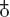 , became associated with the general symbol for “female.”
, became associated with the general symbol for “female.”Introduction
“literature, poison forensics, and psychology:” Another topic I learned about via mercury was meteorology. The final peal of the death knell of alchemy sounded on the day after Christmas in 1759, when two Russian scientists, trying to see how cold they could get a mixture of snow and acid, accidentally froze the quicksilver in their thermometer. This was the first recorded case of solid Hg, and with that evidence, the alchemists’ immortal fluid was banished to the realm of normal matter.
Lately mercury has been politicized as well, as activists in the United States have campaigned vigorously against the (totally unfounded) dangers of mercury in vaccines.
1. Geography Is Destiny
“anything but a pure element”: Two scientists observed the first evidence for helium (an unknown spectral line, in the yellow range) during an eclipse in 1868—hence the element’s name, from helios, Greek for “sun.” The element was not isolated on earth until 1895, through the careful isolation of helium from rocks. (For more on this, see chapter 17.) For eight years, helium was thought to exist on earth in minute quantities only, until miners found a huge underground cache in Kansas in 1903. They had tried to light the gas shooting out of a vent in the ground on fire, but it wouldn’t catch.
“only the electrons matter”: To reiterate the point about atoms being mostly empty space, Allan Blackman, a chemist at the University of Otago in New Zealand, wrote in the January 28, 2008, Otago Daily Times: “Consider the most dense known element, iridium; a sample of this the size of a tennis ball would weigh just over 3 kilograms [6.6 pounds]…. Let’s assume that we could somehow pack the iridium nuclei together as tight as we possibly could, thereby eliminating most of that empty space…. A tennis ball–sized sample of this compacted material would now weigh an astonishing seven trillion tonnes [7.7 trillion U.S. tons].”
As a footnote to this footnote, no one really knows whether iridium is the densest element. Its density is so close to osmium’s that scientists cannot distinguish between them, and in the past few decades they’ve traded places as king of the mountain. Osmium is on top at the moment.
“every quibbling error”: For more detailed portraits of Lewis and Nernst (and many other characters, such as Linus Pauling and Fritz Haber), I highly recommend Cathedrals of Science: The Personalities and Rivalries That Made Modern Chemistry by Patrick Coffey. It’s a personality-driven account of the most important era in modern chemistry, between about 1890 and 1930.
“most colorful history on the periodic table”: Other facts about antimony:
1. Much of our knowledge of alchemy and antimony comes from a 1604 book, The Triumphal Chariot of Antimony, written by Johann Thölde. To give his book a publicity boost, Thölde claimed he’d merely translated it from a 1450 text written by a monk, Basilius Valentinus. Fearing persecution for his beliefs, Valentinus had supposedly hidden the text in a pillar in his monastery. It remained hidden until a “miraculous thunderbolt” split the pillar in Thölde’s time and allowed him to discover the manuscript.
2. Although many did call antimony a hermaphrodite, others insisted it was the essence of femininity—so much so that a version
of the alchemical symbol for antimony, 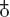 , became associated with the general symbol for “female.”
, became associated with the general symbol for “female.”
3. In the 1930s in China, a poor province made do with what it had and decided to make money from antimony, about the only local resource. But antimony is soft, easily rubbed away, and slightly toxic, all of which makes for poor coins, and the government soon withdrew them. Though worth just fractions of a cent then, these coins fetch thousands of dollars from collectors today.
2. Near Twins and Black Sheep
“really wrote Shakespeare’s plays”: A simpler but less colorful definition of honorificabilitudinitatibus is “with honorableness.” The Bacon anagram for the word is “Hi ludi, F. Baconis nati, tuiti orbi,” which translates to “These plays, born of F[rancis] Bacon, are preserved for the world.”
“anaconda runs 1,185 letters”: There’s some confusion over the longest word to appear in Chemical Abstracts. Many people list the tobacco mosaic virus protein, C785H1220N212O248S2, but a substantial number instead list “tryptophan synthetase α protein,” a relative of the chemical that people (wrongly) suppose makes them sleepy when they eat turkey (that’s an urban legend). The tryptophan protein, C1289H2051N343O375S8, runs 1,913 letters, over 60 percent longer than the mosaic virus protein, and numerous sources—some editions of Guinness World Records, the Urban Dictionary (www.urbandictionary.com), Mrs. Byrne’s Dictionary of Unusual, Obscure, and Preposterous Words—all list tryptophan as the champ. But after spending hours in the dimly lit stacks of the Library of Congress, I never located the tryptophan molecule in Chemical Abstracts. It just doesn’t seem to have appeared in its full, spelled-out form. To be doubly sure, I hunted down the academic paper that announced the decoding of the tryptophan protein (which was separate from the Chemical Abstracts listing), and there the authors chose to abbreviate the amino acid sequence. So its full name has never appeared in print as far as I can tell, which probably explains why Guinness later rescinded the listing for it as the longest word.
I did manage to track down listings for the mosaic virus, which is spelled out twice—first on page 967F of a brownish volume called Chemical Abstracts Formula Index, Jan.–June 1964, then on page 6717F of Chemical Abstracts 7th Coll. Formulas, C23H32–Z, 56–65, 1962–1966. Both books are compendiums that collect data for all the scholarly chemistry papers published between the dates on their covers. That means, contra other references to the world’s longest word (especially on the Web), the mosaic virus listing appeared only when those tomes came out in 1964 and 1966 and not in 1972.
There’s more: the tryptophan paper came out in 1964, and there are other molecules listed in that 1962–1966 Chemical Abstracts compendium with more Cs, Hs, Ns, Os, and Ss than the tobacco mosaic virus. So why aren’t they spelled out? Because those papers appeared after 1965, the year Chemical Abstracts Service, the company in Ohio that collects all this data, overhauled its system for naming new compounds and began discouraging excessively eye-glazing names. But so why did they bother spelling out the tobacco mosaic virus protein in a 1966 compendium? It could have been chopped down but was grandfathered in. And to throw in one more twist, the original 1964 tobacco mosaic virus paper was in German. But Chemical Abstracts is an English-language document, in the fine reference-work tradition of Samuel Johnson and the OED, and it printed the name not to show off but to propagate knowledge, so it sure counts.
Whew.
By the way, I owe Eric Shively, Crystal Poole Bradley, and especially Jim Corning at Chemical Abstracts Service a lot for helping me figure all this out. They didn’t have to field my confused questions (“Hi. I’m trying to find the longest word in English, and I’m not sure what it is…”), but they did.
Incidentally, on top of being the first virus discovered, the tobacco mosaic virus was the first to have its shape and structure analyzed in a rigorous way. Some of the best work in this area was done by Rosalind Franklin, the crystallography expert who generously but naively shared her data with Watson and Crick (see chapter 8). Oh, and the “α” in “tryptophan synthetase α protein” traces back to Linus Pauling’s work on how proteins know how to fold into the proper shape (see chapter 8 again).
“mercifully known as titin”: A few very patient souls have posted the entire amino acid sequence of titin online. Here are the stats: It occupies forty-seven single-spaced pages of a Microsoft Word document in Times New Roman 12-point font. It contains over 34,000 amino acids, and there are 43,781 occurrences of l; 30,710 of y; 27,120 of yl; and just 9,229 of e.
“almost a proof in itself”: From a PBS Frontline piece called “Breast Implants on Trial”: “The silicon content of living organisms decreases as the complexity of the organism rises. The ratio of silicon to carbon is 250:1 in the earth’s crust, 15:1 in humus soil [soil with organic matter], 1:1 in plankton, 1:100 in ferns, and 1:5,000 in mammals.”
“ ‘Bardeen was the brains of this joint organism and Brattain was the hands’ ”: The quote about Bardeen and Brattain being a joint organism comes from the PBS documentary Transistorized!
“a‘genius sperm bank’ ”: Shockley’s “genius sperm bank,” based in California, was officially called the Repository for Germinal Choice. He’s the only Nobel Prize winner to admit publicly that he donated, although the sperm bank’s founder, Robert K. Graham, claimed a number of others did, too.
