

Growth requires many components, all of which must be present simultaneously in the necessary amounts. Growth is limited by the factor that is least abundant relative to the plant’s need for that factor. This is called the Law of the Minimum.
For example, if a pasture has enough of most growth factors to produce 10 tons an acre, but it has only enough zinc to produce 2 tons per acre, it will produce only 2 tons per acre. The pasture manager’s job is to identify the factors limiting production and correct them whenever economical to do so.
The most limiting factor will change throughout the season, even during the course of each day. Early in the growing season, lack of heat or availability of phosphorus may be limiting factors, but moisture may be abundant. Later in the season, heat may be abundant, but moisture may be lacking. Lack of one growth factor may also reduce another growth factor. For example, lack of a mineral nutrient may cause an inability to produce chlorophyll, and then a deficiency of sunlight capture.
There are five growth factors for plants: oxygen, carbon dioxide, sunlight, water, and mineral nutrients (16 have been identified and many more are suspected). We will discuss each growth factor in turn.
Oxygen is necessary for plant respiration. All living plant cells consume oxygen, but most aboveground plant parts are able to access oxygen in more-than-adequate quantities. Belowground, however, oxygen is often the most limiting factor. Plant roots must have at least 10 percent soil oxygen to function, and when the percentage of oxygen in the soil atmosphere drops below that, the roots of most plants cease to grow. Under low-oxygen conditions, root cells begin anaerobic respiration and start to produce ethanol as a by-product of that process. Ethanol eventually reaches toxic levels, and roots begin to die.
There can be many reasons for low levels of soil oxygen. One of the most common is poor internal drainage, in which saturated soil excludes air and root respiration quickly uses up any oxygen dissolved in the water. Compacted soils are another cause of poor soil oxygen levels. Hoof traffic on bare soil can compact its surface layer, which can prevent movement of air into the soil. Vehicle traffic, particularly on wet soil, can cause deep compaction, which restricts rooting depth. It is easier to prevent soil compaction than to remedy compaction after it has occurred.
To prevent compaction:
Maintain adequate levels of surface residue. Surface residue holds up both hooves and tires, similar to a snowshoe keeping a person on top of snow. Overgrazed pastures will invariably become compacted pastures.
Avoid unnecessary traffic, especially in wet weather. Having a place where livestock can be moved to during muddy conditions can spare a lot of pasture damage. If feeding hay on pasture, move all the bales on a dry day and ration out with electric fence. This is discussed more thoroughly in chapter 15.
Trade in the pickup for an ATV. The amount of compaction caused by a vehicle is directly related to the weight of the vehicle. A horse, or your own feet, will cause even less compaction.
Choose plants whose roots can better tolerate low-oxygen conditions. Some plants have a tissue in their roots called aerenchyma, which has open spaces for conducting oxygen from the surface to the roots. Notable examples of forage plants with aerenchyma include Eastern gamagrass and reed canarygrass. These plants can thrive in both compacted and poorly drained soils.
Add drains. Install artificial drainage to remove excess water and allow oxygenated air to enter the soil profile.
Bring in the earthworm troops. Earthworm activity can create air tubes to allow gas exchange, introducing oxygen into the soil and putting carbon dioxide into the plant canopy where it can be used by the plant. Encourage earthworms with a high percentage of legumes and forbs in the plant composition, a high level of plant residue cover, residual plant material left after each grazing, and manure.
Encourage soil fungi. Soil fungi are adept at alleviating compaction. Saprophytic fungi live by eating high-lignin material, such as mature plant stems or woodchips. Mycorrhizal fungi, which colonize the living roots of plants, are available in commercial inoculants. Extract from well-made compost can boost populations of other fungi, but only if a high-lignin food source is available. Leave plant stems uneaten so they can be trampled to the ground to feed fungi and alleviate soil compaction.

