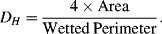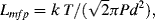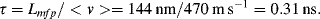Chapter 10
Microfluidics: Dimensional Analysis and Scaling
10.1 Chapter Overview
This chapter describes how dimensional analysis and the use of dimensional parameters can provide the means for simplifying complex physical problems. These tools elucidate the basic relationships between the dependent and independent variables of a physical effect or system and in this way can assist in the planning of experiments and the presentation of measured data designed to better understand them.
After reading this chapter readers will gain an understanding of:
10.2 Dimensional Analysis
Dimensional analysis can be used to reduce complex physical problems to more simple forms prior to attempting quantitative solutions. The basis of this analysis relies on the fact that physical laws are independent of arbitrarily chosen units of measurement, and that they also observe the concept of similarity. Forces acting on a particle do not change if we alter the unit of length from microns to miles, or the unit of time from nanoseconds to years, and so forth. In physical terms similarity refers to equivalence between two disparate phenomena. For example, under certain conditions there is a direct relationship between the forces acting on a log floating down a river and forces acting on a bioparticle in a microfluidic channel. We will also learn later in this chapter that the ‘ball of fire’ created by a nuclear explosion is similar to the behaviour of an air bubble rising in water! Dimensional analysis can elucidate the particular conditions required for the similarity of such phenomena, as well as the relationships between them.
An important aspect of dimensional analyses for microfluidics is an appreciation of how physical quantities scale as a function of the characteristic length L of a system. A simple, but instructive, application of scaling is to ask the question ‘Is it better to be a mouse or an arctic hare in the Arctic tundra?’ The parameter of interest is the ratio of heat loss to body mass. Heat loss is proportional to surface area (L2) and body mass is proportional to volume (L3). The ratio of heat loss to body mass thus scales as L−1. The heat loss suffered by a mouse (length ~ 4 cm) will thus be ten-times larger than that that experienced by a hare of length ~ 40 cm. The energy input (food) required by a mouse will scale accordingly. A polar bear (length ~ 2 m) would have an even more comfortable time than a mouse, by a factor of around 50:1. A scaling law therefore expresses the variation of physical quantities with a change in the size L the system, but maintaining all other quantities such as temperature, pressure and time, for example, constant. We have just considered the scaling of heat loss with body mass in terms of the ratio of a surface effect to a volume effect. We could also consider surface forces such as viscosity, capillary pressure and surface tension, and compare these to volume forces such as inertia and gravity. The basic scaling law for the relative importance of these two classes of force can be expressed as
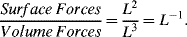
Thus, as L tends to zero the ratio of surface to volume forces approaches infinity. Capillary pressure and surface tension effects scale as L−1, so that with sufficient reduction of size they can overcome gravitational forces. This is why small diameter fluidic channels (capillaries) can pump water to the top of 100 m tall redwood trees, and how some small insects can walk on water, for example. The electric field generated between electrodes of constant voltage difference ΔV also scale as L−1, where L is the electrode gap. This has been exploited in the application of dielectrophoresis (DEP) described in Chapter 3. The DEP force acting on a particle is proportional to the product of the applied electric field and the local field gradient, and so has dimensions of (VL−1)(VL−2) =(V2L−3). Thus, if the effective volume enclosed by the electrodes is reduced 1000-fold, the same DEP force is exerted for a 100-fold reduction of the applied voltage.
A simple example of dimensional analysis is shown in Figure 10.1. The objective is to derive a proof for the theorem of Pythagoras, namely that in any right-angled triangle the square of the hypotenuse is equal to the sum of the squares on the other two sides.
Figure 10.1 Proof of the Pythagorean Theorem using dimensional analysis.
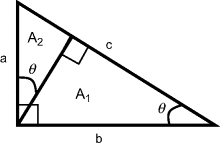
We proceed by recognising that the area At of the whole triangle (area A1 + area A2) shown in Figure 10.1 is a function of angle θ and c2:
| At = f(θ) c2 | |
| We also have | A1 = f(θ) b2 and A2 = f(θ) a2 |
| From the relationship | At = A1 + A2 |
| f(θ) c2 = f(θ) b2 + f(θ) a2 | |
| Cancelling f(θ): | c2 = b2 + a2 |
| QED |
10.2.1 Base and Derived Physical Quantities
Listed in Table 10.1 are the seven base quantities defined for the Système International (SI system), namely: length, time, mass, temperature, current, number of elementary particles, and luminous intensity. The units of length, time and mass are the metre (m), the second (s) and the kilogram (kg), respectively. The SI temperature unit is the kelvin (K), which is defined as the fraction 1/273.16 of the thermodynamic temperature of the triple point of water. The unit for current is the ampere (A) and is defined as the current which, when passed through each of two parallel and infinite, conductors placed one metre apart in vacuum, produces a force of 2 × 10−7 N m−1 on each conductor. The number of elementary particles (e.g. ions, molecules, cells, etc.) is counted in mole units, with one mole equal to Avagadro's number (6.02 × 1023). The candela is the luminous intensity, in a given direction, of a source that emits monochromatic radiation of frequency 540 × 1012 Hz and that has a radiant intensity in that direction of 1/683 watt per solid angle (steradian). 540 THz corresponds to a wavelength of 555 nm, at which the human eye is most sensitive to light.
