CHAPTER 20
Developmental Defects of the Dental Hard Tissues and their Treatment
Ivar Espelid, Dorte Haubek, and Birgitta Jälevik
This chapter focuses on the diagnostic process and classification of disturbances in the dental hard tissues. General treatment principles and treatment planning are discussed. Furthermore, different types of dental defects are described as localized and generalized dental defects including those of genetic origin. The most common and important diseases or conditions are given most attention in the chapter whereas many conditions with low prevalence are mentioned in the boxes.
Aspects related to tooth development
In contrast to bone, enamel and dentin are not remodeled. Therefore disturbances in the function of ameloblasts and/or odontoblasts during tooth development result in permanent defects. In contrast to the ameloblasts, odontoblasts are still functional in a fully formed tooth, capable of producing secondary and tertiary dentin. Irregularities in primary dentin will persist. The tissues under development and mineralization can be reduced in either quantity or quality or both. As the development of the first primary tooth begins in the fourth week in utero and the development of the roots of the third molars is completed around the age of 20 years, teeth serve a role similar to a “flight recorder” that covers a long time period. From this “record” the clinician can roughly judge when the disturbance occurred and the appearance of the defects may in some cases give clues as to the etiologic factor. But one major diagnostic challenge associated with developmental defects of teeth is that the etiologic factor(s) are rarely pathognomonic. The findings are often consistent with many possible etiologic factors and the anamnesis is of crucial importance to achieve the correct diagnosis. On the other hand, some clinical findings are pathognomonic or highly suggestive for a specific condition, such as dental changes due to tetracycline medication during tooth formation (Figure 20.12).
In the body, enamel is the only mineralized tissue of epithelial origin. Once formed, it loses contact with living cells. The basic unit of the enamel is the prism, each prism being the product of the activity of four ameloblasts. The prisms are bundles of crystallites which are much bigger and better organized than other mineralized tissues in the body. Enamel is formed at three stages: (a) deposition and (b) mineralization of the matrix occur simultaneously, and (c) maturation, involving final degradation and transport of the organic constituents, does not begin until the full thickness of enamel has been achieved at a given site. Amelogenins, constituting the major enamel matrix protein species during the secretory stage, are progressively degraded and lost.
The striae of Retzius are incremental growth lines seen in enamel. These striae are results of a halt or a slowing down in enamel growth and end on the enamel surface as perikymata. Microscopically, in cross‐sections, striae of Retzius appear as concentric rings or as a series of dark bands in longitudinal sections. These are incremental lines similar to the annual rings of a tree. The cross‐striations are created by the daily incremental enamel matrix production. The long‐period incremental growth lines are striae of Retzius. These lines are made with a periodicity of 6–11 days [1] (Figures 20.1 and 20.2). The neonatal line is an incremental line which is particularly pronounced due to the stress associated with birth and makes a distinction between the prenatal and postnatal enamel.
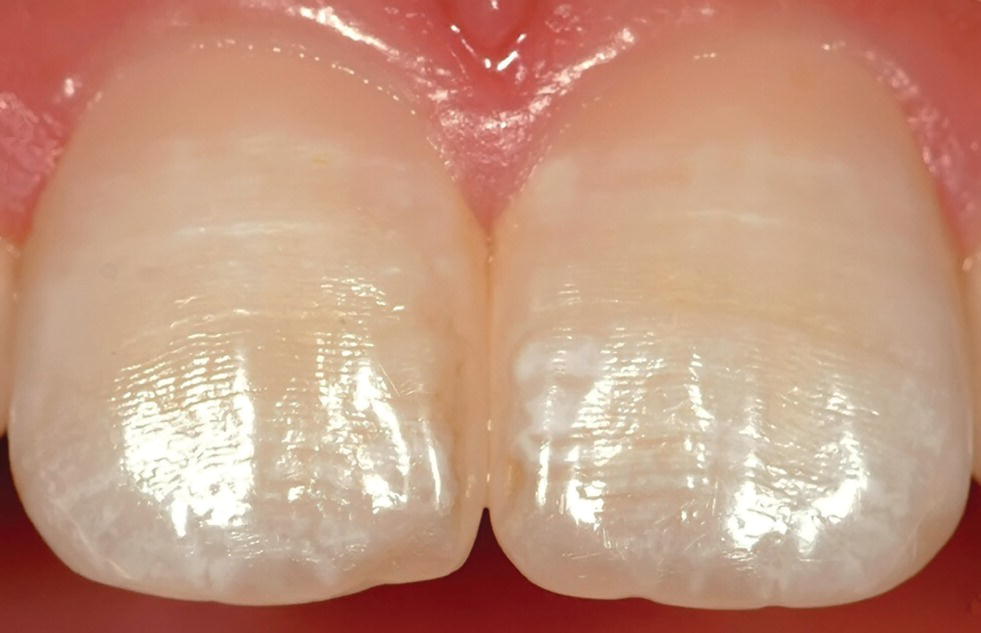
Figure 20.1 Perikymata shown as horizontal lines on buccal surfaces of central incisors. White, opaque patches and lines represent enamel disturbances (mild dental fluorosis). Seven‐year‐old child.

Figure 20.2 Schematic representation of hemi‐sectioned tooth with incremental lines in enamel. SEM images show perikymata on the enamel surface (a) and striae of Retzius (arrows) and cross‐striations of enamel prisms (arrowheads) in an acid‐etched longitudinal section (b). Prism cross‐striations indicate a daily rhythm while striae of Retzius indicate a longer (about 7–10 days) rhythm in enamel formation. Perikymata are the surface representations of the striae of Retzius and run horizontally around the tooth. Prism‐free enamel (PFE) is shown close to enamel surface. P = prism.
Source: S. Risnes. Reproduced with permission of S. Risnes.
Dentin is mineralized connective tissue produced by odontoblasts, which are continually functional cells. The nonmineralized predentin, contiguous with the mineralized dentin, faces the coherent layer of odontoblasts located at the periphery of the pulp. Dentin is traversed by radial tubules, with each tubule housing an odontoblastic process. The main constituent of the organic dentin matrix is type I collagen.
Like dentin, cementum, which is formed by cementoblasts, is mesenchymal in origin. Morphologically, it is divided into the acellular cementum which covers the cervical portion of the root and the cellular cementum overlying the apical portion. Cellular cementum is slowly deposited throughout the life of the tooth.
Etiology of developmental dental defects
Developmental dental defects can be environmentally or genetically derived. Often the reason for the defect(s) in a given patient remains unknown. Genetic defects can be restricted to dental tissues or the dental defect can be a manifestation of generalized tissue/organ involvement. Environmental (also called acquired) dental defects can be divided into those caused by local factors and those caused by systemic factors. Typically, a local factor can be suspected when the enamel defect affects a single tooth or a group of neighboring teeth, or has an asymmetric distribution in the dentition. General symmetric defects are related to the timing of the insult and thus to the sequence of the development of the teeth. These defects are called chronological defects. The etiology of general defects not related to any particular time period during tooth formation is either genetic or due to nongenetic, longstanding environmental influence. Wright et al. [2] has recently reviewed the diverse genetic mechanisms related to hereditary enamel defects. The affected protein functions cover enzymes, regulatory proteins, extracellular matrix proteins, transcription factors, and transmembrane proteins. This diversity is in contrast to the genetically based dentin disturbances which seem to have a more uniform pattern related to fewer proteins.
Figure 20.3 gives a systematic approach to the collection and classification of findings. Boxes 20.4, 20.9, and 20.10 list a number of diseases and etiologic factors which can lead to developmental disturbances in the dental hard tissues.

Figure 20.3 Schematic overview showing a systematic approach to the collection of data and classification of findings related to developmental disturbances of the dental hard tissues.
Collection of information and treatment planning
Anamnestic information
The medical history is of utmost importance for disclosing the etiology of the dental aberration. Often, the nature of the symptoms is not specifically related to a certain cause and differential diagnostic considerations have to be made to form the final diagnosis. Keywords regarding history taking are mapping of family and childhood diseases, previous and current intake of medication, and environmental contamination level during childhood, fluoride exposure, dental history, and symptoms. A checklist of some important questions to ask is given in Box 20.1.
Extraoral examination
A brief examination of the features of the head and face is recommended to record indications of abnormal development (see also Chapter 24). Also, inspection of skin, hair, and nails may in some cases give useful information since they develop from the ectoderm‐like enamel.
Clinical characteristics of developmental dental defect
The classification of abnormalities of the dental hard tissues is governed by clinical characteristics of the affected tooth. However, with the expanding knowledge of the molecular basis of genetically determined dental defects, the need for classification relying on specific molecular defects has become increasingly evident. The most apparent defects are those seen in the enamel, namely enamel opacity and enamel hypoplasia (Figure 20.4). Since dentin is less available to inspection, the fact that dentin may also be involved is often unappreciated, and the dental defects are most often described and referred to as defects of the enamel (Box 20.2).

Figure 20.4 Classification and examples of types of enamel defects due to disturbances in the tooth formation. The natural appearance of the incisor 21 (a) is manipulated to illustrate (b) demarcated opacity (white), (c) diffuse opacities (white) with no well‐defined margins, (d) discolored enamel which has some translucency, (e) single hypoplasia (single deep pit), (f) multiple hypoplastic defects, and (g) posteruptive breakdown due to hypomineralized enamel. Note that very often in cases with enamel defects, several teeth in the dentition will be affected.
Enamel opacity results from incomplete mineralization and is a qualitative defect. Another term frequently used synonymously for this condition is enamel hypomineralization. Opacities are often divided into demarcated and diffuse lesions. Demarcated opacities have a clear and distinct boundary to the adjacent normal enamel and can be white, yellow, or brown in color. Diffuse opacities can have a linear, patchy, or contiguous distribution, but there is no clear boundary to the adjacent normal enamel [3]. Some opacities have significant subsurface porosity which can lead to breakdown of the surface after the tooth has erupted. Tooth attrition and physical stress may result in such defects. These defects should be denoted posteruptive breakdown (Figure 20.4g) and not hypoplasia (Figure 20.4e,f).
Enamel hypoplasia is a consequence of deficient enamel matrix formation. It appears as a surface defect resulting from reduced enamel thickness. Hypoplasia can occur in the form of pits—single or multiple, shallow or deep, or scattered or arranged in horizontal rows; or grooves—single or multiple, narrow or wide, or evident as partial or complete absence of enamel over a considerable area of the tooth crown. The enamel of reduced thickness may be translucent or opaque when the hypoplasia is combined with hypomineralized enamel [3].
It should be noted that the same etiologic factor may cause an opacity or hypoplasia, depending on the timing, duration, and severity of the influence of the disturbing agent and the susceptibility of the individual.
Diagnosis
To make a final diagnosis, it is necessary to combine the information from the patient history and medical record as well as clinical and radiographic findings from the dental examination. In some cases, it may be impossible to reach a final diagnosis or there is more than one likely diagnosis. For example, mild degrees of dental fluorosis and some cases of amelogenesis imperfecta (AI) have similar appearance.
Classification
Historically, symptoms and newly discovered diseases were named and more or less arbitrarily classified according to the current knowledge and beliefs. As medicine and dentistry progress continuously, more knowledge of the etiology and genetics is acquired. Classification of hereditary enamel and dentin diseases, primarily based on molecular etiology, will be an absolute necessity for genetic counseling, but so far not enough knowledge of the genetics for proposal of new classifications describing the relationship between genotype and phenotype is available [4–7].
A classification based primarily on the genetics and secondarily on phenotypic expression has been proposed [4]. The first criterion is information on the defective gene locus and then the biochemical consequences of this failure. This systematic approach is more common in medicine; however, it may over time also lead to reclassification of hereditary dental defects as well.
For hereditary dentin defects, the classification of dentinogenesis imperfecta (DI) and dentin dysplasia (DD) by Shields et al. from 1973 [8] is commonly used (Box 20.3). However, more recent studies indicate that the entities DI‐II and DI‐III are different phenotypic expressions of the same defect in the gene 4q13‐21. Similar gene defects are found in DD‐II, indicating that this is a similar entity. Another problem with Shields’ classification is the DI‐I diagnosis. This is based on dentin alterations in a syndrome while other syndromes, such as Ehlers–Danlos (EDS), are not included. EDS may have phenotypes with dysplastic dentin and obliterated pulp which can mimic DD‐I and DI‐II with variable expressivity, and similar findings may appear in some other syndromes as well [7].
All known diseases with a genetic component are catalogued and described in the electronic database Online Mendelian Inheritance in Man (OMIM). This can be accessed with the Entrez database searcher of the National Library of Medicine in the USA (http://www.ncbi.nlm.nih.gov/sites/entrez?db=OMIM). The database contains updated genetic information about all the hereditary diseases including those with dental implications.
General treatment principles
Patients with developmental disturbances of the teeth constitute a very heterogeneous group with respect to treatment needs. The opinions and instruction from general practitioners as to when and to what extent developmental defects of enamel need treatment vary considerably. The perception of dentists is influenced by type, size, and color of the defects, and in addition by dentist‐related factors [9]. There is no common standard showing when it is correct to perform operative treatment. In relation to cases with mainly esthetic problems, the self‐perceived opinion of the patient is of paramount importance with respect to the treatment need.
The appearance of normal enamel may vary from one individual to another with respect to color, texture, and morphology. Teeth constitute an important part of an individual’s appearance and satisfaction with teeth contributes to well‐being for many people. As professional health workers it is important not to strengthen the tendency in society to judge people by the appearance of their teeth, and dentists should be aware that perceptions of desirable tooth color vary among parents, dentists and children [10]. On the other hand, the dentist has to accept that the “owner” of the teeth has his or her right to dislike the appearance, but the task for the dentist is to decide whether it is within the ethics of the health profession to do the specific treatment considered. The concept of normality has a wide range also with respect to tooth color.
Treatment planning
It is important to identify and address the particular needs of each individual patient. Rapport with parents and child is essential for establishing a long‐term relationship, particularly in cases with an extended treatment and follow‐up period. Systematic collection of clinical experience in relation to rare dental conditions can be made in larger, more specialized clinics in which dentists gain experience from treating a number of these patients. An example is the rare condition dentinogenesis imperfecta in which the dental treatment constitutes a clinical challenge. But there is also reason to claim that the general dental practitioner can do much of the treatment, but preferably under supervision in complex cases to ensure that the patient will receive proper treatment.
The period from diagnosis of the defect through childhood and adolescence can be divided into three phases: acute treatment, observational period and long‐term treatment.
In a child or adolescent with tooth disturbances this may affect the psychosocial health as in a patient with systemic health conditions [11]. Some focus on the parents’ need for support and information is also required [12].
Acute treatment
The treatment of teeth with developmental disturbances that cause pain, teeth with marked disintegration of enamel (chipping), exposed dentin, and/or extreme wear has to be taken care of as soon as possible after eruption. The overall goals of acute treatment are to relieve symptoms and prevent further disintegration of teeth and consequential problems. Diseases where such conditions can be encountered could be severe AI or DI, which as soon as the first tooth has erupted may have problems due to a heavily affected primary dentition. Exposed dentin should be protected to avoid pain and the choice of restorative materials differs with the type of dental problems. Temporary materials as intermediate restorative material (IRM) may be useful and both resin‐modified GICs (RMGIC) and high viscosity GICs may also be used as semi‐permanent restorations. It should also be kept in mind that uncured resin is a potential source for adverse reactions. The eruption of the permanent dentition starts at 5–7 years of age and introduces a new treatment period for the permanent first molar and the permanent front teeth in severe cases. Acute treatment will include handling of the extreme sensitivity of teeth and covering of the exposed dentin.
Young individuals who are severely affected with developmental disturbances are sometimes very sensitive to pain, and it is important to provide proper pain control which often is combined with sedation. Patients with extensive treatment need are in danger of developing dental fear [13]. General analgesia is recommended when young patients have to undergo extensive treatment. A caring attitude with respect for the integrity of the child combined with good pain control and feeling of control will help in prevention of dental fear (see Chapters 6 and 9).
Observational period
After the acute treatment is taken care of there is a transitional period when the permanent front teeth and permanent first molar should be kept under supervision and restorations are maintained according to the individual needs. In this period from about 7 to 11 years of age, the major lines in the final treatment are planned. In complicated cases it is necessary to have contributions from a multidisciplinary team of specialists. In addition to the pediatric dentist, the team may consist of an orthodontist, an oral surgeon, a prosthodontist, a maxillofacial radiologist or other specialists relevant for the specific condition. During the planning it is important to find the most appropriate time for various treatments, such as tooth extractions and prosthetic therapy.