The vigorous Eastern gamagrass on the left was inoculated with mycorrhizal fungi; the stand to the right was not.
To make glucose, the photosynthesis process requires carbon dioxide. It is a necessary factor for all photosynthesizing tissues — usually any plant part that is green. Carbon dioxide in the plant canopy is therefore a good thing, while carbon dioxide in the soil is bad because it excludes oxygen.
Carbon dioxide becomes a limiting factor when all other growth factors are in abundance, such as warm, sunny days with good soil moisture and adequate mineral fertility. Added carbon dioxide is of particular benefit to plants in the C3 photosynthetic pathway. Having additional CO2 in the stomatal cavity can greatly reduce photorespiration and improve the temperature tolerance and summer productivity of cool-season plants (see chapter 2).
Although little can be done to raise the level of carbon dioxide in the atmosphere, it is feasible to raise carbon dioxide levels in the plant canopy. Here are two ways:
Add organic materials to the soil surface and allow them to decay. Manures, composts, sewage sludge, lawn wastes, woodchips, and other materials can all offer carbon for the benefit of both photosynthesis and soil structure. In the best-case scenario, only one-third of the carbon in an organic material becomes part of soil humic materials upon decay. The rest of the carbon eventually escapes to the atmosphere in carbon dioxide.
Apply ground limestone to acidic soils to adjust soil pH to a desirable level. Each ton of calcium carbonate will produce about 900 pounds of CO2. Surface applications on a frequent basis provide a slow, sustained release of CO2 into the canopy.
Mineral fertility (along with soil pH) is often the most limiting factor to pasture growth. There are 13 major mineral nutrients for plant growth that must be obtained from soil (three other nutrients — carbon, hydrogen, and oxygen — are derived from air and water). These soil minerals:
|
|
Other minerals necessary in trace amounts are nickel, silicon, vanadium, and likely many others.
Most of these minerals are also necessary for animal growth, although animals also require sodium and cobalt. Animals require some of these minerals in larger amounts than plants do for optimum growth. Supplemental minerals for livestock help fill in the gaps. One way to do so is to supply these minerals mixed with salt. Another approach is to fertilize pastures with these minerals in excess of plant needs, so that plants take up more than they require (a phenomenon called luxury consumption) so that the plants are rich in minerals required by animals. Note that some micronutrients can be toxic in excess; don’t just spread fertilizer without soil testing, plant tissue testing, and consultation with an agronomist.
Soil testing should form the foundation of a soil fertility management program. There are several types of soil tests, and all have limitations. Traditional soil tests were developed in the period after World War II. Simply put, a soil test mimics the ability of a plant root to extract a nutrient from a soil. The test gives a certain value of extractable nutrient, such as 12 parts per million of phosphorus. The fertilizer recommendation is then developed by strip trials applying gradually increasing amounts of fertilizer to see the plant response over many years.
For example, on a soil testing 10 ppm, there would be a trial with no applied fertilizer, another with 20 pounds of phosphate per acre, another with 40 pounds, another with 60, and so forth. The application rate that gave the most profitable response over several years as an average becomes the recommended amount to apply for soils that test at that level. The same procedure is conducted on soils of different test levels. These tests continue to be useful, but it is important to understand their limitations. The recommendations are based on many decades of trial and error that all occurred in the past, on yields acceptable in that era. If it is desired to exceed those levels of production, then more nutrients may be needed.
Traditional soil tests are very good at measuring pH and predicting responses to phosphorus, potassium, and lime but are poor predictors of nutrient release from carbon-based fertilizers such as manure and compost. In the case of high levels of carbon-based fertilizers, a different test, such as the Haney test (performed by Ward Laboratories in Kearney, Nebraska), can more accurately predict nutrient needs. The Haney test is also good at predicting nitrogen response, as well as phosphorus and potassium, and has a decided edge when high levels of carbon-based fertilizer are used.
Another limitation of soil-test lab recommendations is that all of them are based on someone’s opinion. The exact same soil test level measured by several labs can come back with different recommendations. So which one is correct? The truth is, you won’t know before the season is over. An aggressive fertilizer recommendation may be most profitable in a year of abundant rain, while the most conservative one may be most profitable in a drought year.
Or at least that is what conventional wisdom would tell you. What I have seen, however, is that the additional pasture growth in a drought year from added fertility is far more valuable than the added production in a wet year, when forage is already in ample supply (see box above).
Tissue testing of plants can predict response to sulfur and many micronutrients. Use tissue testing to refine fertility after soil tests. Instructions for taking tissue tests are available from the testing laboratory. Tissue testing is useful for predicting plant responses to fertilizer and identifying mineral needs of grazing livestock.
There are at least 14 nutrients necessary to the growth of forage plants. Their importance varies according to the needs of individual plant species.
Nitrogen is the single most common limiting nutrient. Plants use nitrogen to form protein. All protein contains about 16 percent nitrogen; plants cannot form protein without nitrogen in some form. Do the math — a ton of 12 percent protein forage requires about 40 pounds of nitrogen; a ton of 6 percent protein forage requires 20 pounds of nitrogen. A nitrogen deficiency in plants will result in low-protein forage, manifesting itself in yellow leaves at the bottom of the plants (see photo below).