“Nobel Prize for his integrated circuit”: For information on Kilby and the tyranny of numbers, see the wonderful book The Chip: How Two Americans Invented the Microchip and Launched a Revolution by T. R. Reid.
Oddly, a club DJ using the handle “Jack Kilby” released a CD in 2006 called Microchip EP, with a picture of a very old Kilby on the cover. It features the songs “Neutronium,” “Byte My Scarf,” “Integrated Circuit,” and “Transistor.”
3. The Galápagos of the Periodic Table
“the reality of atoms”: It might seem incredible to us today that Mendeleev refused to believe in atoms, but this was a not uncommon view among chemists at the time. They refused to believe in anything they couldn’t see with their own eyes, and they treated atoms as abstractions—a handy way of doing the accounting, maybe, but surely fictitious.
“at least in history’s judgment?”: The best description of the six scientists competing to form the first systematic arrangement of elements can be found in Eric Scerri’s The Periodic Table. Three other people are generally given credit for coinventing, or at least contributing to, the periodic system.
Alexandre-Emile Béguyer de Chancourtois, according to Scerri, discovered “the single most important step” in developing the periodic table—“that the properties of the elements are a periodic function of their atomic weights, a full seven years before Mendeleev arrived at the same conclusion.” De Chancourtois, a geologist, drew his periodic system on a spiral cylinder, like the thread of a screw. The possibility of his getting credit for the table was dashed when a publisher couldn’t figure out how to reproduce the crucial screw diagram showing all the elements. The publisher finally threw his hands up and printed the paper without it. Imagine trying to learn about the periodic table without being able to see it! Nonetheless, de Chancourtois’s cause as founder of the periodic system was taken up by his fellow Frenchman Lecoq de Boisbaudran, perhaps partly to get Mendeleev’s goat.
William Odling, an accomplished English chemist, seems to have been a victim of bad luck. He got many things right about the periodic table but is virtually forgotten today. Perhaps with his many other chemical and administrative interests, he simply got outworked by Mendeleev, who obsessed over the table. One thing Odling got wrong was the length of the periods of elements (the number of elements that have to appear before similar traits reappear). He assumed all the periods were of length eight, but that’s true only at the top of the table. Because of d-shells, rows three and four require a period of eighteen elements. Because of f-shells, rows five and six require thirty-two.
Gustavus Hinrichs was the only American on the list of codiscoverers (although he was not native-born) and the only one described as both a crank and a maverick genius ahead of his time. He published over three thousand scientific articles in four languages and pioneered the study and classification of elements with the light emissions that Bunsen discovered. He also played with numerology and developed a spiral-arm periodic table that placed many really tough elements in the correct groups. As Scerri sums him up, “The work of Hinrichs is so idiosyncratic and labyrinthine that a more complete study will be required before anyone can venture to pronounce on its real value.”
“Earl Grey ‘eats’ their utensils”: If you’re dying to see the gallium practical joke in action, you can see a spoon of gallium melting into nothing on YouTube. Oliver Sacks also talks about pulling pranks of this sort in Uncle Tungsten, a memoir of his boyhood.
“Streets are named for minerals and elements”: For some of the descriptions of the history and geology of Ytterby and for details about the town today, I consulted Jim Marshall, a chemist and historian at the University of North Texas, who was extremely generous with his time and help. He also sent me wonderful pictures. Jim is currently on a quest to revisit the spot where every element was first discovered, which is why he traveled to Ytterby (easy pickings). Good luck, Jim!
4. Where Atoms Come From
“proved by 1939”: One man who helped figure out the fusion cycles in stars, Hans Bethe, won a $500 prize for doing so, which he used to bribe Nazi officials and spring his mother and, oddly, her furniture from Germany.
“ ‘chemically peculiar stars’ ”: A fun factoid: Astronomers have identified a strange class of stars that manufacture promethium through an unknown process. The most famous is called Przybylski’s star. The truly odd thing is that unlike most fusion events deep inside stars, the promethium must be created on the star’s surface. Otherwise, it’s too radioactive and short-lived to survive the million-year crawl from the fusion-rich core of a star to its outer layers.
“stars govern the fate of mankind”: The two portentous Shakespeare quotes that opened the B2FH paper were as follows:
It is the stars, / The stars above us, govern our conditions.
King Lear, act 4, scene 3
The fault, dear Brutus, is not in our stars, / But in ourselves.
Julius Caesar, act 1, scene 2
“post-ferric fusion”: To be technical, stars don’t form iron directly. They first form nickel, element twenty-eight, by fusing two atoms of silicon, element fourteen, together. This nickel is unstable, however, and the vast majority of it decays to iron within a few months.
“low-watt, brownish light”: Jupiter could ignite fusion with deuterium—“heavy” hydrogen with one proton and one neutron—if it had thirteen times its current mass. Given the rarity of deuterium (1 out of every 6,500 hydrogen molecules), it would be a pretty weak star, but it would still count. To ignite regular hydrogen fusion, Jupiter would need seventy-five times its current mass.
“like microscopic cubes”: And not to be outdone by Jupiter’s or Mercury’s strange weather, Mars sometimes experiences hydrogen peroxide “snow.”
“a siderophile, or iron-loving element”: The siderophiles osmium and rhenium have also helped scientists reconstruct how the moon was formed from a cataclysmic impact between the very early earth and an asteroid or comet. The moon coalesced from the debris that was thrown up.
“later dubbed Nemesis”: The goddess Nemesis punished hubris. She made sure no earthly creature could ever grow too proud by striking down anyone who threatened to grow more powerful than the gods. The analogy to the sun’s companion star was that if earthly creatures (say, dinosaurs) evolved toward true intelligence, Nemesis would wipe them out before they got traction.
“like a carousel as it drifts”: Ironically, the overall motion of the sun, if viewed from afar, would resemble the old wheels-within-wheels cycles and epicycles that ancient astronomers bent backward trying to explain in their pre-Copernican, earth-centered cosmos (it’s just that earth cannot be called the center anymore, not by a long shot). Like Miescher and proteins, this is an example of the cyclical nature of all ideas, even in science.
5. Elements in Times of War
“went on to win the war”: For more details on the history of chemical warfare, especially the experience of American troops, see “Chemical Warfare in World War I: The American Experience, 1917–1918,” by Major Charles E. Heller, part of the Leavenworth Papers published by the Combat Studies Institute, U.S. Army Command and General Staff College, Fort Leavenworth, Kansas, http://www-cgsc.army.mil/carl/resources/csi/Heller/HELLER.asp.
“6.7 billion people today”: Among the many other things we can attribute to Fritz Haber’s ammonia: Charles Townes built the first working maser, the precursor of the laser, by using ammonia as the stimulating agent.
6. Completing the Table . . . with a Bang
“a full and correct list”: Urbain wasn’t the only person Moseley embarrassed. Moseley’s apparatus also dismantled Masataka Ogawa’s claim for discovering nipponium, element forty-three (see chapter 8).
“ ‘most irreparable crimes in history’ ”: For accounts of the bungling orders and battles that led to Moseley’s death, see The Making of the Atomic Bomb by Richard Rhodes. And actually, you should probably just read the whole thing, since it’s the best account of twentieth-century science history yet written.
“as ‘not good for much’ ”: The Time magazine article that mentioned the discovery of element sixty-one also included this tidbit about the question of what to name the element: “One convention wag suggested [naming it] grovesium, after loud-mouthed Major General Leslie R. Groves, military chief of the atom bomb project. Chemical symbol: Grr.”
“Pac-Man style”: Besides the electron-gobbling Pac-Man model of the nucleus, scientists at the time also developed the “plum pudding” model, in which electrons were embedded like raisins in a “pudding” of positive charge (Rutherford disproved this by proving that a compact nucleus existed). After the discovery of fission, scientists discovered the liquid drop model, in which large nuclei split like a drop of water on a surface splitting cleanly into two drops. Lise Meitner’s work was crucial in developing the liquid drop model.
