department for additional guidance. If the patient has a medical emergency and you need to call 911, notify the dispatch personnel that the patient has, or is being evaluated for COVID-19.
• Household members should stay in another room or be separated from the patient as much as possible. Household members should use a separate bedroom and bathroom, if available.
• Prohibit visitors who do not have an essential need to be in the home.
• Household members should care for any pets in the home. Do not handle pets or other animals while sick. For more information, see COVID-19 and Animals.
• Make sure that shared spaces in the home have good air flow, such as by an air conditioner or an opened window, weather permitting.
• Perform hand hygiene frequently. Wash your hands often with soap and water for at least 20 seconds or use an alcohol-based hand sanitizer that contains 60 to 95% alcohol, covering all surfaces of your hands and rubbing them together until they feel dry. Soap and water should be used preferentially if hands are visibly dirty.
• Avoid touching your eyes, nose, and mouth with unwashed hands.
• The patient should wear a facemask when you are around other people. If the patient is not able to wear a facemask (for example, because it causes trouble breathing), you, as the caregiver, should wear a mask when you are in the same room as the patient.
• Wear a disposable facemask and gloves when you touch or have contact with the patient’s blood, stool, or body fluids, such as saliva, sputum, nasal mucus, vomit, urine.
○ Throw out disposable facemasks and gloves after using them. Do not reuse.
○ When removing personal protective equipment, first remove and dispose of gloves. Then, immediately clean your hands with soap and water or alcohol-based hand sanitizer. Next, remove and dispose of facemask, and immediately clean your hands again with soap and water or alcohol-based hand sanitizer.
• Avoid sharing household items with the patient. You should not share dishes, drinking glasses, cups, eating utensils, towels, bedding, or other items. After the patient uses these items, you should wash them thoroughly (see below “Wash laundry thoroughly”).
• Clean all “high-touch” surfaces, such as counters, tabletops, doorknobs, bathroom fixtures, toilets, phones, keyboards, tablets, and bedside tables, every day. Also, clean any surfaces that may have blood, stool, or body fluids on them.
○ Use a household cleaning spray or wipe, according to the label instructions. Labels contain instructions for safe and effective use of the cleaning product including precautions you should take when applying the product, such as wearing gloves and making sure you have good ventilation during use of the product.
• Wash laundry thoroughly.
○ Immediately remove and wash clothes or bedding that have blood, stool, or body fluids on them.
○ Wear disposable gloves while handling soiled items and keep soiled items away from your body. Clean your hands (with soap and water or an alcohol-based hand sanitizer) immediately after removing your gloves.
○ Read and follow directions on labels of laundry or clothing items and detergent. In general, using a normal laundry detergent according to washing machine instructions and dry thoroughly using the warmest temperatures recommended on the clothing label.
• Place all used disposable gloves, facemasks, and other contaminated items in a lined container before disposing of them with other household waste. Clean your hands (with soap and water or an alcohol-based hand sanitizer) immediately after handling these items. Soap and water should be used preferentially if hands are visibly dirty.
• Discuss any additional questions with your state or local health department or healthcare provider.
Interim Guidance for Discontinuation of In-Home Isolation for Patients with COVID-19
Summary Page
Who this is for: Healthcare providers and public health officials managing patients with coronavirus disease (COVID-19) under in-home isolation.
What this is for: To help prevent the spread of COVID-19 in the community.
How to use: Reference to guide healthcare staff and public health officials before discontinuing in-home isolation of patients with COVID-19.
Summary of Recent Changes
Revisions were made on February 11, 2020, to use of laboratory testing results:
• Negative rRT-PCR results from at least 2 consecutive sets of nasopharyngeal and throat swabs collected at least 24 hours apart from a patient with COVID-19 are needed before discontinuing in-home isolation is considered. A total of four negative specimens are needed to meet this requirement.
Limited information is available to characterize the spectrum of clinical illness, transmission efficiency, and the duration of viral shedding for patients with novel coronavirus disease (COVID-19). This guidance is based on available information about COVID-19 and what is known about similar diseases caused by related coronaviruses (MERS-CoV and SARS-CoV). This guidance is subject to change as additional information becomes available.
For Hospitalized Patients, see (Interim Guidance for Discontinuation of Transmission-Based Precautions Among Hospitalized Patients with COVID-19).