Nitrogen deficiency causes a yellowing along the midribs of older leaves.
Nitrogen is unique in that it is not a component of any rock, as other mineral nutrients are. Rather, it originates in the air, where it occurs as dinitrogen gas (N2). This gaseous nitrogen is in a form that is unavailable to plants but can be converted into available forms by lightning, by a man-made process called the Haber-Bosch process (used to manufacture nitrogen fertilizer), or by the action of several forms of microbes, such as those that colonize legume roots. Lightning, accompanying rainfall and snow, usually adds at best a few pounds of nitrogen per acre per year.
On a practical basis, therefore, nitrogen fertility must be supplied through commercial fertilizer, through application of biological materials (such as manure or compost) that contain nitrogen already taken up previously by plants, or from companion legumes in the pasture mixture. Yet another method is to use free-living rhizosphere microbes that fix nitrogen (convert atmospheric nitrogen into forms available to plants), such as Azospirillum and Azotobacter. We will discuss each of these methods.
Synthetic nitrogen fertilizers can be used for rapid effect, as they are in water-soluble form and contain one or both of the two plant-available ions (ammonium or nitrate) that plants can readily use.
Nearly all commercial fertilizer nitrogen sources are now produced by the Haber-Bosch process, in which methane (natural gas) combines with atmospheric nitrogen to form ammonia gas, which can be liquified under pressure. The resulting anhydrous ammonia can be used as fertilizer applied deep into the soil, or it can be used as a feedstock to produce other forms of nitrogen fertilizer.
Ammonia can be combined with carbon dioxide to form a solid nitrogen fertilizer known as urea (sold as 46-0-0), which can be processed further to form another solid fertilizer called ammonium nitrate (34-0-0). Both urea and ammonium nitrate can be mixed with water to form urea-ammonium nitrate (UAN), which is sold as either 32-0-0 or 28-0-0. Ammonia can also combine with phosphatic fertilizers to form monoammonium phosphate (MAP, or 11-52-0) and diammonium phosphate (DAP, or 18-46-0).
Anhydrous ammonia (82-0-0) is almost always the cheapest source of nitrogen fertilizer per pound, but since it becomes a gas once it comes out of the tank it must be injected into the soil or it will escape into the atmosphere. Injecting a gas into soil requires expensive equipment for success, and since many pastures are full of rocks and stumps, this expensive equipment is likely to be damaged during application.
Urea is usually the next cheapest source of nitrogen, but urea can volatilize (turn into gas and escape to the atmosphere) with surface applications such as those on pastures. Volatilization can be minimized by scheduling fertilizer applications prior to a rain, or with certain commercial additives to the fertilizer that prevent volatilization. I hope you have better luck coordinating rain events and fertilizer applications than I do. Volatilization is more of a problem on high-residue pastures, since residue often contains an enzyme called urease that contributes to volatilization. Volatilization is also enhanced by high surface soil pH, as may occur after a recent application of ground limestone. Urea applications and limestone applications should be separated by a rain event, and the urea should preferably precede the limestone.
Ammonium nitrate is another source of nitrogen fertilizer. It is the most effective nitrogen fertilizer on pasture, but since Timothy McVeigh used a truckload of it mixed with diesel fuel to blow up the Alfred P. Murrah Federal Building in Oklahoma City a couple of decades back, there have been so many regulations tied to its use that now it is hard to find and expensive. Most fertilizer dealers don’t want the hassle of complying with all the red tape that comes with selling a potential explosive.
Ammonium sulfate is a highly effective nitrogen fertilizer source and also supplies sulfur, but it is more expensive as a nitrogen source and, if used to supply all the nitrogen needs of a pasture, will supply sulfur far in excess of actual need (sulfur is required by plants in a rough ratio of 1 part sulfur to 10 parts nitrogen).
Urea-ammonium nitrate is a liquid and usually is applied with a spray boom. That is fine on wide-open areas, but in typical pastures with scattered trees, brush, and fence posts, it can be a recipe for a broken sprayer boom. UAN also causes leaf burn, so its use is generally reserved for the dormant season. The urea portion of the mix is also susceptible to volatilization.
Manure, composts, and other biologically derived materials (which I will refer to as carbon-based fertilizers) are also potential sources of nitrogen and most all mineral nutrients required by plants, because they are derived originally from plant materials, either directly or after consumption by animals. These materials can be collected from external sources (such as municipal sewage sludge or lawn clippings) and spread on pastures.
If such carbon-based fertilizers are available locally at reasonable cost, then I strongly suggest you obtain and use them. If you spread a considerable rate of animal manure or plant-based compost, then it is highly likely that every single plant nutrient will be supplied in adequate amounts, in the approximately correct ratios, by that material, and you won’t have to deal with any other fertilizer applications. That means you can skip the entire rest of this chapter and get on to the more interesting parts of the book!
More importantly, carbon-based fertilizers supply a source of energy for microbes that improve soil function and structure, so they enhance soil moisture and oxygen relations, and release valuable carbon dioxide, also a plant nutrient. If you can get carbon-based fertilizers at reasonable cost, by all means use them. If you cannot, then you may find it useful to apply synthetic fertilizer.
It is also important to realize that while every livestock operation generates manure, not all operations derive the same benefit from it. Animals fed in confinement deposit all that fertility in a spot that cannot benefit from it, and it must be spread mechanically to areas that produce vegetation.
Pastured areas have more inherent efficiency of nutrient recycling, because the manure and urine are deposited by the grazing animals right on the pasture. Even so, this is not a perfect system. The nutrients contained in manure are limited to what the animal ate originally, so grazing does not add minerals to the pasture. It does, however, recycle the nutrients back to the pasture rather than export them the way hay or grain farming does, and the active biology in the vicinity of manure often acts to release minerals from soil particles.
