Learning Objectives
After Chapter 10.2, you will be able to:
- Estimate the square root of a given value, like

- Estimate the log value of a given number
- Simplify expressions such as: (a + 2b)3
After Chapter 10.2, you will be able to:

For many students, exponents and logarithms are topics filed away in the depths of memory. While exponential and logarithmic functions are uncommon in everyday life, a number of science topics and equations regularly tested on the MCAT require use of these concepts, as shown in Table 10.1.
| Topic | Equation | Location in Kaplan MCAT Review Series |
| Sound level |
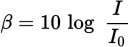
|
Chapter 7 of MCAT Physics and Math Review |
| Exponential decay | n = n0e−λt | Chapter 9 of MCAT Physics and Math Review |
| Arrhenius equation for activation energy |

|
Chapter 5 of MCAT General Chemistry Review |
| Gibbs free energy |

|
Chapter 7 of MCAT General Chemistry Review |
| p scales (pH, pOH, pKa, pKb) | pH = −log [H+] | Chapter 10 of MCAT General Chemistry Review |
| Henderson–Hasselbalch equation |
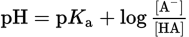
|
Chapter 10 of MCAT General Chemistry Review |
In addition to exponential equations, exponents appear frequently on the MCAT in the context of scientific notation, discussed earlier. Here, we look at the rules of arithmetic with exponents.
Only a basic understanding of exponents is necessary for the MCAT, although it can be helpful to know a few values and basic rules. First, any number to the zeroth power is equal to 1:
When adding or subtracting numbers with exponents, the true value must be calculated before the addition or subtraction can be performed. For example, 32 + 32 ≠ 62; rather, 32 + 32 = 9 + 9 = 18. However, if the base and exponent are the same, we can add the coefficients: 32 + 32 = (1 + 1) × 32 = 2 × 32 = 18.
In cases of multiplication and division, the exponents can be manipulated directly, as long as the base number is the same. When multiplying two numbers with the same base, the exponents are added to determine the new number:
When adding, subtracting, multiplying, or dividing numbers with exponents, the base must be the same.
In division, we subtract the exponent of the denominator from the exponent in the numerator to find the exponent in the quotient, as long as all bases are the same:
For a number that is raised to an exponent and then raised again to another exponent, the two exponents are multiplied:
When a fraction is raised to an exponent, the exponent is distributed to the numerator and denominator:
Negative exponents represent inverse functions:
For fractional exponents, the numerator can be treated as the exponent, and the denominator represents the root of the number:
On Test Day, you may be expected to calculate approximate square roots. To do so, it is useful to be familiar with the values in Table 10.2.
| X | X2 | X | X2 | X | X2 | X | X2 |
| 1 | 1 | 6 | 36 | 11 | 121 | 16 | 256 |
| 2 | 4 | 7 | 49 | 12 | 144 | 17 | 289 |
| 3 | 9 | 8 | 64 | 13 | 169 | 18 | 324 |
| 4 | 16 | 9 | 81 | 14 | 196 | 19 | 361 |
| 5 | 25 | 10 | 100 | 15 | 225 | 20 | 400 |
If you are asked to calculate the square root of any number less than 400, you can approximate its value by determining which two perfect squares it falls between. As an alternative method, you can divide the number given to you by known squares to attempt to reduce it:

One can estimate this value by considering that the square root of five is somewhere
between 2 and 3 (22 = 4 and 32 = 9), and is closer to 2 than 3. If we estimate
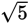 to be about 2.2, then
to be about 2.2, then
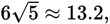 which is congruent with our knowledge that the square root of 180 will be between
13 and 14. The true value of
which is congruent with our knowledge that the square root of 180 will be between
13 and 14. The true value of
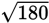 is approximately 13.4.
is approximately 13.4.
If you are using a number in scientific notation, adjust the decimal by one place if necessary so that the exponent is easily divisible by two:
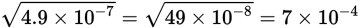
Estimation of square roots and logarithms is generally sufficient to the first decimal place; don’t struggle to become more precise because it won’t be necessary on Test Day.
Finally, it is useful to know the values of
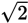 and
and
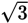 :
:
Logarithms follow many of the same rules as exponents because they are inverse functions. The logarithmic rules are described below:
It is also useful to know that “p” can be shorthand for −log; thus, pH = −log [H+], pKa = −log Ka, and so on.
Logarithms can use any base, but the most common are base ten, as in our decimal system, and base e (Euler’s number, about 2.718). Base-ten logarithms (log10) are called common logarithms, whereas those based on Euler’s number (loge or ln) are called natural logarithms. Both common and natural logarithms obey the rules discussed above, but it can be easier to estimate common logarithms because of our familiarity with the decimal number system. Therefore, it is useful to be able to convert between natural logarithms and common logarithms:
e is Euler’s number, which is 2.718281828459045… . It is also the base for the natural logarithm.
When estimating the logarithm of a number, use scientific notation. An exact logarithmic calculation of a number that is not an integer power of 10 is unnecessary on the MCAT. The testmakers are interested, however, in testing your ability to apply mathematical concepts appropriately in solving certain problems. Fortunately, there is a simple method of approximation that can be used on Test Day. If a value is written in proper scientific notation, it will be in the form n × 10m, where n is a number between 1 and 10. From this fact, we can use logarithm rules to approximate the value:
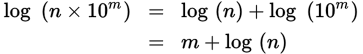
Because n is a number between 1 and 10, its logarithm will be a decimal between 0 and 1 (log 1 = 0 and log 10 = 1). The closer n is to 1, the closer log n will be to 0; the closer n is to 10, the closer log n will be to 1. As a reasonable approximation, one can say that
where 0.n represents sliding the decimal point of n one position to the left (dividing n by ten). For example, log (9.2 × 108) ≈ 8 + 0.92 = 8.92 (actual = 8.96).
A similar concept for estimating logarithms is used in calculations of pH, as described in Chapter 10 of MCAT General Chemistry Review. The shortcut is slightly different because we are working with negative logarithms and a negative exponent in the case of pH: