
Top texture: © Laguna Design / Science Source;
CHAPTER 10: The Replicon: Initiation of Replication
Chapter Opener: © Dr. Gopal Murti/Science Photo Library/Getty Images.
10.1 Introduction
Whether a cell has only one chromosome (as in most prokaryotes) or has many chromosomes (as in eukaryotes), the entire genome must be replicated precisely, once for every cell division. How is the act of replication linked to the cell cycle?
Two general principles are used to compare the state of replication with the condition of the cell cycle:
Initiation of DNA replication commits the cell (prokaryotic or eukaryotic) to a further division. From this standpoint, the number of descendants that a cell generates is determined by a series of decisions about whether to initiate DNA replication. Replication is controlled at the stage of initiation. When replication has begun, it continues until the entire genome has been duplicated.
If replication proceeds, the consequent division cannot be permitted to occur until the replication event has been completed. Indeed, the completion of replication might provide a trigger for cell division. The duplicate genomes are then segregated, one to each daughter cell. The unit of segregation is the chromosome.
The unit of DNA in which an individual act of replication occurs is called the replicon. Each replicon “fires” once, and only once, in each cell cycle. The replicon is defined by its possession of the control elements needed for replication. It has an origin at which replication is initiated. It can also have a terminus at which replication stops. Any sequence attached to an origin—or, more precisely, not separated from an origin by a terminus—is replicated as part of that replicon. The origin is a cis-acting site, able to affect only that molecule of DNA on which it resides.
(The original formulation of the replicon [in bacteria] viewed it as a unit possessing both the origin and the gene coding for the regulator protein. Now, however, “replicon” is usually applied to eukaryotic chromosomes to describe a unit of replication that contains an origin; trans-acting regulator protein[s] might be encoded elsewhere.)
Bacteria and archaea can contain additional genetic information in the form of plasmids. A plasmid is an autonomous circular DNA that constitutes a separate replicon. Each invading phage or virus DNA also constitutes a replicon, and thus is able to initiate many times during an infectious cycle. Perhaps a better way to view the prokaryotic replicon, therefore, is to reverse the definition: Any DNA molecule that contains an origin can be replicated autonomously in the cell.
A major difference in the organization of bacterial, archaeal, and eukaryotic genomes is seen in their replication. A genome in a bacterial cell has a single replication origin and thus constitutes a single replicon; therefore, the units of replication and segregation coincide. Initiation at a single origin sponsors replication of the entire genome, once for every cell division. Each haploid bacterium typically has a single chromosome, so this type of replication control is called single copy. The other prokaryotic domain of life, the archaea, is more complex. Whereas some archaeal species have chromosomes with a bacterial-like situation of a single replication origin, other species initiate replication from multiple sites on a single chromosome. For example, the single circular chromosomes of Sulfolobus species have three origins and thus are composed of three replicons. This complexity is further heightened in eukaryotes. Each eukaryotic chromosome (usually a very long linear molecule of DNA) contains a large number of replicons spaced unevenly throughout the chromosomes. The presence of multiple origins per chromosome adds another dimension to the problem of control: All of the replicons on a chromosome must be fired during one cell cycle. They are not necessarily, however, active simultaneously. Each replicon must be activated over a fairly protracted period, and each must be activated no more than once in each cell cycle. Multiple mechanisms exist to prevent premature reinitiation of replication.
Some signal must distinguish replicated from nonreplicated replicons to ensure that replicons do not fire a second time. Many replicons are activated independently, so another signal must exist to indicate when the entire process of replicating all replicons has been completed.
In contrast with nuclear chromosomes, which have a single-copy type of control, the DNA of mitochondria and chloroplasts might be regulated more like plasmids that exist in multiple copies per bacterium. There are multiple copies of each organelle DNA per cell, and the control of organelle DNA replication must be related to the cell cycle (see the chapter titled Extrachromosomal Replicons).
10.2 An Origin Usually Initiates Bidirectional Replication
Replication begins at an origin by separating or melting the two strands of the DNA duplex. FIGURE 10.1 shows that each of the parental strands then acts as a template to synthesize a complementary daughter strand. This model of replication, in which a parental duplex gives rise to two daughter duplexes, each containing one original parental strand and one new strand, is called semiconservative replication.

FIGURE 10.1 An origin is a sequence of DNA at which replication is initiated by separating the parental strands and initiating synthesis of new DNA strands. Each new strand is complementary to the parental strand that acts as the template for its synthesis.
A molecule of DNA engaged in replication has two types of regions. FIGURE 10.2 shows that when replicating DNA is viewed by electron microscopy, the replicated region appears as a replication bubble within the nonreplicated DNA. The nonreplicated region consists of the parental duplex; this opens into the replicated region where the two daughter duplexes have formed.
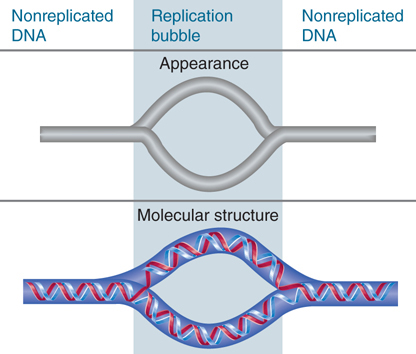
FIGURE 10.2 Replicated DNA is seen as a replication bubble flanked by nonreplicated DNA.
The point at which replication occurs is called the replication fork (also known as the growing point). A replication fork moves sequentially along the DNA from its starting point at the origin. The origin can be used to start either unidirectional replication or bidirectional replication. The type of event is determined by whether one or two replication forks set out from the origin. In unidirectional replication, one replication fork leaves the origin and proceeds along the DNA. In bidirectional replication, two replication forks are formed; they each proceed away from the origin in opposite directions.
The appearance of a replication bubble does not distinguish between unidirectional and bidirectional replication. As depicted in FIGURE 10.3, the bubble can represent either of two structures. If generated by unidirectional replication, the bubble represents one fixed origin and one moving replication fork. If generated by bidirectional replication, the bubble represents a pair of replication forks. In either case, the progress of replication expands the bubble until ultimately it encompasses the whole replicon. When a replicon is circular, the presence of a bubble forms the θ (theta) structure shown in FIGURE 10.4.
