
During the last few years, we have collected a variety of biomarkers that are related to cognitive changes with age. Some of these data are included in the next chapter (on early detection of dementia). This topic now deserves a separate chapter that includes our work on the relation of the APOE gene to psychometric measures of cognition, as well as the impact of biomarkers of inflammatory disease. It also includes some early findings on neuroimaging work now in progress.
There have been a number of interesting findings by geneticists regarding the relation of ApoE allele combinations to the likelihood of occurrence and the time of onset of Alzheimer’s disease. There are six possible APOE genotypes, commonly referred to as ε2/2, ε2/3, ε2/4, ε3/3, ε3/4, and ε4/4. The most common genotype is the ε3/3 combination (Poduslo & Yin, 2001; Saunders, 2000). Specifically, the presence of the ε4 allele has been linked to the disease in a dose-dependent manner. That is, there may be some predictive power for a single ε4 allele, but high predictive power for the presence of the double ε4 allele (cf. Saunders et al., 1993). Moreover, a meta-analysis across a large number of APOE studies found significant associations between the APOE ε4 allele and Alzheimer’s disease in white, African American, Hispanic, and Chinese subpopulations in the United States (Farrer et al., 1997). In addition, presence of the ε2 allele has been linked to lower low-density lipoprotein cholesterol levels as well as lower total/high-density lipoprotein cholesterol ratios; the latter may in turn be related to health outcomes that influence the severity of age-related decline and possibly the likelihood of an eventual diagnosis of dementia.
In this section, I report results of some preliminary analyses of a subsample from the SLS for whom we obtained APOE genotypes and for whom we have at least 7-year longitudinal cognitive data. The analyses were to determine whether there is differential age-related decline in cognitive abilities that depends on membership in a given allele combination (Kennet, McGuire, Willis, Schaie, & Caskie, 2000; Kennet, Revell, & Schaie, 1999; Kennet & Schaie, 1998; Pierre, Schaie, Ryan, & Stiehler, 2008; Stiehler, Ryan, Willis, & Schaie, 2008).
APOE alleles have been determined thus far on 1,042 SLS participants (41.8% male, 58.2% female) who at their last cognitive test in 2005 averaged 65 years of age and for whom ε2/2 = 11 (1.06%), ε2/3 = 129 (12.38%), ε2/4 = 36 (3.45%), ε3/3 = 620 (59.50%), ε3/4 = 222 (21.31%), and ε4/4 = 24 (2.30%). These frequencies are roughly comparable to those reported in other studies of European and North American populations (cf. Parasuraman, Greenwood, & Sunderland, 2002).
Dependent variables in these analyses were the six mental ability factor scores of Inductive Reasoning, Spatial Orientation, Numeric Facility, Verbal Ability, and Verbal Memory (see chapter 3).
Data were analyzed by analyses of covariance (ANCOVAs), controlling for participant age, to examine the effect of APOE allele combinations. APOE combination is treated as a categorical variable (the ε2/2 combination was omitted because of the low frequency of occurrence in our sample).
The ANCOVA yielded a significant overall effect for differences among allele combinations [Rao’s R (df = 30, 2666) = 1.55, p < .03]. Univariate follow-ups resulted in significant allele combination differences for the Inductive Reasoning and Verbal Memory factors (p < .01). There were no significant gender or Gender × Allele combination effects. Age differences among allele combinations were significant, but only for the ε4/4 combination as compared with all others. Seven-year declines in the cognitive factor scores are shown in table 18.1. Greatest decline was shown for both Inductive Reasoning and Verbal memory by the ε2/4 combination; least decline was observed for the ε2/3 combination. On average, there was also a trend for greater decline for the combined group of individuals with at least one ε4 allele.
In conjunction with the genotyping for the APOE alleles, we have also routinely conducted other analyses on the blood samples obtained for that purpose. These analyses include a lipid profile, consisting of measures of triglycerides, total cholesterol, low-density cholesterol, and high-density cholesterol. In addition we have obtained measures of the inflammatory markers of homocysteine and C-reactive protein. Thus far we have found only modest negative correlations between low-density cholesterol measures of perceptual and motor speed. However, we have also been able to store samples of blood plasma with the intention of enabling us to examine other biomarkers that become identified as having promising relationships to changes in adult cognition.
