
MOST READERS WILL HAVE ENCOUNTERED a bird nesting within their garden, perhaps a Blackbird nesting in a shrub or a Blue Tit occupying a nest box erected for the purpose. Many other nesting attempts will have gone unnoticed, the first indication of their occurrence revealed by the appearance of newly fledged youngsters on the garden lawn or at hanging feeders. But just how many birds nest in our gardens, and how do their nesting attempts and breeding success compare with those made by the same species in other habitats? This is something that we will explore through this chapter, which also includes a wider examination of territory, breeding behaviour and nesting opportunities. Courtship behaviour is covered more fully in Chapter 5.
HOW MANY BIRDS BREED IN GARDENS?
As we saw in Chapter 1, data from the BTO/JNCC/RSPB Breeding Bird Survey have been used to produce habitat-based population estimates for many UK species. The population estimates produced for those habitats associated with human sites – urban, suburban and rural habitation – suggest that sizeable proportions of the populations of several species breed alongside us, with many of these birds using the urban green space that is gardens (Newson et al., 2005). That 33 per cent of our Blackbirds and 54 per cent of our Starlings breed alongside us means that how we manage urban green space, including gardens, can have a significant influence on a large component of their UK breeding populations.

FIG 46. Wrens are cavity nesters, but adaptable enough to take advantage of even the most unlikely cavities. (John Harding)
While these habitat-based population estimates are very valuable, they do not tell the whole story; the Breeding Bird Survey involves participants walking transects through randomly selected survey squares. While this approach works well within much of the wider countryside, it may not give a complete picture of the breeding bird populations to be found in urban areas. Since many urban birds will be breeding and feeding in private gardens, hidden from view by hedges, fences and buildings, it seems likely that Breeding Bird Survey observers will miss a proportion of the birds that they pass while carrying out their fieldwork. The method should allow the survey to pick up changes in urban populations over time, which is its purpose, but it might be less good at determining the size of these populations.
Similar criticisms can also be made of the approaches used to derive national population estimates from atlas data (e.g. Gibbons et al., 1993). While atlas fieldwork covers all habitats, it again suffers from the difficulties of working within urban areas (see Chapter 6) and is likely to miss a sizeable proportion of the bird populations breeding there. Recognition of this problem can be seen when Gibbons et al. (1993) rejected their initial population estimate for House Sparrow in favour of one that had been derived largely from urban data presented by Dennis Summers-Smith (Summers-Smith, 1959). Summers-Smith’s estimate took into account direct measures of House Sparrow density, made in urban and suburban sites within London, alongside those from rural areas. This suggests that finding other ways to secure information on the numbers of birds breeding within urban areas, and their associated green spaces and private gardens, could support the production of more robust estimates of national populations.
One approach, adopted by Bland et al. (2004), is to ask householders to survey their gardens for nesting birds and to report back on what they find. Richard Bland and John Tully, both retired teachers living in Bristol, worked with Jeremy Greenwood of the BTO on a garden-nesting survey, carried out in 2000. A questionnaire was sent to all 12,687 households participating in BTO Garden BirdWatch, with 6,035 useable responses returned. From these, and using additional information on the number and type of households present in the UK (taken from the 1991 housing census), it was possible to produce estimates for the garden populations of 17 familiar species. This approach makes a number of assumptions, most notably that survey respondents find all of the nests on their property and that those participating in the survey are representative of wider society. Neither of these assumptions is likely to be true, but it is unclear how big a difference this makes to the resulting estimates. Undercounting the number of nests will mean that the final estimate is lower than it should be; if Garden BirdWatch gardens are better for birds than non-Garden BirdWatch gardens, then it might mean that the final estimate is larger than it should be. Despite this, the estimates produced in the paper suggest that national population figures, derived from other methods, are too low for several species because we have not looked closely at gardens. The five species where the national estimates produced by Gibbons et al. (1993) are highly likely to have been significantly underestimated are Dunnock, Starling, Great Tit, Blue Tit and House Sparrow, with some evidence to suggest that Robin and Blackbird are also too low. The study also provides important information for Swift and House Martin.
While the different approaches used to derive population estimates for urban breeding birds have their individual limitations, it is useful to look across the range of figures produced to see if there are any general patterns (Table 5). Looking at the figures produced by Bland et al. (2004) suggests that domestic properties and their gardens may sustain densities of nesting birds approaching those seen in many woodland habitats, although the diversity of species present in gardens is considerably less than that seen in woodland. Of course, the figures presented in the table are for the most common and familiar species and do not tell us about the less common species that may also make use of gardens for breeding. We do know, however, that where a garden is located and which habitats surround it will also shape the species using it for nesting. A quiet corner in a rural garden, perhaps with long grass or a tangle of nettles and bramble, might support nesting Red-legged Partridge Alectoris rufa or Blackcap, while the flat-roof of a Georgian town house might have a nesting Lesser Black-backed Gull.
TABLE 5. Estimates of numbers of pairs of birds nesting in gardens in Great Britain derived from the BTO Garden Nesting Survey (Bland et al., 2004), compared to those derived from the national Bird Atlas (Gibbons et al., 1993), the latter split into figures calculated for all habitats other than farmland and woodland, and all habitats.

Now that we have a sense of the numbers of breeding birds present within gardens and the wider urban area, we need to turn to the question of their importance. To some extent we have already touched on this by highlighting the numerical importance of the sizeable breeding populations to be found in gardens. However, such populations are only important for the wider population if they are productive and contribute young birds to the next generation. If the failure rates of nests built in gardens are high, or if the numbers of chicks produced is low, then garden nesting may be a very poor option for a bird. If this proves to be the case, then, as some authors have suggested, gardens may be an ‘ecological trap’ (Robb et al., 2008b). This is what we will turn to look at now.
IS NESTING IN A GARDEN A GOOD OPTION?
There have been various studies looking at the breeding ecology of birds within gardens and the wider urban environment. Some of these have only looked at this habitat; some have looked at different types of urban habitat (e.g. urban versus suburban); and others have looked at how urban breeding compares with that seen in more natural habitats. One of the key measures to examine when seeking to determine whether nesting in a garden is a good option or not is productivity, the number of chicks fledged from the nesting attempt. This is a commonly reported measure but doesn’t tell the whole story because it is the number of those fledglings who go on to recruit into the breeding population, and breed themselves, that actually determines future population size. If productivity is good but subsequent survival poor, then you may find a population is no better off than one in which productivity is poor but subsequent survival good. The difficulty in tracking individual birds through from the egg to when they breed explains why the number of young fledged is so commonly used as the measure of productivity. Since it can also be difficult to follow the sometimes multiple breeding attempts made by a pair across the breeding period, productivity tends to be reported as ‘productivity per nesting attempt’ rather than as ‘productivity per season’.
Perhaps a good place to start is with the familiar Blue Tit and Great Tit, since these are the species that most readers are likely to have encountered breeding within their garden. They are also species for which a good deal of information on breeding biology has been collected, both in a variety of woodland habitats and, to a lesser extent, from within gardens and the wider environment. Cowie & Hinsley’s study, carried out in two suburban areas within Cardiff, underlines that the breeding success of these two species is much lower in suburban gardens than it is in natural woodland habitats (Cowie & Hinsley, 1987). In addition to producing smaller clutches, suburban tits only managed to rear, on average, half as many young as their counterparts living in mixed deciduous woodland typically do. A particular characteristic of these suburban nesting attempts is the mortality of nestlings due to starvation, suggestive of a shortage of favoured invertebrate food during the chick-rearing phase. Other UK work with an urban component has been carried out by Roger Riddington and Andy Gosler, working at Oxford University’s Wytham Wood study site and in the surrounding area. Wytham is a mixed deciduous woodland, of the type that is most productive for nesting tits. Riddington & Gosler (1995) compared the breeding success of the Wytham Wood population with those pairs nesting in smaller woods and local gardens. Clutches initiated in Wytham were larger and started earlier, with larger broods, more fledged young and heavier fledglings, all underlining that the mixed deciduous woodland was the better breeding habitat; unfortunately, Riddington and Gosler did not separate out the garden nesting birds from the other habitat types lumped within their ‘marginal habitat’ category. Examination of nestling diet, through analysis of faecal sacs collected during nest monitoring visits, revealed that the woodland chicks received a better-quality diet, dominated by caterpillars; chick diet in the marginal habitats was dominated by adult flies, beetles and spiders.

FIG 47. Gardens support a wide range of breeding birds, including species like Red-legged Partridge. (Jill Pakenham)
The loss of nestlings is something that has also been reported from work carried out on garden-nesting and urban-nesting thrushes. Murray (2004) found that the daily failure rates of nestling Song Thrushes were significantly higher in gardens and parks than they were in nearby woodland sites. It is important to understand whether such nesting failure is the result of low food availability or increased predation risk. Evidence for a shortage of suitable invertebrate prey within gardens comes from a detailed study of House Sparrow populations living in and around the city of Leicester (Peach et al., 2008). This study, led by Kate Vincent, found an average of just 2.02 chicks fledging per nesting attempt, with a quarter of nesting attempts failing to fledge any young at all. High levels of chick mortality were important in driving this poor performance, with 72 per cent of the chick mortality occurring within the first four days after hatching and most likely the result of starvation. Chick survival and chick body mass at 10–12 days old were both strongly related to the quality of chick diet, with chicks receiving a greater proportion of plant material faring worse than those receiving more invertebrates. An examination of the insects being provisioned to the chicks revealed the presence of craneflies, weevils, spiders, ants and aphids, but few of the grasshoppers and moth caterpillars seen in many other studies.