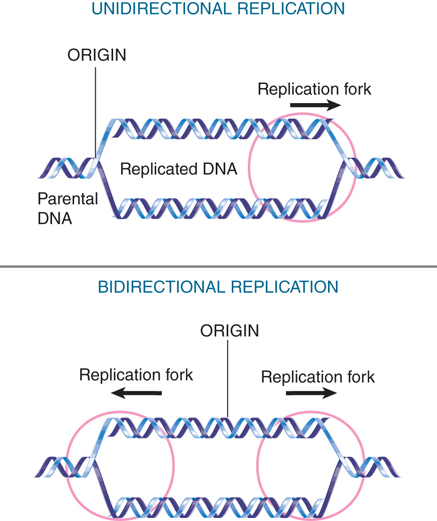
FIGURE 10.3 Replicons can be unidirectional or bidirectional, depending on whether one or two replication forks are formed at the origin.
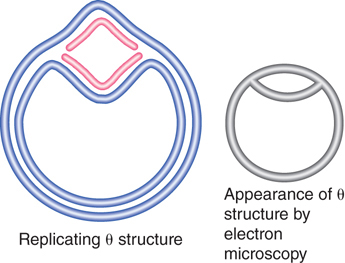
FIGURE 10.4 A replication bubble forms a θ structure in circular DNA.
10.3 The Bacterial Genome Is (Usually) a Single Circular Replicon
Prokaryotic replicons are usually circular, so that the DNA forms a closed circle with no free ends. Circular structures include the bacterial chromosome itself, all plasmids, and many bacteriophages, and are also common in chloroplasts and mitochondrial DNAs. FIGURE 10.5 summarizes the stages of replicating a circular chromosome. After replication has initiated at the origin, two replication forks proceed in opposite directions. The circular chromosome is sometimes described as a θ structure at this stage, because of its appearance. An important consequence of circularity is that the completion of the process can generate two chromosomes that are linked because one passes through the other (they are said to be catenated), and specific enzyme systems may be required to separate them (see the chapter titled Replication Is Connected to the Cell Cycle).

FIGURE 10.5 Bidirectional replication of a circular bacterial chromosome is initiated at a single origin. The replication forks move around the chromosome. If the replicated chromosomes are catenated, they must be disentangled before they can segregate to daughter cells.
The genome of E. coli is replicated bidirectionally from a single unique site called the origin, identified as the genetic locus oriC. Two replication forks initiate at oriC and move around the genome at approximately the same speed to a special termination region (see the chapter titled DNA Replication). One interesting question is this: What ensures that the DNA is replicated right across the region where the two forks meet?
What happens when a replication fork encounters a protein bound to DNA? We assume that repressors, for example, are displaced and then rebind. A particularly interesting question is what happens when a replication fork encounters an RNA polymerase engaged in transcription. A replication fork moves 10 times faster than RNA polymerase. Under the best of conditions, in log phase growth, collisions between the replication machinery and RNA polymerase do occur. In times of stress, such as amino acid starvation, it increases. A set of transcription factors acting as elongation factors interact with RNA polymerase to facilitate replication read through by removing transcription roadblocks, but this requires active transcription. It is not yet clear what the mechanism of action is. Most active transcription units are oriented so that they are expressed in the same direction as the replication fork that passes them. Many exceptions comprise small transcription units that are infrequently expressed. The difficulty of generating inversions containing highly expressed genes suggests that head-on encounters between a replication fork and a series of transcribing RNA polymerases might be lethal.
10.4 Methylation of the Bacterial Origin Regulates Initiation
The bacterial DnaA protein is the replication initiator; it binds sequence specifically to multiple sites (dnaA boxes) in oriC, the replication origin. DnaA is an ATP-binding protein and its binding to DNA is affected depending on whether ATP, ADP, or no nucleotide is bound. One mechanism by which the activity of the replication origin is controlled is DNA methylation. The E. coli oriC contains 11 copies of the sequence, which is a target for methylation at the N6 position of adenine by the Dam methylase enzyme. These sites are also found scattered throughout the genome. Note, though, that several of these methylation sites overlap dnaA boxes, as illustrated in FIGURE 10.6.

FIGURE 10.6 The E. coli origin of replication, oriC, contains multiple binding sites for the DnaA initiator protein. In a number of cases these sites overlap Dam methylation sites.
Before replication, the palindromic target site is methylated on the adenines of each strand. Replication inserts the normal (nonmodified) bases into the daughter strands. This generates hemimethylated DNA, in which one strand is methylated and one strand is unmethylated. Thus, the replication event converts Dam target sites from fully methylated to hemimethylated condition.
What is the consequence for replication? The ability of a plasmid relying upon oriC to replicate in dam− E. coli depends on its state of methylation. If the plasmid is methylated, it undergoes a single round of replication, and then the hemimethylated products accumulate, as described in FIGURE 10.7. The hemimethylated plasmids then accumulate rather than being replaced by unmethylated plasmids, suggesting that a hemimethylated origin cannot be used to initiate a replication cycle.

FIGURE 10.7 Only fully methylated origins can initiate replication; hemimethylated daughter origins cannot be used again until they have been restored to the fully methylated state.
This suggests two explanations: Initiation might require full methylation of the Dam target sites in the origin, or it might be inhibited by hemimethylation of these sites. The latter seems to be the case, because an origin of nonmethylated DNA can function effectively.
Thus hemimethylated origins cannot initiate again until the Dam methylase has converted them into fully methylated origins. The GATC sites at the origin remain hemimethylated for approximately 13 minutes after replication. This long period is unusual because at typical GATC sites elsewhere in the genome, remethylation begins immediately (less than 1.5 minutes) following replication. One other region behaves like oriC: The promoter of the dnaA gene also shows a delay before remethylation begins. Even though it is hemimethylated, the dnaA gene promoter is repressed, which causes a reduction in the level of DnaA protein. Thus, the origin itself is inert, and production of the crucial initiator protein is repressed during this period.
DNA methylation in bacteria serves a second function, as well: It allows the DNA mismatch recognition machinery to distinguish the old template strand from the new strand. If the DNA polymerase has made an error, such as creating an A-C base pair, the repair system will use the methylated strand as a template to replace the base on the nonmethylated strand. Without that methylation, the enzyme would have no way to determine which is the new strand.
10.5 Initiation: Creating the Replication Forks at the Origin oriC
Initiation of replication of duplex DNA in E. coli at the origin of replication, oriC, requires several successive activities. Some events that are required for initiation occur uniquely at the origin; others recur with the initiation of each Okazaki fragment during the elongation phase (see the chapter titled DNA Replication):
Protein synthesis is required to synthesize the origin recognition protein, DnaA. This is the E. coli licensing factor that must be made anew for each round of replication. Drugs that block protein synthesis block a new round of replication, but not continuation of replication.
There is a requirement for transcription activation. This is not synthesis of the mRNA for DnaA, but rather either one of two genes that flank oriC must be transcribed. This transcription near the origin aids DnaA in twisting open the origin.