TABLE 18.1. Cognitive Decline Over 7 Years in T-Scores by Apoprotein E Allele Combination

Cognitive decline and brain atrophy occurring with increasing age can be categorized as either normal or pathologic depending on the age of onset, the rate of decline, and the extent of change (Hedden & Gabrieli, 2004; Raz & Rodrigue, 2006; Raz et al., 2008; Rodrigue & Kennedy, 2011). The association between hippocampal volume and episodic memory and their salience in both the preclinical phase and diagnosis of dementia have been studied extensively (Barnes, Ourselin, & Fox, 2009). Both age-related memory loss and Alzheimer’s disease have been associated with hippocampal volume loss (Bäckmann, 2008; Braak & Braak, 1991), and the rate of hippocampal atrophy is predictive of transition from normal cognition to mild cognitive impairment (MCI) and to dementia (Mungas et al., 2005). However, the level of cognitive performance during the years preceding the diagnosis of AD, that is, preclinical deficits, cannot reliably differentiate lifelong impairments and normal age-related deficits from preclinical disease (Bäckman, Jones, Berger, Laukka, & Small, 2005). Similarly, absolute hippocampal volume during midlife is poorly correlated with memory function and later cognitive decline (Nyberg & Bäckman, 2011; Van Petten, 2004). Thus, while midlife is increasingly recognized as a critical period in the study of both healthy and pathological aging (Finch, 2009), it remains unclear how midlife cognitive abilities relate to cognitive decline and brain atrophy in old age.
Most aging research focuses on normative versus pathological aging, leaving our knowledge of what constitutes optimal aging more limited. Cross-sectional studies suggest that memory, and other cognitive domains, show increasing age differences throughout life beginning in early adulthood (Park & Reuter-Lorenz, 2009; Salthouse, 2009). Longitudinal studies, including our own, suggest that many cognitive abilities remain stable during midlife (defined as ages 40–60) and decline only later in old age (Giambra, Arenberg, Kawas, Zonderman, & Costa, 1995; Hultsch, Hertzog, Dixon, & Small, 1998; Nilsson, Sternang, Ronnlund, & Nyberg, 2009; Schaie, 2005a). However, we have found that some individuals appear to improve or decline on various cognitive measures, including episodic memory (i.e., memory for time-related events and experiences), during midlife (Willis & Schaie, 2010). Thus, using longitudinal data, we sought to determine if differential change (stability, improvement, or decline) in midlife episodic memory is predictive of hippocampal volumes in nondemented adults in middle and old age (Borghesani et al., 2011).
In this study we have used cognitive testing data from the Seattle Longitudinal Study (SLS; Schaie, 2005a; Willis & Schaie, 2005) to retrospectively identify subjects (age 52–87) whose episodic memory improved, declined, or remained stable during midlife (age 43–63). Structural MRIs were then collected in 2006–2007 to determine: (1) if the trajectory of midlife memory change predicted hippocampal and/or brain volume at the end of midlife and later in old age, (2) if individual mid-life memory scores predicted delayed recall performance in middle and old age, and (3) if the apolipoprotein ε4 (APOE ε4) allele, known to be a risk factor for brain atrophy and dementia (Mahley, Weisgraber, & Huang, 2006), and/or demographic factors modified this relationship.
The neuroimaging studies currently involve 161 SLS participants who were selected to undergo MRI scanning in 2006–2007. This subset was selected from a larger group (n = 572) who, (1) had two or three assessments of episodic memory over a 14-year interval during midlife (ages ranged from 43 to 63 yrs), who (2) participated in the 2005 SLS data collection and, (3) for the old age cohort, who had at least one assessment in old age (>64 yrs). Beyond cognitive and imaging data, participants completed health surveys to assess their vascular risk and disease, and they provided blood samples for APOE genotyping (Northwest Lipid Research Laboratories, Seattle WA). Self-reported vascular risk factors (history of smoking, hypertension, diabetes, and/or hyperlipidemia) were common with 67% of the sample having one or more risk factors (Willis & Schaie, 1998).
The trajectory of midlife episodic memory change was characterized as declining, improving, or stable by examining each individual’s immediate and delayed recall scores over two 7-year intervals in midlife (average age intervals 46 to 53 years and 53 to 60 years). Improvement or decline was defined by: (1) > 1 SE of change over a 14-year interval in immediate and delayed recall (cf. Dudek, 1979; Schaie & Willis, 1986b), (2) consistent direction of memory change in each of the two 7-year intervals and, (3) concordant direction of change for immediate and for delayed recall. In the entire sample of SLS participants with midlife data (n = 572), approximately 12% (N = 67) of individuals were identified as improving and 14% (N = 78) declining (Schaie, Willis, & Madhyastha, 2012).