FIG 48. Grasshoppers feature in House Sparrow diet in many wider countryside studies but are largely absent from urban diets. (Mike Toms)
It has been shown that the body condition of House Sparrow chicks is a good predictor of future survival prospects during the period immediately after they have left the nest. Many of the chicks in the Leicester study were small and of low body weight, suggesting their future survival chances were poor. Interestingly, Liker et al. (2008) found that the body size and condition of adult House Sparrows was lower in an urban environment than a rural one, and that this difference was maintained even when captive individuals from the two populations were fed on identical diets. Liker et al. postulated that this was indicative of carry-over effects from a poor nestling diet. If this is the case, then we might predict that the poor condition, small-sized nestlings fledging from those Leicester nest boxes would go on to become poor condition, small-sized adults. It is unclear whether smaller size and poor body condition is common across urban bird populations but it has also been noted in urban tit populations in Germany (Junker-Bornholdt & Schmidt, 1999).
That the body condition of the chicks in the Leicestershire study was strongly and negatively related to local NO2 concentrations, might suggest that pollution levels in this area were impacting the House Sparrows by reducing the availability of favoured prey. If this didn’t result in starvation while they were in the nest, then it might still reduce their chances upon fledging. Peach et al. (2008) carried out some modelling work to see whether the productivity of this House Sparrow population was sufficient to sustain it longer term. In two of the three study years, the average annual production of young per pair was lower than the predicted threshold required to maintain a stable breeding population.
With all of these different studies, looking at different species in different ways, it can be difficult to tease out common patterns and to draw general conclusions. Attempts have been made to do this by using a technique known as meta-analysis; meta-analysis uses a statistical approach to combine the results from multiple studies. We used this approach to look at a suite of studies that had reported on various measures of breeding success from urban and non-urban sites, selecting only those studies that had made a comparison between paired sites – one urban and one non-urban (Chamberlain et al., 2009a). Our meta-analysis revealed that, across the species studied, urban populations started breeding earlier in the year than non-urban populations but they were less productive, with all the different measures of productivity found to be higher in non-urban sites. Only the rate of nest failure was found not to differ between the two different habitat types.
These differences are of particular interest because relatively early breeding in natural or semi-natural habitats often leads to higher productivity; so why do we not see this in urban habitats? It could be that the supplementary food being provided in gardens supports early nesting (see Chapter 2) but that urban sites then lack the invertebrate foods needed by the resulting chicks, leading to lower levels of productivity. The associated question of whether the supplementary food enables females to achieve breeding condition earlier, or merely shapes the timing of breeding by acting as an indicator of food resources available within the environment, might be answered by our finding that clutch size was larger in non-urban sites. Since you might expect clutch size to be determined by the resources that a female has available, discovering that clutches were not bigger in urban areas might suggest that the supplementary food was not shaping breeding condition itself but merely indicating general feeding conditions in the environment, which might be the trigger to initiate a breeding attempt. Of course, supplementary food could enable females to attain breeding condition earlier, but it might be a different resource (such as calcium) that ultimately limits the number of eggs that a female can produce in an urban habitat.
There is also the possibility that other components of the urban landscape might influence the timing of breeding. Later in this book we will look at the impacts of heat pollution on songbird activity at garden feeding stations, but heat pollution also needs a mention here. Urban areas are often a few degrees warmer than the surrounding countryside and this additional warmth might result in birds nesting earlier in the year than they might otherwise do. It has, for example, been shown that nest boxes exposed to warmer temperatures result in earlier laying by Great Tits (Dhondt & Eyckerman, 1979).
As we noted earlier in this book, there is much variety between gardens, and between different types of urban landscape in terms of their suitability for birds. Consequently, we might expect to see variation in breeding success across different types of gardens and between different parts of a larger urban landscape. Using BTO Nest Record Scheme data, Gavin Siriwardena and Humphrey Crick found that the laying dates of House Sparrows and Starlings varied across the urban landscape, being the earliest in urban sites and latest in suburban sites – interestingly, rural sites were intermediate between the two (Siriwardena & Crick, 2002; Crick & Siriwardena, 2002). Siriwardena & Crick’s work also revealed that Starling clutch size was significantly lower in urban sites, something also evident in the work of Mennechez & Clergeau (2001).
That gardens appear to be a sub-optimal habitat for many small birds, particularly those feeding their chicks on caterpillars, is perhaps not that surprising. The pattern of poor breeding success and low fledging weights seen in gardens is similar to that seen in other sub-optimal habitats, such as small and isolated woodlands (Hinsley et al., 1999). Blue Tits and Great Tits breeding in small woodland fragments tend to be less successful and rear lighter young; in some years, the Great Tit populations using these woods suffer a high proportion of complete nest failures at the chick stage (something that also seems to occur in gardens), suggestive of starvation. This tendency is less evident in Blue Tits, which might help to explain why we see more Blue Tits breeding in gardens than Great Tits, despite the latter’s ready use of garden feeding stations in winter and dominance at suitable nest sites.

FIG 49. Nest survival rates for Blackbirds using rural gardens are higher than those in urban and wider countryside sites. (Jill Pakenham)
It is worth just reiterating that gardens vary in their structure, location and in the resources they contain. The consequences of this for nesting birds can be seen quite clearly in a piece of recent work by colleagues at the BTO who have been developing new statistical approaches to look at nesting success (Miller et al., 2017). This work, which uses Blackbird as its model species, has revealed that nest survival rates are higher in rural gardens than in either urban gardens or wider countryside habitats. This suggests that rural gardens offer a better balance between the lower availability of favoured food seen urban areas and the higher predation rates of the wider countryside. This underlines that we need to be careful not to generalise about gardens or to treat them as a single homogenous entity.
THE TIMING OF BREEDING
As we saw in Chapter 2, the presence of supplementary food can lead to earlier breeding, something we might therefore expect to see in the urban populations with access to garden feeding stations. There is evidence to support this from studies carried out here in the UK and elsewhere within Europe. Suburban Great Tit populations – but not the Blue Tit populations – in Cardiff were found to breed earlier than their woodland counterparts, something already alluded to when we discussed the meta-analysis carried out by Chamberlain et al. (2009a). This pattern of earlier breeding in urban environments has also been reported in other garden bird species. In Blackbird, for example, both male and female birds living in an urban area in Munich developed their reproductive organs some three weeks earlier than those breeding in a nearby forest. In both populations, regress in the reproductive organs took place at the same time during mid-summer, providing the urban birds with an extended breeding season (Partecke et al., 2004). Urban Magpie populations have also been found to lay significantly earlier than those in rural locations (Antonov & Atanasova, 2003).
In woodland populations of Blue Tit and Great Tit, breeding early in the spring usually enhances breeding success (Nilsson, 2000). It has been shown, for example, that early hatched nestlings tend to grow more rapidly (Perrins & McCleery, 1989) and have better longer-term survival prospects (Norris, 1993) than those hatched later in the season. Of course, because these two species are so dependent on caterpillars, there is a limit to how early the nesting attempt can be; nest too early and the peak demands of your growing nestlings will fall significantly earlier than the peak in caterpillar abundance, greatly reducing the number of chicks that fledge. Some idea of the forward planning required can be seen from the time between when the decision to begin egg laying is made and when the first eggs hatch – in tits this interval is something like 26 days.
The timing of breeding is something that has been influenced by climate change, with UK birds now starting their breeding attempts earlier than they did just a few decades ago (Crick et al., 1997). Climate change has also altered the arrival and departure dates of UK migrants (Newson et al., 2016), and both of these factors may shape the timing of breeding events within gardens. While we see an earlier start to the breeding season in gardens, for the reasons already outlined in this chapter, we do not necessarily see this leading to increased breeding success. In fact, for most species, we see a lower breeding success than is the case in other habitats. We also see significant variation within urban areas in terms of the success of individual nesting attempts, something that may be shaped by the quality of the parents, by the nature of the nest site selected, or by the resources available to the birds. These are things that we will explore over the following sections of this chapter.
TERRITORY
Most garden birds maintain a defended breeding territory throughout part of the year; in some species this may only extend to the area in the immediate vicinity of the nest site, but for others it can extend over a much larger area. The distribution and size of these territories is typically driven by the availability of food resources, though it can sometimes be shaped by the availability of nest sites. Where food resources are spread relatively evenly throughout the landscape, then the pattern of territories is likely to be fairly regular, but if food resources are clumped or distributed in a way that leads to some areas or habitats holding more, then territory size and spacing becomes irregular. An area such as a small deciduous woodland, with an abundance of favoured caterpillar prey, will potentially support a higher density of breeding territories than an urban park, with few caterpillars. In addition to the territory-holding birds there will be other individuals, either those that have yet to reach breeding age or those that are of breeding age but which have been unable to secure a territory of their own. The presence of the latter group of individuals can be readily proved by removing territory-holding birds; such vacant territories are then quickly filled.

FIG 50. Robin territories are defined and defended through a combination of song and display. (Jill Pakenham)
The ownership of a breeding territory is advertised and maintained through song and display, with individual birds only resorting to direct aggression if these behaviours fail to drive an intruding bird away. Behaviours associated with territorial defence can involve the adoption of particular postures, such as those that show off plumage ornamentation, asserting status and dominance. We will look at these in more detail in Chapter 5 when we examine garden bird behaviour. If resources are not distributed evenly, which is usually the case, then we might expect the most dominant birds to occupy the best territories and to then receive the benefits associated with this (such as increased productivity). A question that then arises is to what extent does territory quality, rather than individual quality, determine breeding performance? While some studies have suggested that territory quality is the more important, others suggest it is that of the individual. Teasing out these different components is difficult and what you really need is a study in which you can follow the fortunes of a series of individuals, nesting across a series of territories and sometimes using different ones in different years. This is something that has been examined on the outskirts of Sheffield for Magpie, with the aim of quantifying the separate effects of individual quality and territory quality on several different measures of breeding performance (Goodburn, 1991). The results of this study revealed that male quality explained 70 per cent of a breeding pair’s success within a breeding season, with female quality explaining over 60 per cent of the variance in clutch size and in the size of the eggs laid. The timing of breeding, however, appeared to be controlled primarily by territory quality – though the effect was relatively weak. These results contrast with those of Högstedt (1980), who also studied Magpies, the different findings most likely the results of the greater variability in territory quality evident within Högstedt’s study area and the more frequent occurrence of territory vacancies.
Garden birds, particularly those occupying gardens within larger urban areas, may have access to ready supplies of food (at least in terms of adults and fledged young). They may also have ready access to nest sites – though this clearly varies with species and the types of nest sites favoured. How then might the structure and distribution of territories within an urban area differ from those seen in other habitats, and how might the ‘quality’ of territory holders compare with those elsewhere? David Snow (1958), working in Oxford, found his Blackbirds to hold territories ranging in size from 0.16 to 0.24 ha, though it is worth just noting that Creighton (2001), working the same site, reported males holding territories of 0.54 ha. Elsewhere, urban Blackbird territories have been reported varying from 0.12 to 0.7 ha (Jackson, 1954; Lind, 1955; Ludvig et al., 1994). By way of comparison, territory sizes in mixed deciduous woodland habitat are typically 2.2–2.7 ha (Tomiałoj, 1992). The smaller territory sizes of urban Blackbird populations are reflected in the very high densities reported in the work of Simms (1965), Batten (1973) and Mason (2000), documenting urban densities of 96, 246 and 186 territories per km2 respectively.
Habitat structure appears to influence the settlement patterns of nesting Blackbirds in wider countryside habitats, with areas occupied by breeding pairs typically having greater habitat complexity than unoccupied sites (Hatchwell et al., 1996b). Settlement behaviour may be important at a more general level; it has, for example, been found that Great Tits settling on urban territories may be smaller on average than those individuals occupying rural territories (Lehikoinen, 1986). Some of this may result from urban-born individuals being structurally smaller than their rural-reared counterparts, but it may also result from size-related success when competing for food resources within woodland during the winter months.
The results of our work on urban House Sparrow populations (Shaw et al., 2011) help to draw out some of the landscape features important to this species during the breeding season. By mapping the presence of chirping male House Sparrows onto a detailed habitat map for each of our survey squares, we were able to determine which urban habitat features were favoured and which were avoided. Habitat use by House Sparrows within the core 50 m around their nest sites was significantly non-random with respect to the availability of habitat types within the survey squares as a whole. House Sparrows consistently selected residential areas with gardens over every other habitat type, regardless of the level of urbanisation in the area. Interestingly, the least preferred habitats were buildings without gardens and (perhaps surprisingly) urban green space.
The importance of houses with gardens (combining nesting and foraging opportunities) has long been recognised for House Sparrow, though it is only more recently that we have discovered that those properties in the more economically deprived areas of towns and cities offer better opportunities for House Sparrows than those in more affluent areas (Shaw et al., 2008). Houses situated within more economically deprived areas are likely to offer more nesting opportunities for House Sparrows because their roofs are likely to be in a poorer state of repair, while their associated gardens are more likely to contain less well-managed vegetation and, potentially, a greater abundance of invertebrate prey. Detailed local work carried out in Oxford by Wilkinson (2006) suggests that gardens with a high density of bushes are more likely to support House Sparrows, as are gardens with a greater proportion of native vegetation. The aversion to green space found in our study was unexpected, given how important it appears to be to House Sparrow populations in other European cities (Murgui, 2009), where private gardens are sometimes less common, but it may be because urban green spaces in the UK tend to be open and rather homogenous in character, reducing their attractiveness to House Sparrows.

