CHAPTER 15
The Manakin’s Story
WHY SO MANY SUBOSCINES?
It is a sultry morning in mid-August. My wife and I are following our guide along a well-worn trail as it snakes around the corrugated slopes of Tobago’s Main Ridge. High above us, the forest’s skyline is engulfed in a swirl of cloud, dark with foreboding. Soon, the surrounding leaves rustle knowingly and, within minutes, the first cold raindrops have become one of the day’s many torrential downpours. All around, spiny palms and bromeliad-adorned tree ferns sway to the water’s tune, while muddy rivulets stream across the path, only to disappear into the void below. From where we stand, huddled beneath a mass of bamboo fronds, we watch as the leafcutter ants struggle with their booty and unknown creepy-crawlies seek refuge from what must have seemed like an impending tsunami. As is typical in the tropics, the clouds soon clear, the sun re-emerges, and the montane rainforest becomes alive again with avian chatter. A high-pitched, drawn-out call soon reveals a Blue-grey Tanager sitting at eye level; a subspecies (nesophilus) more brightly coloured than its cousins that we would soon be seeing on Trinidad. We set off once more, and it’s not long before the guide stops, listens for a moment and then points excitedly to a nearby strangler fig. High up, almost hidden by leaves, is one of the key targets of our trip, a male Blue-backed Manakin. Our group peers intently at this tiny gem of a bird, with its pale blue back and scapulars, vivid red skullcap and orange-red legs, as it hops about in the dense foliage, feeding on fruit and insects. Moments later, an inconspicuous female, sporting a drab olive-green outfit, is spotted in a nearby tree. Then, as if by some prearranged signal, both take to the air and vanish for good higher up the valley.
The rationale for recounting this vignette is not that it was a memorable day’s birding or that the manakin was my first sighting of a suboscine, both of which are true. Rather, it provides a snapshot of an ongoing confrontation between two major avian clades, a microcosm of a continental struggle. The Blue-grey Tanager, a foot-soldier from the newly arrived northern oscines, is competing for resources and territory with a Blue-backed Manakin, a defending suboscine from the south: oscine versus suboscine. But how did these representatives of two great avian suborders come to be facing one another on the island of Tobago?
It is a story that we will address in the remaining chapters of this book.
Ancestral suboscines
Amid the luxuriant foliage of Gondwana, the earliest passerines flourished – seeking mates, building nests, laying eggs and searching for fruit and insects to feed their young. Then, during the early Palaeogene, the ancestral population underwent a major divergence, one of the most important in the evolutionary history of birds. In eastern Gondwana, in an area that would become Australia,1 a population began to evolve a more complex syrinx, a voice box characterised by an increased number of muscles that enabled a greater range of vocalisations. These basal songbirds or oscines may have had an evolutionary advantage, since they appear to have dominated and limited the suboscines to the western areas. Later, the ancestral suboscines underwent a pivotal divergence of their own.2 One lineage, or infraorder, entered South America to become the New World suboscines, while the other took an alternative route north, reaching Asia and then Africa (see The Sapayoa’s Story).
The New World suboscines rapidly colonised South America, by way of a broad continental shelf that linked the continent’s southern tip to Antarctica. These early passerines encountered a vast fertile continent, an eighth of the world’s landmass, inhabited by ancestral toucans, motmots, jacamars and trogons. Despite competition from these well-established families, it would be the momentous climatic and geological upheavals over the next 50 million years that would shape their evolution. So let us look at these processes and see how they led to such a diversity of species.
Initially, South America’s climate was distinctly warmer and more equable than today’s, with warm-temperate zones extending from the tropical equatorial forests towards the poles, where cooler conditions predominated.3 The Andes, which converted Patagonia into a desert by shielding it from the moist Pacific air, would not rise for another 30 million years. At the end of the Palaeocene, the Earth entered a ‘greenhouse’ phase, with temperatures reaching the highest levels experienced at any time during the past 65 million years. Polar seas reached 23 °C; the Earth became free of snow and ice; crocodiles and hippopotamuses wallowed in Arctic waters, and early primate-like mammals and palm trees flourished in the American West. This episode, which began 55 million years ago, is known as the Palaeocene–Eocene Thermal Maximum (PETM) and is likely to have resulted from a sudden release of methane, a potent greenhouse gas.4 The trigger was a rise in the Earth’s temperature that resulted from high atmospheric carbon dioxide levels, caused by volcanic eruptions from tectonic rifting. Methane, which is produced continually by decomposing microbes, is trapped in ice-like structures, called clathrates, under the ocean floor. The addition of large amounts of methane gas to the atmosphere would have caused further increases in the Earth’s temperature and further clathrate destabilisation. In effect, a positive feedback mechanism occurred that led to a runaway increase in temperature. Overall, some 2,500 gigatonnes of carbon were released into the atmosphere and ocean, resulting in a ‘greenhouse’ Earth within a very short time, estimated at between 2,000 and 3,000 years.5 Climatologists are interested in these gas levels, as they are comparable to those predicted from gross anthropogenic emissions by the end of the twenty-first century.6
The ecological consequences of PETM were complex. Deep-water organisms, known as foraminifera, were reduced by 50 per cent, possibly because of the increasing oceanic acidity. Their reduction would have contributed to the global warming, since foraminifera capture atmospheric carbon, in the form of carbon dioxide, and lock it up in their shells. In contrast, many new species of tree and plant evolved, despite a high extinction rate, while insects and mammals thrived – although the latter became much smaller, almost dwarf-like.