In cases where the need for treatment is extensive and may result in partial or complete prosthetic reconstruction, the child may often go through a lot of early, intermediate treatment in the primary and young permanent dentition during childhood. This may include provisional or permanent fillings, microabrasion in an attempt to remove tooth discoloration, insertion of a partial denture or bridge work to replace teeth extracted early, or missing due to hypodontia, tooth retention or eruption disturbances. In many cases, there is no need for further treatment, but the teeth with disturbances have to be paid particular attention at the dental check‐ups due to increased risk for caries, chipping of enamel or attrition.
Long‐term treatment
The long‐term treatment goals are a combination of a well‐functioning dentition, no pain or sensation from teeth and good esthetics. Prosthetic work has a limited durability and a lifelong period with regular dental check‐ups is needed. In cases with generalized severe disturbances full crown therapy as early as the teenage years may represent the optimal treatment. In patients with less severe tooth disturbances, a tooth‐saving philosophy using adhesive techniques may be the best option. A good relationship between the dentist and the patient is beneficial for the clinical results, and the pediatric dentist should put particular effort into the avoidance of dental fear in these patients. In cases with complicated and extensive treatment needs, it is important to be aware relative early on of the need to consult specialists within the field to obtain the best possible treatment outcome. In severe cases, it is beneficial to the patient to have dental treatment under general anesthesia [14].
Environmentally determined dental defects
Localized defects of the dental hard tissues
External damage to developing teeth
A common local trauma to the permanent tooth germ occurs often with avulsion or intrusive luxation in very young children when the primary incisor penetrates the unmineralized or still poorly mineralized permanent tooth (Figure 20.5). It is useful to think of the three‐dimensional localization of the primary teeth in relation to the permanent tooth buds. The root apices of the primary front teeth are placed in a more buccal position compared to the permanent teeth. A trauma to the primary teeth, which brings these into physical contact during trauma or creates an infection in the region, is likely to create some kind of damage to the permanent tooth. The severity may vary from a minor opacity to a hypoplasia with missing enamel and altered morphology of the crown and/or root. Another, local trauma to developing teeth may result from dental mutilation. Dental mutilation has long been an important cultural tradition in various ethnic groups around the world. Enucleation of the primary canine bud is reported among British‐born Somali children, raising important health issues [15]. Removal of primary teeth or attempts to remove tooth germs from their follicles with improper instruments may often damage the developing teeth (Figure 20.6). In the study from the UK, 32% of the subjects showed dental features that could indicate previous removal of canine buds.

Figure 20.5 Hypoplasia and opacity of permanent maxillary left central incisor due to intrusion trauma of primary incisor at the age of 30 months (the child is now 10 years old).
Source: G. Koch, Jönköping, Sweden. Reproduced with permission of G. Koch.
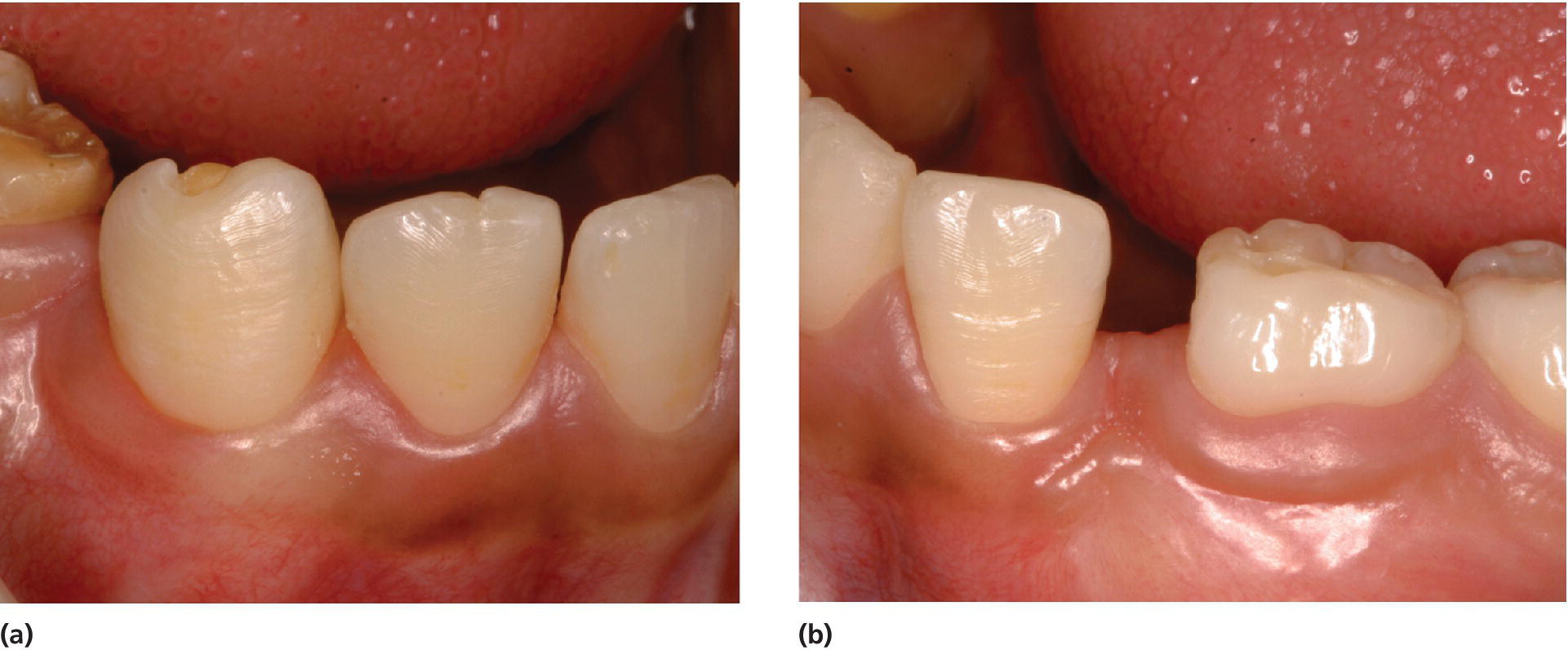
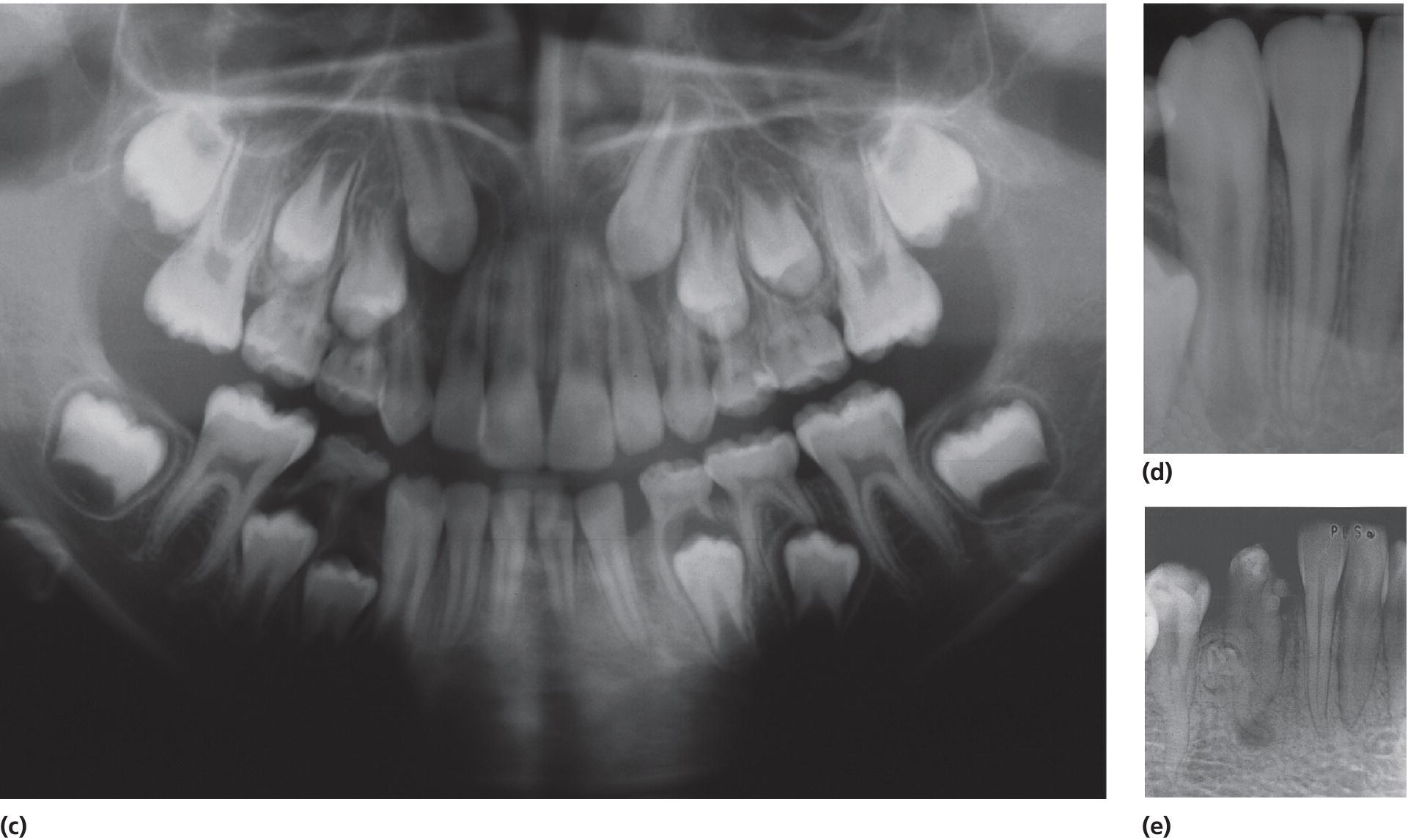
Figure 20.6 Dental mutilation resulting in (a) hypoplasia with exposure of dentin of the right lower permanent canine and (b) absence of the left lower permanent canine due to removal of the tooth germ (germectomy). (c) Panoramic radiograph shows absence of the permanent left canine. (d) Radiograph of the right lower permanent canine shows apparently normal development of the root despite the malformed tooth crown due to the damage previously performed towards the tooth germ. (e) Intraoral radiograph of a malformed and hypoplastic permanent mandibular canine in a 14‐year‐old Ethiopian girl as a result of dental mutilation. An odontoma on the root surface and periapical destruction are present.
A long‐lasting periradicular infection of a primary molar may result in a range of developmental disturbances of the permanent successor (Figure 20.7). These vary from enamel opacities to enamel hypoplasia and to arrest of the development of the permanent tooth germ [16]. Which of these possible and various changes in the permanent tooth occur is determined by the interaction of a number of factors, including the timing, severity and duration of the insult in relation to the development of the permanent tooth, and the susceptibility/resistance of the host. Other examples of possible disturbances of tooth development are therapeutic irradiation or high‐dose chemotherapy. These interventions can severely disturb dental development resulting in enamel defects, microdontia, arrested root development and agenesia of one or more teeth (Figure 20.8).

Figure 20.7 Erupting defective 24 in a 6‐year‐old girl who had 64 extracted 6 months earlier. The extracted tooth had a longstanding, chronic periapical periodontitis.
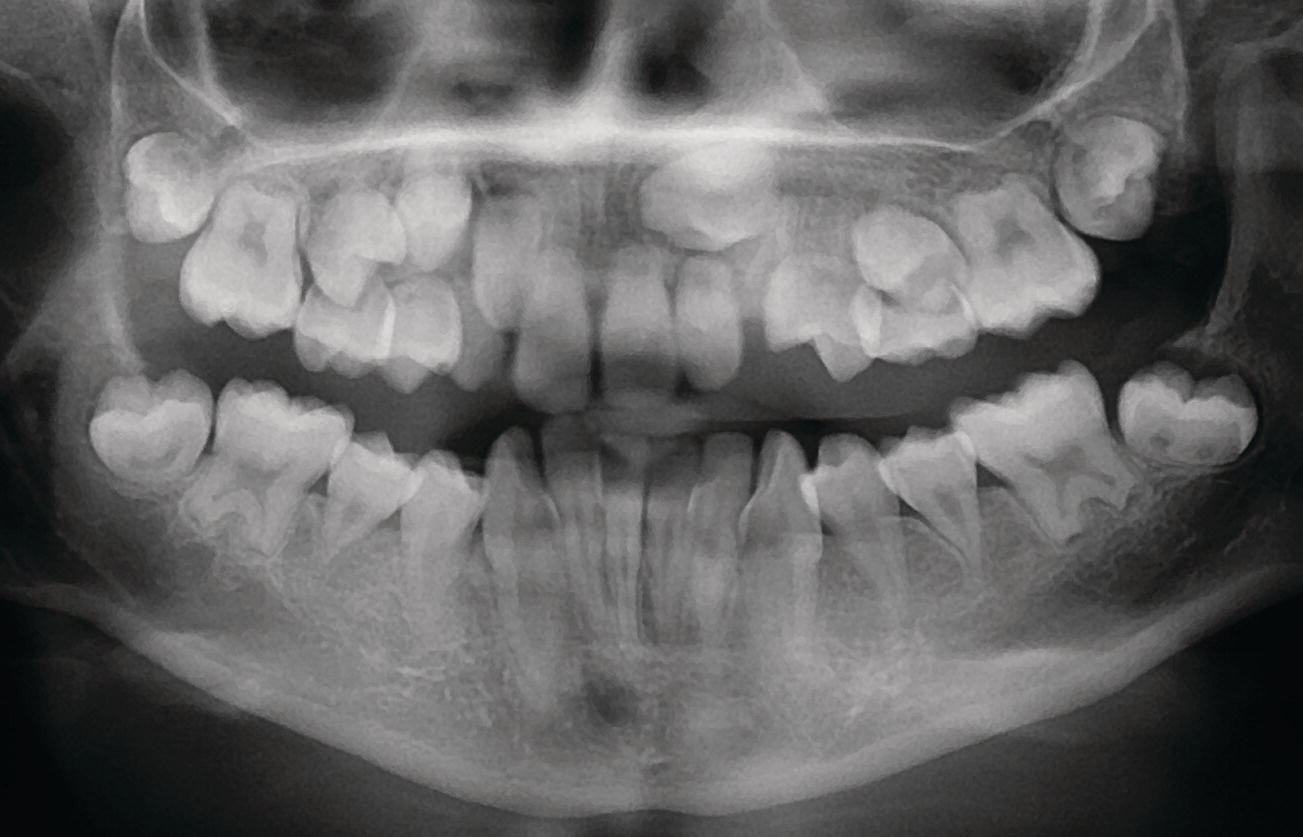
Figure 20.8 Arrested root development in a 13‐year‐old girl who was treated by irradiation (50.4 Gy/28 fractions) at age 6 because of rhabdomyosarcoma in the pharynx.
Generalized defects of the dental hard tissues
Disturbances in the development of the dental hard tissues of systemic origin can occur prenatally, perinatally, postnatally, during infancy or during early childhood. At birth, even the normal change from intrauterine to extrauterine life may have an adverse effect on amelogenesis and dentinogenesis as evidenced by the so‐called neonatal line (Figure 20.9). This pronounced incremental line is seen histologically in all primary teeth. Any stressful event during birth is likely to accentuate this line, resulting in clinically evident enamel defects.

Figure 20.9 Neonatal lines of dentin (left) and enamel (right) demarcate the respective hard tissues formed/mineralized before and after birth.
Other factors associated with developmental defects include ingestion of chemicals (fluoride, tetracycline, thalidomide, aromatic hydrocarbons, such as polychlorinated biphenyls and dioxin); prematurity/low birth weight (Figure 20.10); severe malnutrition, neonatal hypocalcemia, vitamin D deficiency; bilirubinemia (Figure 20.11), thyroid and parathyroid disturbances; maternal diabetes; neonatal asphyxia; certain viral infections; and metabolic disorders.
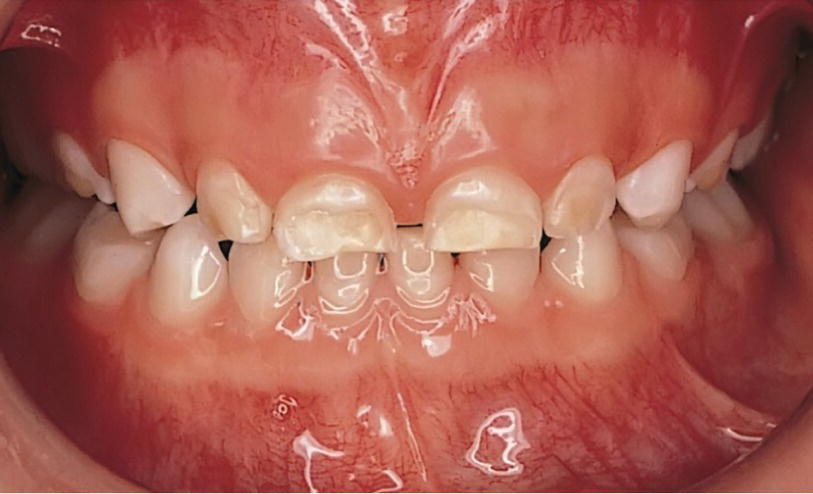
Figure 20.10 Hypoplasia of enamel of a preterm child.
