11
The Hopeful Monoprops
Monopropellants, unlike Gaul, are divided into two parts. Low-energy monoprops are used for auxiliary power on a missile, sometimes for attitude control on a space vehicle (the Mercury capsules, and the X-15 airplane at high altitudes used hydrogen peroxide for attitude control) for tank pressurization and the like. High-energy monoprops, the glamour boys, are intended to compete with bi-propellants for main propulsion.
There haven’t been too many of the first sort, and their development has been more or less straightforward. The first, of course, was hydrogen peroxide, used by Von Braun to drive the turbines of the A-4. He used a solution of calcium permanganate to catalyze its decomposition, but later workers at Buffalo Electrochemical Co. (BECCO) found it more convenient to use a silver screen, coated with samarium oxide to do the job. (I’m not sure whether samarium was chosen as a result of a systematic investigation of all of the rare-earth metals, or because the investigator had some samarium nitrate in his stock room.) The leaders in this work were the people at RMI, who were investigating peroxide, at the same time, as the oxidizer for a “super-performance” engine on a fighter plane. They had one interesting monopropellant application of H2O2 very much on their minds. This was the ROR, or “Rocket on Rotor” concept, by which a very small—perhaps fifty pounds thrust—peroxide motor was mounted on the tip of each rotor blade of a helicopter. The propellant tank was to be in the hub of the rotor, and centrifugal force would take care of the feed pressure. The idea was to improve the performance of the chopper, particularly when it had to lift off in a hurry. (That means when somebody is shooting at you.) The work on this went on from 1952 to 1957, and was a spectacular success. I’ve seen an ROR helicopter operating, and when the pilot cut in his rockets the beast shot up into the air like a goosed archangel. The project was dropped, for some reason, which seems a shame. An ROR chopper would have been awfully helpful in Vietnam, where somebody usually is shooting at you.
At any rate, peroxide is still used as a low-energy monopropellant, and will probably continue to be used in applications where its high freezing point isn’t a disadvantage.
One such application is as a propellant for torpedoes. (After all, the ocean is a pretty good thermostat!) Here it is decomposed to oxygen and superheated steam, the hot gases spin the turbines which operate the propellers, and the torpedo is on its way. But here a little complication sets in. If you’re firing at a surface ship, the oxygen in the turbine exhaust will bubble to the surface, leaving a nice visible wake, which not only gives the intended victim a chance to dodge, but also tells him where you are. BECCO came up with an ingenious solution in 1954. They added enough tetrahydrofuran or diethylene glycol (other fuels could have been used) to the peroxide to use up the oxygen, letting the reaction go stoichiometrically to water and carbon dioxide. The water (steam) is naturally no problem, and CO2, as anybody knows who’s ever opened a can of beer, will dissolve in water with the help of a little pressure. That solved the wake problem, but made the stuff fearfully explosive, and brought the combustion temperature up to a level which would take out the turbine blades. So BECCO added enough water to the mixture to bring the chamber temperature down to 1800°F, which the turbine blades could tolerate, and the water dilution reduced the explosion hazard to an acceptable level.
Another low-energy monopropellant was propyl nitrate, first investigated around 1949 or 1950. It was plugged, enthusiastically, in England by Imperial Chemical Industries, who insisted that it was absolutely harmless and non-explosive. Ha! ERDE (Waltham Abbey) investigated it and its homologues rather extensively, and in this country the Ethyl Corporation and Wyandotte Chemical Co. did the same. The work in England was done on isopropyl nitrate, but in this country, due to a magnificently complicated patent situation, normal propyl nitrate was the isomer used. By 1956, not only Ethyl and Wyandotte, but United Aircraft, JPL, NOTS, Aerojet and the Naval Underwater Ordnance Station (the old Torpedo Station at Newport) were working with it, either as an auxiliary power source or as a torpedo propellant, and either straight or mixed with ethyl nitrate. It was easy to start—either a hot glow bar or a slug of oxygen and a spark plug were enough—burned clean and smoothly, and seemed to be the answer to a lot of problems.
And then it showed its teeth. NPN doesn’t go off on the card-gap tester. You can throw it around, kick it, put bullets through it, and nothing happens. But if there is a tiny bubble of gas in it, and that bubble is compressed rapidly—possibly by a water-hammer effect when a valve is closed suddenly—it will detonate—violently. This is known as “sensitivity to adiabatic compression,” and in this respect it is at least as touchy as nitroglycerine. It was at Newport that it happened. Somebody closed a valve suddenly, the NPN let go, and the explosion not only did a lot of damage but convinced most rocket people that monopropellant was not for them.
Another low-energy monopropellant that got quite a play starting about 1950 was ethylene oxide, C2H4O. It’s commercially available, cheaply and in quantity, since it’s an important chemical intermediate. It’s easy to start—a sparkplug is enough to do it—and decomposes in the reactor to, primarily, methane and carbon monoxide. It has a tendency, however, to deposit coke in the reactor, to an extent which depends upon the nature of the surface of the latter. This effect can be prevented by lining the chamber with silver—the flame temperature is very low—or by adding a sulfur containing compound to the propellant. It is also likely to polymerize in storage, forming gummy polyethylene ethers, which plug up everything. Sunstrand Machine Tool worked with it for several years, using it very successfully to drive a turbine. Experiment Incorporated, Walter Kidde, and Wyandotte Chemical also investigated it, and Forrestal Laboratory, at Princeton, tried it as the fuel of a ram-rocket during 1954 and 1955.
Some work was done on acetylenics, such as methyl acetylene and di-isopropenyl acetylene, by Experiment Incorporated, by Air Reduction, and by Wyandotte between 1951 and 1955 but these were never successful as monopropellants—too much coking, even if they didn’t decide to detonate.
A monopropellant with better staying power was hydrazine. Louis Dunn, at JPL, investigated it in 1948–51, and it’s still with us. It can decompose either to hydrogen and nitrogen, or to ammonia and nitrogen, and the relative importance of the two reactions depends on any number of things: the chamber pressure, catalytic effects, the stay-time of the gases in the chamber, and so on. The reaction is best started by flowing the hydrazine through a catalyst bed into the combustion chamber. Grant, at JPL, in 1953, came up with the first reasonably satisfactory catalyst: iron, cobalt, and nickel oxides deposited on a refractory substrate. The decomposing hydrazine, of course, reduces the oxides to the finely divided metals, which take over the catalytic role after startup. But restarts, if the catalyst bed has cooled, are just about impossible. The Shell Development Company, in recent years (1962 to 1964) has brought out a catalyst which allows restarts—iridium metal deposited on the substrate. But nobody is really happy with it. It’s easy to “drown” the catalyst bed by trying to run too much propellant through it, so that you get incomplete decomposition or none at all, and it works very poorly with the substituted hydrazines, which you have to use for low temperature applications. On top of that, iridium is the rarest of the platinum metals and the catalyst is horribly expensive. And just to make it interesting, the major supplier of iridium is the Soviet Union.
Another way to get restarts is to use a “thermal” instead of a catalytic bed. This has a high heat capacity and is insulated against heat loss, so that it will stay hot for some time after shutdown, and will reignite the propellant on restart simply by heating it. For the original start, the bed is impregnated with iodine pentoxide, I2O5, or with iodic acid, HIO3, either of which are hypergolic with hydrazine. But if the period between shutdown and restart is too long——! All that we can say now is that a satisfactory technique for starting hydrazine decomposition is yet to be developed. It’s still unfinished business.
During the ten years after World War II, a respectable amount of monopropellant work was going on in England. Not only were the British very much interested in peroxide (both as an oxidizer and as a monopropellant), and in propyl nitrate and its relatives, they were also intrigued with the idea of a monopropellant which could compete with bi-propellants for main propulsion. As early as 1945 they fired the German 80/20 mixture of methyl nitrate and methanol, and came to the regretful conclusion that it was something that just couldn’t be lived with, in spite of its respectable performance.
Then the Waltham Abbey people came up with another idea. The “Dithekites” had been developed during the war as liquid explosives, and ERDE thought that they might possibly be good monopropellants. The Dithekites are mixtures comprising one mole of nitrobenzene and five of nitric acid (which makes the mixture stoichiometric to water and CO2) and a varying percentage of water. D-20 contains 20 percent water. Even with the added water, the mixtures weren’t too stable, and the nitrobenzene had a tendency to get nitrated further. But the British tend to be more casual (or braver) about such things than we are in this country, and that didn’t deter them appreciably. Nor did another hazard, peculiar to the Dithekites. They are, of course corrosive, and very rough on the human skin, and to make it worse, the highly poisonous nitrobenzene was absorbed rapidly through the damaged tissue into the anatomy of the victim, subjecting him, as it were, to a one-two punch. However, they persevered and fired the things more or less successfully in 1949–50, only to discover that if you put enough water into them to keep them from blowing your head off the performance you got wasn’t worth the trouble. End of Dithekites.
Another type of monopropellant that they investigated about this time (1947–48) was based on a mixture of ammonium nitrate and a fuel dissolved in water. A typical mixture was AN-1, consisting of
|
26 percent |
|
|
Methyl ammonium nitrate |
50 percent |
|
Ammonium dichromate (combustion catalyst) |
3 percent |
|
Water |
21 percent |
Their performance, unfortunately, was so bad that development was dropped.
In this country, up to 1954, there were two main lines of high energy monopropellant development. One stemmed from the efforts, described in Chapter 3, to reduce the freezing point of hydrazine. As has been related, JPL and NOTS, between 1948 and 1954, had examined mixtures of hydrazine and hydrazine nitrate with a thoroughness which left little to be desired. And it was obvious, of course, that a mixture of hydrazine and hydrazine nitrate would have a better monopropellant performance than straight hydrazine. And when it was tried, which it was by 1950, it was discovered that the obvious was indeed true. There was only one catch. Any mixture which contained enough hydrazine nitrate and little enough water to have a respectable performance was more likely than not to detonate with little or no provocation. So that was not the route to a high energy monopropellant.1
Some years later, in the late 50’s, Commercial Solvents, working with their own money (which is unusual in the propellant business), and in considerable ignorance of what had been done already (which is not unusual in the propellant business) devised a series of monopropellants which were rather similar to the hydrazine mixtures, except that they were based on methyl amine, to which was added ammonium nitrate or hydrazine nitrate or methylammonium nitrate, or lithium nitrate. These were safe enough, but their energy and performance were low.
The other line of high-energy monopropellant work in this country was the development of nitromethane. By 1945, EES, JPL, and Aerojet had worked with it, and had discovered that it could be more or less desensitized by the addition of 8 percent of butanol. JPL did some work with it—finding the optimum injector and chamber design and so on—right after the war, and in 1949 J. D. Thackerey at Aerojet started an intensive study that carried on through 1953. And there was plenty to study!
Ignition was a big problem; it isn’t easy to get the stuff going. Aerojet found that you couldn’t ignite it with a spark unless a stream of oxygen was introduced at the same time. An ordinary pyrotechnic igniter was useless; it had to be one of the thermite type. One esoteric starting technique they developed was to spray a liquid sodium–potassium alloy into the chamber at startup. That reacted with the nitromethane with sufficient enthusiasm to get things going—but it’s not the easiest substance in the world to handle.
Stable and efficient combustion in a reasonably small chamber was another big problem. Aerojet tried dozens of combustion catalyst additives, including such surprising things as uranyl perchlorate, and finally settled on chromium acetylacetonate.
Other additives were tried, to reduce the freezing point and to desensitize the propellant, among them nitroethane and ethylene oxide. They found that the addition of amines, such as aniline, immensely increased the sensitivity, and Fritz Zwicky patented that as an invention in the field of explosives. The final mixture on which they settled comprised 79 percent nitromethane, 19 percent ethylene oxide, and 2 percent chromium acetyl acetonate. They gave it the depressing name of “Neofuel.”
Martin and Laurie had been doing similar work for the Canadian Defense Establishment in 1950. Their approach was to try to upgrade the performance of nitromethane or nitroethane or other nitro-alkyls by mixing in a suitable amount of WFNA. (Note the similarity to the Dithekites.) The performance was improved (nitroethane turned out to be the best nitro-alkyl base to start with) but the sensitivity of the mixture made it impossible to live with.
So, in the spring of 1954, the only reasonably high-energy monopropellant that could be used with reasonable safety was Aerojet’s “Neofuel.” And monopropellant research seemed to be at a dead end.
