Miller-Urey experiment.
As it became clear that the molecules of life obey the same chemical rules as other molecules, the question of how living material had evolved from non-living material moved from the realm of philosophy into the realm of science. As early as 1922, the Russian chemist Alexander Oparin jumped off from the then recent discovery of methane in the atmospheres of the giant planets to suggest that the early Earth had possessed a strongly ‘reducing’ atmosphere containing methane, ammonia, water, and hydrogen, which had provided the raw materials of life molecules, such as proteins, thanks to the input of energy from lightning and ultraviolet radiation from the Sun. Similar ideas were developed independently in the 1920s by the British polymath J. B. S. Haldane, who coined the term ‘primordial soup’ to describe the conditions in the ocean of the young Earth. Both of them, in fact, were thinking along the same lines as Charles Darwin, who had written in a letter to Joseph Hooker in 1871: ‘If (and oh! what a big if!) we could conceive [of] some warm little pond, with all sorts of ammonia and phosphoric salts, light, heat, electricity etc. present, that a protein compound was chemically formed ready to undergo still more complex changes.’46

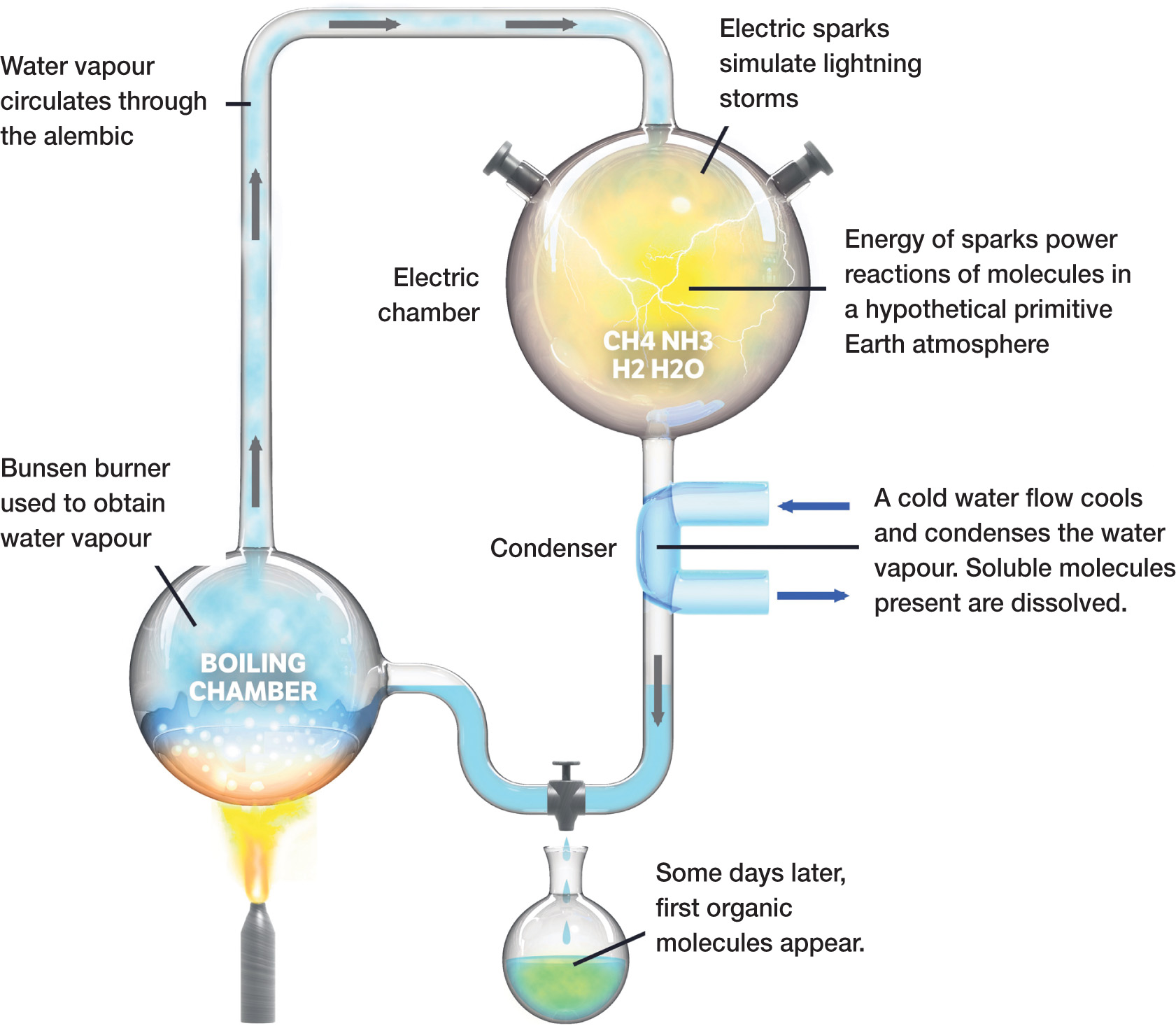
In the 1950s, the American chemist Harold Urey (who had received the Nobel Prize in 1934 for his discovery of deuterium) was working at the University of Chicago. He gave a lecture on the Oparin-Haldane hypothesis, which encouraged a graduate student, Stanley Miller, to ask if he could work on the task of constructing a suitable experiment to test the idea, under Urey’s supervision. The resulting Miller-Urey experiment was a simple closed system in which a 5-litre glass flask containing methane, ammonia, water vapour, and hydrogen was energized by electric sparks to simulate lightning. A half-litre flask half full of steaming hot water provided water vapour to the mixture, with the hot gases leaving the first flask being cooled and circulated back over the boiling water into the flask. Any liquid produced could be caught in a trap, rather like the U-bend under a sink, and collected. The experiment could be run continuously for a week or more before the products were collected and analysed.
