Pauling learned the hardest way that the rules of biology are much more delicate than the rules of chemistry. You can nigh well abuse amino acids chemically and end up with the same bunch of agitated but intact molecules. The fragile and more complex proteins of a living creature will wilt under the same stress, be it heat, acid, or, worst of all, rogue elements. The most delinquent elements can exploit any number of vulnerabilities in living cells, often by masking themselves as life-giving minerals and micronutrients. And the stories of how ingeniously those elements undo life—the exploits of “poisoner’s corridor”—provide one of the darker subplots of the periodic table.
The lightest element in poisoner’s corridor is cadmium, which traces its notoriety to an ancient mine in central Japan. Miners began digging up precious metals from the Kamioka mines in ad 710. In the following centuries, the mountains of Kamioka yielded gold, lead, silver, and copper as various shoguns and then business magnates vied for the land. But not until twelve hundred years after striking the first lode did miners begin processing cadmium, the metal that made the mines infamous and the cry “Itai-itai!” a byword in Japan for suffering.
The Russo-Japanese War of 1904–1905 and World War I a decade later greatly increased Japanese demand for metals, including zinc, to use in armor, airplanes, and ammunition. Cadmium appears below zinc on the periodic table, and the two metals mix indistinguishably in the earth’s crust. To purify the zinc mined in Kamioka, miners probably roasted it like coffee and percolated it with acid, removing the cadmium. Following the environmental regulations of the day, they then dumped the leftover cadmium sludge into streams or onto the ground, where it leeched into the water table.
Today no one would think of dumping cadmium like that. It’s become too valuable as a coating for batteries and computer parts, to prevent corrosion. It also has a long history of use in pigments, tanning agents, and solders. In the twentieth century, people even used shiny cadmium plating to line trendy drinking cups. But the main reason no one would dump cadmium today is that it has rather horrifying medical connotations. Manufacturers pulled it from the trendy tankards because hundreds of people fell ill every year when acidic fruit juice, like lemonade, leached the cadmium from the vessel walls. And when rescue workers at Ground Zero after the September 11, 2001, terrorist attacks developed respiratory diseases, some doctors immediately suspected cadmium, among other substances, since the collapse of the World Trade Center towers had vaporized thousands of electronic devices. That assumption was incorrect, but it’s telling how reflexively health officials fingered element forty-eight.
Sadly, that conclusion was a reflex because of what happened a century ago near the Kamioka mines. As early as 1912, doctors there noticed local rice farmers being felled by awful new diseases. The farmers came in doubled over with joint and deep bone pain, especially the women, who accounted for forty-nine of every fifty cases. Their kidneys often failed, too, and their bones softened and snapped from the pressure of everyday tasks. One doctor broke a girl’s wrist while taking her pulse. The mystery disease exploded in the 1930s and 1940s as militarism overran Japan. Demand for zinc kept the ores and sludge pouring down the mountains, and although the local prefecture (the Japanese equivalent of a state) was removed from actual combat, few areas suffered as much during World War II as those around the Kamioka mines. As the disease crept from village to village, it became known as itai-itai, or “ouch-ouch,” disease, after the cries of pain that escaped victims.
Only after the war, in 1946, did a local doctor, Noboru Hagino, begin studying itai-itai disease. He first suspected malnutrition as the cause. This theory proved untenable by itself, so he switched his focus to the mines, whose high-tech, Western excavation methods contrasted with the farmers’ primitive paddies. With a public health professor’s help, Hagino produced an epidemiological map plotting cases of itai-itai. He also made a hydrological map showing where the Jinzu River—which ran through the mines and irrigated the farmers’ fields miles away—deposited its runoff. Laid over the top of each other, the two maps look almost identical. After testing local crops, Hagino realized that the rice was a cadmium sponge.