For Patients with COVID-19 Under In-Home Isolation:
• The decision to discontinue in-home isolation for patients with COVID-19 should be made on a case-by-case basis in consultation with clinicians and public health officials. This decision should consider disease severity, illness signs and symptoms, and results of laboratory testing for COVID-19 in respiratory specimens. Guidance for discontinuation of in-home isolation precautions is the same as that to discontinue Transmission-Based Precautions for hospitalized patients with COVID-19. Considerations to discontinue in-home isolation include all of the following:
○ Resolution of fever, without use of antipyretic medication
○ Improvement in illness signs and symptoms
○ Negative results of an FDA Emergency Use Authorized molecular assay for COVID-19 from at least two consecutive sets of paired nasopharyngeal and throat swabs specimens collected ≥24 hours apart* (total of four negative specimens—two nasopharyngeal and two throat). See Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Patients Under Investigation (PUIs) for 2019 Novel Coronavirus (2019-nCoV) for specimen collection guidance.
Footnote
* Initial guidance is based upon limited information and is subject to change as more information becomes available. In persons with a persistent productive cough, SARS-CoV-2-RNA might be detected for longer periods in sputum specimens than in upper respiratory tract (nasopharyngeal swab and throat swab) specimens.
Additional Resources
• Interim Guidance for Implementing Home Care of People Not Requiring Hospitalization for 2019 Novel Coronavirus (2019-nCoV)
• Interim guidance for persons who may have 2019 Novel Coronavirus (2019-nCoV) to prevent spread in homes and residential communities
References
• Al-Abdely HM, Midgley CM, Alkhamis AM, et al. Middle East respiratory syndrome coronavirus infection dynamics and antibody responses among clinically diverse patients, Saudi Arabia. Emerg Infect Dis. 2019 Apr;25(4):753–66.
• Al-Abdely HM, Midgley CM, Alkhamis AM, et al. Infectious MERS-CoV isolated from a mildly ill patient, Saudi Arabia. Open Forum Infect Dis. 2018 May 15;5(6):ofy111.
• Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020 Jan 24; 395(10223);514–23. doi: 10.1016/S0140-6736(20)30154-9.
• Chan KH, Poon LL, Cheng VC, et al. Detection of SARS coronavirus in patients with suspected SARS. Emerg Infect Dis. 2004 Feb;10(2):294–9.
• Cheng PK, Wong DA, Tong LK, et al. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004 May 22;363(9422):1699–700.
• Corman VM, Albarrak AM, Omrani AS, et al. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis. 2016 Feb 15;62(4):477–83.
• Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020 Jan 31. doi: 10.1056/NEJMoa2001191. [Epub ahead of print]
• Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Jan 24;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5
• Hung IF, Cheng VC, Wu AK, et al. Viral loads in clinical specimens and SARS manifestations. Emerg Infect Dis. 2004 Sep;10(9):1550–7.
• Liu W, Tang F, Fontanet A, et al. Long-term SARS coronavirus excretion from patient cohort, China. Emerg Infect Dis. 2004 Oct;10(10):1841–3.
• Memish ZA, Assiri AM, Al-Tawfiq JA. Middle East respiratory syndrome coronavirus (MERS-CoV) viral shedding in the respiratory tract: an observational analysis with infection control implications. Int J Infect Dis. 2014 Dec;29:307–8.
• Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 Jan 24. doi: 10.1056/NEJMoa2001017 [Epub ahead of print]
Healthcare Supply of Personal Protective Equipment
FAQ about Personal Protective Equipment
Strategies for Optimizing the Supply of N95 Respirators
Checklist for Healthcare Facilities
Release of Stockpiled N95 Respirators Beyond the Manufacturer-Designated Shelf Life
Personal Protective Equipment Recommendations
Based on the current COVID-19 situation and availability of personal protective equipment (PPE), CDC has specific recommendations, summarized below. As we learn more about COVID-19 and as the needs of the response or availability of PPE within U.S. healthcare facilities changes, we will update our guidance.

Who needs PPE:
Patients with confirmed or possible SARS-CoV-2 infection should wear a facemask when being evaluated medically.
Healthcare personnel should adhere to Standard, Contact, and Airborne Precautions, including the use of eye protection (e.g., goggles or a face shield) when caring for patients with SARS-CoV-2 infection. These precautions include the use of PPE, including NIOSH-approved N95 respirators, gowns, gloves, face shield/eye protection, etc. This includes, but is not limited to, surgical N95 respirators.

Who does not need PPE:
CDC does NOT currently recommend the general public use facemasks. Instead, CDC recommends following everyday preventive actions, such as washing your hands, covering your cough, and staying home when you are sick.

Manufacturers and Distributors:
Cases of COVID-19 are being reported in China as well as other countries. Given decreases in exports from select countries (e.g., China, India, Taiwan) and increases in demand due to the outbreak, manufacturers of select types of PPE are reporting increased volume of orders and challenges in meeting order demands. Plans to surge manufacturing globally are underway.
Webinar for Healthcare Professionals
Strategies for Healthcare Systems Preparedness and Optimizing N95 Supplies.
