After Chapter 5.1, you will be able to:
Charged subatomic particles come in two varieties. One, the proton, has a positive charge; the other, the electron, has a negative charge. While opposite charges exert attractive forces, like charges—those that have the same sign—exert repulsive forces. Unlike the force of gravity, which is always an attractive force, the electrostatic force may be repulsive or attractive depending on the signs of the charges that are interacting.
While many of the particles we discuss in electrostatics are very, very tiny, do not forget that they still do have mass. We can use equations such as the kinetic energy equation when solving problems with charged particles, and the MCAT will sometimes require us to do just that.
Most matter is electrically neutral, as a balance of positive and negative charges ensures a relative degree of stability. When charges are out of balance, the system can become electrically unstable. Even materials that are normally electrically neutral can acquire a net charge as result of friction. When you shuffle your feet across the carpet, negatively charged particles are transferred from the carpet to your feet, and these charges spread out over the total surface of your body. The shock that occurs when your hand gets close enough to a metal doorknob allows that excess charge to jump from your fingers to the knob, which acts as a ground—a means of returning charge to the earth. Static charge buildup or static electricity is more significant in drier air because lower humidity makes it easier for charge to become and remain separated.
The SI unit of charge is the coulomb, and the fundamental unit of charge is
A proton and an electron each have this amount of charge, although the proton is positively charged (q = +e), while the electron is negatively charged (q = −e). Even though the proton and the electron share the same magnitude of charge, they do not share the same mass; the proton has a much greater mass than the electron.
Like mass and energy, electric charge is governed by a law of conservation of charge. This law states that charge can neither be created nor destroyed.
The fundamental unit of charge is e = 1.60 × 10−19 C. A proton and an electron each have this amount of charge; the proton is positively charged (q = +e), while the electron is negatively charged (q = −e).
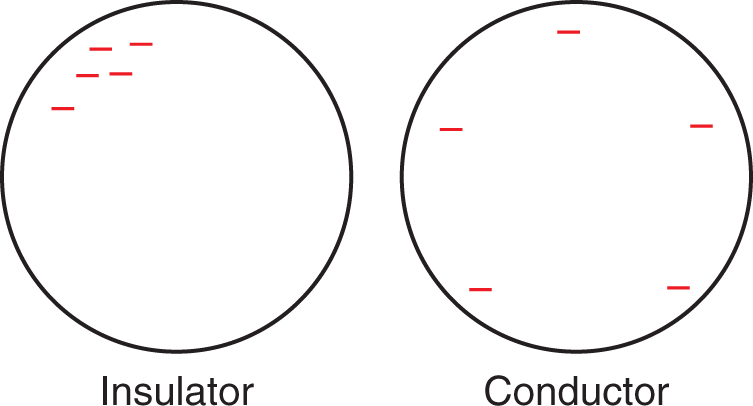
Insulators and conductors vary in their ability to both hold and transfer charges. An insulator will not easily distribute a charge over its surface and will not transfer that charge to another neutral object very well—especially not to another insulator. On a molecular level, the electrons of insulators tend to be closely linked with their respective nuclei. By extension, most nonmetals are insulators. Experimentally, insulators serve as dielectric materials in capacitors, as well as in isolating electrostatic experiments from the environment to prevent grounding.
In contrast, when a conductor is given a charge, the charges will distribute approximately evenly upon the surface of the conductor. Conductors are able to transfer and transport charges and are often used in circuits or electrochemical cells. Conductors are often conceptualized as nuclei surrounded by a sea of free electrons that are able to move rapidly throughout the material and are only loosely associated with the positive charges. Conductors are generally metals, although ionic (electrolyte) solutions are also effective conductors. Figure 5.1 demonstrates the behaviors of an insulator and a conductor when a negative charge is placed on them.