Then it happened. Tom Rice, at the Naval Research Laboratory, had an idea. He knew that pyridine was extremely resistant to nitration. So, he reasoned that if it were dissolved in WFNA it would probably go to pyridinium nitrate rather than a nitro compound, and then, as the salt, should be quite stable in the acid. And, by varying the amount of pyridine in the acid, he could get any oxidizer-fuel ratio he wanted in the mixture—and should have himself a high energy monopropellant. He tried mixing the pyridine with the acid, got some hissing and sputtering but no violent reaction, and had confirmed his first hypothesis. Then he burned some of his mixture in a liquid strand burner,2 and found that it would burn as a monopropellant. He didn’t go any further at that time, since he didn’t have access to a test stand.
Paul Terlizzi, my then boss, had been visiting NOL, and told me what Tom was doing, just as a piece of gossip. I instantly saw the possibilities, and something else that Paul and, apparently, Tom hadn’t thought of. Which was that almost any amine, and not just extremely stable ones like pyridine, could be made into a monopropellant, if its nitrate salt were made first and then dissolved in the acid. And God only knows how many amines there are!
I made a few crude performance calculations, and found that trimethylamine should give a performance somewhat better than that of pyridine. Then I had the gang make a small sample of pyridinium nitrate, and another of trimethylammonium nitrate, and mix them up into propellants. This was no trouble—the salts crystallized nicely, and dissolved in the acid with no fuss. We took a preliminary look at the mixtures, and liked what we saw. And then I sat down and wrote a letter to the Rocket Branch in BuAer, asking that I be authorized to look into the whole business. This was early in June, 1954. I should, of course, have waited for official authorization before I did anything more, but as there didn’t seem to be any particular reason to observe the legalities, we decided to get going immediately, and to make up a hundred or so pounds of each salt before anybody got around to telling us not to. We had a lot of pyridine around the place, and, for some unknown reason, a tank of liquefied trimethylamine. Plus, of course, unlimited nitric acid, so things went fast.
Making the pyridinium nitrate was easy: Just dissolve the pyridine in water, neutralize with nitric acid, boil off most of the water and crystallize. (But once, during the boiling down process, something went wrong, the mixture started to turn brown and evolve ominous NO2 fumes, and the whole thing had to be carried hurriedly outdoors and flooded down with a hose!) When we had the dry salt, we dissolved it in the acid in the proportions which would give the best performance, and sent a sample of the mixture down to Tom Rice to try on his strand burner. It burned better and faster than his had. We investigated the discrepancy, and found that his WFNA had more water in it than ours did.
We named the stuff “Penelope” because we’d been waiting so long for something like it. (Of course, in the original, Penelope did the waiting, but we weren’t inclined to be fussy about details.)
The trimethylammonium nitrate went just as easily—except for one small detail. The highly volatile trimethylamine sticks tenaciously to the skin and clothes, and smells like the Fulton Street fish market on a hot Saturday morning (although some of us used a more earthy comparison) and poor Roger Machinist, who had the job of making the salt, was saluted, for some weeks, by people who held their noses with one hand, pointed at him with the other, and shouted, “Unclean, unclean!” We called that propellant “Minnie,” for reasons which now escape me.
We finally got the authorizing letter from the Rocket Branch at the beginning of September 1954. They insisted that we concentrate our efforts, at first, on the pyridine mixture, so we made up a large batch of Penelope and turned it over to the hardware boys to see what they could do with it.
At this precise juncture Hurricane Hazel dropped the biggest oak tree in New Jersey on me and my MG. We were both out of action for some time and when I got back to work (with a jaw still held together with baling wire) I learned what had happened.
It seems that the engineers had taken a small motor—about fifty pounds thrust—fitted it with a monopropellant injector, and mounted it horizontally on the test stand. They then stuck a pyrotechnic igniter into the nozzle, started it going, and opened the propellant valve. The propellant promptly extinguished the igniter. Two more trials gave the same results. Bert Abramson, who was in charge of the test work, then took an acetylene torch and heated the motor red hot, and opened the prop valve. This time he got ignition, and some half-hearted operation for a few seconds. Inspired to further effort, he crammed about a yard of lithium wire into the chamber, and pushed the button.
Penelope sprayed into the chamber, collected in a puddle in the bottom, and then reacted with the wire. The nozzle couldn’t cope with all the gas produced, the chamber pressure rose exponentially, and the reaction changed to a high order detonation which demolished the motor, propagated through the fuel line to the propellant tank, detonated the propellant there (fortunately there were only a few pounds in the tank) and wrecked just about everything in the test cell. Penelope should have been named Xantippe. She also scared everybody to death—particularly Abramson.
There next occurred what might be called an agonizing reappraisal. It took some months, and then we decided to do what we should have done in the first place. I ordered samples of every reasonably simple amine that I could find on the market, from monomethyl amine to tri hexyl amine. Plus several unsaturated amines, a few aromatics, and some pyridine derivatives.
As soon as the first samples came in I put the gang onto the job of making the amine nitrate salts, which were then made into propellants. At times there would be half a dozen different flasks in the lab each with a different nitrate in it, and all bubbling away at the same time.
That was the case once, when we were all seated around the table in the middle of the lab, having lunch. I glanced up, and noticed that the contents of one flask was turning a little brown. “Who owns that one?” I asked (every man was making a different salt), “Better watch it!” One of them started to get up. The contents of the flask frothed up and then settled, frothed up and settled again, just like a man about to sneeze. I said “Hit the deck!” I got instant obedience when I used that tone, and seven heads met with a crash under the table as the flask and its contents went “Whoosh!” across the top of it. No damage except to the ego of the chemist concerned. But sometimes I wonder how I managed to run that shop for seventeen years without a time-lost accident.
Some of the nitrates couldn’t be made into propellants, but would start to react and heat up rapidly when they were mixed with the acid. The unsaturated amines acted this way, as did some of those with long chains, such as the hexyl amines. These were diluted with water and dumped in a hurry. Once we had to call the fire department to do it for us.
The salts varied madly in their physical properties. Some crystallized nicely; others refused to crystallize under any circumstances, and the solution had to be evaporated to dryness over a steam bath, coming out as a fine powder. And some were liquids, even perfectly dry and at room temperature. Monoethylammonium nitrate was one of these—a clear, viscous, slightly greenish liquid. Molten salts are nothing new, but these were the only ones I ever heard of that were liquid at 25°C. I’ve never found a use for the ethylamine compound, but something with such interesting properties ought to be good for something!
But most of them dissolved in the acid without any fuss. I had them made up to λ = 1.00 (stoichiometric to CO2 and H2O), since I expected their sensitivity to be at a maximum at that mixture ratio, and had them card gapped. (We had acquired an old destroyer gun turret—there were dozens of them lying around the place—and set up a card-gap test rig inside it. The idea of the turret was to contain the fragments from the cup holding the test specimen, and to make it possible to find the witness plate after firing.) A goof-off ensign that I was stuck with was supposedly in charge of the card-gap work, but John Szoke, my madly industrious technician, and one of the best men I’ve ever seen in a lab, did most of the work. And it was a lot of work.
Altogether, he card-gapped about forty different mixtures during that session—and if you can nail down the go-no go point of a single one in less than a dozen shots, you’re lucky.
The results of the tests were surprising. First, Penelope and her relatives (derived from pyridine and related compounds) were among the most sensitive of all the mixtures tried—one of them rated some 140 cards. Second, the propellant made from trimethylamine (the one that I’d wanted to try in the first place) was remarkably insensitive—rating about ten cards. And, as the amine samples came in from the manufacturers, a fascinating pattern started to emerge. If you disregarded things like methylcyclohexyl amine, which didn’t seem to follow any rules, and considered only those propellants made from straight or branched chain aliphatic amines, the card gap sensitivity appeared to be a powerful function of the structure of the molecule. The longer the chain, the more sensitive was the propellant mixture. A propellant made from propylamine was more sensitive than one made form ethyl amine, and one made from tripropylamine was more sensitive than one made from dipropylamine, which, in turn, was more sensitive than the one made from monopropylamine. And one made from isopropylamine was less sensitive than the normal propylamine mixture.
To say that the explanation of these regularities was not obvious would be to understate the matter. But I proceeded in the way a scientist usually does when he’s confronted with a mass of apparently inexplicable numerical data. I worked out an empirical equation relating the card-gap sensitivity to a function, ϕ, which I called “floppiness coefficient,” and calculated from the number of carbon chains in the ammonium ion, their lengths, and their degree of branching. (In deriving it, I had to use logarithms to the base three, which is something so weird as to be unheard of. Fortunately they canceled out, and didn’t appear in the final function!) And from this equation, with the help of the specific heat of the propellant, the size of the ammonium ion, and a few assumptions, I was able to make a guess at the heat of activation or the explosion process. It came out at quite a reasonable figure—some 20 to 30 kilocalories/mole—right in the range of the strength of molecular bonds.
This was interesting, but what was more to the point, my list of candidate propellants was drastically pruned. Starting with thirty-three mixtures, and taking thirty-five cards as an arbitrary sensitivity limit, I had only ten survivors. Some of these I dropped immediately, because the freezing point of the mixture, when made up to the optimum mixture ratio, was too high, or because the dry salt was unstable in storage, or because it was much more expensive than another compound with the same sensitivity.
The final selection was based on thermal stability. Some of the mixtures could be evaporated to the dry crystals over a steam bath, but others, when the acid was almost all gone, would ignite and burn merrily. This was some indication of the relative stability of the propellants, but for more formal—and quantitative—work, we designed and had built a thermal stability tester. This was a small, sealed, stainless steel bomb, with a total volume of approximately 10 cc, equipped with a pressure pickup and a recorder, and with a rupture (or burst) disc failing at approximately 300 psi. The bomb was loaded with 5 cc of propellant and placed in a constant temperature bath, and the pressure buildup was recorded. There was an open “chimney” above the burst disc, which extended above the liquid level of the bath, so that when the disc let go the bath liquid, which was usually old cylinder oil, wouldn’t be spread all over the landscape.
In a typical run, a sample was placed in the bath at 100°. In a very few minutes the pressure rose to about 100 psi, and stayed there for about fifteen hours. Then, it started to increase at an accelerating rate, and the burst disc failed at seventeen hours. When we ran a series of runs, at different temperatures, and then plotted the logarithm of the time-to-burst against the reciprocal of the absolute temperature, we got a gratifyingly straight line, from whose slope it was easy to calculate the heat of activation of the decomposition process. (It turned out to be surprisingly close to that derived from the card gap work!)
Anyhow, we found that, other things being more or less equal, secondary amine mixtures were more stable than primary amine mixtures, and that tertiary amine mixtures were the most unstable of all. And of the propellants which had survived our other screenings, that made from diisopropyl amine had the best thermal stability. So it was Isolde. (It was our custom, by this time, to give our monoprops feminine names—like hurricanes. Sometimes the name was vaguely mnemonic of the amine involved—as Beulah for a butyl amine, for instance—and sometimes it had nothing to do with anything. Roger Machinist had been the one to make diisopropylammonium nitrate, and hence had the inalienable right to name it. And he’d been to the opera the night before.)
That was OK with us. Isolde salt was easy to make, crystallized nicely, and was, in general, a joy to work with.
In the meantime, we’d been trying to invent some way of igniting it without blowing up the motor. That wasn’t easy. You couldn’t set fire to the propellant in the open, even with an oxygen-propane torch. Ordinary pyrotechnic squibs, as we already knew, were useless in a motor. We tried to make some really hot igniters by mixing up powdered aluminum or magnesium, potassium nitrate or perchlorate, and epoxy cement, letting the mess harden in a polyethylene tube, and then cutting off the tube. The results were spectacular. When we lit one of them off (we did it with an electrically heated wire) we got a brilliant white flame, clouds of white smoke, and all sorts of sound effects. We tried them out just outside of the door of the lab, and always had one ready to greet any incautious safety man as he was strolling by. Bert Abramson came in for a demonstration, and when one of them was touched off he tried to extinguish it with a wash bottle. Whereupon the igniter broke in two, with the business end dropping to the floor and chasing him about the lab as everybody cheered. But they wouldn’t do the job—a stream of Isolde would put out the fire.
