CHAPTER 1
The Tinamou’s Story
DEATH OF A PARADIGM
The wintry gusts failed to dampen the great man’s enthusiasm for delivery. Thomas Henry Huxley, aptly nicknamed ‘Darwin’s Bulldog’, was on top form that evening, and he knew it. Standing behind the Royal Institution’s desk, he fixed his ‘hawk-like’ eyes on the assembled socialites and gauged their reaction to his latest ideas. In the raked amphitheatre’s gaslight, Huxley cut an assured figure, dressed in a subfusc frock coat, bow-tie and pince-nez, topped off with a shock of dark hair and matching sideburns – manna for the caricaturists of the day. Nevertheless, his confident mien and evangelical oratory masked a decade of struggle with Darwin’s theory and the genealogical approach to classification. But not now, for Huxley had become convinced that his friend’s radical views offered the best explanation for the emergence of new species. Taxa were not static but evolved from one to another over time, although how long this required remained unclear. Simply put, all organisms are descended from a single common ancestor and therefore related to one another. Armed with his newly acquired conviction, Huxley proceeded to captivate his audience with the pinnacle of his palaeontological studies. His lecture was a tour de force, one that contained the most astonishing of pedigrees – a reptilian ancestry for birds. In doing so, the self-taught biologist became the first to propose that the ancient flightless birds, of which the kiwis and rheas are but ‘scanty modern heirs’, had evolved from dinosaurs.1
The catalyst for Huxley’s proposal was a visit six months earlier, in October 1867, to Oxford University Museum. Here, among the collection of precious relics, he noticed a fossil that had been incorrectly identified as part of the shoulder girdle of a Megalosaurus. Instead, Huxley realised that it was part of the dinosaur’s upper pelvis or ilium: a bone that struck him as ‘so bird-like as to be astonishing’. His belief that dinosaurs and birds are closely related was further bolstered after he had the foresight to reconstruct the British Museum’s Iguanodon as a biped rather than a quadruped. Despite the specimen’s size, approximately 9 metres tall, Huxley noted that there was a ‘considerable touch of a bird about the pelvis and legs!’ Later, he would go much further:
If the whole hindquarters from the ilium to the toes, of a half-hatched chicken could be suddenly enlarged, ossified, and fossilised as they are, they would furnish us with the last step of the transition between Birds and Reptiles, for there would be nothing in their characters to prevent us from referring them to the Dinosauria.2
During the last 10 yearalthough their precise phylogenetics, a wealth of well-preserved feathered dinosaurs and bird fossils from China has helped confirm Huxley’s belief that birds are the descendants of dinosaurs. Indeed, it is not hard to imagine his smug, self-satisfied look were he able to learn of the insights that these and other astounding finds have provided, not just in the linkage of dinosaurs to birds but also concerning the origins of feathers and powered flight.3 Huxley’s prescience, however, was not the result of sudden genius, but rather the outcome of many years of dogged and gritty work, involving the detailed study of thousands of avian bones. Such painstaking anatomical comparisons led him to another crucial insight, one that provides the foundation stone for The Ascent of Birds – the flightless ratites are the most primitive of all modern birds.
Based on the structure of the breastbone, Huxley was able to classify modern birds into two groups, or superorders: the Ratitae, which he considered to be closest to the non-avian dinosaurs, and the Carinatae, which included all other birds. Indeed, his confidence in the basal position of ratites is evident from a letter he wrote to the German biologist and polymath, Ernst Haeckel: ‘I am engaged [in] a revision of the Dinosauria, with an eye to the “Descendenz Theorie.” The road from Reptiles to Birds is by way of Dinosauria to the Ratitae.’4
The term ratite (derived from the Latin ratis, meaning ‘raft’) refers to the shape of the sternum or breastbone – one that is flat because it lacks the median ridge, or keel, needed to anchor the strong flight muscles of flying species. As a result, all ratites are flightless, since their feeble vestigial wings cannot lift their heavy bodies off the ground. Four extant families make up the ratites. The largest are the ostriches (Struthionidae), which live in the savannas and Sahel of Africa. Slightly smaller are the rheas (Rheidae), which are native to the pampas and Chaco forests of South America, and the cassowaries (Casuariidae), the most colourful of all the ratites, which live in the tropical forests of New Guinea and northeastern Australia. They are the most dangerous, and when surprised or cornered can attack with razor-sharp talons. Until recently the Emu, which inhabits inland and coastal regions of Australia, was assigned its own family (Dromaiidae), but it has now been moved to join the cassowaries as a member of the Casuariidae. The most unusual ratites are the nocturnal kiwis (Apterygidae), species endemic to New Zealand that nest in burrows and locate their invertebrate prey using a highly developed sense of smell.
