Melanocytic Tumors
More than most categories of cutaneous disease, the melanocytic tumors are especially dominated by one condition: melanoma. Virtually every melanocytic lesion is accompanied by the specter of melanoma. Although in some cases this possibility is remote, in others it is a more immediate concern. In short, melanoma is the diagnosis against which almost all other melanocytic lesions are judged. This chapter considers the constellation of melanocytic lesions; the histopathologic features that make them unique; and those microscopic changes that overlap, mimic, or can be mimicked by the variants of melanoma.
Non-neoplastic Hyperpigmented Lesions
Clinical Features: Ephelides (singular ephelis, although it is sometimes given as ephelid) is a small hyperpigmented spot typically measuring less than 4 mm in diameter. The epidermal surface is otherwise normal, essentially the same as adjacent uninvolved skin. The spots tend to be light tan in color but may darken with sun exposure. Typically, ephelides are seen in individuals with skin types that burn readily but tan poorly with sun exposure, such as those with Celtic background.1
Microscopic Findings: Biopsy changes are subtle, consisting of a circumscribed area of basilar hyperpigmentation that differs only slightly from the pigmentation of adjacent, uninvolved skin. No discernible melanocytic proliferation is seen; down-growths of rete ridges are not observed; and the melanocytes lack significant morphologic abnormalities, showing only increased amounts of coarse pigment granules. In fact, the numbers of melanocytes per unit area are the same or slightly decreased compared with those in the adjacent epidermis.2
Differential Diagnosis: Lentigines frequently (but not invariably) show slightly exaggerated down-growth of rete ridges and a greater density of basilar pigmentation. In contrast to ephelides, there are increased numbers of melanocytes per unit area in lentigines, which can be nicely demonstrated with techniques such as Melan-A staining. Distinction between the naturally brown melanin pigment and positive immunohistochemical staining can be achieved by using a counterstain for melanin, such as azure B, or by using a chromogen other than the brown-staining diaminobenzidine (e.g., aminoethyl carbazole—a red stain). Café-au-lait macules may have similar degrees of pigmentation but are much larger than ephelides. Giant melanosomes can be found in these macules—a feature not generally described in ephelides.
Mucosal Melanosis
Clinical Features: This condition encompasses an array of variably named lesions that share similar, if not identical, clinical and histopathologic features. These are irregularly shaped, well-demarcated pigmented macules on mucosal surfaces, including the lip (labial melanotic macule), portions of the oral cavity (oral melanosis), and genitalia (penile/vulvar melanosis or genital lentiginosis).3,4 These asymptomatic lesions can cause significant concern for mucosal melanoma in patients and clinicians.
Microscopic Findings: All of these forms of mucosal melanosis show prominent basilar hyperpigmentation with accentuation at the tips of rete ridges (Fig. 28-1A and B). Melanophages are usually found in the papillary dermis/lamina propria of oral lesions and virtually always in genital lesions. The numbers of melanocytes are normal, and there is no nesting of these cells. Significant cytologic atypia is not observed, and no mitoses are identified.3–5
Differential Diagnosis: Many of these lesions display evenly distributed basilar hypermelanosis across a broad front of epidermis, with well-demarcated lateral borders; confluence of junctional melanocytes as seen in lentiginous melanoma is not observed. Exaggerated down-growths of rete ridges as seen in lentigines and (in a more extreme manner) reticulated pigment anomalies are not truly features of these lesions, and based on this difference, some authors prefer to make a distinction between mucosal lentiginosis and mucosal melanosis. Melanoacanthoma is probably a phenomenon rather than a distinct clinicopathologic entity; in contrast to melanotic macules, dendritic melanocytes are found scattered within suprabasilar epidermis, with poor pigment transfer to adjacent keratinocytes.6 Forms of postinflammatory hyperpigmentation can resemble melanotic macules with prominent melanophages in the papillary dermis or lamina propria. However, the discrete nature and sharp demarcation of melanotic macules and lack of significant inflammation argues against hyperpigmentation arising in an inflammatory dermatosis.
Café-au-Lait Macules and Nevus Spilus
Clinical Features: Café-au-lait macules are homogeneously colored, light to dark brown macules or patches. They can arise anywhere on the body surface except for mucous membranes and are actually quite common, with solitary lesions being found in 10% to 20% of the population. Café-au-lait macules can serve as markers for various syndromes, particularly neurofibromatosis types 1 and 2, but also the McCune-Albright syndrome and Watson syndrome.7 Nevus spilus, or speckled lentiginous nevus, features a grouping of small, pigmented macules and papules on a light tan macule. This is a not uncommon incidental finding but can be associated with several types of phakomatosis (defined as a hereditary syndromes with involvement of eye, skin, and brain). The macular form of nevus spilus features hyperpigmented macules that are evenly distributed on a light tan background; it is associated with phakomatosis spilorosea, a combination of nevus spilus and a telangiectatic nevus with a distinctive pale pink, salmon color.8 The papular form of nevus spilus shows uneven distribution of the papules; it is associated with phakomatosis pigmentokeratotica, a combination of nevus spilus and a nonepidermolytic epidermal nevus.9 Spitz nevi may develop in either type of speckled lentiginous nevus, but melanoma has developed much more frequently in the macular form of nevus spilus.10 Blue nevi (including cellular blue nevi) have also occurred in nevus spilus.
Microscopic Findings: In café-au-lait macules, there is an increase in melanin in melanocytes and basilar keratinocytes. Therefore, the findings are similar to those in ephelides. However, increased numbers of melanocytes per square millimeter have been found when the dihydroxyphenylalanine (DOPA) reaction has been applied to skin sections.11,12 Giant melanosomes are sometimes observed in these lesions and are not restricted to those associated with neurofibromatosis. These can be seen on routine examination as rounded, evenly pigmented bodies that are larger than normal melanin granules (ranging in size up to 5 µ in diameter) in basal keratinocytes as well as melanocytes (Fig. 28-2A and B).13 The background tan macule of nevus spilus shows mild junctional melanocytic hyperplasia and, sometimes, exaggerated down-growth of pigmented rete ridges—a feature of lentigo.14–16 The speckles within nevus spilus usually show features of junctional or compound nevi.16 In the macular form the speckles consist of junctional lentiginous nevi, whereas in the papular form these lesions are compound or intradermal nevi.10 As noted, Spitz nevi can be found in either the macular or papular variants. Blue nevi, including those with changes of cellular blue nevi, can also be found in the context of a nevus spilus.17
Differential Diagnosis: Café-au-lait macules can resemble ephelides, lentigo simplex, or solar lentigo but can be differentiated on clinical grounds. In contrast to ephelides, increased numbers of melanocytes per unit area of epidermis can be demonstrated. Although giant melanosomes are features of some café-au-lait macules, they are not always observed in these lesions, even in those associated with neurofibromatosis.18 At the same time, they are not uncommonly found in otherwise conventional lentigines or junctional lentiginous nevi; in fact, on a percentage basis, most of the lesions with giant melanosomes fall within this category. The background tan macules of nevus spilus most often have the microscopic features of lentigo. Other hyperpigmented lesions, such as melasma, could be confused with café-au-lait macules or the background macules of nevus spilus, but they are quite different clinically; the intensity and depth of dermal pigment associated with melasma is the most significant consideration, particularly from a therapeutic standpoint.
Becker Nevus
Clinical Features: Becker nevi are typically unilateral, large (2 to 15 cm in diameter), hyperpigmented patches and plaques located often on the trunk, although they may be found virtually anywhere on the body surface.19 They are usually noted in the second or third decade of life and are more common in males. Hypertrichosis may be noted within the lesion, and although well demarcated, the borders are often irregular. Becker nevi have been associated with a variety of developmental anomalies.20,21 An association with smooth muscle hamartoma has been described in some cases.22,23
Microscopic Findings: The epidermis often shows hyperkeratosis, acanthosis, and papillomatosis. Elongation of the rete ridges is frequently present, and in many cases the ridges are tapered rather than flattened at their tips (Fig. 28-3). There is increased melanin in basal keratinocytes, and the numbers of melanocytes along the basilar layer are normal to slightly increased. Pigment incontinence with ingestion by macrophages is frequently observed. Increased numbers of hair follicles and sebaceous glands may be noted, particularly when compared to normal for the particular anatomic location; however, horizontal sections may be necessary to appreciate the degree of hypertrichosis noted clinically. In examples associated with smooth muscle hamartoma, irregularly distributed, well-differentiated smooth muscle bundles are observed within the dermis.23 If necessary, these can be well demonstrated with trichrome stain or with immunohistochemical methods (actin, desmin).
Differential Diagnosis: The slight papillomatosis and elongation of rete ridges could be confused with epidermal nevus, although usually these changes are milder than seen in the majority of epidermal nevi. Increased numbers or prominence of follicles or sebaceous glands (compared to normal for the anatomic site) may also serve as a clue to Becker nevus. When a smooth muscle hamartoma is present, it may be the predominating feature, and then there are several considerations. First, careful evaluation of the epidermis should be carried out to determine the possible coexistence of Becker nevus. Second, piloleiomyoma should be ruled out. However, the latter usually features a large aggregate of smooth muscle, whereas smooth muscle hamartomas feature scattered smooth muscle bundles within the dermis.
Benign Melanocytic Tumors
Lentigo Simplex and Solar Lentigo
Clinical Features: Lentigo simplex is a brown to black, well-defined, typically uniformly pigmented macule. It often arises in children and young adults with no relationship to ultraviolet (UV) radiation, thereby distinguishing it from solar lentigo. Lentigo simplex lesions can occur anywhere on the body surface. In contrast to ephelides, these lesions do not darken with sun exposure.24 The absence of BRAF, FGFR3, and PIK3CA mutations is a characteristic of lentigo simplex that distinguishes it from solar lentigo as well as melanocytic nevus.25
Solar lentigo is a UV-induced, tan- to dark-brown macule exclusively found on sun-exposed surfaces, especially the face, dorsal hands and arms, and shoulders.26 Often oval-shaped and homogeneous, the macules can also be irregularly defined and mottled in appearance. Solar lentigines occur in middle aged to elderly patients, most commonly in Caucasians. Their incidence increases with age. A clinical variant termed “ink spot” lentigo is characterized by its black color and irregular outline, mimicking a spot of ink on the skin.27
Microscopic Findings: In lentigo simplex, there is an increase in the number of single basilar melanocytes, leading to basilar keratinocyte hyperpigmentation. Mild elongation of rete ridges is noted (Fig. 28-4). Solar lentigo is a benign condition with both keratinocyte and melanocyte proliferative changes. It shows acanthosis with elongation of the rete ridges and characteristic club-shaped or bud-like extensions. There are increased basal layer pigmentation, accentuated along the sides and tips of rete ridges, and an increased number of non-nested melanocytes (Fig. 28-5).28,29 Facial solar lentigines frequently lack rete ridge hyperplasia.28 Solar elastosis can be identified in the underlying dermis.
Differential Diagnosis: Lentigo simplex is distinguished from junctional nevus by the presence of junctional melanocytic nests in the latter. Frequently, lesions are encountered that have predominant features of lentigo but a rare small nest can also be identified. Some authors have preferred to designate the latter lesion “jentigo.”30 The freckle, or ephelis, shows slight basilar hypermelanosis compared to adjacent uninvolved epidermis, but lacks down-growth of rete ridges, and the numbers of melanocytes per unit area are normal or slightly decreased.
Solar lentigo certainly resembles lentigo simplex in some respects, but acanthosis and/or down-growth of rete ridges is often more extensive than in the latter lesion, and changes of solar elastosis are observable in the underlying dermis. The proliferative rete ridges of solar lentigo are sometimes quite pronounced, and small horn cysts, resembling the changes of pigmented, reticulated seborrheic keratosis, can also be identified. As mentioned earlier, a subset of solar lentigines shows loss of the rete ridge pattern; this change is particularly but not exclusively seen in the head and neck region. Some of these lesions also feature enlargement of both keratinocyte nuclei and cytoplasm; such lesions are sometimes called large-cell acanthoma (see discussion in Chapter 18).
Melanocytic Nevi and Variants
Clinical Features: Acquired melanocytic nevi are composed of modified melanocytes (nevomelanocytes). These extremely common skin lesions usually appear during childhood or young adulthood. They are typically well-circumscribed, symmetrical, round to ovoid lesions, generally measuring between 2 and 6 mm in diameter. They are believed to begin as junctional nevi, confined to the epidermis. Later, melanocytes migrate into the dermis, forming compound nevi. Ultimately, all of the cells migrate into the dermis, forming intradermal nevi. The junctional nevus is a brown-black, macular lesion, whereas compound and intradermal nevi display varying degrees of elevation and generally range from brown to flesh-colored. Melanocytic nevi may have papillomatous or verrucous features and can be accompanied by coarse or dark hair. There is evidence that numerous nevi on skin represent a marker for increased risk of cutaneous melanoma. Both nevi and melanoma appear to be induced by intermittent exposure to sunlight.
Microscopic Findings: Junctional nevi, which in pure form are relatively uncommon, feature discrete nests of melanocytes along the dermal-epidermal junction without evidence of confluence and with good cohesion among melanocytes within the nests (Fig. 28-6A). In compound nevi, nests of melanocytes are present both at the dermal-epidermal junction and within the dermis (see Fig. 28-6B), whereas in intradermal nevi, nests, cords, or other groupings of melanocytes are located exclusively within the dermis (see Fig. 28-6C). Many junctional and compound nevi also have changes associated with lentigo: exaggerated down-growth of hyperpigmented rete ridges. Intradermal nevi are sometimes classified into two groups: Unna nevi, in which nevomelanocytes are confined to the papillary and perifollicular dermis, and Miescher nevi, in which nevomelanocytes that diffusely infiltrate adventitial and reticular dermis assume a wedge-shaped configuration.31 Compound and intradermal nevi often show an arrangement of three types of nevus cells: larger, pigmented, type A nevus cells near the junctional zone and in the superficial dermis; a deeper focus of smaller, rounded, nonpigmented type B nevus cells; and spindled type C nevus cells at the base of the lesion. This arrangement, in which superficial nests of larger cells evolve to smaller, more singly dispersed cells in the deeper portion of the specimen, is referred to as maturation descent, a characteristic feature of nevi that is lost or poorly demonstrated in melanomas. Furthermore, nevus cells are separated from one another by a delicate network of reticulin (type III collagen), whereas melanomas tend to show organization of cells as sheets or large aggregates with no intervening reticulin between the cells. Expansile nests or tumor nodules are not observed in nevi, with the exception of some congenital nevi (see later discussion).32 Mitotic figures are rare in conventional melanocytic nevi but can be observed from time to time.33 They were found in 4% of one series of 157 nevi.34 The role of trauma in the induction of mitotic activity in nevus cells is questionable, and in one study of 92 traumatized nevi, only one mitotic figure was identified.35 Type C nevus cells may be arranged to form neuroid structures resembling Wagner-Meissner bodies; this change is sometimes termed neurotization. In some examples of intradermal nevi, type C spindle cells may predominate, creating a resemblance to neurofibroma. It is possible that many isolated “neurofibromas” identified in middle-aged to older adults may actually represent completely neurotized nevi. In fact, type C nevus cells show immunohistochemical as well as morphologic evidence of schwannian differentiation.36 However, lentiginous melanocytic nevi can be identified overlying neurofibromas in type 1 neurofibromatosis.37 Other cytologic features seen in banal melanocytic nevi include sporadic cells with enlarged, hyperchromatic nuclei or intranuclear vacuolization, considered a variant of “ancient” change that can be seen in a variety of tumors in addition to melanocytic nevi. Some compound and intradermal nevi can feature associated epithelial or mesenchymal abnormalities. Thus, nevi can have an associated epidermal cyst, and sometimes rupture of the cyst produces changes that prompt a biopsy of the lesion. Melanocytic nevi sometimes have prominent vessels. However, this should not be confused with pseudovascular changes within a nevus; this results from clefts that form between columns of nevus cells. Immunostaining of such cases demonstrates that the clefts are lined by melanocytes and not endothelial cells.38 Fat cells are commonly seen in melanocytic nevi, although it was shown in one study that these cells do not contain S-100 protein.39,40 Some nevi show “balloon cells,” singly or in aggregates, and lesions composed predominantly of “balloon cells” also exist. The cytoplasm of these cells is finely vacuolated and may compress the nuclei, giving them a scalloped configuration (Fig. 28-7A and B). The change is said to result from a defect in the process of melanogenesis.

Figure 28-6 Common types of melanocytic nevi. A, Junctional nevus. B, Compound nevus. C, Intradermal nevus.