Part of the problem with grazing is that the animal takes in minerals via forage from a large area and deposits them as manure in a very small area. Thus, although most of the minerals in the forage are cycled back through manure, most of the area is impoverished while small areas are grossly enriched.
Over time, you would think this would balance out, but it usually does not. This is because under uncontrolled grazing, there is a movement of fertility to areas where animals rest, such as close to water and under shade. Moving watering sources and shade to underutilized areas can help remedy this problem, as can more frequent rotation, so the manure is deposited more uniformly across the landscape.
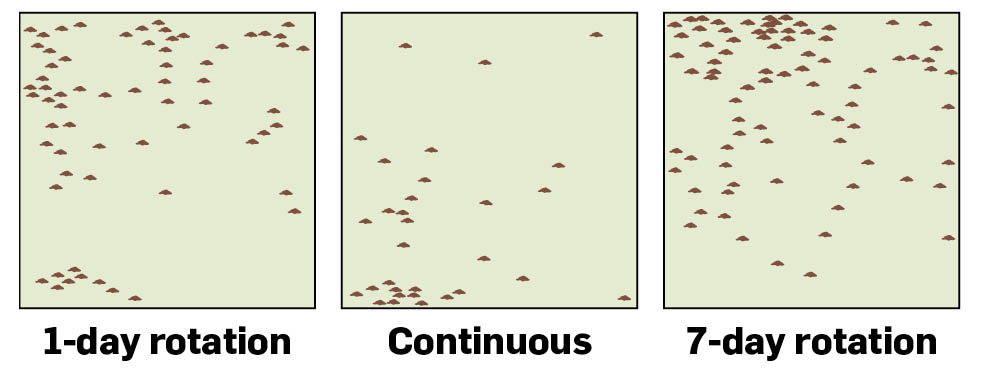
Distribution of manure (represented by dots) is much better with rotational than continuous grazing.
Legumes host bacteria on their roots that are capable of fixing nitrogen in sufficient quantities for the growth of the legume, and in most situations, some of the nitrogen taken in by the legume is exuded from the roots and is made available to other plants in the pasture. If the plants are colonized by mycorrhizal fungi, there can be considerable sharing of nitrogen through the fungi. More information on nitrogen fixation by legumes is covered in chapter 10.
Free-living nitrogen-fixing microbes are not host-specific, like the nitrogen-fixing microbes on legume roots, but instead live in the soil around the roots of many kinds of plants. There are thousands of different nitrogen-fixing microbes, but unfortunately most of these organisms fix only small amounts of nitrogen. Some do hold promise for high levels of fixation, and scientists around the world are enthusiastically trying to isolate and develop better strains of these microbes.
Two microbes that appear to be very useful are Azospirillum and Azotobacter. These gram-negative, nitrogen-fixing soil bacteria are widely used worldwide but are little used in the United States. They have been shown to fix as much as 80 pounds of nitrogen per acre, but a more typical rate is 20 to 30 pounds. This is not enough to produce a profitable grain crop by itself, and if additional nitrogen fertilizer is applied the Azospirillum and Azotobacter use the applied nitrogen instead of expending energy to fix their own.
In a pasture of mixed grass and legumes, however, there is seldom any applied nitrogen fertilizer to avoid reducing nitrogen fixed by legumes. Even 20 to 30 pounds of nitrogen is enough to add an additional ton or so of forage production per acre. This appears to be an ideal niche for free-living nitrogen-fixing microbes. They seem to thrive best in the rhizosphere (soil around the roots) of warm-season grasses, probably because of these grasses’ high rate of both photosynthesis and sugar-rich root exudates.
Phosphorus is often the most limiting plant nutrient in pasture ecosystems and in livestock diets as well. It is a component of ATP, the energy currency of cells, and is a major building block of bones and teeth in animals. Fertilizer phosphates are derived from apatite (rock phosphate) deposits, which are found in only a few locations worldwide. The scarcity of rock phosphate deposits should be cause for eventual concern, as it is estimated that current deposits will be depleted in 100 years or less.
Rock phosphate is sometimes used directly as fertilizer, but it is not water soluble and requires very high rates of application to be effective. It is much more available in acid soil than in alkaline soil. It is also high in fluorine, which can reach toxic levels if applied in high amounts.
Phosphate fertilizers are manufactured by treating rock phosphate with either sulfuric or phosphoric acid to render it water soluble. The most common phosphate fertilizers in the U.S. market today are monoammonium phosphate (MAP), which has an analysis of 11-52-0 (11 percent nitrogen, 52 percent phosphate, and 0 percent potash) and diammonium phosphate (DAP) which has an analysis of 18-46-0. Both supply nitrogen as well as phosphorus, although obviously the DAP has a higher percentage of nitrogen. They are very similar in use.
Finely ground bones are a good source of phosphate and are more available now since the feeding of bone meal became less prevalent due to concerns over prion diseases, such as bovine spongiform encephalopathy. Municipal biosolids (sewage sludge) are a very good source of phosphate, both because the human diet is rich in phosphorus and many detergents contain phosphorus.
One difficulty in phosphorus nutrition for plants is that any water-soluble form of phosphorus is quickly rendered into forms that are progressively less and less available over time. Colonization by mycorrhizal fungi greatly enhances the ability of plants to access phosphorus from otherwise unavailable forms. A phosphorus-deficient plant will be stunted, and the lower leaves often exhibit a purplish coloration.
Potassium functions mainly as an osmotic regulator, important in moving water throughout the plant. Deficient plants often show a burning of the lower leaf edges. The most common potassium fertilizer is potassium chloride, which is mined from several naturally occurring deposits across the West, notably Searles Lake in the Mojave Desert of California.
Potassium chloride is cheap and readily available but has a strong salt effect. The chloride is also a plant nutrient but is required in only small amounts. Other options are potassium phosphate and potassium sulfate, which are agronomically superior but also more expensive.
Other notable sources include wood ashes (usually around 8 percent potassium) and various finely ground rock powders of feldspar materials such as greensand. Wood ashes have a strong alkaline effect and should only be used on acidic soils. They also contain 1 to 2 percent phosphate and about 30 percent calcium, along with many trace minerals and various amounts of charcoal. The rock powders are quite insoluble and should only be valued for their long-term effect unless applied at very high rates (1 ton per acre or so).
Rock powders have very enthusiastic supporters who claim not only improved crop yields but also improved flavor of garden vegetables. Research is less enthusiastic, but well-managed pasture ecosystems with their high level of soil biology may be more able to render the insoluble nutrients into available form.