Table 10.1 The base quantities in the SI system of units.
| Quantity | SI name | SI Symbol |
| Length (L) | metre | m |
| Time (t) | second | s |
| Mass (M) | kilogram | kg |
| Temperature (T) | kelvin | K |
| Electric Current (I) | ampere | A |
| Amount of substance | mole | mol |
| Luminous Intensity | candela | cd |
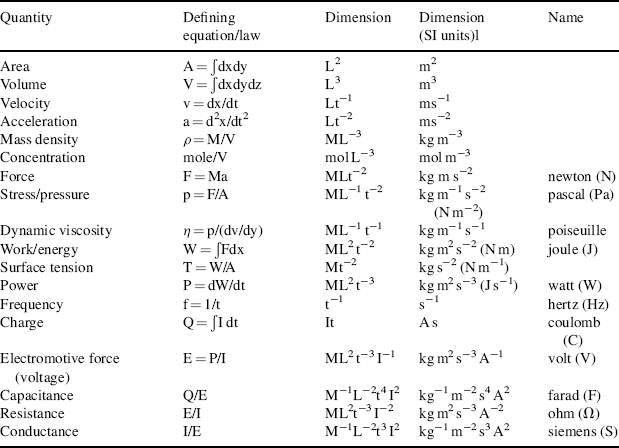
Other physical quantities can be derived and expressed in terms of the base quantities listed in Table 10.1. For example, force is made a derived quantity by writing Newton's law as F = ma. This use of Newton's law is solely for the purpose of deriving the dimensions of force. (Dimensions are not the same as units. For example, the physical quantity, velocity, may be measured in units of metres per second, miles per hour and so on; but regardless of the units used, speed is always a length divided by a time, with dimensions Lt−1.) A particular force being considered, such as that used to define the ampere, need not involve a mass being accelerated. An important theorem in dimensional analysis is the Dimensional Homogeneity Theorem [1] which states that any physical quantity Q is dimensionally a power law monomial of the form:
where the coefficient k and exponents a, b, c, d and e are real dimensionless numbers whose values distinguish one type of derived physical quantity from another. No alternative form will represent a physical quantity – all monomial derived quantities have this power-law form. Some derived physical quantities are presented in Table 10.2.
Table 10.2 Some derived physical quantities, with their defining equation or law, and dimensions.
10.2.2 Buckingham's π-Theorem
The most important exercise in dimensionless analysis is to identify a complete set of independent variable quantities q1, q2, ... qn that determine the value of a dependent variable quantity Q in a defined physical effect or process. Q will be a dependent variable if its value is determined uniquely by the set of independent variable quantities q1, ... qn. We can represent this relationship in the form:
According to Equation (10.1) all the quantities q have dimensions of the form:
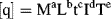
Quantities q1, ... qn will form a complete set if no other quantity can influence the value of Q, and independent if by changing any one of their values this does not alter the value of any other member of the set. As an example of this exercise, consider an experiment to determine the terminal velocity of various solid spherical particles rising or falling freely through various fluids. Our understanding of the basic hydrodynamics involved informs us that the terminal velocity U (our dependent quantity) is attained when the gravitational buoyancy or settling force is equal to the viscous drag force acting on the particle. This would suggest that U depends on the mass (m) and diameter (D) of the particle, the density ρp of the particle and fluid (ρf), and the viscosity η of the fluid (we assume that the gravitational acceleration constant g and temperature remain constant). The five quantities m, D, ρp, ρf, and η form a complete set – but not an independent one. If we define the diameter and density of the particle, the value for the mass m will also be defined. Therefore, either m or ρp must be excluded, to give a total of four independent variables in the set q1, ... qn. If, for example, we require five data points for each determination of U as a function of each of the four independent variables, we would have to make 54 (i.e. 625) measurements. We can reduce the number of required measurements to 125 by making the difference in the particle and fluid density (Δρ) as one of the variables, to replace the two variables ρp and ρf. An important application of dimensional analysis is that it leads to a significant reduction of the number of required experiments, whilst also providing an indication of how the experiments should be designed and the results presented. The way to achieve this is by application of Buckingham's π-Theorem [2].
Sonin [3] gives a clear description of the physical basis of dimensionless analysis and Buckingham's π-Theorem, and shows that an alternative form of Equation (10.2) is:
where π1, π2, ... πn−k is a complete dimensionless subset of the original q1, q2, ... qn given in Equation (10.2). The values of these dimensionless quantities are independent of the sizes of the base units, but the values of q1, ... qk do depend on base unit size. From the principle of Dimensional Homogeneity [1] that any physically meaningful equation must be dimensionally homogeneous, then quantities q1, ... qk must in fact be absent from Equation (10.3). In other words we have a dimensionless equation of the form:
Equation (10.4) encompasses Buckingham's π-Theorem, which states:
For a given physical effect or process where there are n physically relevant variables that can be described by k fundamental dimensions, there are a total of n-k independent, dimensionless, quantities (or ‘Pi groups’) π1, π2, ..., πn−k. The behaviour of the effect or process can be described by dimensionless Equation (10.4).
This theorem can be applied as follows:
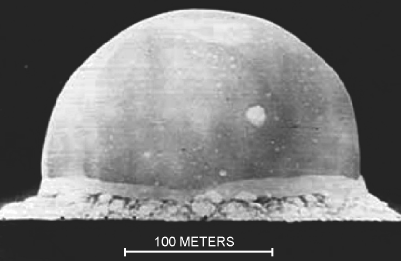
As a demonstration of the procedure we will consider a particularly extreme case of the concept of similarity. The British physicist Sir Geoffrey Taylor employed dimensional analysis [4] to estimate the energy yield of the first atomic bomb explosion (Trinity, New Mexico, 16 July 1945) when this was still classified information. The concept of similarity he used was that the buoyancy of hot air created in the ‘ball of fire’ of the explosion would behave like a rising bubble in water (until the hot air suffers turbulent mixing with the surrounding cold air). An example of one of the photographs he used in this analysis is shown in Figure 10.2.