Find more information on supplies of personal protective equipment
View presentation slides
Frequently Asked Questions about Personal Protective Equipment
This document is intended to address frequently asked questions about personal protective equipment (PPE).
Gowns

• What testing and standards should I consider when looking for CDC-recommended protective clothing?
○ CDC’s guidance for Considerations for Selecting Protective Clothing used in Healthcare for Protection against Microorganisms in Blood and Body Fluids outlines the scientific evidence and information on national and international standards, test methods, and specifications for fluid-resistant and impermeable gowns and coveralls used in healthcare.
○ Many organizations have published guidelines for the use of personal protective equipment (PPE) in medical settings. The American National Standards Institute (ANSI) and the Association of the Advancement of Medical Instrumentation (AAMI): ANSI/AAMI PB70:2012 describes the liquid barrier performance and a classification of surgical and isolation gowns for use in health care facilities.
○ As with any type of PPE, the key to proper selection and use of protective clothing is to understand the hazards and the risk of exposure. Some of the factors important to assessing the risk of exposure in health facilities include source, modes of transmission, pressures and types of contact, and duration and type of tasks to be performed by the user of the PPE. (Technical Information Report (TIR) 11 [AAMI 2005]).
○ For gowns, it is important to have sufficient overlap of the fabric so that it wraps around the body to cover the back (ensuring that if the wearer squats or sits down, the gown still protects the back area of the body).
• What type of gown is recommended for patients with suspected or confirmed COVID-19?
○ Nonsterile, disposable patient isolation gowns, which are used for routine patient care in healthcare settings, are appropriate for use by patients with suspected or confirmed COVID-19.
• What types of gowns are available for healthcare personnel to protect from COVID-19?
○ While the transmissibility of COVID-19 is not fully understood, gowns are available that protect against microorganisms. The choice of gown should be made based on the level of risk of contamination. Certain areas of surgical and isolation gowns are defined as “critical zones” where direct contact with blood, body fluids, and/or other potentially infectious materials is most likely to occur. (ANSI/AAMI PB70).
○ If there is a medium to high risk of contamination and need for a large critical zone, isolation gowns that claim moderate to high barrier protection (ANSI/AAMI PB70 Level 3 or 4) can be used.
○ For healthcare activities with low, medium, or high risk of contamination, surgical gowns (ANSI/AAMI PB70 Levels 1-4), can be used. These gowns are intended to be worn by healthcare personnel during surgical procedures.
○ If the risk of bodily fluid exposure is low or minimal, gowns that claim minimal or low levels of barrier protection (ANSI/AAMI PB70 Level 1 or 2) can be used. These gowns should not be worn during surgical or invasive procedures, or for medium to high risk contamination patient care activities.
• What is the difference between gowns and coveralls?
○ CDC’s guidance for Considerations for Selecting Protective Clothing used in Healthcare for Protection against Microorganisms in Blood and Body Fluids provides additional comparisons between gowns and coveralls.
○ Gowns are easier to put on and, in particular, to take off. They are generally more familiar to healthcare workers and hence more likely to be used and removed correctly. These factors also facilitate training in their correct use.
○ Coveralls typically provide 360-degree protection because they are designed to cover the whole body, including the back and lower legs, and sometimes the head and feet as well. Surgical/isolation gowns do not provide continuous whole-body protection (e.g., they have possible openings in the back, and typically provide coverage to the mid-calf only).
○ The level of heat stress generated due to the added layer of clothing is also expected to be less for gowns when compared to coveralls due to several factors, such as the openings in the design of gowns and total area covered by the fabric.
• How do I put on (don) and take off (doff) my gown?
○ Check to see if your facility has guidance on how to don and doff PPE. The procedure to don and doff should be tailored to the specific type of PPE that you have available at your facility.
○ If your facility does not have specific guidance, the CDC has recommended sequences for donning and doffing of PPE.
○ It is important for Health Care Providers (HCP) to perform hand hygiene before and after removing PPE. Hand hygiene should be performed by using alcohol-based hand sanitizer that contains 60-95% alcohol or washing hands with soap and water for at least 20 seconds. If hands are visibly soiled, soap and water should be used before returning to alcohol-based hand sanitizer.
• Is it acceptable for emergency medical services to wear coveralls as an alternative to gowns when COVID-19 is suspected in a patient needing emergency transport?
○ Unlike patient care in the controlled environment of a healthcare facility, care and transport by EMS present unique challenges because of the nature of the setting. Coveralls are an acceptable alternative to gowns when caring for and transporting suspect COVID-19 patients. While no clinical studies have been done to compare gowns and coveralls, both have been used effectively by healthcare workers in clinical settings during patient care. CDC’s Considerations for Selecting Protective Clothing used in Healthcare for Protection against Microorganisms in Blood and Body Fluids guidance provides a comparison between gowns and coveralls, including test methods and performance requirements. Coveralls typically provide 360-degree protection because they are designed to cover the whole body, including the back and lower legs, and sometimes the head and feet as well. This added coverage may be necessary for some work tasks involved in medical transport. However, coveralls may lead to increased heat stress compared to gowns due to the total area covered by the fabric. Training on how to properly remove (doff) a coverall is important to prevent self-contamination. Comparatively, gowns are easier to put on and, in particular, to take off.