There must be membrane/cell wall synthesis. Drugs (like penicillin) that inhibit cell wall synthesis block initiation of replication.
Initiation of replication at oriC begins with formation of a complex that ultimately requires six proteins: DnaA, DnaB, DnaC, HU, gyrase, and SSB. Of the six proteins, DnaA draws our attention as the one uniquely involved in the initiation process. DnaB, an ATP hydrolysis-dependent 5′ to 3′ helicase, provides the “engine” of initiation after the origin has been opened (and the DNA is single-stranded) by its ability to further unwind the DNA. These events will only happen if the DNA at the origin is fully methylated on both strands.
DnaA is an ATP-binding protein. The first stage in initiation is binding of the DnaA-ATP protein complex to the fully methylated oriC sequence. This takes place in association with the inner membrane. DnaA is in the active form only when bound to ATP. DnaA has intrinsic ATPase activity that hydrolyzes ATP to ADP and thus inactivates itself when the initiation stage ends. This ATPase activity is stimulated by membrane phospholipids and single-stranded DNA. Single-stranded DNA forms as soon as the origin is open. This is part of the mechanism used to prevent reinitiation of replication. The origin of the replication region remains attached to the membrane for about one-third of the cell cycle as another part of the mechanism to prevent reinitiation. While sequestered in the membrane, the newly synthesized strand of oriC cannot be methylated and so oriC remains hemimethylated until DnaA is degraded.
Opening oriC involves action at two types of sequence in the origin: 9-bp and 13-bp repeats. Together the 9-bp and 13-bp repeats define the limits of the 245-bp minimal origin, as indicated in FIGURE 10.8. An origin is activated by the sequence of events summarized in FIGURE 10.9, in which binding of DnaA-ATP is succeeded by association with the other proteins.
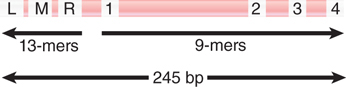
FIGURE 10.8 The minimal origin is defined by the distance between the outside members of the 13-mer and 9-mer repeats.

FIGURE 10.9 A two-state assembly model during initiation. DnaA-ATP monomers in an extended state associate with the high-affinity 13-mer sequences. DnaA-ATP transitions to a compact state as the 9-mer region begins to melt, stabilizing the single-stranded DNA.
Data from: Duderstadt, K. E., et al. 2010. “Origin Remodeling and Opening in Bacteria.” Journal of Biological Chemistry 285:28229–28239, The American Society for Biochemistry and Molecular Biology.
The four 9-bp consensus sequences on the right side of oriC provide the initial binding sites for DnaA-ATP in an extended multimeric state promoted by the accessory protein DiaA, which stimulates cooperative binding of DnaA. DnaA-ATP binds cooperatively to form a helical central core around which oriC DNA is wrapped. DnaA then acts at three A-T–rich 13-bp tandem repeats located on the left side of oriC. In its active form, DnaA-ATP transitions from the extended state to a compact form, twisting open the DNA strands in an unknown manner to form an open bubble complex and stabilizing the single-stranded DNA. All three 13-bp repeats must be opened for the reaction to proceed to the next stage. Transcription of either of the two genes flanking oriC provides additional torsional stress to help snap apart the double-stranded DNA.
Altogether, two to four monomers of DnaA-ATP bind at the origin, and after release of DiaA, they recruit two “prepriming” complexes of the DnaB helicase bound to DnaC-ATP, so that there is one DnaB–DnaC-ATP complex for each of the two (bidirectional) replication forks. The function of DnaC is that of a chaperone to repress the helicase activity of DnaB until it is needed. Each DnaB–DnaC complex consists of six DnaC monomers bound to a hexamer of DnaB. Note that the DnaB helicase cannot open double-stranded DNA; it can only unwind DNA that has already been opened, in this case by DnaA. DnaB binding to single-stranded DNA is the signal to hydrolyze ATP and for release of DnaC.
The prepriming complex generates a protein aggregate of 480 kD, which corresponds to a sphere with a radius of 6 nm. The formation of a complex at oriC is detectable in the form of the large protein blob visualized in Figure 10.9. When replication begins, a replication bubble becomes visible next to the blob. The region of strand separation in the open complex is large enough for both DnaB hexamers to bind, which initiates the two replication forks. As DnaB binds, it displaces DnaA from the 13-bp repeats and extends the length of the open region using its helicase activity. It then uses its helicase activity to extend the region of unwinding. Each DnaB activates a DnaG primase—in one case to initiate the leading strand, and in the other to initiate the first Okazaki fragment of the lagging strand.
Some additional proteins are required to support the unwinding reaction. Gyrase, a type II topoisomerase, provides a swivel that allows one DNA strand to rotate around the other. Without this reaction, unwinding would generate torsional strain (overwinding) in the DNA that would resist unwinding by the helicase. The protein single-strand binding protein (SSB) stabilizes and protects the single-stranded DNA as it is formed and modulates the helicase activity. The length of duplex DNA that usually is unwound to initiate replication is probably less than 60 bp. The protein HU is a general DNA-binding protein in E. coli. Its presence is not absolutely required to initiate replication in vitro, but it stimulates the reaction. HU has the capacity to bend DNA and is involved in building the structure that leads to formation of the open complex.
Input of energy in the form of ATP is required at several stages for the prepriming reaction, and it is required for unwinding DNA. The helicase action of DnaB depends on ATP hydrolysis, and the swivel action of gyrase requires ATP hydrolysis. ATP also is needed for the action of primase and to load the β subunit of Pol III in order to initiate DNA synthesis.
After the prepriming complex is loaded onto the replication forks, the next step is the recruitment of the primase, DnaG, which is then loaded onto the DnaB hexamer. This entails release of DnaC, which allows the DnaB helicase to become active. DnaC hydrolyzes ATP in order to release DnaB. This step marks the transition from initiation to elongation (see the chapter titled DNA Replication).
10.6 Multiple Mechanisms Exist to Prevent Premature Reinitiation of Replication
Replication in bacteria and in eukaryotes is licensed and permitted to occur only once per cell cycle. Each replicon is allowed to fire only once. What mechanisms are in place to ensure reinitiation does not occur? Because it is critical to maintain genomic integrity, multiple mechanisms exist to ensure that each replicon fires once, and only once, during each cell cycle.
As described in the section Methylation of the Bacterial Origin Regulates Initiation earlier in this chapter, the E. coli oriC is fully methylated at the beginning of replication. After semiconservative replication has occurred, oriC is hemimethylated and remains in that condition for approximately 13 minutes. What is responsible for this delay in remethylation at oriC? The most likely explanation is that these regions are sequestered in a form in which they are inaccessible to the Dam methylase.