Several extinct families also belong to the ratites, including the elephant birds (Aepyornithidae) and the moas (Dinornithidae). The flightless elephant birds were huge species, reaching 2–3 metres in height and weighing up to half a tonne. They were widespread on the island of Madagascar up until the thirteenth century, when habitat loss and hunting pressure probably led to their extinction 400 years later. Elephant birds are believed to have been the inspiration for the fabled roc of Sinbad fame, a giant eagle-like bird that reportedly was capable of carrying off and devouring full-grown elephants (hence its name). The species’ eggs were just as impressive, with a circumference of nearly a metre and a capacity of 9 litres – the equivalent of 200 chicken eggs. However, it is the moas of New Zealand that have captured our imagination more than any other ratite, ever since the anatomist Richard Owen famously deduced their existence from a single fragment of bone (Plate 2). Sadly, these herbivores met the same fate as the elephant birds, although this time at the hands of Polynesian settlers who colonised the islands during the thirteenth century. Indeed, by the time Europeans had reached New Zealand, the moas were reduced to ‘mere bone and egg fragments in hunting middens’.5 As we will discuss in The Buzzard’s Story, not long after the moas were eliminated, the largest known bird of prey, the Haast’s Eagle, was also lost. It is now clear that the Māoris had disrupted a food chain, and once the eagle’s primary food source – the moas – had disappeared the raptor was unable to thrive and rapidly became extinct.
Huxley identified another skeletal commonality among ratites, a primitive, reptilian-looking palate.6 In general, ratites possess a more complex, stronger and less flexible palate than the light-boned, flexible forms found in all other birds. Anatomists use the term palaeognathous to describe such a primitive palate and, as a result, Huxley’s two avian superorders are now known as Palaeognathae (‘ancient jaws’) and Neognathae (‘new jaws’). Of all the bird families that Huxley studied, only one caused him a taxonomic headache: the South American tinamous (family Tinamidae) (Plate 3). For these medium-sized ground-dwelling birds seemed to defy his neat taxonomic dichotomy. Tinamous possess a sternal keel, with associated wing muscles. Indeed, as birders know only too well, tinamous, although reluctant to fly, can suddenly rise when disturbed and, with loud, frantic wingbeats, disappear quickly from view. Flight distances are always short, since they possess small hearts in relation to their body size. Tinamous, therefore, are not ratites. However, they do possess a primitive palate, indicating that they are palaeognaths. So where should tinamous be placed – with the palaeognaths, or among the neognaths, or in a division all by themselves? In the end, Huxley opted to group them with the neognaths, as he believed that they were most closely related to the ground-feeding Galliformes, a group that includes grouse and turkeys. Today, as we will highlight, tinamous are firmly placed within the Palaeognathae, although their precise phylogenetic position has been the subject of intense debate.
A role for neoteny
In contrast, Huxley’s conclusion that palaeognaths are the most primitive of modern birds has stood the test of time, although the idea has not been without its critics. A popular counterview was that palaeognaths are not primitive birds but only appear so because of the retention of juvenile features into adulthood, a process called neoteny or paedomorphism. Domesticated animals, for example, are thought to be neotenous versions of their wild counterparts. Compared to wolves, dogs retain many anatomical and behavioural features that are characteristic of puppies: floppy ears, large eyes, as well as playfulness and bouts of affection. The novel idea that neoteny might account for the primitive features of palaeognaths was promulgated by the respected zoologist and comparative embryologist Sir Gavin de Beer. In the 1950s, de Beer argued that ratites had evolved from neognaths and that their downy plumage, unfused cranial bones and primitive palates are merely retained juvenile features.7 Although this hypothesis has not been supported by recent studies, the role of neoteny in avian evolution turns out to be far more profound than de Beer could ever have imagined. For it is now apparent that neoteny enabled the rapid evolution of all modern birds and facilitated their subsequent global success.