The early New World suboscines adapted to the tropical environment and spread throughout the extensive forests of South America, filling many vacant niches. At the height of the greenhouse period, suboscines divided into two independent lineages or parvorders.2 One, the Furnariida, produced the woodcreepers, ovenbirds, tapaculos and antbirds, while the other, the Tyrannida, gave rise to the manakins, cotingas and tyrant flycatchers. Before most of these crown splits had occurred, however, the Earth was subjected to yet another dramatic climatic upheaval.
By the middle Eocene, 40 million years ago, South America had separated from Antarctica and a seaway, the Drake Passage, had opened up between the two continents.7 As a result, the flora and fauna of South America was cut adrift and evolved in isolation until a land bridge was established with North America around 3–4 million years ago. To the east, Australia remained attached to Antarctica for a little longer, delaying the establishment of the Antarctic Circumpolar Current. As a result, the warm waters of the tropical Pacific and Atlantic Oceans could still reach Antarctica and keep the continent warmer than it would otherwise have been. Once Australia broke free, and the Tasman Passage formed, the circum-Antarctic gyre developed – with dramatic consequences for Earth’s climate and life’s evolution. Equatorial heat transfer to the Antarctic fell dramatically, and the cooling of the surrounding seas led to the formation of the south polar ice cap and, eventually, the refrigeration of the whole planet.8 At the same time, the rise of the Himalayas and other mountain chains contributed to the cooling, mainly due to the increased physical and chemical weathering of their rocks. Such processes expose reactive minerals that combine with carbon dioxide, so reducing the atmospheric levels of the greenhouse gas.
The marked cooling of the planet, which reached a nadir around 30 million years ago, triggered the retraction of South America’s extensive tropical forests towards the equator and their replacement by savanna and desert habitats.9 The falling temperatures led to a high biotic turnover. Megatherms – plant species that required stable high temperatures and abundant moisture – disappeared, while crocodiles and lizards retreated to the equatorial tropics. Strange hoofed herbivores evolved, with many developing extreme parallelisms to unrelated forms found on other continents. For example, notoungulates, an order of mammals that became extinct 5,000 years ago, evolved to fill the niches occupied elsewhere by rabbits and hares (among many others), while Litopterna took the place of horses and camels on the grazing lands.
The suboscines were not immune to these climatic changes. The Furnariida, however, were better able to cope, as they were primarily terrestrial and insectivorous, and indeed had already started to diversify by the middle Eocene. The earliest birds may have resembled the crescentchests (Melanopareiidae) which today thrive in the arid scrub and tropical dry forest environments of central South America. In contrast, the Tyrannida, having adapted to a forest lifestyle and a diet of mainly fruit, suffered a severe culling, with many species becoming extinct. Consequently, their diversification was delayed by approximately 15 million years,2 although what forms the lost passerines took, we can only speculate. Robert Ricklefs, a biologist from the University of Missouri, questions whether they might have ‘comprised members of groups we are familiar with today, or, like the notoungulates and many of the marsupials, were they highly unusual local products of South American splendid isolation?’10 In the absence of future fossil finds, we may never know the answer.
A significant and permanent cooling occurred around 14 million years ago, a fact linked to both an increased production of cold Antarctic waters and a major extension of the East Antarctic ice sheet. Curiously, for reasons that are not entirely clear, the temperatures in the low latitudes remained stable and warm.11 As a result, the north–south temperature gradient increased, and the boundaries between climatic zones strengthened. Such changes led to the Earth’s flora and fauna becoming subdivided into reasonably distinct provinces defined by temperature, patterns of seasonality, and precipitation.12 The Middle Miocene Climatic Optimum, as this period is now called, saw the New World suboscines diversify quickly, especially the spinetails and tyrant flycatchers. Most of these species-rich radiations moved into riverine or riparian habitats throughout the tropical lowlands, as well as the more open habitats to the south, before spreading northwards into the tropical Andes.2 Riparian habitats also enabled the tyrant flycatchers to expand up into the mountains along the streams and to adapt to the newly emerging montane forest habitats.13 Eventually, these radiations would account for approximately half of all the New World suboscines. In contrast, the diversification of antbirds, woodcreepers, manakins and some cotingas was restricted to the humid tropical rainforest habitats, predominantly in the forest understorey.
Over millions of years, the suboscines colonised all areas of South America. Then, during the late Pliocene, following the formation of the Panamanian isthmus, they invaded Central America, Mexico, Middle America and the Caribbean. Furthermore, several species of tyrant flycatcher began to migrate further north in summer, with the Alder Flycatcher now regularly reaching central Alaska. The payoff for these energy-sapping migrations is that the northern latitudes offer a greater number of territories, more food and fewer nest-robbing predators than their winter quarters in the tropics.