Figure 10.2 A photograph of the ‘ball of fire’ produced by the Trinity atom bomb taken 0.015 seconds after detonation. The luminous globe rises like a large bubble in water. (G. Taylor, Proc. Roy. Soc. Lond. A201: 175–186, 1950. Reproduced with permission.)
Taylor assumed that the radius R of the ‘equivalent bubble’ depends on the energy yield E of the explosion, the time t after detonation, and the initial density ρ of the air. He found that the only parameter with dimensions of length that can be constructed from these quantities is:
Based on official high-speed photography of the Trinity atom bomb test Taylor used his formula to deduce that the bomb's yield was 16.8 kilotons of TNT – a result close to the official ‘top-secret’ value of 18 kilotons. Equation (10.5) can be derived as follows:
Assuming that the radius R of the atomic ‘cloud’ depends on the explosive energy E, time t after detonation and the initial density ρ of the air, we have:
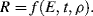
This equation contains four variable parameters, to give n = 4 in Buckingham's π-Theorem. We now construct the following table containing these variables and define their dimensions with the aid of Table 10.2:

In this table there are three different dimensions (M, L, t) to give k = 3, and n-k = 1. Buckingham's π-theorem thus informs us that only one dimensionless parameter is required to describe the initial atomic blast. This dimensionless parameter π is formed by multiplying the dependent variable R by the remaining parameters, each raised to an unknown exponent:
In terms of the dimensions of the quantities in equation (10.6) we have:
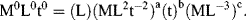
We now solve for a, b and c using the method of exponents:
| M: | 0 = a + c, |
| L: | 0 = 1 + 2a − 3c, |
| t: | 0 =− 2a +b. |
to give a =− 1/5; b =− 2/5; c = 1/5. Equation (10.6) therefore takes the form:
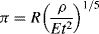
or
In Equation (10.7) the initial air density ρ is known and the bomb's energy yield E is also fixed. Thus, in the time range for which the luminous globe produced by the explosion behaves like a rising bubble in water, we should expect R5/2 ∝ t. Thus, a plot of 2.5 logR against log t should produce a straight line. This plot is shown in Figure 10.3.
Figure 10.3 A logarithmic plot, based on equation (10.12), of the radius of the luminous globe produced by the Trinity atom bomb as a function of time after detonation. (Derived from the dimensionless analysis performed by G. Taylor, Proc. Roy. Soc. Lond. A201: 175–186, 1950. Reproduced with permission.)
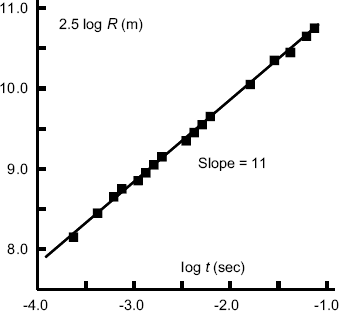
The straight line in Figure 10.3 corresponds with
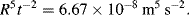
Based on this result Taylor determined the value of the atom bomb's energy yield to be 7.14 × 1013 J. Taking 1gm of TNT as liberating 1000 calories (4.2 kJ), then this energy is equivalent to 16.8 kilotons of TNT. Because of the assumed similarity with a rising air bubble, this energy does not include that part of the energy radiated beyond the ‘ball of fire’. This may partly account for the slight discrepancy between Taylor's result and the official one of 18 kilotons TNT disclosed many years later.
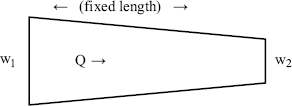
The following worked example is of relevance to the design of microfluidic systems:
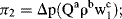
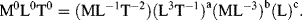
| M: | 0 = 1 + b, |
| L: | 0 =− 1 + 3a −3b + c, |
| t: | 0 =− 2 −a. |
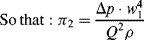
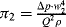 .) (Attempts to find a dimensionless parameter of the form: π2 = Δp(Qaρb) will fail.)
.) (Attempts to find a dimensionless parameter of the form: π2 = Δp(Qaρb) will fail.)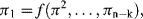
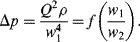
Figure 10.5 A plot of Δp (= Q2 ρ/w14) as a function of (w1/w2) takes the form of a smooth universal plot.
Not only has this dimensionless analysis provided the means for greatly simplifying the presentation of experimental data, it also assists in the planning of the experiments by revealing the basic relationships between the dependent and independent variables.
10.3 Dimensionless Parameters
To be able to design a well-functioning microfluidic device requires an understanding of how fluids flow within channels having micro- and nanoscale dimensions. Although fluids are collections of discrete molecules, each with individual properties, it is usually helpful to define velocity, temperature, density and pressure as the statistically based properties of a continuous material. This may no longer be the case when device dimensions are reduced. As an example, if the physical dimensions of a fluidic channel become so small that there are statistically few molecules near the channel walls at any time, the assumption of zero slip boundary conditions discussed in Chapter 9 can no longer be assumed. Fluid flow behaviour can be better appreciated and predicted by employing dimensionless parameters such as the Knudsen, Peclet, Reynolds, and Bond numbers. These parameters can reveal which physical mechanisms should be used to manipulate the flow and create the functionality desired for a particular microfluidic device. Such ‘top level’ understanding can be obtained without the need to perform in-depth analyses.
Useful physical data to be used in the application of dimensionless parameters are presented in Tables 10.3–10.6.