FIG 51. Nesting opportunities for urban House Sparrows have declined with changes in building regulations and the nature of roof tiles and barge boards. (John Harding)
Nesting opportunities for urban House Sparrows in the UK appear to be mainly restricted to the cavity sites that form under roof tiles or behind wooden fascia boards, both of which have become increasingly scarce following changes to building regulations and the replacement of wooden fascia boards with those made from PVC. Such changes are also thought to have reduced nesting opportunities for Starlings and Swifts. John Tully (2000; 2001) found that the type of roofing tile used on a house influenced whether or not the property was used by breeding House Sparrows, and work on the BTO House Sparrow dataset also indicates that roof tile type, and restoration work on a property, are linked to whether or not the site retains breeding pairs.
You might imagine that the densities of birds breeding within the built environment would be lower than seen in other habitats; while this is true for some species, it isn’t necessarily the case for all, as we have just seen for Blackbird. Great Tit and Blue Tit populations breeding in urban areas in southern Finland, for example, have been recorded breeding at densities of up to 50 pairs per km2 and 14 pairs per km2 respectively, while those breeding in wider countryside habitats are up to 20 pairs per km2 and 5 pairs per km2 respectively (Suhonen & Jokimäki, 1988).
SONG AND THE ADVERTISEMENT OF TERRITORY
The dawn chorus is a feature of the garden bird community that provides a welcome start to the day for many householders, though the extent to which it does this may sometimes be determined by the species that are singing; Blackbirds, Robins and Song Thrushes are typically favoured over Woodpigeon and Collared Dove. The question of why birds sing at this time of day has prompted a good deal of research. There are several theories, and supporting evidence, which coalesce around either energetic or behavioural hypotheses. Singing is energetically expensive, especially at dawn when a bird has been unable to feed for several hours and may well be eating into its energy reserves. Where a bird has greater reserves, it may be able to sing for longer, providing an indication of quality and/or condition (Thomas & Cuthill, 2002). The dawn chorus may also serve to support the defence of the territory and the attraction and protection of a mate. In male Wrens, for example, song output is increased when the song of another male (recorded the previous day) is played back from a speaker located in their breeding territory (Amrhein & Erne, 2006). This suggests that the song is a verbal warning to an individual making a territorial incursion. Work in North America on Black-capped Chickadees has revealed that each male is establishing a communication network, in which he is joined by two or three male competitors (Foote et al., 2010). Jennifer Foote’s work suggests that singing males are involved in high levels of song matching with their neighbours, and that they match multiple individuals both simultaneously and sequentially.
Dawn is the time of the day when vacant territories are most likely to be occupied by wandering individuals, perhaps reflecting overnight mortality of territory holders – Kacelnik & Krebs (1983) noted that c. 5 per cent of territory-holding male tits died between January and May, so singing at dawn provides an opportunity to advertise that a territory is still occupied. It is also just worth mentioning that singing at dawn may benefit from better acoustic conditions, because it is known that there tends to be less wind and turbulence at this time of the day in many habitats. Another feature of dawn is the low light levels, which may make this an inefficient time to seek food, opening up the opportunity to sing instead. Many individuals also sing at dusk, though the combined ‘dusk chorus’ is significantly reduced compared to that taking place at dawn.

FIG 52. Singing is energetically expensive, particularly so at dawn after a long night without feeding. (John Harding)
If song is advertising that a territory is occupied, then it also says something about the singing bird, a characteristic that may be used by the female when assessing the quality of a potential mate. Lambrechts & Dhondt (1986) found that song capacity (which includes measures of song length and repertoire size) in male Great Tits correlated with both their lifetime reproductive success and their social dominance at feeding stations during the winter months. This underlines that song capacity is an honest signal of male quality in this species, something that has also been found in many other birds. An important question is whether song output is similar in urban and garden settings compared to other habitat types, and this is what we will examine next.
NOISE POLLUTION
Our towns and cities can be noisy places, the sounds of human activities sometimes proving a challenge to birds seeking to communicate with each other through song. If a bird cannot be heard, then it may be unable to attract a mate or successfully advertise and defend a breeding territory. This may make some urban areas unsuitable for birds, perhaps leaving gardens close to busy roads or factories, for example, without a resident Blackbird or Robin pair. The impacts of roads on the settlement patterns of small birds are well known, and a number of studies have demonstrated that male birds whose territories are close to noisy roads are unable to attract a mate (Reijen & Foppen, 1994). Traffic noise tends to be pitched at a relatively low frequency, and Rheindt (2003) found that those bird species whose songs were much higher-pitched were less sensitive to road noise than species with lower-frequency songs.
Birds may respond to the challenges of noise pollution by changing their behaviour or even the way in which they sing. Work on urban Blackbirds living close to Madrid Airport found that these birds modified their song, changed its timing and sang for longer in the presence of aircraft noise (Sierro et al., 2017). Although variable, Blackbird song is composed of two main components: a series of loud low-frequency whistles, followed by a final flourish. Javier Sierro and colleagues found that airport Blackbirds were more likely to sing songs without the final flourish than their rural counterparts. The airport Blackbirds sang earlier and increased the time they spent singing during that part of the season where the chorus of song and aircraft traffic noise overlapped; this effect then disappearing later in the season, when the chorus and aircraft traffic schedule became separated by the advancement of the dawn chorus. Great Tits, Blue Tits and Chaffinches exposed to considerable traffic noise also move the timing of their song, starting far earlier than populations in quieter locations.
Noise pollution may not just affect the ability of birds to advertise their presence, attract a mate and defend a breeding territory. A population of Great Tits exposed to traffic noise laid smaller clutches and fledged fewer young in the noisiest areas (Halfwerk et al., 2011), though it is not clear whether confounding factors – such as differences in NO2 levels between the sites – were controlled for sufficiently robustly. A more convincing piece of work demonstrating impacts of noise pollution on reproductive performance comes from a House Sparrow nest box study taking place on the island of Lundy, located in the Bristol Channel (Schroeder et al., 2012). The island relies on a set of generators, running continuously between 6 a.m. and noon. Examination of the breeding success and breeding behaviour of individual pairs nesting at different sites on the island revealed that the noise from the generators was detrimental to those breeding attempts made in nest boxes located close by. House Sparrow chicks reared close to the generators had a lower survival rate between hatching and fledging, and a significantly lower probability of recruiting into the population; they also had a lower body mass at day 12. There are two important aspects to this work: first, the researchers used cross-fostering to control for any variation in the genetic quality of parents, and second, they also examined House Sparrow behaviour during periods when the generators were running and when they were switched off. The behavioural component of the study revealed that female House Sparrows nesting near the generators provisioned their young less frequently than those nesting elsewhere; they also fed their young less frequently when the generators were running than when they were switched off. Schroeder et al. suggested that the females fed their chicks less often when the generators were switched on because they could not hear the begging calls of their chicks, which is thought to provide a stimulus to bring in food. Without this signal, the chicks received less food and so did not develop as well as those from broods nesting elsewhere.
NEST SITES
Garden habitats and their associated buildings provide a range of nesting opportunities for birds, but importantly they lack much of the low scrubby cover favoured by certain species (e.g. Whitethroat Sylvia communis, Blackcap and Chiffchaff Phylloscopus collybita), and they lack the natural cavity sites favoured by others (e.g. Jackdaw, Stock Dove Columba oenas and Tawny Owl Strix aluco). To a certain extent, the lack of natural cavity sites has been addressed by the availability of nest boxes provided by keen householders, but many of these tend to be of a standard design, favouring nesting tits rather than those species that prefer a larger cavity or entrance hole. The presence of evergreen cover may make gardens particularly attractive to early season species like Blackbird and Song Thrush, while the presence of thorn-bearing bushes may help to reduce the unwanted attentions of potential nest predators.
Several studies have shown that bird nests placed higher in trees have a better chance of survival than those placed lower down (Croci et al., 2008). However, this relationship is likely to be influenced by many other factors, including the type of predators present in the area, the availability of cover around the nest and local climatic conditions, meaning that other researchers have found contrasting results. Katherine Kelleher and John O’Halloran, for example, found that nest height in Song Thrushes breeding in Ireland varied with season, and that nest failure rates were greater at nests built in trees than in those placed in hedgerows or bushes. Nest failure in this study was mostly linked to avian predators, raiding the nest at egg stage, so nests placed in trees may have been easier for these predators to find. Within gardens, Jay, Magpie and Carrion Crow are likely to be the main avian nest predators involved, often visiting early in the morning when small birds are active provisioning chicks left unfed overnight, or taking a break from incubation duties. Most small and medium-sized gardens lack taller mature trees and so may not provide the high nest sites favoured by species like Mistle Thrush Turdus viscivorus.

FIG 53. Whitethroat and other species that nest in low cover are uncommon in gardens, even rural ones, because few gardeners tolerate the bramble or nettle beds which these species prefer. (Mike Toms)
Nest site placement has also been shown to influence nesting success in Blackbirds; by measuring the height at which 430 Blackbird nests were placed, their bulk and the degree of cover around each nest, Ben Hatchwell and colleagues were able to determine that the degree of nest exposure was the only feature that differed significantly between successful and failed nests (Hatchwell et al., 1996b). Successful nests were less exposed than failed ones; interestingly, nest exposure was only important during the laying and incubation period and not during the nestling period. Presumably, the presence of chicks in an active nest increases the chances that it will be found by a predator, reducing the beneficial effects of having cover around the nest.
It appears that the presence of people can also influence breeding success of those birds nesting within the built environment. A study of the Blackbird population breeding on the University of Exeter campus found that those nests placed closer to the paths used by students, staff and visitors, suffered lower rates of nest predation (from Jay, Magpie, Carrion Crow and Grey Squirrel) than those located further from away. This finding did not appear to be linked to differences in the vegetation planted on the campus, since nests at different distances had similar site characteristics, suggesting that the presence of people was responsible for keeping predators away from the nests (Osborne & Osborne, 1980).
Site selection may also be shaped by prevailing weather conditions, something demonstrated by work looking at whether tits, Nuthatch and Pied Flycatcher Ficedula hypoleuca show preferences for nest boxes whose entrances are orientated in a particular direction (Gaedecker & Winkel, 2005). Most of the nesting pairs tested by Gaedecker and Winkel selected boxes that faced an easterly direction, either northeast, east or southeast, with the strongest preference exhibited by nesting Pied Flycatchers. As the authors of this work noted, east is the weather-opposing side of a tree, with less moisture and heat inside the box. Interestingly, such preferences may be moderated by what sites are available; the same study found that Starlings using natural cavity sites tended to use those that were orientated southwest. Closer examination of the results revealed that this was a consequence of the high availability of natural sites facing southwest, with fewer facing towards the east.

FIG 54. Blackbird nests may be placed low down at the base of a tree but are more often to be found in a garden hedge or climber. (Mike Toms)
NESTS
Nests are constructed in a particular way and the process of construction usually results in the development of four functionally distinct components: an attachment layer, an outer layer (often decorative in purpose), a structural layer, and a lining (Hansell, 2000). Not all of these elements are present in all nests. In the case of Blackbird, there are three components: an outer layer mostly composed of grass, moss and stems, a layer of mud, and a lining of grass (Biddle et al., 2015). Bullfinch nests have just two layers: an outer layer of stems or small twigs, and an inner layer of fine rootlets and thin grass culms. The different components serve different purposes, delivering sufficient rigidity and support to the female bird and her eggs, providing camouflage and helping to insulate the nest contents. It is not always clear as to the function of particular components, a good example being the hard lining found in the nest of the Song Thrush. In this species, the outer layer of moss and grass is then lined with a smooth layer of wood pulp and mud or dung; there is no additional lining of grass so the eggs rest directly on the hard lining. It has been suggested that the lining might provide some additional insulative properties (Pikula, 1978) or that it might reduce the number of ectoparasites (Reicholf, 2003), but we don’t as yet have a definitive answer.
Nest construction has also been found to vary between species, individuals within a species, within individuals between years and between locations (Biddle et al., 2018). There is certainly evidence that the availability of local materials can shape those used in a nest’s construction: Britt & Deeming (2011), for example, noted an increase in the use of wool by Blue Tits and Great Tits in a year when sheep were being kept within the area local to their study site. Some garden birds spend a significant amount of time constructing their nest; this is certainly true of Long-tailed Tit, whose beautiful domed nest, camouflaged with lichen and lined with very large numbers of feathers, can take a pair more than a month to complete (McGowan et al., 2004). A Long-tailed Tit nest is built in two stages: the pair begins by constructing the outer structure – on average this takes 23 days – before turning their attention to lining the nest with feathers, the latter process taking a further 15 days on average. The length of time spent building a nest tends to decline later in the season, possibly because of the increased urgency to squeeze in a nesting attempt, but it may also reflect changes in environmental conditions, as is the case for Long-tailed Tits. At 11 days, the construction of second nests by Long-tailed Tits is much quicker than for first nests, with the birds reducing the number of feathers incorporated into the nest as the warmer conditions later in the season require less insulation.