Participants were stratified into two birth cohorts: middle age (MA), DOB 1941–1955, N = 49; and old age (OA), DOB 1907–1941, N = 35. All subjects underwent structural MRI in 2006–2007 such that the MA cohort was scanned between ages 53–65 (mean 60.9 years, n = 84) while the OA cohort was imaged between ages 66–87 (mean 74.5 years, n = 77) 7 or more years after their midlife cognitive evaluations. Magnetization prepared rapid gradient echo (MPRAGE) imaging was performed on a Philips 3.0 T Achieva scanner using the following parameters: repetition time (TR) = 7 milliseconds (ms), echo time (TE) = 3.20 ms; flip angle = 8°; matrix size of 240 × 240 and with 160 sagittally collected slices and a slice thickness of 1 mm. Time of collection was approximately 20 minutes.
MPRAGE scans were reconstructed into a 1 × 1 × 1 mm 3D volume using MRIcron software and dcm2nii (http://www.mccauslandcenter.sc.edu/CRNL/). All measurements were performed blinded on deidentified data.
Intracranial Volumes (ICV): The ICV for each individual was calculated using a stereologic Cavalieri technique (Barta, Dhingra, Royall, & Schwartz, 1997) implemented through MEASURE software (http://pni.med.jhu.edu/methods/morph.htm) using the Gundersen formula (Gundersen et al., 1988). In brief, a 3D grid of points overlaying the intracranial cavity, including the cerebrum, cerebellum, sulcal and ventricular CSF, and brainstem superior to the foramen magnum, was manually identified for each subject by a single rater (R.L.E.) and ICV volume calculated.
Whole Brain Volumes. The brain (cerebrum, cerebellum, and brain stem) was skull stripped using FreeSurfer (http://surfer.nmr.mgh.harvard.edu/) and manually reviewed. The FANTASM plug-in for MIPAV (http://medic.rad.jhu.edu/projects/fantasm/ and http://mipav.cit.nih.gov/) was used to segment the stripped brain image into gray matter, white matter, and cerebral spinal fluid, and white and gray matter were added together for total volume.
Hippocampal Volumes. A FreeSurfer + LDDMM (large deformation diffeomorphic metric mapping; Beg, Miller, Trouve, & Younes, 2005) pipeline as described by Khan, Wang, and Beg (2008) and by Wang et al. (2009) was used. In brief, the stripped brains were segmented into subcortical and neocortical structures using FreeSurfer 4.1.0 (Fischl et al., 2002), and these “bounding boxes” were used in limit LDDMM during the template-to-target transformations. A hippocampal template mask (both left and right) was generated by hand from a typical subject by an expert rater (E. H. A.). Confidence maps from the FreeSurfer segmentations (Kahn, Chung, & Beg, 2009) were used to weight the influence of the initial segmentations in a simultaneous LDDMM registration.
Regression methods were used to correct hippocampal and brain volume for head size with the following formula: adjusted volume = raw volume β(ICV − mean ICV). In this formula β is the slope of the regression of ICV to raw volume, and mean ICV is the average ICV from all 161 subjects (Rodrigue & Raz, 2004). Categorical variables included midlife DR trajectory (improve, decline, or stable), gender (male or female), and cohort (MA or OA). Continuous variables included age at MRI (AGEMRI), delayed recall (DR) score at MRI (DRMRI), education (in years), and brain and hippocampal volumes. Hippocampal and brain volumes were used as the dependent variable while all other variables were modeled as predictors.
To determine whether change in midlife (age 43–63) delayed recall (DR) scores were predictive of hippocampal volume, we retrospectively classified two subject cohorts, middle-age (MA, 53–65 yrs) and old-age (OA, 66–87 yrs), as declining, improving, or stable with regard to the trajectory of their midlife DR scores. Within their age cohorts, groups did not differ in regard to gender, education, or MMSE (Table 18.2). Across all participants, decliners were more likely to be carriers of the APOE ε4 allele than improvers (43% vs. 15%; χ2 = 5.1 p < 0.05). However, neither group differed significantly from the stables (25% APOE ε4 carriers), and within cohorts differences in APOE ε4 status did not reach significance (logistic regression in MA: χ2 = 1.3, p = 0.25; and in OA: χ2 = 1.0, p = 0.31). The average age of OA decliners at the time of MRI (AGEMRI) was somewhat greater than other OA groups (Table 18.2). Longitudinal analysis of DR scores from all subjects showed that from average age 60 (range 57–63) and onward, midlife DR decliners had significantly lower DR scores than midlife improvers or stables (see table 18.3).