Table 10.3 Values of viscosity and surface tension for various liquids at 293 K.
| Liquid | η (Pa s) | Ts (N m−1) |
| Water | 1.002 × 10−3 | 7.275 × 10−2 |
| Blood (37 °C) | 3 ~ 4 × 10−3 | 5.5 × 10−2 |
| Ethanol | 1.074 × 10−3 | 2.21 × 10−2 |
| Methanol | 5.94 × 10−4 | 2.27 × 10−2 |
| Mercury | 1.55 × 10−3 | 47.2 × 10−2 |
| Benzene | 6.04 × 10−4 | 2.89 × 10−2 |
| Chloroform | 6.96 × 10−4 | 2.75 × 10−2 |
| Glycerol (100%) | 1.41 | 6.4 × 10−2 |
Table 10.4 Diffusion coefficients for various molecules and ions in water at 298 K.
| Molecule | D (10−9 m2 s−1) |
| Water | 2.26 |
| Sucrose | 0.52 |
| Methanol (CH3OH) | 1.6 |
| Glycine | 1.06 |
| NaCl | 1.7 |
| H+ | 9.3 |
| OH− | 5.3 |
| Na+ | 1.33 |
| K+ | 1.96 |
| Cl− | 2.03 |
Table 10.5 Diffusion coefficients for various macromolecules and particles in water at 293 K. (Derived using the Stokes-Einstein relation: D = kT/(6πηa) where ‘a’ is the hydrodynamic radius of a spherical particle).
| Macromolecule | D (m2 s−1) |
| Ribonuclease | 1.2 × 10−10 |
| Lysozyme | 1.0 × 10−10 |
| Serum albumin | 5.9 × 10−11 |
| Haemoglobin | 6.9 × 10−11 |
| Urease | 3.5 × 10−11 |
| Collagen | 6.9 × 10−12 |
| Viruses, bacteria, cells | 10−13 ~ 10−16 |
Table 10.6 Diffusion coefficients for some molecules in water and air at 293 K. (As a rough guide, a molecule's diffusion coefficient is ~ 104-times greater in air than in water).
| Molecule | In water (10−9 m2/sec) | In air (10−5 m2/sec) |
| H2O | 2.26 | 2.5 |
| CO2 | 1.6 | 1.6 |
| O2 | 2.0 | 2.0 |
10.3.1 Hydraulic Diameter
Most dimensionless parameters include a length scale in their definition. This characteristic dimension L varies with the geometrical shape of the fluidic channel. The concept of a wetted or hydraulic diameter is generally chosen as the appropriate length scale, and two common examples of this are described in Figure 10.6. The hydraulic diameter DH is defined as:
Figure 10.6 The appropriate length scale for a fluidic channel is often given as its wetted or hydraulic diameter (e.g. D or 2wh/(w + h).
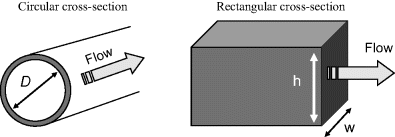
For the case of the channel of circular cross-section DH is equal to the channel diameter D. For rectangular channels:
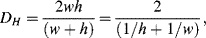
where, as shown in Figure 10.6, h is the channel height and w its width.
For a channel of triangular cross-section and equal sides (a)
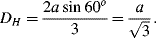
Equilateral triangle cross-sections can be etched into silicon substrates, but are not commonly found in glass or plastic substrates. Two cross-sections that are common in microfluidic devices are the trapezoidal and rounded trapezoidal ones shown in Figure 10.11 for self study problem number 4. The effective hydraulic radius value (DH/2) obtained for a nonstandard cross-section channel (e.g. a trapezoid) can be used with Equations (9.17) and (9.18) of Chapter 9 to provide a rough estimate of the pressure drop required to produce a desired volume flow rate through such a channel. Equations 9.19 to 9.21 should be used to provide a more accurate estimate for circular, semi-circular or rectangular cross-sections.
10.3.2 The Knudsen Number
The Knudsen Number is a dimensionless parameter that compares the characteristic dimensions of a microfluidic device to the intermolecular spacing (mean free path between molecular collisions) of the fluid. It provides an important test of the validity of the continuum approximation, and is defined as:
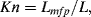
in which Lmfp is the mean free path and L is the characteristic length of the flow-field. L can be taken as the hydraulic diameter defined by Equation (10.10), or the gradient of a bulk property such as density ( ). From Chapter 9, Equation (9.2), the mean free path, for an ideal gas, is given by the formula:
). From Chapter 9, Equation (9.2), the mean free path, for an ideal gas, is given by the formula:
where k is the Boltzmann constant, T is absolute temperature, P is absolute pressure, and d is the molecular diameter. Typical molecular diameters fall in the range 0.2 ~ 0.3 nm. For liquids, since the molecules are always in a collision state, the mean free path is roughly equivalent to the molecular diameter. We can appreciate the difference at the molecular level between a liquid and a gas by noting that one dm3 of liquid nitrogen weighs ~ 800 gm, whilst at STP gaseous nitrogen weighs ~ 1.2 gm per dm3. At the molecular level this informs us that on average a nitrogen molecule in the gas phase occupies ~ 670-times more space than it does as a liquid. The average centre-to-centre separation of molecules in the liquid state is a little larger than its molecular diameter d, and so the average separation of molecules in a gas will be ~ 6701/3d, namely ~ 8.8d. We can imagine a gas consisting of molecular spheres randomly distributed in space, with an average separation close to ten-times their molecular diameter. The molecules will have a distribution of velocities given by the Maxwell-Boltzmann distribution described in Chapter 9, and the distance they travel before colliding with another molecule is given by Equation (10.11).
The range of the Knudson number for gaseous systems is shown in Figure 10.7. As a rough guide we can adopt the continuum model if the characteristic scale of our system is more than 1000-times larger than the molecular mean free path length. At the other end of the scale, where the molecular mean free path length is 10-times larger than the characteristic length of the system, the molecular particles will collide with the physical boundaries of the system more often than they will with one another. We now have to consider the dynamics of the individual molecular particles – we cannot treat them as a homogeneous medium. In the Knudsen number range between the continuum and molecular model we operate in the mesoscale, and methods for modelling at this scale were discussed in Chapter 9.