Apparently it just wasn’t practical to light the stuff off with an external energy source. It would have to produce its own energy, which meant that we had to develop some source of hypergolic ignition. We didn’t want to bother with the plumbing that would be involved if we used a slug of UDMH for instance, to react with the acid in the propellant; that would lead to too much complication. What we wanted was some solid material, which could be placed in the chamber before-hand, and would react with the propellant when it was injected to start the fire going. We tried all sorts of things—powdered magnesium, metallic sodium, and what not. (The candidate was placed in a horizontal one- or two-inch diameter glass tube, the propellant was sprayed in at one end, and the results were monitored with a high-speed camera.) We had no luck for some time, but then we finally hit on a highly improbable mixture that worked—a mixture of lithium hydride and rubber cement. This unlikely sounding mixture was made into a thick dough, spread on a sheet of gauze, and then wrapped around a wooden dowel. The end of the dowel was tapered and screwed into a plug tapped with a 1/8″ pipe thread. The plug, in turn, was screwed into an appropriately threaded hole in the center of the injector, so that when the propellant was injected it would impinge upon, and react with the ignition mixture. The whole device, some six inches long, was kept in a sealed test tube until it was needed, to protect the LiH from atmospheric moisture. The business end of it was a ghastly corpse-gray, and it was the most obscene looking object I have ever seen—and the rocket mechanics christened it accordingly.3
But it worked. We had our first successful run in January 1956, and by April we had a smooth-running and workable system. We got the best results with a propellant made up with anhydrous nitric acid rather than with ordinary WFNA, and with a salt/acid mixture that gave a λ (the ratio of reducing to oxidizing valences in the propellant) of 1.2. So we called it Isolde 120 A (the 120 referring to the mixture ratio and the A to the anhydrous acid) and wrote our reports. And we had something to report—the highest performing monopropellant ever fired anywhere by anybody. Combustion was good—we got close to 95 percent of the theoretical performance with a surprisingly small chamber—and we didn’t need a fancy (and expensive) injector. In fact, the one we used was made from six commercially available oil-burner spray nozzles costing seventy-five cents each.
Our reports (mine on the development of the propellant and the igniter, methods of analyzing the propellant, card-gap results and so on, and the engineering report on the motor work) came out together in November 1956, but everybody in the business had a pretty good idea of what we had done by June. And then all hell broke loose.
Everybody and his uncle wanted a piece of the action, and wrote a proposal to one of the three services, for a research program on monopropellants. RMI, right next door to us, and intimately acquainted with our work, was first off the mark, in March 1956, when they got a Navy contract to develop “Superior Liquid Monopropellants,” but the others weren’t far behind. Wyandotte Chemical had a Navy contract by September, Phillips Petroleum and Stauffer Chemical got into the act early in 1957, and by 1958 Pennsalt, Midwest Research, Aerojet, and Hughes Tool had joined them. In addition to these, all of whom were trying to brew up new propellants, several organizations, including GE, were motor-testing propellants that others had developed, and were trying to apply them to tactical systems. It was a busy time.
Reaction Motors (before long they had not only a Navy monopropellant program, but an Army contract as well) tried two approaches. One was to dissolve a fuel in an oxidizer, and the other was to produce a single-compound propellant, the nitrate or the nitramine of an energetic radical. Propargyl nitrate, propargyl nitramine, glycidyl nitrate, 1,4 dinitrato 2 butyne, and 1,6 dinitrato 2,4 hexadyne are typical of the monstrosities they produced. (Reading the names is enough to dampen a propellant man’s brow!)
I don’t believe that they ever made enough of any of them to do a card-gap, but the results of certain other tests were enough to make one a bit cautious. Joe Pisani phoned me from RMI one day late in 1958, asking me if I’d do a thermal stability run on a sample of propargyl nitrate. I replied that I’d be glad to, but that he’d have to replace anything that got busted, since I didn’t trust the stuff. So he sent his sample up to us. It was only 3 cc (we usually used 5) but maybe we were lucky at that. John Szoke heated the oil bath up to 160°C (the temperature that we used then for routine tests) loaded the sample into the bomb, lowered the bomb into the bath, and scurried back into the lab, closing the door behind him. (For obvious reasons, the setup was outdoors and not in the lab.) He turned on the recorder and watched. Nothing happened for a while. The pressure rose slowly as the sample warmed up, and then seemed to stabilize.
And then it let go, with an ear-splitting detonation. Through the safety glass window we saw a huge red flare as the oil flashed into flame, only to quench immediately as it hit the ice-cold concrete. We cut everything off, and went out to survey the damage. The bomb had fragmented; the burst disc just couldn’t rupture fast enough. The pressure pickup was wrecked, as was the stirrer. The cylindrical stainless steel pot which had held the oil had been reshaped into something that would have looked well under a bed. And the oil—it had been old vacuum pump oil, black and filthy. It had hit the concrete floor of the test area, the wall of the building, and everything else in reach, and had cleverly converted itself (the temperature was well below freezing) into something resembling road tar. I got on the phone.
“Joe? You know that stuff you sent me to test for thermal stability? Well, first, it hasn’t got any. Second, you owe me a new bomb, a new Wianco pickup, a new stirrer, and maybe a few more things I’ll think of later. And third (crescendo and fortissimo) you’ll have a couple of flunkies up here within fifteen minutes to clean up this (—bleep—) mess or I’ll be down there with a rusty hacksaw blade. . . .” I specified the anatomical use to which the saw blade would be put. End of conversation.
And it was the end of the propargyls and their relatives. Washington told Reaction to forget that foolishness and start working on the N-F compounds instead. That story will be told a little later.
The other approach to a monopropellant at RMI was taken by Stan Tannenbaum, who tried mixtures of inert (he hoped) oxidizers and fuels. This was bathtub chemistry, involving little or no synthesis, but requiring strong nerves. It had the advantage, of course, that the stoichiometry could be adjusted ad lib, and wasn’t constrained, as in the one-component monoprops, by the nature of the molecule. And the idea wasn’t exactly new. The French, during World War I, had employed aerial bombs filled with a mixture of N2O4 and benzene. (The stuff was so touchy that the two liquids weren’t mixed together until the bomb had been dropped from the plane!) And, incidentally, some years before I got into the monopropellant business, a hopeful inventor had tried to sell me this same mixture for a monoprop, averring that it was as harmless as mother’s milk. I didn’t buy it.
Stan worked with N2O4 and with perchloryl fluoride. He found that he could mix bicyclooctane or decalin in N2O4 without immediate disaster, but that the mixture was too touchy to live with. He tried tetramethyl silane too, in the hope (unrealized) that it would be safer, but finally and regretfully came to the conclusion, late in 1959, that you could not make a practical monopropellant based on N2O4. Howard Bost, at Phillips Petroleum, who had been working with mixtures of N2O4 and neopentane or 2,2 dinitropropane, came to the same conclusion at about the same time. And if any more evidence were needed, the card-gap values for various N2O4-hydrocarbon mixtures, as determined by McGonnigle of Allied Chemical, furnished it. N2O4-fuel mixtures are not useful monoprops.
He didn’t have any more luck with perchloryl fluoride. He tried first mixing it with amines, but found that if they dissolved at all they immediately reacted with the oxidizer. He could dissolve hydrocarbons or ethers, but the mixtures were touchy and too dangerous to handle. (The same discovery was made at GE, when a mixture of perchloryl fluoride and propane detonated, seriously injuring the operator.) So that approach, too, was hopeless. Nor was it a good idea to try to mix N2F4 with monomethyl hydrazine, as he discovered early in 1959!
If Tannenbaum’s mixtures were bad, that proposed at a monopropellant conference in October 1957 by an optimist from Air Products, Inc., was enough to raise the hair on the head of anybody in the propellant business. He suggested that a mixture of liquid oxygen and liquid methane would be an extra high-energy monopropellant, and had even worked out the phase diagrams of the system.4 How he avoided suicide (the first rule in handling liquid oxygen is that you never, never let it come in contact with a potential fuel) is an interesting question, particularly as JPL later demonstrated that you could make the mixture detonate merely by shining a bright light on it. Nevertheless, ten years later I read an article seriously proposing an oxygen-methane monopropellant! Apparently junior engineers are allergic to the history of their own business.5
The work done at Wyandotte by Charlie Tait and Bill Cuddy wasn’t quite as hairy as that performed at Air Products, but it approached it closely enough to satisfy a reasonably prudent man. For one thing, Bill, like Joe Pisani, synthesized some really fancy organic nitrates, such as 1,2 dinitratopropane, and nitratoacetonitrile, and as might have been expected, discovered that nobody in his right mind would try to use them as propellants. For another, he examined the possibility (admittedly slight) of using alkyl perchlorates, such as ethyl perchlorate, C2H5ClO4, as monoprops. I read in a Wyandotte report that they intended to do this, and phoned Bill to read to him what Sidgwick, in “Chemical Elements and their Compounds,” had to say on the subject of the ethyl compound.
“Hare and Boyle (1841) say [Sidgwick wrote] that it is incomparably more explosive than any other known substance, which still seems to be very nearly true. . . . Meyer and Spormann (1936) say that the explosions of the perchlorate esters are louder and more destructive than those of any other substance; it was necessary to work with minimum quantities under the protection of thick gloves, iron masks [Ha, there, M. Dumas!], and thick glasses, and to handle the vessels with long holders.” But Cuddy (presumably investing in leather gloves and an iron mask first) went ahead anyway. He told me later that the esters were easy enough to synthesize, but that he and his crew had never been able to fire them in a motor, since they invariably detonated before they could be poured into the propellant tank. It is perhaps unnecessary to add that this line of investigation was not further extended.
A system on which they worked for more than two years was based on a solution of a fuel in tetranitromethane—the stuff that had meant nothing but trouble to everybody who had ever had anything to do with it. And Bill and Charlie had their troubles.
One fuel they tried was nitrobenzene. It dissolved nicely in the TNM, to make a propellant with the proper oxygen balance, and the solution seemed reasonably stable. But when they card-gapped it, they found that its sensitivity was over 300 cards. (In my own work, I flatly refused to have anything to do with anything with a card-gap figure much over 30.) Acetonitrile, which they chose as a fuel (they had calculated the performances of dozens of the possibilities, and had tried a few of them) wasn’t quite as bad as the nitrobenzene, but it was bad enough. But about this time some of the people in the monoprop business, when accused of producing something which was insanely hazardous, would answer blithely, “Sure, I know it’s sensitive, but the engineers can design around it.” (The engineers took a dim view of this.)
So they went ahead anyway, and actually managed to fire the stuff in a micro-motor. Most of the time. Sometimes, generally, and embarrassingly, when they were demonstrating it to visitors, it would let go with a frightful bang, demolishing the motor and the instrumentation, and scaring everybody half to death. Tait and Cuddy sweat blood, but they were never able to make the TNM mixtures into reliable propellants, and late in 1958 the thrust of their work shifted to the amine nitrates.
If Tait and Cuddy were fighting a lost cause, Jack, Gould, at Stauffer, must have been smoking Acupulco Gold. His investigations were pure fantasy, to be described properly only by Lewis Carroll. He had a Navy contract to develop “High Energy Monopropellants,” and his efforts in that direction challenge belief. The most sensible thing he tried was to dissolve NH3 in NF3. Both are quite stable compounds, and he might have come up with a high performing and reasonably safe propellant. Unfortunately, the ammonia wouldn’t dissolve in the NF3 to any extent. Otherwise:
- He tried to make nitronium borohydride, NO2BH4, and failed. (The idea of a stable salt with an oxidizing cation and a reducing anion isn’t very plausible on the face of it.)
- He tried to mix pentaborane with nitro-ethyl nitrate. They exploded on contact. (The NEN by itself card-gapped at about 50 cards.)
- He tried to mix NF3 and diborane. They reacted.
- He tried to mix NEN with amine derivatives of various boranes. They reacted or exploded.
And so on indefinitely. And in every quarterly report he would list a lot of hypothetical compounds, like di-imide, H—N=N—H, which would be beautiful propellants if you could only make them. Finally the Rocket Branch, fed up, told him to quit that sort of foolishness and to work on the NF systems instead. Which, starting about the end of 1958, is what he did.
While all this was going on, the amine nitrate monopropellants were very much in the picture—but NARTS was not alone there with them. GE jumped in and started motor work, trying to use Isolde in a novel self-pumping motor that they were developing. (They blew up their setup, which goes to show that it isn’t a good idea to try to develop a new type of motor with an experimental propellant. One unknown at a time is plenty to worry about!)