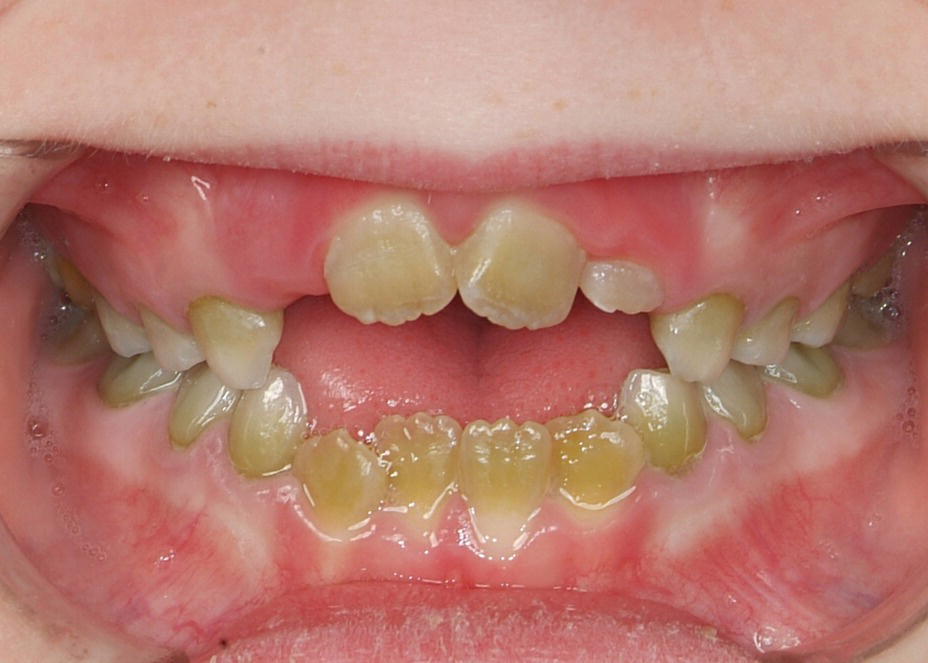
Figure 20.11 A 7‐year old child with pigmentation in both primary and permanent teeth due to bilirubine deposits in teeth caused by biliary atresia in early life.
This list by no means includes all the generalized environmental conditions that have been described as associated with developmental defects of the dental hard tissues. The resultant defect is generally not specific to the generalized environmental insult but depends, as with localized defects, on the timing, severity, and duration of the insult, the stage of development of the dentition and the susceptibility of the host; and the same insult may result in different responses in different individuals. However, there are a few exceptions and among those are discolorations of teeth caused by tetracycline given during the tooth‐forming period (Figure 20.12).
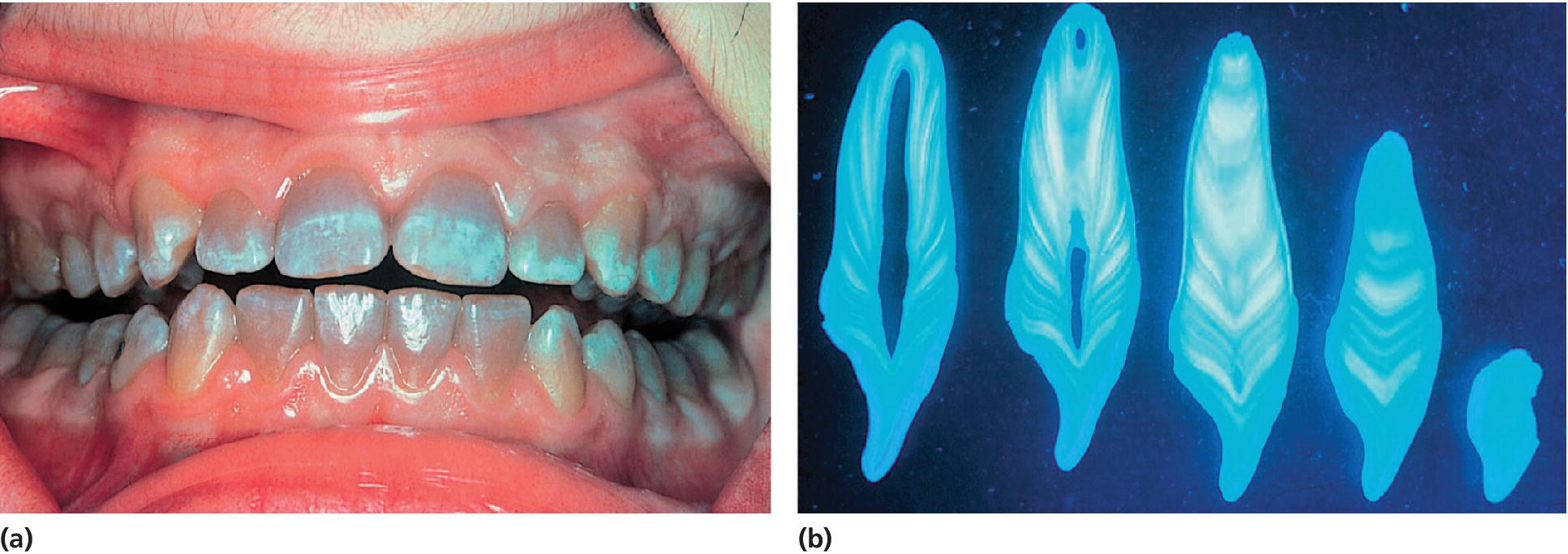
Figure 20.12 (a) A 14‐year‐old girl with cystic fibrosis who was treated with repeated courses of tetracyclines for recurrent chest infection. (b) Histologic sections of a tooth showing the bands of tetracycline staining.
Source: R.W. Fearnhead. Reproduced with permission of R.W. Fearnhead.
Fluoride‐induced defects
The presence of appropriate concentrations of fluorides in the oral environment, whether it originates from naturally occurring fluorides in the water supply or added as part of a public health program, or in oral hygiene products, e.g., toothpastes and mouthrinses, has contributed significantly to the major reduction in dental caries in industrialized countries over the past three decades. However, excess fluoride ingestion can result in enamel disturbances. Clinically, mild fluorosis appears as thin white lines following the perikymata (Figure 20.13). In more severe cases the enamel is chalky, stains easily and has areas of posteruptive breakdown due to the forces of abrasion or attrition (Figure 20.14). The teeth erupt with an intact surface. The breakdown is related to areas of subsurface porosity.
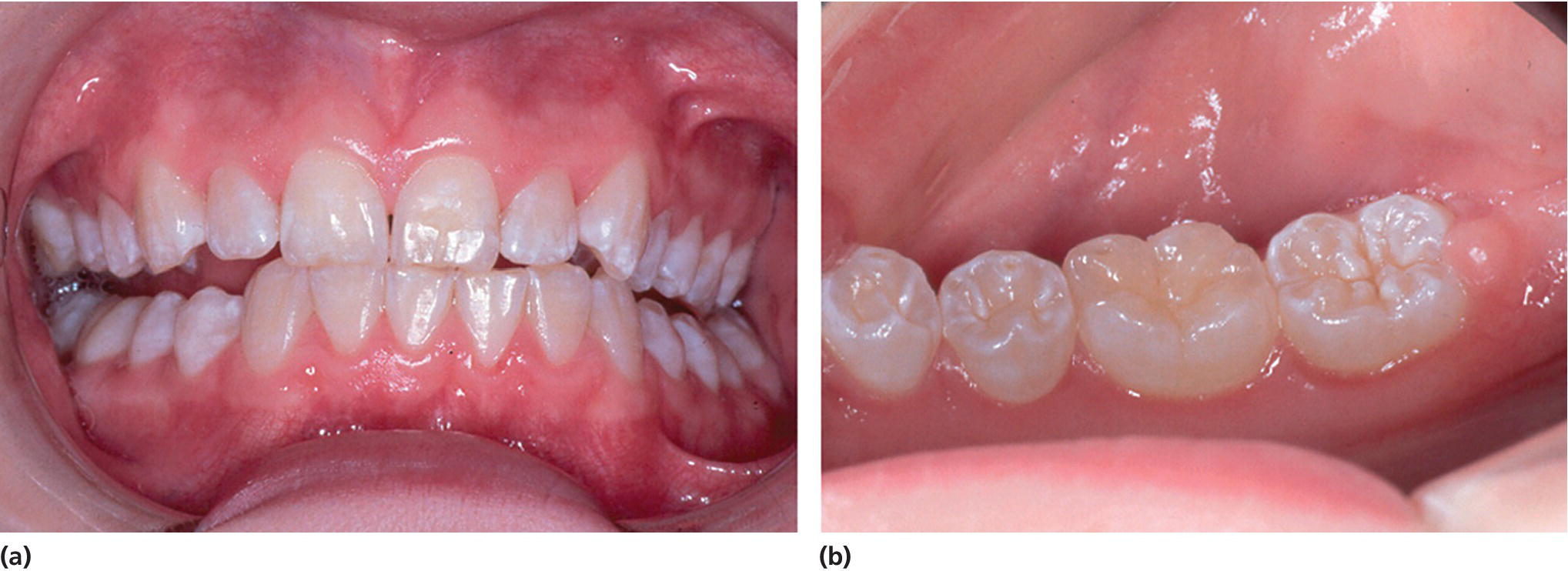
Figure 20.13 Mild fluorosis in a 15‐year‐old girl.
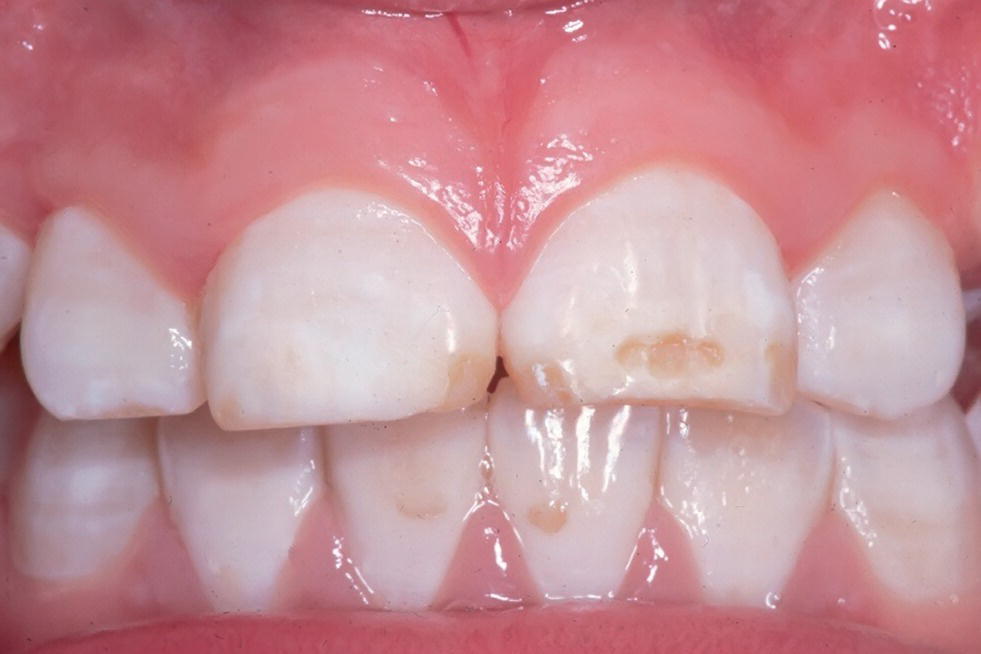
Figure 20.14 Dental fluorosis with posteruptive breakdown of the enamel and tooth wear in a 12‐year‐old boy with natural high (≈5 ppm) fluoride concentration in the drinking water.
It has been suggested that fluoride outside the ameloblasts decreases the concentration of free calcium ions and thereby reduces the activity of the proteases. Thus, fluoride interferes with the degradation of amelogenin, which leads to a higher content of protein in the enamel [17].
Fluoride defects have been studied in areas with naturally occurring high concentrations of fluoride in the water supply. Children who swallowed fluoride toothpaste during early infancy, when the amelogenesis was still proceeding, may have enamel opacities. This is also seen in populations where fluoride tablets are used frequently. The differential diagnosis of these opacities can be difficult as some hypomaturation types of AI may clinically resemble fluorosis. Anamnestic information is crucial for a correct diagnosis.
Treatment of dental fluorosis
For mild and moderate cases, the main problem is poor esthetics affecting the incisors and canines, especially in the upper jaw. The mild opacities are confined to the superficial area of enamel. The appearance of these can be improved by the technique of microabrasion (Figure 20.15). More severe defects extend deeper and may require the use of restorations or veneers. Cases of severe fluorosis require extensive treatment comparable with the treatment of severe AI (Figure 20.16).
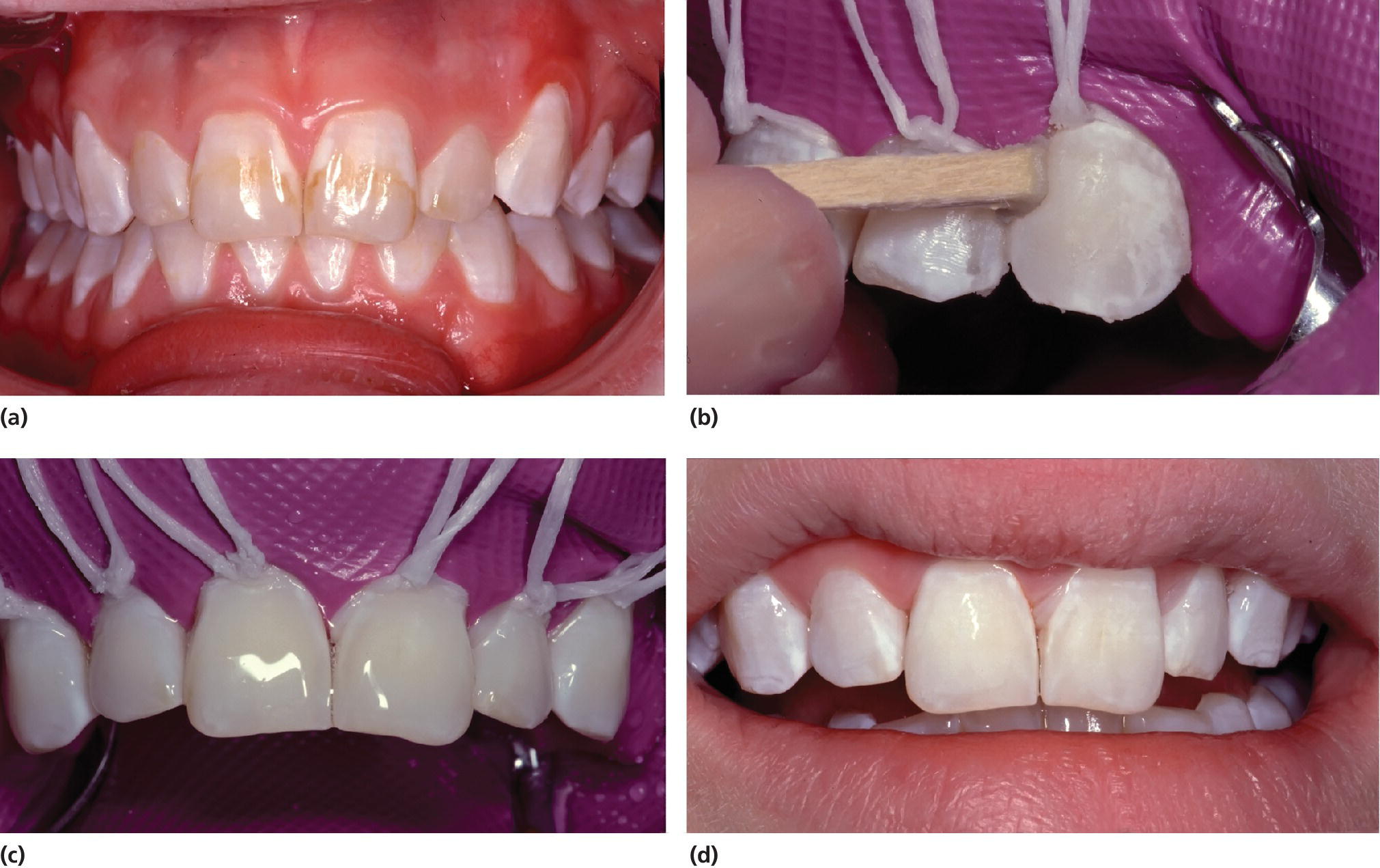
Figure 20.15 (a) Mild dental fluorosis in a 14‐year‐old girl. (b) The surface is rubbed using a wooden pin with 18% hydrochloric acid in pumice. (c) The surfaces are covered by 2% sodium fluoride gel. (d) Treatment result.