Figure 10.7 The range of Knudson numbers for gaseous systems, to show the range (Kn < 0.1) where the continuum model and zero slip boundary conditions apply, and the range (Kn > 10) where discontinuous, dynamic, molecular flow dominates. Between these two ranges there is the meso region.
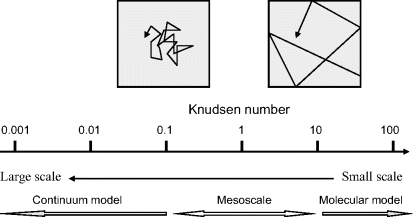
The following ranges of the Knudson number provide a rough guide as to when we can treat a gaseous fluid as a continuum or as an assembly of discrete molecular particles:
The following examples should assist with an understanding of these concepts:
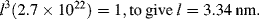
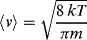
10.3.3 The Peclet Number: Transport by Advection or Diffusion?
The constant motion of molecules in fluids ensures that, when one fluid is placed adjacent to a second fluid, its molecules proceed into that second fluid in a process called diffusion. When we employ the continuum concept, instead of calculating each individual motion, we calculate the average motion of a statistically significant number of molecules. It then becomes convenient to separate the actual diffusion process into two conceptual transport mechanisms: a molecular process modelled as a statistical random walk that is proportional to the degree of kinetic energy in the system and an advective process in which molecules are carried along by the average velocity of the flow. The common practice is to restrict the word diffusion to describe the first process and label the second process advection (convection if heat is being transferred). The relative importance of these two conceptual transport mechanisms is given by the Peclet Number, the ratio of advection and diffusion:
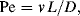
in which v is the fluid velocity, D is the diffusion coefficient of the solute in the solvent, and L is the characteristic dimension of the fluid passage (Figure 10.6). When L is so small that the Peclet number is less than 1000, molecular diffusion becomes an important mechanism for mixing. Stirring may be appropriate for mixing in a macroscale device, but a diffusion-based approach should be used in a low Pe device.
10.3.4 The Reynolds Number: Laminar or Turbulent Flow?
All fluid flow, whether around an object, in pipes, or in a river, can be broadly classified as either laminar or turbulent. These two flow regimes behave markedly differently, with significant implications for mass and heat transport. Whether fluid flow is laminar or turbulent depends on the relative importance of the inertial forces (ρv2/L) versus viscous forces (ηv/L2) in the flow (i.e. ratio of the momentum of the fluid and the friction force imparted by the channel walls). This ratio is defined as the Reynolds Number:
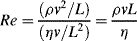
Re (originally proposed by Osborne Reynolds [5] in which v is the bulk velocity of the flow, ρ is fluid density, and η is the fluid's dynamic viscosity) can also be expressed as vL/υ,where υ is the kinematic viscosity (υ = η/ρ) with units of m2/s. The characteristic length L can be taken as the diameter of a fluid channel or pipe, or the diameter of a spherical object in a fluid stream.
A low Reynolds-number flow is a laminar, or layered, flow in which fluid streams flow parallel to each other and mix only through advective and molecular diffusion. Laminar flow is dominated by viscous forces and has fluid velocity at all locations invariant with time when boundary conditions are constant. There is advective mass transport only in the direction of fluid flow. An excellent example of laminar flow can be found with some toothpastes (Figure 10.8). Several brands have two or more different components, typically varying in both colour and composition. When such toothpaste is squeezed out of its tube, the colors do not mix because the paste's high viscosity ensures a low Re and thus laminar flow.
Figure 10.8 Laminar flow in toothpaste, characterised by a low Reynold's number resulting from high viscosity. Mixing of the components takes place by molecular diffusion – a very slow process.

In contrast, a high Reynolds-number flow is a turbulent flow in which inertial forces dominate and various parts of the fluid exhibit motions that are simultaneously random in both space and time. Significant advective mass transport occurs in all directions. This is the kind of flow we can see in rapidly flowing mountain streams, or when we vigorously stir cream into coffee, for example. This difference in the behaviour of laminar and turbulent flow is depicted in Figure 10.9.
Figure 10.9 Schematic representation of laminar flow (Re < ~ 2000) and turbulent flow Re > ~ 2000).
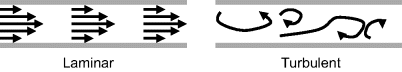
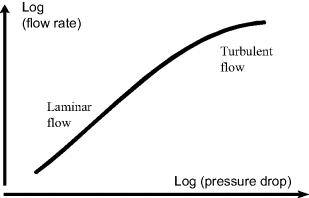
The transition between laminar and turbulent flow typically occurs above Re = 2000, though some experiments [6] suggest transition in gas flows in microchannels may occur at Re as low as 400. A flow can be identified as laminar or turbulent by either experimental or computational methods. From experimental data laminar flow is identified by a linear proportionality between the log of the pressure drop in the channel and the volume flow rate, i.e. a straight line on a log-log plot of pressure loss versus flow rate. If the flow transitions to turbulence at higher flow rates, the same linear proportionality no longer holds and the slope of the line changes at that flow rate, as depicted in Figure 10.10.
Figure 10.10 Laminar flow is identified by a linear proportionality between the logarithm of the pressure drop in a channel and the volume flow rate of the fluid.
Transition to turbulence can also be identified using numerical techniques such as the finite element or finite volume methods described in Chapter 9 to simulate the flow. Turbulence can be defined as irregular flow with random variation of flow properties (e.g. velocity, pressure, etc.) in both time and space coordinates simultaneously. Hence, a numerical simulation based on solving the appropriate conservation of mass and momentum equations will not converge to a steady solution if the flow is randomly varying. Time-averaging of the flow properties or some other technique must be used to model a turbulent flow. By these means, the flow in a microchannel can be accurately differentiated as laminar or turbulent, and analysed accordingly.