“ ‘would go thermonuclear’ ”: The quotes from George Dyson can be found in his book Project Orion: The True Story of the Atomic Spaceship.
“ ‘methodological map’ ”: The quote about the Monte Carlo method being a “netherland at once nowhere and everywhere on the usual methodological map” appears in Peter Louis Galison’s Image and Logic.
7. Extending the Table, Expanding the Cold War
“ ‘Talk of the Town’ section”: The New Yorker item appeared in the April 8, 1950, issue and was written by E. J. Kahn Jr.
“the alarm one last time”: For more details about the experiments that led to elements 94 through 110, and for personal information about the man himself, see Glenn Seaborg’s autobiographies, especially Adventures in the Atomic Age (cowritten with his son Eric). The book is intrinsically interesting because Seaborg was at the center of so much important science and played such a large role in politics for decades. Honestly, though, Seaborg’s cautious writing style makes the book a bit bland at points.
“poisonous nickel smelters”: The information about the lack of trees around Norilsk comes from Time.com, which in 2007 named Norilsk one of the ten most polluted cities in the world. See http://www.time.com/time/specials/2007/article/0,28804,1661031_1661028_1661022,00.html.
“June 2009, copernicium (Cn)”: It covers a bit of the same material as here, but a story I wrote for Slate.com in June 2009 (“Periodic Discussions,” http://www.slate.com/id/2220300/) examines in detail why it took thirteen full years to promote copernicium from provisional element to full member of the periodic table.
8. From Physics to Biology
“they won forty-two”: Besides Segrè, Shockley, and Pauling, the other twelve scientists on the cover of Time were George Beadle, Charles Draper, John Enders, Donald Glaser, Joshua Lederberg, Willard Libby, Edward Purcell, Isidor Rabi, Edward Teller, Charles Townes, James Van Allen, and Robert Woodward.
The Time “Men of the Year” article contained the following words by Shockley on race. He meant them as complimentary, obviously, but his view on Bunche had to have sounded weird even at the time, and in retrospect it’s creepy. “William Shockley, 50, is that rare breed of scientist, a theorist who makes no apology for a consuming interest in the practical applications of his work. ‘Asking how much of a research job is pure and how much applied,’ says Shockley, ‘is like asking how much Negro and white blood Ralph Bunche might have. What’s important is that Ralph Bunche is a great man.’ ”
The article also shows that the legend about Shockley as the main inventor of the transistor was already firmly established:
Hired by Bell Telephone Laboratories right after he graduated from M.I.T. in 1936, theoretical physicist Shockley was one of a team that found a use for what had previously been a scientific parlor stunt: the use of silicon and germanium as a photoelectric device. Along with his partners, Shockley won a Nobel Prize for turning hunks of germanium into the first transistors, the educated little crystals that are fast replacing vacuum tubes in the country’s booming electronics industry.
“of all the damned luck, Ida Noddack”: Overall, Ida Noddack had a spotty run as a chemist. She helped find element seventy-five, but her group’s work with element forty-three was riddled with mistakes. She predicted nuclear fission years before anyone else, but about that same time, she began arguing that the periodic table was a useless relic, because the multiplication of new isotopes was rendering it unwieldy. It’s not clear why Noddack believed that each isotope was its own element, but she did, and she tried to convince others that they should scrap the periodic system.
“ ‘The reason for our blindness is not clear’ ”: The quote from Segrè about Noddack and fission comes from his biography Enrico Fermi: Physicist.
“a malfunctioning molecule”: Pauling (with colleagues Harvey Itano, S. Jonathan Singer, and Ibert Wells) determined that defective hemoglobin causes sickle-cell anemia by running defective cells through a gel in an electric field. Cells with healthy hemoglobin traveled one way in the electric field, while sickle cells moved in the opposite direction. This meant that the two types of molecules had opposite electric charges, a difference that could arise only on a molecular, atom-by-atom level.
Funnily enough, Francis Crick later cited the paper in which Pauling laid out his theory about the molecular basis of sickle-cell anemia as a major influence on him, since it was exactly the sort of nitty-gritty molecular biology that interested Crick.
“a molecular appendix”: Interestingly, biologists are slowly coming back around to their original view from Miescher’s day that proteins are the be-all and end-all of genetic biology. Genes occupied scientists for decades, and they’ll never really go away. But scientists now realize that genes cannot account for the amazing complexity of living beings and that far more is going on. Genomics was important fundamental work, but proteomics is where there’s real money to be made.
“DNA was”: To be scrupulous, the 1952 virus experiments with sulfur and phosphorus, conducted by Alfred Hershey and Martha Chase, were not the first to prove that DNA carries genetic information. That honor goes to work with bacteria done by Oswald Avery, published in 1944. Although Avery illuminated the true role of DNA, his work was not widely believed at first. People were beginning to accept it by 1952, but only after the Hershey-Chase experiments did people such as Linus Pauling really get involved in DNA work.
People often cite Avery—and Rosalind Franklin, who unwittingly told Watson and Crick that DNA was a double helix—as prime examples of people who got locked out of Nobel Prizes. That’s not quite accurate. Those two scientists never won, but both had died by 1958, and no one won a Nobel Prize for DNA until 1962. Had they still been alive, at least one of them might have shared in the spoils.
“James Watson and Francis Crick”: For primary documents related to Pauling and his competition with Watson and Crick, see the wonderful site set up by Oregon State University, which has archived and posted the contents of hundreds of personal papers and letters by Pauling and also produced a documentary history called “Linus Pauling and the Race for DNA” at http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/dna/index.html.
“before Pauling recovered”: After the DNA debacle, Ava Pauling, Linus’s wife, famously scolded him. Assuming that he would decipher DNA, Pauling had not broken much of a sweat on his calculations at first, and Ava lit into him: “If [DNA] was such an important problem, why didn’t you work harder at it?” Even so, Linus loved her deeply, and perhaps one reason he stayed at Cal Tech so long and never transferred his allegiance to Berkeley, even though the latter was a much stronger school at the time, was that one of the more prominent members of the Berkeley faculty, Robert Oppenheimer, later head of the Manhattan Project, had tried to seduce Ava, which made Linus furious.
“the Nobel Prize in Physics”: As one last punch in the gut, even Segrè’s Nobel Prize was later tainted by accusations (possibly unfounded) that he stole ideas while designing the experiments to discover the antiproton. Segrè and his colleague, Owen Chamberlain, acknowledged working with the combative physicist Oreste Piccioni on methods to focus and guide particle beams with magnets, but they denied that Piccioni’s ideas were of much use, and they didn’t list him as an author on a crucial paper. Piccioni later helped discover the antineutron. After Segrè and Chamberlain won the prize in 1959, Piccioni remained bitter about the slight for years and finally filed a $125,000 lawsuit against them in 1972—which a judge threw out not for lack of scientific standing but because it had been filed more than a decade after the fact.
From the New York Times obituary of Piccioni on April 27, 2002: “ ‘He’d break down your front door and tell you he’s got the best idea in the world,’ said Dr. William A. Wenzel, a senior scientist emeritus at Lawrence Berkeley National Laboratory who also worked on the antineutron experiment. ‘Knowing Oreste, he has a lot of ideas; he throws them out a dozen a minute. Some of them are good, some of them aren’t. Nevertheless, I felt he was a good physicist and he contributed to our experiment.’ ”
9. Poisoner’s Corridor
“a gruesome record”: People still die of thallium poisoning today. In 1994, Russian soldiers working at an old cold war weapons depot found a canister of white powder laced with this element. Despite not knowing what it was, they powdered their feet with it and blended it with their tobacco. A few soldiers reportedly even snorted it. All of them came down with a mysterious, entirely unforeseeable illness, and a few died. On a sadder note, two children of Iraqi fighter pilots died in early 2008 after eating a birthday cake laced with thallium. The motive for the poisoning was unclear, although Saddam Hussein had used thallium during his dictatorship.