Gloves
• What type of glove is recommended to care for suspected or confirmed COVID-19 patients in healthcare settings?
○ Nonsterile disposable patient examination gloves, which are used for routine patient care in healthcare settings, are appropriate for the care of patients with suspected or confirmed COVID-19.
• What standards should be considered when choosing gloves?
○ The American Society for Testing and Materials (ASTM) has developed standards for patient examination gloves.
○ Standard specifications for nitrile gloves, natural rubber gloves, and polychloroprene gloves indicate higher minimum tensile strength and elongation requirements compared to vinyl gloves.1,2,3,4
○ The ASTM has developed standards for patient examination gloves. Length requirements for patient exam gloves must be a minimum of 220mm-230mm depending on glove size and material type.1,2,3,4
• Is double gloving necessary when caring for suspected or confirmed CoVID-19 patients in healthcare settings?
○ CDC Guidance does not recommend double gloves when providing care to suspected or confirmed 2019-COVID patients.
• Are extended length gloves necessary when caring for suspected or confirmed COVID-19 patients in healthcare settings?
○ According to CDC Guidance, extended length gloves are not necessary when providing care to suspected or confirmed COVID-19 patients. Extended length gloves can be used, but CDC is not specifically recommending them at this time.
• How I do put on (don) and take off (doff) my gloves?
○ Check to see if your facility has guidance on how to don and doff PPE. The procedure to don and doff should be tailored to the specific type of PPE that you have available at your facility.
○ If your facility does not have specific guidance, the CDC has recommended sequences for donning and doffing of PPE.
○ It is important for HCP to perform hand hygiene after removing PPE. Hand hygiene should be performed by using an alcohol-based hand sanitizer that contains 60-95% alcohol or washing hands with soap and water for at least 20 seconds. If hands are visibly soiled, soap and water should be used before returning to alcohol-based hand sanitizer.
Footnotes
1 ASTM D6319-Standard Specification for Nitrile Examination Gloves for Medical Applications
2 ASTM D3578 Standard Specification for Rubber Examination Gloves
3 ASTM D5250 Standard Specification for Poly(vinyl chloride) Gloves for Medical Application
4 ASTMD 6977 Standard Specification for Polychloroprene Examination Gloves for Medical Application
Respirators
• Should I wear a respirator in public?
○ CDC does not recommend the routine use of respirators outside of workplace settings (in the community). Most often, spread of respiratory viruses from person-to-person happens among close contacts (within 6 feet). CDC recommends everyday preventive actions to prevent the spread of respiratory viruses, such as avoiding people who are sick, avoiding touching your eyes or nose, and covering your cough or sneeze with a tissue. People who are sick should stay home and not go into crowded public places or visit people in hospitals. Workers who are sick should follow CDC guidelines and stay home when they are sick.
• What is a respirator?
○ A respirator is a personal protective device that is worn on the face or head and covers at least the nose and mouth. A respirator is used to reduce the wearer’s risk of inhaling hazardous airborne particles (including infectious agents), gases or vapors. Respirators, including those intended for use in healthcare settings, are certified by the CDC/NIOSH.
• What is an N95 filtering facepiece respirator (FFR)?
○ An N95 FFR is a type of respirator which removes particles from the air that are breathed through it. These respirators filter out at least 95% of very small (0.3 micron) particles. N95 FFRs are capable of filtering out all types of particles, including bacteria and viruses.
• What makes N95 respirators different from facemasks (sometimes called a surgical mask)?
○ Infographic: Understanding the difference between surgical masks and N95 respirators
○ N95 respirators reduce the wearer’s exposure to airborne particles, from small particle aerosols to large droplets. N95 respirators are tight-fitting respirators that filter out at least 95% of particles in the air, including large and small particles.
○ Not everyone is able to wear a respirator due to medical conditions that may be made worse when breathing through a respirator. Before using a respirator or getting fit-tested, workers must have a medical evaluation to make sure that they are able to wear a respirator safely.
○ Achieving an adequate seal to the face is essential. United States regulations require that workers undergo an annual fit test and conduct a user seal check each time the respirator is used. Workers must pass a fit test to confirm a proper seal before using a respirator in the workplace.
○ When properly fitted and worn, minimal leakage occurs around edges of the respirator when the user inhales. This means almost all of the air is directed through the filter media.