10.3.5 Reynolds Number as a Ratio of Time Scales
If a flat plate at rest receives a step-function impulse of force, causing it to move in its own plane at velocity v, a fluid boundary layer will develop at the plate surface due to the nonslip of fluid at this boundary. If L is the characteristic length scale, then the characteristic time τc for transport of material by convection down the resulting fluid flow is τc = L/v. The boundary layer will widen at a rate proportional to the fluid viscosity. The kinematic viscocity υ (υ = η/ρ) has units of m2 s−1 so that the characteristic time τc for a viscosity controlled effect to be transmitted normal to the fluid flow can be given as τvisc = L2/υ. The ratio of viscous to convective time scales is
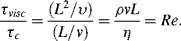
Thus the Reynolds number is a measure of the viscous and convective time scales. A large Reynolds number means that viscous effects propagate slowly into the fluid. This is the reason why boundary layers are thin in high Reynolds number flows because the fluid is being convected along the flow direction at a much faster rate than the spreading of the boundary layer, which is normal to the flow direction.
10.3.6 The Bond Number: How Critical is Surface Tension?
Another flow characteristic that becomes important in microscale channels is the interfacial tension between gas and liquid phases, or between immiscible fluids. In flow in porous media the Capillary number, a ratio of viscous and interfacial tension forces, is important. For droplet breakup the Weber number, a ratio of inertial and interfacial tension forces, is the useful parameter (see next section).
The Bond number is defined as:
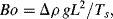
which represents a ratio of gravity and interfacial tension forces, in which Δρ is the density difference between the two fluids, g is the acceleration of gravity, Ts is surface tension, and L is the appropriate length scale (e.g. wetted diameter of a capillary, or the contact radius).
Because L is squared the Bond number decreases rapidly as the flow passage is reduced in size to the microscale. A high Bond number indicates that the system is relatively unaffected by surface tension effects; a low number (typically less than one) indicates that surface tension dominates. A low Bond number flow is more likely to respond to change in surface energy than change in elevation of the free surface between the phases. This is the reason that a liquid will rise in a capillary tube in spite of the gravitational force acting on it. Intermediate Bond numbers indicate a nontrivial balance between the two effects.
A characteristic length λ, known as the capillary length, is defined as
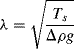
and corresponds to the curved surface or meniscus length of a droplet or fluid in contact with a surface. The Bond number therefore compares the characteristic dimension of the fluid element to the capillary length (Bo = L2/λ2). For water at STP the capillary length λ is ~ 2x10−3 m. A fluid droplet of low Bond number (Bo 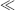 1) deposited on a substrate will appear as a rounded, hemispherical drop, whereas a large drop of fluid (Bo
1) deposited on a substrate will appear as a rounded, hemispherical drop, whereas a large drop of fluid (Bo 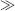 1) will appear more like a flat fluid disk.
1) will appear more like a flat fluid disk.
The Bond number can also be configured to accommodate forces other than gravitational ones. Electrowetting on a dielectric (EWOD) is described in Chapter 3. The deformation of a droplet when exposed to an electric field E can be related to the electric Bond number, given by
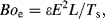
where ε is the dielectric permittivity of the liquid.
10.3.7 Capillary Number: Relative Importance of Viscous and Surface Tension Forces
In small-scale flows where the effect of surface tension is important, the Capillary number Ca is defined as the ratio of the viscous (elongational) force to the surface tension force acting at an interface between a liquid and a gas, or between two immiscible liquids (e.g. oil and water):
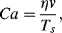
where η is the dynamic viscosity, v is the characteristic velocity and Ts is the surface or interfacial tension between the two fluid phases. The shear stress is given by ηv /L and the capillary force as Ts/L. The ratio of these two forces gives the Capillary number. The characteristic velocity v could be the rate (m s−1) of emergence of oil through an oil-water saturated porous material, or the rate of shear or elongation of a fluid emerging from a nozzle or constriction. As the emerging fluid stream elongates and reduces in diameter, the capillary force (Ts/L) increases until it breaks up the fluid stream into droplets. This occurs at a critical Capillary number typically of the order 0.1 ~ 0.01.
10.3.8 Weber Number: Relative effects of Inertia and Surface Tension
The Weber number We can be used to predict the disruption of the flow of small volumes or thin films formed between the interface between to immiscible fluids or between a fluid and a gas. The quantity determined by the Weber number is the ratio of the fluid's inertial force and surface tension forces:
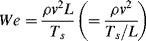
where L is typically the thickness of a fluid film or the diameter of a fluid droplet. The factor ρv2 corresponds to dynamic pressure and Ts/L to capillary pressure. When surface tension forces dominate, a fluid element is likely to take the form of a spherical droplet (i.e. having a convex interface). If inertial forces dominate an interface can assume a rippled structure or form localised concave surfaces that can eventually disrupt the fluid's structural from.
A fluid jet that rapidly ‘atomises’ into a fine spray of spherical droplets corresponds to a low Weber number. Consider the ‘splash’ caused by an object falling into a water-air surface. If the splash mainly takes the form of droplets of water emerging from the surface, we can assume that surface tension forces are dominant and that the Weber number is low. However, if we observe intricate fluid-air surfaces and nonspherical droplets we can assume that a high Weber number is in action.
10.3.9 Prandtl Number: Relative Thickness of Thermal and Velocity Boundary Layers
The Prandtl number Pr is defined as:
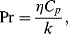
where Cp is the specific heat at constant pressure and k is the coefficient of thermal conduction. It is the ratio of momentum diffusivity (kinematic viscosity) to thermal diffusivity, and can be related to the thickness of the thermal and velocity boundary layers (it is in fact the ratio of these two thicknesses). When Pr = 1, these boundary layers are equal and coincide. When Pr is small, heat diffuses very quickly compared to the fluid velocity (momentum), so that the thickness of the thermal boundary layer is much bigger than the velocity boundary.