FIG 55. The nest of the Song Thrush is lined with a hard layer, made from mud and rotten wood. Its purpose is unclear. (Mike Toms)
An obvious question to raise is whether garden birds, and those living within a wider urbanised environment, use different materials in building their nests or build their nests in different ways from individuals breeding in other habitats. It is known that nests constructed in colder locations tend to be larger than those constructed in warmer locations, which might suggest that the warmer environment of urban areas should favour the construction of smaller nests (Deeming et al., 2012). However, it has also been shown that nests built earlier in the year tend to be larger than those built later – again thought to be linked to temperature differences (Britt & Deeming, 2011); given that some urban birds breed earlier than their rural counterparts, might this counteract the effects of the comparative additional warmth felt in urban areas?
There is evidence from Reading that Blue Tit nests built in boxes in urban areas are lighter than those built in rural areas, but no difference in nest weight was found for the related Great Tit (Hanmer et al., 2017a). Jim Reynolds and colleagues looked at whether the position of nest boxes, used by Blue Tits along an urban gradient in the city of Birmingham, influenced their composition. By taking apart 131 nests removed from nest boxes at the end of the breeding season, the researchers discovered that nest composition did vary significantly depending on where on the urban gradient the box was located. Boxes in areas with a greater degree of built cover contained fewer feathers, while those in areas with more connected tree cover contained more feathers. Perhaps unsurprisingly, given the nature of the habitat, anthropogenic materials were found in nearly three-quarters of the nests examined; however, the inclusion of such material was unrelated to the location of the box on the gradient. Hanmer and his colleagues found a similar pattern in their urban tit populations; both species used anthropogenic material – it was found in 77 per cent of Blue Tit nests examined and 94 per cent of Great Tit nests. Interestingly, roughly a quarter of the materials used by the Great Tits were anthropogenic in origin (highly processed cottons, for example) but the amount used was much lower in Blue Tit, being 16 per cent in garden nest boxes and just 1–2 per cent in areas of urban green space. Hanmer et al. (2017a) failed to find any link between the degree of urbanisation and the use of anthropogenic materials. That Blue Tits make less use of anthropogenic material than Great Tits, a finding also reported by Surgey et al. (2012), might reflect their preference for feathers when lining the nest, something that they do to a greater degree than Great Tit.

FIG 56. Great Tits may use wool, or the material used to coat tennis balls and dog toys in the construction of their nests. (Jill Pakenham)
The inclusion of anthropogenic material might be harmful to nesting birds, perhaps increasing the chances of nestlings becoming entangled (Townsend & Barker, 2014) or suffering from toxic effects. Alternatively, such material might be beneficial, reducing the numbers of ectoparasites, such as fleas, found in the nest. Work on this in Mexico City has revealed that both House Finches and House Sparrows incorporate cigarette butts into their nests, and that this behaviour leads to a reduction in the number of ectoparasites within the nest (Suárez-Rodríguez et al., 2012). Elsewhere, the addition of natural plant material to the nests of several different species has been shown to reduce ectoparasite numbers, so this might appear to be an extension of this behaviour. Worryingly, this behaviour comes with a cost, as more recent work by the same authors has shown (Suárez-Rodríguez et al., 2017). Both the House Finches and House Sparrows studied in Mexico City show increased levels of genotoxic damage, seemingly linked to the amount of contact with cigarette butts.
How far garden birds will travel to collect nesting material is unclear, but work on woodland tit populations suggests that individuals do not always use material from the nearest source to their nest (Surgey et al., 2012). By experimentally providing the tits nesting in Treswell Wood, Nottinghamshire, with a wool-like substance, Joanne Surgey, Chris du Feu and Charles Deeming were able to examine use of the material, colour preferences and the distance birds were willing to travel. Although individual birds might collect material from two, three or even four well-separated sources, very few individuals travelled further than 200 m from their nest box. In Great Tit, the most common species in the boxes, use of the material declined with distance to its source. Use of the material appears to be opportunistic, which might explain why we sometimes find material taken from lost tennis balls – popular with dog walkers – in the Great Tit nests built in our woodland Tawny Owl boxes. Another interesting feature of these boxes, which are significantly larger than the small hole-fronted boxes used by many of Great Tits nesting in gardens, is that the birds cover the entire floor of the box with nesting material, placing the small cup in one corner. This must require significant investment on the part of the female.

FIG 57. Male Wrens make a series of ‘cock nests’, one of which will be chosen by a prospective mate. The more nests a male builds, the more likely he is to attract a mate. (Mike Toms)
Nest size in Great Tits has also been linked to the quality of the individuals involved in the breeding attempt. Female Great Tits are solely responsible for building the nest, and it has been found that the strength of a female’s colouration is related to the size of the nest constructed. Since the strength of plumage colour comes from carotenoids within the diet, the colour serves to indicate an individual’s condition. Perhaps most interestingly, the relationship between plumage colour and nest size is even stronger in the males, which suggests that the better-quality females who mate with brighter-coloured males invest more energy in nest construction (Broggi & Senar, 2009). A bigger nest might provide better insulation for the eggs and chicks.
The signalling of quality might manifest in nest construction itself, particularly in those species (such as Wren, Blackcap and Whitethroat) in which the male constructs a number of nests within his territory, one of which will then be selected by his mate. These male nests are known as ‘cock nests’ and are rarely fully finished. Nevertheless, the effort that a male Wren puts into constructing his nests is substantial, with some individuals building as many as 12 nests within a territory. Male Wrens with more cock nests are more likely to attract a female (Evans & Burn, 1996), and as a male ages so he builds more nests. This appears to be accomplished by starting nest building earlier in the season and by going on building for longer, rather than building the nests more quickly as he gets older. While you might assume that older individuals are more likely to attract a mate than younger ones, this is not necessarily the case. The number of nests constructed is also influenced by the habitat structure present on the territory and by male body mass, with heavier males starting nest construction earlier in the season than lighter males (Evans, 1997). While male Wrens do build more nests per season as they get older, the variation seen within a year between different males is much greater than that seen between years within a male’s lifetime. As Matthew Evans notes in the conclusion to one of his papers, ‘Some males are more accomplished nest builders than others and that while males do improve with age, age cannot compensate for lack of ability.’
NEST BOXES
Nest box designs can be grouped into three main types: those with a small entrance hole (‘hole-fronted’), those with a large, more open entrance (‘open-fronted’) and those of a specialist nature and usually designed for one particular species (‘specialist boxes’). Many of the simple designs can be made yourself, following the cutting plans that appear in books (Cromack, 2018) or online, but more complex designs may be better purchased from specialist suppliers. Nest boxes for common garden birds are readily available from wild bird care companies, garden centres and even supermarkets, though the quality of the design (and indeed the product itself) can vary markedly. For this reason it is worth considering the species you wish to attract and noting down its requirements before you go out to purchase a nest box. Plenty of suitable advice can be found online but there are some general pointers worth following. For a hole-fronted box, check that the diameter of the entrance hole is the right size for the species you wish to attract (see Table 6) and make sure that the distance from the bottom of the entrance hole to the floor of the box is at least 12 cm; any less than this, then you may increase the risk of the nest being predated. Make sure that the box is made from wood that is at least 15 mm thick; the insulative properties of the box may be insufficient if the wood used is thinner than this and the chicks may chill or overheat. Avoid boxes that have a perch; small birds won’t need a perch but a predator might be able to make use of it. Do not purchase a box that is incorporated into the roof of a bird table; any bird that chooses to use the box will spend all of its energy trying to chase away all the birds that arrive to feed at the bird table below. A good nest box will be well made and allow access for cleaning it out at the end of the breeding season or for monitoring the progress of the nesting attempt if you are submitting data to the BTO Nest Record Scheme.
TABLE 6. Suggested box dimensions and entrance hole diameters for hole-fronted boxes used by garden birds. All measurements are in mm.
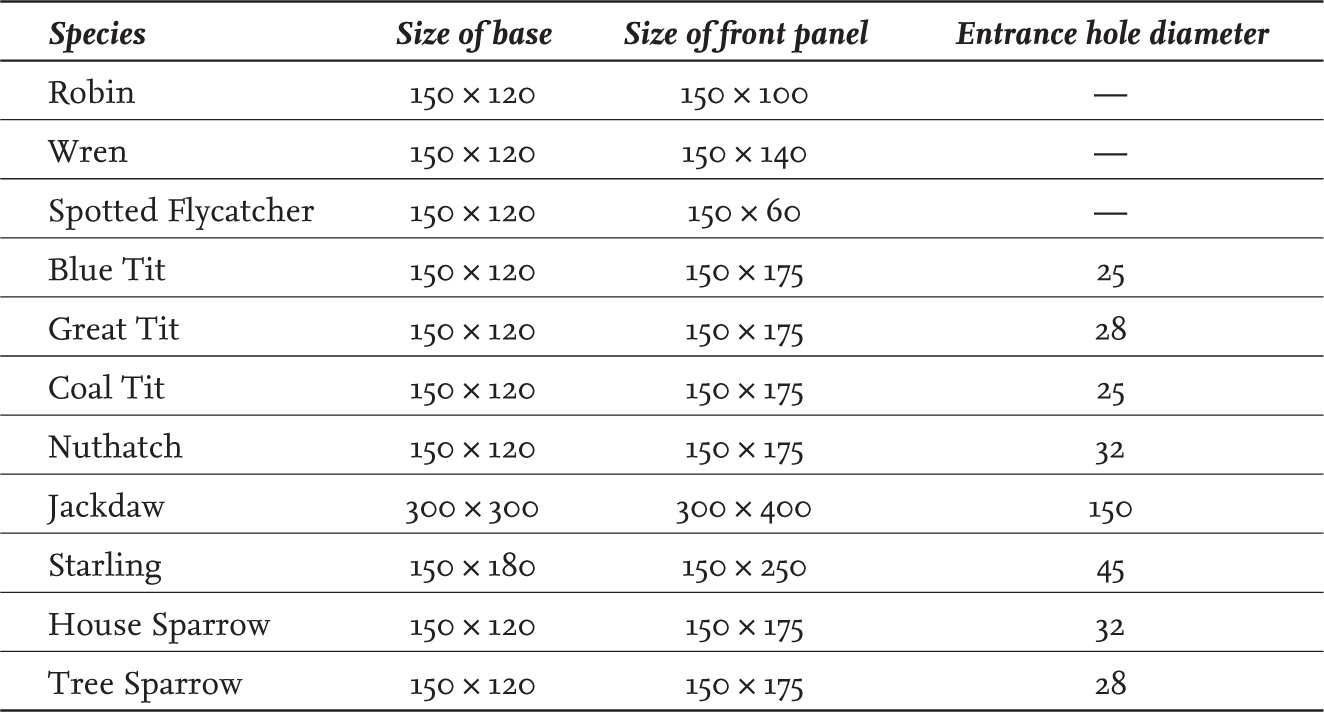
Davies et al. (2009) reported on data collected through the Survey of English Housing and other studies, suggesting that on average at least 16 per cent of UK households had at least one nest box associated with their property. Scaled up, this suggests a minimum of 4.3 million nest boxes are available to UK birds. Of course, not all of these nest boxes will be suitable, perhaps because the entrance hole or the box itself is too small to attract interest. Nest boxes are usually less well insulated than natural cavity sites – though the degree of insulation does vary substantially between different types or makes of nest box – and this may contribute to the finding that nest boxes are sometimes associated with lower rates of nestling survival during periods of inclement weather (Duckworth et al., 2017). Nest boxes may – again depending upon type – suffer from higher levels of predation under certain circumstances. A review by Vincze et al. (2017) revealed that artificial nests (both nest boxes and nesting cups) placed in urban areas were more likely to be predated than was the case for natural nests. Interestingly, the reverse was found to be the case for less urbanised landscapes.
EGGS
The egg is a truly remarkable thing, containing as it does all of the nutrients (except for oxygen) necessary for the developing embryo. Its production requires a female bird to deposit sufficient fat, protein, water and calcium to make the egg viable; for a small bird, with a large clutch of eggs, the accumulation of these materials may be a substantial task. Some of these materials will be easier to secure than others; the 1.3 g of water needed for each Great Tit egg, for example, should not present too much of a challenge, but finding sufficient calcium (see below) may be more difficult. Because of their small body size, most garden birds will be unable to store these materials in the quantities required for a whole clutch of eggs, which implies that they must collect the materials during the period over which the eggs are being formed and laid. There is some evidence that certain materials, for example fat, can be stored in small amounts; it is thought that female House Sparrows can store sufficient fat to service some 30 per cent of the energy required for her clutch of eggs (Schifferli, 1980).