In 2012, a group from Harvard University, headed by Bhart-Anjan Bhullar and Arkhat Abzhanov, concluded that the evolution of modern birds occurred through a neotenous change in the development of dinosaurs.8 With the use of sophisticated x-ray technology, the team scanned juvenile and adult fossilised skulls from non-avian theropod dinosaurs and ancient birds, as well as the skulls of modern birds. Crucially, the researchers had access to fossilised dinosaur eggs that contained developing embryos. After highlighting various ‘landmarks’ on each scan, Bhullar and colleagues were able to track how the skulls had evolved over millions of years. The results were a surprise. It turns out that all dinosaurs, even those that are most closely related to modern birds, underwent dramatic maturational changes in their skull structure. In contrast, the skulls of modern birds remain similar to their juvenile forms throughout life. The researchers concluded that birds evolved from dinosaurs by a block in maturation so that they retained the large brain, big eyes and short face of infantile dinosaurs. Paedomorphism, therefore, enabled modern birds to become smaller and to reach sexual maturity far more rapidly – in as few as 12 weeks in some species – and this process opened up new opportunities for evolutionary experimentation. Being small would have had the added advantages of requiring less food and being less susceptible to predation. Maybe it is not so surprising that the only dinosaurs to have survived the dramatic mass extinction event at the Cretaceous–Palaeogene (K–Pg) boundary were the small neotenous ones (see The Vegavis’s Story).9 According to Abzhanov, ‘what is interesting about this research is the way it illustrates evolution as a developmental phenomenon. By changing the developmental biology in early species, nature has produced the modern bird – an entirely new creature.’10
Neoteny, therefore, provides life with an efficient and rapid evolutionary route, one that works by taking something already available and modifying it, rather than having to develop a whole new set of genetic instructions. Maturation block may also explain why humans are so radically different from their nearest cousins, the chimpanzees and bonobos. The retention of the primate’s juvenile features, including hair distribution, large brain and flat face, may have enabled humans to evolve more rapidly, despite sharing most of the same genes.11 Indeed, the process appears to have been so dramatic that some scientists refer to our species as the ‘neotenous clan of apes’.12
The neotenous switch to a juvenile-like skull shape in dinosaurs allowed the potential of modern birds to be unleashed, as the development of a larger skull-to-body ratio enabled the formation of bigger and more complex brains. More neuronal connections would have allowed early birds to evolve innovative and flexible behaviours to help compensate for any environmental changes, as well as increasing their ability to colonise novel ecological niches. It is ironic, therefore, that, while de Beer’s ideas have been side-lined, neoteny is now seen as a crucial step in the evolution of all modern birds, enabling them to become one of the most successful groups of organisms on the planet.
In theory, the role of neoteny in avian evolution could be confirmed by tweaking the relevant genes in developing embryos and seeing if it resulted in a reversion to a dinosaurian phenotype. Indeed, in 2015, Bhullar, now working at the University of Yale, together with Abzhanov reported the results of a study that suggested that such an approach might be possible. By using small-molecule inhibitors that downregulate protein essential for beak formation, the researchers were able to induce chicken embryos to express a snout and palate similar to those of the small Velociraptor-like dinosaurs.13 Once the genes controlling neoteny have been identified, there is no reason why similar experiments could not be undertaken.
We have discussed the role of neoteny long enough. Let us move on and consider how the entire group of palaeognaths – ratites and tinamous – evolved, and how their unique biogeography can be explained. As I will reveal, the tinamous provided the key that unlocked the group’s evolutionary past, although the story is a labyrinthine one, beset with many twists and turns.
The unravelling of vicariance
From the beginning, scientists were uncertain whether palaeognaths (tinamous and ratites) are merely a hodgepodge of unrelated forms that have followed a parallel line of evolution with multiple ancestral origins (termed ‘polyphyly’) or whether they form a natural group, with a single common ancestor (termed ‘monophyly’). You will recall that Huxley identified tinamous as palaeognaths, although he placed them with the Neognathae: a taxonomy that implied a separate ancestry for ratites and tinamous. He did, however, admit that tinamous were ‘the most struthious [ostrich-like] of all carinate birds.’6 His arch rival, and nemesis, Richard Owen, went much further, suggesting an independent origin for most ratites, since he believed that ostriches were allied to bustards and that the moas and kiwis were close to megapodes. Support for the so-called ‘polyphyly hypothesis’ was still evident in the 1980s when such palaeontological heavyweights as Alan Feduccia and Storrs Olson believed that different ratites could have variously evolved from ducks, geese, cranes and even ibises. Protagonists of the multiple-ancestor theory offered two explanations: either the group’s characteristic anatomy had evolved independently by convergent evolution as adaptations for a flightless cursorial lifestyle, or their key features were due to neotenous changes, as argued by de Beer. The key question, therefore, was whether various neognath families could have given rise to the different ratites, given enough time?