A circuit responsible for controlling reuse of origins is identified by mutations in the gene seqA. The mutants reduce the delay in remethylation at both oriC and dnaA. As a result, they initiate DNA replication too soon, thereby accumulating an excessive number of origins. This suggests that seqA is part of a negative regulatory circuit that prevents origins from being remethylated. SeqA binds to hemimethylated DNA more strongly than to fully methylated DNA. It can initiate binding when the DNA becomes hemimethylated, at which point its continued presence prevents formation of an open complex at the origin. SeqA does not have specificity for the oriC sequence, and it seems likely that this is conferred by DnaA. This would explain the genetic interactions between seqA and dnaA.
As the only member of the replication apparatus uniquely required at the origin, DnaA has attracted much attention. DnaA is a target for several regulatory systems. It might be that no one of these systems alone is adequate to control frequency of initiation, but that when combined they achieve the desired result. Some mutations in dnaA render replication asynchronous, which suggests that DnaA could be the “titrator” or “clock” that measures the number of origins relative to cell mass. Overproduction of DnaA yields conflicting results, which vary from no effect to causing initiation to take place at reduced mass.
The availability of the amount of DnaA for binding at the origin is the result of competition for its binding to other sites on the chromosome. In particular, a locus called dat has a large concentration of DnaA-binding sites. It binds a larger number of DnaA molecules than the origin. Deletion of dat causes initiation to occur more frequently. This significantly increases the amount of DnaA available to the origin, but researchers do not yet understand exactly what role this might play in controlling the timing of initiation.
It has been difficult to identify the protein component(s) that mediate membrane attachment of oriC. A hint that this is a function of DnaA is provided by its response to phospholipids. Phospholipids promote the exchange of ATP with ADP bound to DnaA. Researchers do not know what role this plays in controlling the activity of DnaA (which requires ATP), but the reaction implies that DnaA is likely to interact with the membrane. This would imply that more than one event is involved in associating with the membrane. Perhaps a hemimethylated origin is bound by the membrane-associated inhibitor, but when the origin becomes fully methylated, the inhibitor is displaced by DnaA associated with the membrane.
Because DnaA is the initiator that triggers a replication cycle, the key event will be its accumulation at the origin to a critical level. There are no cyclic variations in the overall concentration or expression of DnaA, which suggests that local events must be responsible. To be active in initiating replication, DnaA must be in the ATP-bound form. Thus, hydrolysis of ATP to ADP by DnaA has the potential to regulate its own activity. Although DnaA has a weak intrinsic ATPase activity that converts the ATP to ADP, this is enhanced by a factor termed Hda. In a conceptually elegant feedback loop, Hda is recruited to a replication origin via the β subunit of the DNA polymerase. Thus, only when the origin has been activated and the full replication machinery assembled is Hda recruited, it acts to switch off DnaA, preventing a second round of replication.
The full scope of the system used to control reinitiation is not clear, but multiple mechanisms are involved: physical sequestration of the origin, delay in remethylation, competition for DnaA binding, hydrolysis of DnaA-bound ATP, and repression of dnaA transcription. It is not immediately obvious which of these events cause the others and whether their effects on initiation are direct or indirect. Indeed, we still have to come to grips with the central issue of which feature has the basic responsibility for timing. The period of sequestration appears to increase with the length of the cell cycle, which suggests that it directly reflects the clock that controls reinitiation. One aspect of the control might lie in the observation that hemimethylation of oriC is required for its association with cell membranes in vitro. This might reflect a physical repositioning to a region of the cell that is not permissive for replication initiation.
10.7 Archaeal Chromosomes Can Contain Multiple Replicons
Archaea are an interesting group of organisms. Like the other prokaryotes, the eubacteria, they have small, circular chromosomes that are not located within a nuclear membrane. However, archaea transcription, translation, and replication, in many respects, more closely resemble that of eukaryotes.
Some archaea chromosomes possess multiple replication origins. Sequence motifs within these origins are recognized and bound specifically by archaeal homologs of the eukaryotic replication initiation factors Orc1 and Cdc6. These proteins bind to several sites in the origin and, in doing so, deform the DNA. In the archaeal species Sulfolobus, all three of its origins are activated within a few minutes of one another. Termination of replication is also similar to that of eukaryotes in that replicons terminate by stochastic fork collisions rather than by discrete terminator sequences as in eubacteria.
10.8 Each Eukaryotic Chromosome Contains Many Replicons
In eukaryotic cells, the replication of DNA is confined to the second part of the cell cycle, called S phase, which follows the G1 phase (see the chapter titled Replication Is Connected to the Cell Cycle). The eukaryotic cell cycle is composed of alternating rounds of growth followed by DNA replication and cell division. After the cell divides into two daughter cells, each must grow back to approximately the size of the original mother cell before cell division can occur again. The G1 phase of the cell cycle is primarily concerned with growth (although G1 is an abbreviation for first gap because the early cytologists could not see any activity). In G1, everything except DNA begins to be doubled: RNA, protein, lipids, and carbohydrate. The progression from G1 into S is very tightly regulated and controlled by a checkpoint. For a cell to be allowed to progress into S phase, there must be a certain minimum amount of growth, which is biochemically measured. In addition, there must not be any damage to the DNA. Damaged DNA or too little growth prevents the cell from progressing into S phase. When S phase is completed, G2 phase commences. There is no control point and no sharp demarcation.
Replication of the large amount of DNA contained in eukaryotic chromatin is accomplished by dividing it into many individual replicons, as shown in FIGURE 10.10. Only some of these replicons are engaged in replication at any point in S phase. Presumably, each replicon is activated at a specific time during S phase, although the evidence on this issue is not decisive. Note that a crucial difference between replication in bacteria and replication in eukaryotes is that in bacteria replication is occurring on DNA, whereas in eukaryotes replication is occurring on chromatin and nucleosomes play a role, so their presence must be taken into account. This is discussed in the chapter titled Chromatin.

FIGURE 10.10 A eukaryotic chromosome contains multiple origins of replication that ultimately merge during replication.
The start of S phase is signaled by the activation of the first replicons. Over the next few hours, initiation events occur at other replicons in an ordered manner. Chromosomal replicons usually display bidirectional replication.
Individual replicons in eukaryotic genomes are relatively small, typically approximately 40 kb in yeast or flies and approximately 100 kb in animal cells. They can, however, vary more than 10-fold in length within a genome. The rate of replication is approximately 2,000 bp/min, which is much slower than the 50,000 bp/min of bacterial replication fork movement, presumably because the chromosome is assembled into chromatin, not naked DNA.