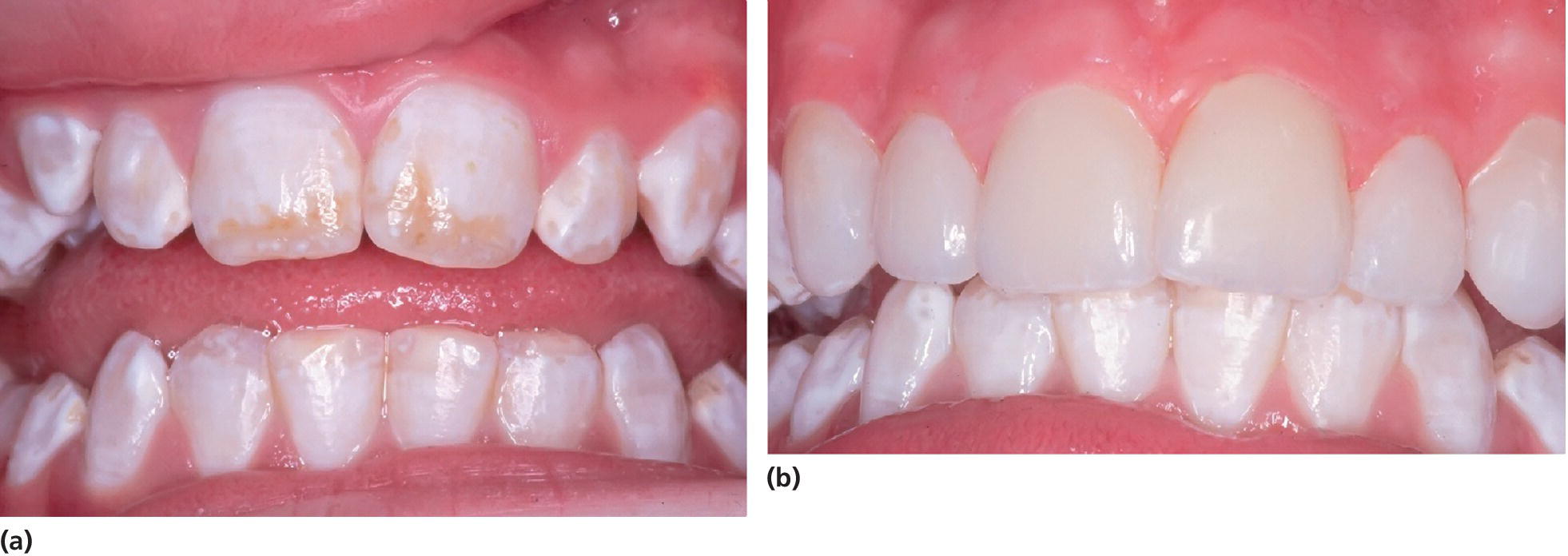
Figure 20.16 Porcelain veneers in a 13‐year‐old girl with moderate dental fluorosis.
Tetracycline defects
Tetracycline has a strong affinity to mineralized tissues, primarily to dentin and bone. Tetracycline oxidation occurs on exposure to light. Since dentin is not remodeled, the dentin defects are persistent. The discolored horizontal bands may appear gray or bluish when observed under ultraviolet light. In natural light the teeth may all have grayish appearance. The discolored enamel usually has some translucency left. The severity of the defect depends on the time of exposure in relation to the stage of tooth development, duration of exposure, dosage and the type of tetracycline preparation. Repeated exposure gives rise to series of bands of tetracycline bound to dentin and within the enamel (Figure 20.12). As tetracycline may cause discoloration of teeth these antibiotics should not be prescribed to children below the age of 8 years, pregnant women or lactating mothers.
Anti‐acne treatment with preparations containing tetracycline (typically minocycline) has been shown to cause discolored enamel if administered before crowns of third molars are completed at the age of 15 [18].
Treatment of discolored teeth due to tetracycline
Management of discolored teeth is difficult as much of the stain originates from dentin. In cases of moderate or severe discoloration, composite veneers may be used as temporary treatment. They may need to be replaced by porcelain veneers or jacket crowns at a later age. However, it has been shown that extended bleaching resulted in tooth whitening, which was stable over a period of 5 years [19].
Molar–incisor hypomineralization
Demarcated opacities in the permanent first molars are common in many child populations. Epidemiologic studies have found prevalence estimates up to 40%. The first permanent molars are particularly affected, but the permanent incisors are often also involved. In the literature, this condition has had many denominations as hypomineralized permanent first molars, idiopathic enamel hypomineralization, nonfluoride hypomineralization in permanent first molars, and cheese molars. In 2001, the term molar–incisor hypomineralization (MIH) was proposed [20] and is currently widely used, although the logic of the terminology of the condition may be disputed.
The enamel defects can be present in one to four first molars and one or more incisors are often also affected concomitantly. Similar lesions can be seen in the second deciduous molars, termed DMH (decidous molar hypomineralization) [21], and in the cuspal parts of the permanent canines, as these teeth are mineralizing in the same period of time as the permanent first molars and incisors. The degree of disturbance in the molars varies from creamy‐white opacities, with a hard, well‐mineralized surface, to yellowish‐brown discoloration and enamel disintegration. The defects in the incisors are described as creamy‐white, sometimes yellowish‐brown opacities. The number of affected teeth varies and the enamel spots do not always appear symmetrically (Figure 20.17). Histomorphological and micro‐CT studies of the enamel in affected permanent first molars reveal areas of porosity in the occlusal half of the crown of varying degrees (Figure 20.18). The enamel in the cervical half of the crown is most often well mineralized [22]. The yellowish‐brown defects are more porous than the creamy‐white demarcated opacities and extend through the whole enamel layer, while creamy‐white demarcated opacities are situated in the inner parts of the enamel. Oral bacteria have been found in dentinal tubules beyond the porous enamel zones as well as inflammatory reactions in the pulp [23].

Figure 20.17 Molar–incisor hypomineralization in an 8‐year‐old boy showing different manifestations in similar teeth: (a) 16 with a defective restoration; (b) 26 with seriously disintegrated enamel and caries; the tooth is very sensitive and toothbrushing is impossible; (c) 46 healthy; (d) 36 disintegrated enamel, but no frank cavitation; (e) demarcated white opacities in the upper front teeth.
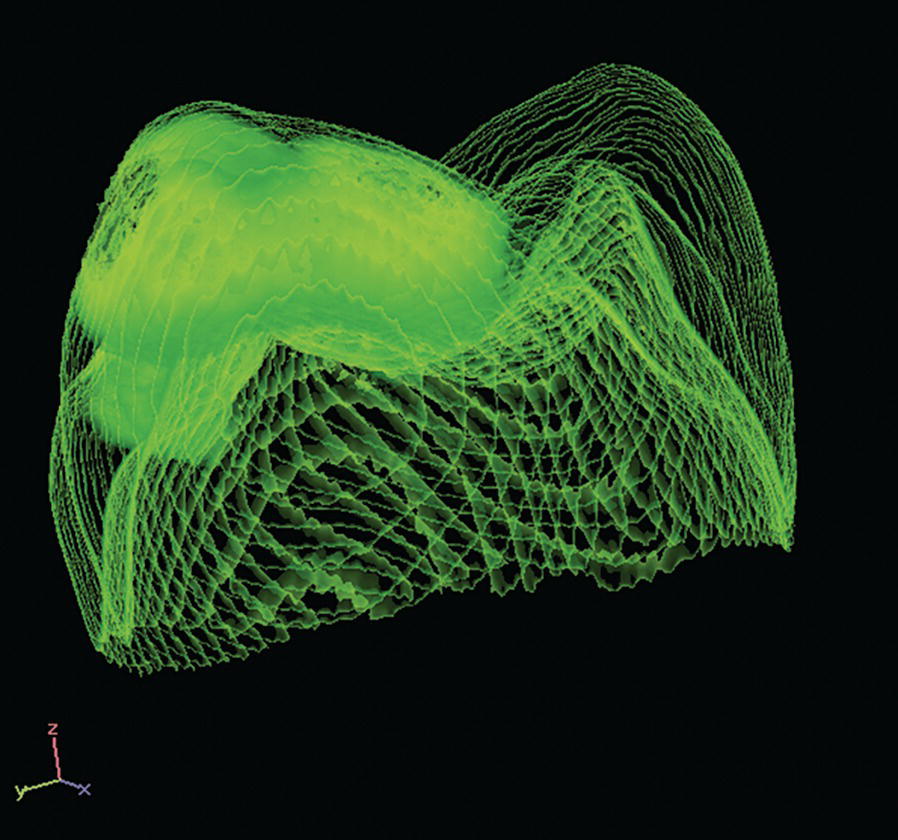
Figure 20.18 X‐ray micro‐computed tomography image of a MIH tooth. The more opaque areas represent hypomineralized enamel.
Source: T. Fagrell. Reproduced with permission of T. Fagrell.
The most severely affected molars often show disintegration of the enamel in the occlusal parts (Figure 20.19). These teeth create problems for patients as well as for dentists. Children often report shooting pain when they are brushing their teeth or even breathing cold air, often shortly after the eruption of the affected teeth has started. At dental examination, these children most often open their mouth reluctantly, and react intensely to air blowing. The severely affected teeth are in need of restoration soon after eruption due to disintegrated enamel and the risk for subsequent caries development [22].
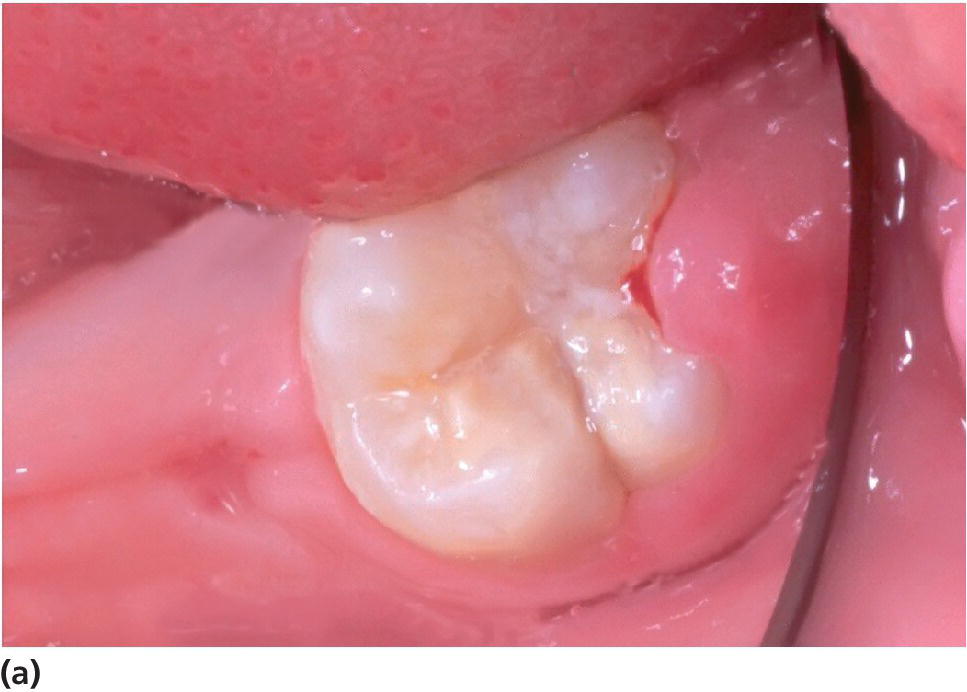
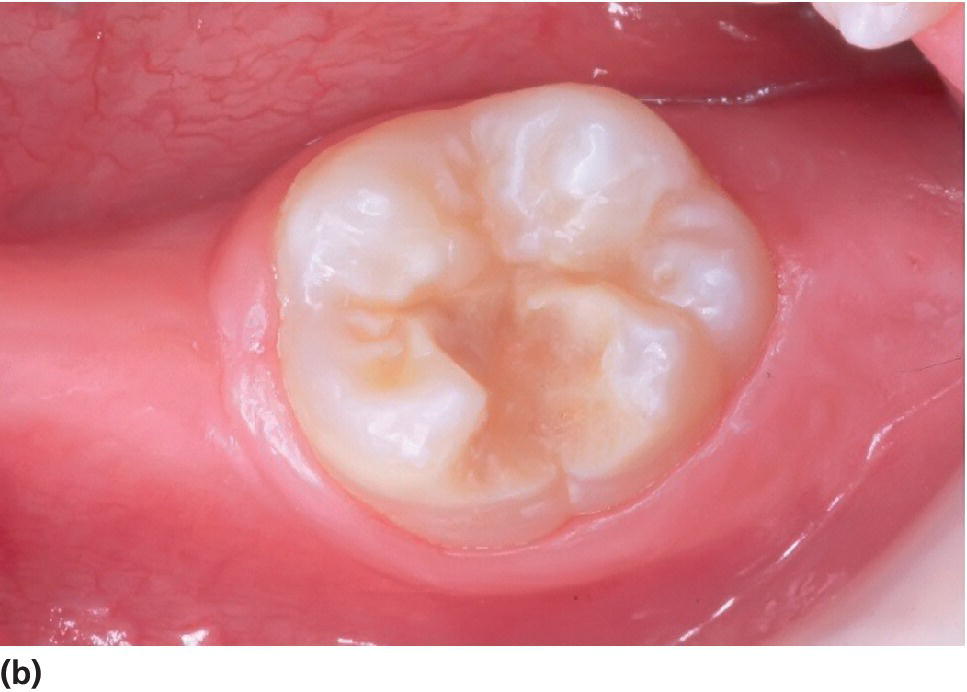
Figure 20.19 (a) A severely hypomineralized lower first molar is erupting. Normal morphology. (b) Heavy disintegration 6 months later.
The etiology of MIH is not yet fully understood. There have been some suggestions of possible etiologic factors. The clinical appearance indicates some specific environmental influence on the development of enamel during a limited period of time. A systematic review of the literature dealing with etiology, concluded that childhood illness is likely to be associated with MIH. [24]. There is some evidence that early childhood fever and respiratory disease may increase the odds of MIH. Asthma/allergy in early childhood is also associated with MIH in some studies. In the review the authors discuss possible biologic mechanisms which hampers the crystal growth of enamel. In addition possible effect of genetic predispositions and epigenetic influence is mentioned [24]. However, a lot of high quality research is needed before any final conclusion can be drawn about MIH etiology.
Treatment of MIH‐affected teeth
The treatment rationale depends on the severity of the lesion. It varies from a small filling to extensive restoration, e.g., onlays and crowns, to more radical treatment as extraction of the affected molars.
When moderate or severe hypomineralized areas in the enamel have been diagnosed in a newly erupted tooth, frequent controls are of the utmost importance in order to determine the future fate of the tooth. Fluoride varnishing prevents caries and may also have some influence on preventing hypersensitivity.
The main clinical problems are the continuing enamel disintegration with subsequent caries and the frequently reported hypersensitivity of the affected tooth. When the lesions comprise large areas of the tooth surface, the filling often will be surrounded by opaque porous enamel. Then, depending on the degree of porosity and the size of the opacity, continuous disintegration of enamel may occur and repeated filling therapy may be needed. Occasionally, an affected tooth is extremely sensitive when it erupts, preventing the child from tooth‐brushing and eating cold food items, e.g., ice‐cream, while other teeth become more and more sensitive, in particular after repeated restorative treatment. It is a main treatment objective to cover exposed dentin to avoid sensitivity, caries development and damage to the pulp. In cases like these with highly sensitive teeth, dentists frequently report considerable difficulties in obtaining pain control. It is reported that increased TRPV1 expression is found within the pulps of hypomineralized teeth even with intact enamel surface [25]. TRPV1 plays a role in signalling related to noxious heat stimuli. Malfunction may provide a major contribution to certain pain conditions. This can possibly also contribute to the understanding of the heat sensitivity experienced by some patients with MIH.
In filling therapy, GICs are the material of choice in the initial treatment, as microscopy studies of affected enamel indicate it is not suitable for etching and thus not composite bonding [26,27] (Figure 20.20). Resin‐modified GICs appear to work well. Pretreatment with sodium hypochlorite can be a good idea, as there is indication of retained organic substance in the porous enamel. In the long run, restorations of composite resins may be durable if the affected enamel is removed and the restoration is bonded to sound enamel. Painless treatment is important. In cases with poor effect of local analgesia, sedation with nitrous oxide or benzodiazepines may help. Use of preoperative analgesics may also be considered.