Painstaking work soon revealed the pathology of cadmium. Zinc is an essential mineral, and just as cadmium mixes with zinc in the ground, it interferes with zinc in the body by replacing it. Cadmium also sometimes evicts sulfur and calcium, which explains why it affected people’s bones. Unfortunately, cadmium is a clumsy element and can’t perform the same biological roles as the others. Even more unfortunately, once cadmium slips into the body, it cannot be flushed out. The malnutrition Hagino suspected at first also played a role. The local diet depended heavily on rice, which lacks essential nutrients, so the farmers’ bodies were starved of certain minerals. Cadmium mimicked those minerals well enough that the farmers’ cells, in dietary desperation, began to weave it into their organs at even higher rates than they otherwise would have.
Hagino went public with his results in 1961. Predictably and perhaps understandably, the mining company legally responsible, Mitsui Mining and Smelting, denied all wrongdoing (it had only bought the company that had done the damage). To its shame, Mitsui also campaigned to discredit Hagino. When a local medical committee formed to investigate itai-itai, Mitsui made sure that the committee excluded Hagino, the world expert on the disease. Hagino ran an end around by working on newfound cases of itai-itai in Nagasaki, which only bolstered his claims. Eventually, the conscience-stricken local committee, despite being stacked against Hagino, admitted that cadmium might cause the disease. Upon appeal of this wishy-washy ruling, a national government health committee, overwhelmed by Hagino’s evidence, ruled that cadmium absolutely causes itai-itai. By 1972, the mining company began paying restitution to 178 survivors, who collectively sought more than 2.3 billion yen annually. Thirteen years later, the horror of element forty-eight still retained such a hold on Japan that when filmmakers needed to kill off Godzilla in the then-latest sequel, The Return of Godzilla, the Japanese military in the film deployed cadmium-tipped missiles. Considering that an H-bomb had given Godzilla life, that’s a pretty dim view of this element.
Still, itai-itai disease was not an isolated incident in Japan last century. Three other times in the 1900s (twice with mercury, once with sulfur dioxide and nitrogen dioxide), Japanese villagers found themselves victims of mass industrial poisonings. These cases are known as the Big Four Pollution Diseases of Japan. In addition, thousands more suffered from radiation poisoning when the United States dropped a uranium and a plutonium bomb on the island in 1945. But the atomic bombs and three of the Big Four were preceded by the long-silent holocaust near Kamioka. Except it wasn’t so silent for the people there. “Itai-itai.”
Scarily, cadmium is not even the worst poison among the elements. It sits above mercury, a neurotoxin. And to the right of mercury sit the most horrific mug shots on the periodic table—thallium, lead, and polonium—the nucleus of poisoner’s corridor.
This clustering is partly coincidence, but there are legitimate chemical and physical reasons for the high concentration of poisons in the southeast corner. One, paradoxically, is that none of these heavy metals is volatile. Raw sodium or potassium, if ingested, would explode upon contact with every cell inside you, since they react with water. But potassium and sodium are so reactive they never appear in their pure, dangerous form in nature. The poisoner’s corridor elements are subtler and can migrate deep inside the body before going off. What’s more, these elements (like many heavy metals) can give up different numbers of electrons depending on the circumstances. For example, whereas potassium always reacts as K+, thallium can be Tl+ or Tl+3. As a result, thallium can mimic many elements and wriggle into many different biochemical niches.
That’s why thallium, element eighty-one, is considered the deadliest element on the table. Animal cells have special ion channels to vacuum up potassium, and thallium rides into the body via those channels, often by skin osmosis. Once inside the body, thallium drops the pretense of being potassium and starts unstitching key amino acid bonds inside proteins and unraveling their elaborate folds, rendering them useless. And unlike cadmium, thallium doesn’t stick in the bones or kidneys, but roams like a molecular Mongol horde. Each atom can do an outsized amount of damage.