Even before the Isolde report was published, Bost and Fox at Phillips Petroleum had made nitrate salts of some of their bi-tertiary amines, and dissolved the salts in nitric acid to come up with AN propellants of their own. They discovered, however, that their thermal stability was extremely poor, which agreed with our own experience with tertiaries. They also discovered that their di-nitrate solutions were extremely viscous, as we had learned, very early in the game, when we tried to make a propellant from ethylene diamine.
At NARTS, the engineers plowed ahead, trying Isolde in high pressure motors—1000 instead of 300 psi chamber pressure—and as a regenerative coolant. It could be used that way, but the process was somewhat precarious. You had to shut down with a water flush through the system, or the propellant left in the cooling passages of the still-hot motor would cook off and probably blow up the works.
This was all very commendable, but not very interesting to anybody except a hardware merchant. Which I was not. So I decided to see whether quaternary ammonium nitrates would make better propellants than secondary ammonium nitrates. We had never investigated the quaternaries, since they were comparatively difficult to make, and there was no a priori reason to believe that they would be any better or any worse than Isolde. But there was only one way to find out.
We had a little tetramethyl ammonium hydroxide in the laboratory, so I made it into the nitrate salt—it crystallized beautifully—and had it made up into propellant. We didn’t have enough for card-gap work, but we tried it in the thermal stability tester. And got a shock.
It was incredibly stable. When Isolde cooked off in fifty minutes at 130°, the new stuff just sat there. And at 160° it would stand up for more than a week with nothing happening. (Isolde lasted two minutes at 160°.)
This was exciting, and we looked around for a way to make more of the stuff. Tetramethyl ammonium nitrate wasn’t commercially available—there was no reason why anybody would have wanted it before—but the chloride was, and I ordered enough to convert into a reasonable amount of the salt we wanted. The conversion was easy enough, even though we used up a lot of expensive silver nitrate doing it (we later went to the trouble of reclaiming the silver, so we could use it over and over) and we soon had enough propellant to do card-gap tests. And our new propellant at λ = 1.2 had a sensitivity of about five cards, which meant that the shock wave pressure that was needed to set if off was more than twice the pressure that would detonate Isolde.6 Immensely cheered, we christened her with the obvious name “Tallulah” (as being practically impervious to shock) and continued on our way. This was early in 1957.
The only trouble with Tallulah was that when it was mixed up to λ = 1.20, the freezing point was much too high—about –22°. (Such a beautifully symmetrical ion can hardly be restrained from crystallizing.) So we next tried the ethyl trimethyl salt (“Portia,” but don’t ask me to explain the convoluted line of reasoning that led to that name!) and the diethyl dimethyl ammonium nitrate. (“Marguerite,” and don’t ask me to explain that one either.) Portia didn’t quite make it—it would meet the freezing point specifications at λ = 1.10, but not at 1.20, and it crystallized poorly and was rather hygroscopic. Marguerite met the freezing point specifications all right, but it crystallized very badly, and was so hygroscopic as to be practically unusable.
These salts we had made for us by outside manufacturers who had the pressure equipment which we did not have, and which is practically indispensable in making quaternaries in any quantity. John Gall at Pennsalt, and Dr. Phyllis Oja of Dow were both remarkably helpful here in talking their respective pilot plants into making the things and absorbing the financial loss involved. (We got the salts for much less than it cost the manufacturers to make them.)
If Marguerite had poor physical properties, a more compact and symmetrical isomer might do the job, and trimethyl isopropyl ammonium nitrate was the next thing we tried. That was fine—good freezing point, excellent thermal stability, a little more sensitive than Tallulah on the card-gap, but not enough to matter, and physical properties that made it a joy to handle. We called it “Phyllis.” (After all, when a lady talks her employers into making 150 pounds of a completely unheard of salt for you, and then doesn’t charge you anything for it, on the grounds that the paper work would cost more than the stuff is worth, the least a gentleman can do is to name it after her!)
At the end of 1957, Phyllis looked like the best bet, but we kept on looking. All through 1957, and for three years more, we scurried about, rounding up likely looking amines, quaternizing them and checking them out. We’d usually make enough at first to check the thermal stability and the melting point, and then, if it passed these tests (most of them didn’t) make a lot big enough for card-gap work. And if a candidate showed up well there, it was time to look for somebody who would make enough for motor work.
In January, 1958, Bost and Fox, of Phillips, with a new Air Force contract, returned to the monopropellant business with a splash. Phillips, of course, had all the fancy equipment that a man could desire, and they could work
fast. For instance, if they wanted the fuel-ion 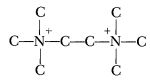 (hydrogens, as usual omitted for simplicity) they would simply react ethylene chloride with trimethylamine, in almost any solvent, under pressure, and have what they wanted. We envied them their equipment and cursed the affluence of the petroleum industry. Anyhow, they synthesized about a dozen different quaternary amine nitrates, dissolved them in nitric acid, and checked out their properties. They did some work with perchlorates, but found that they were entirely too sensitive; and some with N2O4 and N2O4-H2O mixtures, but found that the nitrate salts weren’t sufficiently soluble in N2O4 to make a propellant, and that when enough water was added to make them soluble they lost too much energy. So they resigned themselves to investigating the same sort of systems that we were working on. And for a year or so our two programs went along more or less in parallel—they working on double ended propellants, we working on single enders.
(hydrogens, as usual omitted for simplicity) they would simply react ethylene chloride with trimethylamine, in almost any solvent, under pressure, and have what they wanted. We envied them their equipment and cursed the affluence of the petroleum industry. Anyhow, they synthesized about a dozen different quaternary amine nitrates, dissolved them in nitric acid, and checked out their properties. They did some work with perchlorates, but found that they were entirely too sensitive; and some with N2O4 and N2O4-H2O mixtures, but found that the nitrate salts weren’t sufficiently soluble in N2O4 to make a propellant, and that when enough water was added to make them soluble they lost too much energy. So they resigned themselves to investigating the same sort of systems that we were working on. And for a year or so our two programs went along more or less in parallel—they working on double ended propellants, we working on single enders.
A newcomer in the field was J. Neff, of Hughes Tool. (Yes, Howard Hughes’ company.) Early in 1958, armed with a Navy contract and more than the usual complement of optimism, he started work on the development of a boron-based nitric acid monopropellant. The program lasted about a year and a half, and while it didn’t lead to a useful propellant, it involved some interesting chemistry. His most nearly successful approach was based on the carborane structure, in which two carbon atoms take their place with the ten borons in the open basket decaborane structure to form a twelve-atom closed icosahedral cage. (See the boron chapter.) To one or both of these carbons he would attach a dimethylaminomethyl or dimethylaminoethyl group, then make the nitrate salt of the result, and dissolve the salt in nitric acid. In some cases he managed to get away with it, although ignition was likely if he mixed the components too rapidly. But his solutions were unstable; they would evolve gas if they were warmed up a bit, or would separate into two layers, or do something else to emphasize that this was not the way to make a monopropellant. His work never got to the stage of motor testing.
Nor did Aerojet’s monopropellant work for the Air Force. Late in 1958, M. K. Barsh, A. F. Graefe, and R. E. Yates started investigating certain boron ions, such as [BH2(NH3)2]+, with the intention of making the nitrates and dissolving them in nitric acid. There was a whole family of these ions, sometimes with hydrazine in place of the ammonia, some containing more than one boron atom, and so on. The chloride of the one shown above can be obtained by milling together in a ball-mill lithium borohydride and ammonium chloride. Max Barsh and Co. called these ions the “Hepcats,” from High Energy Producing CATions. (My deplorable habit of giving propellants fancy names was apparently catching!) They made some attempt to synthesize the aluminum analogs of some of the ions, without any notable success. But unfortunately, by the end of July 1959, they had discovered that the Hepcats weren’t stable, even in water, let alone nitric acid or N2O4. End of the Hepcats.
As I have said, Phyllis seemed to be the most promising AN around at the beginning of 1958, and by the end of that year it, as well as Tallulah, had been fired successfully by NARTS and by Spencer King, of Hughes Tool. Howard Bost’s “Ethane” had also been fired, and, as far as performance was concerned, there didn’t seem to be much to choose among them. I don’t think that anybody ever actually fired either Portia or Marguerite. In most of these monopropellant tests, ignition was by a UDMH slug. This was more complicated, of course, than using our “Tristan” igniter, but it was considerably more reliable for test stand work.
The next development was touched off by John Gall, of Pennsalt, who, in the summer of 1958, sent me samples of two amine nitrates for evaluation. The ions are shown below.
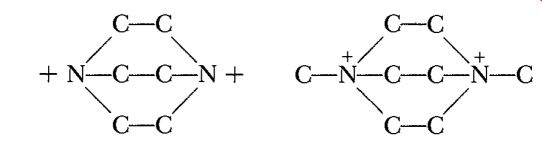
There was some delay—one of my gang goofed and made the test propellants up in the wrong proportions, so we had to ask for another sample—but we finally managed to run off thermal stability tests. The tertiary ammonium nitrate, of course, was no good, but the quaternary appeared to be at least as stable as Tallulah. It then occurred to me that it might be interesting to compare the three different, but very similar, ions shown below, which might be thought of as made of two Tallulah ions joined by one, two, or three links respectively.
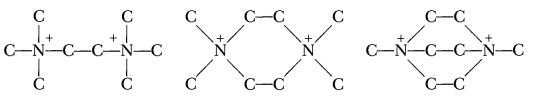
Mike Walsh, of our laboratory, announced our intention of doing this at an ARS meeting in the middle of September, and we started in at once. The nitrate of the first ion was easily available: It was Howard Bost’s “Ethane” salt. For the third, we got another and larger sample from John Gall. The second had never been made, but Jefferson Chemical Co. made N,N′ dimethylpiperazine, and it wasn’t any trick at all to quaternize that and get the salt he wanted. Anyway, we made up the propellants, and tried them in the termal stability tester at 160°. Number 1 lasted for a bit more than two hours. Number 2 lasted about two minutes. And number 3 just sat there, doing nothing, until we got bored with the whole business and cut off the test after three days.
This was verrrry interesting—apparently we had something even tougher than Tallulah. So we made it up to λ = 1.2, and card-gapped it. And discovered, to our astonishment, that it did not detonate even at zero cards. This was more than interesting—it was sensational. The freezing point was bad, –5°C, but we figured that we could get around that somehow, and it didn’t dampen our enthusiasm.
It had to have a good name. Nobody was going to call it by its formal title, 1,4, diaza, 1,4, dimethyl, bicyclo 2,2,2, octane dinitrate, that was for sure. The ion had a nice symmetrical closed cage structure, so I called it “Cavea” (which, after all, has a vaguely feminine sound) after the Latin for cage. Nobody objected—it was easy to remember and to pronounce, although lots of people asked me what it meant!
We went through the usual routine, burning the salt in the calorimeter to get its heat of formation, measuring the heat of solution of the salt in the acid (these two so we could make decent performance calculations) measuring the density and the viscosity of the propellant as a function of temperature, and all the rest. And everything was fine except that freezing point.
One valiant attempt to remedy the situation went on for months, and got exactly nowhere. I reasoned that a single ended ion, such as,
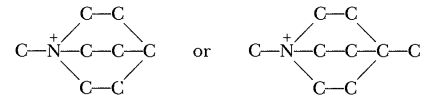
would result in a propellant with a freezing point that would suit anybody. The difficulty lay in getting the nitrates of either of the ions in question. I shopped around for months before I found an outfit that would—or could—make me a sample of the first one. And when it finally came in and was made into a propellant, the thermal stability was abysmally bad. Spurge Mobley, in my own laboratory, synthesized the other (it was the very devil of a job and took him weeks), and it, too, as a propellant, had an impossibly bad thermal stability. Oh, well, it was a good idea, anyway.
In the meantime we (and BuAer) we’re in a hurry to get Cavea into a motor. So we wanted a large amount of Cavea salt—fast.
I knew that John Gall’s people had made it by methylating triethylene diamine  and at the beginning of December he told me that the latter came from Houdry Process Co. It was used for a polymerization catalyst and sold under the peculiarly repulsive trade name “Dabco.” In the meantime, he could furnish me with Cavea salt for about seventy dollars a pound, in ten pound lots. I put in an order, but I wasn’t entirely convinced that the salt couldn’t be made more cheaply than by reacting methyl iodide (which is quite expensive) with triethylene diamine, and then metathesizing with silver nitrate.
and at the beginning of December he told me that the latter came from Houdry Process Co. It was used for a polymerization catalyst and sold under the peculiarly repulsive trade name “Dabco.” In the meantime, he could furnish me with Cavea salt for about seventy dollars a pound, in ten pound lots. I put in an order, but I wasn’t entirely convinced that the salt couldn’t be made more cheaply than by reacting methyl iodide (which is quite expensive) with triethylene diamine, and then metathesizing with silver nitrate.