From the speed of replication, it is evident that a mammalian genome could be replicated in approximately 1 hour if all replicons functioned simultaneously. S phase actually lasts for more than 6 hours in a typical somatic cell, though, which implies that no more than 15% of the replicons are likely to be active at any given moment. There are some exceptional cases, such as the early embryonic divisions of Drosophila embryos, and other organisms that do not have the leisure of placental development, for which the duration of S phase is compressed by the simultaneous functioning of a large number of replicons.
How are origins selected for initiation at different times during S phase? In Saccharomyces cerevisiae, the default appears to be for replicons to replicate early, but cis-acting sequences can cause origins linked to them to replicate at a later time. In other organisms, there is a general hierarchy to the order of replication. Replicons near active genes are replicated earliest and replicons in heterochromatin replicate last.
Available evidence suggests that most chromosomal replicons do not have a termination region like that of bacteria at which the replication forks cease movement and (presumably) dissociate from the DNA. It seems more likely that a replication fork continues from its origin until it meets a fork proceeding toward it from the adjacent replicon. Recall the discussion about the potential topological problem of joining the newly synthesized DNA at the junction of the replication forks.
The propensity of replicons located in the same vicinity to be active at the same time could be explained by “regional” controls, in which groups of replicons are initiated more or less coordinately, as opposed to a mechanism in which individual replicons are activated one by one in dispersed areas of the genome. Two structural features suggest the possibility of large-scale organization. Quite large regions of the chromosome can be characterized as “early replicating” or “late replicating,” implying that there is little interspersion of replicons that fire at early or late times. Visualization of replicating forks by labeling with DNA precursors identifies 100 to 300 “foci” instead of uniform staining; each focus shown in FIGURE 10.11 probably contains greater than 300 replication forks. The foci could represent fixed structures through which replicating DNA must move.

FIGURE 10.11 Replication forks are organized into foci in the nucleus. Cells were labeled with BrdU. The left panel was stained with propidium iodide to identify bulk DNA. The right panel was stained using an antibody to BrdU to identify replicating DNA.
Photos courtesy of Anthony D. Mills and Ron Laskey, Hutchinson/MRC Research Center, University of Cambridge.
10.9 Replication Origins Can Be Isolated in Yeast
Any segment of DNA that has an origin should be able to replicate, so although plasmids are rare in eukaryotes, it might be possible to construct them by suitable manipulation in vitro. Researchers have accomplished this in yeast, but not in multicellular eukaryotes.
S. cerevisiae mutants can be “transformed” to the wild-type phenotype by addition of DNA that carries a wild-type copy of the gene. The discovery of yeast origins resulted from the observation that some yeast DNA fragments (when circularized) are able to transform defective cells very efficiently. These fragments can survive in the cell in the unintegrated (autonomous) state; that is, as self-replicating plasmids.
A high-frequency transforming fragment possesses a sequence that confers the ability to replicate efficiently in yeast. This segment is called an autonomously replicating sequence (ARS). ARS elements are derived from origins of replication.
Although ARS elements have been systematically mapped over extended chromosomal regions, it seems that only some of them are actually used to initiate replication at any one time. The others are silent, or possibly used only occasionally. If it is true that some origins have varying probabilities of being used, it follows that there can be no fixed termini between replicons. In this case, a given region of a chromosome could be replicated from different origins in different cell cycles.
An ARS element consists of an A-T–rich region that contains discrete sites in which mutations affect origin function. Base composition rather than sequence might be important in the rest of the region. FIGURE 10.12 shows a systematic mutational analysis along the length of an origin. Origin function is abolished completely by mutations in a 14-bp “core” region, called the A domain, which contains an 11-bp consensus sequence consisting of A-T base pairs. This consensus sequence (sometimes called the ACS, for ARS consensus sequence) is the only homology between known ARS elements.

FIGURE 10.12 An ARS extends for ~50 bp and includes a consensus sequence (A) and additional elements (B1–B3).
Mutations in three adjacent elements, numbered B1 to B3, reduce origin function. An origin can function effectively with any two of the B elements, as long as a functional A element is present. (Imperfect copies of the core consensus, typically conforming at 9/11 positions, are found close to, or overlapping with, each B element, but they do not appear to be necessary for origin function.)
The origin recognition complex (ORC) is a highly conserved complex found in all eukaryotes. It is composed of six proteins with a mass of approximately 400 kilodaltons (kD). ORC binds to the yeast A and B1 elements on the A-T-rich strand and is associated with ARS elements throughout the cell cycle. This means that initiation depends on changes in its condition rather than de novo association with an origin (see the section Licensing Factor Binds to ORC later in this chapter). By counting the number of sites to which ORC binds, we can estimate that there are about 400 origins of replication in the yeast genome. This means that the average length of a replicon is approximately 35,000 bp. Counterparts to ORC are found in cells of multicellular eukaryotes.
ORC was first found in S. cerevisiae (where it is sometimes called scORC), but similar complexes have now been characterized in Schizosaccharomyces pombe (spORC), Drosophila (DmORC), and Xenopus (XlORC). All of the ORC complexes bind to DNA. Although researchers have not characterized any of the binding sites in the same detail as in S. cerevisiae, in several cases, they are at locations associated with the initiation of replication. It seems clear that ORC is an initiation complex whose binding identifies an origin of replication. Details of the interaction, however, are clear only in S. cerevisiae; it is possible that additional components are required to recognize the origin in the other cases.
The yeast ARS elements satisfy the classic definition of an origin as a cis-acting sequence that causes DNA replication to initiate. The conservation of the ORC suggests that origins are likely to take the same sort of form in other eukaryotes, but in spite of this, there is little to no conservation of sequence among putative origins in different organisms. Difficulties in finding consensus origin sequences suggest the possibility that origins might be more complex (or determined by features other than discrete cis-acting sequences). There are suggestions that some animal cell replicons might have complex patterns of initiation: In some cases, many small replication bubbles are found in one region, posing the question of whether there are alternative or multiple starts to replication and whether there is a small discrete origin. Replication origins are often associated with promoters of genes.
Reconciliation between this phenomenon and the use of ORCs is suggested by the discovery that environmental effects can influence the use of origins. At one location where multiple bubbles are found, there is a primary origin that is used predominantly when the nucleotide supply is high. When the nucleotide supply is limiting, though, many secondary origins are also used, giving rise to a pattern of multiple bubbles. One possible molecular explanation is that ORCs dissociate from the primary origin and initiate elsewhere in the vicinity if the supply of nucleotides is insufficient for the initiation reaction to occur quickly. At all events, it now seems likely that we will be able in due course to characterize discrete sequences that function as origins of replication in multicellular eukaryotes.