The simple answer is no. During the last 10 years, it has become clear that all ratites possess anatomical features not present in any other bird. Furthermore, a large number of molecular studies have consistently shown that ratites and tinamous are more closely related to each other than they are to any other bird family. Palaeognaths, therefore, are monophyletic, and any biogeographical explanation needs to take this into account.
A further controversy, however, proved more intractable: the precise phylogenetic relationships between the various palaeognaths, extant and extinct. Until the end of the twentieth century, the flightless ratites were thought to be monophyletic, with the whole group being sister to the flying tinamous (sister groups are lineages that are each other’s closest evolutionary relatives). Such a conclusion, however, implied that the ratites’ common ancestor must have been flightless – an idea that troubled biogeographers, since a non-flying ancestor could not have crossed the wide ocean barriers to reach the various continents and islands in the southern hemisphere where they are found today.
Then, biogeographers were offered an unexpected lifeline. In 1962, Harry Hess, a Princeton geologist, published what would turn out to be one of the most influential papers in modern science.14 Succinctly entitled ‘History of ocean basins’, it revealed a mechanism for how landmasses could drift apart; it was a seminal publication that laid the foundations for the unifying theory of plate tectonics. Hess’s elegantly compelling concept was understandably seized upon by biogeographers, including the influential American ornithologist and palaeontologist, Joel Cracraft. In 1974, Cracraft proposed that the flightless ancestor of the ratites, which had already split off from the flying tinamous, roamed widely across Gondwana during the late Cretaceous. Gondwana is the name given by geologists to the southern supercontinent which included present-day Antarctica, South America, Africa, Madagascar, India, Australia and New Zealand. As this vast southern landmass broke up, between 130 and 50 million years ago, the early flightless ratites would have been split into several isolated populations, a process termed vicariance. Over time, the ancestral groups were then postulated to have drifted on fragments of the Earth’s crust to reach their current locations, before giving rise to the various species we recognise today.15 Despite several nagging inconsistencies, the convenient serendipity of continental drift proved irresistible, and the ratite story remained a paradigm of vicariance biogeography. Indeed, the idea was regularly rehashed by journalists and popular science writers. Even the renowned biologist Richard Dawkins devoted many pages to continental drift and ratite evolution in the first edition of his popular book The Ancestor’s Tale, published in 2004.16
However, around the turn of the twenty-first century, what had seemed a robust model started to crumble. The first cracks resulted from studies that analysed a greater number of genes and which gave a clearer resolution of ratite relationships. The resultant phylogenies, coupled with better molecular dating, revealed a poor agreement between the branching order of the ratite tree and the sequence of Gondwana break-up. Madagascar, for example, has been an island for at least 88 million years, a landmass that separated from Gondwana well before the arrival of the elephant bird. Similarly, the kiwi lineage reached New Zealand millions of years after the islands’ separation from Gondwana.