10.4 Applying Nondimensional Parameters to Practical Flow Problems
As an instructive exercise we will predict the flow behaviour of water vapour and a liquid in a channel of width 1 mm and height 0.05 mm (50 μm) at STP. We will specify a flow rate of 0.05 μL s−1.
10.4.1 Channel Filled with Water Vapour
To decide to what extent we can assume a continuum model, we calculate the Knudsen number:
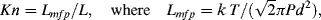
k = 1.3806 × 10−14 nJ/K; diameter of a water molecule d = 0.25 × 10−6 mm.
If we assume absolute pressure P = 1.013 × 105 Pa, T = 293 K, then the mean free path is Lmfp = 1.44 × 10−4 mm.
By using the smallest dimension L, the channel height of 0.05 mm, gives Kn = 0.0029.
We have Kn < 0.1, so according to the discussion of Knudsen number ranges in 10.3.2 we can utilise the continuum approximation in this case – but will need to account for a finite slip between the water vapour and the channel walls.
10.4.2 Channel Filled with a Dilute Electrolyte at 293 K
We are now dealing with a liquid, so we approximate Lmfp with the molecular diameter d. This gives Kn = 0.00025, which allows both the continuum approximation and zero slip boundary conditions. Will diffusion be a major factor? We can answer this by calculating the Peclet number
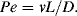
The diffusion coefficient for NaCl in water is D = 1.74 × 10−3 mm2/s. Since the width is an order-of-magnitude larger than the height, the hydraulic diameter concept suggests a characteristic length that is approximately twice the smaller dimension, or L = 0.1 mm.
The bulk fluid velocity v is given by the ratio of the flow rate and the flow area, so v =10−3 m s−1. Thus, the Peclet number is Pe = 57.5, suggesting that diffusion is an effective mass transport mechanism in this case. (Indeed, a diffusion front of NaCl would cross the channel height in less than 2 seconds.)
The Reynolds number will show if the flow is laminar, turbulent, or possibly in transition. Assuming essentially water properties, the fluid density is ρ = 0.001 g/μL and dynamic viscosity is η = 0.001 Pa s.
Thus, Re = ρv L/η = 0.1, and we certainly have laminar flow. Two streams carrying different solutes would flow side-by-side in this channel with their components mixing only by diffusion. The Bond number is given by:
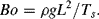
We have g = 9810 mm/s2 and surface tension for water Ts = 72 μN/mm, giving Bo = 0.0014. This suggests that during filling of the channel, gravity will be a weak mechanism compared to the capillary effect.
These examples demonstrate that before we perform any complex analysis or sophisticated numerical simulation of the flow we can predict much about the characteristics of microfuidic flow. In these examples we know that we have a highly laminar flow in which solutes mix only by diffusion. The wetting of the channel will depend on surface energy, and not on elevation change for example. In addition, we also know how changing channel dimensions or fluid properties will impact this flow behaviour.
10.5 Characteristic Time Scales
Various characteristic time scales may be defined for microfluidic systems, and the following four are commonly used:
10.5.1 Convective Time Scale
This is the time taken for a perturbation to propagate in a liquid:
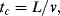
where L is the characteristic dimension and v is the velocity of the liquid. If the fluid is responding to shear stress (see Section 9.3.5 in Chapter 9) the convective time scale is also given by the reciprocal of the shear rate:
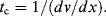
10.5.2 Diffusion Time Scale
This is the time taken for a physical perturbation to propagate (diffuse) in the fluid:
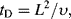
where υ = η/ρ is the kinematic viscosity (η is the dynamic viscosity and ρ the density of the fluid).
10.5.3 Capillary Time Scale
This is the time taken for a physically perturbed interface to regain its original shape against viscous opposition:
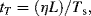
where Ts is the surface tension.
10.5.4 Rayleigh Time Scale
This is the time scale of a physically perturbed interface induced by inertial and surface tension forces:
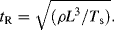
10.6 Applying Micro- and Nano-Physics to the Design of Microdevices
Researchers developing microfluidic devices are frequently confronted by issues that are directly related to the fundamental physics that apply at the micro- and nanoscales. Some practical examples include:
- Fluids that are brought together in a microfluidic circuit do not mix easily. Any mixing that does occur arises mainly from the diffusion of solutes across the boundaries between separate laminar flows.
- Solute particles that are heavier than the surrounding fluid settle to the channel bottom very quickly.
- The devices tend to have a very large surface-to-volume ratio, thus presenting the opportunity for particles to stick to a large surface area for a given volume.
- A small drop of fluid placed in the inlet of a microfluidic device can evaporate very rapidly.
- In microdevices, capillary force and surface energy effects are large forces compared to gravity. Depending on their direction and nature, they may move fluids upwards and sideways, or even block downward fluid movement.
- Small fluid volumes will almost immediately take on the temperature of the environment, and cool down or heat up very quickly.
- Liquids flowing in microchannels often do not have sharply defined frontal and trailing boundaries. This is caused by capillary action, and can cause spatial dispersion during fluid flow that hinder the ability to accurately define the timing of reactions or analyses.
However, at the same time these seemingly undesirable effects can be converted into extremely powerful tools: Examples include:
- Flow is usually laminar, allowing the parallel flow of several layers of fluid. This can be turned to advantage in the design of separation and detection devices based on laminar fluid diffusion interfaces.