FIG 58. A clutch of eggs represents a serious investment of resources for a small bird – here those of a Linnet. (Mike Toms)
Small birds may counter some of the difficulties in sourcing sufficient nutrients by adopting different behaviours during the laying period or modifying existing ones. They may, for example, change the interval over which eggs are laid; this appears to be constrained in tits – which have a large clutch to lay – but is seen in the Swift, which usually lays its eggs two days apart but will increase this interval to three days if weather conditions are poor. Birds may also change the size of their eggs in relation to the available resources (see below); interestingly, female Goldcrests regularly produce larger eggs as the laying sequence progresses, resulting in the last egg being as much as 20 per cent larger than the first to be laid (Haftorn, 1986). A third option is for the male to provide his mate with additional resources, a behaviour referred to as ‘courtship feeding’. This behaviour is seen in a number of garden bird species, and in Blue Tit these additional resources are estimated to match the increase in energy demand that the female experiences during the egg laying period (Krebs, 1970). In addition to providing extra resources for the female, courtship feeding may help to cement the pair bond or even provide the female with information about her mate’s ability to provision nestlings.
CALCIUM AND EGG FORMATION
Snails are likely to be an important source of the dietary calcium needed by small birds for the formation of their eggshells, the importance of which is demonstrated by a piece of work carried out in the Netherlands. Drent & Woldendorp (1989), working in the Buunderkamp Forest, observed that about a third of the female Great Tits in their study population produced eggs with thin or porous shells, defects that were also evident from other forest study sites located on calcium-poor soils. Using an experimental approach, Drent was able to quantify the rates of eggshell defects and their impacts on breeding success, before going on to counter them through the provision of supplemented sources of calcium (Graveland & Drent, 1997). Providing some of the Great Tits with an additional source of calcium in the form of snail shells and chicken eggshells reduced the number of females who did not produce any eggs, lowered the frequency of clutch desertion – which had been as high as 48 per cent in females laying one or more defective eggs – and reduced the proportion of nests with defective eggs. A study of Great Tit populations breeding in mixed deciduous woodland here in the UK found that females nesting in low-calcium areas, where snails were known to be scarce, laid thinner-shelled eggs than did females breeding in high-calcium areas (Gosler et al., 2005).

FIG 59. Snail shells, such as these scattered on a Song Thrush anvil, may be an important source of the calcium used by small garden birds to form their clutch of eggs. (Mike Toms)
Human-induced acid deposition was thought to be responsible for the increased levels of defective eggs seen by Graveland and Drent – and presumably for a decline in the abundance of the small snails on which the Great Tits were dependent for dietary calcium. However, the availability of small snails may also vary between habitat types, with differences in underlying geology, or because of the chemical control of snail populations through the use of molluscicides. This could mean that some gardens – particularly those within highly urbanised landscapes – support fewer small snails and, as a consequence, have less dietary calcium available to small birds.
While larger birds may be able to utilise some of their skeletal calcium to produce eggshells in areas of low environmental calcium, this is not an option for a small bird like a Great Tit or Blue Tit. A Blue Tit will need to find 0.25 g of calcium for a clutch of eggs but has just 0.175 g present within her 0.5 g skeleton. Some 90 per cent of the calcium required by a female Great Tit for her clutch of 6–9 eggs is consumed during the egg laying period. Small birds may have to respond to a decrease in calcium supply by increasing the amount of time that they put into searching for suitable sources (Holford & Roby, 1993). Female Great Tits faced with such shortages may spend 43 per cent of available daylight hours searching for calcium during the egg laying period, almost double the amount of time spent by females with sufficient calcium available to them (Graveland & Berends, 1997). The presence of snail shells in the crops and gizzards of female House Sparrows during the egg laying period demonstrates that these birds take small snails for their calcium, because this a prey type that does not normally feature in their diet. Spotted Flycatchers appear to make use of calcium-rich woodlice, coming down to the ground to catch them. As we have just noted, experimental studies reveal that the addition of calcium-rich supplementary food can counter the impacts of calcium shortage in tits (Tilgar et al., 2002). This is of particular relevance to gardens because some of the bird foods marketed by wild bird care companies include oyster shell grit, which may provide egg laying birds with a ready source of accessible calcium.
The eggshell thickness of Song Thrushes, Mistle Thrushes and Blackbirds in the UK has been examined by Rhys Green (1998). Using material from museum collections, Green found that there had been widespread declines in eggshell thickness in all three species since the nineteenth century. Although the cause of these declines is unknown, the evidence highlights that eggshell thinning in these species began before the widespread use of the organochlorine pesticide DDT, whose introduction led to eggshell thinning in birds of prey and fish-eating birds from 1947 onwards. Green suggested that the underlying cause may have been acid deposition, leading to a reduction in snail populations. Declines in the pH of undisturbed soil samples, taken from southern England and archived, provides supporting evidence for this (Goulding & Blake, 1993). The question of whether there are differences in eggshell thickness in populations of garden bird species living within urban and rural habitats has been little studied, though Bailly et al. (2016) found no difference in eggshell thickness for either Blue Tit or Great Tit when they compared urban populations with those in forest habitat. Brahmia et al. (2013) also failed to find any difference in eggshell thickness in their study populations of Blue Tits, again looking at rural and urban sites.
Eggshell thickness has also been examined in relation to the pigmentation that is found on the eggs of many garden bird species. Eggshell patterning is a feature of many small birds, though it is much less common in non-passerines, which typically have unpatterned eggs. Patterning may have a signalling role, revealing female condition or quality (Moreno et al., 2004), or act to camouflage the egg against its surroundings. It may also be used by females to reduce the chances of successful egg-parasitism by a Cuckoo Cuculus canorus (Davies & Brooke, 1989). More recent work (Gosler et al., 2005) has postulated that eggshell patterning may have a structural role, since the deposition of protoporphyrin pigments in Great Tit eggs correlates with shell thickness. Thinner areas of the shell are marked by the addition of pigment, and there is a relationship between the darkness of the pigment deposited and the degree of thinning. The pattern of pigment spread in Great Tit eggs from Gosler’s long-running study has changed over time, matching a decline in soil calcium levels and a decline of 6.5 per cent in eggshell thickness, adding further evidence for a structural role (Gosler & Wilkin, 2017). Another response to reduced calcium availability may be a change in egg shape, with spherical eggs more common in Great Tit populations living in areas with lower calcium availability in the soil. A spherical shape is stronger than a less spherical shape.
Eggs are not just variable in their patterning and shape, they can also vary in size and not just between species. Variation in size (and patterning) within a clutch of eggs is common and while such variation is usually quite small, there can be significant differences. Perrins (1996), for example, reports on the variation in a sample of 4,752 Great Tits eggs, weighed before incubation had been initiated. The smallest egg recorded weighed 1.0 g, while the largest was 2.1 g. There are some general patterns relating to egg size, teased out from various studies across a number of species over many years. Eggs tend to be slightly smaller in larger clutches, in clutches laid early in the season and when formed during periods of low temperatures. They are also smaller when population density is high, which, in the case of Great Tit, can include the high density of competing species (in this case Blue Tit). In Great Tit, it also appears that egg size may be influenced by the nature of the nest box in which the bird is breeding, as Perrins (1996) found a stepped decline in egg size following the replacement of his project nest boxes with a new ‘anti predator’ design. Egg size is important because it has consequences for later life, having been demonstrated to influence hatching success, fledging success and fledging weight, the latter linked to subsequent recruitment into the breeding population.
CLUTCH SIZE, PARENTAGE AND LAYING ORDER
Clutch size is likely to be shaped by how many eggs a female bird can produce and incubate successfully; it is also likely to be shaped by the number of chicks that can be supported once the clutch has hatched. The typical clutch sizes of a range of garden bird species are shown in Table 7. Within single-brooded species, clutch size tends to decline over the course of the season (Kluijver, 1951), while for multi-brooded species it tends to peak in the middle of the season (Ludvig et al., 1995).
TABLE 7. The breeding traits of some common garden birds. Data derived from the BTO Nest Record Scheme.

Our meta-analysis of demographic differences between urban and rural populations included 46 clutch size comparisons across 19 different species. In general, we found that clutch size was larger in non-urban landscapes. The clutch sizes of Blackbird, Great Tit, Blue Tit and Starling were all consistently larger in non-urban than urban landscapes. However, no significant differences were revealed in studies on Magpie (Eden, 1985; Antonov & Atanasova, 2003), Song Thrush (Kelleher & O’Halloran, 2007) or House Sparrow (Crick & Siriwardena, 2002). Clutch size in urban Great Tit and Blue Tit populations within southern Finland has been found to be some 9.8 to 17.3 per cent smaller in urban areas than in rural habitats (Solonen, 2001), a pattern also seen within UK populations of these species (Perrins, 1965; Cowie & Hinsley, 1987). While there is good evidence to support the suggestion that urban-nesting attempts begin earlier because of the supplementary food available at garden feeding stations, it is less clear as to why urban clutch sizes are generally lower than those seen in wider countryside habitats. Possible causes include a greater proportion of young/lower quality individuals within the urban breeding populations or a lack of suitable foods for the female birds producing the eggs. It is even possible that the smaller urban clutch sizes are an adaptive response to the poorer feeding conditions faced by birds seeking to rear chicks within this environment. Of these possibilities, it is the greater proportion of young or lower quality individuals within urban populations that is perhaps most likely to be driving the difference.
In many garden birds that appear to pair with a single partner – this is referred to as being socially monogamous – it is often the case that the males will seek to mate with paired females who are not their partner. Such matings are referred to as extra-pair copulations and they result in a brood of chicks of mixed parentage. Extra-pair paternity in birds has been investigated in over 150 studies, encompassing nearly as many species (see Griffith et al., 2002 for a review). Females may also solicit extra-pair copulations, as we’ll discover later in this book when we take a more detailed look at the sex life of the Dunnock. In Great Tit, the frequency of extra-pair paternity has been estimated at between 5.4 per cent and 14 per cent of offspring (Blakey, 1994; Lubjuhn et al., 1999). In one long-term study of Great Tits, it was revealed that in each year 27.8–44.2 per cent of broods contained at least one nestling that derived from a male other than its social father. Work on Blue Tits in Belgium revealed that extra-pair paternity occurred in 31–47 per cent of all nests and accounted for 11–14 per cent of all offspring (Kempenaers et al., 1997).

