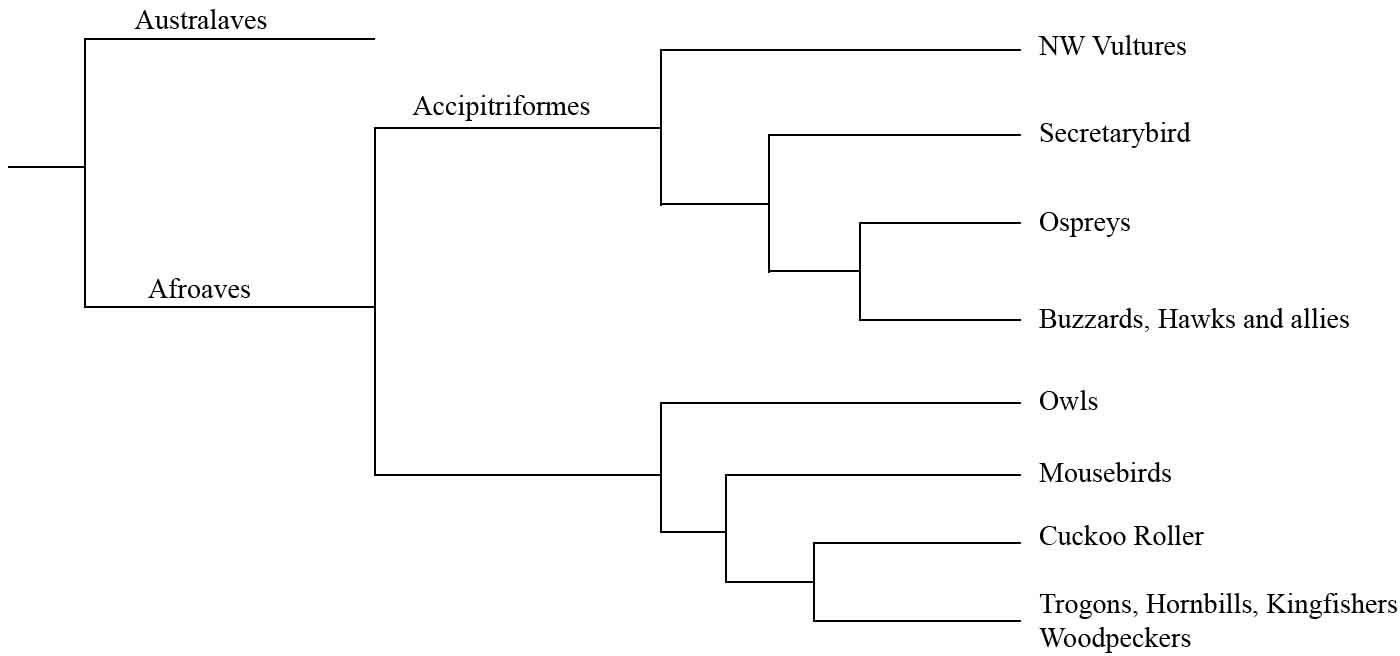
Figure 9.1 Phylogenetic relationships of the Afroaves. It is likely that the ancestor of all landbirds (Afroaves and Australaves) was an apex predator. Modified from Prum et al. (2015).2
After the break-up of Gondwana, landbirds diverged into two major clades – Afroaves and Australaves – that then underwent marked radiations to give rise to nearly all the landbirds living today.1 Since carnivorous species occupy the basal branches of both groups – diurnal birds of prey in the case of Afroaves (Figure 9.1), and seriemas for the Australaves (Figure 13.1) – it is likely that the ancestor of all the core landbirds was an apex predator, and that the raptorial trait was lost twice during their evolution. The birds of prey (family Accipitridae) comprise a global radiation of 256 birds that includes kites, hawks, buzzards, eagles and harriers. They all belong to a single clade or order, known as the Accipitriformes, whose earliest branches spawned the New World vultures, the Secretarybird, and the ospreys (Figure 9.1).
Falcons, however, are not part of the clade, as they evolved later and are sister species to the parrots and passerines (see The Parrot’s Story). Indeed, the evolutionary distance between the Accipitridae and falcons should not be a surprise, since the two families are very different in morphology and behaviour. Falcons lack the distinctive brow ridges of the hawks and tend to have longer and pointed wings. Accipiters also tend to prefer forests, where they capture and subdue struggling prey using their taloned feet, while falcons favour open country and kill swiftly with their beaks.