“in his mother’s backyard”: Various newspapers in Detroit have tracked David Hahn over the years, but for the most detailed account of Hahn’s story, see Ken Silverstein’s article in Harper’s magazine, “The Radioactive Boy Scout” (November 1998). Silverstein later expanded the article into a book of the same name.
10. Take Two Elements, Call Me in the Morning
“a cheaper, lighter copper nose”: In addition to studying the crust around Brahe’s fake nose, the archaeologists who dug him up also found signs of mercury poisoning in his mustache—probably a result of his active research into alchemy. The usual story of Brahe’s demise is that he died of a ruptured bladder. One night at a dinner party with some minor royalty, Brahe drank too much, but he refused to get up and go to the bathroom because he thought leaving the table before his social superiors did would be rude. By the time he got home, hours later, he couldn’t pee any-more, and he died eleven excruciating days later. The story has become a legend, but it’s possible that mercury poisoning contributed as much or more to the astronomer’s death.
“are copper-coated”: The elemental compositions of U.S. coins: New pennies (since 1982) are 97.5 percent zinc but have a thin copper coating, to sterilize the part you touch. (Old pennies were 95 percent copper.) Nickels are 75 percent copper, the balance nickel. Dimes, quarters, and half-dollars are 91.67 percent copper, the balance nickel. Dollar coins (besides special-issue gold coins) are 88.5 percent copper, 6 percent zinc, 3.5 percent manganese, and 2 percent nickel.
“one-oared rowboats”: Some further facts about vanadium: Some creatures (no one knows why) use vanadium in their blood instead of iron, which turns their blood red or apple green. It can also turn human tongues green. When sprinkled into steel, vanadium greatly strengthens the alloy without adding much weight (much like molybdenum and tungsten; see chapter 5). In fact, Henry Ford once boomed: “Why, without vanadium there would be no automobiles!”
“forced to double up”: The bus metaphor for how electrons fill their shells one at a time until “someone” is absolutely forced to double up is one of the best in chemistry, both folksy and accurate. It originated with Wolfgang Pauli, who discovered the Pauli “exclusion principle” in 1925.
“surgical strikes without surgery”: Besides gadolinium, gold is often cited as the best hope for treating cancer. Gold absorbs infrared light that otherwise passes through the body, and grows extremely warm as it does so. Delivering gold-coated particles into tumor cells could allow doctors to fry the tumors without damaging the surrounding tissue. This method was invented by John Kanzius, a businessman and radio technician who underwent thirty-six rounds of chemotherapy for leukemia beginning in 2003. He felt so nauseated and beaten up by the chemo—and was so filled with despair at the sight of the children with cancer he encountered in his hospital—that he decided there had to be a better way. In the middle of the night, he came up with the idea of heating metal particles, and he built a prototype machine using his wife’s baking pans. He tested it by injecting half of a hot dog with a solution of dissolved metals and placing it in a chamber of intense radio waves. The tampered-with side of the hot dog fried, while the other half remained cold.
“selling it as a supplement”: In the May 2009 issue of Smithsonian, the article “Honorable Mentions: Near Misses in the Genius Department” describes one Stan Lindberg, a daringly experimental chemist who took it upon himself “to consume every single element of the periodic table.” The article notes, “In addition to holding the North American record for mercury poisoning, his gonzo account of a three-week ytterbium bender… (‘Fear and Loathing in the Lanthanides’) has become a minor classic.”
I spent a half hour hungrily trying to track down “Fear and Loathing in the Lanthanides” before realizing I’d been had. The piece is pure fiction. (Although who knows? Elements are strange creatures, and ytterbium might very well get you high.)
“self-administer ‘drugs’ such as silver once more”: Wired magazine ran a short news story in 2003 about the online reemergence of “silver health scams.” The money quote: “Meanwhile, doctors across the country have seen a surge in argyria cases. ‘In the last year and a half, I’ve seen six cases of silver poisoning from these so-called health supplements,’ said Bill Robertson, the medical director of the Seattle Poison Center. ‘They were the first cases I’d seen in fifty years of medical practice.’ ”
“only one handedness, or ‘chirality’ ”: It’s a bit of a stretcher to claim that people are exclusively left-handed on a molecular level. Even though all of our proteins are indeed left-handed, all of our carbohydrates, as well as our DNA, have a right-handed twist. Regardless, Pasteur’s main point remains: in different contexts, our bodies expect and can only process molecules of a specific handedness. Our cells would not be able to translate left-handed DNA, and if we were fed left-handed sugars, our bodies would starve.
“the boy lived”: Joseph Meister, the little boy Pasteur saved from rabies, ended up becoming the groundskeeper for the Pasteur Institute. Tragically, poignantly, he was still groundskeeper in 1940 when German soldiers overran France. When one officer demanded that Meister, the man with the keys, open up Pasteur’s crypt so that he, the officer, could view Pasteur’s bones, Meister committed suicide rather than be complicit in this act.
“by I. G. Farbenindustrie”: The company Domagk worked for, I. G. Farbenindustrie (IGF), would later become notorious around the world for manufacturing the insecticide Zyklon B, which the Nazis used to gas concentration camp prisoners (see chapter 5). The company was broken up shortly after World War II, and many of its directors faced war crimes charges at Nuremberg (United States v. Carl Krauch, et al.) for enabling the Nazi government in its aggressive war and mistreating prisoners and captured soldiers. IGF’s descendants today include Bayer and BASF.
“ ‘the chemistry of dead matter and the chemistry of living matter’ ”: Nevertheless, the universe seems to be chiral on other levels, too, from the subatomic to the supergalactic. The radioactive beta decay of cobalt-60 is an asymmetric process, and cosmologists have seen preliminary evidence that galaxies tend to rotate in counterclockwise spiral arms above our northern galactic pole and in clockwise spirals beneath Antarctica.
“the most notorious pharmaceutical of the twentieth century”: A few scientists recently reconstructed why thalidomide’s devastating effects slipped through clinical trials. For nitty-gritty molecular reasons, thalidomide doesn’t cause birth defects in litters of mice, and the German company that produced thalidomide, Grünenthal, did not follow up mouse trials with careful human trials. The drug was never approved for pregnant women in the United States because the head of the Food and Drug Administration, Frances Oldham Kelsey, refused to bow to lobbying pressure to push it through. In one of those curious twists of history, thalidomide is now making a comeback to treat diseases such as leprosy, where it’s remarkably effective. It’s also a good anticancer agent because it limits the growth of tumors by preventing new blood vessels from forming—which is also why it caused such awful birth defects, since embryos’ limbs couldn’t get the nutrients they needed to grow. Thalidomide still has a long road back to respectability. Most governments have strict protocols in place to make sure doctors do not give the drug to women of childbearing age, on the off chance that they might become pregnant.
“don’t know to make one hand or the other”: William Knowles unfolded the molecule by breaking a double bond. When carbon forms double bonds, it has only three “arms” coming out of it: two single bonds and a double. (There are still eight electrons, but they are shared over three bonds.) Carbon atoms with double bonds usually form triangular molecules, since a tricornered arrangement keeps its electrons as far apart as possible (120 degrees). When the double bond breaks, carbon’s three arms become four. In that case, the way to keep electrons as far apart as possible is not with a planar square but with a three-dimensional tetrahedron. (The vertices in a square are 90 degrees apart. In a tetrahedron, they’re 109.5 degrees apart.) But the extra arm can sprout above or below the molecule, which will in turn give the molecule either left- or right-handedness.
11. How Elements Deceive
“in underground particle accelerators”: A professor of mine from college once held me captive with a story about how a few people died from nitrogen asphyxiation in a particle accelerator at Los Alamos in the 1960s, under circumstances very similar to the NASA accident. After the deaths at Los Alamos, my professor added 5 percent carbon dioxide to the gaseous mixtures in the accelerators he worked on, as a safety measure. He later wrote to me, “Incidentally I did put it to the test about a year later, when one of our graduate student operators did exactly the same thing [i.e., forgot to pump the inert air out and let oxygenated air back in]. I entered the pressure vessel with it full of inert gas…. But not really, [because] by the time I got my shoulders through the hole I was already in desperation, panting due to ‘breathe more!’ commands from my breathing center.” Air is normally 0.03 percent CO2, so one breath of the doped air was about 167 times more potent.