Suboscine diversity
A birder’s first visit to South America can be a daunting experience, given the continent’s unparalleled avian diversity. Peru, for example, has the highest concentration of bird life on Earth, with over 1,800 species. Many bird tours see over 500 species in two weeks, while one company has recorded a staggering 1,000 species in less than a month in Colombia. Such diversity is reflected in the New World suboscines, a radiation that has given rise to 1,289 extant species, comprising predominantly tyrant flycatchers (435 species), ovenbirds and woodcreepers (314 species) and antbirds (235 species).14 To put this in perspective, the number of species in each one of these families greatly exceeds the total number of species that regularly breed in the United Kingdom. But how is it that so many suboscine species have evolved in South America, and how can their local distributions be explained? It is certainly not just a consequence of the continent’s large area. Sub-Saharan Africa has only half the number of species, despite being of equal size. The answer to these questions, which have intrigued evolutionary biologists for over a century, turns out to be much more complicated than one might first imagine. As we will highlight, suboscine biodiversity results from the interplay of many vicariant events, including riverine barriers, marine transgressions, vegetative shifts and Andean uplift. Also, their adaptive radiations were facilitated by the length of time available for speciation, their anatomical and behavioural plasticity, and the emergence of many novel ecological niches.
Alfred Russel Wallace, an intrepid adventurer, collector and co-author of the theory of evolution, was the first to consider the role of geographical barriers, or vicariance, to explain Neotropical biodiversity. During his travels in South America, Wallace was struck by how often different species inhabited the opposite sides of rivers. He wrote:
During my residence in the Amazon district I took every opportunity of determining the limits of species, and I soon found that the Amazon, the Rio Negro, and the Madeira formed the limits beyond which certain species never passed.15
Importantly, he added: ‘I have only referred to the monkeys, but the same phenomena occur both with birds and insects, as I have observed in many instances.’ It was an impressive piece of detective work, as his observations were rather patchy, and he had to rely on the reports of several other naturalists. A year later, in 1853, Wallace ventured a theory to explain his conclusions in a book entitled A Narrative of Travels on the Amazon and Rio Negro.16 He imagined that continuous populations of species inhabiting the low-lying plains of South America had become suddenly partitioned by the development of the area’s three main rivers: the Amazon, the Rio Negro and the Rio Madeira. Eventually, these rivers would have formed natural dividing lines, beyond which certain species never passed. But he also observed that near their narrower sources, the rivers ceased to be boundaries and the ranges of species overlapped.17
Wallace’s astute deductions laid the foundations for what is now termed the ‘river-barrier’ hypothesis of speciation. This idea supposes that previously widespread ancestral species became separated when the Amazon and its major tributaries formed during the late Miocene. Originally, the western area of Amazonia was a million-square-kilometre marshland – the Pebas mega-wetland – consisting of shallow lakes and swamps that drained northwards towards the Caribbean (Figure 15.1). As South America drifted away from Africa, forming the Atlantic Ocean in the process, it moved across a subduction zone, where slabs of the Earth’s crust sink into the softened mantle. These tectonic processes resulted in an intense uplift along the continent’s Pacific edge that created the Andes, the world’s longest mountain range, extending over six countries. But the uplift affected more than just the plate boundary, and the whole of the western half of South America became elevated. At the same time, the continent’s northeastern areas subsided by as much as 400 metres. The net effect was that South America tilted like a giant seesaw, and the resultant ‘slide’ redirected the waters of western Amazonian over 6,400 kilometres into the Atlantic Ocean.18 The newly formed river systems then fragmented the early bird populations, so that many became isolated and subjected to different selection pressures, genetic drift and mutations which eventually led to the evolution of new species.
Figure 15.1 (Left) Thirteen million years ago, the Pebas mega-wetland covered over 1 million square kilometres of what is now the Amazonian basin and drained northwards into the Caribbean. (Right) Marine transgressions (dark grey) resulted in allopatric speciation of many taxa by vicariance.
The late Ernst Mayr, a doyen of neo-Darwinism, was the first to extend Wallace’s deliberations, concluding that the Amazonian rivers could have ‘initiated the first steps of speciation.’19 Biologists had long known that the distribution of many suboscine taxa appears to coincide with the course of the Amazon and its tributaries. White-breasted Antbirds, for example, never cross the Tapajós. In northeast Peru, the Golden-headed Manakin is restricted to the forest understorey to the north of the river while the Red-headed Manakin replaces it to the south. Such riverine delineations can also apply to the ranges of subspecies. The red-crowned race of the Blue-backed Manakin abuts the Amazon’s north bank, while the yellow-crowned subspecies resides only on the south side. These and other field observations imply that the ancestors of many antbirds and manakins must have inhabited the area before the river was formed.20 Recently, Angelo Capparella documented marked genetic differences between morphologically identical populations of Blue-crowned Manakin that inhabit the opposite banks of the Amazon. This finding confirmed for the first time the river’s potential effectiveness as an impediment to gene flow.21 Interestingly, this effect appears unique to the Amazon and its major tributaries; the Amazon’s headwaters and the continent’s second-largest river, the Paraquay–Paraná system, fail to provide adequate barriers.