○ Unlike NIOSH-approved N95s, facemasks are loose-fitting and provide only barrier protection against droplets, including large respiratory particles. No fit testing or seal check is necessary with facemasks. Most facemasks do not effectively filter small particles from the air and do not prevent leakage around the edge of the mask when the user inhales.
○ The role of facemasks is for patient source control, to prevent contamination of the surrounding area when a person coughs or sneezes. Patients with confirmed or suspected COVID-19 should wear a facemask until they are isolated in a hospital or at home. The patient does not need to wear a facemask while isolated.
• What is a Surgical N95 respirator and who needs to wear it?
○ A surgical N95 (also referred as a medical respirator) is recommended only for use by healthcare personnel (HCP) who need protection from both airborne and fluid hazards (e.g., splashes, sprays). These respirators are not used or needed outside of healthcare settings. In times of shortage, only HCP who are working in a sterile field or who may be exposed to high velocity splashes, sprays, or splatters of blood or body fluids should wear these respirators, such as in operative or procedural settings. Most HCP caring for confirmed or suspected COVID-19 patients should not need to use surgical N95 respirators and can use standard N95 respirators.
○ If a surgical N95 is not available for use in operative or procedural settings, then an unvalved N95 respirator may be used with a faceshield to help block high velocity streams of blood and body fluids.
• My employees complain that Surgical N95 respirators are hot and uncomfortable – what can I do?
○ The requirements for surgical N95 respirators that make them resistant to high velocity streams of body fluids and help protect the sterile field can result in a design that has a higher breathing resistance (makes it more difficult to breath) than a typical N95 respirator. Also, surgical N95 respirators are designed without exhalation valves which are sometimes perceived as warmer inside the mask than typical N95 respirators. If you are receiving complaints, you may consider having employees who are not doing surgery, not working in a sterile field, or not potentially exposed to high velocity streams of body fluids wear a standard N95 with an exhalation valve.
• My N95 respirator has an exhalation valve, is that okay?
○ An N95 respirator with an exhalation valve does provide the same level of protection to the wearer as one that does not have a valve. The presence of an exhalation valve reduces exhalation resistance, which makes it easier to breathe (exhale). Some users feel that a respirator with an exhalation valve keeps the face cooler and reduces moisture build up inside the facepiece. However, respirators with exhalation valves should not be used in situations where a sterile field must be maintained (e.g., during an invasive procedure in an operating or procedure room) because the exhalation valve allows unfiltered exhaled air to escape into the sterile field.
• How can I tell if a respirator is NIOSH-approved?
○ The NIOSH approval number and approval label are key to identifying NIOSH-approved respirators. The NIOSH approval label can be found on or within the packaging of the respirator or sometimes on the respirator itself. The required labeling of NIOSH-Approved N95 filtering facepiece respirators includes the NIOSH name, the approval number, filter designations, lot number, and model number to be printed on the respirator. You can verify that your respirator approvals are valid by checking the NIOSH Certified Equipment List (CEL).
• How do I know if my respirator is expired?
○ NIOSH does not require approved N95 filtering facepiece respirators (FFRs) be marked with an expiration date. If an FFR does not have an assigned expiration date, you should refer to the user instructions or seek guidance from the specific manufacturer on whether time and storage conditions (such as temperature or humidity) are expected to have an effect on the respirator’s performance and if the respirators are nearing the end of their shelf life.
• What do I do with an expired respirator?
○ In times of increased demand and decreased supply, consideration can be made to use N95 respirators past their intended shelf life. However, the potential exists that the respirator will not perform to the requirements for which it was certified. Over time, components such as the strap and nose bridge may degrade, which can affect the quality of the fit and seal. Prior to use of N95 respirators, the HCP should inspect the respirator and perform a seal check. Additionally, expired respirators may potentially no longer meet the certification requirements set by NIOSH. For further guidance, visit Release of Stockpiled N95 Filtering Facepiece Respirators Beyond the Manufacturer-Designated Shelf Life: Considerations for the COVID-19 Response.
• What methods should healthcare facilities consider in order to avoid unintentional loss of PPE during COVID-19?
○ Monitoring PPE supply inventory and maintaining control over PPE supplies may help prevent unintentional product losses that may occur due to theft, damage, or accidental loss. Inventory systems should be employed to track daily usage and identify areas of higher than expected use. This information can be used to implement additional conservation strategies tailored to specific patient care areas such as hospital units or outpatient facilities. Inventory tracking within a health system may also assist in confirming PPE deliveries and optimizing distribution of PPE supplies to specific facilities.
Healthcare Professionals: Frequently Asked Questions and Answers
See also the general COVID-19 Novel Coronavirus FAQ.
Q: What are the clinical features of COVID-19?