Figure 20.20 Scanning electron microscopy. The cut surface has been etched with 30% phosphoric acid for 30 s. (a) Normally mineralized enamel. (b) Hypomineralized enamel. (c) The border between normal and hypomineralized enamel.
In cases with extensive disintegration, troublesome hypersensitivity, and/or increasing dental anxiety, crown therapy or extraction of the tooth must be considered. The choice of treatment alternative should be made in collaboration with both the child and the parents after the permanent dentition has been mapped and the development of occlusion analyzed. When crown therapy is preferred, a stainless‐steel crown can be used as a semipermanent restoration until the adjacent permanent teeth erupt to the occlusal plane, when it is replaced by a cast‐metal crown. However, in many cases extraction is to be preferred, when, for example, crowding is expected, or when the child is not able to manage crown therapy. One advantage of extraction is that the troublesome treatment‐consuming tooth is gone, since similar defects in the bicuspids or the second molars are rarely seen. Extraction of the permanent first molars generally presents no problems as regards the maxilla when performed prior to eruption of the second molar. In general, the best result was found when extractions were performed prior to eruption of the second permanent molar. Crowding in the relevant quadrant is also found to be favorable for a good orthodontic result as well as presence of the third molar. A careful follow‐up and easy access to orthodontic expertise are mandatory in all cases of extraction of permanent teeth due to developmental defects.
The age of the child, the cooperative skills, and “dental age” are some of the factors necessary to take into consideration before extensive preparation of the teeth. The exact age when it is possible to perform this type of treatment is difficult to establish on a general basis. In the treatment of young individuals the dentist may need to take intermediary types of treatment into consideration. Examples of this treatment plan could be the use of restorative materials and techniques requiring minimal preparation such as using an adhesive bonding technique (Figures 20.21, 20.22, and 20.23).
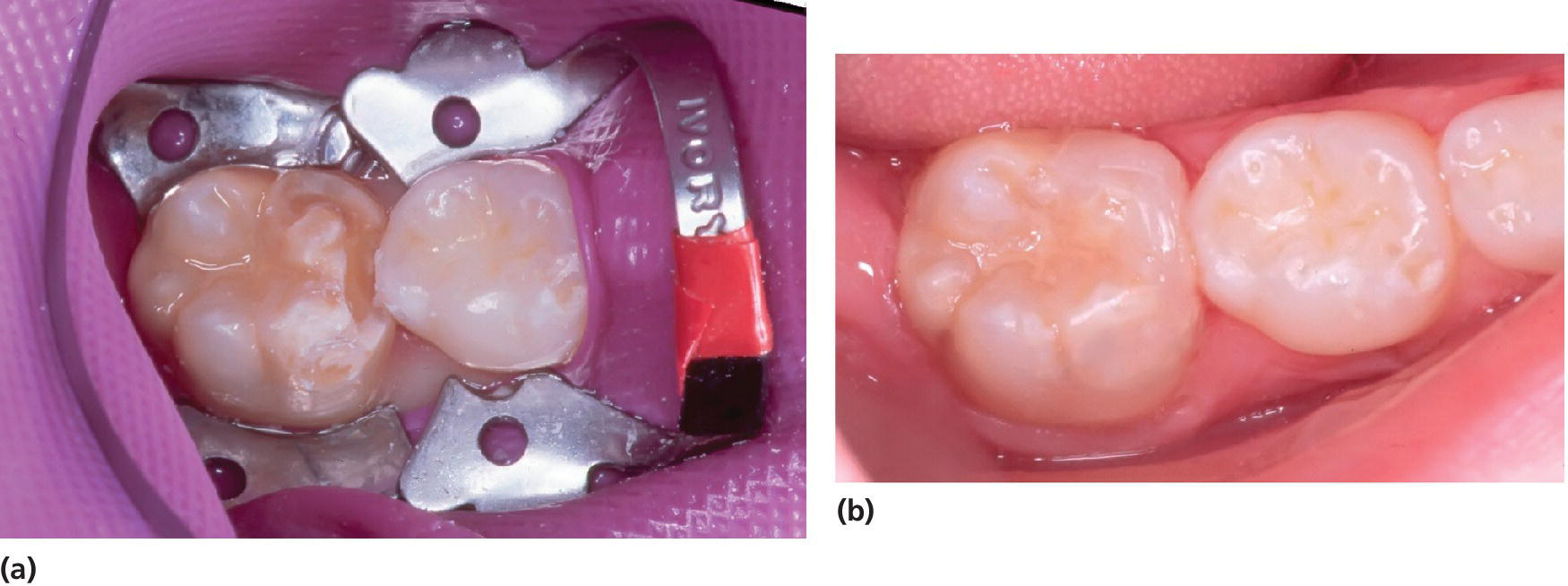
Figure 20.21 (a) An 8‐year‐old girl with an MIH‐affected molar to be restored. Porous, soft enamel was removed by a bur until hard, sound enamel appeared. (b) After acid etch of the cavity walls, dentin–enamel bonding agent was applied and the tooth was restored with composite.
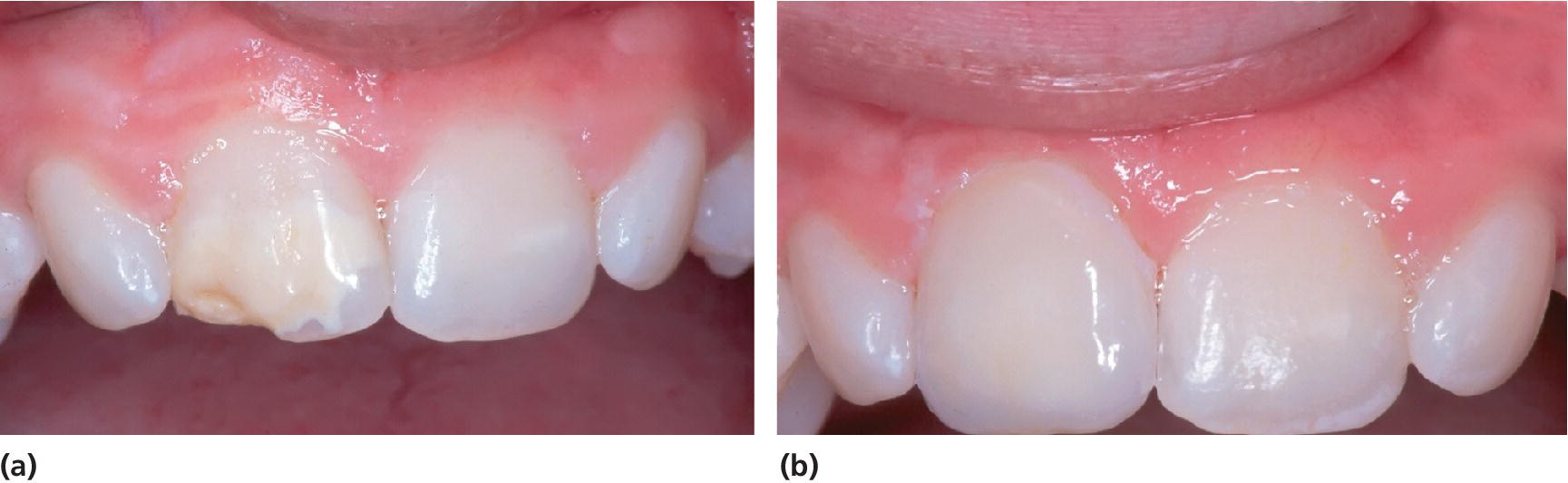
Figure 20.22 (a) A 13‐year‐old boy with MIH. (b) Enamel disintegration in 11 was restored with a porcelain veneer.

Figure 20.23 (a) Hypomineralized permanent first molars with opacities, posteruptive breakdown of enamel and insufficient conservative treatment, i.e., posteruptive enamel breakdown along the margins of composite fillings. (b) Treatment with cast‐gold copings after minimal preparation due to the scarce thickness required to be able to make a cast‐gold coping. (c) A cast‐gold coping on the cast. (d) The cast‐gold coping can be made with surface roughening by use of sugar crystal impression method with the purpose of improving the retention.
Inherited defects of the enamel
Amelogenesis imperfecta
Amelogenesis imperfecta (AI) is a genetic disease characterized by clinical as well as genetic heterogeneity [2,5,6,28–30] (Figure 20.24). Crawford et al. [29] have defined the disease:
AI represents a group of conditions, genomic in origin, which affect the structure and clinical appearance of the enamel of all or nearly all the teeth in a more or less equal manner, and which may be associated with morphologic or biochemical changes elsewhere in the body. AI is a developmental condition of the dental enamel (characterized by hypoplasia and/or hypomineralization) that shows autosomal dominant, autosomal recessive, sex‐linked and sporadic inheritance patterns, as well as sporadic cases.
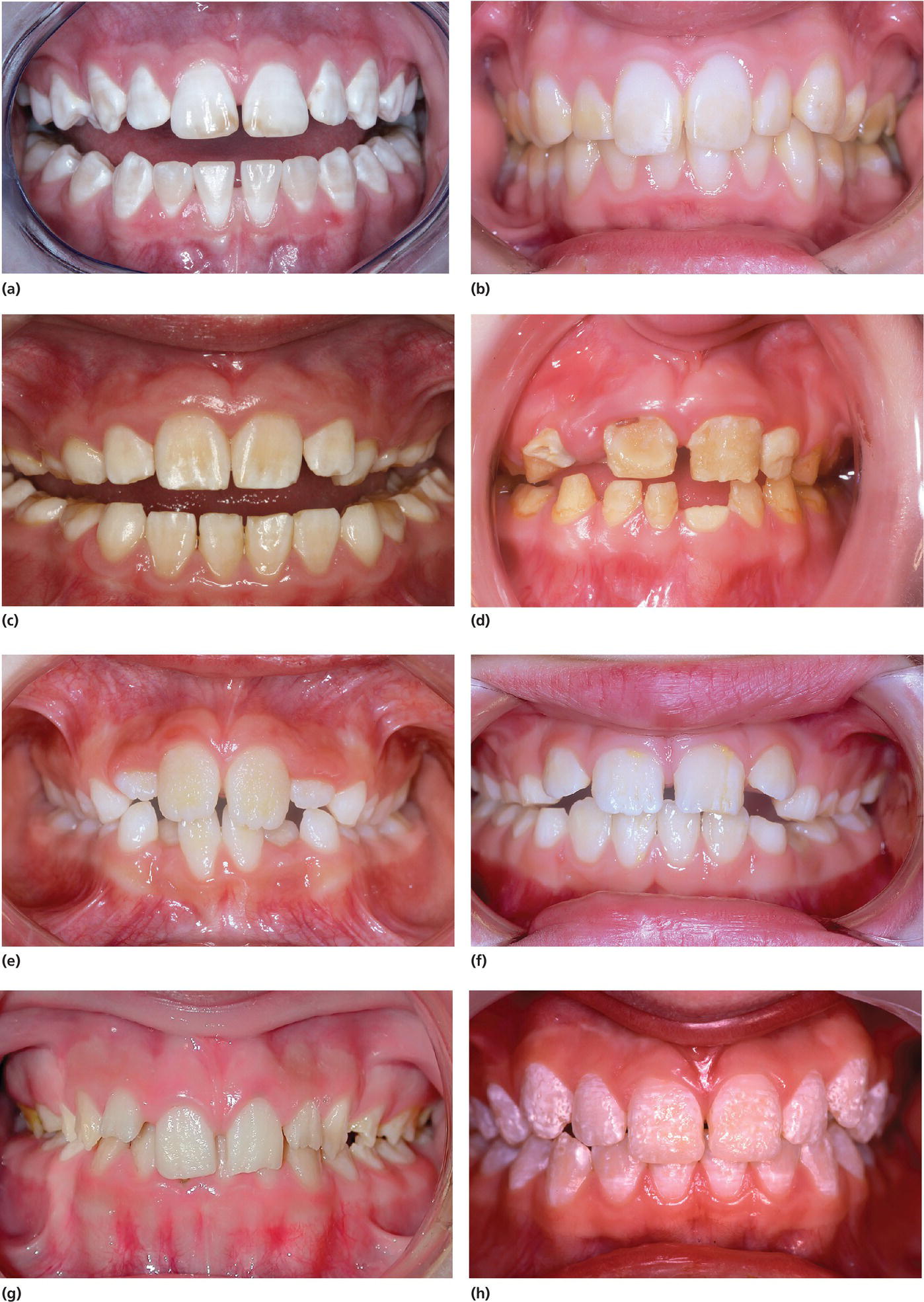
Figure 20.24 Clinical manifestation of amelogenesis imperfecta. (a) Hypomaturation type (generalized white opacities with brownish discolorations on upper central incisors). (b) Hypomaturation type (generalized yellowish opacities). (c) Hypomaturation type, mixed dentition. Chipping of enamel. (d) Hypocalcified type. (e) Hypoplastic type (rough, pitted). (f) Hypoplastic type (rough, vertical grooves). (g) Hypoplastic type (rough, thin enamel)
(courtesy of J. Daugaard‐Jensen).
(h) Combination of hypomaturation and hypoplastic type
(courtesy of J. Daugaard‐Jensen).
(g) and (h)
reproduced with permission of J. Daugaard‐Jensen.
AI is caused by mutations in the genes controlling amelogenesis and follows patterns of autosomal dominant, autosomal recessive or X‐linked inheritance. Occasionally, new mutations occur in genes controlling amelogenesis. In such cases a family history or pattern of inheritance relevant to the disease may not be possible to identify. AI is seen in both the primary and the permanent dentition (Figures 20.24 and 20.25). The disease may be less clinically evident in the primary dentition in some AI phenotypes.
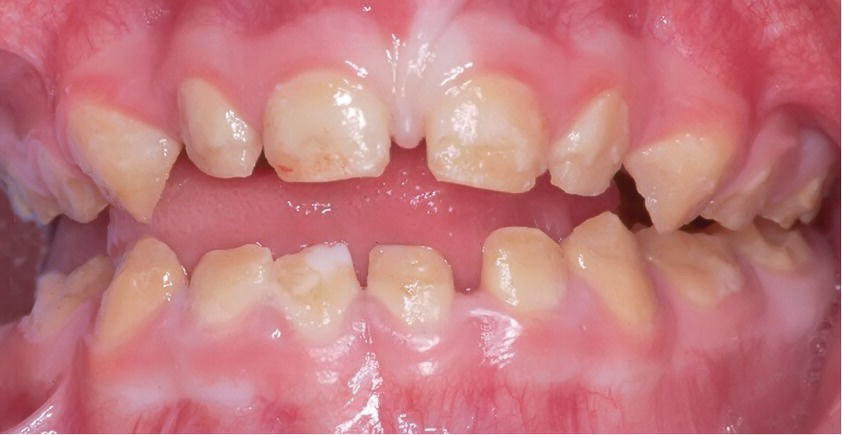
Figure 20.25 Hypocalcified amelogenesis imperfecta (AI) in the primary dentition of a 3‐year‐old girl.
Different prevalence figures have been reported from different parts of the world, being from one in 14,000 in Michigan, to one in 700 in the north of Sweden. The majority of reports are descriptions of cases and only a few epidemiologic studies reporting prevalence data are available [31–33].
The clinical picture of AI is highly variable and numerous variants have been described [34]. In the literature three main types have been focused on, one named hypoplastic type (Figure 20.24e‐g) and two hypomineralized types, called hypomaturated (Figure 20.24a‐c) and hypocalcified types (Figure 20.24d). Furthermore, a fourth type mixing hypoplastic and hypomaturated traits (Figure 20.24h) and, in addition, characterized by taurodontism has been described [34,35]. The characteristics of the three main types of AI are given in Boxes 20.5, 20.6 and 20.7. In the majority of teeth with AI the mineral content is lower than in unaffected teeth. Even in AI teeth with hypoplasia, various degrees of hypomineralization in areas of the enamel are found [36]. As mentioned above, taurodontism may be a feature in some variants of AI. An association between AI and anterior open bite of skeletal origin is found, but no exact reason for this co‐occurrence has been reported. In some cases of AI delayed eruption and/or retention of teeth are seen (Box 20.7).
The numerous AI classification systems suggested over time have been based primarily or exclusively on clinical manifestation (phenotype). In the 1990s, a classification of AI based on the molecular defect, biochemical results, mode of inheritance and phenotype was suggested [37]. The presently intense research activity related to the understanding of the genetics of AI will inevitably increase the use of genetic testing in the diagnostics of AI. Genetic counseling and molecular identification of specific mutations that cause the inherited disease in AI patients and their families may be more easily accessible and turn out to be an integrated part of diagnosis of AI in the future. The increasing body of information on the genetics of AI is also thought to be an integrated part of the classification of AI in the future [6].