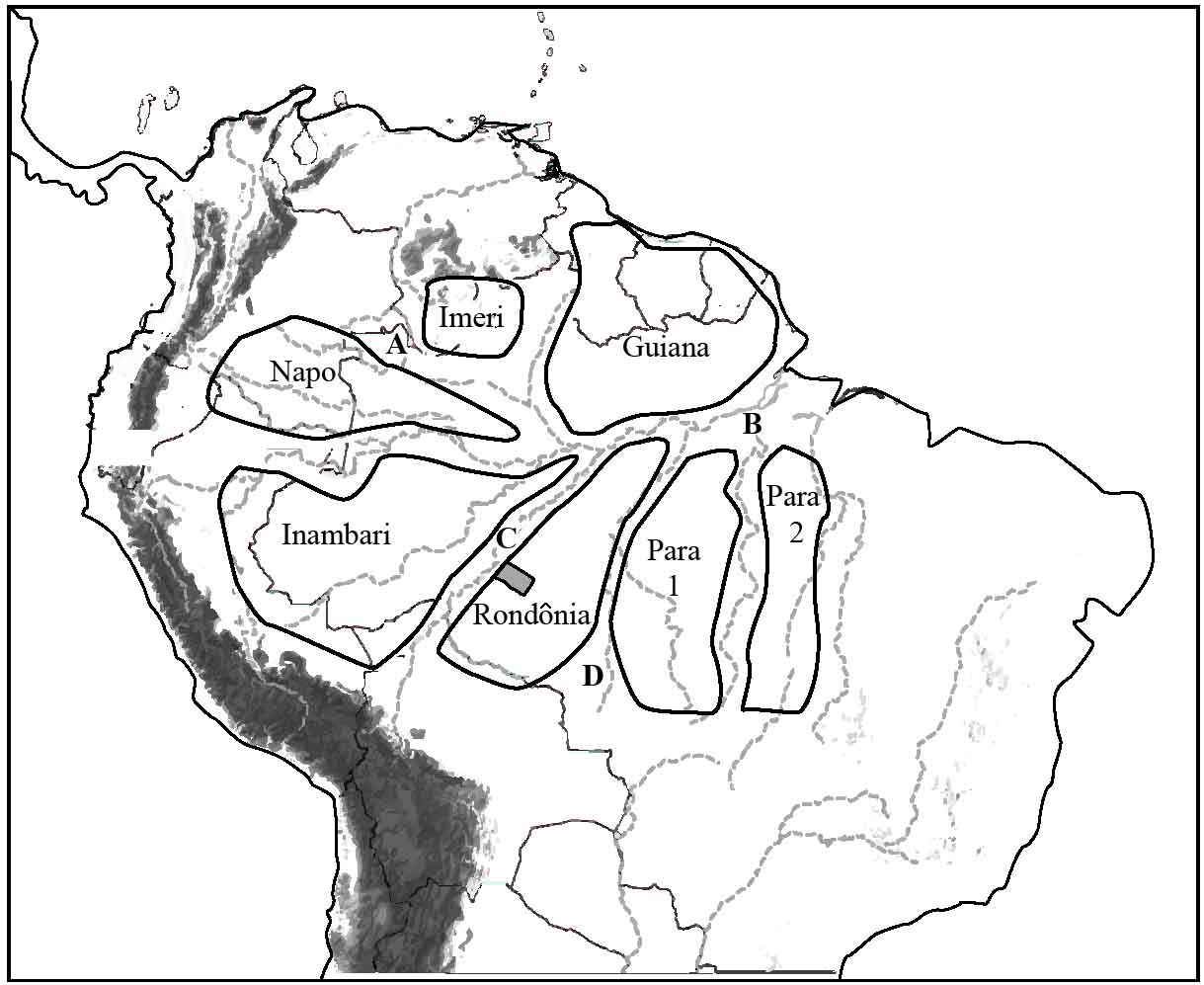
In 1985, Joel Cracraft, a biologist working at the Field Museum of Natural History in Chicago, proposed the existence of 33 areas of avian endemism in South America.22 This seminal work, based on the distribution charts of species, provided indirect evidence for the river-barrier hypothesis. In essence, Cracraft superimposed the range maps for most of the continent’s endemics and theorised that the areas of maximal overlap were major sites of speciation. As shown in Figure 15.2, all the endemic centres he identified within Amazonia are bordered by major rivers; for example, the Rondȏnia area lies between the Madeira and Tapajós, while the Imeri is between the Rio Negro and the Rio Branco.
Figure 15.2 Location of the Amazonian areas of avian endemism, based on Cracraft (1985).22 Major rivers: A, Negro; B, Amazon; C, Madeira; D, Tapajós. The ‘mini-interfluvial’ area (grey block) inhabited by the Rondonia Warbling Antbird lies between the Aripuaña and Jiparaná rivers.