A: The clinical spectrum of COVID-19 ranges from mild disease with non-specific signs and symptoms of acute respiratory illness, to severe pneumonia with respiratory failure and septic shock. There have also been reports of asymptomatic infection with COVID-19. See also Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease 2019 (COVID-19).
Q: Who is at risk for COVID-19?
A: Currently, those at greatest risk of infection are persons who have had prolonged, unprotected close contact with a patient with symptomatic, confirmed COVID-19 and those who live in or have recently been to areas with sustained transmission.
Q: Who is at risk for severe disease from COVID-19?
The available data are currently insufficient to identify risk factors for severe clinical outcomes. From the limited data that are available for COVID-19 infected patients, and for data from related coronaviruses such as SARS-CoV and MERS-CoV, it is possible that older adults, and persons who have underlying chronic medical conditions, such as immunocompromising conditions, may be at risk for more severe outcomes. See also See also Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease 2019 (COVID-19).
Q: When is someone infectious?
A: The onset and duration of viral shedding and period of infectiousness for COVID-19 are not yet known. It is possible that SARS-CoV-2 RNA may be detectable in the upper or lower respiratory tract for weeks after illness onset, similar to infection with MERS-CoV and SARS-CoV. However, detection of viral RNA does not necessarily mean that infectious virus is present. Asymptomatic infection with SARS-CoV-2 has been reported, but it is not yet known what role asymptomatic infection plays in transmission. Similarly, the role of pre-symptomatic transmission (infection detection during the incubation period prior to illness onset) is unknown. Existing literature regarding SARS-CoV-2 and other coronaviruses (e.g. MERS-CoV, SARS-CoV) suggest that the incubation period may range from 2–14 days.
Q: Which body fluids can spread infection?
A: Very limited data are available about detection of SARS-CoV-2 and infectious virus in clinical specimens. SARS-CoV-2 RNA has been detected from upper and lower respiratory tract specimens, and SARS-CoV-2 has been isolated from upper respiratory tract specimens and bronchoalveolar lavage fluid. SARS-CoV-2 RNA has been detected in blood and stool specimens, but whether infectious virus is present in extrapulmonary specimens is currently unknown. The duration of SARS-CoV-2 RNA detection in upper and lower respiratory tract specimens and in extrapulmonary specimens is not yet known but may be several weeks or longer, which has been observed in cases of MERS-CoV or SARS-CoV infection. While viable, infectious SARS-CoV has been isolated from respiratory, blood, urine, and stool specimens, in contrast – viable, infectious MERS-CoV has only been isolated from respiratory tract specimens. It is not yet known whether other non-respiratory body fluids from an infected person including vomit, urine, breast milk, or semen can contain viable, infectious SARS-CoV-2.
Q: Can people who recover from COVID-19 be infected again?
A: The immune response to COVID-19 is not yet understood. Patients with MERS-CoV infection are unlikely to be re-infected shortly after they recover, but it is not yet known whether similar immune protection will be observed for patients with COVID-19.
Q: How should healthcare personnel protect themselves when evaluating a patient who may have COVID-19?
A: Although the transmission dynamics have yet to be determined, CDC currently recommends a cautious approach to persons under investigation (PUI) for COVID-19. Healthcare personnel evaluating PUI or providing care for patients with confirmed COVID-19 should use Standard Precautions, Contact Precautions, Airborne Precautions, and use eye protection (e.g., goggles or a face shield). See the Interim Infection Prevention and Control Recommendations for Patients with Known or Patients Under Investigation for Coronavirus Disease 2019 (COVID-19) in Healthcare Settings.
Q: Should any diagnostic or therapeutic interventions be withheld due to concerns about transmission of COVID-19?
A: Patients should receive any interventions they would normally receive as standard of care. Patients with suspected or confirmed COVID-19 should be asked to wear a surgical mask as soon as they are identified and be evaluated in a private room with the door closed, ideally an airborne infection isolation room, if available. Healthcare personnel entering the room should use Standard Precautions, Contact Precautions, Airborne Precautions, and use eye protection (e.g., goggles or a face shield).
Q: How do you test a patient for SARS-CoV-2, the virus that causes COVID-19?
A: See recommendations for reporting, testing, and specimen collection at Interim Guidance for Healthcare Professionals.
Q: Will existing respiratory virus panels, such as those manufactured by Biofire or Genmark, detect SARS-CoV-2, the virus that causes COVID-19?
A: No. These multi-pathogen molecular assays can detect a number of human respiratory viruses, including other coronaviruses that can cause acute respiratory illness, but they do not detect COVID-19.
Q: How is COVID-19 treated?