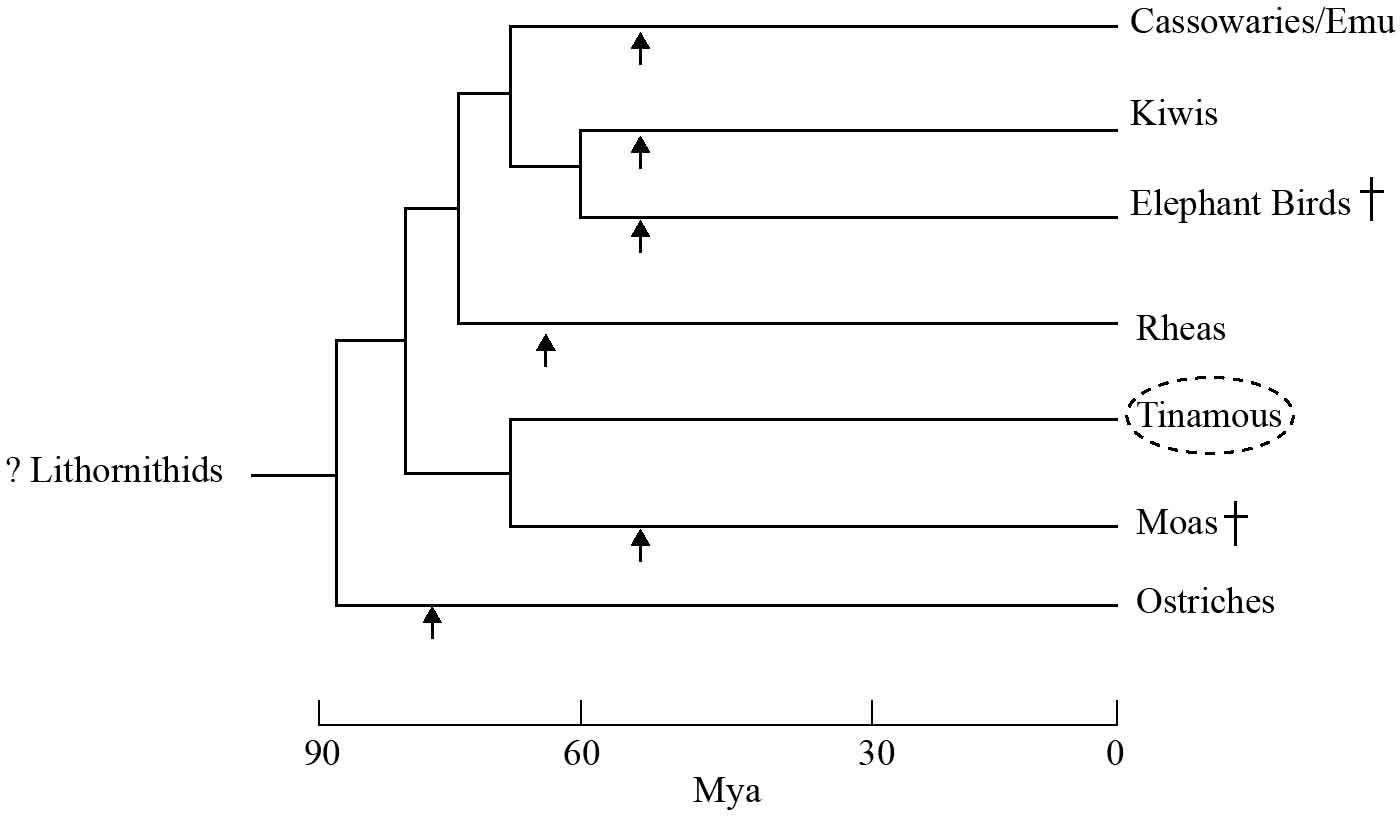
It was an international study in 2008, however, led by John Harshman at the Field Museum of Natural History in Chicago, that shook the model’s foundations.17 The American team analysed genetic material from 18 species of palaeognath, including all the living ratites and four tinamou taxa. Instead of relying on just one or two regions of DNA, they sequenced 20 stretches that were widely dispersed throughout the genome. Contrary to the prevailing view, Harshman showed that it is the ostrich and not the tinamou that is the sister group to all the other ratites. Indeed, the flying tinamous lie deeply embedded within the ratites’ family tree (Figure 1.1). The implications of Harshman’s study are profound: the common ancestor of the palaeognaths must have been a flying bird, capable of long-distance dispersal, which crossed the southern oceans to reach the different continents. It was only after their arrival that the various ratite lineages – ostrich, rhea, kiwi, cassowary, elephant bird and moa – lost the ability to fly. The group’s characteristic anatomy, therefore, was the result of convergent evolution, in which each lineage evolved similar features in response to comparable selection pressures. The alternative scenario in which tinamous regained the ability to fly, while not impossible, is highly unlikely since there are no examples of birds (as far as we know) that have lost and regained flight. Indeed, flight has only evolved three times during 500 million years of vertebrate evolution: first by the pterosaurs (ancient reptiles that existed up until the end of the Cretaceous), then by birds, and finally by bats. In contrast, loss of flight is not uncommon in the avian world, having occurred in 18 extant families, especially the rails (family Rallidae), and many more times in extinct groups.