FIG 60. Offspring quality may be shaped by the order in which an egg has been laid, by the food provided at the nest and by the sex of the individual that has hatched. (John Harding)
One other aspect of the reproductive behaviour of garden birds is worth a mention here and that is laying order. The order in which eggs are laid can have an effect on the survival chances of the chicks that will emerge. In a way, we have already touched on this in our discussions of egg size and the resources put into eggs. There can be another effect of laying order and that is offspring sex. It has been shown that some female birds can skew the primary sex ratio of their offspring in response to breeding conditions. Female Blue Tits, for example, tend to produce more sons when mated to attractive males (Sheldon et al., 1999), while Tawny Owls produce more daughters when there is a high availability of food resources (Appleby et al., 1997). In Tawny Owl the female is the larger sex, and a female chick may require more food resources than a male chick. More recently it has been found that female Blue Tits may adjust the sex of their offspring according to the order in which their eggs are laid (Cicho et al., 2003). It appears that female Blue Tits may favour daughters in the first eggs laid, with the proportion of sons quickly increasing after this. There is a small size difference between male and female Blue Tits, and having the smaller sex (female) in the early eggs may balance out the relative disadvantage of being a smaller individual within a brood of nestlings.
INCUBATION BEHAVIOUR IN GARDEN BIRDS
Most small garden birds lay one egg each day, usually early in the morning. It has generally been assumed that cavity-nesting birds like Blue Tits and Great Tits remain in the nest cavity overnight, and there is good evidence that this is the case (Pendlebury & Bryant, 2005). Such behaviour probably helps to reduce the energetic costs to the female, because conditions inside the box will be better than those outside, and it probably also reduces her risk of being predated or of her nesting attempt being usurped by another bird. We know that tits do not begin to incubate their clutch until the last egg (or the penultimate egg) has been laid; if they did, then the eggs would all hatch at different times. So how does the female avoid initiating incubation while remaining in the box overnight? By filming nesting Great Tits, Chris Pendlebury and David Bryant were able to show that most female Great Tits spend the night settled in the nest cup, head pushed down into the nesting material or tucked under a wing. Temperature probes inserted into the nest revealed that the nest cup temperature did not increase above the value at which incubation would begin, so the females must have been able to prevent their body heat reaching the eggs. Whether they did this by placing nest material between the eggs and themselves, by not allowing their brood patch to come into contact with the eggs, or because the brood patch was not yet fully developed is unclear.
Another reason why female tits may remain within the nest box overnight is to prevent damage to the developing egg. The shell of each new egg is laid down over several hours, often beginning at midday and being completed by 6 a.m. the following morning (Schifferli, 1979). During the initial stages of this process, the shell is soft and less readily damaged, as Schifferli (1977) demonstrated for female House Sparrows – those handled during the day produced undamaged eggs, while a large proportion of those handled overnight produced damaged eggs. By remaining in the nest box and not moving around, the female tit may reduce the chances of damage to her developing egg. It should just be noted that not all birds lay at dawn; Blackbirds, for example, appear to lay somewhat later into the morning (Perrins, 1996).
Incubation is usually the responsibility of the female but in a few garden bird species the male may also get involved, though usually to a lesser degree. In Woodpigeon, the two sexes split incubation between them, with each sex taking a significant shift. In other species, males may cover the eggs while the female is away from the nest feeding, perhaps reducing the amount of heat lost but not actually incubating the eggs. The length of incubation bouts varies between species and in relation to time of day and weather conditions. Typically, an incubating female will leave the nest every 30–40 minutes in order to feed, returning some 5–10 minutes later. The male may accompany her or, as we have just seen, sit on the nest or remain nearby. Interestingly, the males of some garden birds provision the female while she is incubating, a behaviour seen in Long-tailed Tit during incubation but not during egg laying. It has been shown that the provision of food to incubating female Long-tailed Tits enables them to incubate for longer in a given incubation bout (Hatchwell et al., 1999a). Male Magpies meet most of the food needs of their mate while she is incubating, with urban males often relying on the availability of scraps and discarded food; this resource, which is common in most towns and cities, may be one reason why urban Magpies breed earlier than their rural counterparts (Antonov & Atanasova, 2003).

FIG 61. Bouts of incubation are typically broken by periods when the female leaves the nest to feed; in some species (including Collared Dove) both sexes may incubate the eggs. (Mike Toms)
Incubation can be a dangerous period for nesting birds, with incubating females and their eggs facing obvious risks from nest predators. Rates of nest failure during the incubation period are very high for Long-tailed Tits, with Hatchwell et al. (1999) recording losses of 47 per cent for their study population, and this is also true of other garden bird species. Working in the Hungarian city of Pécs, Kornélia Kurucz found that the daily survival rates of her study Blackbird nests were significantly higher once the eggs had hatched than was the case during the incubation period; this appeared to result from a mixture of egg predation and clutch abandonment (Kurucz et al., 2012). Apparent clutch abandonment may be the result of predation – the adult bird being taken from the nest or while away foraging – or it may be a genuine case of the parent bird deserting the eggs. It is known that short-term changes in food supply or weather conditions can result in a clutch of eggs being abandoned. Failure of the eggs to hatch may also result in clutch abandonment but, while a common problem, it is unusual for an entire clutch to fail to hatch when the incubation duties of the parent bird have been successfully delivered.
HATCHING SUCCESS
A study of House Sparrow populations along an urban gradient (Peach et al., 2008) revealed that, from an average clutch size of 4.04 eggs, 77 per cent of eggs hatched and 65 per cent of chicks fledged. This resulted in an average of 2.02 chicks fledging from each nesting attempt. However, a quarter of the nesting attempts made failed to fledge any chicks. Examination of the eggs that failed to hatch suggested that in most cases this was due to the eggs being infertile. A more detailed study of egg and chick mortality in House Sparrow (and Tree Sparrow) revealed similar levels of egg mortality (30 per cent for House Sparrow and 25 per cent for Tree Sparrow). The same study also found that nestling mortality ranged from 24–30 per cent for both sparrow species (Pinowski et al., 1994). A large proportion of both eggs and chicks from this study were found to be infected with disease microorganisms, with Escherichia coli (E.coli) predominating. Work in Norway has looked at the bacterial loads present on Magpie eggs, revealing that those laid in urban nests harbour a greater abundance of E.coli and other microbes than rural ones; the differences in microbial loads being related to the difference in hatching success and nestling survival in these two environments, with urban populations exhibiting generally lower hatching success (Lee et al., 2017).
The presence of ectoparasites within the nest may also influence hatching success; work on a nest box population of Great Tits, for example, found hatching success to be lower in boxes with higher levels of ectoparasites present, something that individual birds attempted to counter by preferentially selecting boxes with lower numbers of parasites where they could (Oppliger et al., 1994). Other external factors may also exert an influence on hatching success. Work examining the impacts of the Chernobyl nuclear accident have, for example, highlighted that elevated levels of background radiation can reduce hatching success in Great Tits (Møller et al., 2008), and other work has demonstrated the impacts of heavy metal pollution on Pied Flycatchers and Great Tits (Eeva & Lehikoinen, 1995).
Other factors influencing hatching success may relate to the parent birds themselves, such as their age, condition or fertility, or to other aspects of the breeding attempt. Lower rates of hatching success have been reported in young individuals in a number of longer-lived species, but this is something that is less readily determined for short-lived garden birds like tits and thrushes. Condition, perhaps influenced by disease, parasite load or available food resources, may shape an individual’s ability to produce viable eggs or alter the pattern of incubation behaviour in ways that reduce the chances of eggs hatching (Arcese & Smith, 1988). Clutch size may also play a role, as determined experimentally for Starlings by Reid et al. (2000), who found that eggs incubated within experimentally enlarged clutches exhibited lower levels of hatching success than those incubated within natural-sized clutches. Even within a breeding pair, hatching success may vary with the timing of their nesting attempt. It has already been noted that breeding attempts made in the middle of the season by multi-brooded species like Blackbird should be more successful, but this is not always the case. Working on an urban Blackbird population in Budapest, Hungary, Ludvig et al. (1995) found that while clutch size peaked during the middle of the breeding season, hatching success declined, reducing the potential productivity gain from having a larger clutch. Looking more closely at their data, together with that on local weather conditions, it became clear that food shortage during the period of egg formation (brought about by dry weather reducing earthworm availability) led to the production of lower quality eggs, resulting in lower hatching success.

FIG 62. House Martin nesting attempts may fail for many different reasons, from the nest being taken over by House Sparrows through to the loss of the adult birds to Hobby. (John Harding)
BROOD SIZE
Brood size is influenced by the number of eggs laid, but it is also shaped by hatching success and subsequent levels of chick mortality. In particular, the lack of suitable invertebrate prey within gardens and the wider urban landscape may impact brood size during the latter stages of the nestling period. Nutritional stress has been put forward as the most plausible explanation for the reduction in average brood size in the extensive sample of House Sparrow nests monitored annually through the BTO Nest Record Scheme. Since clutch sizes tend to be smaller in urban populations than those of the wider countryside, it follows that brood sizes also tend to be smaller. The smaller clutch sizes seen in urban tit populations should make it easier for the parent birds to provision their chicks with sufficient food, thereby mitigating some of the negative effects of breeding within an urban environment and emerging through reduced clutch size. However, this does not seem to be the case, at least for some of the tit populations studied. Solonen’s study in southern Finland, mentioned earlier in this chapter, revealed that urban clutches of Great Tit and Blue Tit were some 9.8 to 17.3 per cent smaller than those seen in rural areas; however, fledging success proved to be as much as 19.3 to 21.5 per cent lower, suggesting that the smaller number of chicks hatched did not compensate for the smaller clutch sizes. It seems that these urban breeding birds faced additional challenges.
It has been suggested that female birds might seek to manipulate the age structure of their broods in order to best meet the resource constraints faced during the chick-rearing period. Writing in 1947, David Lack suggested that differences in nestling size, resulting from asynchronous hatching, can lead to competitive differences which allow the efficient reduction of brood size during times of food shortage. This behaviour is well known in owls and other raptors, but it can also occur in small birds like Blackbird and Magpie (Magrath, 1990; 1992). If food proves to be scarce, then the smallest chicks, which are likely to be the ones last to hatch, are outcompeted by their larger siblings and quickly die. The brood size becomes reduced until it reaches the optimal size for the resources available. Were all of the chicks to hatch at the same time, they would be equally competitive, leaving a shortage of food to jeopardise the whole brood rather than just the youngest nestling. While hatching asynchrony is well known, supporting Lack’s hypothesis, there have been numerous studies which demonstrate that parents can successfully raise additional offspring to independence (e.g. Boyce & Perrins, 1987, for Great Tit).

FIG 63. The success of a breeding attempt may depend on an adult Great Tit’s ability to find sufficient invertebrate prey from the garden and its surroundings. (John Harding)
There are, of course, trade-offs between brood size and offspring quality, such that while a pair of Great Tits may be able to rear a large brood, the quality of the individual chicks may be lower than would have been the case were the birds to have produced a smaller brood. Smith et al. (1989) demonstrated this by artificially manipulating the brood size of Great Tit pairs within their study population, finding that nestlings in enlarged broods grew more slowly in the nest and suffered higher levels of mortality, both within the nest and after leaving it. Raising a larger brood may also have implications for the parent birds, perhaps even reducing their future survival prospects. Smith et al.’s parent Great Tits, rearing the artificially enlarged broods, experienced higher levels of summer mortality than those with smaller broods; similar effects have been found for Blue Tit (Nur, 1984a; 1984b).
THE PROVISION OF PREY
It has been possible to examine the rates of food provision to broods of Blue Tits and Great Tits in suburban gardens through the use of nest box cameras (Cowie & Hinsley, 1988b). These reveal that, on average, Great Tits make 22.6 visits per hour, while Blue Tits average 50.9 visits per hour, both of which are comparable to the visit rates made by their woodland counterparts. It is also possible to calculate the weight of prey provisioned; in the case of the Great Tits studied by Cowie & Hinsley, the mean fresh weight provisioned is 105.1 mg, which again is comparable to woodland populations (105 mg: van Balen, 1973; 122 mg: Gibb & Betts, 1963). The mean fresh weight of prey delivered to Blue Tit nests studied by Cowie & Hinsley, which was calculated to be 32.0 mg, is substantially below that seen in woodland (65 mg: Gibb & Betts, 1963). Cowie & Hinsley’s suburban tits were feeding mostly small invertebrates, with spiders (Great Tit) and aphids (Blue Tit) seemingly important, something that contrasts with the high proportion of caterpillars taken in the diets of both of these species when breeding within mixed deciduous woodland (Riddington & Gosler, 1995). It would be valuable to secure additional studies on the visits rates seen in urban tits and other garden birds.