Not all patients with COVID-19 will require medical supportive care. Clinical management for hospitalized patients with COVID-19 is focused on supportive care of complications, including advanced organ support for respiratory failure, septic shock, and multi-organ failure. Empiric testing and treatment for other viral or bacterial etiologies may be warranted.
Corticosteroids are not routinely recommended for viral pneumonia or ARDS and should be avoided unless they are indicated for another reason (e.g., COPD exacerbation, refractory septic shock following Surviving Sepsis Campaign Guidelines).
There are currently no antiviral drugs licensed by the U.S. Food and Drug Administration (FDA) to treat COVID-19. Some in-vitro or in-vivo studies suggest potential therapeutic activity of some agents against related coronaviruses, but there are no available data from observational studies or randomized controlled trials in humans to support recommending any investigational therapeutics for patients with confirmed or suspected COVID-19 at this time. Remdesivir, an investigational antiviral drug, was reported to have in-vitro activity against COVID-19. A small number of patients with COVID-19 have received intravenous remdesivir for compassionate use outside of a clinical trial setting. A randomized placebo-controlled clinical trial of remdesivir for treatment of hospitalized patients with COVID-19 respiratory disease has been implemented in China. A randomized open label trial of combination lopinavir-ritonavir treatment has been also been conducted in patients with COVID-19 in China, but no results are available to date. trials of other potential therapeutics for COVID-19 are being planned. For information on specific clinical trials underway for treatment of patients with COVID-19 infection, see clinicaltrials.gov.
See Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease 2019 (COVID-19)
Q: Should post-exposure prophylaxis be used for people who may have been exposed to COVID-19?
A: There is currently no FDA-approved post-exposure prophylaxis for people who may have been exposed to COVID-19. For more information on movement restrictions, monitoring for symptoms, and evaluation after possible exposure to COVID-19 See Interim US Guidance for Risk Assessment and Public Health Management of Persons with Potential Coronavirus Disease 2019 (COVID-19) Exposure in Travel-associated or Community Settings and Interim U.S Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease 2019 (COVID-19).
Q: Whom should healthcare providers notify if they suspect a patient has COVID-19?
A: Healthcare providers should consult with local or state health departments to determine whether patients meet criteria for a Persons Under Investigation (PUI). Providers should immediately notify infection control personnel at their facility if they suspect COVID-19 in a patient.
Q: Do patients with confirmed or suspected COVID-19 need to be admitted to the hospital?
A: Not all patients with COVID-19 require hospital admission. Patients whose clinical presentation warrants in-patient clinical management for supportive medical care should be admitted to the hospital under appropriate isolation precautions. Some patients with an initial mild clinical presentation may worsen in the second week of illness. The decision to monitor these patients in the inpatient or outpatient setting should be made on a case-by-case basis. This decision will depend not only on the clinical presentation, but also on the patient’s ability to engage in monitoring, the ability for safe isolation at home, and the risk of transmission in the patient’s home environment. For more information, see Interim Infection Prevention and Control Recommendations for Patients with Known or Patients Under Investigation for Coronavirus Disease 2019 (COVID-19) in a Healthcare Setting and Interim Guidance for Implementing Home Care of People Not Requiring Hospitalization for Coronavirus Disease 2019 (COVID-19).
Q: When can patients with confirmed COVID-19 be discharged from the hospital?
A: Patients can be discharged from the healthcare facility whenever clinically indicated. Isolation should be maintained at home if the patient returns home before the time period recommended for discontinuation of hospital Transmission-Based Precautions described below.
Decisions to discontinue Transmission-Based Precautions or in-home isolation can be made on a case-by-case basis in consultation with clinicians, infection prevention and control specialists, and public health based upon multiple factors, including disease severity, illness signs and symptoms, and results of laboratory testing for COVID-19 in respiratory specimens.
Criteria to discontinue Transmission-Based Precautions can be found in: Interim Considerations for Disposition of Hospitalized Patients with COVID-19
Q: What do I need to know if a patient with confirmed or suspected COVID-19 asks about having a pet or other animal in the home?
A: See COVID-19 and Animals.
Waste Management QAs
Q: What do waste management companies need to know about wastewater and sewage coming from a healthcare facility or community setting with either a known COVID-19 patient or person under investigation (PUI)?
A: Waste generated in the care of PUIs or patients with confirmed COVID-19 does not present additional considerations for wastewater disinfection in the United States. Coronaviruses are susceptible to the same disinfection conditions in community and healthcare settings as other viruses, so current disinfection conditions in wastewater treatment facilities are expected to be sufficient. This includes conditions for practices such as oxidation with hypochlorite (i.e., chlorine bleach) and peracetic acid, as well as inactivation using UV irradiation.
Q: Do wastewater and sewage workers need any additional protection when handling untreated waste from healthcare or community setting with either a known COVID-19 patient or PUI?