For these reasons, thallium is known as the poisoner’s poison, the element for people who derive an almost aesthetic pleasure from lacing food and drinks with toxins. In the 1960s, a notorious British lad named Graham Frederick Young, after reading sensationalized accounts of serial killers, began experimenting on his family by sprinkling thallium into their teacups and stew pots. He was soon sent to a mental institution but was later, unaccountably, released, at which point he poisoned seventy more people, including a succession of bosses. Only three died, since Young made sure to prolong their suffering with less-than-lethal doses.
Young’s victims are hardly alone in history. Thallium has a gruesome record* of killing spies, orphans, and great-aunts with large estates. But rather than relive darker scenes, maybe it’s better to recall element eighty-one’s single foray into (admittedly morbid) comedy. During its Cuba-obsessed years, the Central Intelligence Agency hatched a plan to powder Fidel Castro’s socks with a sort of talcum powder tainted with thallium. The spies were especially tickled that the poison would cause all his hair, including his famous beard, to fall out, which they hoped would emasculate Castro in front of his comrades before killing him. There’s no record of why this plan was never attempted.
Another reason thallium, cadmium, and other related elements work so well as poisons is that they stick around for aeons. I don’t just mean they accumulate in the body, as cadmium does. Rather, like oxygen, these elements are likely to form stable, near-spherical nuclei that never go radioactive. Therefore, a fair amount of each still survives in the earth’s crust. For instance, the heaviest eternally stable element, lead, sits in box eighty-two, a magic number. And the heaviest almost-stable element, bismuth, is its neighbor, in box eighty-three.
Because bismuth plays a surprising role in poisoner’s corridor, this oddball element merits a closer look. Some quick bismuth facts: Though a whitish metal with a pinkish hue, bismuth burns with a blue flame and emits yellow fumes. Like cadmium and lead, bismuth has found widespread use in paints and dyes, and it often replaces “red lead” in the crackling fireworks known as dragon’s eggs. Also, of the nearly infinite number of possible chemicals you can make by combining elements on the periodic table, bismuth is one of the very few that expands when it freezes. We don’t appreciate how bizarre this is because of common ice, which floats on lakes while fish slide around below it. A theoretical lake of bismuth would behave the same way—but almost uniquely so on the periodic table, since solids virtually always pack themselves more tightly than liquids. What’s more, that bismuth ice would probably be gorgeous. Bismuth has become a favorite desktop ornament and decorative knickknack for mineralogists and element nuts because it can form rocks known as hopper crystals, which twist themselves into elaborate rainbow staircases. Newly frozen bismuth might look like Technicolor M. C. Escher drawings come to life.
Bismuth has helped scientists probe the deeper structure of radioactive matter as well. For decades, scientists couldn’t resolve conflicting calculations about whether certain elements would last until the end of time. So in 2003, physicists in France took pure bismuth, swaddled it in elaborate shields to block all possible outside interference, and wired detectors around it to try to determine its half-life, the amount of time it would take 50 percent of the sample to disintegrate. Half-life is a common measurement of radioactive elements. If a bucket of one hundred pounds of radioactive element X takes 3.14159 years to drop fifty pounds, then the half-life is 3.14159 years. After another 3.14159 years, you’d have twenty-five pounds. Nuclear theory predicted bismuth should have a half-life of twenty billion billion years, much longer than the age of the universe. (You could multiply the age of the universe by itself and get close to the same figure—and still have only a fifty-fifty shot of seeing any given bismuth atom disappear.) The French experiment was more or less a real-life Waiting for Godot. But amazingly, it worked. The French scientists collected enough bismuth and summoned enough patience to witness a number of decays. This result proved that instead of being the heaviest stable atom, bismuth will live only long enough to be the final element to go extinct.