Intriguingly, smaller areas of endemism are now thought to exist within some of these larger zones. Mario Cohn-Haft, a scientist working at the National Institute for Amazonian Research (INPA), reported that several species in the Rondȏnia area have ranges delineated by the Madeira’s smaller tributaries. Indeed, the pattern was so apparent that his research team referred to the areas as ‘mini-interfluves’.23 A typical example is that of the warbling antbird complex, a group that has been split recently into six distinct populations. One of these, the Rondonia Warbling Antbird, is confined to a small area between the Aripuaña and Jiparaná rivers (see Figure 15.2).24 Mini-interfluvial or fine-scale endemism has also been described for two members of the Tyrannidae that inhabit the Rondȏna zone: the Sucunduri Flycatcher and Chico’s Tyrannulet.25
As in the manakins, genetic differences have also been documented between morphologically indistinguishable populations of tody-tyrants, in particular, the Snethlage’s Tody-Tyrants that reside on the opposite banks of the Aripuaña and Jiparaná rivers.26 A more detailed analysis of three typical understorey suboscines, the Wedge-billed Woodcreeper, the Spot-backed Antbird and the Chestnut-tailed Antbird, produced similar results.27 Collectively, the above findings raise two important issues. First, cryptic endemism appears to be much more widespread than previously appreciated. Indeed, half the 16 lineages of Wedge-billed Woodcreeper described by Alexandre Fernandes and his team are morphologically indistinguishable. Four of these inhabit the Madeira–Tapajós interfluvium, where they replace each other on opposite banks of the Madeira’s tributaries.28 Fine-scale endemism also has significant conservation implications, because many of the critical mini-interfluvial areas have been earmarked for agricultural development. Sadly, it may be too late for the Rondonia Warbling Antbird, as rapid deforestation has already destroyed most of its habitat. Second, the highlighted studies seem to imply that the smaller Aripuaña and Jiparaná rivers have been more effective barriers to gene flow than the main river, the Madeira. This paradox is resolved if the direction and flow rates of the tributaries changed markedly in the past – events that are now thought to have occurred.
Many rivers provide only ‘leaky’ barriers, and most species will eventually find a way across, either in their narrower headwaters or by using islands as stepping stones. Nevertheless, the principal rivers of Amazonia seem to represent formidable barriers for many understorey inhabitants,29 especially manakins, antbirds and woodcreepers. Why this should be so remains unclear. It is possible that such species have adapted over millennia to low levels of light and, as a result, avoid crossing large open spaces. In the case of antbirds, there is an additional factor, one suggested by foraging theory, a model that helps predict how species behave when searching for food. For the ‘professional’ antbird, meals are a smörgåsbord of grasshoppers, cockroaches, praying mantises, scorpions and centipedes, all flushed by army ants on the move. To locate such an event and to compete with the hordes of other ant-followers takes both time and effort. The more successful an individual bird is, in terms of the energy expended, the greater its fitness and the greater the likelihood of it passing on its genes to the next generation. For an obligate antbird, it is more cost-effective to follow insects on one side of the river than to cross over and search for new swarms on the opposite bank. Swarms are uncommon, and antbirds will not know the whereabouts of ants’ bivouacs in unfamiliar territory, and the chances of encountering a swarm by accident are slight.30
The influence of rivers on suboscine diversity, however, extends beyond vicariant speciation. In Amazonia, rivers have created a range of unique habitats adjacent to terra firma, a term biogeographers use for forested areas that never flood. These include sandbar scrubs, river-edge forests and várzea, or seasonally flooded forests – each of which supports its specialist taxa.31 For example, the Scaled Spinetail is endemic to várzea bordering the east Amazon, while the poorly known Klages’s Antwren is restricted to a few isolated areas of lowland várzea. In contrast, the widespread River Tyrannulet, a small grey flycatcher, is confined to the sandbar scrubs of the Orinoco and Amazon watersheds. Intriguingly, such riverine endemism appears unique to Amazonia.
Rivers are not the only means by which water can isolate or segregate avian populations. Manuel Nores, an Argentinian biogeographer, has noted that the Amazon basin was flooded 10–15 million years ago when sea levels rose by up to 100 metres. By studying relief maps of South America, he concluded that two broad marine incursions would have formed via the Amazon and Orinoco rivers, as well as a narrower one from the smaller Branco. Most of southern Amazonia would have been flooded with brackish water, but two major areas were spared: one located in Venezuela and another straddling the borders of Guyana and northern Brazil (see Figure 15.1). Interestingly, these two ‘islands’ correspond roughly to the Imeri and Guiana areas of endemism identified previously by Cracraft (see Figure 15.2). According to the ‘marine transgression’ hypothesis, as it is now called, many species with continuous distributions became separated and experienced an interruption of genetic exchange, an event that led to allopatric speciation by vicariance. The striking Crimson Fruitcrow, a member of the cotinga family, and the Spot-backed Antwren, a diminutive canopy specialist, are thought to have evolved in this way.32
Pleistocene refugia
A popular view during the mid-twentieth century was that the rich Amazonian biodiversity was merely the consequence of ecological stability. Species never became extinct, but merely accumulated in a static environment. However, this ‘museum’ model was challenged in 1969 by a remarkable amateur ornithologist, Jürgen Haffer. Born in Berlin in 1932, Haffer became fascinated by birds as a youngster, having found a dead bird that had been ringed. After taking it to the local Zoological Institute, he was introduced to Erwin Stresemann, one of the pioneers of modern ornithology. Haffer was inspired by this chance meeting and later studied at the Institute, although he soon realised that zoology wouldn’t pay the bills and he switched to geology and palaeontology. Once qualified, he worked for Mobil Oil as a field geologist and spent the next eight years surveying the rainforests of remote northern Colombia. It was while out in the field, often involving arduous expeditions by mule and canoe, that Haffer became intrigued by the variety and distribution of Neotropical birds. He collected an impressive array of skins and drew up detailed range maps that highlighted where distinct and unique sets of birds were found. He also puzzled over the existence of hybrids that appeared to be limited to narrow contact zones often located far away from any contemporary barrier. Seeking an explanation, he realised that Pleistocene climatic fluctuations could be the missing link, an idea that culminated in his landmark paper published in the journal Science, and entitled ‘Speciation in Amazonian forest birds’.33
Haffer’s novel hypothesis envisaged the Amazonian rainforest to have cycled through several periods of expansion and contraction during the Pleistocene and post-Pleistocene epochs. Glacially driven, colder, drier intervals allowed the forests to contract and fragment into numerous smaller, isolated units or refugia. Birds stranded within these forest tracts were separated by impassable areas of open grassland and underwent allopatric speciation. As the temperature and rainfall increased again during the interglacials, the forest patchwork merged back to its original state, but now with a high level of species diversity and endemism. Since the alternating glacial advances and retreats occurred many times, with each cycle lasting up to 80,000 years, there would have been plenty of scope for new species to evolve. In summary, Haffer was proposing a species pump driven by glacial fluctuations.
