
John Tyndall (1820–1893).
At the end of the 1850s, the Irish physicist John Tyndall was working at the Royal Institution, where his career overlapped with the declining years of Michael Faraday. Tyndall became interested in the ‘radiant heat’ (infrared radiation) discovered by William Herschel (see here). Previous scientists, notably the Frenchman Joseph Fourier, had speculated that the atmosphere of the Earth acts like a blanket, trapping heat and keeping the surface of the planet warmer than it would otherwise be. But Tyndall put these ideas on a secure footing by carrying out experiments to measure the capacity of different atmospheric gases to absorb infrared radiation.

In his experiments, the gas being investigated was in a long brass tube, sealed at each end with transparent rock crystal, which does not absorb in the infrared. One end of the tube was in contact with a heat source. Infrared radiation that had passed through the gas in the tube emerged from the other end onto a sensitive detector called a thermopile, which converts temperature differences into electricity. The other end of the thermopile was in contact with another standard heat source. The amount of electricity produced by the thermopile depended on the difference in temperature between the two ends, and was measured with a galvanometer, to reveal how much (or how little) infrared heat had been absorbed by the gas in the tube. The apparatus, known as a ratiospectrophotometer, was so sensitive that the warmth of a human body would have disturbed the experiment, so the deflection of the needle of the galvanometer had to be read using a telescope from the far side of the laboratory. As Tyndall described it in a lecture at the Royal Institution: ‘My assistant stands several feet off. I turn the thermopile towards him. The heat from his face, even at this distance, produces a deflection of 90 degrees. I turn the instrument towards a distant wall, judged to be a little below the average temperature of the room. The needle descends and passes to the other side of zero, declaring by this negative deflection that the pile feels the chill of the wall.’18
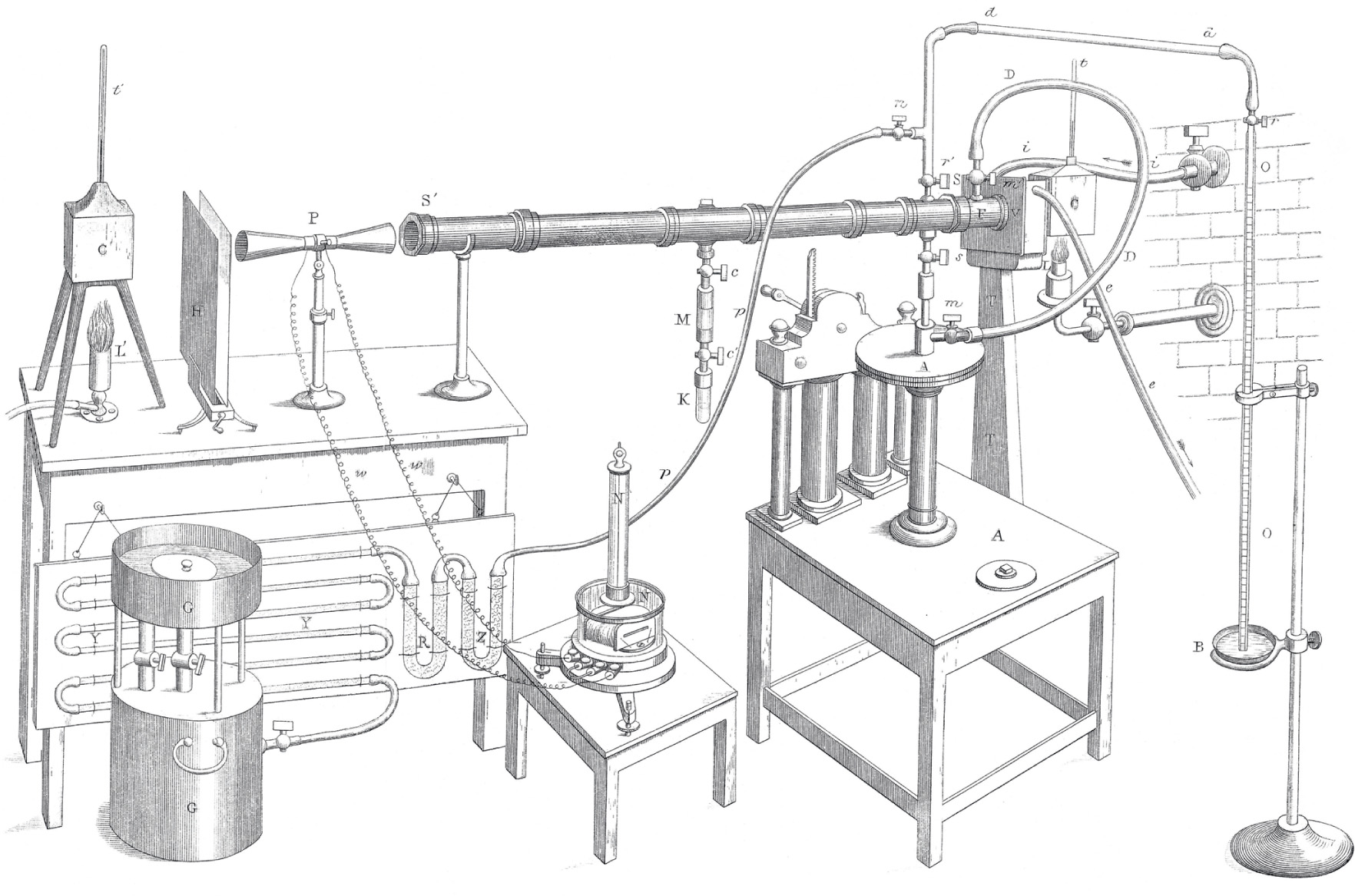