Figure 28-7 “Balloon cell” nevus. This lesion was composed predominantly of “balloon cells.” A, Quick low-power inspection can raise the possibility of other clear cell tumors. B, On higher magnification, it can be seen that the cytoplasm is finely vacuolated and that some of the nuclei have scalloped borders.
Differential Diagnosis: An old saying is that nevus cells (nevomelanocytes) are best recognized by the company they keep; they tend to be arranged in nests, cords, and aggregates of various kinds. When nevus cells are arranged in single units within the dermis, especially when intermingled with lymphocytes or macrophages, they can be quite difficult to identify without special stains, and in some instances, on low-power examination, small-cell nevi can be difficult to distinguish from inflammatory infiltrates. Even then, these cells often tend to aggregate in pairs or small clusters. Their readily recognizable cytoplasm and somewhat larger nuclei allow distinction from lymphocytes in most instances. Type C nevus cells closely resemble those of neurofibroma, and when all cells in a lesion have this appearance, distinction from neurofibroma may be impossible. This usually has little clinical import, unless determination of a form of neurofibromatosis hinges on the distinction. Staining for factor XIIIa or myelin basic protein can be helpful, because these can be demonstrated in neurofibroma but not in neurotized nevi.41 In a case of pigmented plexiform neurofibroma, polygonal pigmented melanocytes in the superficial dermis and within neurofibromatous tissue stained for Melan-A and PNL2, whereas glial fibrillary acidic protein and leu7 (CD57) stained only the slender spindled cells in plexiform foci.42 Nevi with pseudovascular change should be distinguished from hemangiomas, although a more common problem is the distinction of coexisting nevus and hemangioma from pseudovascular nevus. Ordinarily, examination of pseudovascular nevi shows that the cells lining spaces within the dermis are identical to the more obvious nevus cell elements elsewhere in the specimen, but if necessary, differential staining can be carried out, for example using S-100 or Melan-A and endothelial markers such as CD31. Distinction between melanocytic nevi of the common type and melanoma is usually not difficult, and some of the criteria have already been pointed out: maturation descent is demonstrable in nevi but not in melanomas; nevus cells are separated from one another by delicate reticulin, whereas melanomas are arranged in sheets or nodules with no intervening reticulin; expansile nests or tumor nodules are features of melanoma but not of conventional nevi; and mitotic figures are rare in ordinary nevi but common in melanoma. A more challenging problem is the recognition of nevoid melanoma, a subtype of melanoma that can have a close morphologic resemblance to nevus, particularly at low magnification. That distinction is explored in more detail in a later section.
Halo Nevus
Clinical Features: The halo nevus is clinically described as a centrally located pigmented melanocytic nevus surrounded by a depigmented zone.43 These lesions undergo four stages of evolution: (1) appearance of the halo; (2) loss of pigment within the centrally located nevus; (3) disappearance of the nevus; and (4) disappearance of the halo, with return of the patient’s normal pigment. These are solitary or multiple lesions, often occurring on the trunk of young adults. Halo nevi are associated with vitiligo in some cases and can also occur in patients with melanoma.44 Halo nevi represent an immunologic response, with both cell-mediated and humoral elements, to antigens expressed on nevomelanocytes.45 Halo nevi can also develop in the setting of melanoma, including occult melanoma.46
Microscopic Findings: Fully evolved halo nevi are characterized by a dense, diffuse lymphocytic infiltrate surrounding and infiltrating melanocytes at the junctional zone and/or in the dermis. The infiltrate is typically well demarcated both laterally and at its base. It is often so dense that it largely obscures the melanocytes (Fig. 28-8A), but clusters of melanocytes can often be identified in the midst of the field of lymphocytes (see Fig. 28-8B), and they can also be identified with special stains such as S-100 and Melan-A. The latter is preferred, in that the infiltrate may contain prominent S-100–positive Langerhans cells. The number of nevus cells depends on the stage at which the biopsy was taken, and they may appear swollen, variable in size, and demonstrate mild to moderate and, sometimes, severe cytologic atypia.47 Occasional mitotic figures can be found. If the specimen is of sufficient size, it may be possible to demonstrate an absence of junctional melanocytes adjacent to the lesion with stains such as Melan-A. However, it should be noted that there is often discordance between the presence of an inflammatory infiltrate and the clinical appearance of a halo. Thus, there may be microscopic inflammatory change without a clinical halo (so-called “halo nevus without halo”),48 or a clinical halo without inflammation.49 The former is the more frequent finding.
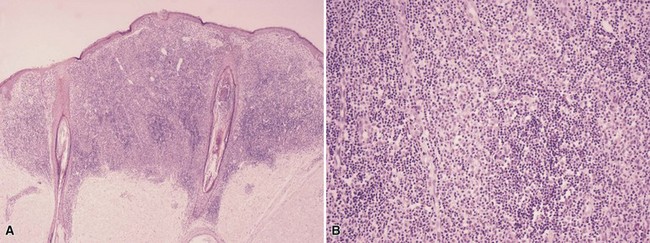
Figure 28-8 Halo nevus. A, There is a dense inflammatory infiltrate, sharply demarcated laterally and at its base, that partly obscures dermal melanocytes. B, On higher magnification, clusters of somewhat larger, paler cells can be identified; these are nevus cells, and their melanocytic origin can be confirmed with special staining.
Differential Diagnosis: White rings have been found around a variety of lesions, including dermatofibroma50 and basal cell carcinoma,51 and “halo dermatitis” or “eczema” has been noted to surround a variety of lesions, including seborrheic keratoses, keloids, lentigines, arthropod bites, and basal and squamous cell carcinomas as well as melanocytic nevi. However, this “halo dermatitis” is clinically distinct from a white halo and more closely resembles the Meyerson phenomenon (see subsequent discussion). It is not clear that the white rings around such lesions as dermatofibroma and basal cell carcinoma represent the same mechanism as associated with a true halo nevus—that is, a temporary loss of junctional melanocytes. One possible explanation could be vasoconstriction due to the application of topical corticosteroids. Another could be a phenomenon related to Woronoff ring, a white ring around psoriatic plaques that frequently follows coal tar and UV therapy. That ring has been shown to result from a local inhibitor of prostaglandin synthesis not found in normal skin.52 Halo nevi must also be distinguished from melanoma, particularly with early regressive change. This can be a difficult task, but diagnosis should depend on a careful assessment of the melanocytic lesion that underlies the inflammation, with attention being paid to features such as degree of symmetry, junctional melanocytic confluence, presence or absence of pagetoid intraepidermal change, and cytologic atypia, with particular reference to maturation with descent (see “Melanoma—Differential Diagnosis”). In contrast to halo nevus, regression in melanoma is generally accompanied by coarse fibrosis. The reasons for this difference are not entirely clear, but halo nevi have been found to have higher tumor necrosis factor-alpha expression than melanoma; this cytokine is known to have antifibrotic properties.53
Meyerson Nevus
Clinical Features: In 1971, Meyerson reported two cases of what he described as “a peculiar papulosquamous eruption” involving pigmented nevi.54 Since that time, there have been a number of reports of melanocytic nevi surrounded by a rim of spongiotic (eczematous) dermatitis.55,56 The condition has been observed frequently in young adults but has also been described in children.57 Although generally associated with acquired melanocytic nevi, it has been found to occur in congenital nevi as well.57,58 Lesions are prone to develop over the trunk and proximal upper extremities. The eczematous changes eventually resolve, either spontaneously or with topical corticosteroid therapy. Unlike halo nevi, the nevi involved by eczematous changes do not appear to involute.59 However, transition to a halo nevus has been reported,60 as has coexistence with halo nevi.61 The Meyerson phenomenon has been associated with dysplastic nevi62,63 and melanoma lesions.59,62,64 In one case, a patient developed multiple Meyerson nevi, one of which involved what proved to be melanoma; following excision of the melanoma, the Meyerson phenomenon subsided.59
Microscopic Findings: In the typical case, a junctional or compound nevus is associated with spongiosis, parakeratosis, and a perivascular infiltrate composed of lymphocytes, macrophages, and varying numbers of eosinophils (Fig. 28-9).65 The infiltrate is composed mainly of CD3+ lymphocytes, the majority of which are CD4+, although there are also CD8+ cells.65,66 Limited upward epidermal migration of melanocytes is observed, even in otherwise nondysplastic nevi.62 It has been suggested that interaction of CD4+-lymphocytes with increased epidermal expression of intercellular adhesion molecule-1 (ICAM-1) might explain some of the changes.66
Differential Diagnosis: In the absence of a clinical history, Meyerson nevus is difficult to distinguish from a nevus that has simply been secondarily eczematized or is present in the midst of an eczematous dermatitis that has developed for other reasons (e.g., atopic or allergic contact dermatitis). Eczematous changes have also been described within or around other types of lesions, including molluscum contagiosum, dermatofibroma, seborrheic keratosis, nevus flammeus, and smooth muscle hamartoma.67–70 However, these should be distinguishable from Meyerson nevi either clinically or microscopically.
“Special Site” Nevi
Clinical Features: Over the years, it has been recognized that melanocytic nevi from certain anatomic sites share particular histopathologic features, some of which may be regarded as “atypical” and raise concerns for malignancy. This has led to the alternate designation “nevi with site-related atypia.” Nevertheless, these nevi appear to be biologically bland and do not signify an increased risk of melanoma. The reasons for the distinctive features of special site nevi are not entirely clear and cannot be explained simply by anatomy or mechanical factors, because most nevi that occur in these sites have conventional characteristics indistinguishable from those arising anywhere on the cutaneous surface.71
Few unique clinical characteristics are associated with these nevi. Acral lesions often show irregular borders with uniform pigmentation and have a distinctive dermoscopic pattern in which pigment is distributed along the sulci of ridges constituting the dermatoglyphics of the palms and soles.72 Atypical genital nevi in women are more apt to occur in mucosal sites as opposed to traditional, architecturally disordered nevi, which mainly occur in hair-bearing areas.73 A subset of nevi along the milk line have polypoid features.74 The following microscopic descriptions emphasize the key features in each of these special site nevi.
Microscopic Findings: The microscopic characteristics of “special site” nevi have been well described in a highly useful review by Hosler and colleagues.71 Acral nevi often show significant upward epidermal migration of melanocytes (Fig. 28-10), a finding that has earned the acronym MANIAC, or melanocytic acral nevus with intraepidermal ascent of cells.75 Other features include bridging of rete ridges, fibroplasia, significant inflammation, pigment in the stratum corneum, and nested melanocytes in the dermis with “skip areas” and syringotropism.71,76
Genital (particularly, vulvar) nevi can be large, nodular lesions with variability of size and shapes of nests along the rete ridges (not always at the tips) and in the dermis, loss of cohesion of melanocytes within nests, sometimes severe atypia on the basis of cellular enlargement but with a preserved nuclear-to-cytoplasmic ratio, and rare mitoses.71,73 Upward epidermal migration of melanocytes and adnexal involvement are sometimes observed,77 and the stromal changes tend to be nonspecific,78 although diffuse fibrosis can sometimes be seen, especially in polypoid lesions (Fig. 28-11A-C).71 Symmetry and preservation of maturation descent are features that further support the diagnosis of nevus in these lesions.

Figure 28-11 Genital nevus. This example shows several characteristic features of this type of nevus. A, There is loss of cohesion within melanocytic nests. B, Adnexal involvement is frequently observed. C, A rare mitosis is identified in the dermis.
Nevi from the breast have similar features in men and women, and the characteristic findings tend to be more prevalent in young adults.71 Findings include a garland-like arrangement of junctional nests, enlarged but uniform melanocytes with preservation of normal nuclear-to-cytoplasmic ratios and generous amounts of clear to dusty cytoplasm, and maturation with descent in the form of more bland-appearing cells arranged singly and in smaller aggregates.71 Rongioletti and associates found suprabasilar melanocytes, cytologic atypia, and papillary dermal fibroplasia.79
Rongioletti and associates also performed a study of flexural nevi. They found that about 50% of patients displayed confluence of large nests, varying in size, shape, and location along the junctional zone, as well as loss of cohesion of melanocytes within nests. These changes are similar to the ones in genital nevi.80
Nevi of the scalp often display changes associated with other “special site” nevi,81 including large nests of different sizes, distributed at varying sites along the junctional zone (not always at tips of rete ridges), loss of cohesion within nests, adnexal involvement, suprabasilar melanocytes, and inconspicuous stromal changes. Cytologic atypia is often observed.71
Lesions of the ears are often considered “special site” nevi and share some features with such nevi in other anatomic sites. Lazova and coworkers described several particularly common findings among these lesions: “shouldering” of the junctional component, poor lateral circumscription, elongated rete ridges with bridging, dermal lymphocytic infiltration, and cytologic atypia.82 Saad and colleagues also found poor lateral circumscription and cytologic atypia in their cases, and in addition noted suprabasilar melanocytes (Fig. 28-12).83

Figure 28-12 Nevus from the ear. These lesions show poor lateral circumscription, occasional suprabasilar melanocytes, and a degree of cytologic atypia.
Conjunctival nevi may create diagnostic problems because of the common highly cellular dermal components of these lesions and recognizable cytologic atypia. Clues to the location of these lesions include goblet cells and epithelial-related cystic and pseudoglandular spaces intermingled with melanocytes (Fig. 28-13). Symmetry, circumscription, and pigment stratification with descent tend to support a benign interpretation.
Differential Diagnosis: When confronted with diagnosing a lesion from a “special site,” one has the dual task of not overinterpreting the findings as melanoma while not underinterpreting a lesion as benign in view of the potentially dire consequences of missing a melanoma. In particular, this is the case with lesions of the genitalia or the ears; in these locations, melanomas can be particularly aggressive. Finding some of the common features among “special site” nevi can be reassuring about the benign nature of a lesion, but several of these—particularly, upward epidermal migration of cells, loss of cohesion within nests, and cytologic atypia—can be troubling. Findings that should be present in nevi are overall symmetry and circumscription, a lack of atypia (at least, a lack of cells with abnormal nuclear-to-cytoplasmic ratios), and an absence of atypical or deep mitoses. Any upward epidermal migration of melanocytes should consist of cells without atypia, limited to the central portion of the lesion. Caution also should be exercised when the submitted lesion represents only a partial biopsy, and lentiginous changes extend to the specimen margin. This raises the possibility that there might be features of melanoma extending beyond the site of the tissue sample; re-excision should be recommended in such circumstances.71
Deep Penetrating Nevus and Plexiform Spindle Cell Nevus
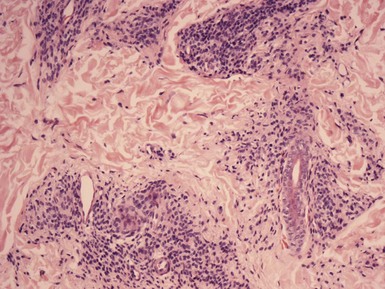
Clinical Features: In 1989, Seab and Graham reported a group of lesions that they termed deep penetrating nevus (DPN). These typically present as well-circumscribed, hyperpigmented nodules of the face, upper trunk, or proximal extremities in young persons (in the range of 10 to 30 years). Their microscopic findings can create concern because of the depth of dermal involvement and frequent lack of maturation with descent, leading to an erroneous diagnosis of melanoma. Several years later, Barnhill and associates from the Massachusetts General Hospital reported a group of cases that they described as plexiform spindle cell nevus (PSCN).84 These lesions are also described as bluish or black and occur most commonly on the shoulders or back of young adults—characteristics that are similar to those described for DPN. These lesions have microscopic changes that can create problems with interpretation, and although recurrences or metastases typically do not occur, rare examples of lymph node metastasis have been reported.85
Microscopic Findings: DPN are generally well circumscribed. They may show junctional nesting. The dermal component of the lesion displays a wedge-shaped configuration, with the “point” of the wedge (which may actually be rounded) extending into the deep dermis or subcutis.86 Pigmented spindled cells are more loosely organized superficially and are grouped into nests or fascicles in the deep dermis, often distributed along neurovascular structures or adnexal epithelia.87 Melanophages frequently accompany the lesion. Maturation with descent is often not convincingly demonstrated, and cytologic atypia can be observed in the form of enlarged nuclei or prominent nucleoli (Fig. 28-14A and B). Mitotic figures can occasionally be found88; they are usually not atypical or found in deeper portions of the lesion. The findings in PSCN are similar, except that the predominant feature is the tracking along neurovascular plexuses and adnexal structures in a plexiform configuration (Fig. 28-15A and B).84 The general trend is to regard these as microscopic variants of the same lesion.89
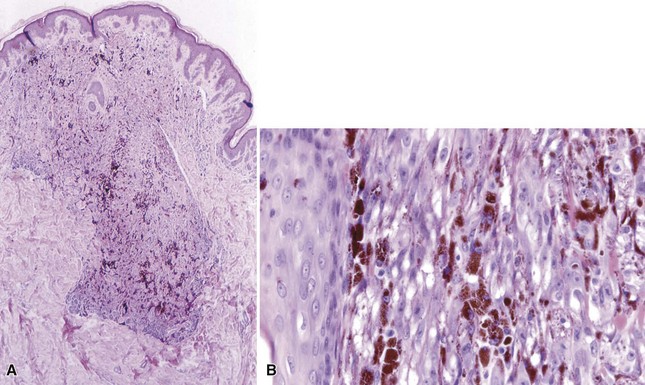
Figure 28-14 Deep penetrating nevus. A, The dermal component displays a wedge-shaped configuration, with extension deep into the dermis. B, Deeper dermal cells adjacent to a follicular unit. Note that maturation descent is not convincingly demonstrated, and cytologic atypia is observed in this portion of the lesion.

Figure 28-15 Plexiform spindle cell nevus and “clonal” nevus. A, The lesion shows deep extension but has a distinctly plexiform configuration. B, Cells proliferate along neurovascular plexuses. C, An example of a combined, or “clonal” nevus, with superficial features of conventional compound nevus and a deep component resembling deep penetrating or plexiform spindle cell nevus.
An association of these changes with conventional melanocytic nevus is common.89 In addition, it has been recognized for some time that aggregates of pigmented, epithelioid to fusiform cells resembling those of DPN or PSCN can be confined to the superficial dermis in association with conventional compound or congenital-type nevi. Such lesions have been variously termed “clonal” or “combined” melanocytic nevi or “nevus with focal atypical epithelioid component” (see Fig. 28-15C).90 These lesions also have a favorable outcome and may well represent variant forms of DPN or PSCN.
Differential Diagnosis: DPN and PSCN share some microscopic features with Spitz nevus and cellular blue nevus. Although they can generally be distinguished from the latter nevi by their typical clinical presentation and wedge-shaped or plexiform microscopic arrangements, they may be within the same family of disorders because of overlapping cytologic characteristics. Differentiation from melanoma is the most important consideration. Features arguing against melanoma include smaller size, high degree of symmetry, sharp circumscription, generally low-grade cytologic atypia, and low-to-absent mitotic activity.
Persistent/Recurrent Melanocytic Nevus
Clinical Features: Persistent/recurrent melanocytic nevi occur after an incomplete sampling of a nevus, especially following a shave biopsy.91 Clinically, these lesions often develop within 6 months of the surgical procedure and show pigmentation arising within the scar; this pigment is macular and often irregularly distributed. In fact, the changes may be such that they violate the usual “ABCD” role of dermoscopic examination: namely, asymmetry, irregularity of borders, color variation, and differential dermoscopic structures (pigment streaks, dots and globules), thereby creating concerns for melanoma.92 The back is a common site, and the phenomenon is more frequently observed in young women.91,93 Despite the unusual clinical features and the microscopic difficulties created by this phenomenon, recurrent nevi are benign lesions amenable to complete excision.93
Microscopic Findings: Typically, the epidermis is effaced, and there is junctional melanocytic proliferation, in a singly dispersed or lentiginous configuration, confined to the region overlying a dermal scar. Residual intradermal nevomelanocytes can sometimes be identified deep to the scar (Fig. 28-16A). It is believed that the junctional melanocytes result from proliferation of melanocytes left behind within the epidermis or adnexal epithelia following the previous biopsy procedure (see Fig. 28-16B).94,95 King and coworkers described four different microscopic images associated with persistent/recurrent nevi, all associated with dermal scar: junctional and compound melanocytic hyperplasia with effacement of rete ridges, and junctional and compound melanocytic hyperplasia associated with lentiginous down-growth of rete ridges.96 Pagetoid upward migration of melanocytes through the epidermis is sometimes noted.97 Melanocytes at the junction are variably pigmented and show mild to moderate cytologic atypia in terms of nuclear size and appearance of nucleoli. Although this is the “classic” image of persistent/recurrent nevus, recurrent blue nevi are known to extend beyond the scarred site,98,99 and recurrent Spitz nevi may display nodular or sclerotic (desmoplastic) features.100,101 Persistent/recurrent nevi with dermal components show maturation with descent and “stratification” of HMB-45 or tyrosinase staining (i.e., decreasing with depth in the dermis) and a low proliferative index using Ki-67 staining.102

Figure 28-16 Persistent/recurrent melanocytic nevus. A, There are rete ridge effacement, underlying dermal scar, and patchy chronic inflammation. Clusters of residual intradermal melanocytes can be seen on the right side of the figure. B, Junctional melanocytes may result, in part, from proliferation of remaining melanocytes from within adnexal epithelia.
Differential Diagnosis: The differential diagnosis includes atypical nevus with stromal fibroplasia, melanoma with regressive changes, and recurrent melanoma. A history of a recent surgical procedure at the site, confinement of the lesion to a zone overlying the dermal scar, and careful assessment of the cytologic features should lead to a correct diagnosis. However, caution should be advised, especially when assessing a possible recurrent blue nevus or Spitz nevus, because these do not always “obey the rules” (i.e., extension beyond the confines of the scar or nodular recurrences). Significant problems can also arise when attempting to distinguish recurrent nevus from recurrent melanoma.100 Confirmation of prior biopsy, evaluation of the initial biopsy specimen when possible, and assurance of complete removal of the recurrent lesion are therefore recommended.
Other Variants of Acquired Nevi
The ancient melanocytic nevus is often a dome-shaped lesion that features a population of cells with large pleomorphic nuclei and occasional mitoses, among other, smaller cells with an arrangement suggesting a conventional or congenital nevus. Lesions are often strictly intradermal, but a junctional component is sometimes present. The large cells resemble those of epithelioid Spitz nevus (see later discussion). Stromal degenerative changes are also identified, including hemorrhage, fibrosis, or mucin deposition. Despite the atypical features, these nevi show a low proliferative index with Ki-67 staining. Recurrences or metastases have not been reported.103–105
The neurotized nevus consists of type C spindled nevus cells, sometimes with organization into Wagner-Meissner–like bodies (Fig. 28-17A).106 Typically, these changes occur in the setting of an otherwise conventional melanocytic nevus or congenital nevus, but occasionally they may be the predominating feature and therefore difficult to distinguish from neurofibroma (see Fig. 28-17B). In such circumstances, immunohistochemical staining may be helpful. Factor XIIIa staining is strongly positive in neurofibromas but negative in neurotized nevi,107 and the neurotized areas of melanocytic nevi in one study were uniformly positive for Melan-A, in contrast to the negative staining for this marker in neurofibromas.108