- At microdimensions, diffusion becomes a viable approach to move particles, mix fluids, and control reaction rates. Typical low molecular weight biomolecules can diffuse more than 10−3 m s−1 at 298 K in aqueous solutions. This allows the establishment of controlled concentration gradients in flowing systems, as well as complete equilibration of the molecule across a 100 μm channel in less than one minute, for example.
- Unaided by centrifugation, sedimentation becomes a viable means to separate dispersed particles by density across small channel dimensions. For example, red blood cells will sediment in a 100 μm deep channel in about 1 minute and generate a 50 μm layer of plasma in the process.
- In microchannels, the diffusion distance can be made extremely small, particularly if fluid streams are hydrodynamically focused. Thus, diffusion-controlled chemical reactions occur more rapidly than in comparable macroscopic reaction vessels. For example, microfluidic immunoassays can be completed in less than 25 seconds, as opposed to more typical immunoassay reaction times of 10 minutes or more.
- Evaporation of small quantities of fluids can be extremely rapid because of a typically large surface-to volume ratio. This effect can be used for the concentration of sample particles.
- Active particle transportation and separation methods, such as capillary electrophoresis, show greatly enhanced separation performance in small channels.
Other positive characteristics of microfluidic devices that are derived from economics, convenience, and safety include:
- Plastic microfluidic structures can be mass-produced at very low unit cost, allowing them to be made disposable.
- Microfluidic devices are amenable to high throughput by processing multiple samples and assays in parallel. For example, massively parallel processing can speed DNA, RNA, protein, immunologic, and other tests to reduce time intervals for drug discovery and medical diagnosis.
- Microdevices require only small volumes of sample and reagents, and produce only small amounts of waste, which can often be contained within the disposable device.
- The small scale of the various components of microfluidic systems allows them to be integrated into total-analysis systems (Micro-TAS) capable of handling all steps of the analysis on-chip, from sampling, sample processing, separation and detection steps to waste handling. This integration also makes complex analyses potentially simpler and safer to perform.
- It is possible to design passive fluidic devices that utilise inherent properties of the fluid and its microenvironment (capillary force, evaporation, wicking, heat transfer, diffusion, etc.) for fluid movement, mixing, heating, cooling, and catalysing chemical reactions. Thus, disposable standalone devices can be designed that require no external power source or instrumentation, yet still perform many, if not all, of the functions typically associated with full-scale automated chemical analysis devices containing pumps, mixers, heat elements, readout signals utilising fluorescence, for example. The phenomenon of ‘wicking’ occurs when a fluid and channel wall have a higher degree of affinity, as for example an aqueous solution and a hydrophilic surface. This effect can be used to modify fluid frontal boundaries through surface treatments such as the hydrophobic coating of a channel wall.
10.1. Determine the dimensions of the following quantities:
(a) Sin(30°), (b) π.log102, (c) Thermal conductance (defined as the quantity of heat that passes in unit time through a plate of particular area and thickness when its opposite faces differ in temperature by one kelvin).
10.2. For y = ekt, where t is time, what are the dimensions of the factor ‘k?’
10.3. Determine if the following equations are dimensionally correct:
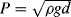
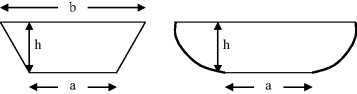
10.4. Calculate the hydraulic diameter for the two quite common channel cross-sections shown in Figure 10.11.
Figure 10.11 Self-study Problem 10.4: Calculate the hydraulic diameter for the trapezoidal (left) and rounded trapezoidal (right) channel cross-sections.
10.5. The drag force on a spherical particle of radius r immersed in a fluid of flow velocity v and dynamic viscosity η is given by Stokes' Law as:
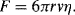
Use this equation to determine the dimensions of dynamic viscosity.
The force on a body immersed in a flowing fluid depends on a channel dimension L, as well as the fluid density ρ, viscosity η and velocity v.
Express the relationship between these parameters in the form:
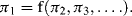
One of these π parameters takes the form of a well-known dimensionless parameter. Name this parameter and explain how its magnitude can be helpful in fluid flow analysis.
The drag force acting on a rough sphere is a function of its diameter D, the average depth k of grooves made into its surface, the density ρ, viscosity η and velocity v of the fluid. Express the relationship between these parameters in the form:
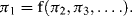
A flowing fluid will exert a force on any object that it encounters. Assume, under the conditions of interest, that this drag force F depends on the speed v of the fluid relative to the object, the fluid density ρ, the viscosity η of the fluid, and the size of the body (expressed in terms of its forward-facing cross-sectional area A).
1. Bridgman, P.W. (1931) Dimensional Analysis, 2nd edn, Yale University Press, New Haven.
2. Buckingham, E. (1914) On Physically Similar Systems; Illustrations of the Use of Dimensional Analysis. Physical Review, 4, 345–376.
3. Sonin, A.A. (2001) The Physical Basis of Dimensional Analysis, 2nd edn, MIT, Cambridge, MA.
4. Taylor, G. (1950) The Formation of a Blast Wave by a Very Intense Explosion. II. The Atomic Explosion of 1945. Proceedings of the Royal Society of London, A201, 175–186.
5. Reynolds, O. (1883) An experimental investigation of the circumstances which determine whether the motion of water in parallel channels shall be direct or sinuous and of the law of resistance in parallel channels. Philosophical Transactions of the Royal Society, 174, 935–982.
6. Wu, P. and Little, W.A. (1983) Measurement of friction factors for the flow of gases in very fine channels used for micro-miniature Joule-Thomson refrigerators. Cryogenics, 23, 273–277.
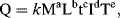
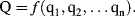
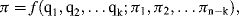
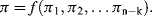
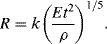
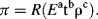
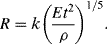
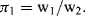
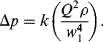
 versus
versus  should produce a single
should produce a single