After Chapter 9.2, you will be able to:
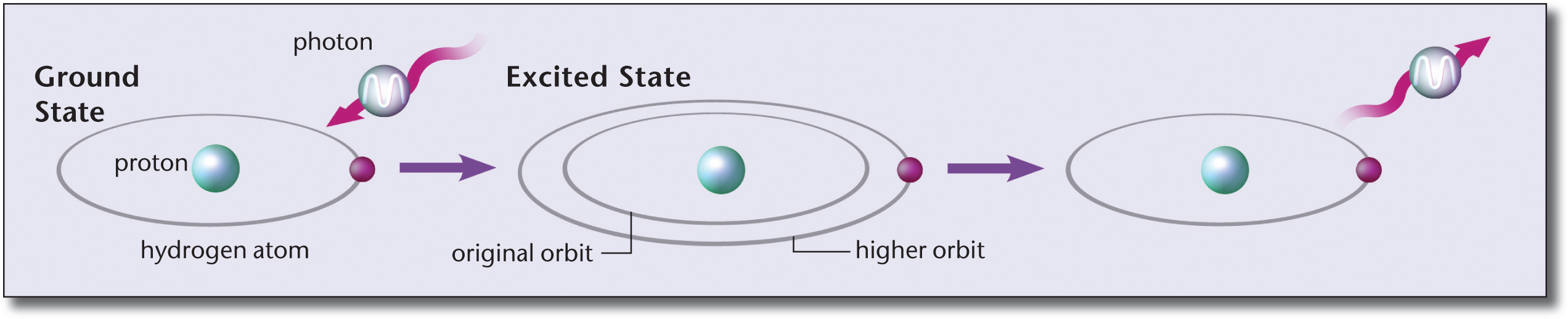
In Chapter 1 of MCAT General Chemistry Review, we explored the Bohr model of the atom. As a reminder, the Bohr model states that electron energy levels are stable and discrete, corresponding to specific orbits. An electron can jump from a lower-energy to a higher-energy orbit by absorbing a photon of light of precisely the right frequency to match the energy difference between the orbits (E = hf). If a photon does not carry enough energy, then the electron cannot jump to a higher energy level. When an electron falls from a higher-energy level to a lower-energy level, a photon of light is emitted with an energy equal to the energy difference between the two orbits. These processes of atomic absorption and emission are shown in Figure 9.2.

While information about a single electron is a great foundation for Test Day, in the real world we’ll often be handling more complex structures. In organic chemistry, we use infrared (IR) spectroscopy to determine chemical structure because different bonds will absorb different wavelengths of light. UV–Vis spectroscopy takes this one step further, looking at the absorption of light in the visible and ultraviolet range. Absorption spectra may be represented as a color bar with peak areas of absorption represented by black lines. It can also be shown as a graph with the absolute absorption as a function of wavelength. This is shown in Figure 9.3, which shows the absorption spectrum for the atmosphere across the entire electromagnetic spectrum.

Changes in molecular structure can cause dramatic shifts in the absorption patterns of a substance. Consider indicators like phenolphthalein. This indicator has a clear appearance in its acidic state, and thus does not absorb any visible light. In its basic state, it is a bright pink, and thus is absorbing all but the longer wavelengths of visible light—remember that we see the colors that are not absorbed. Most indicators contain large organic compounds that have strikingly different absorption patterns based solely on the protonation state of the compound. These compounds often have conjugated double bonds or aromatic ring systems, as this permits the absorption of light from photons in the visible range.
Another phenomenon related to absorption and emission of visible light is fluorescence. If one excites a fluorescent substance (such as a ruby, an emerald, or the phosphors found in fluorescent lights) with ultraviolet radiation, it will begin to glow with visible light. Photons of ultraviolet light have relatively high frequencies (short wavelengths). After being excited to a higher energy state by ultraviolet radiation, the electron in the fluorescent substance returns to its original state in two or more steps. By returning in two or more steps, each step involves less energy, so at each step, a photon is emitted with a lower frequency (longer wavelength) than the absorbed ultraviolet photon. If the wavelength of this emitted photon is within the visible range of the electromagnetic spectrum, it will be seen as light of the particular color corresponding to that wavelength. The wide range of colors in fluorescent lights, from the whitish-green of office lighting to the glaring colors of neon signs, is the result of the distinct multi-step emission spectra of different fluorescent materials.