Maybe I could do the synthesis differently. Instead of starting with 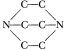 and putting the carbons on the ends to get
and putting the carbons on the ends to get 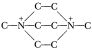 , might it not be possible to start with Jefferson Chemical’s N,N′ dimethylpiperazine,
, might it not be possible to start with Jefferson Chemical’s N,N′ dimethylpiperazine, 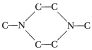 and plug in a two carbon bridge
and plug in a two carbon bridge
to get the same thing? I could use ethylene bromide (which is a whole lot cheaper than methyl iodide) as the bridging agent, and would come up with the bromide rather than the iodide salt.
We tried it, and the reaction worked beautifully, giving us about a 95 percent yield on the first try. The next thing to do was to find a cheap way to convert from the bromide to the nitrate.
I knew that it was quite easy to oxidize the bromide ion to free bromine, but that it was considerably tougher to force it up to the bromate. And I was pretty sure that nitric acid would do the first and not the second. If I added the Cavea bromide to fairly strong, say 70 percent, nitric acid, the reaction should go
2Br− + 2HNO3 → 2HBr + 2NO3−
and then
2HBr + 2HNO3 → 2NO2 + 2H2O + Br2.
And if I ran a stream of air through the mixture, to blow out the bromine and the NO2, I should be left with a solution of Cavea nitrate in fairly dilute nitric acid.
We tried it, and it worked. But we discovered that if we added the salt to the acid too fast, or let the bromine concentration build up, we got a brick-red precipitate of Cavea tribromide—the salt of the anion, and it took hours of blowing before that would dissociate and release the bromine. I had heard vaguely of the possibility of such an anion, but that was the first time I ever saw one of its salts, Anyway, we dried out the nitrate over a steam bath, recrystallized it from water (it crystallized in beautiful hexagonal crystals) and had our Cavea salt by a simple route that didn’t require any expensive reagents. By this time it was the middle of February 1959, and we had learned that Howard Bost, working with his di-quaternaries, had independently hit upon Cavea, and had, like us, decided that it was the best propellant to work with. So now our two programs had converged completely. This was emphasized at a symposium on the AN monopropellants, held at NARTS on the first and second of April. Never have I met such unanimous agreement in such a high-powered group. (The nineteen guests comprised eighteen PhD’s and one drunken genius.) And we were all convinced that the future belonged to Cavea, possibly with some structural modification that would get us a better freezing point.
One new development, however, was reported by Dr. Wayne Barrett, of W. R. Grace Co. He had methylated UDMH, to get the ion, 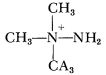 and had made a monoprop with the nitrate of that. Besides the trimethyl compound shown, he had also made the triethyl and the tripropyl, and was so new and innocent in the propellant business that he didn’t even start to run when, as he mixed up the propyl salt with the acid, the stuff had started to warm up and give off NO2 fumes! Anyway, I had my gang immediately make up some of his propellant and give it the treatment. We learned about it on Thursday the second of April, had the synthesis and purification completed and the methyl propellant made up by Tuesday the seventh, and on the eighth saw it wreck our thermal stability tester all over again. (It sat there quietly for fourteen minutes and then detonated—but violently.) I phoned Barrett, and warned him against his brain-child, but he decided to go ahead anyway and repeat our stability test. After some weeks he phoned me and reported that his sample had lasted for seventeen minutes before blowing up the place, and did I consider that a good check!
and had made a monoprop with the nitrate of that. Besides the trimethyl compound shown, he had also made the triethyl and the tripropyl, and was so new and innocent in the propellant business that he didn’t even start to run when, as he mixed up the propyl salt with the acid, the stuff had started to warm up and give off NO2 fumes! Anyway, I had my gang immediately make up some of his propellant and give it the treatment. We learned about it on Thursday the second of April, had the synthesis and purification completed and the methyl propellant made up by Tuesday the seventh, and on the eighth saw it wreck our thermal stability tester all over again. (It sat there quietly for fourteen minutes and then detonated—but violently.) I phoned Barrett, and warned him against his brain-child, but he decided to go ahead anyway and repeat our stability test. After some weeks he phoned me and reported that his sample had lasted for seventeen minutes before blowing up the place, and did I consider that a good check!
In the meantime, I had been taking steps toward getting Houdry and Jefferson interested in manufacturing Cavea salt. I had phoned them both on February 19, describing the salt I wanted, and asking if they were interested in bidding on a hundred pounds of it. At Houdry, apparently everybody took off on cloud nine. (This was early in 1959, remember, the cold war was on, everybody was excited over missiles and space, and apparently everybody was convinced—falsely—that there was a lot of money to be made in the rocket propellant business.) Anyhow, they phoned me back several times, and when I got home that evening they had the cleaning lady on the phone. And the next day they had their director of research up from Philadelphia to talk to me. Before I got through with them I told him that Jefferson Chemical was in on the bidding, too, and hinted that perhaps Jefferson could make the stuff cheaper than Houdry could.
The response of Jefferson Chemical was not quite so hysterical, but enthusiastic enough. I got their director of research in Houston, Dr. McClellan, and described the dimethyl piperazine-ethyl bromide reaction to him—he didn’t really believe it until he tried it himself—and asked him what he could do. I also hinted to him that perhaps Houdry could give me a better price than he could. This is the procedure known as playing both ends against the middle.
Both companies delivered preliminary samples for approval within a month, and I discovered that McClellan had come up with an interesting method of getting rid of the bromide. He would acidify the Cavea bromide cold, with nitric acid, and then blow ethylene oxide through the solution. It reacted, C2H4O + HBr → HOC2H4Br, with the HBr to form ethylene bromohydrin, which was just blown out of the system. I considered this a neat trick.7
Anyway, both companies eventually came up with bids, and, as I suspected would be the case, Jefferson’s was the better. They wanted fifteen dollars a pound for Cavea salt, while the best that Houdry could do, at that time, was seventy-five, although their research people thought that they might be able to argue their business end down to fifty. So now there was no supply problem
to cope with. Howard Bost did some work  with which had a better freezing point than Cavea, and Charlie Tait phoned me on June 10 with some interesting news. Apparently Wyandotte had decided to give up the TNM monoprops as a lost cause, and to shift to the AN family. And they had some assorted substituted piperazines available, such as
with which had a better freezing point than Cavea, and Charlie Tait phoned me on June 10 with some interesting news. Apparently Wyandotte had decided to give up the TNM monoprops as a lost cause, and to shift to the AN family. And they had some assorted substituted piperazines available, such as  And so Charlie bridged that with ethylene bromide, and came up with
And so Charlie bridged that with ethylene bromide, and came up with 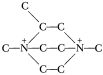 The propellant had a freezing point well below –54°, and card-gapped at only three cards. Otherwise, it was just like Cavea. It was called Cavea B (the Rocket Branch thought that “2 methyl Cavea” would be too revealing a name!)
The propellant had a freezing point well below –54°, and card-gapped at only three cards. Otherwise, it was just like Cavea. It was called Cavea B (the Rocket Branch thought that “2 methyl Cavea” would be too revealing a name!)
Wyandotte had made other similar compounds, some with two extra methyl groups, variously arranged, but Cavea B was the simplest and hence the best, and the others got nowhere. And Spurge Mobley had found himsel a seven-membered piperazine-like compound 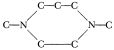 and had bridged that one to produce the odd structure
and had bridged that one to produce the odd structure 
Spurge was highly indignant at me because, while his creation had the right freezing point, I noted that it was no improvement over Cavea B and cost several times as much, and so wouldn’t push it.
Cavea B was the winner, and seemed to be the ideal monopropellant. And by the end of the year it had been fired successfully by NARTS, GE, Wyandotte, and Hughes Tool, with JPL soon to follow. It performed very well in a motor, yielding 94 or so percent of the theoretical impulse with a comparatively small chamber. Combustion was remarkably smooth—better than with the original Cavea (now called Cavea A) which was apparently just too symmetrical and stable to offer the combustion process any place to take hold. And there was no difficulty with supply. Wyandotte had any amount of the piperazine raw material.
Although the AN’s were firmly planted in center stage at this time, other monopropellant systems were vigorously elbowing their way toward the spot light. For instance, Kenneth Aoki, at Wyandotte, made  the diamine oxide of triethylenediamine, and dissolved that in nitric acid. But he found that the heat of solution was so high (the acid probably decomposed the oxide and formed the nitrate instead) that any possible performance advantage over Cavea A or B was negated. He also made
the diamine oxide of triethylenediamine, and dissolved that in nitric acid. But he found that the heat of solution was so high (the acid probably decomposed the oxide and formed the nitrate instead) that any possible performance advantage over Cavea A or B was negated. He also made  intending to dissolve it in N2O4 orTNM, but found that it was too insoluble in those oxidizers to be of any use.
intending to dissolve it in N2O4 orTNM, but found that it was too insoluble in those oxidizers to be of any use.
A more interesting approach to monopropellant development derived from the B–N propellant system, described in the boron chapter. As has been related, bipropellant B–N work had been plagued by combustion problems, and it was hypothesized—or hoped—that these might be alleviated if the boron and the nitrogen were combined in the same propellant—or even the same molecule.
McElroy and Hough, at Callery Chemical Co. started work on what they called the “Monocals.” These consisted of an adduct, or addition compound of decaborane and two or three mulecules of monomethyl hydrazine, all dissolved in about seven molecules of hydrazine. The mixing of the MMH with the decaborane had to be done in solution, or the product would explode when it warmed up. The propellants weren’t particularly uniform, and varied considerably from lot to lot, for reasons not well understood, if at all. They were extremely viscous, but didn’t appear to be particularly sensitive. Wyandotte tried them on the test stand, in a 50-thrust motor, with discouraging results. Bill Cuddy and his crew got off five runs altogether, four of them either starting or ending with a detonation and a wrecked motor. The Monocals died, unlamented, in 1960.
“Dekazine” lasted a little longer. In June of 1958, H. F. Hawthorne’s group at Rohm and Haas prepared B10H12 ∙ 2NH3 by reacting bis (acetonitrile) decaborane with hydrazine. They had hoped to incorporate the N–N group of the hydrazine into the decaborane molecule, so as to get the closed cage carborane structure, but found that their product retained the open decaborane basket, and had no N-N bonds. At any rate, they dissolved one mole of it in about 7.5 of hydrazine (they couldn’t get it to dissolve in fewer) and thought that they had a monopropellant. It wasn’t the easiest thing in the world to live with. First, it picked up oxygen from the air. Second, it was thermally rather sensitive, starting to decompose exothermically at 127°. Its card-gap value was low—about 4 cards—but it was indecently sensitive to adiabatic compression, rating, on that test, between normal propyl nitrate and nitroglycerine. But they managed to get it fired, over the next couple of years, by Spencer King at Hughes and by Bob Ahlert at Rocketdyne at the 500-pound thrust level. Nobody ever got more than 75 percent of its theoretical performance out of it, and nobody could seem to find a way to prevent it from detonating (usually near the injector in a motor run) when it felt in the mood. Which was frequently—Ahlert survived some really impressive explosions. And so by the end of 1960, everybody gave up Dekazine as a bad job, and it was tenderly laid out on the marble slab next to that occupied by the late Monocal.
In 1959, however, Hawthorne of Rohm and Haas made an interesting observation, which was to lead to a different approach to the problem of a B-N monopropellant. He observed that when he reacted the bis (acetonitrile) (An) adduct of decaborane, in benzene at room temperature, with triethylamine (NEt3) the reaction went mainly as below.