FIG 64. Bullfinches feed their young by regurgitation, so won’t be seen carrying food to their nest site. (John Harding)
Both time of year and time of day can influence the prey delivered to nest boxes. Great Tits, for example, provision more craneflies and hoverflies early in the morning to boxes located in suburban gardens than is the case later in the day, presumably because these insects are more sluggish early in the day and become harder to catch as it warms up (Cowie & Hinsley, 1988b). Provisioning rates may also be shaped by the number of chicks in the brood, something evident from work on Blackbirds (Chamberlain et al., 1999), which demonstrates that parental provisioning rates increase with brood size. The diet of suburban Blackbirds is dominated by earthworms, typically taken from areas of short turf, such as lawns. The availability of these worms close to the soil’s surface – where they can be taken by the birds – is influenced by soil moisture, with recent rainfall greatly aiding the bird’s chances of taking worms. During periods of dry weather, and with earthworms unavailable, provisioning becomes much more difficult and whole Blackbird broods may starve. Dry conditions of this kind appear to be less of an issue for Song Thrush, which is able to switch to snails. Recent work on Blackbirds, using new statistical methods (Miller et al., 2017), underlines the importance of rainfall for urban populations feeding on soil invertebrates. Mark Miller and colleagues found that nest success was influenced by the amount of recent precipitation in urban habitats, but that it was the longer-term availability of soil moisture which was important in wider countryside habitats, suggesting that the urban environment is more challenging in this regard.
The delivery of prey to Long-tailed Tit nestlings is often boosted by the presence of ‘helpers’; these are individuals who are usually a close relative of one of the parent birds (Russell & Hatchwell, 2001). About half of all Long-tailed Tit broods studied by Ben Hatchwell and his colleagues (Hatchwell et al., 2004) had helpers, 86 per cent of which were male. The presence of the helpers has long-term benefits for the recruitment of young from these breeding attempts, thereby delivering indirect fitness benefits for the helpers, if related, though these are substantially lower than the benefits that the helpers would have achieved had they managed a successful breeding attempt of their own. This suggests that the helpers are making the best of a bad job, their own breeding attempt having failed. Other work by the team at Sheffield (McGowan et al., 2003) has revealed that failed breeders who become helpers have a higher survival probability than those failed breeders who do not; however, they also have a lower probability of successfully breeding in a subsequent year, suggesting there are additional costs and benefits to becoming a helper. Interestingly, Hatchwell & Russell (1996) showed experimentally that parents with helpers reduce their own provisioning effort. The presence of helpers does not always result in a reduction in parental provisioning, as can be seen from work on Dunnocks (Byle, 1990), where ‘beta’ males only help to provision a female’s chicks if they have copulated with her; in fact, the greater the access a ‘beta’ male has to the female, the greater proportion of feeds he will provide.
Garden birds exhibit a considerable degree of variation in the extent to which male parents contribute to the care of their young (Silver et al., 1985). The need for male provisioning (and indeed social monogamy) may have evolved in circumstances where two parents are required to rear young successfully, perhaps because of the challenges in finding sufficient food. This being the case, one would expect paternal care to increase chick survival and/or growth (Aho et al., 2009). The level of parental care invested can also be viewed as an evolutionary strategy, balancing the costs and benefits of parental investment. If a bird invests time and energy in parental care, then it may be sacrificing other opportunities to improve its reproductive success, such as by pursing other matings. If a species makes multiple breeding attempts in a season, then the costs and benefits of deserting a brood of chicks and an existing mate also come into play. This is something that has been examined in the Treecreeper (see Chapter 7).

FIG 65. Magpies provide a wide range of invertebrate prey to their chicks, with some prey items more likely to be prepared by the adult before feeding. (Jill Pakenham)
Some garden birds prepare food items before giving them to their nestlings, perhaps because some parts may be dangerous (e.g. the sting of a bee) or even toxic; others may be too large for the chick to consume and so need to be butchered to a more manageable size. A study of Great Tits, for example, has revealed that prey preparation is mainly focussed on larger prey items, with the degree of preparation falling as the chicks grow in size (Barba et al., 1996). Blue Tit parents may prepare certain food types more than others, the work of Jerzy Bañbura and colleagues revealing that while caterpillars are processed quickly, spiders and grasshoppers receive much more detailed treatment (Bañbura et al., 1999). Magpies also prepare some of the invertebrate prey that they feed to their nestlings more than others (Ponz et al., 1999).
CHICK GROWTH
It is amazing just how quickly a tiny chick, fresh out of the egg, can grow; in Blackcap, for example, a chick is capable of leaving the nest at just over a week old, though it usually fledges at 11 or 12 days old. A Blackbird chick usually leaves the nest at 12 to 15 days of age, though will then follow its parent around for another couple of weeks before it gains independence. Woodpigeon chicks, on the other hand, don’t fledge until they are a month old or more. The risk of predation places a selection pressure on time spent in the nest, with resources directed to those parts of the chick’s body that will enable it to leave the nest as soon as it can. Investment in those parts of the body, such as body feathering, that are less important at this stage is reduced. The chicks of some birds are able to leave the nest immediately after hatching; this ability is a feature of gamebirds and waders, but is not generally seen in those species nesting within gardens – Pheasant Phasianus colchicus, Red-legged Partridge and Mallard being the obvious exceptions.
The growth rates of chicks are influenced by food availability and by the provisioning rates of their parents. When the weather is poor, such as during periods of rain, invertebrate prey may be harder to find and the female parent may have to spend time brooding the chicks rather than foraging. It has been shown that the presence of daytime rain, falling at above the rate of 1 mm per hour, results in a 10 to 20 per cent reduction in Great Tit chick growth rates compared to what it would have been had it been dry (Keller & van Noordwijk, 1994). Provisioning rates may also vary with the time of day, being greatest early in the morning. One of the drivers for provisioning rate is the level of begging behaviour of the chicks, something to which we alluded earlier in this book when discussing the impact of background noise on House Sparrow feeding rates. Higher rates of begging tend to lead to increased rates of food provision in garden birds like Blue Tit (Grieco, 2002).
In House Sparrow, the body mass of chicks at day 13 has been found to be a good predictor of post-fledging survival probability (Hole, 2001). Chick growth may be influenced by environmental conditions, including the weather and the possible effects of pollutants on prey availability. In one urban House Sparrow population, body condition at 2–6 days and body mass at 10–12 days of age were both found to be strongly and negatively related to the local NO2 concentration (Peach et al., 2008). As we have seen, the weight at fledging is important for chicks because reproductive performance in later life has been found to be positively related to weight at fledging (Haywood & Perrins, 1992). Elsewhere, comparisons have been made between urban and rural populations of House Sparrows, examining the types of food provided and the patterns of nestling growth. These studies suggest that an inadequate diet is responsible for poor nestling growth and that where chicks are cross-fostered, or reared in captivity, these differences disappear.
CHICK MORTALITY
Chick mortality is a common feature of many nesting attempts, with much of this mortality occurring within the first few days after hatching. Levels of mortality at the chick stage appear to be lower than that seen at the egg stage, at least in the small number of cases where this has been studied in detail within urban populations (e.g. Ebenman & Karlsson, 1984). Most of the chick mortality seen in an urban House Sparrow population in Leicester was found to occur within the first four days after hatching, and appeared to be the result of starvation (Peach et al., 2008), a pattern also seen in a study of North American House Sparrows (Mock et al., 2009). Douglas Mock’s study revealed that chick mortality occurred in 42 per cent of the broods studied, occurring more frequently in larger broods than smaller ones. The first chicks to die are typically the individuals that were the last to hatch (Veiga, 1990), and mortality appears to be linked to starvation, occurring most frequently when parents are only able to provision at low levels to large broods. Studies of Finnish Starlings found that chick mortality was greatest where mean chick weights were low and between-sibling weight differences large, indicating that small chicks are outcompeted by larger siblings (Tiainen et al., 1989).
These studies on House Sparrows and Starlings do not provide a full picture, however, because these species are cavity nesters and therefore less susceptible to the levels of nest predation seen in open-nesting species like Blackbird. Typically, nest predation results in the loss of the entire brood rather than just some of the chicks. Predators like Jay, Magpie and Weasel Mustela nivalis may make several visits to a nest, taking one chick after another, resulting in brood reduction over a few minutes or hours. Chicks may die for other reasons, perhaps because of disease, injury or through an accident. Individuals may fall from the nest or, in the case of a Dunnock nest that has been parasitised by a Cuckoo, be deliberately ejected. Others may become entangled in nest material, such as horsehair, resulting in an individual developing normally but ultimately starving because it is unable to fledge from the nest.