Figure 28-17 Neurotized melanocytic nevus. A, This example shows structures resembling Wagner-Meissner bodies. B, Nevus cells can also take on an appearance closely resembling neurofibroma.
About 10% of benign melanocytic nevi show pseudovascular spaces, suggesting on initial inspection a vascular lesion.109 Erythrocytes can sometimes be found in these spaces. However, they appear to be lined by nevus cells and not by endothelium, and basement membrane material is not identified (Fig. 28-18). Immunohistochemical staining shows that the lining cells are in fact melanocytes and not endothelial cells.38,110 It is generally assumed that this change is an artifact resulting from the mechanical stress of the biopsy procedure and/or subsequent tissue processing.
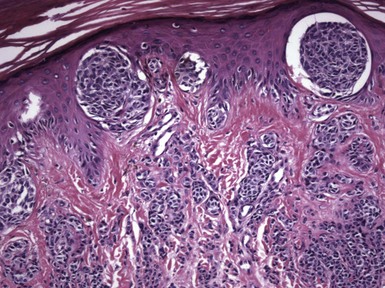
Spindle and Epithelioid Cell Nevus (Spitz Nevus)
Clinical Features: The spindle and epithelioid cell nevus is a benign melanocytic lesion that is most commonly encountered in children and young adults. Its chief importance is its microscopic resemblance to melanoma. In fact, the 1948 paper by Sophie Spitz set apart a group of “melanomas of childhood” that shared certain histopathologic features but had a better than expected clinical outcome. An interesting facet of this study, and one that is pertinent to current diagnostic issues regarding Spitz nevi, is that one of the 13 reported cases had an aggressive clinical course resulting in death of a patient. Spitz postulated that a histopathologic feature allowing distinction of “juvenile melanoma” from true melanoma was the presence of giant cells in the former.111 Since that time, there have been refinements in the diagnostic criteria for this category of nevus,112 and molecular analysis holds the promise of more reliable distinction of Spitz nevus from melanoma.
As mentioned, Spitz nevus is most frequently a lesion encountered in young persons and may present as a congenital lesion,113 but examples in adults have also been reported with varying frequencies.112,114,115 There are reports of patients with Spitz nevi in the eighth decade of life,115 but certainly, caution must be advised in making this diagnosis in older adults. Most often, the Spitz nevus presents as a solitary, dome-shaped, flesh-colored to pink nodule, although pigmented variants definitely occur.116 Abrupt onset or rapid growth is frequently described. Groupings of these lesions, called agminate Spitz nevi, can also occur,117,118 sometimes following biopsy of a solitary Spitz nevus, and a rare disseminated variant has also been reported.119,120 These are typically benign lesions, but atypical variants occur that are biologically aggressive.121 In particular, a group of childhood lesions bearing resemblances to Spitz nevus involve regional lymph nodes but appear to have a favorable clinical course.122 Some examples of spitzoid tumors defy accurate categorization on histopathologic grounds alone, and widespread metastases have occasionally been reported in recurrent Spitz nevi.123 For these reasons, it has been recommended that all Spitz nevi be completely excised.124
An important variant of Spitz nevus is the desmoplastic Spitz nevus. The authors’ group refers to this lesion as sclerotic Spitz nevus, both to avoid confusion with desmoplastic melanoma and to diffuse the inevitable criticism that the term might be used as an evasion in difficult diagnostic circumstances. These lesions manifest as firm papules or nodules and frequently occur on the extremities.112,125 However, it is clear that not all desmoplastic nevi have cytologic characteristics of Spitz nevi.125,126 Desmoplastic Spitz nevi are reported most often in young adults, although children can also present with these lesions. The authors have encountered a number of them in patients in the fifth to sixth decades of life—significantly more often than is the case for conventional Spitz nevi. A subtype of desmoplastic Spitz nevus has prominent vasculature and has been designated angiomatoid Spitz nevus.127,128 These lesions have a favorable outcome following complete excision.
Microscopic Findings: There is a constellation of characteristic findings in Spitz nevi, but variations occur that can make accurate diagnosis particularly challenging. Typically, the lesions are sharply circumscribed and symmetrical, dome-shaped nodules. The epidermis is often acanthotic. The majority of Spitz nevi are compound, but junctional or intradermal types are also seen.129 The constituent cells are either spindled or epithelioid in type, and sometimes a mixture of both types is seen. It has been suggested that Spitz nevi in older adults are spindled rather than epithelioid in type. Junctional nests tend to be sharply demarcated from the adjacent epidermis, and spindled forms are oriented perpendicularly to the epidermis. Transepidermal elimination of nests does occur, resulting in the finding of nests in upper levels of the epidermis (Fig. 28-19A and B); pagetoid migration of single melanocytes through upper portions of the epidermis is not common but does occur in some examples.130,131 In Spitz nevi with a dermal component, maturation descent can often be demonstrated; this does not always include diminution of cell and nuclear size but may instead show mainly progression from nesting in the superficial dermis to single dispersion of cells in the deeper dermis (Fig. 28-20). Mitoses are uncommon but can be identified in these nevi. When present, they lack atypical characteristics and are usually not found in the lower third of the lesion. Accompanying stromal changes include papillary dermal edema and telangiectasia. Kamino bodies are pale pink globules that are found at the base of the epidermis in many Spitz nevi, often at the juncture of epidermis and melanocytic nests (see Fig. 28-19B).132 They are composed of basement membrane components and do not represent cellular apoptosis.133,134 Although Kamino bodies can be found in both melanomas and conventional melanocytic nevi,135 they tend to be more prominent in Spitz nevi; however, they may be missed if only a single section is examined.135 Desmoplastic Spitz nevi also have a dome-shaped configuration on low power, although they are sometimes plaquelike. The epidermis may be either acanthotic or effaced, and junctional involvement is often absent or limited in extent (Fig. 28-21A). Plump spindled to epithelioid cells tend to be organized into groupings, or nests, in the superficial dermis but are dispersed singly between thickened collagen bundles in the deeper dermis (see Fig. 28-21B). Nucleoli are variable in size but tend to be less conspicuous in the deeper dermis, and mitotic figures are uncommon. Small vessels are prominent in those cases classified as angiomatoid Spitz nevi.127,128 Lymphocytic inflammation is patchy and perivascular or diffuse and symmetrical; it tends to be inconspicuous in desmoplastic Spitz nevi.

Figure 28-19 Spitz nevus. A, This example is mainly junctional. Note irregular acanthosis, sharp demarcation of nests with a “raining down” configuration of some spindle cells within nests, and transepidermal elimination of nests. B, Transepidermal elimination of a melanocytic nest. Note the pale pink globule (Kamino body) at the juncture of the nest and the epidermis.

Figure 28-20 Spitz nevus. Maturation descent of dermal melanocytes is demonstrated by the transition from larger nests in the more superficial dermis (top) to smaller groups of cells and, sometimes, singly dispersed cells in the deeper dermis.

Figure 28-21 Desmoplastic Spitz nevus. A, There is an acanthotic epidermis with aggregates of spindled to epithelioid cells in a sclerotic matrix. Note absence of junctional involvement in this example. B, Nevus cells are singly dispersed within thickened collagen of the deeper dermis.
Problematic are those cases designated “atypical Spitz nevus” or “atypical Spitz tumor.”121,136 Histopathologic features of these lesions have varied, but some of the most important are ulceration, asymmetry and poor lateral circumscription, disordered intraepidermal melanocytic proliferation, expansile dermal proliferation of “back-to-back” cells, numerous and/or atypical mitoses, cell necrosis, and patchy dermal lymphocytic inflammation with a plasma cell component.
Immunohistochemistry has two major uses: to distinguish the occasional Spitz nevus that lacks obvious nesting from other lesions with epithelioid features (Spitz nevi are positive for S-100 and often for MART-1/Melan-A, in contrast to true epithelial or “histiocytic” lesions) and to aid in the distinction of Spitz nevi from melanomas with Spitz-like features. Spitz nevi show stratification of HMB-45 staining (reduced expression toward the lesional base)137 and reduced Ki-67 staining compared with melanoma.138 Positivity for p16 has also been found to distinguish desmoplastic Spitz nevi from desmoplastic melanomas, which are either negative or only weakly positive for this marker.139
Differential Diagnosis: As noted previously, nesting of cells (particularly at the dermal-epidermal junction) and, if present, pigment production, can allow the distinction of Spitz nevi from other lesions composed of epithelioid cells, including squamous cell carcinomas and “histiocytic” lesions (e.g., xanthogranuloma, reticulohistiocytoma, epithelioid cell histiocytoma). When the typical morphology of a melanocytic lesion is not present, differential immunostaining can be performed, using melanocytic markers (S-100, Melan-A) and other pertinent stains such as keratin, CD68, and factor XIIIa (realizing, however, that melanocytes can sometimes express CD68). However, the major diagnostic difficulty is clearly the distinction between some Spitz nevi, particularly atypical Spitz tumors, from melanoma. As mentioned, immunostaining can be of help here, in that, in contrast to melanomas, Spitz nevi show diminished HMB-45 staining toward the lesional base, decreased Ki-67 expression (indicating diminished proliferative capacity), and usually p16 positivity. It is well known, however, that some spitzoid tumors defy accurate morphologic classification. In this case, molecular analysis may prove helpful. For example, using comparative genomic hybridization, a subset of Spitz nevi shows gains of chromosome 11p, whereas melanomas are prone to feature chromosome 9 deletion.140
Pigmented Spindle Cell Nevus of Reed
Clinical Features: This nevus typically presents as a well-demarcated, darkly pigmented papule or plaque on the proximal lower extremity of young adult women.141,142 It is seen in other locations, such as the back, and does occur in men.143 Lesions are about 3 to 6 mm in diameter. It is thought by some that this nevus may represent a pigmented variant of Spitz nevus, but it has such a characteristic clinical and microscopic appearance that a specific diagnosis can usually be made. The rapid onset of these lesions raises concerns about melanoma, but they are benign lesions that respond well to complete excision.
Microscopic Findings: Pigmented spindle cell nevi are typically well demarcated and have a somewhat flat, platelike low-power configuration. The thickness of the epidermal component of the lesion is quite uniform. Melanocytic proliferation is mainly junctional (although there may be cell aggregates within the papillary dermis). This is composed of elongated spindled cells, arranged in nests that tend to lack sharp demarcation from the adjacent epidermis and often blend imperceptibly with bordering keratinocytes. Heavy pigmentation, of course, defines the entity. When melanocytes are present in the dermis, maturation descent can usually be demonstrated (Fig. 28-22). Cytologic atypia is minimal and nucleoli are usually inconspicuous, but mitoses can be identified among junctional melanocytes.142,143 Kamino bodies can be found. Although they were common in one study,144 they may not be seen in a given section and therefore additional levels are often necessary to find them. Atypical variants of pigmented spindle cell nevus have been described. These are characterized by such changes as lateral extension of junctional melanocytic hyperplasia, pagetoid spread to a midepidermal level, and/or bridged or horizontally confluent nests.145,146 Nevertheless, these lesions maintain overall symmetry and display limited cytologic atypia.
Differential Diagnosis: Pigmented spindle cell nevi are sometimes considered variants of Spitz nevi but differ from most Spitz nevi by the usual epidermocentric profile, abundance of pigment, and the slender appearance of the spindled cells. In contrast to pigmented spindle cell nevi, atypical (“dysplastic”) nevi are more prone to have lateral spread or “shouldering” of melanocytic proliferation along the junctional zone beyond the main body of the lesion, elongation of the rete ridges, stromal fibroplasia, mixed cell types (i.e., epithelioid or conventional-appearing melanocytes in addition to, or instead of, spindled forms), and horizontally bridged nests,147 although there may be a greater degree of overlap with atypical variants of pigmented spindle cell nevus. Pigmented spindle cell nevi differ from melanoma in that they are generally smaller, more symmetrical, show nests that are prone to be oriented perpendicularly to the epidermis, have limited pagetoid change (which, when present, tends to reach only to a midepidermal level), demonstrate maturation with descent, and (in contrast to some invasive melanomas) lack infiltration into the reticular dermis.
Blue Nevus and Variants
Clinical Features: The common blue nevus is a well-circumscribed, blue or blue-black macule or dome-shaped papule, usually less than 5 mm in diameter. It is most commonly located on acral surfaces, face, and scalp but can be found in virtually any location.148 The lesions are said to most commonly arise in childhood or adolescence, but biopsies of blue nevi are also frequently obtained from older adults. Multiple and agminated blue nevi have been described,149,150 and there is a rare association with nevus spilus.17,151
The cellular blue nevus is larger than the common blue nevus, generally 1 to 3 cm in diameter, and typically located on the buttocks, sacrococcygeal area, scalp,152 and distal extremities. These lesions can present at any age, although adults younger than 40 years of age are most commonly affected.153
The epithelioid blue nevus was first described in patients with the Carney complex. The latter term refers to a group of autosomal dominant conditions that include myxomas of heart and skin, endocrine dysfunction, schwannomas, and varying types of pigmented cutaneous lesions, including ephelides and lentigines. One variant, the LAMB syndrome (lentigines, atrial myxoma, mucocutaneous myxomas, blue nevi) also includes blue nevi with microscopic features of epithelioid blue nevi.154 However, this type of blue nevus has also been seen in patients who do not have evidence for the Carney complex.155 Clinical descriptions indicate that these present as darkly pigmented nodules or plaques that may range between 1 and 2 cm in diameter.155 Epithelioid blue nevi have a favorable clinical course, but “metastasis” to regional lymph nodes has been reported.156,157 For that reason, it has been proposed that epithelioid blue nevi be grouped with so-called “animal type” melanoma under the rubric “pigmented epithelioid melanocytoma,” which is believed to better characterize the intermediate biologic activity that both of these tumors possess152 (see subsequent discussion).
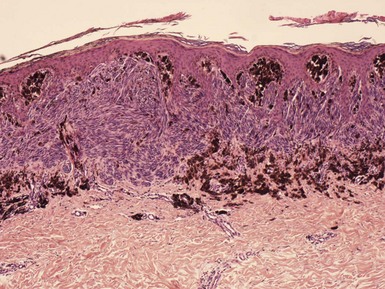
Microscopic Findings: The common blue nevus is composed of circumscribed aggregates of spindled to dendritic intradermal melanocytes containing fine granules of melanin (Fig. 28-23). Although pigmentation of these lesions is usually obvious, a hypopigmented to amelanotic variant of common blue nevus has been described (Fig. 28-24A and B).158,159 Junctional aggregates of dendritic melanocytes have also been reported, although this is distinctly uncommon.160 The melanocytes show a slender, branching network of dendritic processes with small, elongated, and hyperchromatic nuclei without significant atypia or mitotic figures.152 Melanophages and sclerosis of collagen are often present. The dendritic melanocytes of blue nevus stain positively for S-100, HMB-45, and MART-1/Melan-A,161,162 although one study found that HMB-45 staining was negative or weakly positive in a group of amelanotic blue nevi.158

Figure 28-23 Blue nevus. This is a conventional blue nevus, composed of circumscribed aggregates of heavily pigmented dendritic melanocytes.

Figure 28-24 Hypopigmented blue nevus. A, At low magnification, there appears to be a proliferation of small, bland-appearing cells that could be confused with neurofibroma or dermatofibroma. B, On higher magnification, it can be seen that the dendritic cells contain small amounts of melanin. This can be confirmed with Fontana-Masson stain or with melanocytic immunohistochemical markers.
The cellular blue nevus demonstrates a biphasic appearance with classic areas of common blue nevus and distinctly cellular areas densely packed with spindled to oval melanocytes with clear or finely pigmented cytoplasm (Fig. 28-25A-C).163 Cytologic atypia is minimal, cell necrosis is not observed, and mitoses only rarely encountered. An “ancient” form of cellular blue nevus does possess pleomorphic melanocytes, but there are also degenerative changes, including edema, myxoid change, hyalinization of stroma, and “angiomatous” features.164 There is also an atypical blue nevus, characterized by asymmetry, infiltrative margins, and/or cytologic atypia with mitotic activity; these lesions tended to show a higher proliferative index using proliferating cell nuclear antigen (PCNA) and Ki-67 but demonstrated a similar natural history to that of conventional cellular blue nevus.165

Figure 28-25 Cellular blue nevus. A, The superficial portion of this lesion resembles a common blue nevus, whereas the deeper portion is composed of cellular islands that contain spindled to oval melanocytes. B, In this example, the deeper cellular islands have dispersed following removal and processing. C, Often, the deep component of a cellular blue nevus displays a well-demarcated, rounded or “pushing” border.
The epithelioid blue nevus is a darkly pigmented, symmetrical dermal melanocytic proliferation. There are three cell types: dendritic, pigmented polygonal, and large epithelioid. The latter cells are also polygonal, and they have generous amounts of cytoplasm and centrally located, rounded, vesicular nuclei with prominent nucleoli. Maturation descent is not convincingly displayed in these lesions.154,155
Differential Diagnosis: Common blue nevi are usually not difficult to recognize. However, two particular problems can occur. First, cutaneous melanoma metastases can resemble blue nevi of either conventional or epithelioid type. An example is provided in a report of such lesions in a patient with ocular melanoma.166 Confirmation of the diagnosis in that case was aided by the findings of nuclear pleomorphism and mitotic activity. Fluorescent in situ hybridization analysis can also be helpful, in that significant, differentiating chromosomal aberrations can be found in metastatic melanoma lesions, particularly related to gains in 6p25 relative to centromere 6.167 The hypomelanotic common blue nevus should be distinguished from other benign spindle cell tumors, such as dermatofibroma, neurofibroma, or dermal scar. Fontana-Masson staining may reveal subtle melanin pigment not seen on hematoxylin and eosin (H&E)-stained sections, and the cells stain for other melanocytic markers, including S-100, HMB-45, and Mel-5.158,159,163 Sclerotic lesions could be confused with desmoplastic melanoma, but significant nuclear pleomorphism or mitotic activity is not observed and HMB-45 staining is not usually seen in desmoplastic melanoma. Cellular blue nevi should be distinguished from the so-called malignant blue nevus, probably better designated melanoma arising in or resembling a blue nevus.168 In contrast to cellular blue nevus, melanomas arising in or resembling blue nevus are larger and more asymmetrical, irregularly lobulated, and hypercellular, and have greater degrees of cytologic atypia, tumor necrosis, and mitotic activity.169 A more difficult problem is the separation of atypical cellular blue nevi from these forms of melanoma, where the differences are largely a matter of degree.165,170 Both lesions require complete excision and close clinical follow-up.
Other Dermal Melanocytoses
Clinical Features: The term dermal melanocytosis is most often applied to lesions that are macular, bluish in color, and show widely scattered pigment-containing melanocytes in the reticular dermis with few other abnormalities. The entities included in this group are lumbosacral melanocytosis, also termed Mongolian spot; nevus of Ota (nevus fuscoceruleus ophthalmomaxillaris); nevus of Ito (nevus fuscoceruleus acromiodeltoideus); and acquired dermal melanocytosis. Most are congenital or begin in early childhood (with the exception of acquired dermal melanocytosis). Each of these presents as bluish macules in the corresponding anatomic locations; acquired dermal melanocytosis frequently occurs over the face and extremities.171 Nevus of Ota corresponds to the distribution of the trigeminal nerve and often involves the ipsilateral sclera; bilateral examples have been described.172 There is also a rare report of bilateral nevus of Ito.173 Dermal melanocytoses are most commonly, but not exclusively, encountered in dark-skinned individuals.174 Nevus of Ota is much more common in women than in men.175 Although these are generally considered benign lesions, melanoma has been reported to arise in association with nevus of Ota, variously developing in ocular tissues, skin, and meninges.176–178 Melanoma has only rarely occurred within a nevus of Ito179 or in acquired dermal melanocytosis associated with a nevus spilus.180 Lasers and/or pulsed light systems have been used to treat uncomplicated dermal melanocytoses.181,182
Microscopic Findings: Each of the dermal melanocytoses features widely scattered cells with delicate dendritic process within the reticular dermis. These contain melanin granules, although they are not necessarily heavily pigmented.183 In nevus of Ota and nevus of Ito, melanocytes tend to be more numerous and may be found in the papillary as well as the reticular dermis184,185 and around appendages (Fig. 28-26A and B).186 The more superficial location of these cells may account for the shades of brown pigment sometimes seen clinically in these lesions. Nodular foci display more concentrated dendritic melanocytes, thereby closely resembling blue nevi.187 Acquired dermal melanocytosis features melanocytes within the upper to mid-dermis, and there appears to be no significant difference in distribution of these cells between acquired bilateral nevus of Ota-like macules and other forms of acquired dermal melanocytosis.188

Figure 28-26 Dermal melanocytosis (nevus of Ito). A, The low-power view shows minimal abnormality, although this example did display basilar hypermelanosis with pigment incontinence, creating a focally brown appearance of the clinical lesion. B, Occasional dendritic melanocytes in the deep dermis show cytoplasmic pigment.
Differential Diagnosis: In lesions with few dermal melanocytes that are widely distributed and only lightly pigmented, it may be difficult to make a diagnosis of dermal melanocytosis in the absence of a strong index of suspicion or clinical guidance. This is particularly the case for Mongolian spots. Therefore, dermal melanocytosis should be included as one of the potential “invisible dermatoses.” Blue nevus differs by having more concentrated aggregates of pigmented dendritic melanocytes, although again, occasional nodular foci within dermal melanocytoses can be virtually indistinguishable from blue nevi.
Neurocristic Hamartoma
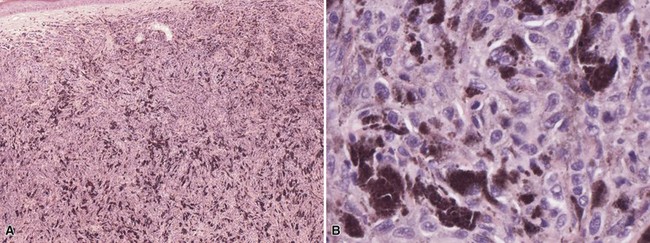
Clinical Features: Neurocristic cutaneous hamartomas are rare pigmented skin lesions that may be congenital or acquired. Although some are based in the deep subcutaneous tissue, a variant termed “pilar neurocristic hamartoma” is more superficial and perifollicular in location. These hamartomas are believed to result from aberrant development of the neuromesenchyme. In addition to a dermal/subcutaneous melanocytic component, they can also contain neurosustentacular and neuromesenchymal elements and can undergo malignant transformation. Some lesions present as bluish macules and can have a widespread distribution,189 whereas others are plaquelike.190 Pilar neurocristic hamartoma can present as a patch composed of perifollicular papules.191 Melanoma can arise in neurocristic hamartoma,192 but the risks of occurrence and degree of biologic aggressiveness are not predictable. It does appear that melanomas arising in these lesions are biologically distinct from more common cutaneous melanomas in that, whereas they demonstrate local invasion, distant metastasis is often a late event.192,193
Microscopic Findings: Neurocristic hamartomas show intermingling of nests of cuboidal cells, fascicles of spindled cells, and heavily pigmented, dendritic cells. Perivascular pseudorosettes demonstrate nuclear palisading of the pigmented cells. Mixtures of Schwann cells and neuromesenchyme are also seen, but not invariably. Much of this process is seen within the deep dermis and subcutaneous tissue (Fig. 28-27A-D),189 although the perifollicular distribution of pilar neurocristic hamartoma is distinctive.191 Immunohistochemical staining is positive for S-100, HMB-45 (variably), and Melan-A.189 Some foci are positive for S-100 and leu7 and negative for HMB-45, sometimes surrounded by spindled cells positive for epithelial membrane antigen; this suggests neural differentiation.