B10H12An2 + 2NEt3 → B10H12(NEt3)2 + 2An
However, if he ran the reaction at the boiling (refluxing) point of benzene, the reaction went almost quantitatively this way:
B10H12An2 + 2NEt3 → (HNEt3+)B10H10= + 2An
The open decaborane basket had closed up to the 10-cornered, 16-faced closed-cage structure of what was later called the “perhydrodecaborate ion.” This was a remarkably stable structure, the anion of a very strong acid—almost as strong as sulfuric acid. It was no trick to get the hydrazine salt of this acid (several simple routes were discovered that same year), (N2H5)2B10H10. The unsolvated salt was shock-sensitive, but when crystallized with either one or two molecules of hydrazine—it was easy to get either form—it was safe and easy to handle. And it could be dissolved in hydrazine to make a propellant.8
Unfortunately, you couldn’t dissolve enough of it in hydrazine to get the number of B atoms in the mixture to equal the number of N atoms, which is what you want with a B–N monopropellant. Lou Rapp, at Aerojet (he had recently moved there from Reaction Motors) somewhere around the beginning of 1961, thought that if he substituted one of the “Hepcat” cations for the hydrazinium ions in the salt, he might be able to remedy that deficiency, I had the same idea at the same time—and I could move faster than he could. My outfit was small, and I didn’t have to pay any attention to contracts, since I didn’t have any, the brass seldom paid any attention to what I was doing, and I could usually try whatever I wanted to try before anybody in authority could get around to telling me not to. So it went like a breeze.
I had Mobley make up a few grams of the Hepcat chloride by milling ammonium chloride and lithium borohydride together, as has already been described. And I had him make up some potassium perhydrodecaborate. Then he dissolved the two in liquid ammonia, and mixed them together in the proper proportions. The reaction went
K2B10H10 + 2[BH2(NH3)2]Cl →
[BH2(NH3)2]2B10H10 + 2KCl.
The potassium chloride precipitated, and was filtered off, the ammonia was allowed to evaporate, and I had the Hepcat perhydrodecaborate. After making sure that I had what I thought I had—using IR and so on for diagnosis—I had him add one mole of it to six moles of hydrazine. Four of the hydrazines displaced the ammonias in the cations and it bubbled off, and two were left over as solvent. So I finally had [BH2(N2H4)2]2Bi10H10 + 2N2H4. Here I had two borons in the cations, and ten in the anion. There were eight nitrogens in the cations, and four in the solvent. And finally, there were twenty hydrogens in the cations, ten in the anion, and eight in the solvent, so that the whole mess balanced to 12BN + 19H2. And miraculously, the thing was liquid at room temperature, and not too viscous, and didn’t appear to be particularly sensitive. We had only a few cc of the stuff, but it looked interesting.
The psychological payoff came at a monopropellant meeting in August. Lou Rapp described what he was trying to do, and I then took a sadistic delight (I was chairman of that session) in pulling the rug out from under him by pointing out that we had already done it, and describing how. Lou Rapp and I were good friends, but it wasn’t often that I had a chance to do him in the eye, and it was too good an opportunity to miss.
However, that was about as far as that propellant ever got. The Army brass (the Navy had moved out a year before, and the Army had taken over NARTS, which became the Liquid Rocket Propulsion Laboratory of Picatinny Arsenal) passed down the word that the Army had no interest in BN monopropellants, and to knock it off. I believe that their decision was the right one. My monster would have been horribly expensive to make, its density was by no means impressive, and there was no a priori reason to believe that it would perform any better than the other B–N monoprops. The stuff was not a practicable propellant. The whole performance had been a tour de force designed to show that a balanced B–N monoprop could be made. It was a lot of fun, but it was the end of the B–N monopropellants.
All this interest in monopropellants had led to the formation, at the first monopropellant conference in November 1953, of the Monopropellant Test Methods Committee, which was operated first under the sponsorship of BuAer, then under the American Rocket Society, and then under Wright Air Development Center. In November 1958 its field was extended to cover all liquid propellants, and the Liquid Propellant Information Agency took it over, and it’s still in operation. I served on it, on and off, for several years.
The original reason for its formation was the inherent instability of monopropellants. Any monopropellant with a reasonable amount of energy in it can be detonated if you go about it the right way. Everybody in the business had his own pet method of measuring the sensitivity of the monoprops he was working with. The only difficulty was that no two methods were alike, and it was just simply impossible to compare the results from one laboratory with those from another. In fact, it was just about impossible to define, say, shock sensitivity, at all. Even the relative sensitivities of two propellants might depend—and often did—upon the apparatus used to make the measurement. The job of the committee was to examine all the methods used, to pick out those which gave more or less reproducible results, or to talk people into developing such methods, then to standardize these, and finally to try to persuade the people in the field to use those methods. Then, it was hoped, even if none of our tests or results made sense we would be unanimous in our fantasies and could talk to each other with something approaching coherence, and with luck, comprehension by the hearer.
The first test that was adopted in July 1955 was the card-gap test, previously described. It had first been developed by the Waltham Abbey People, and then streamlined by NOL, and we spent a lot of time chasing down anomalous results. I remember Joe Herrickes, of the Bureau of Mines, and I firing our two card-gap setups side by side for a whole day, trying to discover why, with one monopropellant they agreed beautifully, and with another disagreed wildly. We finally discovered, after interchanging practically everything in the setups, that the trouble lay in the sample cups. Joe’s were made of aluminum, mine of iron pipe. The committee standardized on the iron pipe, and we published. Putting a final report together was sometimes something of a Donnybrook. The committee, as might be imagined, was composed of highly self-confident and howlingly articulate individualists, and there were always at least six of us present each of whom considered himself a master of English prose style. Whew!
A test that took a lot longer to decide on was the “Drop-Weight.” For years, people in the explosive business had been dropping weights on samples of their wares, and rating their sensitivity on the basis of how far you had to drop how big a weight in order to make the sample go off. We looked into the matter and discovered, to our dismay, that the JPL tester disagreed with the Picatinny tester, which disagreed with the Hercules apparatus, whose results could not be compared with those of the Bureau of American Railroads, which, in turn, contradicted those of the Bureau of Mines. Furthermore, none of them was any good at all with liquids.
Bill Cuddy, of Wyandotte, in March 1957 described a tester designed specifically for liquids; it was modified by Don Griffin of Olin Mathieson and finally evolved into what is known as the OM drop weight tester.9 Actually it was a device for measuring adiabatic sensitivity. The falling weight suddenly compressed a tiny and standardized air bubble above the one drop sample, and the adiabatic heating of this bubble set the thing off—or wasn’t enough to do it, as the case might be. It was, and is, quite a satisfactory instrument once you got used to its little foibles. For instance, it has to be on a really solid foundation if you hope to get reproducible results. We ended up with the instrument bolted to a three-foot square of three-inch armor plate, which was in turn bolted to a six-foot cube of concrete which rested on bedrock—granite. That way, it worked fine.
Al Mead, of Air Reduction, came up in 1958 with the standard thermal stability tester, another very useful instrument. In it, a small sample was heated at a constant rate, and the temperature at which the sample started to warm up faster than the heating bath was taken as the takeoff point. We used it, along with our own, for years. They really measured different things, and we could use them both.
Other tests were standardized and published, but these were the more useful and most often used. One subject that was investigated for years was detonation velocity, the critical diameter for detonation, and the construction of detonation traps. If you have a monopropellant blow in your motor, that’s one thing. But if that detonation propagates back (at some 7000 meters per second, usually) through the propellant line to the propellant tank, and that blows, then you can be in real trouble. If the diameter of the propellant line is small enough, the detonation will not propagate and dies out—the limiting diameter being called the “critical diameter.” It varies with the nature of the propellant, the material of which the line is made (steel, aluminum, glass, etc.) with the temperature, and maybe with a few more things. (When we found that a detonation in Isolde would propagate nicely through hypodermic-needle tubing, our hair stood on end, and we perspired gently.)
Starting around 1958, and carrying on into 1962, a lot of work on detonation propagation and trapping was carried on at Rocketdyne, Wyandotte, JPL, BuMines, GE, Hughes, Reaction Motors, and NARTS-LRPL, and valiant efforts—some of them successful—were made to find a way to stop a detonation in its tracks. Because that can be done, sometimes, by putting a detonation trap in the line. Designing such a thing is not a scientific matter; it’s a piece of empirical engineering art. And the various designs showed it. One early trap consisted simply of a loop in the line—like so: When the detonation came barreling down the line, blowing up the tubing as it came, it would cut the other part of the line where they crossed, leaving the detonation no place to go. This wasn’t too reliable. The bang that cut the line might start another, brand new, detonation going. Bob Ahlert did very well by putting a section of Flex-Hose, a Teflon tube reinforced with a metal wire mesh, in his line. The detonation would simply blow this weak section out, and then have nowhere to go. And Mike Walsh, of our group, devised a trap that worked beautifully with Cavea B, as well as with several other monopropellants. Cavea B would not propagate a detonation through a 0.25-inch line, but would through a one-inch line. So Mike inserted a one-foot long piece of two-inch piping into his one-inch line, and filled this section with a cylindrical bar of a plastic that would resist the propellant for a reasonable time. (Polystyrene was good.) And he drilled sixteen 0.25-inch holes longitudinally through this cylindrical plug, so that he had the same area for flow as he had in the one-inch main line. And when he checked it out, the detonation rolled down to the trap, blew up about the first third of that, and stopped cold.
But detonation traps aren’t always the complete answer. We discovered that when, in the summer of 1960, we tried to fire a 10,000-pound thrust Cavea B motor. We didn’t have Mike’s trap at that time, so we inserted a battery of sixteen 0.25-inch loop traps in the line. Well, through a combination of this and that, the motor blew on startup. We never discovered whether or not the traps worked—we couldn’t find enough fragments to find out. The fragments from the injector just short-circuited the traps, smashed into the tank, and set off the 200 pounds of propellant in that. (Each pound of propellant had more available energy than two pounds of TNT.) I never saw such a mess. The walls of the test cell—two feet of concrete—went out, and the roof came in. The motor itself—a heavy, workhorse job of solid copper—went about 600 feet down range. And a six-foot square of armor plate sailed into the woods, cutting off a few trees at the root, smashing a granite boulder, bouncing into the air and slicing off a few treetops, and finally coming to rest some 1400 feet from where it started. The woods looked as though a stampeding herd of wild elephants had been through.10
As may be imagined, this incident tended to give monopropellants something of a bad name. Even if you could fire them safely—and we soon saw what had gone wrong with the ignition process—how could you use them in the field? Here you have a rocket set up on the launching stand, under battlefield conditions; and what happens if it gets hit by a piece of shrapnel? LRPL came up with the answer to that. You keep your monoprop in the missile in two compartments: one full of fuel-rich propellant made up to λ = 2.2 or 2.4, and the other containing enough acid to dilute it to λ = 1.2. Just before you fire, a can-opener arrangement inside the missile slits open the barrier separating the two liquids, you allow a few seconds for them to mix, and then push the button. The idea—it was called the “quick mix” concept—worked fine. We couldn’t use a double-ended compound like Cavea A or B for the propellant—made up to 2.4 the freezing point was too high—so we first tried “Isobel,” the dimethyldiisopropyl ammonium nitrate, I phoned a chemical company in Newark to have them make us a hundred pounds or so of the salt, and was assured by their director of research that it couldn’t be done, since the compound was sterically impossible. I protested that I was staring at that moment at a bottle of the salt sitting on my desk in front of me, but I couldn’t convince him. Isobel, however, didn’t quite meet the freezing point limitations, so we shifted to Isobel E and Isobel F, the diethyldipropyl, and the ethyltripropyl salts, respectively, which did. These didn’t have quite the thermal stability I wanted (usually the higher the λ the worse the stability) so I finally came up with Isobel Z, diethyldiisopropyl ammonium nitrate, which was immensely better. (If that character in Newark thought that Isobel was impossible, I wonder what he would have thought of Isobel Z! There actually isn’t any trace of steric hindrance in the ion, but it’s a near thing. Not one more carbon atom could have been crammed into the space around the nitrogen.) LRPL tried the fuel-rich Isobels, at 2.4 or so, as low-energy monopropellants, for APU work and so on, and found that they worked very well in such a role. That work was pretty well cleaned up by 1962.
Dave Gardner, of Pennsalt, didn’t anticipate any detonation problems when he started work in May of 1958. What the Air Force wanted from him was a low-energy monoprop for APU work, and one with such thermal stability that it could survive at 200–300°C, and Dave naturally figured that a low-energy compound with that sort of stability wasn’t going to give him much trouble. He worked for about three years on the job—Sunstrand Machine Tool did his motor work—at first with mixtures of extremely stable—and hence low-energy—fuels in various acid oxidizers. His fuels were sometimes salts; the tetramethyl ammonium salt of sulfuric acid, or of fluorosulfonic acid, or of trifluoromethane sulfonic acid, or the pyridine salt of sulfuric acid. Sometimes he used methane or ethane sulfonic acid itself as a fuel, or the fluorinated CF3CH2SO3H. His oxidizers were either perchloric acid dihydrate, or a mixture of nitrosylpyrosulfate and sulfuric acid, sometimes with some nitric acid added. Performance—which he wasn’t looking for—was naturally poor, but he had some remarkably heat-resistant propellants on his hands.