FIG 66. Jay is major predator of open-nesting songbirds, often returning to a nest over several visits to remove all of the chicks in the brood. (Jill Pakenham)
NEST PREDATION
Nest predation by avian predators is the main cause of nesting failure for many garden bird species, particularly those that build open nests within vegetation. Avian predators like Magpies and Jays are likely to be more important nest predators than domestic cats, though the latter are likely to have a far greater impact on chicks once they have left the nest, at least in a garden setting. Nests may be located by visual or auditory cues and it has long been supposed that nests containing chicks are more likely to be predated than those containing eggs because of the increased levels of activity at the nest and the begging calls of the young. This is not always the case, however, since work by Will Cresswell on Blackbirds found no evidence that begging behaviour increased predation rates in his study population; nest conspicuousness appeared to be the more important factor (Cresswell, 1997). Other work on Blackbirds, this time by Ibáñez-Álamo & Soler (2017), has revealed how parents may respond to the threat of nest predation by reducing the number of visits they make to a nest containing chicks; this is a pattern of behaviour also documented for this species by the same researchers during the incubation period. Most garden birds are wary when they approach their nest and may avoid returning if they are aware of a potential nest predator nearby. Some species, such as Robin, greatly modify their nest-visiting behaviour in the presence of a potential nest predator. Robins will drop the food they are carrying or even make false feeding visits to an alternative site, well away from their active nest, if they believe they are being watched.
Research examining whether levels of nest predation vary in relation to the degree of urbanisation has yielded mixed results, with apparently contradictory findings evident between many studies. A meta-analysis, published in 2017 by Ernö Vincze, revealed the reason why such differences tended to occur. Vincze found that while the survival of natural nests tended to increase with the level of urbanisation, that of ‘artificial’ nests – well used by ecologists when studying nest predation – tended to decrease (Vincze et al., 2017). It seems that the methodology used has an effect, perhaps because artificial nests are not as well hidden as natural ones; perhaps because they do not have natural activities (such as nest defence) taking place at them; or possibly because artificial nest studies usually focus on open-nesting species rather than cavity-nesting ones – and we know that cavity-nesting and open-nesting species show different relationships between predation risk and the degree of urbanisation. Cavity nests are predated significantly less in urban sites than rural ones, but open nests show no such pattern.
So why should natural nests be less likely to be predated in urban areas than non-urban ones? Is it simply down to the greater numbers of cavity nesters breeding in nest boxes, or does the different suite of nest predators have a role to play? Certainly, urban sites tend to lack some key nest predators, such as Weasel and snakes, but they can have high densities of avian predators like Magpie, which is known to be a significant predator of urban Blackbird nests, for example (Groom, 1993). We will look at Magpies and their role in nest predation in more detail in the next chapter.
Levels of nest predation in urban areas appear to show a seasonal pattern with, for example, the first nesting attempts made by Blackbirds in April showing lower levels of nest predation than the second attempts taking place in June (Kurucz et al., 2010). It is thought that this may be linked to the energy demands of the nest predators (notably Jays in this study), which peak in June when they have their own chicks to feed. Kathi Borgmann, working on Dusky Flycatchers Empidonax oberholseri, also found a seasonal effect linked to variation in the food available to nest predators, in addition to which the seasonal variation in foliage density (providing nesting cover) was apparent. We’ve already noted the importance of vegetation cover around the nest earlier in this chapter and the role that evergreen or non-native vegetation can play, particularly for early season nests. While non-native shrubs, in the form of ornamental conifers, may provide early season nesting cover in many UK gardens, the use of non-native vegetation is sometimes associated with increased levels of nest predation. Work in North America, looking at Northern Cardinal and American Robin nesting in non-native honeysuckles and roses, found that nests placed in these exotic shrubs suffered from higher rates of nest predation (Borgmann & Rodewald, 2004).
Energetic demands and food availability may also shape levels of nest predation via the behaviour of the birds whose nests are targeted. We have already alluded to the suggestion that chick begging behaviour might increase predation risk, though noting that Will Cresswell found no evidence for this in his Blackbird population. Work in North America on Song Sparrows has revealed a tendency for the vulnerability of active nests to diurnal nest predators to decrease when additional food is made available to the parent birds (Rastogi et al., 2006). This may enable the parent birds to spend more time in anti-predator behaviours, such as nest defence or increased vigilance, but probably does not have an effect on chick begging rates, which did not differ between food-supplemented and non-supplemented nests in Rastogi’s study. Anne Rastogi also found that food supplementation reduced the levels of nest predation during the incubation stage, by enabling incubating females to reduce the length of their off-nest foraging bouts and increase the time spent at the nest.
Birds may potentially reduce their exposure to potential nest predators by selecting breeding areas that have a lower abundance of such predators, or by reducing the investment they make in a nesting attempt made in an area with a high abundance of predators. This has been tested through work on Blackbirds breeding within the city of Sheffield. Colin Bonnington and colleagues found no evidence that these urban Blackbirds responded to variations in predator abundance by adjusting the location of their breeding territory or by reducing their clutch size. This could suggest that these urban Blackbirds were caught in an ‘ecological trap’ (Bonnington et al., 2015).
FLEDGING AND GAINING INDEPENDENCE
The sight of young Great Tits being coaxed from their garden nest box by the calls of their parents is one of the most rewarding moments for those who have put up a nest box for these birds. Leaving the relative security of the nest box brings each chick into a more dangerous world, where it will need to gain its independence rather quickly. The weight at which a chick fledges has important consequences for its chances of survival as it gains independence, something to which we have already alluded in our discussions of chick weight in House Sparrows. Work on Great Tits also underlines the importance of leaving the nest at a good weight. The work of Chris Perrins, for example, has revealed that a greater proportion of the individuals leaving their nest box with a fledging weight of 19 g recruit into the breeding population, compared to those with a fledging weight of 18 g (Perrins, 1965). Young birds often leave the nest site in a staggered manner, with some individuals perhaps slow to leave because their development has been slower than that of their siblings. On occasion, all of the chicks may leave at the same time, a behaviour that may be seen in Blackcaps or Blackbirds when the nest has been disturbed by a nest predator. This latter behaviour is an anti-predator strategy, the chicks leaving the nest prematurely but having better survival prospects than had they remained in the nest to face the predator.

FIG 67. Once a young Great Tit has left the nest, it faces a whole suite of challenges as it moves towards independence. (Jill Pakenham)
Once the chicks have left the nest, the amount of time that they then remain with their parents will be determined by a number of factors, foremost of which is species-specific behaviour. Some pairs may be waiting to begin another breeding attempt and so will drive their young away within a few days or even hours; in other species, the chicks may remain with their parents for much longer. We know from work with BTO Garden BirdWatchers that adult Great Spotted Woodpeckers guide their chicks to garden feeding stations, accompanying the youngsters as they first discover reliable sources of food, to which they will return on their own as they move towards full independence. Male Great Tits also guide their broods to good foraging sites, but the amount of time they then spend with them is shaped by individual personality traits – both of the parent and the chicks. ‘Fast exploring’ male Great Tits, for example, have been shown to be more aggressive towards their fledged offspring than slow-exploring fathers, resulting in the chicks moving away from their natal territory more rapidly (Dingemanse et al., 2003).
Parent birds may divide responsibility for their brood between the two members of the pair, a behaviour sometimes referred to as ‘brood division’ and studied in several garden bird species, including Robin (Harper, 1985a), Blackbird (Edwards, 1985) and Dunnock (Byle, 1990). In both Robin and Dunnock, the incidence of brood division is greater later in the season than it is early in the season, with food availability thought to play a role in whether or not a brood is divided. Once this period of parental support comes to its end, the fledglings are on their own and likely to move away from their natal territory, a process that is termed natal dispersal. While the fledglings may endeavour to secure a territory of their own close to where they were raised, they will usually be disadvantaged in terms of age and experience when competing with those individuals already established as part of the local breeding population. In a non-migratory species like Great Tit, how far a chick disperses may well depend on the local availability of suitable breeding territories and whether or not they are already held by established birds. For those individuals raised as part of a genuine second brood, competition will not just be with established adults, it will also involve young birds from earlier breeding attempts. This may explain why young Great Tits leaving their nest later in the breeding season disperse a greater distance than chicks fledging earlier in the year (Dhondt & Huble, 1968). One of the main reasons for natal dispersal is the avoidance of potential inbreeding and the damaging effects that it can have on subsequent generations.
SECOND BROODS AND REPLACEMENT CLUTCHES
Many species will initiate a second nesting attempt if the first one fails, though the chances of this happening may depend on when the first attempt failed and for what reasons. A smaller number of species will make multiple breeding attempts during the course of their breeding season, and it is these attempts – rather than the replacement clutches just mentioned – that are referred to as ‘second broods’ (or ‘third broods’, etc.). Some species will almost always make several breeding attempts during the course of the season, while for others the usual number of breeding attempts is just one, with a second brood an exception rather than the rule.
Second broods are uncommon in UK Great Tit populations, perhaps because of the short period over which the peak availability of their favoured caterpillar prey occurs; however, Cowie & Hinsley (1987) found that 18 per cent of their study Great Tits in suburban Cardiff managed to attempt a second brood. This may have been facilitated by the earlier start to the breeding season noted in the Great Tits monitored in this study – the early start and finish to the first attempt opening up the opportunity to begin another one. To some extent there is the potential for having multiple broods to compensate for the lower productivity of individual nesting attempts seen in urban and garden habitats. This appears to have been the case in a study of urban Great tits, where Junker-Bornholdt & Schmidt (1999) found that despite the very low clutch size and breeding success of their study population (compared to those in nearby woodland), the urban population remained stable. In this case, the high proportion of replacement clutches and second broods, coupled with reduced levels of adult mortality, appeared to compensate for the low breeding success of individual nesting attempts.
One of the challenges faced by those monitoring the nesting attempts made by garden birds is the difficulty in determining the reproductive success of a pair of birds over the course of their breeding season. This isn’t really an issue for those studying single-brooded species, unless they are unable to locate replacement clutches following a failed nesting attempt, but it can prove problematic for those working with multi-brood species. We have already touched on this in our discussions about the definition of breeding success, noting that what is often meant by researchers is the productivity of a single nesting attempt rather than for the season as a whole. Getting at season-long productivity is an interesting challenge, but one we have been able to look at in a slightly unusual way for House Sparrow. Using information from the weekly BTO Garden BirdWatch survey, and noting the sedentary nature of House Sparrow populations, we were able to derive an index of annual productivity for this species by looking at the change in mean peak counts using garden feeding stations between the start of the breeding season and the time of the post-breeding season peak. Our measure of annual productivity was similar in both rural and urban gardens, but showed regional variation that matched what was happening to breeding populations. Our measure was lower in the south and the east of Britain (at 1.32 fledglings per adult), where Breeding Bird Survey data show populations are declining, than in the north and west where populations are stable (1.37 fledglings per adult). Comparison with data from the Nest Record Scheme also revealed similar regional variation in its per nesting attempt measure of clutch and brood size (Morrison et al., 2014). It is not clear that this approach would work for other, more mobile species.
SENESCENCE
As noted already, the age and experience of an individual may have a bearing on its breeding success, something that is perhaps more apparent in longer-lived species than those typical of many garden bird communities. In general, those birds breeding for the first time tend start their breeding attempt later in the season; they also produce smaller eggs and smaller clutches, and have lower hatching and fledging success than older breeders (Dhondt, 1989; Saether, 1990). Work on Blackbirds breeding in the city of Cambridge, for example, has revealed that clutch-initiation date, breeding-season length and clutch size all vary with age. First-year females started breeding the latest, had the shortest breeding seasons and produced the smallest clutches.
Increasing reproductive success with age is thought to be the result of the early death of low quality individuals, coupled with improving experience and increased reproductive effort (Forslund & Pärt, 1995). However, those reproductive traits that improve with age at the beginning of life also tend to deteriorate as an individual approaches the end of its life (Dhondt, 1989), something referred to as senescence. In the Treecreeper, for example, senescence starts particularly early – this is a very short-lived species – and egg size in the second year of life is smaller than that seen in the first (Enemar & Nilsson, 2008). A recent study of the life histories of 431 urban Blackbirds has revealed evidence of senescence in this species. While the average life expectancy of individuals in this population was just 3.7 years, reproductive success in females was found to peak at 4 years – supporting what we were saying earlier about the short lives of many garden birds truncating possible evidence for senescence. Male reproductive success remained stable until the fifth year of life, with subsequent breeding success beyond years four and five declining for females and males respectively (Jankowiak et al., 2018).

FIG 68. Treecreeper breeding success has been shown to decline rapidly with age, even though it is a short-lived species. (John Harding)
CONCLUDING REMARKS
As this chapter has demonstrated, the success or failure of an individual nesting attempt may be determined by a range of different factors. Failure may happen at any point during the breeding attempt and for different reasons. A long-term study of tit populations breeding in nest boxes in a woodland site in central England has, for example, revealed that different tit species may suffer nest failure more often at different parts of the nesting cycle from one another. Deeming & Du Feu (2011) found that while Coal Tits tended to suffer the greatest losses because of unhatched eggs, the failures seen in Great Tits were more often seen during the chick-rearing phase; Blue Tits appeared to struggle with both incubation and chick rearing. Similar differences may be found within garden-breeding populations, but are more difficult to study here because of the reasons outlined in Chapter 6.
At the start of this chapter, we explored whether nesting within a garden was a good option for a breeding bird. We have seen that gardens provide some opportunities, such as the presence of nest boxes and the absence of certain nest predators, but they also present some significant challenges, not least the lower availability of those invertebrate prey species that are so important for growing chicks. The nature of the vegetation present within our gardens may alter the vulnerability of open nests, and our domestic pets may take an increasing toll on newly fledged young. The combination of these different opportunities and threats has led some authors to suggests that gardens may be an ecological trap, tempting birds to nest within them, or tempting them to nest earlier than they should, but then catching them out later in the breeding cycle through higher levels of nest failure and chick starvation. As we have noted elsewhere in this book, we still need to collect more information on the lifetime reproductive success of individuals before we can say for certain whether choosing to nest in a garden is a bad thing. Until then, all we can do is continue to collect the evidence and take advantage of emerging technologies that enable us to better study the individual birds with which we share our gardens.