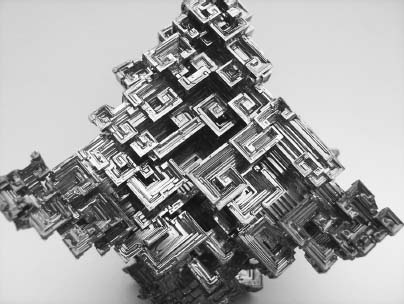
The wild, Technicolor swirls of a hopper crystal form when the element bismuth cools into a staircase crystalline pattern. This crystal spans the width of an adult hand. (Ken Keraiff, Krystals Unlimited)
(A similarly Beckettesque experiment is running right now in Japan to determine whether all matter will eventually disintegrate. Some scientists calculate that protons, the building blocks of elements, are ever-so-slightly unstable, with a half-life of at least 100 billion trillion trillion years. Undaunted, hundreds of scientists set up a huge underground pool of ultra-pure, ultra-still water deep inside a mineshaft, and they surrounded it with rings of hair-trigger sensors, in case a proton does split on their watch. This is admittedly unlikely, but it’s a far more benevolent use of the Kamioka mines than previously.)
It’s time to confess the full truth about bismuth, though. It’s technically radioactive, yes, and its coordinates on the periodic table imply that element eighty-three should be awful for you. It shares a column with arsenic and antimony, and it crouches among the worst heavy-metal poisons. Yet bismuth is actually benign. It’s even medicinal: doctors prescribe it to soothe ulcers, and it’s the “bis” in hot-pink Pepto-Bismol. (When people got diarrhea from cadmium-tainted lemonade, the antidote was usually bismuth.) Overall, bismuth is probably the most misplaced element on the table. That statement might cause chagrin among chemists and physicists who want to find mathematical consistency in the table. Really, it’s further proof that the table is filled with rich, unpredictable stories if you know where to look.
In fact, instead of labeling bismuth a freakish anomaly, you might consider it a sort of “noble metal.” Just as peaceful noble gases cleave the periodic table between two sets of violent—but differently violent—elements, pacific bismuth marks the transition of poisoner’s corridor from the conventional retching-and-deep-pain poisons discussed above to the scorching radioactive poisons described below.
Lurking beyond bismuth is polonium, the poisoner’s poison of the nuclear age. Like thallium, it makes people’s hair fall out, as the world discovered in November 2006 when Alexander Litvinenko, an ex–KGB agent, was poisoned by polonium in a London sushi restaurant. Past polonium (skipping over, for now, the ultra-rare element astatine) sits radon. As a noble gas, radon is colorless and odorless and reacts with nothing. But as a heavy element, it displaces air, sinks into the lungs, and discharges lethal radioactive particles that lead inevitably to lung cancer—just another way poisoner’s corridor can nip you.
Indeed, radioactivity dominates the bottom of the periodic table. It plays the same role the octet rule does for elements near the top: almost everything useful about heavy elements derives from how, and how quickly, they go radioactive. Probably the best way to illustrate this is through the story of a young American who, like Graham Frederick Young, grew obsessed with dangerous elements. But David Hahn wasn’t a sociopath. His disastrous adolescence sprang from a desire to help people. He wanted to solve the world’s energy crisis and break its addiction to oil so badly—as badly as only a teenager can want something—that this Detroit sixteen-year-old, as part of a clandestine Eagle Scout project gone berserk in the mid-1990s, erected a nuclear reactor in a potting shed in his mother’s backyard.*
David started off small, influenced by a book called The Golden Book of Chemistry Experiments, written in the same wincingly earnest tone as a 1950s reel-to-reel educational movie. He grew so excited about chemistry that his girlfriend’s mother forbade him to speak to guests at her parties because, in the intellectual equivalent of talking with his mouth full, he’d blurt out unappetizing facts about the chemicals in the food they were eating. But his interest wasn’t just theoretical. Like many prepubescent chemists, David quickly outgrew his box chemistry set, and he began playing with chemicals violent enough to blow his bedroom walls and carpet to hell. His mother soon banished him to the basement, then the backyard shed, which suited him fine. Unlike many budding scientists, though, David didn’t seem to get better at chemistry. Once, before a Boy Scout meeting, he dyed his skin orange when a fake tanning chemical he was working on burped and blew up in his face. And in a move only someone ignorant of chemistry would try, he accidentally exploded a container of purified potassium by tamping it with a screwdriver (a baaaad idea). An ophthalmologist was still digging plastic shards out of his eyes months later.