Our a priori hypothesis was that the trajectory of midlife DR change would predict hippocampal volume differently in the MA and OA cohorts. An analysis of covariance (ANCOVA) revealed significant main effects of cohort (F8,74 = 33.5, p < 0.001), DR trajectory (F8,74 = 3.8, p < 0.05) and their interaction (F8,74 = 3.6, p < 0.05) on hippocampal volume (with or without current DR scores [DRMRI], education, or AGEMRI gender as covariates; hippocampal and brain volumes corrected for intracranial volumes via regression methods). Post hoc analyses revealed that hippocampal volumes in OA improvers were 13% larger than OA stables and decliners while hippocampal volumes in OA improvers did not differ from MA subjects (Figure 18.1).
Similar ANCOVAs using total brain volumes as the independent variable revealed only cohort (F8,74 = 27.5, p < 0.001) as a significant main effect while midlife DR scores and trajectory were unrelated to total brain size. Post hoc analyses revealed the MA cohort had 6% larger brains than did the OA cohort (1128 cm3 vs. 1060 cm3, t-test, p < 0.001). Complementary regression analysis without stratifying subjects into MA and OA cohorts revealed a significant AGEMRI × midlife DR trajectory interaction (F9, 73 = 4.6, p < 0.05) with improvers showing less hippocampal volume loss with age (with or without demographic or DRMRI covariates).
TABLE 18.2. Sample Demographics for Midlife Memory and Hippocampal Change Study
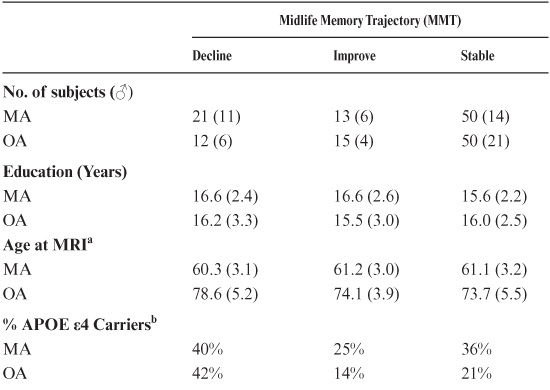
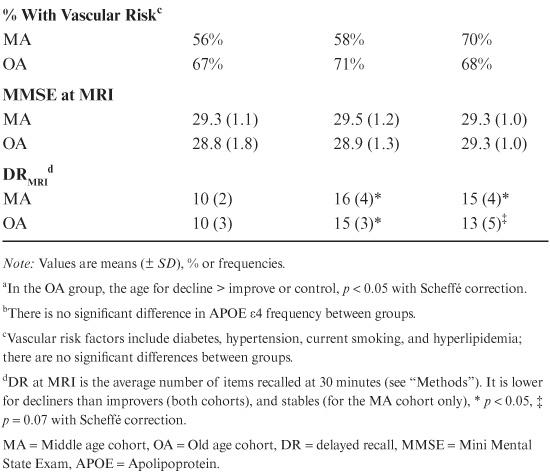
In the preceding analysis, the inclusion of DRMRI as a covariate did not affect the significance of midlife DR trajectory on hippocampal volume in OA, nor did the DRMRI attain significance in the ANCOVA (DRMRI beta, p = 0.69). This suggests that current DR scores (i.e., DRMRI) were a poor predictor of hippocampal volumes. To determine if this were true at all ages, and if past DR scores were predictive of hippocampal volumes, linear regressions using DR scores as the sole dependent variable were performed independently for the oldest subjects (average age 78 ± 3.7, range 73–87, n = 25), younger subjects (the MA cohort), and those in between (average age 69 ± 2.4, range 66–72, n = 10). In our oldest subjects, DRMRI scores and DR scores 7 years prior to the MRI were significantly associated with hippocampal volumes (For DRMRI, F1,23 = 8.1, p < 0.01, adjR2 = 0.23; and for 7 years prior, F1,23 = 5.7, p < 0.05, adjR2 = 0.16) while earlier scores (i.e., scores from 14 or 21 years prior to the MRI) were not associated with hippocampal volume. In contrast, in neither the MA cohort nor those slightly older were DRMRI scores or any prior DR scores significantly predictive of hippocampal volumes (data not shown). Finally, in our population, hippocampal volume was not associated with APOE ε4 status in either the MA or OA cohorts (no main effects of being an APOE ε4 carrier in an ANCOVA with cohort, education, gender, and DRMRI as covariates). Given the low frequency of APOE ε4 carriers who were improvers (n = 2 in both MA and OA cohorts), the possible interaction between APOE status and midlife DR trajectory could not be clarified.