“scales up very quickly to toxic”: To its shame and embarrassment, the U.S. government admitted in 1999 that it had knowingly exposed up to twenty-six thousand scientists and technicians to high levels of powdered beryllium, to the extent that hundreds of them developed chronic beryllium disease and related ailments. Most of the people poisoned worked in aerospace, defense, or atomic energy—industries the government decided were too important to arrest or impede, so it neither improved safety standards nor developed an alternative to beryllium. The Pittsburgh Post-Gazette ran a long and damning front-page exposé on Tuesday, March 30, 1999. It was titled “Decades of Risk,” but one of the subtitles captures the pith of the story better: “Deadly Alliance: How Industry and Government Chose Weapons over Workers.”
“and calcium”: However, scientists at the Monell Chemical Senses Center in Philadelphia believe that in addition to sweet, sour, salty, bitter, and savory (umami), humans have a separate, unique taste for calcium, too. They’ve definitely found it in mice, and some humans respond to calcium-enriched water as well. So what does calcium taste like? From an announcement about the findings: “ ‘Calcium tastes calciumy,’ [lead scientist Michael] Tordoff said. ‘There isn’t a better word for it. It is bitter, perhaps even a little sour. But it’s much more because there are actual receptors for calcium.’ ”
“like so much sand”: Sour taste buds can also go flat. These taste buds respond mostly to the hydrogen ion, H+, but in 2009 scientists discovered that they can taste carbon dioxide as well. (CO2 combines with H2O to make a weak acid, H2CO3, so perhaps that’s why these taste buds perk up.) Doctors discovered this because some prescription drugs, as a side effect, suppress the ability to taste carbon dioxide. The resulting medical condition is known as the “champagne blues,” since all carbonated beverages taste flat.
12. Political Elements
“killed Pierre”: Pierre might not have lived long anyway. In a poignant memory, Rutherford once recalled watching Pierre Curie do an astounding glow-in-the-dark experiment with radium. But in the feeble green glow, the alert Rutherford noticed scars covering Pierre’s swollen, inflamed fingers and saw how difficult it was for him to grasp and manipulate a test tube.
“her rocky personal life”: For more details about the Curies especially, see Sheilla Jones’s wonderful book The Quantum Ten, an account of the surprisingly contentious and fractious early days of quantum mechanics, circa 1925.
“pre-seeped bottles of radium and thorium water”: The most famous casualty of the radium craze was the steel tycoon Eben Byers, who drank a bottle of Radithor’s radium water every day for four years, convinced it would provide him with something like immortality. He ended up wasting away and dying from cancer. Byers wasn’t any more fanatical about radioactivity than a lot of people; he simply had the means to drink as much of the water as he wished. The Wall Street Journal commemorated his death with the headline, “The Radium Water Worked Fine Until His Jaw Came Off.”
“its spot on the table”: For the true story of hafnium’s discovery, see Eric Scerri’s The Periodic Table, a thorough and superbly documented account of the rise of the periodic system, including the often strange philosophies and worldviews of the people who founded it.
“special ‘heavy’ water”: Hevesy performed heavy-water experiments on goldfish as well as himself, and he ended up killing a number of them.
Gilbert Lewis also used heavy water in a last-ditch effort to win the Nobel Prize in the early 1930s. Lewis knew that Harold Urey’s discovery of deuterium—heavy hydrogen with an extra neutron—would win the Nobel Prize, as did every other scientist in the world, including Urey. (After a mostly lackluster career that included ridicule from his in-laws, he came home right after discovering deuterium and told his wife, “Honey, our troubles are over.”)
Lewis decided to hitch himself to this no-miss prize by investigating the biological effects of water made with heavy hydrogen. Others had the same idea, but Berkeley’s physics department, headed by Ernest O. Lawrence, happened to have the world’s largest supply of heavy water, quite by accident. The team had a tank of water it had been using for years in radioactivity experiments, and the tank had a relatively high concentration of heavy water (a few ounces). Lewis begged Lawrence to let him purify the heavy water, and Lawrence agreed—on the condition that Lewis give it back after his experiments, since it might prove important in Lawrence’s research, too.
Lewis broke his promise. After isolating the heavy water, he decided to give it to a mouse and see what happened. One queer effect of heavy water is that, like ocean water, the more you drink, the more throat-scratchingly thirsty you feel, since the body cannot metabolize it. Hevesy ingested heavy water in trace amounts, so his body really didn’t notice, but Lewis’s mouse gulped all the heavy water in a few hours and ended up dead. Killing a mouse was hardly a Nobel Prize–worthy exercise, and Lawrence went apoplectic when he learned a lousy rodent had peed away all his precious heavy water.
“blocked him for personal reasons”: Kazimierz Fajans’s son Stefan Fajans, now a professor emeritus of internal medicine at the University of Michigan’s medical school, kindly supplied information to me in an e-mail:
In 1924 I was six years old, but either then and certainly in the years to follow I did hear from my father of some aspects of the Nobel Prize story. That a Stockholm newspaper published a headline “K. Fajans to Receive Nobel Prize” (I do not know whether it was in chemistry or physics) is not rumor but fact. I remember seeing a copy of that newspaper. I also remember seeing in that newspaper a photo of my father walking in front of a building in Stockholm (probably taken earlier) in somewhat formal dress but not [formal] for that time…. What I did hear was that an influential member of the committee blocked the award to my father for personal reasons. Whether that was rumor or fact is impossible to know unless someone could look at the minutes of these meetings. I believe they are secret. I do know as a fact that my father expected to receive the Nobel Prize as intimated to him by some people in the know. He expected to receive it in the years to follow…. but it never happened, as you know.
“ ‘protactinium’ stuck”: Meitner and Hahn actually named their element “protoactinium,” and only in 1949 did scientists shorten it by removing the extra o.
“ ‘disciplinary bias, political obtuseness, ignorance, and haste’ ”: There’s a wonderful dissection of Meitner, Hahn, and the awarding of the Nobel Prize in the September 1997 issue of Physics Today (“A Nobel Tale of Postwar Injustice” by Elisabeth Crawford, Ruth Lewin Sime, and Mark Walker). The article is the source of the quote about Meitner losing the prize because of “disciplinary bias, political obtuseness, ignorance and haste.”
“the peculiar rules for naming elements”: Once a name has been proposed for an element, the name gets only one shot at appearing on the periodic table. If the evidence for the element falls apart, or if the international governing body of chemistry (IUPAC) rules against an element’s name, it is blacklisted. This might feel satisfying in the case of Otto Hahn, but it also means that no one can ever name an element “joliotium” after Irène or Frédéric Joliot-Curie, since “joliotium” was once an official candidate name for element 105. It’s unclear whether “ghiorsium” has another shot. Perhaps “alghiorsium” would work, although IUPAC frowns on using first and last names, and in fact once rejected “nielsbohrium” in favor of plain “bohrium” for element 107—a decision that didn’t please the West German team that discovered 107, since “bohrium” sounds too much like boron and barium.
13. Elements as Money
“in Colorado in the 1860s”: The fact that gold-tellurium compounds were discovered in the mountains of Colorado is reflected in the name of a local mining town, Telluride, Colorado.
“It’s called fluorescence”: To clarify some easily (and often) confused terms, “luminescence” is the umbrella term for a substance absorbing and emitting light. “Fluorescence” is the instantaneous process described in this chapter. “Phosphorescence” is similar to fluorescence—it consists of molecules absorbing high-frequency light and emitting low-frequency light—but phosphorescing molecules absorb light like a battery and continue to glow long after the light shuts off. Obviously, both fluorescence and phosphorescence derive from elements on the periodic table, fluorine and phosphorus, the two most prominent elements in the molecules that first exhibited these traits to chemists.
“the silicon semiconductor revolution eighty years later”: Moore’s law says that the number of silicon transistors on a microchip will double every eighteen months—amazingly, it has held true since the 1960s. Had the law held for aluminium, Alcoa would have been producing 400,000 pounds of aluminium per day within two decades of starting up, not just 88,000. So aluminium did well, but not quite well enough to beat its neighbor on the periodic table.
“Alcoa shares worth $30 million”: There’s some discrepancy about the magnitude of Charles Hall’s wealth at his death. Thirty million dollars is the high end of the range. The confusion may be because Hall died in 1914 but his estate was not settled until fourteen years later. One-third of his estate went to Oberlin College.
“spelling disagreement”: Aside from differences between languages, other spelling discrepancies within a language occur with cesium, which the British tend to spell “caesium,” and sulfur, which many people still spell “sulphur.” You could make a case that element 110 should be spelled mendeleevium, not mendelevium, and that element 111 should be spelled röntgenium, not roentgenium.
14. Artistic Elements
“Sybille Bedford could write”: The Sybille Bedford quote comes from her novel A Legacy.
“a hobby”: Speaking of strange hobbies, I can’t not share this in a book full of quirky stories about elements. This anagram won the Special Category prize for May 1999 at the Web site Anagrammy.com, and as far as I’m concerned, this “doubly-true anagram” is the word puzzle of the millennium. The first half equates thirty elements on the periodic table with thirty other elements:
hydrogen + zirconium + tin + oxygen + rhenium + platinum + tellurium + terbium + nobelium + chromium + iron + cobalt + carbon + aluminum + ruthenium + silicon + ytterbium + hafnium + sodium + selenium + cerium + manganese + osmium + uranium + nickel + praseodymium + erbium + vanadium + thallium + plutonium = nitrogen + zinc + rhodium + helium + argon + neptunium + beryllium + bromine + lutetium + boron + calcium + thorium + niobium + lanthanum + mercury + fluorine + bismuth + actinium + silver + cesium + neodymium + magnesium + xenon + samarium + scandium + europium + berkelium + palladium + antimony + thulium
That’s pretty amazing, even if the number of ium endings mitigated the difficulty a little. The kicker is that if you replace each element with its atomic number, the anagram still balances.
1 + 40 + 50 + 8 + 75 + 78 + 52 + 65 + 102 + 24 + 26 + 27 + 6 + 13 + 44 + 14 + 70 + 72 + 11 + 34 + 58 + 25 + 76 + 92 + 28 + 59 + 68 + 23 + 81 + 94 = 7 + 30 + 45 + 2 + 18 + 93 + 4 + 35 + 71 + 5 + 20 + 90 + 41 + 57 + 80 + 9 + 83 + 89 + 47 + 55 + 60 + 12 + 54 + 62 + 21 + 63 + 97 + 46 + 51 + 69 = 1416
As the anagram’s author, Mike Keith, said, “This is the longest doubly-true anagram ever constructed (using the chemical elements—or any other set of this type, as far as I know).”
Along these lines, there’s also Tom Lehrer’s incomparable song “The Elements.” He adapted the tune from Gilbert and Sullivan’s “I Am the Very Model of a Modern Major-General,” and in it he names every element on the periodic table in a brisk eighty-six seconds. Check it out on YouTube: “There’s antimony, arsenic, aluminum, selenium…”
“ ‘Plutonists’ ”: Plutonists were sometimes called Vulcanists, too, after the fire god Vulcan. This moniker emphasized the role of volcanoes in the formation of rocks.
“Döbereiner’s pillars”: Döbereiner called his groupings of elements not triads but affinities, part of his larger theory of chemical affinities—a term that gave Goethe (who frequently attended Döbereiner’s lectures at Jena) the inspiration for the title Elective Affinities.
“inches close to majesty”: Another majestic design inspired by elements is the wooden Periodic Table Table, a coffee table built by Theodore Gray. The table has more than one hundred slots on top, in which Gray has stored samples of every extant element, including many exclusively man-made ones. Of course, he has only minute quantities of some. His samples of francium and astatine, the two rarest natural elements, are actually hunks of uranium. Gray’s argument is that somewhere buried deep inside those hunks are at least a few atoms of each one, which is true and honestly about as good as anyone has ever done. Besides, since most of the elements on the table are gray metals, it’s hard to tell them apart anyway.
“ruthenium began capping every Parker 51 in 1944”: For the details about the metallurgy of the Parker 51, see “Who Was That Man?” by Daniel A. Zazove and L. Michael Fultz, which appeared in the fall 2000 issue of Pennant, the house publication of the Pen Collectors of America. The article is a wonderful instance of dedicated amateur history—of keeping alive an obscure but charming bit of Americana. Other resources for Parker pen information include Parker51.com and Vintagepens.com.
The famed tip on the Parker 51 was actually 96 percent ruthenium and 4 percent iridium. The company advertised the nibs as being made of super-durable “plathenium,” presumably to mislead competitors into thinking that expensive platinum was the key.
“which Remington turned around and printed anyway”: The text of the letter Twain sent to Remington (which the company printed verbatim) is as follows:
GENTLEMEN: Please do not use my name in any way. Please do not even divulge the fact that I own a machine. I have entirely stopped using the Type-Writer, for the reason that I never could write a letter with it to anybody without receiving a request by return mail that I would not only describe the machine, but state what progress I had made in the use of it, etc., etc. I don’t like to write letters, and so I don’t want people to know I own this curiosity-breeding little joker.
Yours truly,
Saml. L. Clemens
15. An Element of Madness
“pathological science”: Credit for the phrase “pathological science” goes to chemist Irving Langmuir, who gave a speech about it in the 1950s. Two interesting notes on Langmuir: He was the younger, brighter colleague whose Nobel Prize and impudence at lunch might have driven Gilbert Lewis to kill himself (see chapter 1). Later in life, Langmuir grew obsessed with controlling the weather by seeding clouds—a muddled process that skirted awfully close to becoming a pathological science itself. Not even the great ones are immune.
In writing this chapter, I departed somewhat from Langmuir’s description of pathological science, which was rather narrow and legalistic. Another take on the meaning of pathological science comes from Denis Rousseau, who wrote a top-rate article called “Case Studies in Pathological Science” for American Scientist in 1992. However, I’m also departing from Rousseau, mostly to include sciences such as paleontology that aren’t as data driven as other, more famous cases of pathological science.
“Philip died at sea”: Philip Crookes, William’s brother, died on a vessel laying some of the first transatlantic cables for telegraph lines.
“supernatural forces”: William Crookes had a mystical, pantheistic, Spinozistic view of nature, in which everything partakes of “one sole kind of matter.” This perhaps explains why he thought he could commune with ghosts and spirits, since he was part of the same material. If you think about it, though, this view is quite odd, since Crookes made a name for himself discovering new elements—which by definition are different forms of matter!
“manganese and the megalodon”: For more details on the link between the megalodon and manganese, see Ben S. Roesch, who published an article evaluating how unfeasible it is to think that the megalodon survived in The Cryptozoology Review (what a word—“cryptozoology”!) in the autumn of 1998 and revisited the topic in 2002.
“The pathology started with the manganese”: In another strange link between the elements and psychology, Oliver Sacks notes in Awakenings that an overdose of manganese can damage the human brain and cause the same sort of Parkinson’s disease that he treated in his hospital. It’s a rare cause of Parkinson’s, to be sure, and doctors don’t quite understand why this element targets the brain instead of, like most toxic elements, going after other vital organs.
“a dozen African bull elephants”: The bull elephant calculation works as follows. According to the San Diego Zoo, the hugest elephant ever recorded weighed approximately 24,000 pounds. Humans and elephants are made of the same basic thing, water, so their densities are the same. To figure out the relative volume if humans had the appetite of palladium, we can therefore just multiply the weight of a 250-pound man by 900 and divide that number (225,000) by the weight of an elephant. That gives 9.4 elephants swallowed. But remember, that was the biggest elephant ever, standing thirteen feet at the shoulders. The weight of a normal bull elephant is closer to 18,000 pounds, which gives about a dozen swallowed.
“a better, more concise description of pathological science”: David Goodstein’s article on cold fusion was titled “Whatever Happened to Cold Fusion?” It appeared in the fall 1994 issue of the American Scholar.
16. Chemistry Way, Way Below Zero
“proved an easier thing to blame”: The theory that tin leprosy doomed Robert Falcon Scott seems to have originated in a New York Times article, although the article floated the theory that what failed was the tins themselves (i.e., the containers) in which Scott’s team stored food and other supplies. Only later did people start to blame the disintegration of tin solder. There’s an incredibly wide variation, too, in what historians claim that he used for solder, including leather seals, pure tin, a tin-lead mixture, and so on.
“and go roaming”: Plasma is actually the most common form of matter in the universe, since it’s the major constituent of stars. You can find plasmas (albeit very cold ones) in the upper reaches of the earth’s atmosphere, where cosmic rays from the sun ionize isolated gas molecules. These rays help produce the eerie natural light shows known as the aurora borealis in the far north. Such high-speed collisions also produce antimatter.
“blends of two states”: Other colloids include jelly, fog, whipped cream, and some types of colored glass. The solid foams mentioned in chapter 17, in which a gas phase is interspersed throughout a solid, are also colloids.
“with xenon in 1962”: Bartlett performed the crucial experiment on xenon on a Friday, and the preparation took him the entire day. By the time he broke the glass seal and saw the reaction take place, it was after 7:00 p.m. He was so keyed up that he burst into the hallway in his lab building and began yelling for colleagues. All of them had already gone home for the weekend, and he had to celebrate alone.
“Schrieffer”: In a macabre late-life crisis, one of the BCS trio, Schrieffer, killed two people, paralyzed another, and injured five more in a horrific car accident on a California highway. After nine speeding tickets, the seventy-four-year-old Schrieffer had had his license suspended, but he decided to drive his new Mercedes sports car from San Francisco to Santa Barbara anyway, and had revved his speed well into the triple digits. Despite his speed, he somehow managed to fall asleep at the wheel and slammed into a van at 111 mph. He was going to be sentenced to eight months in a county jail until the victims’ families testified, at which point the judge said that Schrieffer “need[ed] a taste of state prison.” The Associated Press quoted his erstwhile colleague Leon Cooper muttering in disbelief: “This is not the Bob I worked with…. This is not the Bob that I knew.”
“almost”: Now, to back off my rigid stance a little, there are a few good reasons why many people conflate the uncertainty principle with the idea that measuring something changes what you’re trying to measure—the so-called observer effect. Light photons are about the tiniest tools scientists have to probe things, but photons aren’t that much smaller than electrons, protons, or other particles. So bouncing photons off them to measure the size or speed of particles is like trying to measure the speed of a dump truck by crashing a Datsun into it. You’ll get information, sure, but at the cost of knocking the dump truck off course. And in many seminal quantum physics experiments, observing a particle’s spin or speed or position does alter the reality of the experiment in a spooky way. However, while it’s fair to say you have to understand the uncertainty principle to understand any change taking place, the cause of the change itself is the observer effect, a distinct phenomenon.
Of course, it seems likely that the real reason people conflate the two is that we as a society need a metaphor for changing something by the act of observing it, and the uncertainty principle fills that need.
“than the ‘correct’ theory”: Bose’s mistake was statistical. If you wanted to figure the odds of getting one tail and one head on two coin flips, you could determine the correct answer (one-half) by looking at all four possibilities: HH, TT, TH, and HT. Bose basically treated HT and TH as the same outcome and therefore got an answer of one-third.
“the 2001 Nobel Prize”: The University of Colorado has an excellent Web site dedicated to explaining the Bose-Einstein condensate (BEC), complete with a number of computer animations and interactive tools: http://www.colorado.edu/physics/2000/bec/.
Cornell and Wieman shared their Nobel Prize with Wolfgang Ketterle, a German physicist who also created the BEC not long after Cornell and Wieman and who helped explore its unusual properties.
Unfortunately, Cornell almost lost the chance to enjoy his life as a Nobel Prize winner. A few days before Halloween in 2004, he was hospitalized with the “flu” and an aching shoulder, and he then slipped into a coma. A simple strep infection had metastasized into necrotizing fasciitis, a severe soft tissue infection often referred to as flesh-eating bacteria. Surgeons amputated his left arm and shoulder to halt the infection, but it didn’t work. Cornell remained half-alive for three weeks, until doctors finally stabilized him. He has since made a full recovery.
17. Spheres of Splendor
“to study blinking bubbles full-time”: Putterman wrote about falling in love with sonoluminescence and his professional work on the subject in the February 1995 issue of Scientific American, the May 1998 issue of Physics World, and the August 1999 issue of Physics World.
“bubble science had a strong enough foundation”: One theoretical breakthrough in bubble research ended up playing an interesting role in the 2008 Olympics in China. In 1993, two physicists at Trinity University in Dublin, Robert Phelan and Denis Weaire, figured out a new solution to the “Kelvin problem”: how to create a bubbly foam structure with the least surface area possible. Kelvin had suggested creating a foam of polygonal bubbles, each of which had fourteen sides, but the Irish duo outdid him with a combination of twelve- and fourteen-sided polygons, reducing the surface area by 0.3 percent. For the 2008 Olympics, an architectural firm drew on Phelan and Weaire’s work to create the famous “box of bubbles” swimming venue (known as the Water Cube) in Beijing, which hosted Michael Phelps’s incredible performance in the pool.
And lest we be accused of positive bias, another active area of research these days is “antibubbles.” Instead of being thin spheres of liquid that trap some air (as bubbles are), antibubbles are thin spheres of air that trap some liquid. Naturally, instead of rising, antibubbles sink.
18. Tools of Ridiculous Precision
“calibrate the calibrators”: The first step in requesting a new calibration for a country’s official kilogram is faxing in a form (1) detailing how you will transport your kilogram through airport security and French customs and (2) clarifying whether you want the BIPM to wash it before and after it has done the measurements. Official kilograms are washed in a bath of acetone, the basic ingredient in fingernail polish remover, then patted dry with lint-free cheesecloth. After the initial washing and after each handling, the BIPM team lets the kilogram stabilize for a few days before touching it again. With all the cleaning and measuring cycles, calibration can easily drag on for months.
The United States actually has two platinum-iridium kilograms, K20 and K4, with K20 being the official copy simply because it has been in the United States’ possession longer. The United States also has three all-but-official copies made of stainless steel, two of which NIST acquired within the past few years. (Being stainless steel, they are larger than the dense platinum-iridium cylinders.) Their arrival, coupled with the security headache of flying the cylinders around, explains why Zeina Jabbour isn’t in any hurry to send K20 over to Paris: comparing it to the recently calibrated steel cylinders is almost as good.
Three times in the past century, the BIPM has summoned all the official national kilograms in the world to Paris for a mass calibration, but there are no plans to do so again in the near future.
“those fine adjustments”: To be scrupulous, cesium clocks are based on the hyperfine splitting of electrons. The fine splitting of electrons is like a difference of a halftone, while the hyperfine splitting is like a difference of a quarter tone or even an eighth tone.
These days, cesium clocks remain the world standard, but rubidium clocks have replaced them in most applications because rubidium clocks are smaller and more mobile. In fact, rubidium clocks are often hauled around the world to compare and coordinate time standards in different parts of the world, much like the International Prototype Kilogram.
“numerology”: About the same time that Eddington was working on alpha, the great physicist Paul Dirac first popularized the idea of inconstants. On the atomic level, the electrical attraction between protons and electrons dwarfs the attraction of gravity between them. In fact, the ratio is about 10^40, an unfathomable 10,000 trillion trillion trillion times larger. Dirac also happened to be looking at how quickly electrons zoom across atoms, and he compared that fraction of a nanosecond with the time it takes beams of light to zoom across the entire universe. Lo and behold, the ratio was 10^40.