Figure 28-27 Neurocristic hamartoma. This is an unusually extensive example of subcutaneous pigmentation. A, Epithelioid to fusiform cells with extensive surrounding pigment. B, Higher power view showing cellular detail. C, A discrete subcutaneous nodule, found on positron emission tomography, is composed of bland-appearing, small cuboidal cells with relatively sparse pigmentation. D, Ki-67 activity is sparse within this nodule.
Differential Diagnosis: Neurocristic hamartomas have many features in common with variants of blue nevus, including cellular blue nevi, and with some congenital nevi. Yet the distribution of cells and depth and extent of involvement are quite different from most examples of those lesions.194
Congenital Nevus
Clinical Features: Congenital nevi are often classified by size and can be divided into three groups: giant (20 cm or more in greatest diameter), intermediate (1.5 to 19.9 cm in greatest diameter), and small (<1.5 cm in greatest diameter). The uncommon giant variety, as the name implies, appears at birth and particularly involves areas of the trunk such as the chest, back, shoulders, sacrum, and buttocks (hence the term “bathing trunk nevus”), but lesions can involve other areas as well, such as the scalp or hand. Leptomeningeal involvement occurs with some large or extensive congenital nevi.195 These nevi are frequently elevated, may be verrucous, and sometimes possess considerable amounts of hair. Smaller satellite lesions also occur, representing café-au-lait spots, connective tissue nevi, or smaller versions of congenital nevus. Incidence estimates range between 0.2% and 6% overall (0.0005% for giant congenital nevi.97,196,197 Smaller congenital nevi can appear virtually identical to acquired melanocytic nevi; they are usually round to ovoid, symmetric, and well delineated. Additional features of congenital nevi can include perifollicular hyperpigmentation and hypopigmentation, papular or rugose texture, asymmetry, and color variation.198 Major clinical issues with congenital nevi are cosmetic disfigurement (particularly with larger nevi) and the potential risks of the development of melanoma. Current studies suggest that there is minimal risk of melanoma arising in small-sized or intermediate-sized congenital nevi—probably about the same risk as that for acquired nevi.199,200 The risks of melanoma arising in giant congenital nevi vary depending on the study, and higher percentages are no doubt often influenced by referral biases; however, the lifetime risk has been estimated to be about 6.3%.201 Excision is recommended for small, changing lesions, and staged excisions are often carried out for the larger congenital nevi, along with close and long-term clinical follow-up.
Microscopic Findings: Intermediate and giant congenital nevi demonstrate considerable radial extent and depth of involvement, sometimes involving the subcutis and fascia. There is often a grenz zone separating diffusely distributed dermal nevus cells from the overlying epidermis, which may appear almost normal or show lentiginous changes. At times, more superficial dermal melanocytes are pigmented, whereas there may be diminution or absence of pigment in deeper portions of the lesion (Fig. 28-28). Nevus cells appear to splay the dermal collagen bundles and surround, or be found within, adnexal epithelia, nerves, and vessels. Spindled cells are found, particularly at the base of some lesions, and neuroid elements can sometimes be identified.202 Although all of these features taken together clearly indicate a congenital nevus, there appears to be no one completely pathognomonic change that ensures that a particular lesion is congenital, with the possible exception of nevus cell aggregates within sebaceous lobules (Fig. 28-29).203–205 Pagetoid changes can be seen within the epidermis in some congenital nevi in childhood; cytologic atypia tends to be minimal.203 Proliferative nodules are sometimes observed, particularly in giant congenital nevi but occasionally in smaller lesions as well. These consist of one or more cellular aggregates that are composed of spindled or epithelioid cells and are variably pigmented. Features indicating that these nodules are benign include limited cytologic atypia, coarse pigmentation (as opposed to finely divided pigmentation), a lack of cell necrosis or mitotic activity, and evidence that the cells within the nodule blend with surrounding melanocytes at the periphery (Fig. 28-30A and B).186,203 Smaller congenital nevi may be indistinguishable from common acquired nevi. Splaying of the collagen by nevus cells that also surround adnexal structures is considered suggestive of congenital nevus but not specific (Fig. 28-31),204,206 whereas the finding of nevus cells with adnexal epithelia or vessels in deeper portions of a lesion is stronger evidence of congenital origin.207

Figure 28-28 Giant congenital nevus. Virtually all the cells seen in the dermis in this specimen are melanocytes. The superficial dermal melanocytes are heavily pigmented. In this example, there is a grenz zone separating the dermal melanocytes from the overlying epidermis.

Figure 28-29 Giant congenital nevus. Involvement of a sebaceus gland is considered by some to be a pathognomonic finding of congenital nevus.

Figure 28-30 Proliferative nodule in a giant congenital nevus. A, This rather large nodule blended with the surrounding melanocytes. It is composed of small, monotonous cells with minimal pigmentation. B, Detail of the cells within the nodule, showing a lack of significant cytologic atypia, cell necrosis, or mitotic activity.
Differential Diagnosis: The most important diagnostic issue in congenital nevus is ruling in (or out) the development of melanoma in these lesions. As noted earlier, pagetoid change can occur in congenital nevi, but in contrast to melanoma, the upwardly migrating cells do not reach the epidermal surface and display a limited degree of atypia. Melanomas arising in giant congenital melanocytic nevi are found in the dermis and are composed of epithelioid, spindled, or small round cells that should display pleomorphism, necrosis, and mitotic activity.193,208,209 Melanoma tumor nodules also have a sharp margin at their interface with the surrounding nevus; in contrast, the benign proliferative nodules tend to be smaller and blend with the surrounding nevus cells.186
Dysplastic Nevus (Atypical Nevus; Architecturally Disordered Nevus with or without Cytologic Atypia)
Clinical Features: For some years it has been recognized that there is a group of melanocytic lesions having similar microscopic characteristics that deviate to varying degrees from what are considered to be ordinary, banal melanocytic nevi. Lesions with these histopathologic features can be seen in the dysplastic nevus syndrome, which has largely been defined by a particular clinical phenotype and a familial history. Over the years, there have been objections to use of the term “dysplastic nevus,” partly on etymologic grounds and partly because lesions with the set of microscopic features to be described below are not uniquely associated with the dysplastic nevus syndrome. Thus, in 1989, Clark stated that the preferred diagnosis is “nevus with melanocytic dysplasia of the type which may be seen in the dysplastic nevus syndrome.”210 As a result, there have been several efforts to come up with alternative terms for this particular type of nevus. One has been to use eponymic designations (i.e., Clark nevus in place of dysplastic nevus, along with Unna nevus for exophytic, papillomatous nevi, Miescher nevus for dome-shaped lesions with wedge-shaped extension into the reticular dermis, and Spitz nevus for lesions with the microscopic features described earlier in this chapter).211 Another has been to replace dysplastic nevus with “atypical nevus” or “architecturally disordered nevus, with or without cytologic atypia.” With those caveats, the author uses “atypical nevus” in this section, not to take sides in the controversy but mainly as a matter of convenience.
Atypical nevi are acquired lesions that are rare in prepubertal children. They are typically between 5 and 12 mm in diameter. They are usually macular, although there may be a papular component as well. The borders of these lesions are slightly irregular and ill defined, and there are often color variations (shades of tan, dark brown, or pink).212 This description is similar to that for melanomas, pointing out as it does lesions of a certain size, border irregularity, asymmetry, and color variation, but these changes are of lesser degree than expected in melanoma. However, it must be pointed out that the correlation between clinical and microscopic atypia is generally poor.213,214 Dysplastic nevi grow for a time and then become stable; changes in previously stable nevi should prompt careful evaluation and sampling for microscopic evaluation. Nevertheless, the frequency of progression to melanoma is uncertain. Clearly, atypical nevi are not obligate precursors to melanoma.
Microscopic Findings: There is elongation of rete ridges with increased numbers of singly dispersed and nested melanocytes along the dermal-epidermal junction. The nests are variable in size and can be found along the sides and tips of rete ridges and sometimes between them. Horizontal bridging of nests is a common feature. This constellation of features is sometimes referred to as lentiginous. When a dermal component is present, it is usually flanked by the intraepidermal component, which extends laterally beyond the intradermal nevus, forming “shoulders” of junctional lentiginous change. Beneath the epidermis is a zone of papillary dermal fibroplasia, which may either follow the rete ridges (concentric fibroplasia), or show horizontal layers of fibrosis separated by cleftlike spaces (lamellar fibroplasia) (Fig. 28-32A and B). Either can predominate, or both can be observed within the same lesion. Dermal vasculature may be prominent. Patchy lymphocytic inflammation is also present in the papillary dermis, mainly in a perivascular distribution but sometimes in a bandlike configuration, without evidence that the lesion has been irritated. Cells in the junctional zone or in the superficial papillary dermis can appear essentially normal, or there may be degrees of nuclear enlargement, contour irregularity, or hyperchromasia. Spindled or epithelioid cells are sometimes observed. In terms of size, mildly atypical nuclei are about the same size or slightly larger than basilar keratinocyte nuclei, whereas moderately atypical nuclei are one and one half to two times the diameter of a keratinocyte nucleus and severely atypical nuclei are greater than two times the size of a keratinocyte nucleus. Other atypical cytologic features include fine, dusky, gray-brown cytoplasmic pigment; prominent nucleoli, sometimes with perinucleolar halos; and coarse chromatin. Often, cytologic atypia is sporadic and focal, but uniform atypia may suggest evolution to melanoma.215–218 Mitoses should be few and limited to the junctional zone; dermal mitoses are rare, and maturation of melanocytes with descent into the dermis is preserved.

Figure 28-32 Atypical (dysplastic) nevus. Two examples of this type of lesion are shown. A, This lesion shows irregular junctional melanocytic proliferation, singly and in small nests. Lamellar fibroplasia can be identified, and there is a dermal inflammatory infiltrate. B, This example shows concentric fibroplasia following the outlines of the rete ridges. Note that there are sporadic atypical junctional melanocytes, with enlarged nuclei and irregular nuclear contours.
Differential Diagnosis: The distinction between an atypical nevus, particularly one with moderate to severe cytologic atypia, and melanoma can be difficult, and there are no doubt examples where a definitive diagnosis cannot be reached. However, some microscopic features favor a diagnosis of melanoma. These include effacement of the rete ridge pattern, prominent pagetoid intraepidermal melanocytic proliferation, loss of cohesion of melanocytes within nests, confluent junctional melanocytic proliferation sufficient to fill an entire high-power field, substantial cytologic atypia among most melanocytes, a lack of maturation of the dermal component, dermal mitoses, and bandlike lymphocytic dermal inflammation. Unfortunately, there is no hard-and-fast rule about which, or how many, of these features need to be present to ensure a diagnosis of melanoma, but a search for these changes often leads to a “tipping point” at which the pathologist becomes convinced that a particular lesion is malignant. In those situations where there is still doubt, it is best to acknowledge that a lesion, although severely atypical in some respects, is of uncertain biologic potential and that conservative local re-excision is the best course of action.
Malignant Melanocytic Tumors
Clinical Features: Melanoma is a malignancy that arises from the melanocyte system of the skin as well as from melanocytes in other locations, such as the eye and vagina. It accounts for about 5% of all skin cancers but, according to the American Cancer Society, causes 77% of all skin cancer–related deaths. As of 2008, United States cancer statistics showed that melanoma of the skin was the fifth most common cancer in men (after cancers of the prostate, lung and bronchus, colon and rectum, and urinary bladder) and the seventh most common cancer in women (after cancers of the breast, lung and bronchus, colon and rectum, corpus and uterus, thyroid, and non-Hodgkin lymphoma).219 However, it should be noted that those statistics typically do not include basal cell carcinoma and squamous cell carcinoma of the skin. Based on 2005–2009 data, the age-adjusted incidence rate for cutaneous melanoma is 21.0 per 100,000 men and women per year. The highest incidence of melanoma is during the fifth to seventh decades of life, although more young adults are now presenting with the lesion (increases have been noted in every age group except prepubertal children).
Melanoma occurs in any cutaneous location but is especially common on the head and neck, upper back, and lower extremities. Persons at greatest risk fall into Fitzpatrick skin types I or II. Type I individuals have very fair skin with red or blond hair, light eyes, and freckles, always burn with UV exposure (are unable to tan), and type II individuals usually have fair skin, burn easily, and tan minimally with UV exposure. However, individuals with any of the six skin types can develop cutaneous melanoma. There is evidence that occasional intense UV exposure is a greater risk factor for melanoma than is chronic, long-term exposure. Genetic predisposition to melanoma is another significant risk factor, an example being the dysplastic nevus syndrome.
Melanomas arise spontaneously but can also clearly develop within or adjacent to preexisting nevi, including congenital and atypical (dysplastic) nevi. They are generally divided into four major clinical types, each of which has particular histopathologic features (as will be discussed later). However, it must also be emphasized that there can be clinical as well as histopathologic overlap among these four types. The superficial spreading melanoma is typically a slightly elevated lesion with an irregular distribution of colors, including black, brown, red, and occasionally blue. White areas may also be seen, and these are believed to represent areas of regression. The borders of superficial spreading melanomas are usually irregular and notched. Lentigo maligna melanoma appears in sun-exposed skin, especially on the face of older individuals (however, an equivalent lesion also arises in mucous membranes, called mucosal lentiginous melanoma). This melanoma arises from a precursor, in situ lesion called lentigo maligna, a flat, brown-to-black macule with irregular borders. Lentigo maligna has the longest horizontal growth phase of any of the types of melanoma, and it may undergo extensive lateral spread for many years before developing the nodularity that signals dermal invasion. Nodular melanoma arises as a deeply pigmented blue-black lesion with an irregular border and, sometimes, surrounding erythema. These tumors have little or no radial growth prior to dermal invasion. Nodular melanomas frequently ulcerate and bleed. Acral lentiginous melanoma presents as an irregular, pigmented, macular or slightly elevated lesion on the palms, soles, and digits. It is particularly common among darker skinned individuals, including African-American and Inuit peoples.
The “warning signs” of melanoma have been widely publicized and outline certain key clinical features that should prompt more detailed inspection and possible biopsy or excision. These are commonly labeled the ABCDEs of melanoma and refer to the following characteristics: asymmetry, border irregularity, color variation, diameter (usually greater than 6 mm), and evolving (enlargement over time). These signs are considered to have semiologic value,220 but unfortunately not all melanomas display all or even some of these features. Examples include amelanotic, or “red” melanomas221 and desmoplastic melanomas,222 which can present as nondescript papules or small nodules.
The prognosis of melanoma varies, depending on its growth phase at the time of detection and other microscopic criteria, which are discussed subsequently. In situ or radial growth phase lesions tend to have an excellent prognosis, whereas thicker tumors in vertical growth phase have a more guarded prognosis. Regarding anatomic location of the primary tumor, there was some preliminary evidence to suggest that melanomas arising in the so-called “BANS” area (upper back, posterolateral upper arm, posterolateral neck, or posterior scalp) may have a worse prognosis than equivalent lesions in other locations, but this has not been borne out by subsequent analyses.223–225 Invasive tumors with certain adverse prognostic features (including thickness greater than 1 mm at the authors’ institution) often prompt a sentinel lymph node biopsy, which is widely considered to be of value in determining prognosis and possible further management (including complete lymph node dissection, immunotherapy, and/or chemotherapy).226
Microscopic Findings: The vast majority of cutaneous melanomas derive from melanocytic proliferations within epithelium, which in the skin includes epidermis and adnexal epithelia. In concert with the four clinical types of melanoma described previously, there are correlating histopathologic features, largely related to the configuration of the intraepidermal changes that will be described below. At this point, it is useful to introduce the stages of melanoma tumor progression: radial growth phase and vertical growth phase.216 A radial growth phase lesion is one in which the net growth of the lesion is in a radial, or horizontal, direction. This is the case for tumors confined to the epidermis (in situ lesions) and those that extend into the papillary dermis (e.g., as single cells or small clusters). A vertical growth phase lesion is one in which the net growth of the lesion is in a vertical direction through the dermis. This is most easily demonstrated by a primary melanoma with a nodular, expansile dermal component. Lesions that fill the papillary dermis in such an expansile fashion, or that demonstrate “accretive” growth within the dermis, show intradermal nests larger than any junctional nest, or have mitotic activity, are generally included as vertical growth phase lesions.227 As might be surmised, radial growth phase lesions tend to have more favorable outcomes than vertical growth phase lesions, which are more likely to be associated with recurrence or metastasis.
The following descriptions pertain to the four major types of melanoma and represent an overview of the most common presentations of melanoma in the skin. Subsequent sections focus on some unique varieties of melanocytic neoplasia.
Superficial spreading melanoma has been considered the most common form of melanoma (about 80% of cases),228 although it is now rivaled by lentigo maligna melanoma, possibly in part because of the aging population. The most characteristic feature is pagetoid spread of singly dispersed and nested melanocytes through the epidermis (Fig. 28-33). The cells are often epithelioid in type, and they feature finely divided cytoplasmic melanin pigment, nuclear contour irregularities, and prominent acidophilic nucleoli. The dermal component is usually quite similar, although fusiform or spindled cells can sometimes be found; mitoses can be identified, and maturation with descent is not observed.