TABLE 18.3. Midlife Memory Trajectory (MMT) Predicts Hippocampal Volumes in Old Agea
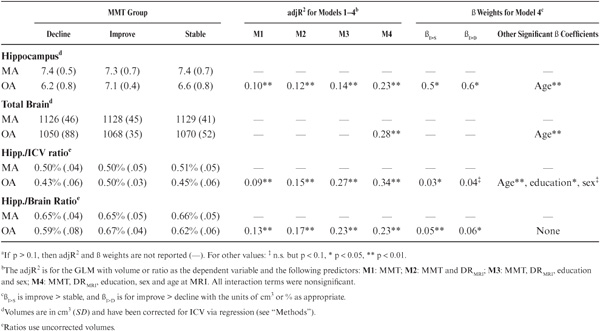

FIGURE 18.1. Hippocampal volume for decliners, stables, and gainers in midlife and old age corrected for intracranial volume.
The above findings confirm the a priori hypothesis that the trajectory of midlife memory change predicts hippocampal volumes in old age (OA). Midlife memory improvement was associated with larger hippocampal volumes in OA, in comparison with OA decliners or stables, while total brain volumes were similar among the improvers, decliners, and stables in both the middle-age (MA) and OA cohorts. Overall, hippocampal volumes in OA improvers were no different from MA subjects. In contrast, episodic memory scores at any single age during midlife did not predict hippocampal volumes (in either MA or OA) demonstrating that memory change, not scores from an individual assessment, are important for predicting future hippocampal volumes. In fact, in this cognitively normal aging sample, only in the oldest subgroup (age > 73) did current and delayed recall (DR) scores from 7 years prior correlate with hippocampal volume.
Hippocampal volume loss in older individuals has been estimated to be ~1% annually (Resnick, Pham, Kraut, Zonderman, & Davatzikos, 2003); it may accelerate with age (Raz et al., 2004) and is associated with age-related episodic memory decline and cognitive impairment (Jack et al., 1999). In contrast, during midlife the association between hippocampal volume and episodic memory is more ambiguous, with some research even suggesting a negative relationship between hippocampal volume and episodic memory (Van Petten, 2004). Findings that DR scores were only associated with hippocampal volume in old age are consistent with past research. Only a few studies have explored the longitudinal association between midlife cognitive changes and hippocampal volume (Persson et al. 2006; Raz et al., 2005). Raz reported that individuals whose cognitive abilities declined over a decade had reduced hippocampal volume, decreased white matter integrity, and altered brain function. This is somewhat at odds with our finding that OA midlife stables and decliners had similar hippocampal volumes in spite of differences in DR scores. One explanation for this finding would be that OA stables, who actually had very good memory in midlife, were able to compensate for hippocampal volume loss through recruitment of alternative cortical networks (Park & Reuter-Lorenz, 2009).
Short-term cognitive training has been shown to significantly enhance cognitive performance and modify brain structure (Fullerton et al., 2000) and function (Thomas et al., 2009). Unfortunately, the effects of these interventions are generally of short duration unless the mental stimulation is continued and becomes part of the individual’s lifestyle (Valenzuela & Sachdev, 2009). In contrast, the effects of midlife memory improvement, which represented an average change of 0.75 standard deviations over a 14-year period in midlife, had long-term outcomes. On average, OA improvers were functioning at a level higher than their performance level in early midlife (Figure 18.1). Similar to studies of cognitive interventions (Papp, Walsh, & Snyder, 2009), episodic memory improvement seen in OA improvers was generally ability specific. That is, on average, memory improvers did not demonstrate comparable gains on other abilities, such as executive functioning in midlife (data not shown). Thus, a major challenge for our future research is examining the specific lifestyle and activities of memory improvers, particularly in midlife, to identify factors associated with this sustained cognitive enhancement.