A: Wastewater workers should use standard practices including basic hygiene precautions and wear the recommended PPE as prescribed for their current work tasks when handling untreated waste. There is no evidence to suggest that employees of wastewater plants need any additional protections in relation to COVID-19.
Q: Should medical waste or general waste from healthcare facilities treating PUIs and patients with confirmed COVID-19 be handled any differently or need any additional disinfection?
A: Medical waste (trash) coming from healthcare facilities treating COVID-2019 patients is no different than waste coming from facilities without COVID-19 patients. CDC’s guidance states that management of laundry, food service utensils, and medical waste should be performed in accordance with routine procedures. There is no evidence to suggest that facility waste needs any additional disinfection.
More guidance about environmental infection control is available in section 7 of CDC’s Interim Infection Prevention and Control Recommendations for Patients with Confirmed COVID-19 or Persons Under Investigation for COVID-19 in Healthcare Settings.
Footnotes
1 Fever may be subjective or confirmed
2 For healthcare personnel, testing may be considered if there has been exposure to a person with suspected COVID-19 without laboratory confirmation. Because of their often extensive and close contact with vulnerable patients in healthcare settings, even mild signs and symptoms (e.g., sore throat) of COVID-19 should be evaluated among potentially exposed healthcare personnel. Additional information is available in CDC’s Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease 2019 (COVID-19).
3 Close contact is defined as—
a) being within approximately 6 feet (2 meters) of a COVID-19 case for a prolonged period of time; close contact can occur while caring for, living with, visiting, or sharing a healthcare waiting area or room with a COVID-19 case
– or –
b) having direct contact with infectious secretions of a COVID-19 case (e.g., being coughed on)
If such contact occurs while not wearing recommended personal protective equipment or PPE (e.g., gowns, gloves, NIOSH-certified disposable N95 respirator, eye protection), criteria for PUI consideration are met.
Additional information is available in CDC’s updated Interim Infection Prevention and Control Recommendations for Patients with Confirmed COVID-19 or Persons Under Investigation for COVID-19 in Healthcare Settings.
Data to inform the definition of close contact are limited. Considerations when assessing close contact include the duration of exposure (e.g., longer exposure time likely increases exposure risk) and the clinical symptoms of the person with COVID-19 (e.g., coughing likely increases exposure risk as does exposure to a severely ill patient). Special consideration should be given to healthcare personnel exposed in healthcare settings as described in CDC’s Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with COVID-19.
4 Documentation of laboratory-confirmation of COVID-19 may not be possible for travelers or persons caring for COVID-19 patients in other countries.
5 Affected areas are defined as geographic regions where sustained community transmission has been identified. For a list of relevant affected areas, see CDC’s Coronavirus Disease 2019 Information for Travel.
1. Fever may not be present in some patients, such as those who are very young, elderly, immunosuppressed, or taking certain medications. Clinical judgement should be used to guide testing of patients in such situations.
* Note: In addition to cough and shortness of breath, nonspecific symptoms such as sore throat, myalgia, fatigue, nausea, and diarrhea have been noted as initial symptoms in some cases of COVID-19. These symptoms can have several alternative explanations; however, failure to identify and implement proper precautions in a healthcare setting for persons infected with COVID-19 can contribute to widespread transmission in that facility due to the presence of susceptible patients and close interactions with healthcare personnel. For this reason, a lower temperature of 100.0oF and the inclusion of mild and non-specific symptoms should be used by healthcare settings evaluating these patients to increase the ability to detect even mild cases of COVID-19.
* Fever is either measured temperature >100.0oF or subjective fever. Note that fever may be intermittent or may not be present in some patients, such as those who are elderly, immunosuppressed, or taking certain medications (e.g., NSAIDs). Clinical judgement should be used to guide testing of patients in such situations. Respiratory symptoms consistent with COVID-19 are cough, shortness of breath, and sore throat. Medical evaluation may be recommended for lower temperatures (>100.0oF) or other symptoms (e.g., muscle aches, nausea, vomiting, diarrhea, abdominal pain headache, runny nose, fatigue) based on assessment by public health authorities.
1 Isolation is defined as the separation or restriction of activities of an ill person with a contagious disease from those who are well.
1 Home healthcare personnel should refer to Interim Infection Prevention and Control Recommendations for Patients with Known or Patients Under Investigation for Coronavirus Disease 2019 (COVID-19) in a Healthcare Setting.
2 Close contact is defined as—
a) being within approximately 6 feet (2 meters) of a COVID-19 case for a prolonged period of time; close contact can occur while caring for, living with, visiting, or sharing a health care waiting area or room with a COVID-19 case
– or –
b) having direct contact with infectious secretions of a COVID-19 case (e.g., being coughed on).