Potassium deficiency affects lower leaves first, browning the leaf edges.

Sulfur deficiency shows up as a yellowing of young leaves.

Calcium deficiency results in stunted growth and reduced leaf area.
Sulfur is intimately associated with nitrogen, because they both are vital to the formation of protein. Plants use sulfur in an approximate ratio of 1 part sulfur to 10 parts nitrogen. A sulfur deficiency will result in low-protein plants, shown by a general yellowing of the youngest leaves. But sulfur deficiency can also manifest itself in more insidious effects. If plants take up nitrogen in the nitrate form and lack sufficient sulfur to convert that nitrogen into protein, the nitrate remains unchanged in the plants. If animals consume these plants in this condition, it can contain potentially toxic levels of nitrate.
The most common form of sulfur fertilizer is elemental sulfur, which is 90 percent or more total sulfur. It must be oxidized into sulfate form for plants to utilize, a process performed by the bacteria Thiobacillus thiooxidans. It often takes as much as a year after application of elemental sulfur for it to be completely oxidized. Other fertilizers, such as ammonium sulfate or zinc sulfate, already contain sulfur in available forms. Any carbon-based fertilizer will also contain sulfur to varying degrees, with higher-protein materials being higher in sulfur.
Calcium functions mainly as a component of cell walls in plants and of bones and teeth in animals. It is also beneficial to earthworms and legumes. Livestock tend to prefer plants with adequate calcium.
When I was a kid, my father spread ag lime (calcium carbonate, the usual source of calcium on acidic soils) on a field to be planted to corn and turned the tractor around on a grassed waterway. That fall, when he turned the cows out on the stalks, the cows began grazing first on the waterway. The first day he checked on the cows, he saw a curious sight. The half-moon-shaped patterns where the lime spreader had turned around in the waterway were eaten completely to the ground, while the rest of the grass was untouched. Perhaps the improvement in palatability came from an adjustment in pH, or perhaps it was from the calcium.
Limestone supplies calcium but also raises soil pH. Gypsum (calcium sulfate) is another source of calcium, and of sulfur. Gypsum does not affect soil pH and is useful on soils that are neutral or alkaline; it is particularly useful on soils impaired by excess sodium.
Iron functions in the formation of chlorophyll in plants. Iron is present in large quantities in virtually every soil, but the availability of that iron to plants depends greatly on the pH of the soil. Iron is most available in acidic soils, and much less so in alkaline soils.