Treatment of amelogenesis imperfecta
In general, the main clinical problems of AI are poor esthetics, sensitivity of teeth, chewing difficulties and the loss of tooth substance due to chipping and attrition often resulting in loss of occlusal vertical dimension [38]. The severity of each symptom depends on the age of the patient, the type of AI and the quality of the enamel. The psychosocial impact of AI on affected individuals has been studied [11]. AI was found to have a marked impact on overall psychosocial health, which should be kept in mind when treating AI patients.
Treatment of AI is ongoing during childhood and adolescence. Advanced restorative care and eventually complete prosthetic reconstruction are in some cases the only long‐term treatment solution (Figure 20.26). However, many intermediate phases in treatment may be necessary during childhood before the complete reconstruction can be performed.
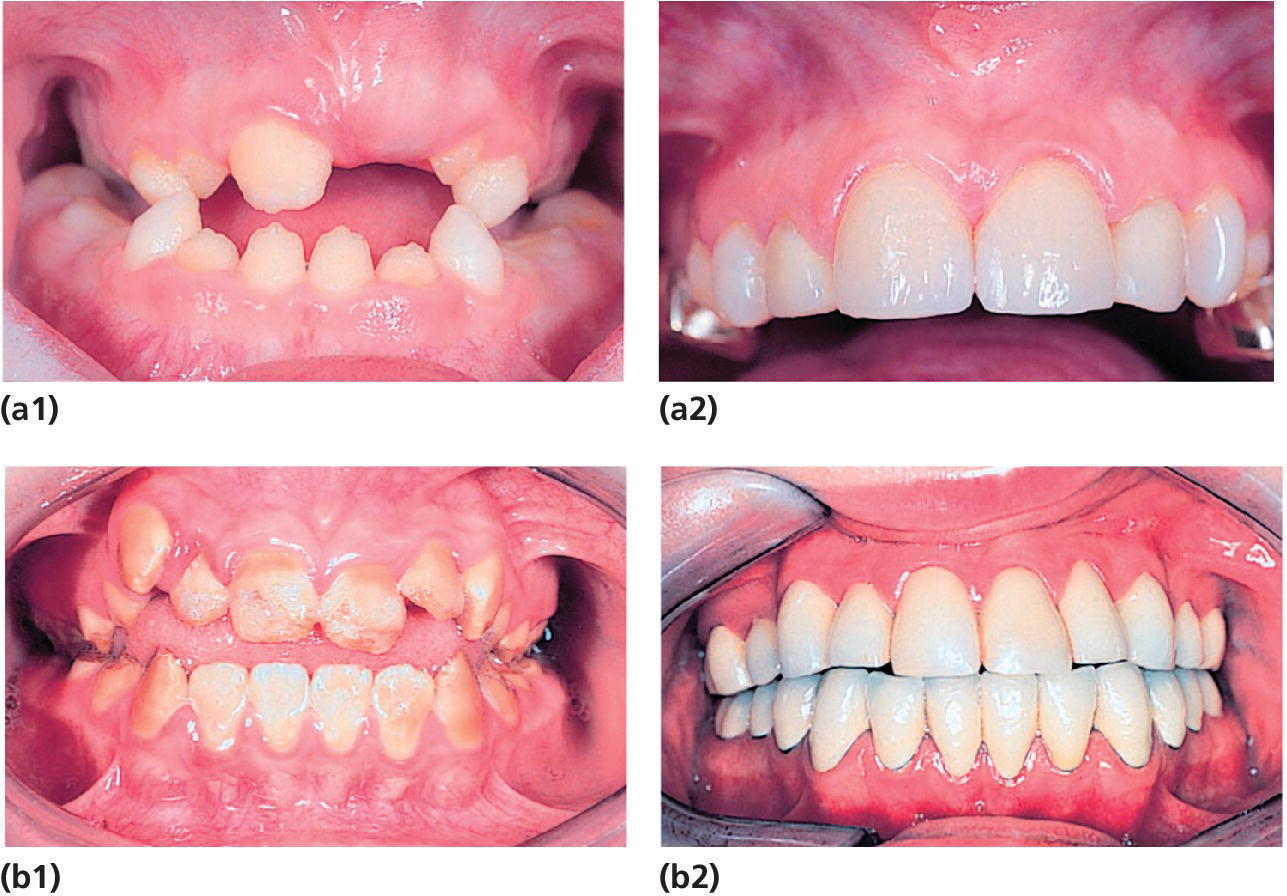
Figure 20.26 (a1) A 9‐year‐old girl with AI (hypoplastic type). (a2) Same girl at the age of 14 years. Maxillary incisors restored with ceramic veneers.
(courtesy of I. Andersson‐Wenckert)
(b1) Dentition of a 12‐year‐old boy with AI (hypocalcified type) before orthodontic treatment and placement of crowns. (b2) Same boy at the age of 20 years with single metalloceramic crowns in the mandible and single conventional gold crowns with composite facades in the maxillary teeth
(courtesy of H. Holming). (a) reproduced with permission of I. Andersson‐Wenckert. (b) reproduced with permission of H. Holming.
Both before and after initiation of treatment of AI patients, the imperfect enamel necessitates well‐functioning prevention. Professional prevention, including fluoride therapy and fissure sealing, should support the oral health care maintained at home.
In the primary dentition, the severity of the enamel defects is often less extensive. In spite of that, the sensitivity of primary teeth may be troublesome, occlusal attrition considerable, and restorative care complicated. In those situations the recommended and most long‐lasting therapy is to cover the primary molars with stainless‐steel crowns (Figure 20.27). In less severe cases, regular supervision and fluoride treatment to reduce sensitivity can be an adequate therapy.

Figure 20.27 A boy with teeth affected by hypocalcified AI: (a) 3.5 years, problems with sensitivity and chipping of enamel; (b) 3.5 years, the molars are restored by stainless‐steel crowns; (c, d) 9 years, time for dressing the permanent molars with steel crowns due to chipping and attrition of enamel.
In the young permanent dentition the severity of the enamel defect influences the choice of treatment. Some patients with milder hypomaturation types of AI may not need other treatment than the one necessary take care of their cosmetic needs. In more severe cases with extensive degrees of hypomineralization of the enamel, more advanced treatment may be needed to ensure proper functioning, e.g., function‐stabilizing treatment on permanent first molars, including stainless‐steel crowns, composite or porcelain onlays, partial or full‐cast gold crowns or copings with a need for minimum preparation (Figure 20.23). Apart from reducing thermal sensitivity these types of treatment serve to maintain normal vertical and mesiodistal dimensions. In less severe cases where the loss of vertical dimension is not as pronounced, dressing of the molars with composite or similar materials with adequate binding properties can be sufficient (Figure 20.28). Not all forms of AI respond favorably to enamel bonding and, for example, for the hypocalcified type of AI, enamel bonding may not be sufficient to keep the filling in place [38]. However, a bonding to enamel is possible in most patients with AI. Despite that, the etching patterns may vary in different AI variants [39]. The etchability of the enamel may depend on which gene is affected [40]. Therefore, the dentist should proceed tentatively with bonding restorative materials to enamel and check the quality of the restorations regularly. Sometimes there is not sufficient enamel to ensure proper retention. As the structure of the enamel may be unfavorable to obtain a reliable etching pattern of the enamel, bonding to exposed dentin has to be utilized. If the capacity of the child to cooperate is limited, the material of choice is the one that is least technique‐sensitive and demanding. Retreatment of the child may be needed as the cooperation of the child improves. To reduce discoloration, AI cases with severe hypoplasia or PEB should be sealed soon after eruption (Figure 20.28). As the child gets older, appearance becomes more important and poor esthetics demand consideration. Direct restoration of the incisors with composites or, later on, porcelain veneers or crowns may be indicated to improve the esthetics sufficiently. Continued eruption and discoloration of the filling margins necessitate continuous control and adjustment. The esthetic problems of children and adolescents with enamel defects must be taken seriously and sometimes require that extensive restorative procedures are carried out at a younger age than might usually be considered. A recent study showed that the longevity of dental composite restorations was significantly lower in patients with AI compared to a matched control group [41]. The crown therapy worked very well in the AI‐group. In AI patients with hypomineralized enamel, restorations had shorter longevity than in those with hypoplastic enamel. The study indicates that it is important to establish an early permanent therapy plan for patients with AI to avoid unnecessary dental treatment and retreatment.
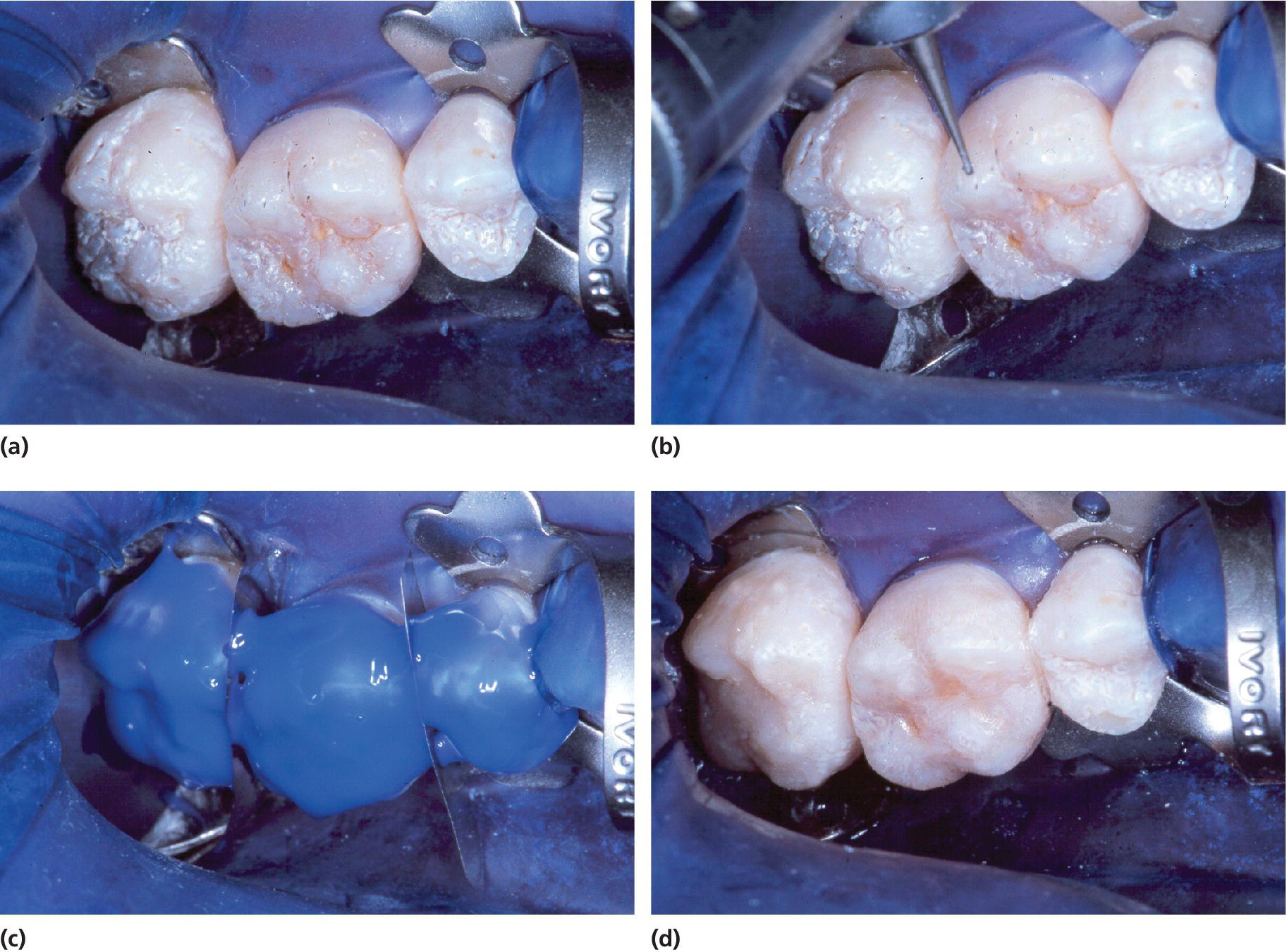
Figure 20.28 Treatment of hypoplastic, rough pitted AI. (a) Prior to treatment, (b) pits are cleaned by the bur, (c) etching, and (d) the teeth are dressed with flowable composite.
Inherited defects of the dentin
Dentinogenesis imperfecta
Shields et al. [8] have subgrouped three different types of DI and de La Dure‐Molla et al. [42] has proposed a new classification based on the fact that DI‐II, DI‐III, and DD‐II are caused by mutations in the same gene and represent different phenotypes of different clinical severities (Box 20.8). The dentinogenesis is affected in all types. Clinically for both DI‐I (associated with osteogenesis imperfecta, OI) and DI‐II, primary teeth tend to be more severely affected than permanent teeth. Later‐formed permanent teeth are less severely affected than early‐formed ones. The teeth are opalescent with bluish‐brown discoloration of primary as well as permanent teeth. Enamel has often microcracks or infractions and tends to chip away, exposing the abnormal, soft dentin. Thus, teeth wear rapidly. Primary teeth may be worn down to the gingival margin within a few years after eruption if not protected (Figure 20.29). Pulp exposure and pulp complication may occur. In the permanent dentition, pulpal involvement is rare due to the rapid obliteration of the pulp chambers. The crowns appear bulbous and the roots are often short and thin on radiographs (Figure 20.30). Initially, normal pulp chambers obliterate soon after eruption and root canals are gradually narrowed. Among patients with mild signs of OI, examination for dental defects may contribute towards establishing the final, medical diagnosis [43].
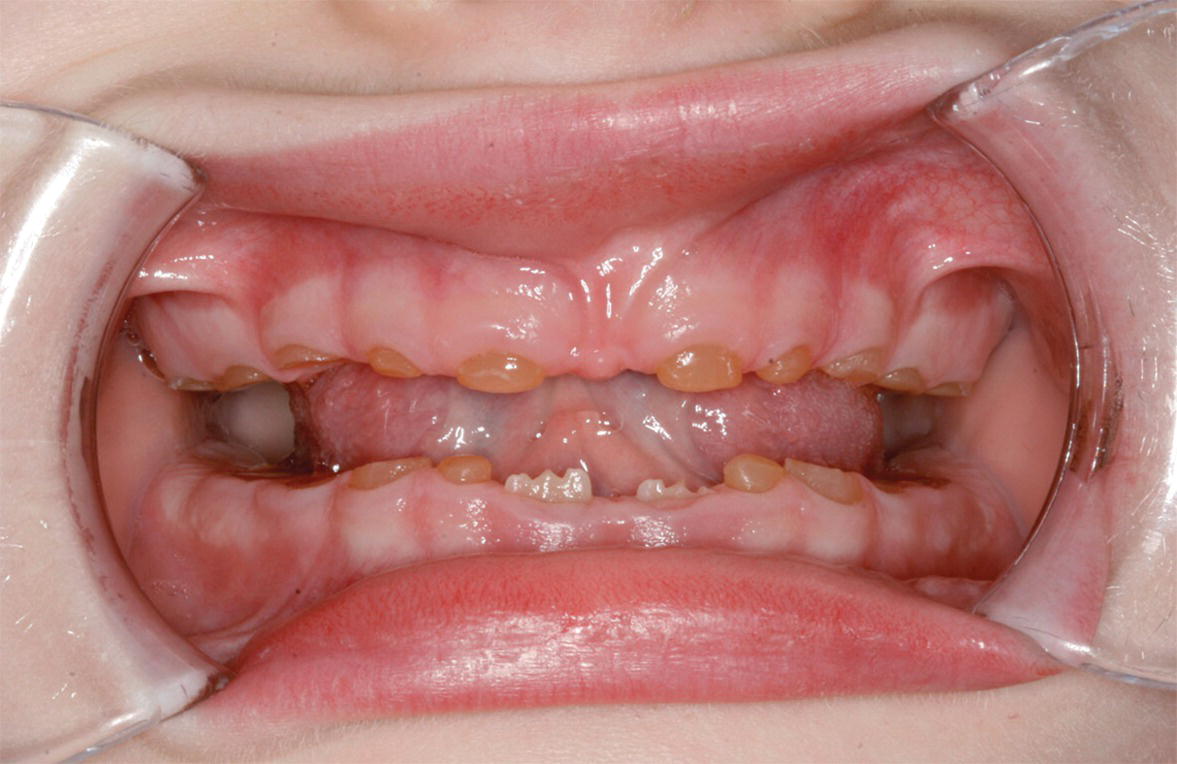
Figure 20.29 A 5‐year‐old child with DI showing extreme tooth wear in the primary molars. No operative treatment has been offered so far, although the child would have benefited from stainless‐steel crowns in the molar regions to keep the occlusion. Permanent lower central incisors are in eruption.