Predictably, the more Dirac looked for it, the more that ratio popped up: the size of the universe compared to the size of an electron; the mass of the universe compared to the mass of a proton; and so on. (Eddington also once testified that there were approximately 10^40 times 10^40 protons and electrons in the universe—another manifestation.) Overall, Dirac and others became convinced that some unknown law of physics forced those ratios to be the same. The only problem was that some ratios were based on changing numbers, such as the size of the expanding universe. To keep his ratios equal, Dirac hit upon a radical idea—that gravity grew weaker with time. The only plausible way this could happen was if the fundamental gravitational constant, G, had shrunk.
Dirac’s ideas fell apart pretty quickly. Among other flaws that scientists pointed out was that the brightness of stars depends heavily on G, and if G had been much higher in the past, the earth would have no oceans, since the overbright sun would have boiled them away. But Dirac’s search inspired others. At the height of this research, in the 1950s, one scientist even suggested that all fundamental constants were constantly diminishing—which meant the universe wasn’t getting bigger, as commonly thought, but that the earth and human beings were shrinking! Overall, the history of varying constants resembles the history of alchemy: even when there’s real science going on, it’s hard to sift it from the mysticism. Scientists tend to invoke inconstants to explain away whatever cosmological mysteries happen to trouble a particular era, such as the accelerating universe.
“Australian astronomers”: For details about the work of the Australian astronomers, see an article that one of them, John Webb, wrote for the April 2003 issue of Physics World, “Are the Laws of Nature Changing with Time?” I also interviewed a colleague of Webb’s, Mike Murphy, in June 2008.
“a fundamental constant changing”: In other alpha developments, scientists have long wondered why physicists around the world cannot agree on the nuclear decay rates of certain radioactive atoms. The experiments are straightforward, so there’s no reason why different groups should get different answers, yet the discrepancies persist for element after element: silicon, radium, manganese, titanium, cesium, and so on.
In trying to solve this conundrum, scientists in England noted that groups reported different decay rates at different times of the year. The English group then ingeniously suggested that perhaps the fine structure constant varies as the earth revolves around the sun, since the earth is closer to the sun at certain times of the year. There are other possible explanations for why the decay rate would vary periodically, but a varying alpha is one of the more intriguing, and it would be fascinating if alpha really did vary so much even within our own solar system!
“from the beginning”: Paradoxically, one group really rooting for scientists to find evidence for a variable alpha is Christian fundamentalists. If you look at the underlying mathematics, alpha is defined in terms of the speed of light, among other things. Although it’s a little speculative, the odds are that if alpha has changed, the speed of light has changed, too. Now, everyone, including creationists, agrees that light from distant stars provides a record, or at least appears to provide a record, of events from billions of years ago. To explain the blatant contradiction between this record and the time line in Genesis, some creationists argue that God created a universe with light already “on the way” to test believers and force them to choose God or science. (They make similar claims about dinosaur bones.) Less draconian creationists have trouble with that idea, since it paints God as deceptive, even cruel. However, if the speed of light had been billions of times larger in the past, the problem would evaporate. God still could have created the earth six thousand years ago, but our ignorance about light and alpha obscured that truth. Suffice it to say, many of the scientists working on variable constants are horrified that their work is being appropriated like this, but among the very few people practicing what might be called “fundamentalist physics,” the study of variable constants is a hot, hot field.
“impish”: There’s a famous picture of Enrico Fermi at a blackboard, with an equation for the definition of alpha, the fine structure
constant, appearing behind him. The queer thing about the picture is that Fermi has the equation partly upside down. The actual
equation is alpha = e2/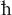 c, where e = the charge of the electron,
c, where e = the charge of the electron, 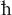 = Planck’s constant (h) divided by 2π, and c = the speed of light. The equation
in the picture reads alpha =
= Planck’s constant (h) divided by 2π, and c = the speed of light. The equation
in the picture reads alpha = 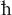 2/ec. It’s not clear whether Fermi made an honest mistake or was having a bit of fun with the photographer.
2/ec. It’s not clear whether Fermi made an honest mistake or was having a bit of fun with the photographer.
“Drake originally calculated”: If you want a good look at the Drake Equation, here goes. The number of civilizations in our galaxy that are trying to get in touch with us, N, supposedly equals
N = R* × fP × ne × fl × fi × fc × L
where R* is the rate of star formation in our local galaxy; fP is the fraction of stars that conjure up planets; ne is the average number of suitable home planets per conjuring star; fl, fi, and fc are, respectively, the fractions of hospitable planets with life, intelligent life, and sociable, eager-to-communicate life; and L is the length of time alien races send signals into space before wiping themselves out.
The original numbers Drake ran were as follows: our galaxy produces ten stars per year (R* = 10); half of those stars produce planets (fP = ½); each star with planets has two suitable homes (ne = 2, although our own solar system has seven or so—Venus, Mars, Earth, and a few moons of Jupiter and Saturn); one of those planets will develop life (fl = 1); 1 percent of those planets will achieve intelligent life (fi = 1/100); 1 percent of those planets will produce post-caveman life capable of beaming signals into space (fc = 1/100); and they will do so for ten thousand years (L = 10,000). Work all that out, and you get ten civilizations trying to communicate with earth.
Opinions about those values differ, sometimes wildly. Duncan Forgan, an astrophysicist at the University of Edinburgh, recently ran a Monte Carlo simulation of the Drake Equation. He fed in random values for each of the variables, then computed the result a few thousand times to find the most probable value. Whereas Drake figured that there were ten civilizations trying to get in touch with us, Forgan calculated a total of 31,574 civilizations just in our local galaxy. The paper is available at http://arxiv.org/abs/0810.2222.
19. Above (and Beyond) the Periodic Table
“one force gains the upper hand, then the other”: The third of the four fundamental forces is the weak nuclear force, which governs how atoms undergo beta decay. It’s a curious fact that francium struggles because the strong nuclear force and the electromagnetic force wrestle inside it, yet the element arbitrates the struggle by appealing to the weak nuclear force.
The fourth fundamental force is gravity. The strong nuclear force is a hundred times stronger than the electromagnetic force, and the electromagnetic force is a hundred billion times stronger than the weak nuclear force. The weak nuclear force is in turn ten million billion billion times stronger than gravity. (To give you some sense of scale, that’s the same number we used to compute the rarity of astatine.) Gravity dominates our everyday lives only because the strong and weak nuclear forces have such short reach and the balance of protons and electrons around us is equal enough to cancel most electromagnetic forces.
“un·bi·bium”: After decades of scientists having to build super-heavy elements laboriously, atom by atom, in 2008 Israeli scientists claimed to have found element 122 by reverting to old-style chemistry. That is, after sifting through a natural sample of thorium, the chemical cousin of 122 on the periodic table, for months on end, a team led by Amnon Marinov claimed to have identified a number of atoms of the extra-heavy element. The crazy part about the enterprise wasn’t just the claim that such an old-fashioned method resulted in a new element; it was the claim that element 122 had a half-life of more than 100 million years! That was so crazy, in fact, that many scientists got suspicious. The claim was looking shakier and shakier, but as of late 2009, the Israelis hadn’t backed off from their claims.
“once-dominant Latin in science”: Regarding the decline of Latin, except on the periodic table: for whatever reason, when a West German team bagged element 108 in 1984, they decided to name it hassium, after the Latin name for part of Germany (Hesse), instead of naming it deutschlandium or some such thing.
“rectilinear shapes”: It’s not a new version of the periodic table, but it’s certainly a new way to present it. In Oxford, England, periodic table taxicabs and buses are running people around town. They’re painted tires to roof with different columns and rows of elements, mostly in pastel hues. The fleet is sponsored by the Oxford Science Park. You can see a picture at http://www.oxfordinspires.org/newsfromImageWorks.htm.
You can also view the periodic table in more than two hundred different languages, including dead languages like Coptic and Egyptian hieroglyphic, at http://www.jergym.hiedu.cz/~canovm/vyhledav/chemici2.html.