10.10 Licensing Factor Controls Eukaryotic Rereplication
A eukaryotic genome is divided into multiple replicons, and the origin in each replicon is activated once, and only once, in a single division cycle. This could be achieved by the provision of some rate-limiting component that functions only once at an origin or by the presence of a repressor that prevents rereplication at origins that have been used. The critical questions about the nature of this regulatory system are how the system determines whether any particular origin has been replicated and what protein components are involved.
Insights into the nature of the protein components have been provided by using a system in which a substrate DNA undergoes only one cycle of replication. Xenopus eggs have all the components needed to replicate DNA—in the first few hours after fertilization they undertake 11 division cycles without new gene expression—and they can replicate the DNA in a nucleus that is injected into the egg. FIGURE 10.13 summarizes the features of this system.

FIGURE 10.13 A nucleus injected into a Xenopus egg can replicate only once unless the nuclear membrane is permeabilized to allow subsequent replication cycles.
When a sperm or interphase nucleus is injected into the egg, its DNA is replicated only once. (This can be followed by use of a density label, just like the original experiment of Messelson and Stahl that characterized semiconservative replication; see the chapter titled Genes Are DNA and Encode RNAs and Polypeptides.) If protein synthesis is blocked in the egg, the membrane around the injected material remains intact and the DNA cannot replicate again. In the presence of protein synthesis, however, the nuclear membrane breaks down just as it would for a normal cell division, and in this case subsequent replication cycles can occur. The same result can be achieved by using agents that permeabilize the nuclear membrane. This suggests that the nucleus contains a protein(s) needed for replication that is used up in some way by a replication cycle, so even though more of the protein is present in the egg cytoplasm, it can enter the nucleus only if the nuclear membrane breaks down. The system can in principle be taken further by developing an in vitro extract that supports nuclear replication, thus allowing the components of the extract to be isolated and the relevant factors identified.
FIGURE 10.14 explains the control of reinitiation by proposing that this protein is a licensing factor. It is present in the nucleus prior to replication. One round of replication either inactivates or destroys the factor, and another round cannot occur until additional factor is provided. Factor in the cytoplasm can gain access to the nuclear material only at the subsequent mitosis when the nuclear envelope breaks down. This regulatory system achieves two purposes. By removing a necessary component after replication, it prevents more than one cycle of replication from occurring. It also provides a feedback loop that makes the initiation of replication dependent on passing through the cell cycle.

FIGURE 10.14 Licensing factor in the nucleus is inactivated after replication. A new supply of licensing factor can enter only when the nuclear membrane breaks down at mitosis.
10.11 Licensing Factor Binds to ORC
The key event in controlling replication is the behavior of the ORC complex at the origin. Recall that in S. cerevisiae, ORC is a 400-kD complex that binds to the ARS sequence (see the section Replication Origins Can Be Isolated in Yeast earlier in this chapter). Its origin (ARS) consists of the A consensus sequence and three B elements (see Figure 10.12). The ORC complex of six proteins (all of which are encoded by essential genes) binds to the A and adjacent B1 element. Orc1 binds first, in G1 phase of the cell cycle and acts as a nucleating center; next, Orc2–5 binds strongly; Orc6 binds weakly and has a nuclear localization signal that must be activated by the cyclin/CDK kinase during the G1 to S transition (see the chapter titled Replication Is Connected to the Cell Cycle). ATP is required for the binding, but is not hydrolyzed until a later stage. The transcription factor ABF1 binds to the B3 element; this assists initiation by affecting chromatin structure, but it is the events that occur at the A and B1 elements that actually cause initiation. Most origins are localized in regions between genes, which suggests that it might be important for the local chromatin structure to be in a nontranscribed condition.
The striking feature is that ORC remains bound at the origin through the entire cell cycle. However, changes occur in the pattern of protection of DNA as a result of binding of other proteins to the ORC-origin complex.
At the end of the cell cycle, ORC is bound to A–B1 elements of the origin. There is a change during G1 that results from the binding of Cdc6 and Cdt1 proteins to the ORC. In yeast, Cdc6 is a highly unstable protein, with a half-life of more than 5 minutes. It is synthesized during G1 and typically binds to ORC between the exit from mitosis and late G1. Its rapid degradation means that no protein is available later in the cycle. In mammalian cells, Cdc6 is controlled differently; it is phosphorylated during S phase, and as a result it is degraded by the ubiquitination pathway. Cdt1 is initially stabilized by the protein Geminin, which prevents its degradation, and subsequent Geminin binding prevents its reuse. These features make Cdc6 and Cdt1 the key licensing factors. These two proteins also provide the connection between ORC and a complex of proteins that is involved in initiation of replication. Cdc6 has an ATPase activity that is required for it to support initiation.
In yeast, the replication helicase MCM2-7 (minichromosome maintenance) complexes enter the nucleus as inactive double hexamers during mitosis. The presence of Cdc6 and Cdt1 at the yeast origin allows the two MCM complexes to bind to each of the two replication forks in G1 in the inactive state. Their presence is necessary for initiation. FIGURE 10.15 summarizes the cycle of the events that follow at the origin. The origin enters S phase in the condition of a prereplication complex, which contains ORC, Cdc6, Cdt1, and the inactive helicase, the MCM proteins. The MCM2–7 proteins form a six-member ring-shaped complex around DNA. MCM2,3,5 are regulatory, whereas MCM4,6,7 have the helicase activity. When initiation occurs, Cdc6 and Cdt1 are displaced, returning the origin to the state of the postreplication complex, which contains only ORC. Cdc6 is rapidly degraded during S phase and, as a result, it is not available to support reloading of MCM proteins. Thus, the origin cannot be used for a second cycle of initiation during S phase. In mammalian cells, Cdt1 is targeted for degradation by the action of a protein complex that is recruited to the origin of replication by PCNA, the eukaryotic counterpart of the bacterial β clamp.
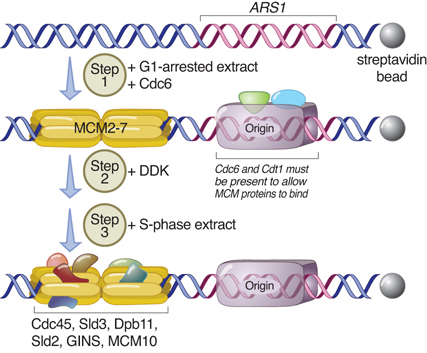
FIGURE 10.15 Proteins at the origin control susceptibility to initiation.
Data from: Heller, R. C., et al. 2011. Cell 146:80–91.