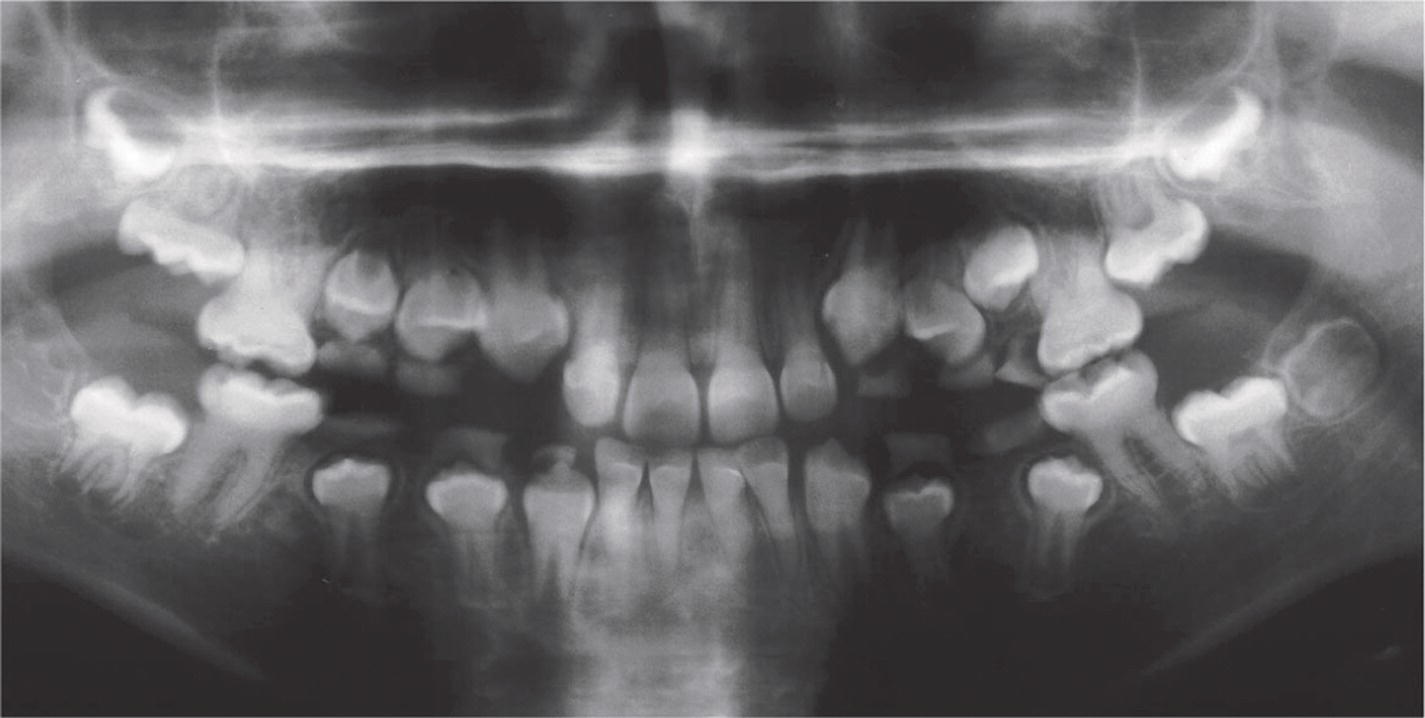
Figure 20.30 Panoramic radiograph of a 10‐year‐old boy with DI inherited as a single trait (type II). Note the obliterated pulp chambers and the cervical constrictions of the crowns.
DI is a single‐gene disorder. Although the phenotypes of DI‐I and DI‐II are similar, the genetics associated with the respective types differ. While DI‐II has an inheritance pattern which is autosomal dominant with high penetrance, OI (DI‐I) is a genetically heterogeneous group of generalized connective tissue diseases. The vast majority of known mutations related to OI are located in the genes encoding the two type I procollagen chains. The main clinical symptom of OI is bone fragility, but the disease may affect sclera, tendons, meninges, dentin, and dermis (Figure 20.31). Bones may break spontaneously and for no known reason. OI can also cause weak muscles, a curved spine, and hearing loss. OI is subdivided into many types of different severity.

Figure 20.31 Osteogenesis imperfecta. (a) Blue sclerae in a 4‐year‐old boy. (b) Characteristic dentin defect (DI type I) in the same boy.
Treatment of dentinogenesis imperfecta
As in AI, the treatment of DI is ongoing through childhood and adolescence. Poor esthetics and the attrition of the teeth constitute the main problems. If untreated, attrition may affect facial height, appearance, and masticatory function. Severe attrition of the primary teeth allows attrition of the permanent first molars soon after eruption and a vicious circle is initiated (Figure 20.29).
In the primary dentition for the long‐term maintenance of the vertical dimension, stainless‐steel crowns on the molars are the treatment of choice. Treatment should be started as soon as excessive attrition or spontaneous fractures of the enamel are observed, when there is still sufficient tooth substance left for retention of the crowns (Figure 20.32a). An early diagnosis is therefore important as well as frequent follow‐ups. Necrotic primary teeth should be extracted.
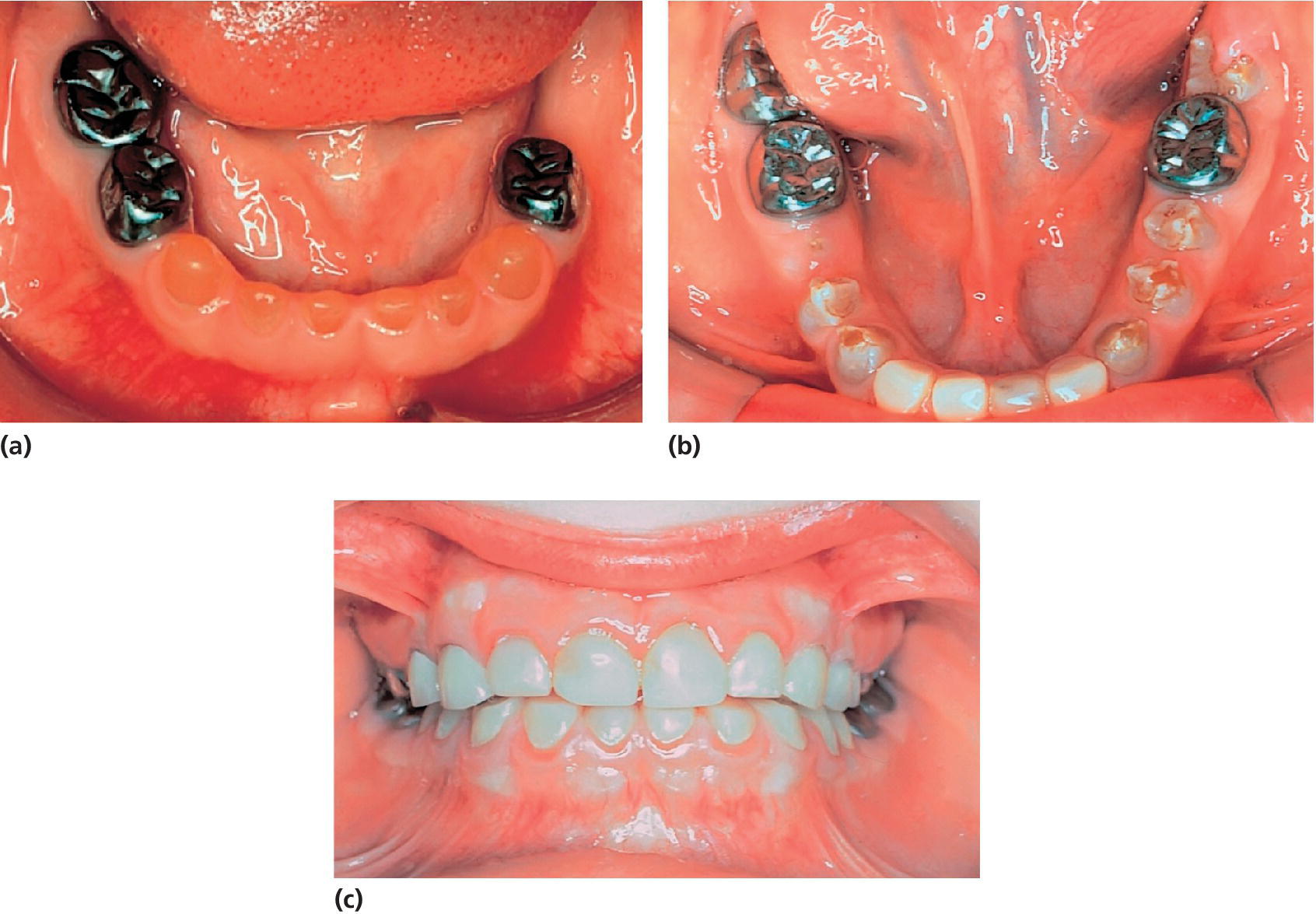
Figure 20.32 A boy with DI type II. (a) Mandibular teeth at the age of 3 years 5 months. The primary left second molar was infected due to attrition and had to be extracted. Stainless‐steel crowns have been made to prevent further attrition. (b) The permanent molars have been fitted with stainless‐steel crowns and the incisors with laboratory‐made composite crowns
(courtesy of J. Waltimo).
(c) Same patient at the age of 10 years 4 months
(courtesy of J. Waltimo). Reproduced with permission of J. Waltimo.
In the young permanent dentition in DI, in contrast to AI, the defects are less severe. In cases with severe attrition of the primary teeth, the vertical dimensions have to be increased to allow permanent molars and incisors to erupt normally. To achieve this, stainless‐steel crowns can be placed on the primary molars even in cases with extensive attrition of these teeth. The use of a customized guard at night‐time may be useful for preventing enamel cracking and attrition in young permanent teeth. In cases of cracking and/or attrition, stainless‐steel crowns or cast‐gold onlays with minimal preparation, GICs, or composites can be used (Figure 20.32b,c). As the child matures, ceramic crowns are alternative restorations for the molars and direct composites or ceramic crowns for the front teeth.
Dentin dysplasia
According to Shields’ classification (Box 20.3), there are two types of DD. Type I (DD‐I) is also called radicular dentin dysplasia or rootless teeth, which is an incomplete tooth formation. Clinically, tooth crowns appear normal, but radiographic examination will often show incomplete root formation and a conical or missing root. In addition, periapical lesions in relation to teeth without caries are typical. Total obliteration of pulp chambers of primary teeth usually occurs. In permanent teeth, crescent‐shaped pulpal remnants of crown pulp may be seen on radiographs. Furthermore, tooth exfoliation may occur. It is a rare autosomal dominant condition (one in 100,000), but so far it is unknown whether the condition is also an allelic disorder of the dentin sialophosphoprotein (DSPP) gene [7].
DD type II (DD‐II) is a rare autosomal dominant condition. The findings are different in the two dentitions. The primary dentition is opalescent with a gray or brownish discoloration as seen in DI. Pulp obliteration is seen on radiographs in primary teeth, while the permanent teeth appear to be normal. However, thistle‐tube pulp chambers with pulp stones are seen radiographically (Figure 20.33). From a genetic point of view, there is reason to believe that DD‐II and DI‐II and III phenotypes are the same disease, but of different severity [7]. DD‐II is the mild form and DI‐III the most severe type. The proposed classification by de La Dure‐Molla et al. [42] is based on genotypical findings of hereditary dentin defects (Box 20.3).
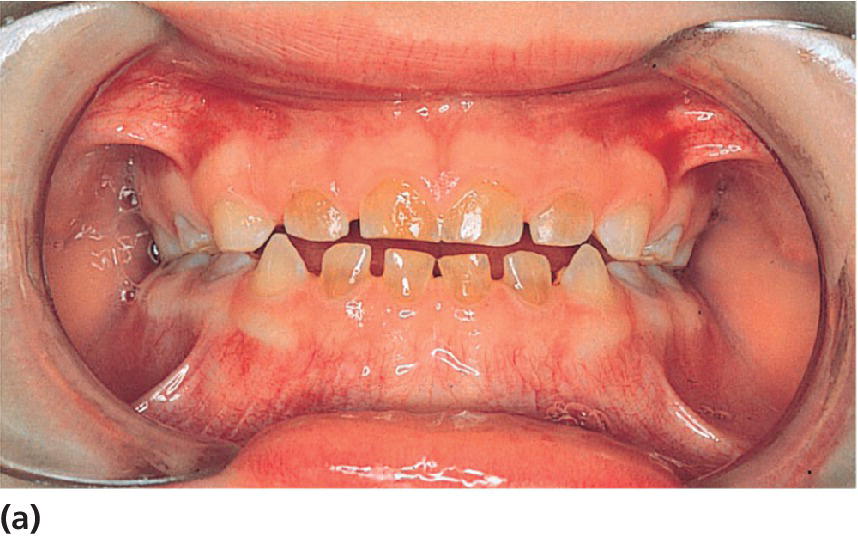
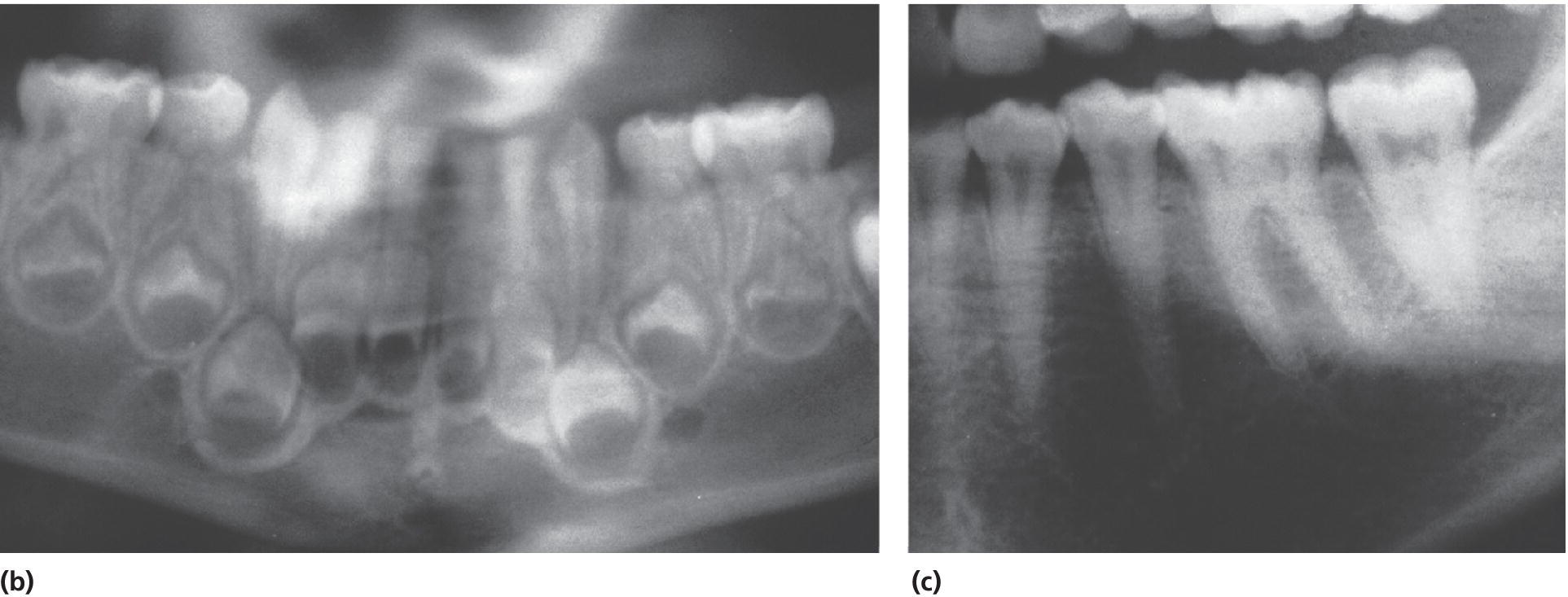
Figure 20.33 Dentin dysplasia type II. (a) A 3‐year‐old boy with DD type II. (b) Panoramic radiograph showing pulpal obliteration in the primary molars. (c) Radiograph of the mother showing characteristic thistle‐tube‐shaped pulp chambers and narrowed root canals.
Disturbances in calcium/phosphate metabolism with relevance to mineralization of teeth
Nutritional rickets
Nutritional rickets (vitamin D deficiency rickets) most frequently arise as a consequence of longstanding vitamin D deficiency [44]. It might be inborn due to vitamin D deficiency in utero or may becaused by a low calcium intake. Nutritional rickets may be subdivided into acquired primary vitamin D deficiency rickets, acquired vitamin D deficiency rickets secondary to other diseases and acquired calcium deficiency rickets.
