After Chapter 8.1, you will be able to:
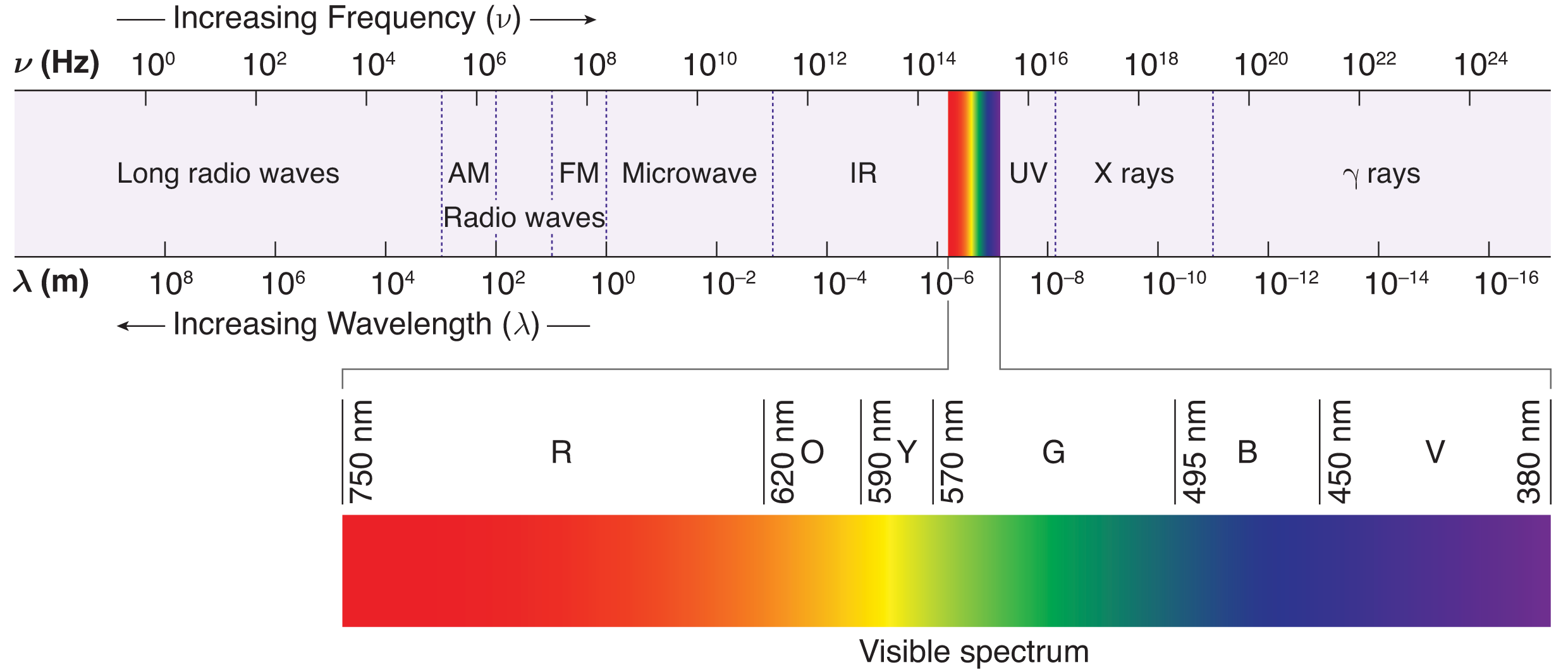
The full electromagnetic spectrum includes radio waves on one end (long wavelength, low frequency, low energy) and gamma rays on the other (short wavelength, high frequency, high energy). Between the two extremes, we find, in order from lowest energy to highest energy, microwaves, infrared, visible light, ultraviolet, and x-rays. This chapter will focus primarily on the range of wavelengths corresponding to the visible spectrum of light (400 nm to 700 nm).