Figure 28-33 Superficial spreading melanoma. There is pagetoid intraepidermal proliferation of atypical melanocytes.
The typical profile of lentigo maligna is an effaced, atrophic epidermis with singly dispersed melanocytes lined up at the junctional zone and extending along outer root sheaths of follicular units or eccrine sweat ducts (Fig. 28-34). Junctional melanocytic nuclei are often angulated and hyperchromatic. Mihm and colleagues have made a distinction between lentigo maligna, which they regard as a precursor to melanoma in situ characterized by intraepidermal melanocytic hyperplasia, and malignant melanoma in situ, lentigo maligna type (lentiginous melanoma in situ), which also features nested and single melanocytes at different levels of the epidermis, confluence of melanocytes essentially replacing the basilar layer of epidermis, and uniform cytologic atypia of the singly dispersed and nested melanocytes.229,230 This is an appealing concept, in that it recognizes an earlier stage of tumor progression that pathologists frequently see but have difficulty forcing into the category of unequivocal melanoma in situ. At the melanoma in situ stage, there may be sufficient confluence of uniformly atypical melanocytes to result in loss of cohesion and artifactual subepidermal separation (the “zipper sign”). Once invasive, lesions are frequently termed “lentigo maligna melanoma” and are prone to have a spindle cell configuration (Fig. 28-35).231 Adnexal involvement can extend deep to areas of apparent dermal invasion, and excisions should be planned to encompass these involved structures.

Figure 28-34 Lentiginous melanoma in situ. Atypical melanocytes are present in a confluent arrangement along the junctional zone of an effaced epidermis. They are both singly dispersed and in nests.

Figure 28-35 Lentigo maligna melanoma. The invasive component of this tumor has a partly spindled configuration. Note that the solar elastotic band has been “pushed” deeper in the dermis by the melanocytic proliferation, and some tumor islands are “breaking through” this band to extend into the deeper dermis and subcutis.
Nodular melanoma has the configuration of an expansile dermal growth with minimal involvement of the junctional zone.228,232 Nested or pagetoid intraepidermal changes can be seen overlying the dermal tumor, but there is minimal lateral spread of the intraepidermal process—arbitrarily set at fewer than three rete ridges beyond the dermal component (Fig. 28-36A and B). In some instances, epidermal connections appear to be lost, and both effacement of the rete ridge pattern overlying the dermal tumor and a thin grenz zone composed of sclerotic collagen are evident; this image suggests that there may have been a kind of superficial regressive change at the dermal-epidermal interface. In any event, the aforementioned features suggest that there is a minimal, short-lived radial growth phase component in nodular melanomas, rapidly followed and replaced by vertical growth. The dermal tumor is usually composed of overtly atypical cells that tend to be epithelioid in type but may be spindled. Diagnostic problems may arise in the occasional circumstance that cytologic atypia is more subtle, creating a “nevoid” appearance (see later discussion of nevoid melanoma).

Figure 28-36 Nodular melanoma. A, This lesion shows expansile dermal growth; junctional involvement is evident immediately overlying the dermal tumor. B, In this example of a polypoid melanoma, the lesion is sharply demarcated at the lateral margin and does not extend laterally beyond the intradermal component of the tumor.
Acral lentiginous melanomas consist of confluent individual melanocytes along the junctional zone of an epidermis that is often hyperkeratotic (even for acral skin) and acanthotic with proliferative rete ridges, intraepidermal nests, prominent pagetoid change (a feature also of acral nevi, although the latter lacks significant cytologic atypia) and, sometimes, significant melanocytic proliferation along eccrine sweat ducts. The proliferative melanocytes may display elongated, pigmented dendritic processes (Fig. 28-37A and B). The changes may be more subtle in early lesions, particularly at their periphery, because junctional confluence may not be complete and uniform cytologic atypia is not identified. Dermal fibrosis and inflammation that is sometimes lichenoid are additional features. The invasive component may be nested or in sheets and composed of cells that are small, epithelioid, or spindled.233–236

Figure 28-37 Acral lentiginous melanoma. A, Confluent, atypical melanocytes proliferate along the junctional zone and focally extend into more superficial epidermis. B, Some examples show heavily pigmented cells with prominent dendritic processes.
Regression is a change sometimes seen in primary melanomas, believed to represent a host immune response to tumor. Regression consists of early, dense inflammation, followed by disruption of tumor, fibrous tissue and new vessel formation, and eventually a loss of tumor with replacement fibrosis. These changes can be focal or occupy an entire lesion (Fig. 28-38). Circumstances arise in which an area of skin is biopsied, either because of its unusual appearance or pigmentation or because metastatic melanoma has been detected in the absence of a known primary lesion; the clinician initiates a search for a possible source of the metastasis. In either event, a biopsy may show only a zone of fibrosis, with scattered inflammatory cells, dilated vessels, and dermal pigment deposition (Fig. 28-39A and B). In other situations, a hyperpigmented cutaneous lesion may show a dermal plaque or nodule containing numerous heavily pigmented polygonal cells. With melanin bleaching and application of an immunohistochemical panel, these cells turn out to be melanophages rather than neoplastic melanocytes—a condition known as tumoral melanosis (Fig. 28-40). The above are examples of a completely regressed primary melanoma.237 In the setting of established metastatic melanoma, finding such changes can be strong presumptive evidence of a primary lesion, but on the other hand their discovery in isolation can be unsettling, leading to an evaluation for possible metastatic disease.
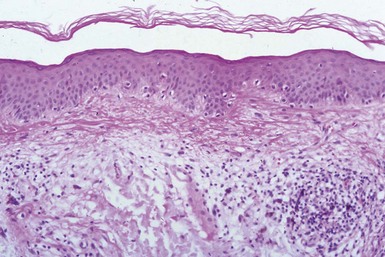
Figure 28-38 Regression in melanoma. This was considered a “radial growth phase” lesion that metastasized to a regional lymph node. Reevaluation of the original lesion showed a zone of fibrosis associated with inflammation, pigment deposition, and vasodilatation, indicating regressive change.

Figure 28-39 Regression in melanoma: the “unknown primary” lesion. A, A patient first presented with metastatic melanoma in a cervical lymph node. Search by the clinician revealed a vaguely pigmented, fibrotic lesion in the vicinity. Sections showed a zone of fibrosis, inflammation, and pigmentation beneath the epidermis and adjacent to a follicular unit. B, On one level, several atypical cells were identified that proved to be positive for S-100. This was an almost completely regressed primary melanoma.
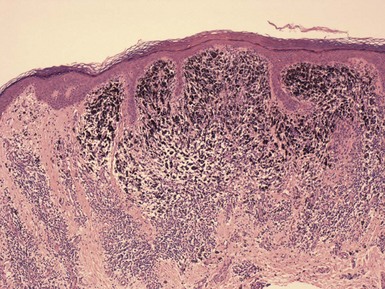
Figure 28-40 Tumoral melanosis. The heavy pigment in this lesion proved to be within macrophages and not melanocytes. This change was also found at the site of a regressed primary melanoma.
Cutaneous melanoma metastases are sometimes biopsied, and they often have certain histopathologic characteristics that should be recognizable. They can be deeply located in the dermis and subcutis (Fig. 28-41) or concentrated in the superficial dermis, sometimes surrounded in part by an epidermal collarette resembling a “ball-and-claw” arrangement. Tumor aggregates are often sharply circumscribed nodules, but they may form sheets or patches. Pigmentation is variable, and of course the cell types may vary from epithelioid to spindled to small-cell type. The cells tend to be “monotonously” atypical, and there is often minimal to no inflammation. Diagnostic problems can arise with an epidermotropic metastasis, in which there are tumor aggregates not only in the papillary dermis but also along the junctional zone and scattered within the dermis, sometimes in a pagetoid fashion, thereby mimicking a primary melanoma (Fig. 28-42). However, in contrast to a true primary melanoma, significant radial growth is not observed, and there is most often a substantial dermal tumor underlying a relatively small focus of epidermal involvement. The monotony of the atypical cells, mitotic activity, and lack of a host response (both stromal and inflammatory) are also clues that a lesion is metastatic rather than primary.238,239
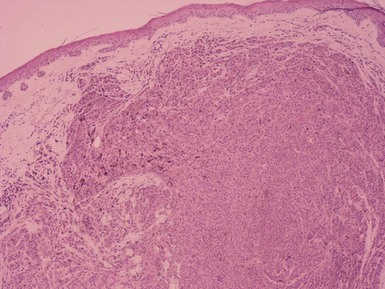
Figure 28-41 Metastatic melanoma. This is a poorly demarcated nodule of metastatic melanoma. Note the lack of junctional involvement, the essential absence of inflammation, and the monotony of tumor cells.

Figure 28-42 Metastatic melanoma. This is an example of an epidermotropic metastasis. It is sharply demarcated, but dermal cells are limited in extent and there is a host inflammatory reaction, in contrast to the classic image of cutaneous melanoma metastases.
Immunohistochemical staining has become an important adjunct to the diagnosis of melanoma. Although perhaps not necessary in obvious lesions with pigment, cytologic atypia, and intraepidermal and intradermal nesting, immunostaining can be helpful in circumstances (1) where there is limited epidermal involvement, (2) where atypical epithelioid or spindled cells raise a significant group of differential diagnostic possibilities, (3) where it is necessary to distinguish between a possible nevus or a nevoid melanoma, or (4) where it is necessary to evaluate intraepidermal melanocytic proliferations for degrees of confluence or pagetoid spread. S-100 continues to be useful stain because, despite a lack of specificity, it reliably stains most melanocytic lesions, particularly those desmoplastic or spindled melanomas where other “melanocytic” markers are frequently negative. MART-1 and Melan-A are sensitive markers and more specific for melanocytes than S-100. Additional useful markers include HMB-45, tyrosinase, microphthalmia transcription factor (MITF), and PNL2. Ki-67 is a proliferation marker that can be helpful in recognizing the proliferative capacity of a tumor and, therefore, its likelihood to be malignant rather than benign. In compound melanocytic neoplasms (especially with spitzoid features) where there is a question of malignancy, staining with HMB-45 and Ki-67 is sometimes used. In nevi, HMB-45 often demonstrates “stratification,” in which there is staining of junctional and papillary dermal melanocytes but not deeper dermal components. Melanomas often demonstrate spotty staining throughout the lesion.240 Ki-67 shows significant positivity in melanomas, particularly in deeper portions of the lesion, and may also provide important (adverse) prognostic information.241 Cyclin-dependant kinase inhibitor 2A (p16) or multiple tumor suppressor 1 (MTS-1) is a tumor suppressor protein that is expressed in benign melanocytic lesions but is often negative in melanomas. This marker has been used successfully to distinguish Spitz nevi from childhood spitzoid melanomas,242 desmoplastic Spitz nevi from desmoplastic melanomas,139 and nevus cells in lymph nodes from nodal melanoma metastases.243 Desmoplastic melanomas can mimic numerous spindle cell tumors, but unfortunately with the most commonly used markers they stain only for S-100 and vimentin. Several stains have been used to circumvent this problem and allow for a more specific diagnosis. These include p75 nerve growth factor receptor,244 KBA62 (which recognizes an unknown antigen expressed by melanomas),245 and SOX-10 (a nuclear transcription factor involved in schwannian and melanocytic differentiation).246
A number of histopathologic features offer significant prognostic information. Once a diagnosis of primary cutaneous melanoma has been established, the next step is to provide the clinician with these important data, which can have an important bearing on further staging and clinical management. The most significant prognostic indicator is tumor thickness, developed by Breslow.247 With a precalibrated ocular micrometer, a measurement is obtained from the top of the epidermal granular cell layer to the deepest extent of the tumor, and the resulting figure is expressed in millimeters. The thickness numbers correlate with survival such that, for example, a tumor less than 0.76 mm has a 5-year survival rate of 100%, whereas a tumor more than 4 mm in thickness has a 5-year survival of 50%. Problems that arise with measuring thickness include ulceration or absence of epidermis overlying the melanoma (measurements are obtained from the topmost viable tumor cell as an approximation) or circumstances where tumor is cut off at the base of a specimen (the figure then becomes an “at least” measurement). Tumor cells in the adventitia of adnexal structures or in the form of microsatellites are not included in thickness measurements, although their presence should be noted in the report. The previous means of assessing tumor depth (Clark levels) has been supplanted by thickness measurements, but these levels can be useful in particular circumstances. For example, for lesions of comparable thickness, a Clark level IV melanoma (extending into the reticular dermis) may have a worse prognosis than a Clark level III melanoma (confined to the papillary dermis). Ulceration has been considered an independent, adverse prognostic variable, but a more significant prognostic factor as a proxy for tumor proliferation may be mitotic rate248; this appears to be supported by the increasing importance of mitoses in current American Joint Committee on Cancer (AJCC) staging criteria. Despite some controversy surrounding the subject, regression has been considered an adverse prognostic variable in some studies, particularly those looking at the association with metastasizing thin melanomas.249,250 Tumor-infiltrating lymphocytes appear to also have a bearing on prognosis, in that brisk tumor-infiltrating lymphocytes (permeating a tumor) confer an improved prognosis when compared to nonbrisk or absent tumor-infiltrating lymphocytes.251 Lymphovascular invasion (in which tumor cells are actually found within vessel walls) is also considered an adverse prognostic variable252; vessel invasion can sometimes be mimicked by tissue retraction artifact around clusters of tumor cells.253 Perineural invasion has also been linked to an adverse prognosis.254 Another adverse prognostic variable is the finding of microscopic satellites; these are islands of tumor measuring at least 0.05 mm in diameter and separated from the main body of the tumor by normal connective tissue.255 Pathology reports of primary cutaneous melanomas should contain information about each of these factors, either in narrative form or in a synoptic report.
Differential Diagnosis: Of great importance in the differential diagnosis of melanoma is its differentiation from melanocytic nevi. Although this may not be difficult in florid examples of melanoma or well-organized, conventional nevi, it may be a more difficult task in cases of atypical, or dysplastic, nevi. There is currently no easy solution to this conundrum using standard morphologic interpretation or even (sometimes) immunohistochemical staining. The basic approach is to examine each section carefully, at both low-power and high-power magnification, and to obtain additional levels in difficult cases. Features the authors look for in favoring melanoma as opposed to a nevus include prominent pagetoid change (realizing, however, that such changes can be seen focally in conventional nevi, variants of Spitz nevus, and “special site” nevi, especially those in acral locations), rete ridge effacement, confluence of junctional melanocytes sufficient to fill entire high-power fields, bandlike dermal inflammation, and adnexal involvement (which can also be seen in some nevi, particularly in special sites such as the breast). Close attention to the degree, type, and extent of cytologic atypia is also important. Having said this, there are no doubt certain lesions for which a clear distinction between atypical nevus and melanoma is not possible. In those cases, reports should indicate that this is an atypical melanocytic lesion of uncertain biologic potential, and a recommendation for complete excision and close clinical follow-up is warranted.
Superficial spreading melanomas should also be distinguished from other pagetoid lesions, such as Paget and extramammary Paget disease and pagetoid Bowen disease. The first two disorders are composed of cells with abundant, pale cytoplasm (positive for mucin stains) that are often separated from, and may compress, the epidermal basilar layer. Bowen disease shows atypia and loss of maturation of nonpagetoid as well as pagetoid cells. Differential immunostaining can resolve difficult cases. Melanoma cells mark with the usual melanocyte stains, both Paget disease and Bowen disease cells express keratins, and Paget cells are reactive for carcinoembryonic antigen and gross cystic disease fluid protein-15. Although they can sometimes contain melanin, those Paget cells do not express melanocytic markers. Lentigo maligna and lentiginous melanoma in situ should be distinguished from solar lentigo, in which there may be substantial junctional melanocytic hyperplasia. So-called “starburst” giant cells are sometimes seen in lentigo maligna and are not encountered in melanocytes in sun-damaged skin,256 although they may be seen in benign melanocytic lesions.257 A diagnosis of lentigo maligna or lentiginous melanoma in situ requires finding significant confluence of junctional melanocytes as well as cytologic atypia; that search can be aided by close examination of multiple levels, and, in the authors’ experience, by immunostaining. The authors’ favorite approach is to stain for MART-1/Melan-A (using the diaminobenzidine method) with azure B counterstaining (which stains melanin blue-green).258 Nodular melanomas, by definition, lack (or may have minimal evidence for) the type of radial growth phase component seen in other types of melanoma, and therefore the dermal tumor may resemble a variety of other lesions. These include Spitz nevi, conventional melanocytic nevi in the case of nevoid melanoma (see subsequent discussion), and cellular blue nevi; spindle cell variants at times resemble spindle cell squamous cell carcinoma or atypical fibroxanthoma. Cytologic atypia, mitotic activity, and lack of maturation with descent should help distinguish nodular melanoma from benign melanocytic lesions, whereas differential immunostaining helps rule out squamous cell carcinoma or atypical fibroxanthoma. Acral lentiginous melanomas have some features in common with acral nevi, including pagetoid intraepidermal involvement, intradermal nesting patterns, syringotropism, inflammation, and fibroplasia. However, acral lentiginous melanomas have higher grade cytologic atypia and mitotic activity, and they lack evidence of maturation of dermal melanocytes with transition to scattered single cells at the lesional base—features generally observed in acral nevi.
Other Malignant Melanocytic Tumors
Desmoplastic melanoma is a difficult lesion to diagnose, both clinically and microscopically. It usually presents as a flesh-colored papule or small nodule, most commonly in the head and neck region of elderly adults. However, it can be seen in other locations and in a range of age groups. The diagnosis is often not expected clinically. Excision is difficult in that margins are not easy to determine. There is evidence that microscopically “pure” desmoplastic melanomas are less likely to metastasize to regional lymph nodes, in contrast to other types of melanoma or to desmoplastic melanomas that are mixed with spindle cell, epithelioid, or other melanoma types. The prognosis appears to be the same as for other melanomas of equivalent thickness, but it is unclear if “pure” desmoplastic lesions have a more favorable outcome. Microscopically, the majority of these lesions are associated with overlying epidermal changes of lentiginous melanoma, but about 20% have no evidence of epidermal involvement or a preceding radial growth phase (Fig. 28-43A–C). Widely scattered spindled cells with nuclear pleomorphism and mitotic activity within a sclerotic matrix are present. The borders of the lesion are ill defined and asymmetrical. Patchy dermal lymphocytic inflammation is often present, sometimes forming nodular aggregates within the deep dermis. Although some lesions are entirely desmoplastic, there are frequently other lesional types, including solid spindle cell areas with neurotropic features, and, sometimes, epithelioid elements can be found (Fig. 28-44). The cells of spindled neurotropic melanoma not only infiltrate perineurium but also form swirled structures that mimic nerve and suggest neural differentiation (Fig. 28-45). As noted above, desmoplastic melanomas are usually positive for S-100 but fail to stain with other traditional melanocytic markers. Therefore, these lesions can be confused with other spindle cell lesions, ranging from dermal scar to soft tissue sarcomas. Some of the newer immunohistochemical stains, such as KBA62 and SOX-10, may help in making an accurate diagnosis and provide assurance of clear surgical margins.

Figure 28-43 Desmoplastic melanoma. A, In this example, there is evidence for a lentiginous melanoma in situ. Nonpigmented spindled cells are scattered in the dermis within a sclerotic matrix. B, In about 20% of cases, there is no evidence for a preceding radial growth phase. C, Widely scattered atypical spindle cells are present within a fibrotic and partly mucinous matrix. These cells are positive for S-100.

Figure 28-44 Desmoplastic melanoma. This is a mixed type of lesion, with more desmoplastic changes seen on the right and a solid, epithelioid to fusiform proliferation on the left. Such lesions may have a different clinical course from purely desmoplastic melanomas.