The ‘Pleistocene refugia’ hypothesis, a synthesis of palaeoclimatology, biogeography and the concept of allopatric speciation by vicariance, gained widespread acceptance. The model’s elegance encouraged many other workers to propose similar explanations for the speciation of frogs, butterflies, lizards and even plants. It was also used to explain population patterns in other parts of the world, including Africa, Eurasia and America. But the idea had its limitations: the precise locations of the putative refugia were unknown, and there remained the difficulty of discriminating between alternative hypotheses. Nevertheless, the concept of Pleistocene refugia was readily adopted by the scientific community and went untested for several decades. Untested, that is, until the ecologist Paul Colinvaux took an interest.
Colinvaux, a Professor Emeritus at Ohio State University, predicted that if Haffer’s theory were correct, then it should be relatively easy to prove, because grass pollen extracted from lake cores laid down during the glacial periods should be increased. This seemingly straightforward project took Colinvaux and his team nearly 10 years to complete, because informative historical lakes, ones that had existed in the lowlands with sediments undisturbed by rivers, were extremely hard to find. Eventually, a number of likely candidates were identified, and the results were a surprise: ice-age deposits failed to show an increase in grass pollen.34 In other words, Colinvaux’s findings did not support the fragmentation of Amazonia, the keystone of Haffer’s widely accepted theory.
In the past decade, scientists from the Deep Ocean Drilling Project have come to the same conclusion after studying core samples of ocean sediment obtained from the mouth of the Amazon.35 This river system acts as a vast pollen trap and carries its cargo to the Atlantic coast, where it is deposited into the ocean. Analysis of pollen counts, or their surrogate markers, shows no alteration throughout the Pleistocene. It seems that the Amazonian rainforest, far from being ephemeral, is quite resilient, and has adapted well to past climate changes. Colinvaux’s uncompromising conclusion, to paraphrase Thomas Huxley, was that the refugia hypothesis was just another ‘beautiful theory … destroyed by an ugly fact’. However, his response may have been an over-reaction. Pleistocene climate changes are widely accepted to have increased savanna at the expense of rainforest in tropical Africa. Furthermore, the results from a recent genetic study of South American leafcutter ants favours a combination of refugia and marine incursions to explain their biodiversity.36
Scientists have also considered other possible interpretations of Haffer’s hypothesis. Could the colder glacial periods, with their reduced carbon dioxide concentrations, have produced significant changes in the composition of forests, leading to the isolation of species even in the absence of refugia? Or could the Pleistocene climatic fluctuations have resulted in effective refugia, not by the formation of savanna during the colder times, but by producing wider rivers during the warmer periods. In other words, could the increased interglacial rainfall have altered not just the size and discharge of rivers, but also their courses, resulting in enhanced barriers to genetic transfer during the wetter periods?
While Haffer’s hypothesis may now be thought suspect by many, his legacy has been an acceptance that past climatic fluctuations significantly influenced Amazonian biodiversity, and that this helped shape present avian distributions.