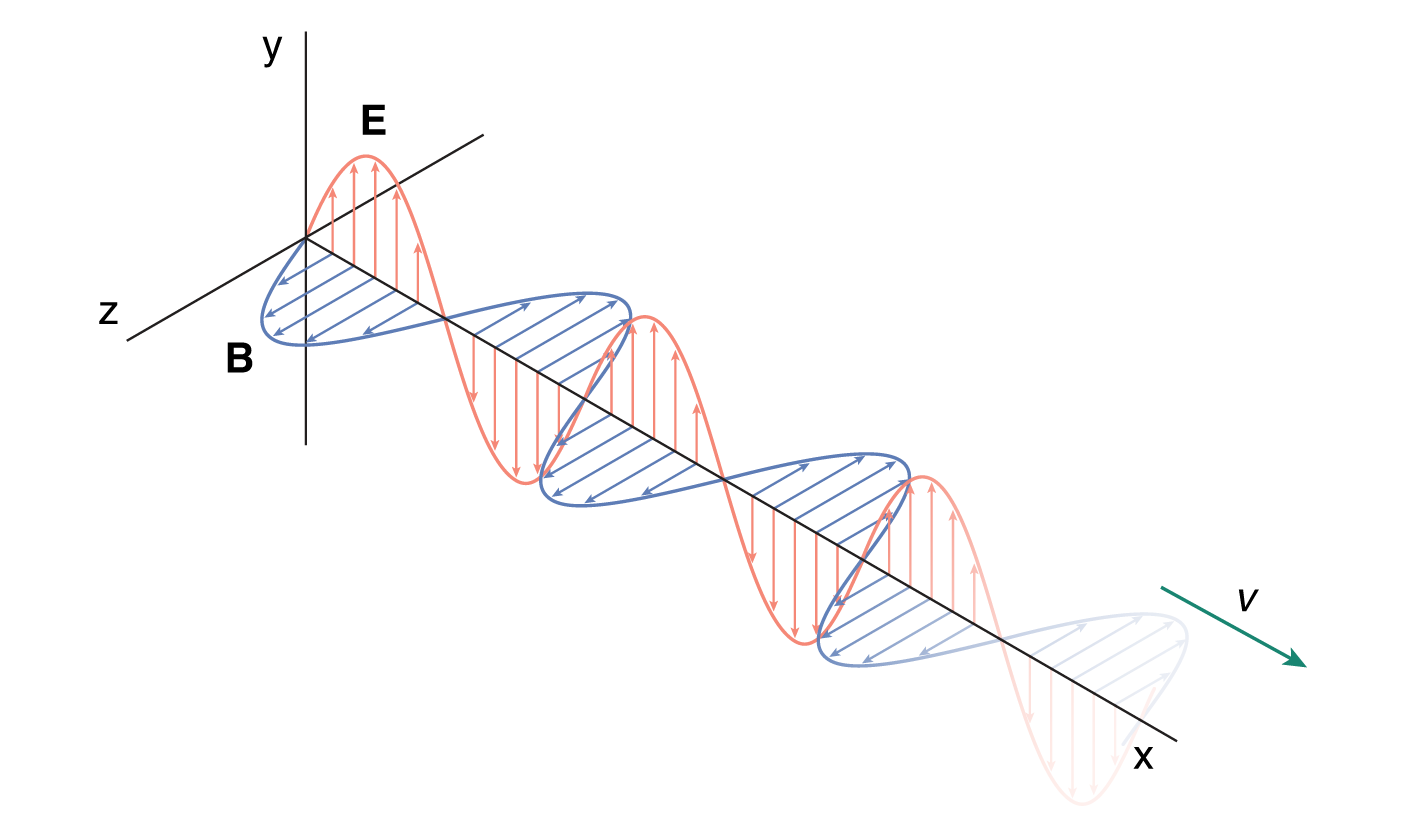
A changing magnetic field can cause a change in an electric field, and a changing electric field can cause a change in a magnetic field. Because of the reciprocating nature of these two fields, we can see how electromagnetic waves occur in nature. Each oscillating field causes oscillations in the other field completely independent of matter, so electromagnetic waves can even travel through a vacuum.
Electromagnetic waves are transverse waves because the oscillating electric and magnetic field vectors are perpendicular to the direction of propagation. The electric field and the magnetic field are also perpendicular to each other. This is illustrated in Figure 8.1.

The electromagnetic spectrum describes the full range of frequencies and wavelengths of electromagnetic waves. Wavelengths are often given in the following units: mm (10–3 m), µm (10–6 m), nm (10–9 m), and Å (ångström, 10–10 m). The full spectrum is broken up into many regions, which in descending order of wavelength are radio (109–1 m), microwave (1 m–1 mm), infrared (1 mm–700 nm), visible light (700–400 nm), ultraviolet (400–50 nm), x-ray (50–10–2 nm), and γ-rays (less than 10–2 nm). The electromagnetic spectrum is depicted in Figure 8.2.
Electromagnetic waves vary in frequency and wavelength, but in a vacuum, all electromagnetic
waves travel at the same speed, called the speed of light. This constant is represented by c and is approximately
 To a first approximation—and for the purposes of all MCAT-related equations—electromagnetic
waves also travel in air with this speed. In reference to electromagnetic waves, the
familiar equation ν = fλ becomes
To a first approximation—and for the purposes of all MCAT-related equations—electromagnetic
waves also travel in air with this speed. In reference to electromagnetic waves, the
familiar equation ν = fλ becomes
where c is the speed of light in a vacuum and, to a first approximation, also in air, f is the frequency, and λ is the wavelength.
To recall the order of the colors in the visible spectrum, remember the grade-school “rainbow” of ROY G. BV (red, orange, yellow, green, blue, violet).
The only part of the spectrum that is perceived as light by the human eye is the visible region. Within this region, different wavelengths are perceived as different colors, with violet at one end of the visible spectrum (400 nm) and red at the other (700 nm).
Wavelengths in the visible range are common on the MCAT. Remembering the boundaries of the visible spectrum (about 400–700 nm) will save you time and energy on Test Day.
Light that contains all the colors in equal intensity is perceived as white. The color of an object that does not emit its own light is dependent on the color of light that it reflects. Thus, an object that appears red is one that absorbs all colors of light except red. This implies that a red object under green illumination will appear black because it absorbs the green light and has no light to reflect. The term blackbody refers to an ideal absorber of all wavelengths of light, which would appear completely black if it were at a lower temperature than its surroundings.