Figure 28-45 Neurotropic melanoma. Elements of neurotropic melanoma may be found in desmoplastic lesions. These lesions can both infiltrate nerve and form structures that suggest neural differentiation.
Malignant blue melanoma is a lesion that may arise in association with a cellular blue nevus, conventional blue nevus, or a form of dermal melanocytosis but may also occur de novo. This lesion does not show epidermal involvement. Two significant case series have been reported.169,259 On average, patients presenting with malignant blue melanoma are in the fifth decade of life, and the majority are men. Tumors have been found on the trunk, extremities, and face, but overall the scalp appears to be the most common location. Most examples have originated from cellular blue nevi, and in fact cases selected for study by Martin and associates were required to have either foci of blue nevus (generally, cellular blue nevus) or areas resembling blue nevus. In-transit and distant metastases occurred in a significant percentage of patients. However, in one study, there was no significant difference in the development of lymph node metastases or overall mortality between patients with malignant blue melanoma and matched controls with melanomas of comparable thickness.259 Microscopically, the characteristics of malignant blue melanoma are lack of epidermal involvement; a large dermal tumor composed of densely cellular nodules of pigmented, spindled melanocytes that extend into the reticular dermis and subcutis; nuclear pleomorphism; and variable degrees of necrosis and mitotic activity (Fig. 28-46A and B).169 Lymph node metastases from these lesions are composed of overtly atypical cells that tend to disrupt the nodal architecture, in contrast to the smaller, “benign” lymph node metastases associated with some cellular blue nevi.260 These lesions should be distinguished from metastatic melanomas and a somewhat controversial group of lesions, termed atypical cellular blue nevi, which have more subtle degrees of cytologic atypia, greater cellularity and/or mitotic activity, and an uncertain clinical course.165,261

Figure 28-46 Malignant blue melanoma. A, This low-power view shows elements of conventional blue nevus but in addition an area of tumor necrosis (left), and tumor islands of dense cellularity (right). B, The densely cellular areas show nuclear pleomorphism and mitotic activity.
Nevoid melanoma is among the most troublesome melanocytic lesions to interpret. Although some authorities regard the use of this term as a form of equivocation, nevoid melanoma is indeed a melanoma, but one whose microscopic configuration very closely resembles that of a melanocytic nevus. This creates a very real risk of misdiagnosis, and for that reason, nevoid melanoma has been aptly described as a “triggered trap.”262 The best defense against these lesions is to maintain a high index of suspicion and to force oneself to inspect bland-appearing melanocytic lesions a bit more carefully, at a higher magnification.
Nevoid melanoma may represent one example of so-called “minimal deviation melanoma,” a term that is best regarded as a concept rather than a clinicopathologic entity.263 Levene described “verrucous and pseudonevoid malignant melanoma” in a 1980 paper on histologic diagnosis and prognosis of malignant melanoma,264 and the first known published use of the term was in a 1985 paper by Schmoeckel.265 Wong,266 McNutt,267 and Zembowicz268 have written other reviews of this topic. Nevoid melanomas occur in individuals of all ages but mainly in those in the fifth decade of life. They arise mainly on the trunk and lower extremities. Some are dome shaped, but verrucous variants also occur. Benign nevus has been the clinical impression in some cases,269 but other examples have presented clinically as an expanding pigmented lesion270 or, in one case, as a whitish mass protruding into the vaginal lumen.271 Despite their frequently bland clinical and microscopic features (at least, in the latter case, at low-power magnification), these lesions have a high recurrence rate,268,269 a metastatic rate from 25% to 50%,265,268 and mortality that may exceed 25%.268
Regarding microscopic findings, lesions are typically raised and either (1) dome shaped or (2) papillomatous and verrucous.266 In addition, lesions resembling (3) lentiginous nevi have been described in sun-exposed skin of older adults,272 and a variant with (4) prominent intraepidermal nesting has also been reported.265,268 At low-power magnification, nevoid melanomas are often sharply circumscribed and give the impression of symmetry, although often they are slightly asymmetrical. There is typically a limited intraepidermal component, except in the fourth variant mentioned, and junctional or intraepidermal changes characteristic of established radial growth phase melanomas are uncommonly seen. The cells may be small, superficially resembling those of a conventional nevus, or sometimes like those of Spitz nevus. There can be a suggestion of maturation, with cells appearing smaller toward the base of the lesion. However, when examined more closely, melanocytes may not form orderly nested arrangements, and foci displaying sheets and cords of cells may be observed.273 Although maturation descent is suggested, it is often incomplete; single-cell infiltration may be present at the base,274 and, when nested, tumors may not demonstrate decreasing size of nests with increasing depth.275 Infiltration of adnexal structures can be observed.265 On high magnification, even small nuclei may be pleomorphic and hyperchromatic, display angulated or irregular contours, and have prominent nucleoli. Mitotic figures are usually present, particularly in deeper portions of the lesion (in one recent study by Jensen and coworkers, only 4% of melanocytic nevi displayed mitoses34) (Fig. 28-47A and B). Inflammation can be variable in these tumors; some show virtually no inflammation, whereas others may display moderately dense, patchy inflammation or tumor-infiltrating lymphocytes.275 In those lesions resembling Spitz nevus, there is often continuous proliferation of melanocytes along the junctional zone, differing from the usual configuration of Spitz nevus.273 Perineural infiltration can be found in some cases. McKee suggests that a nevoid lesion with a warty configuration, seen in an elderly adult, should be a clue to nevoid melanoma, because true nevi from patients in this age group more typically show a dome-shaped configuration.275 Verrucoid lesions are also apt to show continuous proliferations of melanocytes along the junctional zone and sheets of melanocytes in the dermis without convincing maturation.266 Barnhill points out the thickening of nuclear membranes as an atypical cytologic feature.274 The authors also frequently observe prominent nucleoli with perinucleolar halos, even within the small nuclei in the deeper portions of these lesions.