The impact of the Andean orogeny on avian biodiversity also needs consideration. The familiar snow-daubed sentinels, separated by high plateaus, that form a scoliotic spine down the continent’s western rim were not always a feature of South American geography. Rather, they are the ongoing progeny of a relentless and tumultuous slide of one tectonic plate beneath another. Each peak, from the lofty Aconcagua to the plethora of lesser mountains, is the result of the Nazca oceanic plate sinking beneath the lighter South American plate. Despite the initial uplift beginning before the Gondwanan break-up, by the mid-Miocene they were only 1,200–1,800 metres high, still covered with tropical vegetation and populated by lowland birds. It is only during the last 10 million years that the central Andes have risen to heights that are likely to have divided ancestral populations and provided new ecological niches. In support of this view, d’Horta and colleagues have shown that between 5 and 6 million years ago a species of leaftosser became separated on either side of the Andean uplift. As a result, the Black-tailed Leaftosser, the darkest and dullest of the genus, is found to the west while the Scaly-throated Leaftosser is restricted to the east.37 However, most species-level diversity appears to have occurred after the major Andean orogeny in the Neogene, which suggests that dispersal into the mountains from the lowlands, rather than vicariance, may have been the predominant driver for speciation.38
Focusing on single vicariant mechanisms may be overly simplistic. A team from Illinois State University, led by Angelo Capparella, undertook a detailed study of the Blue-crowned Manakin and found its past evolutionary history to be highly complex. Indeed, the geographical distributions of its many subspecies were best explained by the combined effect of three events: Andean uplift, river barriers and climate-induced shifts in vegetation.39
While these and other hypotheses fail to explain all of South America’s suboscine speciation events, they do help clarify why the immense biodiversity and high endemism in Amazonia are not randomly distributed. Lowland Amazonia is a mosaic of large endemic areas, each of which has its own uniform avifauna, but which differ from other apparently similar interfluvial zones. World birders seeking big lists will be well aware of this, since to see most of Amazonia’s birds it is necessary to visit many different areas of seemingly comparable habitat.
Evolutionary shifts in behaviour can serve as an ecological release and facilitate diversification into new habitats. For example, speciation in the ovenbird–woodcreeper assemblage, in particular the spinetails and their allies (genus Synallaxini), was aided by a switch from building ‘closed’ cavity nests to the construction of vegetative nests.40 It is likely that closed nests were the ancestral, or plesiomorphic, condition within the whole parvorder. Cavities, however, cannot be readily concealed, and numerous predators exploit their vulnerability. Also, natural holes or ground areas suitable for excavating tunnels are often limited in open landscapes, and the ability to build a vegetative nest would have provided a competitive advantage in such environments. Despite shifts in nest construction being rare events, it appears to have happened on at least three occasions during the early evolution of the Furnariida, involving the horneros, spinetails and foliage-gleaners. Indeed, the spinetails, which are adapted to dry habitats and build exposed vegetative nests, show the highest diversification rate of all the major clades.41 The move to building vegetative nests is likely to have been a gradual process, as many basal furnariid species still adopt a combined approach. For example, the Sharp-tailed Streamcreeper and the bartails (genus Premnoplex) build domed mossy nests within underground cavities, while the cup-shaped nests of rayaditos (genus Aphrastura) partially cover the walls of their cavities. Although such an energy-demanding ‘double approach’ may have evolved to reduce underground nest humidity, it undoubtedly facilitated the subsequent diversification into more open habitats.
Ecological factors
The importance of ecological factors as drivers of suboscine biodiversity should not be underestimated, especially the role of epiphytes, palms, bamboos, vines, dead leaves and ants. In contrast to the forests of temperate climates, these Neotropical features offer a range of ‘all-year-round’ food resources. As a result, many suboscines evolved specialist foraging strategies unique to the New World that, in turn, led to further speciation.
Up to half of all plants in the Neotropics are epiphytes, species that include mosses, ferns, liverworts, orchids and bromeliads. Epiphytes grow non-parasitically on other plants and rely on specialised aerial root systems to absorb water and nutrients directly from the air. Most are found carpeting tree branches or rooting in pockets of humus and rotting leaves, and provide rainforests with an extra dimension of biodiversity. Canopy soil and detritus, collectively known as crown humus, and non-vascular epiphytes (bryophytes and lichens) are an important food source for birds as they support a diverse invertebrate community.42 Amazingly, epiphytes account for 40 per cent of the rainforest’s entire biomass, so it is not surprising that many birds have evolved to exploit their microhabitats. Indeed, a study conducted in Costa Rica documented that nearly 200 species regularly make use of their offerings for nesting material, water and food (including fruit, nectar and invertebrates). Unlike their hosts, epiphytes can provide resources throughout the year, a fact exploited by several insectivorous birds that have become epiphyte specialists. The Spot-crowned Woodcreeper, for example, favours foliose lichens, while the Ruddy Treerunner and the Buffy Tuftedcheek specialise in bryophytes and arboreal bromeliads respectively.43
Another unique Amazonian niche is provided by the swamp palm, notably the elegant Moriche Palm (Maurita flexuosa). This tall species favours wet areas, either in savannas or deep within forests, where it can reach high densities, known locally as aguajale. Although patchily distributed, such groves provide a home for some species, including two suboscines: the Point-tailed Palmcreeper, a member of the ovenbird family (Furnariidae), and the Sulphury Flycatcher, a tyrant flycatcher (Tyrannidae). The Point-tailed Palmcreeper, as its name suggests, has evolved to become the ultimate palm specialist, spending its whole life among the tree’s fronds, where it can remain frustratingly inconspicuous. Characteristically, it rummages about in the basal pleats of the palm’s fan-shaped leaves, where it is often found hanging upside down feeding acrobatically on insects. The rather noisy and excitable Sulphury Flycatcher, while not an obligate specialist, rarely strays far from the palms, where it pursues its sallying lifestyle.