Figure 1.1 Phylogeny of palaeognaths, showing that the flying tinamous (dotted circle) lie deeply within the ratites (a paraphyletic group): a finding that implies a loss of flight on at least six separate occasions (arrows). Mya = millions of years ago. † = extinct species. Adapted from Baker et al. (2014),18 Mitchell et al. (2014),19 and Christidis and Boles (2008).20
The final blow for the vicariance model came in 2014, with the publication of two studies that incorporated ancient DNA (aDNA). Intriguingly, both papers revealed that the ratites’ geographical neighbours are not their closest evolutionary relatives. The first study, led by Alan Barker from the Royal Ontario Museum, included aDNA extracted from a small bone that belonged to the little bush moa.18 After careful preparation in a specially designed clean room, the specimen yielded a small amount of purified aDNA suitable for amplification and analysis. The results were unexpected. Not only did the genetic sequences confirm that the tinamous are deeply nested within the ratites, but they also showed that their closest relatives are the New Zealand moas and not the South American rheas, as scientists had predicted. Ratites, therefore, are neither monophyletic nor polyphyletic but rather paraphyletic – a term used to describe a group that does not include all the descendants (in this case the tinamous) of their common ancestor (Figure 1.1).
The second study, led by Kieren Mitchell and Alan Cooper from the Australian Centre for Ancient DNA in Adelaide, analysed mitochondrial DNA from various ratite lineages, including samples from bones of two different species of elephant bird.19 By aligning the genetic sequences, they were able to show that the nearest relatives of the herbivorous diurnal giants from Madagascar are not the African ostriches, but the New Zealand kiwis, a clade of secretive, shy, nocturnal omnivores. The resultant phylogeny implies that flightlessness must have evolved a minimum of six times and gigantism at least five times during the early phase of ratite evolution. Furthermore, the common ancestor of the kiwi and elephant bird existed millions of years after New Zealand and Madagascar had separated from Gondwana. Ratites, therefore, were not transported by continental drift following the break-up of Gondwana, but flew across the oceans to reach the southern continents and islands.
It is now believed that the flying ancestor of palaeognaths most likely evolved from an extinct group of birds known as the lithornithids, possibly within Western Gondwana. According to Kieren Mitchell, ‘they would have been quite small, unassuming birds, probably the size of a chicken or quail.’ Interestingly, lithornithids were once widespread and probably an extremely mobile group of species, since their fossils have been recovered from Europe and North America: places that lack ratites today.21
Finally, what is it about the ratites that made the species so prone to flightlessness? Professor Cooper believes that the group’s anatomical modification relates to the ecological vacuum that followed the mass extinction event that wiped out the dinosaurs. Since the surviving mammals were small and unspecialised, and remained so for the next 10 million years, there was a unique opportunity for the evolution of huge, flightless herbivores among the continental birds. The abundance of early grasses and plants throughout the southern hemisphere and the absence of large herbivorous mammals offered rich pickings for any adaptable birds. The ratites rapidly filled the vacant niches and, in doing so, lost the ability to fly. It was a winning strategy that only failed with the devastating arrival of Homo sapiens. Later, the mounting competition from increasingly large herbivores prevented any further flightlessness, except on islands that remained devoid of mammals – the classic example being the Dodo on Mauritius. Cooper also has an explanation for why the kiwis and tinamous remained small, in contrast to their closest relatives. He believes that the first palaeognaths to arrive would have monopolised the available niches, forcing any subsequent arrivals to remain small and to adopt alternative lifestyles. It is for this reason that the tinamous retained their wings and the kiwis became insectivorous and nocturnal.
In his 1870 presidential address to the British Association for the Advancement of Science, Thomas Huxley stated that ‘the great tragedy of science is the slaying of a beautiful hypothesis by an ugly fact.’22 Little could he have known just how apt this statement would prove to be in the field of palaeognath evolution. For the Tinamou’s story has destroyed the concept of Gondwanan vicariance, a theory cherished by many biogeographers for over 25 years.
But just how old are the palaeognaths? To try and answer that question, we need to take a trip to Antarctica.