A little later, impressed by the remarkable stability of the—CF(NO2)2 group he synthesized the monopropellant CH3CF(NO2)2, which he called “Daphne.” (I never discovered what the name celebrated—or commemorated.) The performance wasn’t particularly startling—that C-F bond is awfully strong—but the material appeared to be stable to just about everything. But, alas, Daphne, too, was a woman, and could betray him. And took out most of the test cell when she did.
Incidental to his monopropellant work, Dave produced a pair of rather interesting high-density oxidizers, by fluorinating potassium nitroformate, KC(NO2)3 and similar compounds. They were F—C(NO2)3 which he called D-11, and  called D-112. He measured their heat of formation by reacting them in a bomb calorimeter with carbon monoxide, and when he reported his results insisted on putting my name on the paper as coauthor (which I was not) because I’d tried that reaction first, with tetranitromethane, and had suggested it to him as a good way to get a handle on the thermochemistry of highly oxygenated compounds. The oxidizers appeared, unfortunately, just in time to be made obsolete by ClF5.
called D-112. He measured their heat of formation by reacting them in a bomb calorimeter with carbon monoxide, and when he reported his results insisted on putting my name on the paper as coauthor (which I was not) because I’d tried that reaction first, with tetranitromethane, and had suggested it to him as a good way to get a handle on the thermochemistry of highly oxygenated compounds. The oxidizers appeared, unfortunately, just in time to be made obsolete by ClF5.
One of the strangest, and certainly the hairiest, chapter in the propellant story had its start in two unrelated but almost simultaneous events. First, the Advanced Research Projects Administration (ARPA) initiated “Project Principia” early in June 1958. The idea was to get certain large chemical companies into the business of developing improved solid propellants. “To get the benefit of their fresh thinking,” was the phrase. American Cyanamid, Dow Chemical, Esso R and D, Minnesota Mining and Minerals (3M), and Imperial Chemical Industries were those invited to enter the field.
The old line propellant people—Reaction Motors, Aerojet, Rocketdyne—not unnaturally raised a row. In fact, they screamed from the rooftops. “These newcomers may have all sorts of fancy equipment, and they may be hot on molecular orbital theory and pi bonds, but they don’t know a propellant from a panty raid, and what you’ll get is a lot of expensive and useless chemical curiosities. We, on the other hand, have been living with propellants for years, and know what they have to do, and we’re not such bad chemists ourselves, and just get us the same fancy equipment and anything they can do we can do better!” But their protests fell on unheeding ears, and “Principia” got under way. (In the end, the professional propellant people turned out to be pretty good prophets.)
The second event was the announcement by W. D. Niederhauser of Rohm and Haas, at a propellant meeting late in September, that N2F4, in a gas phase reaction, would add across an olefinic bond, forming the structure  and that this was a general reaction, applicable to practically any olefin. He also announced that HNF2 could be prepared by reacting N2F4 with AsH3.
and that this was a general reaction, applicable to practically any olefin. He also announced that HNF2 could be prepared by reacting N2F4 with AsH3.
So all the companies concerned in Principia started reacting N2F4 with various olefins to see what would happen. (Frequently what happened was that the reactor blew up.) Rohm and Haas was, of course, already doing this sort of thing, and at the beginning of 1959 both Reaction Motors and Jack Gould at Stauffer dropped the monopropellant systems they had been investigating and joined the new field of “N-F” chemistry. And Callery Chemical Co. quickly did the same. So there were soon at least nine organizations involved in this sort of research.
A few glitches interfered with the march of science, and the progress of the solid propellant program. The first was that practically none of the new compounds discovered were solids. Most of them were liquids, and rather volatile liquids at that. ARPA bowed to fate and the facts of life, and in November of 1960 rewrote the contracts to include work on liquids. The second glitch could not, unfortunately, be cured by making a mark on a piece of paper.
Practically every compound discovered turned out to be indecently sensitive and violently explosive. For instance, take the case of the simplest NF compound, the first one discovered. Thermodynamically, it tends to react  And given the slightest provocation, it will do just that. And the formation of four molecules of HF produces enough energy to make the resulting explosion really interesting. The things were shock sensitive. Many of them were heat sensitive. And some were so spark sensitive that they couldn’t be poured. When you tried it, the tiny static charge formed was frequently enough to trigger a detonation. The only way to reduce their sensitivity, apparently, was to make a compound with a large molecule and a small number of NF2 groups. Which was not good for its energy content.
And given the slightest provocation, it will do just that. And the formation of four molecules of HF produces enough energy to make the resulting explosion really interesting. The things were shock sensitive. Many of them were heat sensitive. And some were so spark sensitive that they couldn’t be poured. When you tried it, the tiny static charge formed was frequently enough to trigger a detonation. The only way to reduce their sensitivity, apparently, was to make a compound with a large molecule and a small number of NF2 groups. Which was not good for its energy content.
Another thing that was bad for their (prospective, and eventually, maybe) propellant performance was the fact that on decomposition they produced considerable quantities of free carbon, which, as has been explained, is not good for performance. The obvious thing to do was to incorporate enough oxygen in the molecule to burn the carbon to CO. Dow, in the spring of 1960, synthesized such a compound, balanced to CO by the direct fluorination of urea. Its structure was  and it is perhaps just as well that it was produced in very small quantities, as it was indecently spark sensitive. Jack Gould, in 1961, reported on F2N—CH2—OH and it was synthesized by I. J. Solomon of the Armour Research Institute, early in 1963, by the mole for mole reaction of HNF2 and formaldehyde. Again—impossibly sensitive. Allied Chemical Co., in 1962, and the Peninsular Chemical Co., about four years ago, tried to synthesize its isomer CH3—O—NH2. If they had succeeded, it would have served them right. I know from my own experience that methoxy amine, CH3—O—NH2, which can decompose at worst and most energetically, only to CO and NH3 plus a little hydrogen, is quite sensitive on the OM drop-weight apparatus. What a similar compound, whose decomposition would lead to the formation of two HF molecules would be likely to do, simply boggles the mind.
and it is perhaps just as well that it was produced in very small quantities, as it was indecently spark sensitive. Jack Gould, in 1961, reported on F2N—CH2—OH and it was synthesized by I. J. Solomon of the Armour Research Institute, early in 1963, by the mole for mole reaction of HNF2 and formaldehyde. Again—impossibly sensitive. Allied Chemical Co., in 1962, and the Peninsular Chemical Co., about four years ago, tried to synthesize its isomer CH3—O—NH2. If they had succeeded, it would have served them right. I know from my own experience that methoxy amine, CH3—O—NH2, which can decompose at worst and most energetically, only to CO and NH3 plus a little hydrogen, is quite sensitive on the OM drop-weight apparatus. What a similar compound, whose decomposition would lead to the formation of two HF molecules would be likely to do, simply boggles the mind.
Esso synthesized a number of nitrate and nitramine derivatives of NF compounds, of which 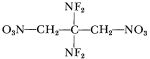 firstdescribed early in 1962, is typical. Some few of the series were solids rather than liquids, but all were impossibly sensitive. This compound is interesting, however, in having two NF2 groups on the same carbon atom. This structure was made possible by a reaction discovered by the Rohm and Haas people in 1960–61. If HNF2 is reacted with an aldehyde or ketone in concentrated sulfuric acid, the reaction goes
firstdescribed early in 1962, is typical. Some few of the series were solids rather than liquids, but all were impossibly sensitive. This compound is interesting, however, in having two NF2 groups on the same carbon atom. This structure was made possible by a reaction discovered by the Rohm and Haas people in 1960–61. If HNF2 is reacted with an aldehyde or ketone in concentrated sulfuric acid, the reaction goes 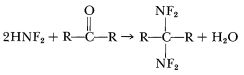 and you are left with two NF2 groups on the same carbon. The reaction was quite general, and a wide variety of “geminal” difluoramino compounds were synthesized. They were just as sensitive as the “vicinal” or
and you are left with two NF2 groups on the same carbon. The reaction was quite general, and a wide variety of “geminal” difluoramino compounds were synthesized. They were just as sensitive as the “vicinal” or 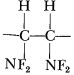 type.
type.
Another way to get oxygen into the monopropellant was to mix the NF compound with an oxygen-type oxidizer. Jack Gould (Stauffer) came up in 1961 with a concoction he called “Hyena,” which consisted of an NF (usually F2NC2H4NF2) dissolved in nitric acid. J. P. Cherenko, of Callery, produced similar mixtures (called “Cyclops” this time) but he sometimes used N2O4 or tetranitromethane instead of the acid, and sometimes tranquilized the propellant (he hoped) by adding pentane. Hyena and Cyclops were both unmitigated disasters. The man who was determined to make an NF monopropellant work, or to prove, definitely, that it couldn’t be done, was Walt Wharton of the Army Missile Command, at Huntsville, and from the middle of 1961 to the end of 1964 he and Joe Connaughton valiantly and stubbornly pursued that objective. His chosen compound was IBA, the IsoButylene Adduct of N2F4, made by Rohm and Haas by the reaction ![]() If the compound is mixed with N2O4 (1.5 molecules of the latter to one of IBA) the mixture is a monopropellant with a good density and a fairly attractive (theoretical) performance—293 seconds. Other compounds containing more NF2 groups would have given more, but the idea was to get any NF to work at all.
If the compound is mixed with N2O4 (1.5 molecules of the latter to one of IBA) the mixture is a monopropellant with a good density and a fairly attractive (theoretical) performance—293 seconds. Other compounds containing more NF2 groups would have given more, but the idea was to get any NF to work at all.
IBA, straight, was extremely sensitive on the OM drop-weight apparatus, and Wharton was immensely encouraged, at first, to discover that the addition of a very small amount of N2O4—less than 1 percent—cut this sensitivity down to practically nothing. But then he started burning-rate studies, in a liquid strand burner. He had ignition problems—a hot wire wasn’t too reliable—and he discovered that the burning rate of the material was vastly sensitive to the bomb pressure. (A trace of ferric chloride would decrease the rate, and one of carbon tetrachloride would increase it.) He furthermore discovered that the material had a lamentable tendency to detonate in the bomb or in a motor, that a glass tube detonation trap wasn’t particularly helpful, and he was made a bit thoughtful by the discovery that the critical diameter for detonation propagation was less than 0.25 millimeters—less than 0.01 inch.
Most of his early work was done with a “T” motor, little more than an injector and a nozzleless chamber, with observation ports so that the ignition process could be observed with a high-speed camera. They tried a slug of ClF3 for ignition, and got a detonation instead. They eventually settled on a slug of antimony pentachloride—of all things—which gave a smooth and reliable start. By this time they were working with an “expendable” motor with no nozzle and a low chamber pressure, about two atmospheres, and were mixing the N2O4 and IBA remotely right on the test stand. It was fortunate that they worked remotely, since 150 cc of the mixture detonated during a run and wrecked the setup.
In the winter of 1962–63 they sent a sample of IBA (dissolved in acetone so it could be transported more or less safely) up to LRPL for card-gap work. We gently distilled off the acetone, and made the tests. (Mixing the IBA and the N2O4 was a precarious business.) Straight IBA wasn’t particularly sensitive on card-gap, about ten cards, and the material with 1 percent of N2O4 in it was about the same. But when mixed up for maximum performance—one mole of IBA to 1.5 of N2O4—the sensitivity was more than 96 cards. We never discovered how much more; our interest in the subject had evaporated.11
These figures did not encourage further work with the IBA-N2O4 mixture. There was some talk of using the combination as a bi-propellant, but that would have been rather pointless. Wharton and Connaughton fired the straight IBA as a monopropellant, at the 250-pound thrust level, but there was so much free carbon in the exhaust that they never got more than 80 percent even of its rather low specific impulse. They were driven, reluctantly, to the conclusion that an NF monopropellant was not practical politics, and abandoned the whole idea late in 1964.