Figure 9.1 Phylogenetic relationships of the Afroaves. It is likely that the ancestor of all landbirds (Afroaves and Australaves) was an apex predator. Modified from Prum et al. (2015).2
One of the morphologically most primitive groups of Accipitriformes to evolve was that of the New World vultures (family Cathartidae). They first appeared in the early Palaeogene, although their crown group did not diversify until much later, during the middle Miocene, around 14 million years ago. At this time, there were many more species than today, as the presence of large herbivores and predatory mammals in open environments provided an abundant supply of carrion. During the late Pleistocene, a dramatic decline of the American megafauna occurred, which reduced the opportunities for scavenging and led to the extinction of many species.3 As a result; there are only seven extant taxa: two condors and five vultures. The California and Andean Condors are the biggest flying birds, with wingspans of around 3 metres and weights of up to 12 kilograms. Perhaps the most remarkable vultures were the teratons (albeit usually placed in their own family, the Teratornithidae), which included Argentavis magnificens from the late Miocene of Argentina. This species weighed up to 70 kilograms and possessed an extraordinary wingspan of 5–6 metres. It is by far the largest known flying bird and exploited the thermals for soaring across the pampas in search of its large prey. Although the Cathartidae resemble the Old World vultures, they are not closely related, and any phenotypic or behavioural similarities are the result of convergent evolution.
The monotypic Secretarybird (family Sagittariidae), unlike most birds of prey, is a terrestrial species that hunts its prey of lizards, snakes and small mammals on foot.4 They have long, pink, scaled legs and typically kick and stamp on their prey’s head until it is killed or incapacitated, particularly if it is a large lizard or a venomous snake. Despite this behaviour, Secretarybirds can fly and soar very well, especially during their nuptial displays, when they may undertake acrobatic flights at a great height. The species’ common name derives from the crest of long black-tipped feathers that gives it the appearance of an old-fashioned clerk with pens tucked behind the ear. An endemic of sub-Saharan Africa, the Secretarybird diverged around 40 million years ago, after the New World vultures and before the osprey and Accipitridae lineages. While confined to sub-Saharan Africa today, fossil evidence from France and the Middle East indicates that Secretarybirds had a much wider distribution in the past.
Ospreys (family Pandionidae) have recently been split into two species – the Western and Eastern Osprey – although their global population consists of four clades with clear genetic differences. This knowledge has enabled biologists to work out the birds’ evolutionary history and to determine their route of global spread. A specialised, fish-eating raptor, the osprey appeared in North America during the Miocene, although there are older fossils from the late Eocene and early Oligocene of Europe and Africa.5 The New World population first dispersed, via the Pacific coast of Asia, to colonise the Indo-Australian regions. Then, from Pleistocene refugia located in Indonesia– Oceania, a rapid range expansion occurred, with populations settling in eastern Asia and the western Palaearctic.6 Interestingly, each of the four clades exhibits a low genetic variability, which suggests that the colonisation of each new area involved only a few individuals with a limited number of genetic variants compared to the ancestral pool (known as a ‘founder effect’).
The phylogeny of the Accipitridae has been difficult to resolve, but it is likely that the basal branch gave rise to the kites. The latter group includes the genus Elanus (Black-winged Kite, Black-shouldered Kite, White-tailed Kite and Letter-winged Kite) that inhabit savanna-like habitat in temperate and arid areas. Like owls, they possess a velvety-comb structure to their upper wing feathers for dampening flight sound, vibrissae around the beak, and disproportionately large, frontally placed eyes. The four species disperse over long distances to feed on cyclic populations of small mammals, and produce many broods each year. These typical owl-like traits are unusual among the Accipitridae and are again the result of convergent evolution.7
The largest raptors belong to the harpy eagle clade (Crested Eagle, Harpy Eagle and Papuan Eagle), found in the Americas and New Guinea. The Philippine Eagle was once regarded as a sister species, but a study in 2005 found that this Asian raptor evolved much earlier and that its nearest relatives are the snake eagles, such as the Bateleur.4 All harpy eagles are immense, hunting carnivores that feed on monkeys and other medium-sized mammals, and, as such, lie near the apex of their respective food chains. The largest of all is the Harpy Eagle, which weighs up to 10 kilograms, and, with a wingspan of over 2 metres, possesses the power and lift to carry a 7-kilogram monkey. As with many birds of prey, the females are larger than the males and tend to take larger prey. And yet, surprisingly, these impressive birds are not the largest eagles to have ever lived (see below).