Figure 28-47 Nevoid melanoma. A, The low-power configuration suggests a melanocytic nevus, including limited junctional involvement and diminishing size of dermal nests. However, the lesion is asymmetrical. B, On higher power, some areas show “back-to-back” cells, subtle nuclear pleomorphism, and mitotic activity.
McNutt and colleagues emphasized the use of HMB-45 staining in these tumors. Frequently, HMB-45 is strongly positive in the dermal component of nevoid melanomas, whereas there is usually a stratification of staining (most prominent in the junctional component, less in the upper dermal component, and absent in the deep component) in nevi.267 However, Weedon has described several examples of metastasizing nevoid melanomas that were negative for HMB-45.273 The tumor suppressor gene product p53, the oncogene product BCL2, and proliferation markers such as cyclin D1 and Ki-67 can also be helpful in these tumors. In contrast to melanocytic nevi, these antibodies tend to produce positive staining throughout the lesion, including the base, in nevoid melanomas,267,270,273–275 although not in every case. Kossard and colleagues studied nucleolar organizer regions in superficial spreading melanomas, nevoid melanomas, and melanocytic nevi; they found that nevoid melanomas had numbers of argyrophilic nucleolar organizer regions (AgNORs) per nucleus between (and significantly different from) those of superficial spreading melanomas and intradermal nevi).276 The recent development of an immunohistochemical stain for nucleolin may prove to be useful in further assessing this nucleolar component in melanocytic lesions, including nevoid melanomas.277
Pigmented epithelioid melanocytoma is a term proposed by Zembowicz and associates156 that defines a group of lesions previously classified as epithelioid blue nevus and animal-type melanoma in humans. These lesions can arise sporadically or may be associated with Carney complex. As a group, they are considered borderline melanocytic lesions that may represent low-grade melanomas. They most typically present in young adults but have a wide age range, and they have been diagnosed in a variety of ethnic groups.278 Lesions manifest as dark nodules that slowly enlarge. Metastases to sentinel lymph nodes occur in slightly less than 50% of cases, but further spread is rare; generally, the prognosis is considered more favorable than that of conventional melanomas of comparable depth.157 Recently, it has been reported that there is loss of expression of protein kinase A regulatory subunit type 1 alpha in pigmented epithelioid melanocytoma (mutations of this subunit are also identified in a majority of patients with the Carney complex) but not in melanoma, suggesting that this is in fact a distinct melanocytic tumor.279 Microscopically, lesions are heavily pigmented and have infiltrative borders. Junctional components have been seen in some cases, consisting of dendritic melanocytes but occasionally having features of a conventional nevus. The constituent dermal cells are epithelioid and spindled, with a tendency for epithelioid cells to occupy the central portions of a lesion.156 They proliferate along adnexal structures and neurovascular plexuses and reach into the subcutis. The cells have vesicular nuclei and prominent nucleoli, and scattered mitoses can be identified (Fig. 28-48A and B). Lymphocytic infiltration is sometimes observed. S-100 staining is positive but variable in intensity; MART-1 and HMB-45 are also positive in many cases.156 Pigmented epithelioid melanocytoma resembles cellular blue nevus but differs because of its epithelioid cell component. In contrast to pigmented epithelioid melanocytoma, malignant blue melanoma usually shows evidence of origination in a preexisting blue nevus or cellular blue nevus and displays greater degrees of nuclear pleomorphism, mitotic activity, and tumor necrosis. On low-power inspection, tumoral melanosis shows similarly intense pigmentation and cellularity but can be distinguished by its macrophagic lineage and negativity for melanocytic markers.
References
1. Brues, AM. Linkage of body build with sex, eye color, and freckling. Am J Hum Genet. 1950;2(3):215–239.
2. Breathnach, AS. Melanocyte distribution in forearm epidermis of freckled human subjects. J Invest Dermatol. 1957;29(4):253–261.
3. Revuz, J, Clerici, T. Penile melanosis. J Am Acad Dermatol. 1989;20(4):567–570.
4. Rudolph, RI. Vulvar melanosis. J Am Acad Dermatol. 1990;23(5 Pt 2):982–984.
5. Barnhill, RL, Albert, LS, Shama, SK, et al. Genital lentiginosis: a clinical and histopathologic study. J Am Acad Dermatol. 1990;22(3):453–460.
6. Maize, JC. Mucosal melanosis. Dermatol Clin. 1988;6(2):283–293.
7. Landau, M, Krafchik, BR. The diagnostic value of café-au-lait macules. J Am Acad Dermatol. 1999;40(6 Pt 1):877–890. [quiz 891-892].
8. Happle, R. Phacomatosis pigmentovascularis revisited and reclassified. Arch Dermatol. 2005;141(3):385–388.
9. Torrelo, A, Zambrano, A. What syndrome is this. Phakomatosis pigmentokeratotica (Happle). Pediatr Dermatol. 1998;15(4):321–323.
10. Happle, R. Speckled lentiginous naevus: which of the two disorders do you mean? Clin Exp Dermatol. 2009;34(2):133–135.
11. Benedict, PH, Szabo, G, Fitzpatrick, TB, et al. Melanotic macules in Albright’s syndrome and in neurofibromatosis. JAMA. 1968;205(9):618–626.
12. Johnson, BL, Charneco, DR. Café au lait spot in neurofibromatosis and in normal individuals. Arch Dermatol. 1970;102(4):442–446.
13. Morris, TJ, Johnson, WG, Silvers, DN. Giant pigment granules in biopsy specimens from cafe au lait spots in neurofibromatosis. Arch Dermatol. 1982;118(6):385–388.
14. Cohen, HJ, Minkin, W, Frank, SB. Nevus spilus. Arch Dermatol. 1970;102(4):433–437.
15. Stewart, DM, Altman, J, Mehregan, AH. Speckled lentiginous nevus. Arch Dermatol. 1978;114(6):895–896.
16. Vaidya, DC, Schwartz, RA, Janniger, CK. Nevus spilus. Cutis. 2007;80(6):465–468.
17. Ishibashi, A, Kimura, K, Kukita, A. Plaque-type blue nevus combined with lentigo (nevus spilus). J Cutan Pathol. 1990;17(4):241–245.
18. Silvers, DN, Greenwood, RS, Helwig, EB. Café au lait spots without giant pigment granules. Occurrence in suspected neurofibromatosis. Arch Dermatol. 1974;110(1):87–88.
19. Cohen, PR. Becker’s nevus. Am Fam Physician. 1988;37(5):221–226.
20. Danarti, R, Konig, A, Salhi, A, et al. Becker’s nevus syndrome revisited. J Am Acad Dermatol. 2004;51(6):965–969.
21. Glinick, SE, Alper, JC, Bogaars, H, et al. Becker’s melanosis: associated abnormalities. J Am Acad Dermatol. 1983;9(4):509–514.
22. Thappa, DM, Sirka, CS, Srikanth, S. Smooth muscle hamartoma associated with bilateral Becker’s nevus. Indian J Dermatol Venereol Leprol. 1997;63(6):387–389.
23. Urbanek, RW, Johnson, WC. Smooth muscle hamartoma associated with Becker’s nevus. Arch Dermatol. 1978;114(1):104–106.
24. Ber Rahman, S, Bhawan, J. Lentigo. Int J Dermatol. 1996;35(4):229–239.
25. Hafner, C, Stoehr, R, van Oers, JM, et al. The absence of BRAF, FGFR3, and PIK3CA mutations differentiates lentigo simplex from melanocytic nevus and solar lentigo. J Invest Dermatol. 2009;129(11):2730–2735.
26. Hodgson, C. Senile lentigo. Arch Dermatol. 1963;87:197–207.
27. Bolognia, JL. Reticulated black solar lentigo (‘ink spot’ lentigo). Arch Dermatol. 1992;128(7):934–940.
28. Andersen, WK, Labadie, RR, Bhawan, J. Histopathology of solar lentigines of the face: a quantitative study. J Am Acad Dermatol. 1997;36(3 Pt 1):444–447.
29. Montagna, W, Hu, F, Carlisle, K. A reinvestigation of solar lentigines. Arch Dermatol. 1980;116(10):1151–1154.
30. Marchesi, L, Naldi, L, Di Landro, A, et al. Segmental lentiginosis with “jentigo” histologic pattern. Am J Dermatopathol. 1992;14(4):323–327.
31. Yus, ES, del Cerro, M, Simon, RS, et al. Unna’s and Miescher’s nevi: two different types of intradermal nevus: hypothesis concerning their histogenesis. Am J Dermatopathol. 2007;29(2):141–151.
32. Xu, X, Bellucci, KS, Elenitsas, R, et al. Cellular nodules in congenital pattern nevi. J Cutan Pathol. 2004;31(2):153–159.
33. Gottlieb, GJ, Ackerman, AB. Mitotic figures may be seen in cells of banal melanocytic nevi. Am J Dermatopathol. 1985;7(Suppl):87–91.
34. Jensen, SL, Radfar, A, Bhawan, J. Mitoses in conventional melanocytic nevi. J Cutan Pathol. 2007;34(9):713–715.
35. Selim, MA, Vollmer, RT, Herman, CM, et al. Melanocytic nevi with nonsurgical trauma: a histopathologic study. Am J Dermatopathol. 2007;29(2):134–136.
36. Aso, M, Hashimoto, K, Eto, H, et al. Expression of Schwann cell characteristics in pigmented nevus. Immunohistochemical study using monoclonal antibody to Schwann cell associated antigen. Cancer. 1988;62(5):938–943.
37. Ball, NJ, Kho, GT. Melanocytic nevi are associated with neurofibromas in neurofibromatosis, type I, but not sporadic neurofibromas: a study of 226 cases. J Cutan Pathol. 2005;32(8):523–532.
38. Modlin, RL, Gottlieb, B, Taylor, C, et al. Identification of cells lining pseudovascular spaces of benign pigmented nevi. Am J Dermatopathol. 1984;6(Suppl):25–29.
39. Barnhill, RL, Penneys, NS, Landau-Price, D, et al. Fat cells in intradermal nevus do not contain S100 protein. Arch Dermatol. 1985;121(2):169–170.
40. Eng, W, Cohen, PR. Nevus with fat: clinical characteristics of 100 nevi containing mature adipose cells. J Am Acad Dermatol. 1998;39(5 Pt 1):704–711.
41. Penneys, NS, Mogollon, R, Kowalczyk, A, et al. A survey of cutaneous neural lesions for the presence of myelin basic protein. An immunohistochemical study. Arch Dermatol. 1984;120(2):210–213.
42. Schaffer, JV, Chang, MW, Kovich, OI, et al. Pigmented plexiform neurofibroma: distinction from a large congenital melanocytic nevus. J Am Acad Dermatol. 2007;56(5):862–868.
43. Kolm, I, Di Stefani, A, Hofmann-Wellenhof, R, et al. Dermoscopy patterns of halo nevi. Arch Dermatol. 2006;142(12):1627–1632.
44. Bergman, W, Willemze, R, de Graaff-Reitsma, C, et al. Analysis of major histocompatibility antigens and the mononuclear cell infiltrate in halo nevi. J Invest Dermatol. 1985;85(1):25–29.
45. Tokura, Y, Yamanaka, K, Wakita, H, et al. Halo congenital nevus undergoing spontaneous regression. Involvement of T-cell immunity in involution and presence of circulating anti-nevus cell IgM antibodies. Arch Dermatol. 1994;130(8):1036–1041.
46. Pellegrini, JR, Wagner, RF, Jr., Nathanson, L. Halo nevi and melanoma. Am Fam Physician. 1984;30(2):157–159.
47. Mooney, MA, Barr, RJ, Buxton, MG. Halo nevus or halo phenomenon? A study of 142 cases. J Cutan Pathol. 1995;22(4):342–348.
48. Happle, R, Echternacht, K, Schotola, I. [“Halonevus without the halo”]. Hautarzt. 1975;26(1):44–46.
49. King, LA, King, DT. Halo congenital nevus without histological inflammation. Arch Dermatol. 1978;114(8):1242.
50. Berman, A. Halo around a histiocytoma. Arch Dermatol. 1978;114(11):1717–1718.
51. Pembroke, AC, Liddell, K. Basal cell epithelioma with a hypopigmented halo. Arch Dermatol. 1981;117(6):317.
52. Penneys, NS, Ziboh, V, Simon, P, et al. Pathogenesis of Woronoff ring in psoriasis. Arch Dermatol. 1976;112(7):955–957.
53. Moretti, S, Spallanzani, A, Pinzi, C, et al. Fibrosis in regressing melanoma versus nonfibrosis in halo nevus upon melanocyte disappearance: could it be related to a different cytokine microenvironment? J Cutan Pathol. 2007;34(4):301–308.
54. Meyerson, LB. A peculiar papulosquamous eruption involving pigmented nevi. Arch Dermatol. 1971;103(5):510–512.
55. Worret, WI. [Halo eczema and nevus cell nevi (Meyerson nevi)]. Hautarzt. 1990;41(5):262–264.
56. Nicholls, DS, Mason, GH. Halo dermatitis around a melanocytic naevus: Meyerson’s naevus. Br J Dermatol. 1988;118(1):125–129.
57. Rolland, S, Kokta, V, Marcoux, D. Meyerson phenomenon in children: observation in five cases of congenital melanocytic nevi. Pediatr Dermatol. 2009;26(3):292–297.
58. Legoux, B, Pawin, H, Prigent, F, et al. [Meyerson’s nevus on congenital pigmented nevus]. Ann Dermatol Venereol. 1991;118(11):873–874.
59. Ferneiny, M, Panse, I, Schartz, N, et al. [Disseminated perinaevic Meyerson phenomenon revealing melanoma]. Ann Dermatol Venereol. 2012;139(2):137–1341.
60. Ramon, R, Silvestre, JF, Betlloch, I, et al. Progression of Meyerson’s naevus to Sutton’s naevus. Dermatology. 2000;200(4):337–338.
61. Petit, A, Viney, C, Gaulier, A, et al. Coexistence of Meyerson’s with Sutton’s naevus after sunburn. Dermatology. 1994;189(3):269–270.
62. Pizem, J, Stojanovic, L, Luzar, B. Melanocytic lesions with eczematous reaction (Meyerson’s phenomenon)—a histopathologic analysis of 64 cases. J Cutan Pathol. 2012;39(10):901–910.
63. Kus, S, Ince, U, Candan, I, et al. Meyerson phenomenon associated with dysplastic compound nevi. J Eur Acad Dermatol Venereol. 2006;20(3):350–351.
64. Rodins, K, Byrom, L, Muir, J. Early melanoma with halo eczema (Meyerson’s phenomenon). Australas J Dermatol. 2011;52(1):70–73.
65. Cook-Norris, RH, Zic, JA, Boyd, AS. Meyerson’s naevus: a clinical and histopathological study of 11 cases. Australas J Dermatol. 2008;49(4):191–195.
66. Loh, J, Kenny, P. Meyerson phenomenon. J Cutan Med Surg. 2010;14(1):30–32.
67. Matsuda, M, Hamada, T, Ishii, N, et al. Acquired smooth muscle hamartoma of the patchy follicular variant with Meyerson phenomenon. Arch Dermatol. 2011;147(10):1234–1235.
68. Hofer, T. Meyerson phenomenon within a nevus flammeus. The different eczematous reactions within port-wine stains. Dermatology. 2002;205(2):180–183.
69. Dawn, G, Burden, AD. Meyerson’s phenomenon around a seborrhoeic keratosis. Clin Exp Dermatol. 2002;27(1):73.
70. Gallais, V, Lacour, JP, Perrin, C, et al. [Halo eczema around a histiocytofibroma: the Meyerson phenomenon]. Ann Dermatol Venereol. 1993;120(9):617–620.
71. Hosler, GA, Moresi, JM, Barrett, TL. Nevi with site-related atypia: a review of melanocytic nevi with atypical histologic features based on anatomic site. J Cutan Pathol. 2008;35(10):889–898.
72. Saida, T, Oguchi, S, Miyazaki, A. Dermoscopy for acral pigmented skin lesions. Clin Dermatol. 2002;20(3):279–285.
73. Gleason, BC, Hirsch, MS, Nucci, MR, et al. Atypical genital nevi. A clinicopathologic analysis of 56 cases. Am J Surg Pathol. 2008;32(1):51–57.
74. Massi, G, LeBoit, PE. Polypoid nevus of pregnancy and milk line nevi. Histologic Diagnosis of Nevi and Melanoma. Darmstadt, Germany: Steinkopff, Verlag; 2004. [329].
75. LeBoit, PE. A diagnosis for maniacs. Am J Dermatopathol. 2000;22(6):556–558.
76. Boyd, AS, Rapini, RP. Acral melanocytic neoplasms: a histologic analysis of 158 lesions. J Am Acad Dermatol. 1994;31(5 Pt 1):740–745.
77. Christensen, WN, Friedman, KJ, Woodruff, JD, et al. Histologic characteristics of vulvar nevocellular nevi. J Cutan Pathol. 1987;14(2):87–91.
78. Clark, WH, Jr., Hood, AF, Tucker, MA, et al. Atypical melanocytic nevi of the genital type with a discussion of reciprocal parenchymal-stromal interactions in the biology of neoplasia. Hum Pathol. 1998;29(1 Suppl 1):S1–S24.
79. Rongioletti, F, Urso, C, Batolo, D, et al. Melanocytic nevi of the breast: a histologic case-control study. J Cutan Pathol. 2004;31(2):137–140.
80. Rongioletti, F, Ball, RA, Marcus, R, et al. Histopathological features of flexural melanocytic nevi: a study of 40 cases. J Cutan Pathol. 2000;27(5):215–217.
81. Fabrizi, G, Pagliarello, C, Parente, P, et al. Atypical nevi of the scalp in adolescents. J Cutan Pathol. 2007;34(5):365–369.
82. Lazova, R, Lester, B, Glusac, EJ, et al. The characteristic histopathologic features of nevi on and around the ear. J Cutan Pathol. 2005;32(1):40–44.
83. Saad, AG, Patel, S, Mutasim, DF. Melanocytic nevi of the auricular region: histologic characteristics and diagnostic difficulties. Am J Dermatopathol. 2005;27(2):111–115.
84. Barnhill, RL, Mihm, MC, Jr., Magro, CM. Plexiform spindle cell naevus: a distinctive variant of plexiform melanocytic naevus. Histopathology. 1991;18(3):243–247.
85. Pulitzer, DR, Martin, PC, Cohen, AP, et al. Histologic classification of the combined nevus. Analysis of the variable expression of melanocytic nevi. Am J Surg Pathol. 1991;15(12):1111–1122.
86. Seab, JA, Jr., Graham, JH, Helwig, EB. Deep penetrating nevus. Am J Surg Pathol. 1989;13(1):39–44.
87. Mehregan, DA, Mehregan, AH. Deep penetrating nevus. Arch Dermatol. 1993;129(3):328–331.
88. Luzar, B, Calonje, E. Deep penetrating nevus: a review. Arch Pathol Lab Med. 2011;135(3):321–326.
89. Cooper, PH. Deep penetrating (plexiform spindle cell) nevus. A frequent participant in combined nevus. J Cutan Pathol. 1992;19(3):172–180.
90. High, WA, Alanen, KW, Golitz, LE. Is melanocytic nevus with focal atypical epithelioid components (clonal nevus) a superficial variant of deep penetrating nevus? J Am Acad Dermatol. 2006;55(3):460–466.
91. Sommer, LL, Barcia, SM, Clarke, LE, et al. Persistent melanocytic nevi: a review and analysis of 205 cases. J Cutan Pathol. 2011;38(6):503–507.
92. Marghoob, AA, Kopf, AW. Persistent nevus: an exception to the ABCD rule of dermoscopy. J Am Acad Dermatol. 1997;36(3 Pt 1):474–475.
93. Kornberg, R, Ackerman, AB. Pseudomelanoma: recurrent melanocytic nevus following partial surgical removal. Arch Dermatol. 1975;111(12):1588–1590.
94. Park, HK, Leonard, DD, Arrington, JH, III., et al. Recurrent melanocytic nevi: clinical and histologic review of 175 cases. J Am Acad Dermatol. 1987;17(2 Pt 1):285–292.
95. Sexton, M, Sexton, CW. Recurrent pigmented melanocytic nevus. A benign lesion, not to be mistaken for malignant melanoma. Arch Pathol Lab Med. 1991;115(2):122–126.
96. King, R, Hayzen, BA, Page, RN, et al. Recurrent nevus phenomenon: a clinicopathologic study of 357 cases and histologic comparison with melanoma with regression. Mod Pathol. 2009;22(5):611–617.
97. Haupt, HM, Stern, JB. Pagetoid melanocytosis. Histologic features in benign and malignant lesions. Am J Surg Pathol. 1995;19(7):792–797.
98. Harvell, JD, White, WL. Persistent and recurrent blue nevi. Am J Dermatopathol. 1999;21(6):506–517.
99. Munoz, C, Quintero, A, Sanchez, JL, et al. Persistent blue nevus simulating melanoma. J Am Acad Dermatol. 2004;50(5 Suppl):S118–S120.
100. Harvell, JD, Bastian, BC, LeBoit, PE. Persistent (recurrent) Spitz nevi: a histopathologic, immunohistochemical, and molecular pathologic study of 22 cases. Am J Surg Pathol. 2002;26(5):654–661.
101. Omura, EF, Kheir, SM. Recurrent Spitz’s nevus. Am J Dermatopathol. 1984;6(Suppl):207–212.
102. Hoang, MP, Prieto, VG, Burchette, JL, et al. Recurrent melanocytic nevus: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2001;28(8):400–406.
103. Kerl, H, Soyer, HP, Cerroni, L, et al. Ancient melanocytic nevus. Semin Diagn Pathol. 1998;15(3):210–215.
104. Kerl, H, Wolf, IH, Kerl, K, et al. Ancient melanocytic nevus: a simulator of malignant melanoma. Am J Dermatopathol. 2011;33(2):127–130.
105. Massone, C, Soyer, HP, Hofmann-Wellenhof, R. A dermoscopic pitfall: ancient melanocytic nevus. J Am Acad Dermatol. 2008;59(2 Suppl 1):S42–S43.
106. Weedon, D. Unusual features of nevocellular nevi. J Cutan Pathol. 1982;9(5):284–292.
107. Gray, MH, Smoller, BR, McNutt, NS, et al. Immunohistochemical demonstration of factor XIIIa expression in neurofibromas. A practical means of differentiating these tumors from neurotized melanocytic nevi and schwannomas. Arch Dermatol. 1990;126(4):472–476.
108. Chen, Y, Klonowski, PW, Lind, AC, et al. Differentiating neurotized melanocytic nevi from neurofibromas using Melan-A (MART-1) immunohistochemical stain. Arch Pathol Lab Med. 2012;136(7):810–815.
109. Soderstrom, KO. Angiomatous type of intradermal nevi. Am J Dermatopathol. 1987;9(6):549–551.
110. Collina, G, Eusebi, V. Naevocytic naevi with vascular-like spaces. Br J Dermatol. 1991;124(6):591–595.
111. Spitz, S. Melanomas of childhood. Am J Pathol. 1948;24(3):591–609.
112. Paniago-Pereira, C, Maize, JC, Ackerman, AB. Nevus of large spindle and/or epithelioid cells (Spitz’s nevus). Arch Dermatol. 1978;114(12):1811–1823.
113. Palazzo, JP, Duray, PH. Congenital agminated Spitz nevi: immunoreactivity with a melanoma-associated monoclonal antibody. J Cutan Pathol. 1988;15(3):166–170.
114. Weedon, D, Little, JH. Spindle and epithelioid cell nevi in children and adults. A review of 211 cases of the Spitz nevus. Cancer. 1977;40(1):217–225.
115. Coskey, RJ, Mehregan, A. Spindle cell nevi in adults and children. Arch Dermatol. 1973;108(4):535–536.
116. Echevarria, R, Ackerman, LV. Spindle and epitheloid cell nevi in the adult. Clinicopathologic report of 26 cases. Cancer. 1967;20(2):175–189.
117. Renfro, L, Grant-Kels, JM, Brown, SA. Multiple agminate Spitz nevi. Pediatr Dermatol. 1989;6(2):114–117.
118. Hamm, H, Happle, R, Brocker, EB. Multiple agminate Spitz naevi: review of the literature and report of a case with distinctive immunohistological features. Br J Dermatol. 1987;117(4):511–522.
119. Mazzurco, J, Menssen, K, Schapiro, B, et al. Eruptive disseminated Spitz nevi in a 26-year-old African-American woman. Int J Dermatol. 2012;51(10):1270–1271.
120. Levy, RM, Ming, ME, Shapiro, M, et al. Eruptive disseminated Spitz nevi. J Am Acad Dermatol. 2007;57(3):519–523.
121. Smith, KJ, Barrett, TL, Skelton, HG, III., et al. Spindle cell and epithelioid cell nevi with atypia and metastasis (malignant Spitz nevus). Am J Surg Pathol. 1989;13(11):931–939.
122. Urso, C, Borgognoni, L, Saieva, C, et al. Sentinel lymph node biopsy in patients with “atypical Spitz tumors.” A report on 12 cases. Hum Pathol. 2006;37(7):816–823.
123. Barnhill, RL, Argenyi, ZB, From, L, et al. Atypical Spitz nevi/tumors: lack of consensus for diagnosis, discrimination from melanoma, and prediction of outcome. Hum Pathol. 1999;30(5):513–520.
124. Casso, EM, Grin-Jorgensen, CM, Grant-Kels, JM. Spitz nevi. J Am Acad Dermatol. 1992;27(6 Pt 1):901–913.
125. Barr, RJ, Morales, RV, Graham, JH. Desmoplastic nevus: a distinct histologic variant of mixed spindle cell and epithelioid cell nevus. Cancer. 1980;46(3):557–564.
126. Sherrill, AM, Crespo, G, Prakash, AV, et al. Desmoplastic nevus: an entity distinct from spitz nevus and blue nevus. Am J Dermatopathol. 2011;33(1):35–39.
127. Diaz-Cascajo, C, Borghi, S, Weyers, W. Angiomatoid Spitz nevus: a distinct variant of desmoplastic Spitz nevus with prominent vasculature. Am J Dermatopathol. 2000;22(2):135–139.
128. Tetzlaff, MT, Xu, X, Elder, DE, et al. Angiomatoid Spitz nevus: a clinicopathological study of six cases and a review of the literature. J Cutan Pathol. 2009;36(4):471–476.
129. Zaenglein, AL, Heintz, P, Kamino, H, et al. Congenital Spitz nevus clinically mimicking melanoma. J Am Acad Dermatol. 2002;47(3):441–444.
130. Han, MH, Koh, KJ, Choi, JH, et al. Pagetoid Spitz nevus: a variant of Spitz nevus. Int J Dermatol. 2000;39(7):555–557.
131. Busam, KJ, Barnhill, RL. Pagetoid Spitz nevus. Intraepidermal Spitz tumor with prominent pagetoid spread. Am J Surg Pathol. 1995;19(9):1061–1067.
132. Kamino, H, Misheloff, E, Ackerman, AB, et al. Eosinophilic globules in Spitz’s nevi: new findings and a diagnostic sign. Am J Dermatopathol. 1979;1(4):323–324.
133. Skelton, HG, Miller, ML, Lupton, GP, et al. Eosinophilic globules in spindle cell and epithelioid cell nevi: composition and possible origin. Am J Dermatopathol. 1998;20(6):547–550.
134. Wesselmann, U, Becker, LR, Brocker, EB, et al. Eosinophilic globules in spitz nevi: no evidence for apoptosis. Am J Dermatopathol. 1998;20(6):551–554.
135. Arbuckle, S, Weedon, D. Eosinophilic globules in the Spitz nevus. J Am Acad Dermatol. 1982;7(3):324–327.
136. Barnhill, RL, Flotte, TJ, Fleischli, M, et al. Cutaneous melanoma and atypical Spitz tumors in childhood. Cancer. 1995;76(10):1833–1845.