Iron deficiency causes a yellowing of the upper leaves, while the leaf veins remain green, a condition called interveinal chlorosis.
Iron deficiency causes a yellowing of the leaf tissue between the veins of the upper (youngest) leaves; this condition is called IDC, an acronym for iron deficiency chlorosis. IDC affects millions of acres of soils in the western Great Plains and the upper Midwest (see box at right).
Grass cover crops can be used preceding legume crops, and legume crops can be used prior to grass cash crops. This has been the single most effective method to enhance iron availability.
Other aids to improve iron availability include:
Magnesium has a major function in plants — it is the central atom in a chlorophyll molecule. While magnesium is occasionally deficient for plant growth, it is most often a problem when deficient in animal diets. This usually occurs in early spring on lush pastures of cereals and cool-season grasses (see chapter 10). Magnesium deficiency in plants shows symptoms similar to iron deficiency, with green leaf veins and yellow tissue in between, but affects the older lower leaves rather than the youngest leaves.
Magnesium can be supplied by Epsom salts (magnesium sulfate), or by the more soluble magnesium nitrate. It is also available as dolomitic limestone (magnesium carbonate), which raises soil pH like calcium carbonate–dominated limestone; it should be reserved for acidic soils.

Magnesium deficiency resembles that of iron but occurs on lowest leaves.

Zinc deficiency is often expressed as white striping on the upper leaves.

Copper deficiency results in yellowing and curling of young leaves.
Copper is an occasional deficient plant nutrient, most often on soils very high in organic matter. This puts it in the minority, as most nutrients increase in availability as organic matter increases. Copper is rarely deficient for plant growth to a great degree in North America but can be limited in livestock diets, and so is a common component of livestock mineral supplements.
The usual fertilizer source is copper sulfate. Copper sulfate is also used as an ingredient in walk-through foot baths to prevent foot rot. Copper deficiency is easily diagnosed in black-haired animals — it causes a reddish tinge of the hair coat.
Zinc seems to be intimately associated with organic matter, and eroded areas with missing topsoil are often deficient. Zinc is also less available in soils with pH higher than 7.0. Corn and sorghum seem particularly susceptible to zinc deficiency. Zinc is also frequently deficient in livestock diets and thus a common ingredient in livestock mineral supplements.
Molybdenum is often deficient on acidic soils in the southeastern United States. It is more often lacking in legumes than in other plants, perhaps because it is also required by nitrogen-fixing bacteria.
Boron is often a limiting factor for growth, particularly for legumes like alfalfa. If deficient, it impacts seed production more than forage production. Boron is necessary for the expansion of cells, so a deficiency manifests itself as unusually small upper leaves. It can be remedied by applications of borax. Borax can be toxic to plants if applied in excess, so do not apply unless called for by either soil test or tissue test.
Chlorine is required by plants but is very seldom lacking in pastures because it is a component of common salt, fed to livestock and excreted in urine. The amount fed in salt and recycled back to the soil is almost always in excess of plant needs by a wide margin.
Manganese deficiency is seldom seen in most pasture plants, but is now common in corn and soybeans, due to the widespread use of genetically modified plants resistant to the herbicide glyphosate (Roundup). Roundup-resistant crops have difficulty taking up manganese, because the enzyme systems used to detoxify glyphosate and to take up manganese are intimately linked (the shikimate pathway). If you are planting Roundup-resistant crops, it may be necessary to use either soil or foliar applications of manganese, particularly on higher-pH soils.

Boron deficiency causes stunted growth in upper leaves.