Even after that, the disasters continued, although, in his defense, David did take on increasingly complicated projects, like his reactor. To get started, he applied the little knowledge he’d gleaned about nuclear physics. This knowledge didn’t come from school (he was an indifferent, even a poor, student) but from the glowingly pro–nuclear energy pamphlets he wrote away for and from correspondence with government officials who believed sixteen-year-old “Professor Hahn’s” ruse about wanting to devise experiments for fictitious students.
Among other things, David learned about the three main nuclear processes—fusion, fission, and radioactive decay. Hydrogen fusion powers stars and is the most powerful and efficient process, but it plays little role in nuclear power on earth, since we can’t easily reproduce the temperatures and pressures needed to ignite fusion. David instead relied on uranium fission and the radioactivity of neutrons, which are by-products of fission. Heavier elements such as uranium have trouble keeping positive protons bound in their tiny nuclei, since identical charges repel, so they also pack in neutrons to serve as buffers. When a heavy atom fissions into two lighter atoms of roughly equal size, the lighter atoms require fewer neutron buffers, so they spit the excess neutrons out. Sometimes those neutrons are absorbed by nearby heavy atoms, which become unstable and spit out more neutrons in a chain reaction. In a bomb, you can just let that process happen. Reactors require more touch and control, since you want to string the fission out over a longer period. The main engineering obstacle David faced was that after the uranium atoms fission and release neutrons, the resulting lighter atoms are stable and cannot perpetuate the chain reaction. As a result, conventional reactors slowly die from lack of fuel.
Realizing this—and going obscenely far beyond the atomic energy merit badge he was originally seeking (really)—David decided to build a “breeder reactor,” which makes its own fuel through a clever combination of radioactive species. The reactor’s initial source of power would be pellets of uranium-233, which readily fissions. (The 233 means the uranium has 141 neutrons plus 92 protons; notice the excess of neutrons.) But the uranium would be surrounded with a jacket of a slightly lighter element, thorium-232. After the fission events, the thorium would absorb a neutron and become thorium-233. Unstable thorium-233 undergoes beta decay by spitting out an electron, and because charges always balance in nature, when it loses a negative electron, thorium also converts a neutron to a positive proton. This addition of a proton shifts it to the next element on the table, protactinium-233. This is also unstable, so the protactinium spits out another electron and transforms into what you started with, uranium-233. Almost magically, you get more fuel just by combining elements that go radioactive in the right way.
David pursued this project on weekends, since he lived only part-time with his mom after his parents’ divorce. For safety’s sake, he acquired a dentist’s lead apron to protect his organs, and anytime he spent a few hours in the backyard shed, he discarded his clothes and shoes. (His mom and stepdad later admitted that they’d noticed him throwing away good clothes and thought it peculiar. They just assumed David was smarter than they were and knew what he was doing.)
Of all the work he did, probably the easiest part of the project was finding the thorium-232. Thorium compounds have extremely high melting points, so they glow extra-bright when heated. They’re too dangerous for household lightbulbs, but in industrial settings, especially mines, thorium lamps are common. Instead of wire filaments as wicks, thorium lamps use small mesh nets called mantles, and David ordered hundreds of replacement mantles from a wholesaler, no questions asked. Then, showing improvement in his chemistry, he melted down the mantles into thorium ash with sustained heat from a blowtorch. He treated the ash with $1,000 worth of lithium he had obtained by cutting open batteries with wire cutters. Heating the reactive lithium and ash over a Bunsen burner purified the thorium, giving David a fine jacket for his reactor core.