137. Bergman, R, Dromi, R, Trau, H, et al. The pattern of HMB-45 antibody staining in compound Spitz nevi. Am J Dermatopathol. 1995;17(6):542–546.
138. Bergman, R, Malkin, L, Sabo, E, et al. MIB-1 monoclonal antibody to determine proliferative activity of Ki-67 antigen as an adjunct to the histopathologic differential diagnosis of Spitz nevi. J Am Acad Dermatol. 2001;44(3):500–504.
139. Hilliard, NJ, Krahl, D, Sellheyer, K. p16 expression differentiates between desmoplastic Spitz nevus and desmoplastic melanoma. J Cutan Pathol. 2009;36(7):753–759.
140. Harvell, JD, Kohler, S, Zhu, S, et al. High-resolution array-based comparative genomic hybridization for distinguishing paraffin-embedded Spitz nevi and melanomas. Diagn Mol Pathol. 2004;13(1):22–25.
141. Reed, RJ, Ichinose, H, Clark, WH, Jr., et al. Common and uncommon melanocytic nevi and borderline melanomas. Semin Oncol. 1975;2(2):119–147.
142. Sagebiel, RW, Chinn, EK, Egbert, BM. Pigmented spindle cell nevus. Clinical and histologic review of 90 cases. Am J Surg Pathol. 1984;8(9):645–653.
143. Sau, P, Graham, JH, Helwig, EB. Pigmented spindle cell nevus: a clinicopathologic analysis of ninety-five cases. J Am Acad Dermatol. 1993;28(4):565–571.
144. Wistuba, I, Gonzalez, S. Eosinophilic globules in pigmented spindle cell nevus. Am J Dermatopathol. 1990;12(3):268–271.
145. Barnhill, RL, Barnhill, MA, Berwick, M, et al. The histologic spectrum of pigmented spindle cell nevus: a review of 120 cases with emphasis on atypical variants. Hum Pathol. 1991;22(1):52–58.
146. Barnhill, RL, Mihm, MC, Jr. Pigmented spindle cell naevus and its variants: distinction from melanoma. Br J Dermatol. 1989;121(6):717–725.
147. Elder, DE, Murphy, GF. Benign melanocytic tumors (nevi). Atlas of Tumor Pathology: Melanocytic Tumors of the Skin. Washington, DC: Armed Forces Institute of Pathology; 1991. [49-51, 57].
148. Leopold, JG, Richards, DB. The interrelationship of blue and common naevi. J Pathol Bacteriol. 1968;95(1):37–46.
149. Velez, A, del-Rio, E, Martin-de-Hijas, C, et al. Agminated blue nevi: case report and review of the literature. Dermatology. 1993;186(2):144–148.
150. Shenfield, HT, Maize, JC. Multiple and agminated blue nevi. J Dermatol Surg Oncol. 1980;6(9):725–728.
151. Park, YM, Kang, H, Cho, BK. Plaque-type blue nevus combined with nevus spilus and smooth muscle hyperplasia. Int J Dermatol. 1999;38(10):775–777.
152. Zembowicz, A, Phadke, PA. Blue nevi and variants: an update. Arch Pathol Lab Med. 2011;135(3):327–336.
153. Rodriguez, HA, Ackerman, LV. Cellular blue nevus. Clinicopathologic study of forty-five cases. Cancer. 1968;21(3):393–405.
154. Carney, JA, Ferreiro, JA. The epithelioid blue nevus. A multicentric familial tumor with important associations, including cardiac myxoma and psammomatous melanotic schwannoma. Am J Surg Pathol. 1996;20(3):259–272.
155. Moreno, C, Requena, L, Kutzner, H, et al. Epithelioid blue nevus: a rare variant of blue nevus not always associated with the Carney complex. J Cutan Pathol. 2000;27(5):218–223.
156. Zembowicz, A, Carney, JA, Mihm, MC. Pigmented epithelioid melanocytoma: a low-grade melanocytic tumor with metastatic potential indistinguishable from animal-type melanoma and epithelioid blue nevus. Am J Surg Pathol. 2004;28(1):31–40.
157. Mandal, RV, Murali, R, Lundquist, KF, et al. Pigmented epithelioid melanocytoma: favorable outcome after 5-year follow-up. Am J Surg Pathol. 2009;33(12):1778–1782.
158. Bhawan, J, Cao, SL. Amelanotic blue nevus: a variant of blue nevus. Am J Dermatopathol. 1999;21(3):225–228.
159. Carr, S, See, J, Wilkinson, B, et al. Hypopigmented common blue nevus. J Cutan Pathol. 1997;24(8):494–498.
160. Kamino, H, Tam, ST. Compound blue nevus: a variant of blue nevus with an additional junctional dendritic component. A clinical, histopathologic, and immunohistochemical study of six cases. Arch Dermatol. 1990;126(10):1330–1333.
161. Sun, J, Morton, TH, Jr., Gown, AM. Antibody HMB-45 identifies the cells of blue nevi. An immunohistochemical study on paraffin sections. Am J Surg Pathol. 1990;14(8):748–751.
162. Zembowicz, A, Mihm, MC. Dermal dendritic melanocytic proliferations: an update. Histopathology. 2004;45(5):433–451.
163. Zembowicz, A, Granter, SR, McKee, PH, et al. Amelanotic cellular blue nevus: a hypopigmented variant of the cellular blue nevus: clinicopathologic analysis of 20 cases. Am J Surg Pathol. 2002;26(11):1493–1500.
164. Cerroni, L, Borroni, RG, Massone, C, et al. “Ancient” blue nevi (cellular blue nevi with degenerative stromal changes). Am J Dermatopathol. 2008;30(1):1–5.
165. Tran, TA, Carlson, JA, Basaca, PC, et al. Cellular blue nevus with atypia (atypical cellular blue nevus): a clinicopathologic study of nine cases. J Cutan Pathol. 1998;25(5):252–258.
166. Wieselthier, JS, White, WL. Cutaneous metastasis of ocular malignant melanoma. An unusual presentation simulating blue nevi. Am J Dermatopathol. 1996;18(3):289–295.
167. Pouryazdanparast, P, Newman, M, Mafee, M, et al. Distinguishing epithelioid blue nevus from blue nevus-like cutaneous melanoma metastasis using fluorescence in situ hybridization. Am J Surg Pathol. 2009;33(9):1396–1400.
168. Barnhill, RL. Malignant melanoma. In: Barnhill RL, Piepkorn M, Busam KJ, eds. Pathology of Melanocytic Nevi and Malignant Melanoma. New York: Springer; 2004:322–327.
169. Connelly, J, Smith, JL, Jr. Malignant blue nevus. Cancer. 1991;67(10):2653–2657.
170. Avidor, I, Kessler, E. ‘Atypical’ blue nevus—a benign variant of cellular blue nevus. Presentation of three cases. Dermatologica. 1977;154(1):39–44.
171. Hidano, A, Kaneko, K. Acquired dermal melanocytosis of the face and extremities. Br J Dermatol. 1991;124(1):96–99.
172. Tateishi, C, Ozawa, T, Shirakawa, M, et al. Bilateral nevus of Ota: a rare manifestation congenital type in a boy. Osaka City Med J. 2011;57(1):45–48.
173. Trindade, F, Santonja, C, Requena, L. Bilateral nevus of Ito and nevus spilus in the same patient. J Am Acad Dermatol. 2008;59(2 Suppl 1):S51–S52.
174. Mizoguchi, M, Murakami, F, Ito, M, et al. Clinical, pathological, and etiologic aspects of acquired dermal melanocytosis. Pigment Cell Res. 1997;10(3):176–183.
175. Kono, T, Kurome, H, Shibuya, Y, et al. Ocular findings in Japanese women with nevus of Ota. Graefes Arch Clin Exp Ophthalmol. 1995;233(11):667–671.
176. Theunissen, P, Spincemaille, G, Pannebakker, M, et al. Meningeal melanoma associated with nevus of Ota: case report and review. Clin Neuropathol. 1993;12(3):125–129.
177. Dompmartin, A, Leroy, D, Labbe, D, et al. Dermal malignant melanoma developing from a nevus of Ota. Int J Dermatol. 1989;28(8):535–536.
178. Roy, PE, Schaeffer, EM. Nevus of Ota and choroidal melanoma. Surv Ophthalmol. 1967;12(2):130–140.
179. Wise, SR, Capra, G, Martin, P, et al. Malignant melanoma transformation within a nevus of Ito. J Am Acad Dermatol. 2010;62(5):869–874.
180. Yoneyama, K, Kamada, N, Mizoguchi, M, et al. Malignant melanoma and acquired dermal melanocytosis on congenital nevus spilus. J Dermatol. 2005;32(6):454–458.
181. Kagami, S, Asahina, A, Watanabe, R, et al. Laser treatment of 26 Japanese patients with Mongolian spots. Dermatol Surg. 2008;34(12):1689–1694.
182. Kouba, DJ, Fincher, EF, Moy, RL. Nevus of Ota successfully treated by fractional photothermolysis using a fractionated 1440-nm Nd:YAG laser. Arch Dermatol. 2008;144(2):156–158.
183. Cole, HN, Jr., Hubler, WR, Lund, HZ. Persistent, aberrant mongolian spots. Arch Derm Syphilol. 1950;61(2):244–260.
184. Burkhart, CG, Gohara, A. Dermal melanocyte hamartoma. A distinctive new form of dermal melanocytosis. Arch Dermatol. 1981;117(2):102–104.
185. Mishima, Y, Mevorah, B. Nevus Ota and nevus Ito in American Negroes. J Invest Dermatol. 1961;36:133–154.
186. Elder, DE, Murphy, GF. Benign melanocytic tumors (nevi). Atlas of Tumor Pathology: Melanocytic Tumors of the Skin. Washington, DC: Armed Forces Institute of Pathology; 1991. [64-78].
187. Dorsey, CS, Montgomery, H. Blue nevus and its distinction from Mongolian spot and the nevus of Ota. J Invest Dermatol. 1954;22(3):225–236.
188. Lee, JY, Lee, JS, Kim, YC. Histopathological features of acquired dermal melanocytosis. Eur J Dermatol. 2010;20(3):345–348.
189. Denlinger, CE, Slingluff, CL, Jr., Mihm, MC, Jr., et al. Extensive neurocristic hamartoma with skeletal muscle involvement. J Cutan Pathol. 2007;34(8):634–639.
190. Bevona, C, Tannous, Z, Tsao, H. Dermal melanocytic proliferation with features of a plaque-type blue nevus and neurocristic hamartoma. J Am Acad Dermatol. 2003;49(5):924–929.
191. Tuthill, RJ, Clark, WH, Jr., Levene, A. Pilar neurocristic hamartoma: its relationship to blue nevus and equine melanotic disease. Arch Dermatol. 1982;118(8):592–596.
192. Pearson, JP, Weiss, SW, Headington, JT. Cutaneous malignant melanotic neurocristic tumors arising in neurocristic hamartomas. A melanocytic tumor morphologically and biologically distinct from common melanoma. Am J Surg Pathol. 1996;20(6):665–677.
193. Hendrickson, MR, Ross, JC. Neoplasms arising in congenital giant nevi: morphologic study of seven cases and a review of the literature. Am J Surg Pathol. 1981;5(2):109–135.
194. Mezebish, D, Smith, K, Williams, J, et al. Neurocristic cutaneous hamartoma: a distinctive dermal melanocytosis with an unknown malignant potential. Mod Pathol. 1998;11(6):573–578.
195. D’Argenio, A, David, P, Engohan, C, et al. Neurocutaneous melanosis in a newborn with giant congenital melanocytic nevus. J Neuroradiol. 2007;34(4):272–275.
196. Ingordo, V, Gentile, C, Iannazzone, SS, et al. Congenital melanocytic nevus: an epidemiologic study in Italy. Dermatology. 2007;214(3):227–230.
197. Castilla, EE, da Graca Dutra, M, Orioli-Parreiras, IM. Epidemiology of congenital pigmented naevi: I. Incidence rates and relative frequencies. Br J Dermatol. 1981;104(3):307–315.
198. Zayour, M, Lazova, R. Congenital melanocytic nevi. Clin Lab Med. 2011;31(2):267–280.
199. Sahin, S, Levin, L, Kopf, AW, et al. Risk of melanoma in medium-sized congenital melanocytic nevi: a follow-up study. J Am Acad Dermatol. 1998;39(3):428–433.
200. Swerdlow, AJ, English, JS, Qiao, Z. The risk of melanoma in patients with congenital nevi: a cohort study. J Am Acad Dermatol. 1995;32(4):595–599.
201. Rhodes, AR, Wood, WC, Sober, AJ, et al. Nonepidermal origin of malignant melanoma associated with a giant congenital nevocellular nevus. Plast Reconstr Surg. 1981;67(6):782–790.
202. Solomon, L, Eng, AM, Bene, M, et al. Giant congenital neuroid melanocytic nevus. Arch Dermatol. 1980;116(3):318–320.
203. Barnhill, RL, Fleischli, M. Histologic features of congenital melanocytic nevi in infants 1 year of age or younger. J Am Acad Dermatol. 1995;33(5 Pt 1):780–785.
204. Rhodes, AR, Silverman, RA, Harrist, TJ, et al. A histologic comparison of congenital and acquired nevomelanocytic nevi. Arch Dermatol. 1985;121(10):1266–1273.
205. Stenn, KS, Arons, M, Hurwitz, S. Patterns of congenital nevocellular nevi. A histologic study of thirty-eight cases. J Am Acad Dermatol. 1983;9(3):388–393.
206. Rhodes, AR, Sober, AJ, Day, CL, et al. The malignant potential of small congenital nevocellular nevi. An estimate of association based on a histologic study of 234 primary cutaneous melanomas. J Am Acad Dermatol. 1982;6(2):230–241.
207. Maize, JC, Ackerman, AB. Pigmented lesions of the skin. Clinicopathologic correlations. Philadelphia: Lea & Febiger; 1987.
208. Reed, WB, Becker, SW, Sr., Becker, SW, Jr., et al. Giant pigmented nevi, melanoma, and leptomeningeal melanocytosis: a clinical and histopathological study. Arch Dermatol. 1965;91:100–119.
209. Mancianti, ML, Clark, WH, Hayes, FA, et al. Malignant melanoma simulants arising in congenital melanocytic nevi do not show experimental evidence for a malignant phenotype. Am J Pathol. 1990;136(4):817–829.
210. Clark, WH, Jr., Ackerman, AB. An exchange of views regarding the dysplastic nevus controversy. Semin Dermatol. 1989;8(4):229–250.
211. Ackerman, AB, Milde, P. Naming acquired melanocytic nevi. Common and dysplastic, normal and atypical, or Unna, Miescher, Spitz, and Clark? Am J Dermatopathol. 1992;14(5):447–453.
212. Consensus conference: Precursors to malignant melanoma. JAMA. 1984;251(14):1864–1866.
213. Meyer, LJ, Piepkorn, M, Goldgar, DE, et al. Interobserver concordance in discriminating clinical atypia of melanocytic nevi, and correlations with histologic atypia. J Am Acad Dermatol. 1996;34(4):618–625.
214. Barnhill, RL, Roush, GC, Ernstoff, MS, et al. Interclinician agreement on the recognition of selected gross morphologic features of pigmented lesions. Studies of melanocytic nevi V. J Am Acad Dermatol. 1992;26(2 Pt 1):185–190.
215. Barnhill, RL, Roush, GC, Duray, PH. Correlation of histologic architectural and cytoplasmic features with nuclear atypia in atypical (dysplastic) nevomelanocytic nevi. Hum Pathol. 1990;21(1):51–58.
216. Clark, WH, Jr., Elder, DE, Guerry, DT, et al. A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Hum Pathol. 1984;15(12):1147–1165.
217. Elder, DE. The dysplastic nevus. Pathology. 1985;17(2):291–297.
218. Friedman, RJ, Heilman, ER, Rigel, DS, et al. The dysplastic nevus. Clinical and pathologic features. Dermatol Clin. 1985;3(2):239–249.
219. Group USCSW. United States Cancer Statistics: 1999-2008 Incidence and Mortality Web-based Report. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2012.
220. Thomas, L, Tranchand, P, Berard, F, et al. Semiological value of ABCDE criteria in the diagnosis of cutaneous pigmented tumors. Dermatology. 1998;197(1):11–17.
221. McClain, SE, Mayo, KB, Shada, AL, et al. Amelanotic melanomas presenting as red skin lesions: a diagnostic challenge with potentially lethal consequences. Int J Dermatol. 2012;51(4):420–426.
222. Wharton, JM, Carlson, JA, Mihm, MC, Jr. Desmoplastic malignant melanoma: diagnosis of early clinical lesions. Hum Pathol. 1999;30(5):537–542.
223. Rogers, GS, Kopf, AW, Rigel, DS, et al. Influence of anatomic location on prognosis of malignant melanoma: attempt to verify the BANS model. J Am Acad Dermatol. 1986;15(2 Pt 1):231–237.
224. Cascinelli, N, Vaglini, M, Bufalino, R, et al. BANS. A cutaneous region with no prognostic significance in patients with melanoma. Cancer. 1986;57(3):441–444.
225. Woods, JE, Taylor, WF, Pritchard, DJ, et al. Is the BANS concept for malignant melanoma valid? Am J Surg. 1985;150(4):452–455.
226. Ribuffo, D, Gradilone, A, Vonella, M, et al. Prognostic significance of reverse transcriptase-polymerase chain reaction-negative sentinel nodes in malignant melanoma. Ann Surg Oncol. 2003;10(4):396–402.
227. Guerry, DT, Synnestvedt, M, Elder, DE, et al. Lessons from tumor progression: the invasive radial growth phase of melanoma is common, incapable of metastasis, and indolent. J Invest Dermatol. 1993;100(3):342S–345S.
228. Clark, WH, Jr., Elder, DE, Van Horn, M. The biologic forms of malignant melanoma. Hum Pathol. 1986;17(5):443–450.
229. Tannous, ZS, Lerner, LH, Duncan, LM, et al. Progression to invasive melanoma from malignant melanoma in situ, lentigo maligna type. Hum Pathol. 2000;31(6):705–708.
230. Flotte, TJ, Mihm, MC, Jr. Lentigo maligna and malignant melanoma in situ, lentigo maligna type. Hum Pathol. 1999;30(5):533–536.
231. Clark, WH, Jr., Mihm, MC, Jr. Lentigo maligna and lentigo-maligna melanoma. Am J Pathol. 1969;55(1):39–67.
232. Heenan, PJ, Holman, CD. Nodular malignant melanoma: a distinct entity or a common end stage? Am J Dermatopathol. 1982;4(5):477–478.
233. Dwyer, PK, Mackie, RM, Watt, DC, et al. Plantar malignant melanoma in a white Caucasian population. Br J Dermatol. 1993;128(2):115–120.
234. Barnhill, RL, Mihm, MC, Jr. The histopathology of cutaneous malignant melanoma. Semin Diagn Pathol. 1993;10(1):47–75.
235. Krementz, ET, Feed, RJ, Coleman, WP, III., et al. Acral lentiginous melanoma. A clinicopathologic entity. Ann Surg. 1982;195(5):632–645.
236. Coleman, WP, III., Loria, PR, Reed, RJ, et al. Acral lentiginous melanoma. Arch Dermatol. 1980;116(7):773–776.
237. Barr, RJ. The many faces of completely regressed malignant melanoma. Pathology (Phila). 1994;2(2):359–370.
238. Piana, S, Valli, R, Ricci, C. Epidermotropic chondroid metastasis of melanoma: report of a case of metastatic melanoma with previously unreported morphological features. Am J Dermatopathol. 2009;31(3):301–304.
239. Bengoechea-Beeby, MP, Velasco-Oses, A, Mourino Fernandez, F, et al. Epidermotropic metastatic melanoma. Are the current histologic criteria adequate to differentiate primary from metastatic melanoma? Cancer. 1993;72(6):1909–1913.
240. Smoller, BR, McNutt, NS, Hsu, A. HMB-45 staining of dysplastic nevi. Support for a spectrum of progression toward melanoma. Am J Surg Pathol. 1989;13(8):680–684.
241. Moretti, S, Spallanzani, A, Chiarugi, A, et al. Correlation of Ki-67 expression in cutaneous primary melanoma with prognosis in a prospective study: different correlation according to thickness. J Am Acad Dermatol. 2001;44(2):188–192.
242. Al Dhaybi, R, Agoumi, M, Gagne, I, et al. p16 expression: a marker of differentiation between childhood malignant melanomas and Spitz nevi. J Am Acad Dermatol. 2011;65(2):357–363.
243. Mihic-Probst, D, Saremaslani, P, Komminoth, P, et al. Immunostaining for the tumour suppressor gene p16 product is a useful marker to differentiate melanoma metastasis from lymph-node nevus. Virchows Arch. 2003;443(6):745–751.
244. Lazova, R, Tantcheva-Poor, I, Sigal, AC. P75 nerve growth factor receptor staining is superior to S100 in identifying spindle cell and desmoplastic melanoma. J Am Acad Dermatol. 2010;63(5):852–858.
245. Aung, PP, Sarlomo-Rikala, M, Lasota, J, et al. KBA62 and PNL2: 2 new melanoma markers-immunohistochemical analysis of 1563 tumors including metastatic, desmoplastic, and mucosal melanomas and their mimics. Am J Surg Pathol. 2012;36(2):265–272.
246. Ramos-Herberth, FI, Karamchandani, J, Kim, J, et al. SOX10 immunostaining distinguishes desmoplastic melanoma from excision scar. J Cutan Pathol. 2010;37(9):944–952.
247. Breslow, A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172(5):902–908.
248. Barnhill, RL, Katzen, J, Spatz, A, et al. The importance of mitotic rate as a prognostic factor for localized cutaneous melanoma. J Cutan Pathol. 2005;32(4):268–273.
249. Guitart, J, Lowe, L, Piepkorn, M, et al. Histological characteristics of metastasizing thin melanomas: a case-control study of 43 cases. Arch Dermatol. 2002;138(5):603–608.
250. Cook, MG, Spatz, A, Brocker, EB, et al. Identification of histological features associated with metastatic potential in thin (<1.0 mm) cutaneous melanoma with metastases. A study on behalf of the EORTC Melanoma Group. J Pathol. 2002;197(2):188–193.
251. Clemente, CG, Mihm, MC, Jr., Bufalino, R, et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77(7):1303–1310.
252. Straume, O, Akslen, LA. Independent prognostic importance of vascular invasion in nodular melanomas. Cancer. 1996;78(6):1211–1219.
253. Petersson, F, Diwan, AH, Ivan, D, et al. Immunohistochemical detection of lymphovascular invasion with D2-40 in melanoma correlates with sentinel lymph node status, metastasis and survival. J Cutan Pathol. 2009;36(11):1157–1163.
254. Baer, SC, Schultz, D, Synnestvedt, M, et al. Desmoplasia and neurotropism. Prognostic variables in patients with stage I melanoma. Cancer. 1995;76(11):2242–2247.
255. Leon, P, Daly, JM, Synnestvedt, M, et al. The prognostic implications of microscopic satellites in patients with clinical stage I melanoma. Arch Surg. 1991;126(12):1461–1468.
256. Cohen, LM. The starburst giant cell is useful for distinguishing lentigo maligna from photodamaged skin. J Am Acad Dermatol. 1996;35(6):962–968.
257. Katz, SK, Guitart, J. Starburst giant cells in benign nevomelanocytic lesions. J Am Acad Dermatol. 1998;38(2 Pt 1):283.
258. Wiltz, KL, Qureshi, H, Patterson, JW, et al. Immunostaining for MART-1 in the interpretation of problematic intra-epidermal pigmented lesions. J Cutan Pathol. 2007;34(8):601–605.
259. Martin, RC, Murali, R, Scolyer, RA, et al. So-called “malignant blue nevus”: a clinicopathologic study of 23 patients. Cancer. 2009;115(13):2949–2955.
260. Lambert, WC, Brodkin, RH. Nodal and subcutaneous cellular blue nevi. A pseudometastasizing pseudomelanoma. Arch Dermatol. 1984;120(3):367–370.
261. Barnhill, RL, Argenyi, Z, Berwick, M, et al. Atypical cellular blue nevi (cellular blue nevi with atypical features): lack of consensus for diagnosis and distinction from cellular blue nevi and malignant melanoma (“malignant blue nevus”). Am J Surg Pathol. 2008;32(1):36–44.
262. McNutt, NS. “Triggered trap”: nevoid malignant melanoma. Semin Diagn Pathol. 1998;15(3):203–209.
263. Reed, RJ. Minimal deviation melanoma. In: Mihm MC, Murphy GF, Kaufman N, eds. Pathobiology and recognition of malignant melalanoma. Baltimore: Williams & Wilkins; 1988:110.
264. Levene, A. On the histological diagnosis and prognosis of malignant melanoma. J Clin Pathol. 1980;33(2):101–124.
265. Schmoeckel, C, Castro, CE, Braun-Falco, O. Nevoid malignant melanoma. Arch Dermatol Res. 1985;277(5):362–369.
266. Wong, TY, Suster, S, Duncan, LM, et al. Nevoid melanoma: a clinicopathological study of seven cases of malignant melanoma mimicking spindle and epithelioid cell nevus and verrucous dermal nevus. Hum Pathol. 1995;26(2):171–179.
267. McNutt, NS, Urmacher, C, Hakimian, J, et al. Nevoid malignant melanoma: morphologic patterns and immunohistochemical reactivity. J Cutan Pathol. 1995;22(6):502–517.
268. Zembowicz, A, McCusker, M, Chiarelli, C, et al. Morphological analysis of nevoid melanoma: a study of 20 cases with a review of the literature. Am J Dermatopathol. 2001;23(3):167–175.
269. Wong, TY, Duncan, LM, Mihm, MC, Jr. Melanoma mimicking dermal and Spitz’s nevus (“nevoid” melanoma). Semin Surg Oncol. 1993;9(3):188–193.
270. Cassarino, DS, Fullen, DR, Sondak, VK, et al. Metastatic nevoid melanoma in a 4 1/2-year-old child. J Cutan Pathol. 2003;30(10):647–651.
271. Fulciniti, F, Ascierto, PA, Simeone, E, et al. Nevoid melanoma of the vagina: report of one case diagnosed on thin layer cytological preparations. Cytojournal. 2007;4:14.
272. Kossard, S, Wilkinson, B. Small cell (naevoid) melanoma: a clinicopathologic study of 131 cases. Australas J Dermatol. 1997;38(Suppl 1):S54–S58.
273. Weedon, D. Lentigines, nevi, and melanomas. In: Weedon D, ed. Skin Pathology. London: Churchill Livingstone; 2002:828–829.
274. Barnhill, RL. Malignant melanoma. In: Barnhill RL, Piepkorn M, Busam KJ, eds. Pathology of Melanocytic Nevi and Malignant Melanoma. New York: Springer; 2004:303–311.
275. McKee, PH. Histological variants of melanoma. In: McKee PH, Calonje E, Granter SR, eds. Pathology of the Skin with Clinical Correlations. Philadelphia: Elsevier; 2005:1331–1333.
276. Kossard, S, Wilkinson, B. Nucleolar organizer regions and image analysis nuclear morphometry of small cell (nevoid) melanoma. J Cutan Pathol. 1995;22(2):132–136.
277. Mourmouras, V, Cevenini, G, Cosci, E, et al. Nucleolin protein expression in cutaneous melanocytic lesions. J Cutan Pathol. 2009;36(6):637–646.
278. Ito, K, Mihm, MC. Pigmented epithelioid melanocytoma: report of first Japanese cases previously diagnosed as cellular blue nevus. J Cutan Pathol. 2009;36(4):439–443.
279. Zembowicz, A, Knoepp, SM, Bei, T, et al. Loss of expression of protein kinase a regulatory subunit 1alpha in pigmented epithelioid melanocytoma but not in melanoma or other melanocytic lesions. Am J Surg Pathol. 2007;31(11):1764–1775.