If Cdc6 is made available to bind to the origin during G2 (by ectopic expression), MCM proteins do not bind until the following G1, which suggests that there is a secondary mechanism to ensure that they associate with origins only at the right time. This could be another part of licensing control. At least in S. cerevisiae, this control does not seem to be exercised at the level of nuclear entry, but this could be a difference between yeasts and animal cells. Some of the ORC proteins have similarities to replication proteins that load DNA polymerase onto DNA. It is possible that ORC uses hydrolysis of ATP to load the MCM ring onto DNA. In Xenopus extracts, replication can be initiated if ORC is removed after it has loaded Cdc6 and MCM proteins. This shows that the major role of ORC is to identify the origin to the Cdc6 and MCM proteins that control initiation and licensing.
As the transition from G1 to S phase begins, CDK/cyclins recruit cdc45 and the GINS complex to the MCM helicase, which then becomes known as the CMG complex (for Cdc45-MCM-GINS) for activation. This marks the transition from initiation to DNA replication, that is, the elongation phase of replication that entails the two different modes of synthesis on the leading (forward) strand and the lagging (discontinuous) strand. The MCM proteins, when activated, are required for elongation as well as for initiation, and they continue to function at the two bidirectional replication forks as the replication helicase.
Summary
Replicons in bacterial or eukaryotic chromosomes have a single unifying feature: Replication is initiated at an origin once, and only once, in each cell cycle. The origin is located within the replicon, and replication typically is bidirectional, with replication forks proceeding away from the origin in both directions. Replication is not usually terminated at specific sequences, but continues until DNA polymerase meets another DNA polymerase halfway around a circular replicon, or at the junction between two linear replicons.
An origin consists of a discrete sequence at which replication of DNA is initiated. Origins of replication tend to be rich in A-T base pairs. A eubacterial chromosome contains a single origin, which is responsible for initiating replication once every cell cycle. The oriC in E. coli is a sequence of 245 bp. Any DNA molecule with this sequence can replicate in E. coli. Replication of the circular bacterial chromosome produces a θ structure, in which the replicated DNA starts out as a small replicating eye. Replication proceeds until the eye occupies the whole chromosome. The bacterial origin contains sequences that are methylated on both strands of DNA. Replication produces hemimethylated DNA, which cannot function as an origin. There is a delay before the hemimethylated origins are remethylated to convert them to a functional state, and this is responsible for preventing improper reinitiation.
Several sites that are methylated by the Dam methylase are present in the E. coli origin, including those of the 13-mer binding sites for DnaA. The origin remains hemimethylated and is in a sequestered state for ~10 minutes following initiation of a replication cycle. During this period, it is associated with the membrane and reinitiation of replication is repressed.
The common mode of origin activation involves an initial limited melting of the double helix, followed by more general unwinding to create single strands. Several proteins act sequentially at the E. coli origin. Replication is initiated at oriC in E. coli when DnaA binds in an elongated form to a series of 9-bp repeats. This is followed by binding to a series of 13-bp repeats, where it uses hydrolysis of ATP to catalyze the transition to a compact form to separate the DNA strands. The prepriming complex of DnaC–DnaB displaces DnaA. DnaC is released in a reaction that depends on ATP hydrolysis; DnaB is joined by the replicase enzyme, and replication is initiated by two forks that set out in opposite directions.
The availability of DnaA at the origin is an important component of the system that determines when replication cycles should initiate. Following initiation of replication, DnaA hydrolyzes its ATP under the stimulus of the β sliding clamp, thereby generating an inactive form of the protein.
A eukaryotic chromosome is divided into many individual replicons. Replication occurs during a discrete part of the cell cycle called S phase. Not all replicons are active simultaneously, though, so the process can take several hours. Eukaryotic replication is at least an order of magnitude slower than bacterial replication. Origins sponsor bidirectional replication and are probably used in a fixed order during S phase. Each replicon is activated only once in each cycle. Origins of replication were isolated as ARS sequences in yeast by virtue of their ability to support replication of any sequence attached to them. The core of an ARS is an 11-bp A-T–rich sequence that is bound by the ORC protein complex, which remains bound throughout the cell cycle. Utilization of the origin is controlled by several licensing factors that associate with the ORC and recruit the MCM helicase proteins.
After cell division, nuclei of eukaryotic cells have licensing factors that are needed to initiate replication. In yeast, their destruction after initiation of replication prevents further replication cycles from occurring. Licensing factor cannot be imported into the nucleus from the cytoplasm, and can be replaced only when the nuclear membrane breaks down during mitosis (or when resynthesized and imported into the nucleus during G1 in yeast, in which the nuclear membrane never breaks down).
The origin in yeast is recognized by the ORC proteins, which in yeast remain bound throughout the cell cycle. The proteins Cdc6 and Cdt1 are available only at S phase. In yeast, they are synthesized during S phase and rapidly degraded. In animal cells, they are synthesized continuously, but are exported from the nucleus during S phase. The presence of Cdc6 and Cdt1 allow the MCM proteins to bind to the origin. The MCM proteins are required for initiation (and then for elongation as the replicative helicase). The combined action of Cdc6, Cdt1, and the MCM proteins provides the licensing function.
References
10.1 Introduction
Research
Costa, A., et al. (2013). Mechanisms for initiating cellular DNA replication. Annu. Rev. Biochem. 82, 25–54.
Jacob, F., et al. (1963). On the regulation of DNA replication in bacteria. Cold Spring Harbor Symp. Quant. Biol. 28, 329–348.
10.2 An Origin Usually Initiates Bidirectional Replication
Review
Brewer, B. J. (1988). When polymerases collide: replication and transcriptional organization of the E. coli chromosome. Cell 53, 679–686.
Research
Cairns, J. (1963). The bacterial chromosome and its manner of replication as seen by autoradiography. J. Mol. Biol. 6, 208–213.
Iismaa, T. P., and Wake, R. G. (1987). The normal replication terminus of the B. subtilis chromosome, terC, is dispensable for vegetative growth and sporulation. J. Mol. Biol. 195, 299–310.
Liu, B., et al. (1994). A transcribing RNA polymerase molecule survives DNA replication without aborting its growing RNA chain. Proc. Natl. Acad. Sci. USA 91, 10660–10664.
Steck, T. R., and Drlica, K. (1984). Bacterial chromosome segregation: evidence for DNA gyrase involvement in decatenation. Cell 36, 1081–1088.
Zyskind, J. W., and Smith, D. W. (1980). Nucleotide sequence of the S. typhimurium origin of DNA replication. Proc. Natl. Acad. Sci. USA 77, 2460–2464.
10.3 The Bacterial Genome Is (Usually) a Single Circular Replicon
Research
Tehranchi, A. K., et al. (2010). The transcription factor DksA prevents conflicts between DNA replication and transcription machinery. Cell 141, 595–605.