Just as Wharton was starting his IBA work, there occurred one of the weirdest episodes in the history of rocket chemistry A. W. Hawkins and R. W. Summers of Du Pont had an idea. This was to get a computer, and to feed into it all known bond energies, as well as a program for calculating specific impulse. The machine would then juggle structural formulae until it had come up with the structure of a monopropellant with a specific impulse of well over 300 seconds. It would then print this out and sit back, with its hands folded over its console, to await a Nobel prize.
The Air Force has always had more money than sales resistance, and they bought a one-year program (probably for something in the order of a hundred or a hundred and fifty thousand dollars) and in June of 1961 Hawkins and Summers punched the “start” button and the machine started to shuffle IBM cards. And to print out structures that looked like road maps of a disaster area, since if the compounds depicted could even have been synthesized, they would have, infallibly, detonated instantly and violently. The machine’s prize contribution to the cause of science was the structure, 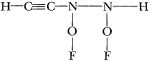 to which it confidently attributed a specific impulse of 363.7 seconds, precisely to the tenth of a second, yet. The Air Force, appalled, cut the program off after a year, belatedly realizing that they could have got the same structure from any experienced propellant man (me, for instance) during half an hour’s conversation, and at a total cost of five dollars or so. (For drinks. I would have been afraid even to draw the structure without at least five Martinis under my belt.)
to which it confidently attributed a specific impulse of 363.7 seconds, precisely to the tenth of a second, yet. The Air Force, appalled, cut the program off after a year, belatedly realizing that they could have got the same structure from any experienced propellant man (me, for instance) during half an hour’s conversation, and at a total cost of five dollars or so. (For drinks. I would have been afraid even to draw the structure without at least five Martinis under my belt.)
The NF programs led to some interesting, if eventually unproductive, oxidizer work. It was obvious, very early in the game, that if you could tie enough NF2 groups to a carbon atom, the result would be more a fluorine-type oxidizer than a monopropellant. Cyanamid, late in 1959, took the first step in this direction when they synthesized 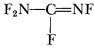 Then 3M, in the spring of 1960, synthesized “Compound M,” F2C(NF2)2 by the direct fluorination of ammeline, and a little later came up with “Compound R,” FC(NF2)3 by the same route. Dow and 3M, in 1960, both synthesized perfluoroguanidine, or “PFG” FN=C(NF2)2 by the reaction of fluorine diluted with nitrogen on guanidine. And finally, in 1963, “Compound Δ” (delta) or “T” or “Tetrakis”—from tetrakis (difluoramino)methane—C(NF2)4 was synthesized at Cyanamid by Frank, Firth, and Myers, and by Zollinger at 3M. The former had fluorinated the NH3 adduct of PFG, the latter had used the HOCN adduct.
Then 3M, in the spring of 1960, synthesized “Compound M,” F2C(NF2)2 by the direct fluorination of ammeline, and a little later came up with “Compound R,” FC(NF2)3 by the same route. Dow and 3M, in 1960, both synthesized perfluoroguanidine, or “PFG” FN=C(NF2)2 by the reaction of fluorine diluted with nitrogen on guanidine. And finally, in 1963, “Compound Δ” (delta) or “T” or “Tetrakis”—from tetrakis (difluoramino)methane—C(NF2)4 was synthesized at Cyanamid by Frank, Firth, and Myers, and by Zollinger at 3M. The former had fluorinated the NH3 adduct of PFG, the latter had used the HOCN adduct.
All these compounds were difficult to make—only R ever achieved synthesis in pound lots—and incredibly expensive. Their calculated performances, with suitable fuels, was impressive enough, but their sensitivity was even more so. None of them could be lived with. Attempts were made to tranquilize them by mixing them with less temperamental oxidizers, but the results were not happy. Wharton worked for some time with a mixture of R and N2O4, and Aerojet tried some mixtures (called “Moxy”), comprising R, N2F4 and ClO3F, or Δ, N2F4 and ClO3F. But it was hopeless. When the NF oxidizer was sufficiently diluted to be safe, all its performance advantage had gone with the wind.
There was some thought that an OF structure attached to the carbon would be more stable than the NF2 structure; and in 1963 W. C. Solomon of 3M showed the way to such structures by reacting fluorine with oxalates suspended in perfluorokerosene, in the presence of a transition metal, to get F2C(OF2)2. Three years later, Professor George Cady’s group, at the University of Washington, synthesized the same compound, neatly and elegantly, by reacting fluorine and carbon dioxide, at room temperature, in the presence of cesium fluoride. But the very mildness of the conditions for its synthesis showed that it was too stable to be of much use as an oxidizer. And, finally, as has been mentioned in the halogen chapter, the group at Allied Chemical, reacted ONF3 with a perfluoro olefin, such as tetrafluoroethylene, to get CF3—CF2—ONF2 or one of its cousins. But an ONF2 group attached to a heavy and remarkably stable fluorocarbon residue isn’t very useful in the rocket business.
So in the long run, NF programs didn’t lead to much in the way of practical liquid propellants, brilliant as was some of the chemistry exhibited. The record of this chemistry is now being collected, to be embalmed safely in a definitive text, so that nobody will ever, ever, have to risk his neck doing it again.
As for the original object of Principia: solid propellant grains containing NF2 groups have been made—and fired. But they have a long way to go and if they are operational before 1980 or so I, for one, will be surprised.
And as for the future of the high-energy monopropellants: I’m afraid that it’s in the past. We all worked for years trying to reconcile properties which we finally and sadly concluded were irreconcilable—high energy and stability. For all our efforts, no high energy monoprop has made the grade to operational status. Cavea B almost made it, but “almost” is not success. But it was a damned good try!
1. But it seemed to be the way to a liquid gun propellant. Even a low-energy monopropellant has more energy in it per gram than does smokeless ball powder, and a great deal more energy per cubic centimeter. (A liquid is much more closely packed than a heap of small grains.) So, if a liquid propellant were used, either packaged in the cartridge as the solid propellant is, or pumped separately into the gun chamber behind the bullet, it should be possible to get a much higher muzzle velocity without any increase in weight. Hydrazine–hydrazine nitrate–water mixtures have been the usual propellants in the liquid gun programs, although NPN sometimes mixed with ethyl nitrate, has been used at times. These programs have been running, on and off, since about 1950, but have never been carried through. The military demands a weapon, programs are started and run for a few years, then money or interest runs out, and the whole thing ends, only to start all over five or six years later. I’ve seen three cycles since I got into the business. JPL, Olin Mathieson, Detroit Controls, as well as various Army and Air Force installations, have been involved. The main problems are more in the engineering than in the chemistry.
2. A liquid strand burner is a gadget which will give you some idea of the burning rate of a monopropellant. It is a pressurized container (bomb), usually with a window. The monopropellant is burned in a narrow (a few millimeters diameter) vertical glass tube. If the tube is not too wide, the propellant will burn straight down like a cigarette, and the rate can be observed and measured. The bomb is pressurized with nitrogen, to pressures similar to those in a rocket combustion chamber, and the burning rate is measured as a function of pressure. It was developed from the strand burner used for solid propellants. Dr. A. G. Whittaker, at NOTS, burned, among other things, a mixture of nitric acid and 2 nitropropane. He was the first to make much use of it. That was in the early 50’s.
3. Since the propellant was named “Isolde” it seemed only reasonable to call the igniter “Tristan.” Then somebody pointed out that the missile using the system would naturally be called the “King Mark.” But when somebody else added that the advanced model of the missile would of course be the “King Mark II” the engineering officer started muttering wistfully about flogging at the gratings, keel-hauling, and the uses of yardarms, and the “Tristan” idea died an untimely death.
4. His idea was to set up a liquid oxygen plant alongside a natural gas well, tank up your ICBM on the spot, and push the button.
5. Sometime later, Irv Glassman, of the Forrestal Laboratories, conceived of an interesting and entirely different type of cryogenic monopropellant. The idea was to use a mixture of acetylene and excess liquid hydrogen. When they reacted, the product would be methane, which, with the excess hydrogen, would be the working fluid, while the heat of decomposition of the acetylene plus that of formation of the methane would be the energy source. Considering the theoretical performance, the chamber temperature would have been remarkably low. The idea, however, has not yet been tested experimentally.
6. About this time I got curious as to whether or not the structure-sensitivity relationships I had found in the amine nitrate monoprops applied to other systems, particularly since McGonnigle had remarked to me that straight chain hydrocarbons in N2O4 were more sensitive than branched chains. I knew that Tallulah at λ = 1.0 with the fuel ion structure 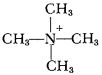 card-gapped at some eight cards, while the propellant with the isomeric fuel ion NH3+—CH2—CH2—CH2—CH3 gapped 58—a difference of fifty cards due to structure alone. So I got some normal pentane, CH3—CH3—CH2—CH2—CH2—CH3 and some neopentane
card-gapped at some eight cards, while the propellant with the isomeric fuel ion NH3+—CH2—CH2—CH2—CH3 gapped 58—a difference of fifty cards due to structure alone. So I got some normal pentane, CH3—CH3—CH2—CH2—CH2—CH3 and some neopentane 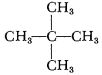 and had them both made up with N2O4 to λ = 1.0, and had them card-gapped. And the normal pentane had a sensitivity of about 100 cards, and the neopentane, 50 cards. Again a difference of 50 cards, which meant that the ratio of the critical shock wave pressures due to structure was the same in both systems. I was fascinated by this coincidence, but never had the chance to carry the work any further. It’s recommended to the attention of some future investigator.
and had them both made up with N2O4 to λ = 1.0, and had them card-gapped. And the normal pentane had a sensitivity of about 100 cards, and the neopentane, 50 cards. Again a difference of 50 cards, which meant that the ratio of the critical shock wave pressures due to structure was the same in both systems. I was fascinated by this coincidence, but never had the chance to carry the work any further. It’s recommended to the attention of some future investigator.
7. He visited me some weeks later, and I asked him what Jefferson’s substituted piperazines were used for. He answered, in a drawl as flat as Texas, “Well, they’re a lot of farmers down our way, and they raise a lot of hawgs. And the hawgs get intestinal worms, and don’t fatten up the way they should. So the farmer puts some of the piperazine in their feed, and the worm goes to sleep and forgets to hold on. And when he wakes up the hawg isn’t there any more!”
8. I had the bright idea that it might also be used to make a peroxide-based monopropellant. I had some of the ammonium salt of the perhydrodecaborate ion made, and put a few milligrams of it on a watch glass. I then put a drop of concentrated H2O2 next to the salt, and tilted the watch glass to bring the two into contact. There was a brilliant green-white flash, and the sharpest detonation I have ever heard. The watch glass was reduced, literally, to a fine powder. End of bright idea.
9. Don Griffin, a free soul if I ever knew one, then took a year’s vacation from rocket propulsion, spending it in the Hula-Hoop business. He said it made more sense.
10. When I got down to the test area after the bang, one of the rocket mechanics galloped up and demanded, “My God, Doc! What the Hell did you send us this time?” The only response of which I was capable was to light a cigarette and remark, “Now, really, Johnny! You should see my Martinis!” What got me though, was the remark by an officer from Picatinny, after viewing the mess. This was just before NARTS was due to be “disestablished” and taken over by the Army, and this character (metaphorically) held his nose and stated to nobody in particular, “Now I know what the Navy means by ‘disestablishment.’” I wanted to kill him.
11. Two people can operate the card-gap apparatus, and three operators is optimum. But when LRPL did this particular job (the feather-bedding at Picatinny was outrageous) there were about seven people on the site—two or three engineers, and any number of rocket mechanics dressed (for no particular reason) in acid-proof safety garments. So there was a large audience for the subsequent events. The old destroyer gun turret which housed our card-gap setup had become a bit frayed and tattered from the shrapnel it had contained. (The plating on a destroyer is usually thick enough to keep out the water and the smaller fish.) So we had installed an inner layer of armor plate, standing off about an inch and a half from the original plating. And, as the setup hadn’t been used for several months, a large colony of bats—yes, bats, little Dracula types—had moved into the gap to spend the winter. And when the first shot went off, they all came boiling out with their sonar gear fouled up, shaking their heads and pounding their ears. They chose one rocket mechanic—as it happens, a remarkably goosy character anyway—and decided that it was all his fault. And if you, gentle reader, have never seen a nervous rocket mechanic, complete with monkey suit, being buzzed by nine thousand demented bats and trying to beat them off with a shovel, there is something missing from your experience.