The sources of fat‐soluble vitamin D (prohormone) are either diet or production in sun‐exposed skin. Vitamin D regulates the absorption of calcium from the jejunum and is very important for the mineralization of bone and dental hard tissue. Hypocalcemia leads to skeletal and dental deformities. Rickets affects the structural organization of enamel and the mineralization of it. In cases of nutritional rickets, teeth have typical chronological, symmetrically hypoplastic defects related to the time period when the hypocalcemia was prominent. Dental hard tissue formed before and after that period is normal (Figure 20.34).
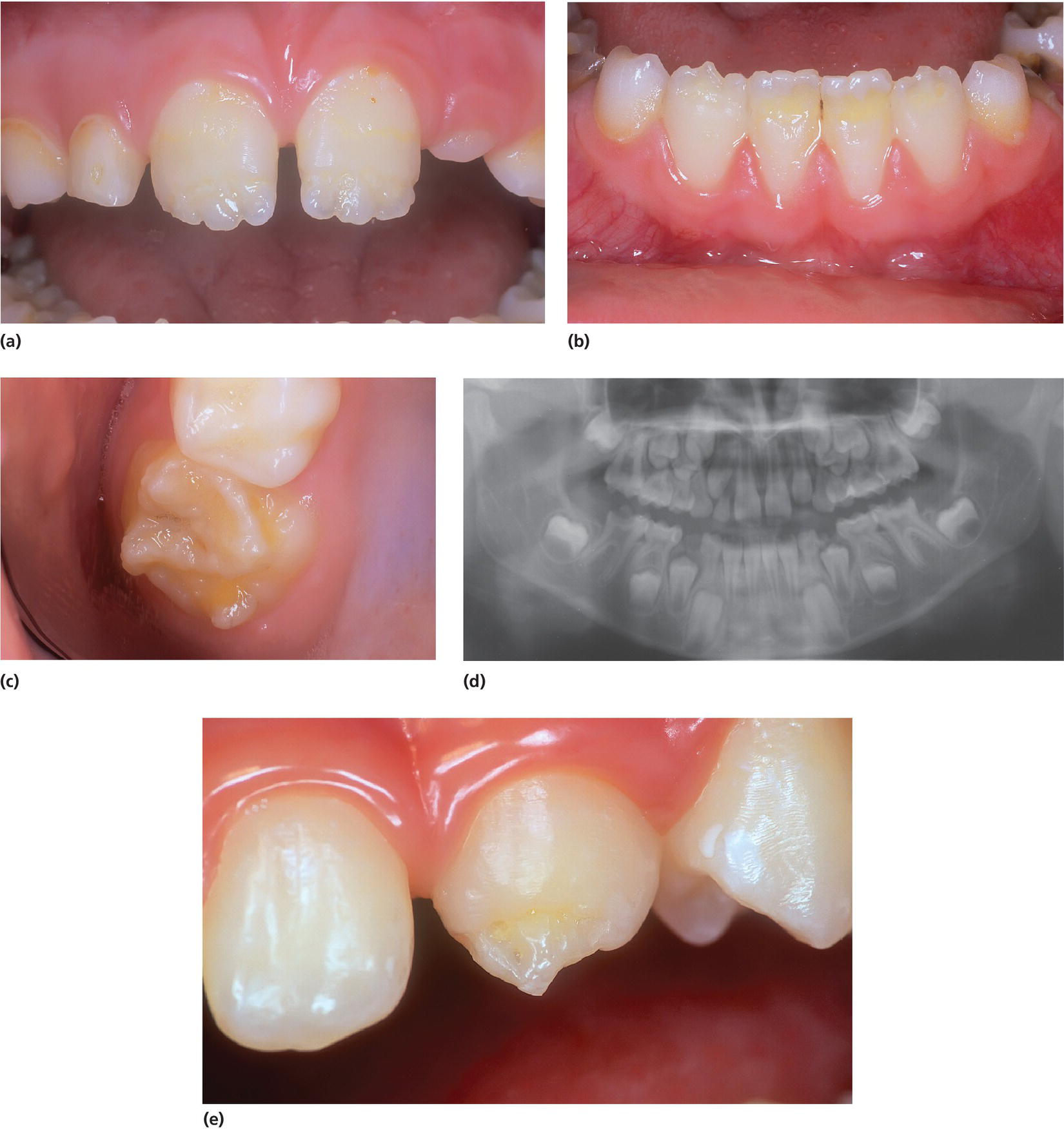
Figure 20.34 Vitamin D deficiency rickets (nutritional rickets). (a–c) Chronological and symmetrical hypoplasia in permanent incisors and first molars in a breastfed 8‐year‐old boy who was not given a vitamin D supplement in the first year of life. (d) Defects can often be seen on a panoramic radiograph. (e) An 11‐year‐old boy with severe hypoplasia on upper permanent incisors due to vitamin D deficiency rickets. Often cusp tips of permanent canines are affected.
Treatment of nutritional rickets
In most cases, the affected teeth presenting with hypoplasia can be treated by restorative therapy, in the incisor region often for esthetic reasons.
Hereditary rickets
An increasing number of genes involved in the phenotypically heterogeneous group of hereditary forms of rickets are known today [44]. Hereditary rickets is a group of rare inherited disorders, and some of the more well‐known subtypes are termed 1‐alfa‐hydroxylase deficiency (VDDR type I) and vitamin D‐resistant rickets (VDDR type II, familial hypophosphatemia) in addition to FGF (fibroblast growth factor)‐23‐associated and non‐FGF‐23‐associated hypophosphatemic rickets.
Often an impairment of the reabsorption of phosphate in the kidneys leading to low levels of phosphate in serum, is a part of the pathophysiology. Dentin may have globular defects and be hypomineralized with enlargement of the pulp. Bacterial invasion through enamel cracks and dentinal defects of the teeth may lead to abscesses arising from seemingly sound teeth (Figure 20.35). Abscess formation is seen, particularly in the primary dentition, and one abscess may predict future abscesses [45]. It is difficult to prevent abscess formation.

Figure 20.35 Familial hypophosphatemia. (a) A 6‐year‐old boy with several infected primary teeth. (b) Radiograph of mandibular primary teeth in a 4‐year‐old boy. Note the enlarged pulp chambers. (c) Microradiograph of a primary molar. Note the enlarged pulp chamber and pulp horns extending to dentino–enamel junction. Defective circumpulpal dentin, mantle dentin seemingly normal. (d) Panoramic radiograph showing periapical radiolucency in the lower incisor region and two lower incisors that have already been endodontically treated in a 14‐year‐old boy. Note also the enlarged pulp chambers and the extended pulp horns in the permanent dentition. (e) Clinical picture of the lower jaw of the same 14‐year‐old boy.
Celiac disease
Celiac disease will in some cases give rise to hypoplastic enamel in permanent teeth formed during acute episodes of the disease at a very young age [46]. The villi in small intestine are destroyed due to intolerance to gluten that is found in wheat, rye, and barley. The patient will be malnourished and too little calcium is absorbed. The treatment is a lifelong gluten‐free diet. Susceptibility to celiac disease is associated with human leukocyte antigen genes. People with celiac disease tend to have other autoimmune diseases such as dermatitis herpetiformis (DH). Symptoms are severe itching and the extensive eruption of vesicles and groups of papules which appear on the elbows, knees, and other pressure points. A gluten‐free diet may also help to control this disease. A higher frequency of enamel defects in patients with DH has been reported [47] and the author suggests that it should be considered whether symmetric, unexplained dental defects may be the only detectable sign of a gluten‐sensitive enteropathy. It is hypothesized that a gluten‐induced immunologic reaction during the tooth formation period may give rise to defective enamel [48]. So far, no scientific evidence confirms this theory. The reported prevalence of dental defects in celiac disease patients varies from 10 to 96% in the literature [49]. However, a case–control study of 50 celiac disease patients was not able to confirm a higher prevalence among them [49].
References
- 1. Risnes S. Growth tracks in dental enamel. J Hum Evol 1998;35:331–50.
- 2. Wright JT, Carrion IA, Morris C. The molecular basis of hereditary enamel defects in humans. J Dent Res 2015;94:52–61.
- 3. Commission on Oral Health RE. A review of the developmental defects of enamel index (DDE Index). Report of an FDI Working Group. Int Dent J 1992;42:411–26.
- 4. Aldred MJ, Savarirayan R, Crawford PJ. Amelogenesis imperfecta: a classification and catalogue for the 21st century. Oral Dis 2003;9:19–23.
- 5. Stephanopoulos G, Garefalaki ME, Lyroudia K. Genes and related proteins involved in amelogenesis imperfecta. J Dent Res 2005;84:1117–26.
- 6. Wright JT. The molecular etiologies and associated phenotypes of amelogenesis imperfecta. Am J Med Genet A 2006;140:2547–55.
- 7. Kim JW, Simmer JP. Hereditary dentin defects. J Dent Res 2007;86:392–9.
- 8. Shields ED, Bixler D, el‐Kafrawy AM. A proposed classification for heritable human dentine defects with a description of a new entity. Arch Oral Biol 1973;18:543–53.
- 9. Wong HM, McGrath C, King NM. Dental practitioners' views on the need to treat developmental defects of enamel. Community Dent Oral Epidemiol 2007;35:130–9.
- 10. Shulman JD, Maupome G, Clark DC et al. Perceptions of desirable tooth color among parents, dentists and children. J Am Dent Assoc 2004;135:595–604; quiz 54–5.
- 11. Coffield KD, Phillips C, Brady M et al. The psychosocial impact of developmental dental defects in people with hereditary amelogenesis imperfecta. J Am Dent Assoc 2005;136:617–30.
- 12. Sneller J, Buchanan H, Parekh S. The impact of amelogenesis imperfecta and support needs of adolescents with AI and their parents: an exploratory study. Int J Paediatr Dent 2014;24:409–16.
- 13. Jalevik B, Klingberg GA. Dental treatment, dental fear and behaviour management problems in children with severe enamel hypomineralization of their permanent first molars. Int J Paediatr Dent 2002;12:24–32.
- 14. Ridell K, Borgstrom M, Lager E et al. Oral health‐related quality‐of‐life in Swedish children before and after dental treatment under general anesthesia. Acta Odontol Scand 2015;73:1–7.
- 15. Rodd HD, Davidson LE. ‘Ilko dacowo’: canine enucleation and dental sequelae in Somali children. Int J Paediatr Dent 2000;10:290–7.
- 16. Cordeiro MM, Rocha MJ. The effects of periradicular inflamation and infection on a primary tooth and permanent successor. J Clin Pediatr Dent 2005;29:193–200.
- 17. Aoba T, Fejerskov O. Dental fluorosis: chemistry and biology. Crit Rev Oral Biol Med 2002;13:155–70.
- 18. Antonini LG, Luder HU. Discoloration of teeth from tetracyclines–even today? Schweiz Monatsschr Zahnmed 2011;121:414–31.
- 19. Matis BA, Wang Y, Eckert GJ et al. Extended bleaching of tetracycline‐stained teeth: a 5‐year study. Oper Dent 2006;31:643–51.
- 20. Weerheijm KL, Jalevik B, Alaluusua S. Molar–incisor hypomineralisation. Caries Res 2001;35:390–1.
- 21. Elfrink ME, Schuller AA, Weerheijm KL et al. Hypomineralized second primary molars: prevalence data in Dutch 5‐year‐olds. Caries Res 2008;42:282–5.
- 22. Jalevik B, Noren JG. Enamel hypomineralization of permanent first molars: a morphological study and survey of possible aetiological factors. Int J Paediatr Dent 2000;10:278–89.
- 23. Fagrell TG, Lingstrom P, Olsson S et al. Bacterial invasion of dentinal tubules beneath apparently intact but hypomineralized enamel in molar teeth with molar incisor hypomineralization. Int J Paediatr Dent 2008;18:333–40.
- 24. Silva MJ, Scurrah KJ, Craig JM et al. Etiology of molar incisor hypomineralization ‐ A systematic review. Community Dent Oral Epidemiol 2016;44:342–53.
- 25. Rodd HD, Morgan CR, Day PF et al. Pulpal expression of TRPV1 in molar incisor hypomineralisation. Eur Arch Paediatr Dent 2007;8:184–8.
- 26. Jalevik B, Dietz W, Noren JG. Scanning electron micrograph analysis of hypomineralized enamel in permanent first molars. Int J Paediatr Dent 2005;15:233–40.
- 27. William V, Burrow MF, Palamara JE et al. Microshear bond strength of resin composite to teeth affected by molar hypomineralization using 2 adhesive systems. Pediatr Dent 2006;28:233–41.
- 28. Kim JW, Simmer JP, Lin BP et al. Mutational analysis of candidate genes in 24 amelogenesis imperfecta families. Eur J Oral Sci 2006;114 Suppl 1:3–12; discussion 39–41, 379.
- 29. Crawford PJ, Aldred M, Bloch‐Zupan A. Amelogenesis imperfecta. Orphanet J Rare Dis 2007;2:17.
- 30. Hart PS, Hart TC. Disorders of human dentin. Cells Tissues Organs 2007;186:70–7.
- 31. Backman B, Holm AK. Amelogenesis imperfecta: prevalence and incidence in a northern Swedish county. Community Dent Oral Epidemiol 1986;14:43–7.
- 32. Sundell S, Koch G. Hereditary amelogenesis imperfecta. I. Epidemiology and clinical classification in a Swedish child population. Swed Dent J 1985;9:157–69.
- 33. Witkop CJ. Hereditary defects in enamel and dentin. Acta Genet Stat Med 1957;7:236–9.
- 34. Witkop CJ, Jr. Amelogenesis imperfecta, dentinogenesis imperfecta and dentin dysplasia revisited: problems in classification. J Oral Pathol 1988;17:547–53.
- 35. Winter GB, Brook AH. Enamel hypoplasia and anomalies of the enamel. Dent Clin North Am 1975;19:3–24.
- 36. Backman B, Angmar‐Mansson B. Mineral distribution in the enamel of teeth with amelogenesis imperfecta as determined by quantitative microradiography. Scand J Dent Res 1994;102:193–7.
- 37. Aldred MJ, Crawford PJ. Amelogenesis imperfecta‐‐towards a new classification. Oral Dis 1995;1:2–5.
- 38. Seow WK. Taurodontism of the mandibular first permanent molar distinguishes between the tricho‐dento‐osseous (TDO) syndrome and amelogenesis imperfecta. Clin Genet 1993;43:240–6.
- 39. Seow WK, Amaratunge A. The effects of acid‐etching on enamel from different clinical variants of amelogenesis imperfecta: an SEM study. Pediatr Dent 1998;20:37–42.
- 40. Simmer JP, Hu JC. Dental enamel formation and its impact on clinical dentistry. J Dent Educ 2001;65:896–905.
- 41. Pousette Lundgren G, Dahllof G. Outcome of restorative treatment in young patients with amelogenesis imperfecta. a cross‐sectional, retrospective study. J Dent 2014;42:1382–9.
- 42. de La Dure‐Molla M, Philippe Fournier B, Berdal A. Isolated dentinogenesis imperfecta and dentin dysplasia: revision of the classification. Eur J Hum Genet 2015;23:445–51.
- 43. Malmgren B. Clinical, histopathologic and genetic diagnosis in osteogenesis imperfecta and dentinogenesis imperfecta. Stockholm: Karolinska Institutet; 2004.
- 44. Beck‐Nielsen SS. Rickets in Denmark. Dan Med J 2012;59:B4384.
- 45. McWhorter AG, Seale NS. Prevalence of dental abscess in a population of children with vitamin D‐resistant rickets. Pediatr Dent 1991;13:91–6.
- 46. Rasmussen P, Espelid I. Coeliac disease and dental malformation. ASDC J Dent Child 1980;47:190–2.
- 47. Aine L. Coeliac‐type permanent‐tooth enamel defects. Ann Med 1996;28:9–12.
- 48. Maki M, Aine L, Lipsanen V et al. Dental enamel defects in first‐degree relatives of coeliac disease patients. Lancet 1991;337:763–4.
- 49. Procaccini M, Campisi G, Bufo P et al. Lack of association between celiac disease and dental enamel hypoplasia in a case–control study from an Italian central region. Head Face Med 2007;3:25.
- 50. Dong J, Amor D, Aldred MJ et al. DLX3 mutation associated with autosomal dominant amelogenesis imperfecta with taurodontism. Am J Med Genet A 2005;133:138–41.
- 51. MacDougall M, Dong J, Acevedo AC. Molecular basis of human dentin diseases. Am J Med Genet A 2006;140:2536–46.
- 52. van den Bos T, Handoko G, Niehof A et al. Cementum and dentin in hypophosphatasia. J Dent Res 2005;84:1021–5.
- 53. Crawford PJ, Aldred MJ. Regional odontodysplasia: a bibliography. J Oral Pathol Med 1989;18:251–63.