Tyndall measured the relative infrared absorbing power of nitrogen, oxygen, water vapour, carbon dioxide (then known as carbonic acid), ozone, methane, and other hydrocarbons. He found that the best absorbers of infrared radiation are water vapour, carbon dioxide, and hydrocarbons such as methane. Nitrogen, the main component of the Earth’s atmosphere, does not significantly absorb infrared, and nor does the oxygen we breathe, although the tri-atomic form of oxygen, ozone, does. His results were presented to the Royal Society in the Bakerian Lecure of 1861, and later elaborated on in his books and other lectures.
This led Tyndall to develop one of the first scientific explanations for the occurrence of ice ages. Water vapour is the most powerful absorber of infrared radiation, but Tyndall suggested that the amount of water vapour in the air is affected by the amount of carbon dioxide. If there is more carbon dioxide, more heat is trapped and the planet warms a little. That causes more water vapour to be evaporated from the oceans, which enhances the warming. Without the presence of these gases in the air, he said, the Earth would be ‘held fast in the iron grip of frost’. This, he thought, could be the explanation for the Ice Age described by Louis Agassiz (see here). In the past, there had been less carbon dioxide and less water vapour in the air, and the planet had been colder as a result. Although not the whole story, this is one component of the modern understanding of Ice Age cycles (see here), in which small changes in temperature are magnified by such ‘feedback’ processes.
Tyndall also correctly explained the fall in temperature at night and the formation of dew or frost as a result of heat being lost by radiation, and invented a technique for measuring the amount of carbon dioxide breathed out in each human breath. This technique is still used in hospitals to monitor anaesthetized patients.
The truth of Tyndall’s remark about the iron grip of frost is shown by comparing the temperature of the Earth with the temperature of the Moon. The airless Moon receives the same amount of solar light and heat on each square metre of its surface, on average, as the surface of the Earth does. But the average temperature of the Moon (averaged over the entire surface area, day and night) is –18 ºC, whereas the similarly averaged temperature of the Earth is +15 ºC. Although a little heat does leak out from the Earth’s interior, that difference, 33 ºC, is almost entirely due to the warming effect of the atmosphere through the trapping of infrared radiation, a process often known today as the greenhouse effect.