Unfortunately, or perhaps fortunately, however well David learned radioactive chemistry, the physics escaped him. David first needed uranium-235 to irradiate the thorium and turn it, the thorium, into uranium-233. So he mounted a Geiger counter (a device that registers radioactivity with a click-click-click-click) on the dashboard of his Pontiac and cruised rural Michigan, as if he’d just stumble onto a uranium hot spot in the woods. But ordinary uranium is mostly uranium-238, which is a weak source of radioactivity. (Figuring out how to enrich ore by separating uranium-235 and uranium-238, which are chemically identical, was in fact a major achievement of the Manhattan Project.) David eventually scored some uranium ore from a sketchy supplier in the Czech Republic, but again it was ordinary, unenriched uranium, not the volatile kind. Eventually abandoning this approach, Hahn built a “neutron gun” to irradiate his thorium and get the uranium-233 kindling that way, but the gun barely worked.
A few sensational media stories later implied that David almost succeeded in building a reactor in the shed. In truth, he wasn’t close. The legendary nuclear scientist Al Ghiorso once estimated that David started with at least a billion billion times too little fissionable material. David certainly gathered dangerous materials and, depending on his exposure, might well have shortened his life span. But that’s easy. There are many ways to poison yourself with radioactivity. There are very, very few ways to harness those elements, with proper timing and controls, to get something useful from them.
Still, the police took no chances when they uncovered David’s plan. They found him late one night poking around a parked car and assumed he was a punk stealing tires. After detaining and harassing him, they searched his Pontiac, which he kindly but stupidly warned them was full of radioactive materials. They also found vials of strange powder and hauled him in for questioning. David was savvy enough not to mention the “hot” equipment in the potting shed, most of which he’d already dismantled anyway, scared that he was making too much progress and might leave a crater. While federal agencies wrangled about who was responsible for David—no one had tried to illegally save the world with nuclear power before—the case dragged on for months. In the meantime, David’s mother, fearing her house would be condemned, slipped into the laboratory shed one night and hauled almost everything in there to the trash. Months later, officials finally stormed across the neighbors’ backyards in hazmat gear to ransack the shed. Even then, the leftover cans and tools showed a thousand times more radioactivity than background levels.
Because he had no malevolent intentions (and September 11 hadn’t happened yet), David was mostly let off the hook. He did argue with his parents about his future, however, and after graduating from high school, he enlisted in the Navy, itching to work on nuclear submarines. Given David’s history, the Navy probably had no choice, but instead of letting him work on reactors, it put him on KP and had him swab decks. Unfortunately for him, he never got the chance to work on science in a controlled, supervised setting, where his enthusiasm and nascent talent might, who knows, have done some good.
The denouement of the story of the radioactive Boy Scout is sad. After leaving the military, David drifted back to his suburban hometown and bummed around without much purpose. After a few quiet years, in 2007 police caught him tampering with (actually stealing) smoke detectors from his own apartment building. With David’s record, this was a significant offense, since smoke detectors run on a radioactive element, americium. Americium is a reliable source of alpha particles, which can be channeled into an electric current inside detectors. Smoke absorbs the alpha particles, which disrupts the current and sets off the shrill alarm. But David had used americium to make his crude neutron gun, since alpha particles knock neutrons loose from certain elements. Indeed, he’d already been caught once, when he was a Boy Scout, stealing smoke detectors at a summer camp and had been kicked off the grounds.
In 2007, when his mug shot was leaked to the media, David’s cherubic face was pockmarked with red sores, as if he had acute acne and had picked every pimple until it bled. But thirty-one-year-old men usually don’t come down with acne. The inescapable conclusion was that he’d been reliving his adolescence with more nuclear experiments. Once again, chemistry fooled David Hahn, who never realized that the periodic table is rife with deception. It was an awful reminder that even though the heavy elements along the bottom of the table aren’t poisonous in the conventional way, the way that elements in poisoner’s corridor are, they’re devious enough to ruin a life.