10.5 Initiation: Creating the Replication Forks at the Origin oriC
Review
Kaguni, J. M. (2006). DnaA: controlling the initiation of bacterial DNA replication and more. Annu. Rev. Microbiol. 60, 351–375.
Research
Bramhill, D., and Kornberg, A. (1988). Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell 52, 743–755.
Davey, M. J., et al. (2002). The DnaC helicase loader is a dual ATP/ADP switch protein. EMBO. J. 21, 3148–3159.
Duderstadt, K. E., et al. (2010). Origin remodeling and opening in bacteria rely on distinct assembly states of the DnaA initiator. J. Biol. Chem. 285, 28229–28239.
Erzberger, J. P., et al. (2006). Structural basis for ATP-dependent DnaA assembly and replication-origin remodeling. Nat. Struct. Mol. Biol. 13, 676–683.
Fuller, R. S., et al. (1984). The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell 38, 889–900.
Funnell, B. E., and Baker, T. A. (1987). In vitro assembly of a prepriming complex at the origin of the E. coli chromosome. J. Biol. Chem. 262, 10327–10334.
Hiasa, H., and Marians, K. J. (1999). Initiation of bidirectional replication at the chromosomal origin is directed by the interaction between helicase and primase. J. Biol. Chem. 274, 27244–27248.
Kasho, K., and Katayama, T. (2013). DNA binding locus data promotes DnaA-ATP hydrolysis to enable cell cycle–coordinated replication initiation. Proc. Natl. Acad. Sci. USA 110, 936–941.
Keyamura, K., et al. (2009). DiaA dynamics are coupled with changes in initial origin complexes leading to helicase loading. J. Biol. Chem. 284, 25038–25050.
Molt, K. L., et al. (2009). A role for the nonessential domain II of initiator protein, DnaA, in replication control. Genetics 183, 39–49.
Sekimizu, K., et al. (1987). ATP activates dnaA protein in initiating replication of plasmids bearing the origin of the E. coli chromosome. Cell 50, 259–265.
Wahle, E., et al. (1989). The dnaB-dnaC replication protein complex of Escherichia coli. II. Role of the complex in mobilizing dnaB functions. J. Biol. Chem. 264, 2469–2475.
10.6 Multiple Mechanisms Exist to Prevent Premature Reinitiation of Replication
Research
Keyamura, K., and Katayama, T. (2011). DnaA protein DNA-binding domain binds to Hda protein to promote inter-AAA+ domain interaction involved in regulatory inactivation of DnaA. J. Biol. Chem. 286, 29336–29346.
10.7 Archaeal Chromosomes Can Contain Multiple Replicons
Review
Barry, E. R., and Bell, S. D. (2006) DNA replication in the archaea. Micro. Mol. Biol. Rev. 70, 876–887.
Research
Cunningham Dueber, E. L., et al. (2007). Replication origin recognition and deformation by a heterodimeric archaeal Orc1 complex. Science 317, 1210–1213.
Duggin, I. G., et al. (2011). Replication termination and chromosome dimer resolution in the archaeon Sulfolobus solfataricus. EMBO J. 30, 145–153.
10.8 Each Eukaryotic Chromosome Contains Many Replicons
Reviews
Fangman, W. L., and Brewer, B. J. (1991). Activation of replication origins within yeast chromosomes. Annu. Rev. Cell. Biol. 7, 375–402.
Masai, H., et al. (2010). Eukaryotic chromosome replication: where, when, and how? Annu. Rev. Biochem. 79, 89–130.
Research
Blumenthal, A. B., et al. (1974). The units of DNA replication in D. melanogaster chromosomes. Cold Spring Harbor Symp. Quant. Biol. 38, 205–223.
10.9 Replication Origins Can Be Isolated in Yeast
Reviews
Bell, S. P., and Dutta, A. (2002). DNA replication in eukaryotic cells. Annu. Rev. Biochem. 71, 333–374.
DePamphlis, M. L. (1993). Eukaryotic DNA replication: anatomy of an origin. Annu. Rev. Biochem. 62, 29–63.
Gilbert, D. M. (2001). Making sense of eukaryotic DNA replication origins. Science 294, 96–100.
Kelly, T. J., and Brown, G. W. (2000). Regulation of chromosome replication. Annu. Rev. Biochem. 69, 829–880.
Research
Anglana, M., et al. (2003). Dynamics of DNA replication in mammalian somatic cells: nucleotide pool modulates origin choice and interorigin spacing. Cell 114, 385–394.
Chesnokov, I., et al. (2001). Functional analysis of mutant and wild-type Drosophila origin recognition complex. Proc. Natl. Acad. Sci. USA 98, 11997–12002.
Ghosh, S., et al. (2011). Assembly of the human origin recognition complex occurs through independent nuclear localization of its components. J. Biol. Chem. 286, 23831–23841.
Marahrens, Y., and Stillman, B. (1992). A yeast chromosomal origin of DNA replication defined by multiple functional elements. Science 255, 817–823.
Wyrick, J. J., et al. (2001). Genome-wide distribution of ORC and MCM proteins in S. cerevisiae: high-resolution mapping of replication origins. Science 294, 2357–2360.
10.11 Licensing Factor Binds to ORC
Review
Tsakalides, V., and Bell, S. P. (2010). Dynamics of pre-replicative complex assembly. J. Biol. Chem. 285, 9437–9443.
Research
Costa, A., et al. (2011). The structural basis for MCM2–7 helicase activation by GINS and Cdc45. Nat. Str. & Mol. Bio. 18, 471–477.
Heller, R. C., et al. P. (2011). Eukaryotic origin-dependent DNA replication in vitro reveals sequential action of DDK and S-CDK kinases. Cell 146, 80–91.
Kara, N., et al. (2015). Orc1 binding to mitotic chromosomes precedes special patterning during G1 phase and assembly of the origin recognition complex in human cells. J. Biol. Chem. 290, 12355–12369.
Ode, K. L., et al. (2011). Inter-origin cooperativity of geminin action establishes an all-or-none switch for replication origin licensing. Genes to Cells 16, 380–396.
Remus, D., et al. (2009). Concerted loading of Mcm2–7 double hexamers around DNA during DNA replication origin licensing. Cell 139, 719–730.
Sheu, Y. J., and Stillman, B. (2010). The Dbf4–Cdc7 kinase promotes S phase by alleviating an inhibitory activity in Mcm4. Nature 463, 113–117.
Ticau, S., et al. (2015). Single molecule studies of origin licensing reveal mechanisms ensuring bidirectional helicase loading. Cell 161, 513–525.