Genetic and vascular risk factors have been found to impact both cognitive aging and hippocampal atrophy (Raz et al., 2005; Small, Rosnick, Fratiglioni, & Bäckman, 2004). Cognitive decline, particularly episodic memory and executive functioning, begins almost a decade earlier in carriers of the APOE ε4 allele, a genetic risk for MCI and AD (Mahley, Weisgraber, & Huang, 2006) as compared with normative samples (Small et al., 2004). Likewise, beginning in midlife, APOE ε4 carriers are reported to have smaller hippocampal volume and a greater accumulation of senile plaque (Kok et al., 2009; Small et al., 2004). In our sample, significantly more midlife memory decliners (41%) were APOE ε4 carriers in comparison with improvers (18%). Overall, 27% of our participants were APOE ε4 carriers, similar to the expected prevalence in community samples (Fullerton et al., 2000). However, APOE ε4 status was not a significant predictor of hippocampal volume in either our MA or OA cohorts. Likewise, vascular risk has been found to exert a negative influence on age-sensitive cognitive abilities and to be related to hippocampal atrophy (Cohen et al., 2006; Gianaros, Greer, Ryan, & Jennings, 2006; Rodrigue & Raz, 2004). Of particular interest are the four improvers who were APOE ε4 carriers and reported no vascular risk factors. This is in accordance with findings that suggest that the detrimental effects of APOE ε4 can be attenuated by monitoring of blood pressure and cholesterol (Kivipelto et al., 2002).
Limitations of this study include both sample and technological issues. First, our sample size is relatively small, generally well educated, and racially homogeneous, and thus the generalizability to lower socioeconomic groups who have increased risk for cognitive decline is unclear. Second, semiautomated volumetric techniques require careful checking and can variably missegment cortical structures. To address this problem, each hippocampal mask generated by the LDDMM procedure was visually inspected, and systematic errors were addressed by reprocessing the entire sample. How/whether to correct hippocampal volumes for head size is debated. Uncorrected volumes, structure-of-interest to brain ratios, and covariance with ICV are all routinely employed, and we chose to use regression methods to correct hippocampal and brain volumes for ICV. In fact, if gender is not considered to be a primary question or confound, then the method of correction, or correcting at all, appears to make little difference when calculating age-related cerebral atrophy (Greenberg et al., 2008).
While stability of cognitive performance is normative in midlife, this study illustrates the importance of changes in midlife cognition for predicting cognitive functioning and hippocampal volume in old age. Declining memory abilities could be an early manifestation of disease, but this would be at odds with the lack of an association between memory decline and hippocampal volume in midlife and inconsistent with the similar hippocampal volumes found in old age in midlife decliners and stables. While decline in midlife cognition and its implications for normal and pathological aging have been reported (Persson et al., 2006), this study is one of the first, to our knowledge, to examine memory improvement in midlife and its impact on cognitive and brain aging. Although little is known about the specifics of cognitive engagement and stimulation that are assumed to be associated with development of cognitive reserve, midlife memory improvement appears to be a marker for optimal hippocampal aging, suggesting that interventions aimed at improving brain health, be they lifestyle or pharmacologic, should be begun during middle age.
This chapter provides initial results for our work with the ApoE genetic marker of dementia as it relates to cognitive decline. We confirmed excess decline over 7 years in persons over age 60 years who possess the ε2/4 allele combination on the cognitive abilities of Inductive Reasoning and Verbal Memory, with a trend toward excess decline in Perceptual Speed. The ε2/3 allele combination, moreover, showed less-than-average decline on these abilities.
While stability of cognitive performance is normative in midlife, we report in this chapter a study of the importance of changes in midlife cognition for predicting cognitive functioning and hippocampal volume in old age. Declining memory abilities could be an early manifestation of disease, but this would be at odds with the lack of an association between memory decline and hippocampal volume in middle age and inconsistent with the similar hippocampal volumes found in old-age midlife decliners and stables. While decline in midlife cognition and its implications for normal and pathological aging have been reported (Persson et al., 2006), this study is one of the first, to our knowledge, to examine memory improvement in midlife and its impact on cognitive and brain aging.
In this chapter we describe our initial efforts to include markers of biological influences that may affect individual differences in cognitive development through adulthood. We first examine the effect of different allele combinations of the Apoliprotein E and show that both homozygous carriers of the ε4 allele and heterozygous carriers of the ε42 combination show greater negative cognitive changes beginning in midlife. We next describe the identification of lipid profiles, noting a modest negative correlation between high-density cholesterol and measures of perceptual and psychomotor speed, as well as measures of the inflammatory markers of homocysteine and C-reactive protein.
The remainder of the chapter provides an initial report of an MRI study of a subset of 192 SLS participants who were classified as having been stables, gainers, or decliners during midlife from the 40s to the early 60s. Participants who decline on memory and executive functions in midlife showed excess hippocampal atrophy in old age, while the hippocampi of midlife gainers remained equivalent to those measured during midlife.