‘True’ or aquiline eagles diverged after the harpy eagles, around 12–15 million years ago.8 Originating in the Old World, the ancestral population gave rise to a single colonisation event of the Neotropics, via Asia and North America, and produced the four hawk-eagle species in the genus Spizaetus. The only other American dispersal was a recent one by the Golden Eagle, which entered the continent via Beringia. Over time, the New World Golden Eagles differentiated into the subspecies (canadensis), which is fully diagnosable from its Palaearctic relatives by molecular analysis. However, the main diversity of aquiline eagles was centred in Africa and Asia, from which two lineages entered Australasia at the end of the Pliocene to give rise to the Little Eagle and the much larger Wedge-tailed Eagle.
Island gigantism
In the spring of 1871, Frederick Fuller, a taxidermist working at Christchurch Museum, New Zealand, recovered some bones in a dried-up swamp that belonged to a large unknown raptorial bird. The fossils, including a femur, a few claw bones and a rib, were associated with a considerable quantity of moa remains. Fuller passed on his finds to the curator of Canterbury Museum, Julius von Haast, who published the first scientific description of the extinct species and named it Harpagornis moorei.9 Subsequent discoveries from dozens of sites in the South Island have added to our knowledge and allowed scientists to reconstruct the raptor’s anatomy and lifestyle.
Despite spanning 3 metres, the wings of Haast’s Eagle, as it is now called, were relatively short for its massive weight (up to 18 kilograms) and better suited to flying among trees than soaring in search of carrion. Its tail was relatively large, and the extra surface area partly compensated for the reduced wing size. Although it possessed a sharp, vulture-like beak, its talons were as large and as lethal as modern tiger claws. The predicted wing aerodynamics suggest that it probably hunted from a forest perch, waiting for a moa to walk by, before swooping down like a hawk and hitting its prey at great speed from the side (Plate 2). Damage on moa bones indicates that its bill was able to reach the ratite’s internal organs, while its talons could grasp their pelvic bones. Indeed, Harpagornis moorei has the distinction of being the only known raptor to have become the top predator in a complex ecosystem. Such a conclusion supports Māori mythology of the legendary pouakai or hokioi, a huge bird that attacked mountain people and could kill small children. And yet, by 500–600 years ago, the largest eagle the world had ever seen disappeared for good as Polynesian settlers destroyed its habitat and hunted its prey to extinction.
Since many New Zealand birds have sister species in Australia, it was assumed that Haast’s Eagle had evolved from one of the continent’s large raptors, most probably the Wedge-tailed Eagle. However, in 2005, Michael Bunce and Richard Holdaway analysed DNA from two 3,000-year-old Harpagornis moorei bones, housed in the Museum of New Zealand.10 The results were a surprise. The genetic sequences bore little resemblance to the Wedge-tailed Eagle’s but were remarkably similar to the DNA from two Hieraaetus eagles that weigh only a kilogram: the Little Eagle from Australasia and the Booted Eagle from Europe. The small number of base changes between the three species’ DNA indicates that the Haast’s Eagle diverged from the Hieraaetus clade as recently as 0.7–1.8 million years ago. These results imply that the ancestors of Haast’s Eagle were small when they arrived in New Zealand and increased rapidly in size, by a factor of 10 to 20, during the next million years. Such gigantism was only possible because the ecological niche usually occupied by large meat-eating mammals was vacant in New Zealand. While moas occupied the place of grazing ungulates such as deer or cattle for millions of years, the Haast’s Eagle became the apex predator that hunted the grazers. According to researchers Paul Scofield and Ken Ashwell, the increase in the eagle’s body size was so rapid that the development of the brain and some sensory systems, including sight and smell, lagged behind – evidence, they believe, that the species was a killing machine and not a vulturine scavenger as originally thought.11
The rate and magnitude of the Haast’s Eagle’s increase in size are unique within the vertebrates, particularly as this occurred in a species that retained the ability to fly. While other large predatory birds have evolved on islands free of competitors, notably the extinct giant eagles and owls on Cuba, the dramatic phenotypic changes of Haast’s Eagle remain unrivalled. Given its evolutionary origins, it seems sensible that the species’ Latin name should be amended to Hieraaetus moorei, since, in the words of Colin Tudge, ‘the world’s mightiest eagle belongs among the little ones.’12
Accidental speciation
The genus Buteo was the final Accipitridae clade to evolve. With origins in South America, it gave rise to a large group of soaring hawks with long, broad wings and relatively short tails and legs. They spread rapidly throughout the Americas to reach the Arctic regions, where a population became isolated in a Beringian refugium during the Pleistocene. Later, under the influences of cyclical climatic changes during the ice ages, descendants of this population invaded the Old World. This eastern offshoot gave rise to two African branches (Madagascan and Red-necked Buzzards), a cluster of Asian species (Eastern, Himalayan and Upland Buzzards) and the most recent offshoot, a superspecies that includes the Common and Steppe Buzzards.13
The story of the genus Buteo, including their relationships and geographical dispersal routes, was deduced by Martin Riesing at the Museum of Natural History in Vienna, after comparing the base sequences of their mitochondrial DNA. But, unbeknown to Riesing and his colleagues, an unexpected evolutionary clue lay hidden among their raw data – one that was revealed eight years later by Professor Mark Pagel’s team from Reading University.