Manganese deficiency shows up as pale stripes on crop leaves.
Cobalt has not been shown to be necessary for plants, but it is the central atom in vitamin B-12 and is needed by both animals and bacteria, particularly the bacteria responsible for nitrogen fixation. Only minute amounts are necessary.
Water is obviously a limiting factor much of the year on many pastures and rangelands. There is little we can do to alter rainfall, but there are hundreds of things we can do to improve the capture of rainfall and its infiltration into the soil. I have authored another book, called The Drought-Resilient Farm, that thoroughly details steps to create more rainfall-efficient pastures. The single best management practice to encourage rainfall infiltration is to leave adequate ground cover after grazing. The table below illustrates how effective higher amounts of residual forage increase infiltration.
Overgrazing creates a drought-susceptible pasture by reducing water infiltration, increasing evaporation, and reducing root growth of forage plants. I cannot stress enough the critical importance of maintaining adequate residual leaf area in pastures. It is the single most important action a pasture manager can make, and I will repeat that same chorus loud and often throughout this book.

Overgrazed pastures turn brown during drought, while properly grazed pastures remain green even with the same minimal rainfall.
Sunlight appears to be something we can do nothing about, but in fact it is a growth factor that we can indeed manage. Although we cannot control the sunlight we receive, we can affect how much we are able to capture.
Anytime we allow sunlight to hit bare ground or dead leaves we are losing productivity potential. The major cause of reduced sunlight capture is overgrazing. The removal of so much mulch that sunlight hits bare ground is the main cause of poor pasture productivity. It takes leaves to make leaves.
Another reason we fail to capture all the available sunlight is that a high percentage of our annual sunlight falls on dormant or nonfunctioning leaves. Sunlight falling on a monoculture of warm-season grasses in spring or fall is wasted potential, because the warm-season grass is dormant at that time. Sunlight falling on a monoculture of cool-season grasses in late summer is often wasted because the grass is photorespirating. Having a diverse mix of pasture species that include both warm-season and cool-season plants can extend the period of effective photosynthesis and increase pasture productivity.

In a balanced pasture ecosystem, an abundance of predators — like this wolf spider — prevents an overabundance of insect pests.
Insect predation can (literally) take a bite out of pasture production. Every plant is eaten by some insect, and some can be devastating, such as the alfalfa weevil on alfalfa.
When I first planted my alfalfa-grass pasture mixes, alfalfa weevils were a terrible scourge. I sprayed for them every year, and every year they seemed to get worse. Then I talked to some producers who told me they were in a similar situation. Every year the weevils got worse; they went from spraying once for weevils to twice for weevils. And every year that they sprayed for weevils, it seemed like another insect problem that also required spraying would show up later in the season. It seemed that they could not kill weevils, no matter how many times they sprayed.
Then they decided to just quit spraying. The weevils were bad the first year, but the next year they were not as bad, and by year three with no spray, they seemed to not be a problem at all. The other later-season insects also ceased to be problematic. Hearing their stories, I thought to myself, What do I have to lose, except a very high spray bill?
I gave up spraying, and just endured the damage in year one. In year two, the damage was much less, and just as predicted, in year three the damage was almost nonexistent. I was surprised, but the reason became apparent one night when I walked through this pasture with a flashlight.
Everywhere I shined the light, I saw little glowing dots. When I looked closer, the glowing dots turned out to be the eyes of wolf spiders. There were thousands and thousands of wolf spiders, ranging from little bitty ones to huge hairy ones. No wonder all my pest insects were disappearing. I made a mental note to not lie down in this pasture, especially at night.
The long-term solution to most insect pests is not a jug with a skull-and-crossbones on it; it is diversity. Most pests have a very narrow range of plants on which they feed. The alfalfa weevil, long the bane of my grazing operation, feeds only on alfalfa. When I made my pastures more diverse, my weevil problems greatly diminished. Most pests prefer a monoculture of their preferred host plant, not complex mixtures.
Shortterm, there are approaches to alfalfa weevil control that do not require chemical intervention. Since alfalfa weevils lay their eggs in alfalfa fields in late fall, grazing or burning these fields in the winter will remove the eggs. Including grasses in a mixture with alfalfa can provide fuel for a fire, and also provide for better winter grazing than a pure stand of alfalfa.
Also, an early haying or grazing, much earlier than normal harvest time, will kill most weevils, as the larvae climb to the top leaves and are exposed to being eaten or crushed by the hay crimper. This will cause a small reduction in alfalfa yield but less than what the weevil can cause, and this is also the time when alfalfa is best able to recover from damage.
I have also heard that spraying a molasses solution on alfalfa will kill alfalfa weevils. The theory is that alfalfa weevils cannot process simple sugars in their digestive system (alfalfa leaves are very low in sugar), so the molasses brings on a severe digestive disturbance. I have not tried this approach, since my weevils now appear to be well under control by their natural enemies, but I wish I had known about this when I was still struggling with them.
Diversity of plants also means a diversity of pests, which means a season-long buffet for things that eat pests, such as wolf spiders and mantids and orioles. Monocultures have large numbers of a few species of pests that appear for just a short period of time; they do not build predator populations because the food supply is not consistent for a long enough period of time. A variety of pests that provide a constant supply of food throughout the growing season can build predator populations and provide a measure of protection against most pest outbreaks.