Bamboo contributes to the region’s biodiversity by supporting a taxonomically and ecologically diverse suite of birds. Indeed, the American ornithologist Ted Parker listed over 100 species of Neotropical birds that are associated with bamboo microhabitats.44 The synchronous production of seeds by many bamboos results in an abundant and nutritious food source for granivores, while their fast growth, hollow stems and densely tangled habitat support a wealth of arthropods that provide subsistence for insectivorous birds. The latter forage among both live and dead bamboo shoots, where they prise open the internodes or probe in holes, as well as capturing insects in flight between the stands.45 Although bamboo clumps can be many kilometres apart, a seminal study recorded 19 bamboo-specialists at just one site in western Amazonia, including foliage-gleaners, antbirds and pygmy tyrants.46 The contribution of bamboo to avian diversity may be even greater than previously appreciated. According to Kristina Cockle and Juan Areta, ‘in the past decade, new species of bamboo-specialist birds have been described, known species have been identified as bamboo-specialists, and bamboo species have been studied in greater detail, revealing relationships with specific species of bamboo.’45
Canopy and mid-storey liana tangles are a resource that takes on considerable importance in the Neotropics. Indeed, ecologists regard the presence of large vines as an indicator of pristine, undisturbed rainforest. Lianas, or climbing vines, are rooted in the ground and use trees to climb to the canopy to gain access to well-lit areas of forest. They provide year-round cover and foraging habitats for a variety of vine-gleaning and vine-inhabiting specialists including foliage-gleaners, treerunners and some antshrikes.
Dead leaves are also a rich food source and are typically found trapped among the vine tangles or hanging in situ from branches. They harbour more insects than green leaves but are much less abundant and more patchily distributed. Extreme dead-leaf specialists, therefore, spend more time and energy searching and handling prey, as extracting insects from curled dead leaves requires probing and acrobatic skills not necessary when gleaning green leaves.47 Most of the dead-leaf specialists are suboscines: foliage-gleaners and antwrens, which are restricted to the Neotropics where leaves are replaced at a low but relatively constant rate throughout the year. A typical example is the Checker-throated Antwren,48 a species which spends all its time foraging dead leaves caught in dense vine tangles for arthropods, many of which are unique to aerial leaf litter.49 Interestingly, phylogenetic studies indicate that all such specialist antwrens are related, and that they evolved separately from other antwrens after diverging around 9 million years ago. It seems that dead-leaf foraging is a primitive trait within the antwren group and appeared well before the radiation of modern species.50
Following army ants is a foraging strategy unique to the tropical regions, with no ecological counterpart in temperate zones. Contrary to popular opinion, antbirds do not eat ants. Instead, the ants increase the birds’ foraging efficiency by acting as ‘beaters’ to drive out hidden prey from beneath the leaf litter. Such activity is parasitic since the birds significantly reduce the ants’ success rate in capturing prey.51 All antbirds favour a single species of ant: the diurnal, swarm-raiding Eciton burchelli, found from southern Mexico to southern Peru and Brazil. A second species, Labidus praedator, is also followed, but they are less predictable and tend to swarm only after heavy rains.52 Obligate army-ant followers have evolved a variety of specialised traits not seen in other species. They can sit ‘sideways’ on small vertical branches above the swarm, snapping up prey from their perches. They do not defend exclusive territories, and they undertake ‘bivouac-checking’, the regular sampling of army-ant activity: a behaviour that reflects the unpredictability of ant swarm activity. But how and when did this specialised foraging strategy evolve?
Robb Brumfield at the American Museum of Natural History thinks it likely that antbirds underwent an evolutionary progression from the least to most the specialised.53 The ancestral antbirds only foraged around swarms as they moved through their territories. This behaviour then progressed, via a stage of regularly attending swarms outside territories, to the obligate ant-following strategy observed today. Although both Eciton burchelli and Labidus praedator have existed for around 20–40 million years,54 it was not until the late Miocene, some 6 million years ago, that the ant-following behaviour of birds evolved.53 Then, intense competition, a characteristic of extant obligate antbirds, would have encouraged their early diversification. This trait, coupled with the hierarchical division of advancing ant swarms into a series of concentric feeding zones, would have favoured the evolution of differently sized antbirds. Today, the largest ant-followers are found at a swarm’s productive leading edge, while the smallest species occupy the trailing edge.
The disarmingly simple question of why there are so many South American passerines turns out to be an unexpectedly complex one. As we have highlighted, the answer is in part the result of the combined effects of prolonged geographical isolation, geological upheavals, climate change, vicariance, and the presence of many unusual ecological niches. Also, as the gap between South and North America reduced during the late Miocene and Pliocene, the continent’s avian diversity dramatically increased due to an invasion of oscines from the north: a collection of songbirds that included jays, wrens, thrushes, wood-warblers, vireos, finches and tanagers.
Life for the suboscines, including the manakins of Tobago, would never be the same again, for they now faced competition from these new arrivals. But before we discuss how songbirds came to dominate the avian world, there is one further New World suboscine worthy of mention: the Sapayoa. For its evolutionary tale is as strange as that of any bird.