The evolutionary relationships of the Accipitridae, as well as those of most of the bird families discussed in this book, have been inferred from phylograms: family trees drawn by comparing the DNA sequences from different species. When constructed, the root of the tree represents the common ancestor, while the branch tips depict that ancestor’s descendants. As you proceed from the root to the tip you are moving forward in time, and the longer the branch, the greater the amount of genetic change and the longer the species’ evolutionary history. The unit of time, therefore, is not a direct measurement but usually inferred from the number of nucleotide substitutions per site and presented either as the number of base changes divided by the sequence length or as the percentage change. Phylograms and their branch lengths are valuable tools, as they provide evolutionary biologists with information about diversifications of lineages, patterns and rates of trait evolution, and the timings of speciation. But, if Mark Pagel is correct, branch lengths also provide valuable information about not just when and where taxa arose, but also how they evolved.
One of the great mysteries of evolution is how subsets of species can suddenly become sexually incompatible and form new species. While mechanisms leading to reproductive isolation are known, such as vicariance (The Manakin’s Story), allochrony (The Storm Petrel’s Story) and hybridisation (The Sparrow’s Story), they are unlikely to account for the majority of speciation events. Instead, it has long been accepted that natural selection is key: a mechanism that leads to the gradual accumulation of many small changes that will, at some critical point, result in a population that can no longer mate with its kin. Although Darwin made a convincing case for natural selection over 150 years ago, no one has been able to devise a means to prove it: that is, until Mark Pagel.
Ten years ago, Pagel realised that if speciation results from the addition of lots of small changes, then there should be statistical evidence hidden within the species’ phylograms. His reasoning was as follows. If a large number of small factors summate to produce an outcome – for example, the combination of environmental and genetic factors that influence blood pressure, height, weight or blood sugar levels – then the population values for each of these measurements will describe a bell-shaped curve when plotted against frequency. In other words, such biological values are normally distributed. Similarly, if speciation is the outcome of many small evolutionary changes, as was believed, then Pagel predicted that the individual branch lengths in a given phylogram should also be normally distributed and describe a bell-shaped curve.
To see if this was correct, Pagel and two colleagues, Chris Venditti and Andrew Meade, plotted a histogram of branch lengths from a wide range of published phylogenetic trees. This task was no easy matter, as it involved obtaining and analysing the primary DNA sequence data that had been used to construct the original phylograms. Importantly, Pagel did not choose the trees randomly but selected only those that contained a narrow taxonomic range of species with similar life histories, morphology and ecology, to reduce any bias from different rates of speciation. Eventually, the available trees were whittled down to 101, including data sets for bumblebees, cats, turtles and roses – as well as birds. In addition to Riesing’s data on buzzards, there were trees for frigatebirds, shags and cormorants, swiftlets, vangas, thrushes and seedeaters. Working with each phylogram separately, Pagel and his team noted the number of base changes between each successive speciation event and used this as a measure of its branch length. If speciation results from many small changes, as they expected, then the branch lengths should fit one of two forms of bell-shaped curve: a normal distribution curve if the changes summate until they reach a threshold level for speciation, or a lognormal curve if individual changes multiply together and the threshold is attained more quickly.