Grasshoppers are often more plentiful in dry years.
One glaring exception to this “diversity provides protection against pests” rule is that general plant feeders, such as grasshoppers and armyworms, can still have outbreaks. Having a healthy population of insect-eating birds can provide some protection, but even the healthiest system can experience grasshoppers. Grasshoppers are typically worse in dry years and overgrazed conditions but can occur in any year and in any pasture.
There are several methods to control grasshoppers. The most obvious is to spray them with an insecticide. This does a great job of killing grasshoppers, but it also kills all the other insects in the pasture, which can have undesirable long-term consequences. The other methods have fewer negative consequences, but it is essential to use them when grasshoppers are small.
The first “less negative” method is to spray with a product called Dimilin. Dimilin is a growth regulator that prevents molting, and thus affects insects that mature through metamorphosis — shedding their exoskeleton at regular intervals. It has fewer off-target effects than broad-spectrum insecticides.
Another method is to spread a wheat bran–based bait containing the common garden insecticide carbaryl (Sevin). This only affects insects that eat the bait. It is very effective against most grasshoppers and crickets, which find wheat bran irresistible. It can also control some of the pest caterpillar species such as armyworms. The amount of insecticide applied to the acre is a tiny fraction of the amount applied in a broadcast spray.
The final method is to use a different wheat bran–based bait, containing the grasshopper protozoan parasite Nosema locustae. This affects only grasshoppers and crickets and has virtually no off-target effects. It is safe for any birds or other insects that consume the dead or dying grasshoppers, maintaining populations of these beneficial organisms. This method is very slow, often requiring as much as 6 weeks to begin killing grasshoppers, so it must be applied very early.
It has an additional advantage that probably makes it the overall best grasshopper control method, if applied early. Since grasshoppers are cannibalistic, consuming their dead comrades, this parasite passes from grasshopper to grasshopper, spreading across the pasture and reproducing itself over time. Thus, one application can not only kill grasshoppers during the year in which it is applied to a particular pasture, but it can also kill grasshoppers in adjacent areas, sometimes for several years into the future. It is also economical, too, costing only a couple of dollars an acre. Keeping grasshoppers at a low level can prevent a great deal of herbivory and preserve more forage for livestock.
Salt in the soil creates a powerful attraction for water, more powerful than the pull that plants can exert to obtain water. The effect of excess salt in the soil is to starve plants for water, though the soil itself may be moist.
The process to remove salt from soil is simple, but usually prohibitively expensive. It involves the installation of underground drainage tubing to give the salt an exit, and enough of either rainfall or surface irrigation water to flush the salt out of the soil. An application of gypsum may also be necessary to keep sodium from attaching to the soil colloids on its way out. Flushing out is effective but slow and costs thousands of dollars an acre; it requires both topography that features a nearby low point to receive the drainage and a source of irrigation water that is low in salt. Since these two conditions seldom occur in areas used for pasture, this is not the typical solution.
Millions of acres of currently irrigated cropland are experiencing salt buildup due to poor drainage and irrigation with salty water sources. Other areas are experiencing saline seeps, when farmers fallow semiarid cropland to build up soil moisture. This can cause the movement of underground salt, which then exits the soil somewhere downhill, creating a salty deposit. Few annual crops have good salt tolerance, but many perennial forage crops do. Often the most economical solution is to simply plant the area to forage crops that are tolerant of salt. See Salt-Tolerant Forages.
Nematodes can greatly reduce plant productivity. Nematodes are most often a problem in monoculture stands of forage. Rarely do nematodes become a problem when there is a diversity of plant species from multiple plant families. Some nematodes only affect grasses, others only attack legumes, and still others only attack forbs. Having all three groups of plants reduces the impact of any one nematode species. Having a diverse mix of forages also means there will be a diverse population of nematodes, which will attract nematode-eating predators.
Most plant diseases affect a single species of plant. Diversity is important to reduce the odds of any one disease causing catastrophic yield loss. An active and healthy soil microbial community will be very helpful in reducing disease outbreaks. A healthy soil microbial community can be achieved as a natural beneficial by-product of the processes used to improve pasture productivity.