Within a day, the liquid held in the trap had turned pink. After a week of operating the experiment, the pink liquid was drained off and Miller found that between 10 and 15 per cent of the carbon present in the original mixture of gases had been incorporated into organic compounds, which included 13 of the 22 amino acids which themselves form the building blocks of proteins in living organisms. He had not created life, but he had produced what were regarded as precursors of life.
The results of the first version of the experiment were published in the journal Science in May 1953, and Miller got his PhD. He continued with research building on this work right up until his death in 2007, using improved equipment and more sophisticated chemical analysis technique, to produce a wide variety of complex organic molecules. By that time, however, it was thought that the atmosphere of the early Earth had probably not been the mixture of gases he had originally assumed, but was dominated by carbon dioxide, with gases such as nitrogen and sulphur dioxide released from volcanoes. But similar experiments with this kind of mixture of gasses, plus water vapour, give similar results to the Miller-Urey experiment.
The key discovery of the original experiment is that relatively simple raw materials plus a supply of energy can produce life molecules. It may not even be necessary for this process to occur on Earth. Astronomers have now detected, using spectroscopy, many complex organic molecules in clouds of gas and dust in space, including things such as formaldehyde and propylene, and a molecule known as iso-propyl cyanide, which has a structure similar to amino acids. And a meteorite that fell near Murchison, Victoria, in Australia on 28 September 1969, was later found to contain many amino acids, including 19 that are found in living things. It now seems likely that complex molecules of this kind, perhaps even including amino acids themselves, were brought down to Earth by comets soon after the planet formed, lacing Darwin’s ‘warm little pond’ with compounds at least as complex as those produced by the Miller-Urey experiment. As Miller himself once said, ‘The fact that the experiment is so simple that a high school student can almost reproduce it is not a negative at all. That fact that it works and is so simple is what is so great about it.’ If a high-school student can manufacture the precursors of life in a week, it is no surprise that the Universe has been able to manufacture life in a few billion years.