To their amazement, neither of the expected curves matched the data. It turned out that the distribution of branch lengths from 78 per cent of the trees exhibited an exponential curve, while only 8 per cent of the data sets were lognormal and not one was normally distributed (Figure 9.2).14 Mathematicians are familiar with exponential curves, and such curves have a straightforward explanation. They are the pattern you obtain when you wait for some single, infrequent event to happen, for example, the time between the ejection of gamma particles from radioactive elements or the intervals between supernova in the Andromeda Galaxy. A more prosaic example, and Chris Venditti’s favourite, is the distance you have to travel between roadkills on a motorway. But the finding that phylogram branch lengths describe an exponential curve has profound and unsettling implications for biology. It implies that new species emerge from single events, each rare but individually sufficient to cause speciation. As Pagel told me, ‘It isn’t the accumulation of many small events that causes speciation, it’s the result of a single random event falling, as it were, from the sky.’ Nevertheless, their findings were so at odds with conventional wisdom that they spent the next two-and-a-half years trying to ‘make the exponential curves go away’. But, no matter how they reanalysed their data; the results were always the same. Even the inclusion of biases that were expected to favour the prevailing view produced little effect: the exponential curves could not be made to disappear.

Figure 9.2 Mechanisms of speciation deduced from phylogram branch lengths, showing the percentage of branch-length distributions fitting each curve type. (A) Exponential curve reflecting a single random and rare speciation event. (B) Variable rate curve produced when bursts of speciation occur. (C) Lognormal curve, indicating that many changes have multiplied together to reach a threshold for speciation. (D) Normal distribution bell curve, reflecting the slow addition of many small changes. Adapted from Venditti et al. (2010).14
Another type of curve, the variable rate curve, provided the best fit for 6 per cent of phylograms and indicates that speciation events have occurred in bursts. Textbook examples include the adaptive radiations of Darwin’s finches and Hawaiian honeycreepers, clades whose phylograms possess lots of branches at irregular intervals, reflecting the availability of many unfilled ecological niches. However, such rapid diversifications are not dependent on rare and random events but result from the omnipresent pull of natural selection. A further 6 per cent of phylogram branch lengths took the form of a Weibull curve, a distribution commonly used in reliability engineering and failure analysis. However, in the context of Pagel’s study, a Weibull curve implies that the probability of speciation is time-dependent. In other words, as the branch length of a tree increases so does the likelihood of speciation.
The key message from Pagel’s statistical approach is that evolution is unpredictable and that all species are subject to rare random events that may lead to reproductive isolation. Whatever form this takes – alterations in mating preferences for plumage colour or song, or genetic changes that result in incompatibility – the results are the same. The affected individuals become isolated from other family members and, in doing so, evolve into new species. Of course, natural selection still has a major role to play, as Darwin envisaged, but one restricted to the moulding and shaping of any new species to the particular conditions it experiences.
Evolutionary biologists have long argued whether rewinding the tape of life and replaying it would produce similar results, or whether chance events dictate the outcome and force evolution down novel and unpredictable paths. These two opposing views yield very different scenarios for the history of life. For example, some scientists argue that you can rerun the tape of life as many times as you like, and the outcome will always be very much the same. The leading advocate, the Cambridge Professor of Palaeobiology Simon Conway Morris, even believes that human-like, self-conscious intelligence is an inevitable product of evolution, rather than a historical accident or a fluke as neo-Darwinians typically assume. In contrast, supporters of the late Stephen Jay Gould, who popularised the tape-of-life metaphor, argue that if the clock were rewound, evolution would not repeat itself. Instead, the world would look quite different, with an absence of familiar life forms, including humans.
The results of Pagel’s branch-length analyses favour Gould’s model and add weight to the central role of contingency in evolutionary transformation. While not all biologists fully embrace this idea, no one has yet been able to find a flaw in Pagel’s scientific approach. Furthermore, in 2015, an ambitious project, involving the construction of a ‘timetree of life’ based on data from 2,274 separate studies and representing over 50,000 species, came to the same conclusion: ‘that speciation and diversification are processes dominated by random events and that adaptive change is largely a separate process.’15
As readers may have guessed, the exponential curve provides the best fit for the branch lengths from the buzzards’ phylogram. If Pagel and his team are correct, then the world’s buzzards, along with most other avian taxa, are the result of the unpredictable hand of fate: a reflection of the utter arbitrariness of speciation.