CHAPTER 22
The Thrush’s Story
SWEEPSTAKE DISPERSALS
To this day, the song of the Common Blackbird evokes memories of my halcyon days of childhood (Plate 31). Lying tucked up in bed with the windows open and the curtains fluttering gently in the evening breeze, I would fall asleep to the males’ languid and fluty tones as each bird sang in defence of its home patch. Now and then, a loud and persistent metallic ‘chook chook chook’ – most likely in response to next door’s cat – would gain my full attention. Indeed, it seemed as though every garden in our neighbourhood possessed a resident ‘Blackie’, and their measured phrases dominated the spring’s soundscape. Only later would I learn that these avian performances often reflect an ongoing struggle with newly arrived competitors from the continent. The English poet William Ernest Henley (1849–1903) encapsulated my youthful sentiment when he wrote:
The nightingale has a lyre of gold,
The lark’s is a clarion call,
And the blackbird plays but a boxwood flute,
But I love him the best of all.
I am convinced that it was the blackbirds’ beguiling refrains, which emanated from nearby rooftops, hedges and television aerials, that kindled my nascent passion for the natural world. And yet the birds’ love of our suburban gardens is intriguing – for, as we will discuss, their drab colouration and low-frequency song appears to have evolved millions of years ago in the tropical rainforests of Central America.
Blackbirds are members of the thrush family (Turdidae), all of which are characterised by an elongated muscular structure, the turdine thumb, that protrudes from the syrinx.1 This strange anatomical feature, as well as their cryptic spotted juvenile plumage, is shared by the Old World flycatchers (family Muscicapidae) and suggests a close evolutionary relationship. Most thrushes are proficient songsters, and some have vocalisations that rank among the most beautiful in the world. All 169 recognised species are plump, small to medium-sized passerines that belong to nearly 20 genera. The largest of these, Turdus, with 87 species, is one of the most widespread, with representatives on all continents except Australia and Antarctica (although nineteenth-century colonists did introduce Common Blackbirds to Australia and New Zealand).
Phylogenetic studies by Gary Voelker’s team at the University of Memphis favour a Eurasian origin for the Turdus thrushes. It is most likely they emerged within the western Palaearctic, since the oldest extant taxa, the Mistle Thrush and the Song Thrush, are distributed throughout the area.2 Such a conclusion is not altogether surprising, as the Turdidae are known to have evolved from the large Passerida radiation that dispersed to Asia from the proto-Papuan archipelago (see Figure 21.1). However, the thrushes’ subsequent evolutionary history turns out to be unexpectedly complex.3 Around 6.6 million years ago, an intercontinental dispersal occurred that led to the colonisation of Africa and the evolution of a limited number of species on islands in both the Atlantic and Indian Oceans, including São Tomé, Principe and the Comoros.
There then followed a remarkable biogeographical event. A population of the early African thrushes succeeded in making an east–west crossing of the Atlantic Ocean, against the prevailing winds and currents, to colonise the Caribbean basin.3 So unlikely was this that scientists refer to it as a ‘sweepstake dispersal’, a term coined by the late American palaeontologist George Gaylord Simpson.4 Although originally used to explain mammalian biogeography, the description is now applied to any chance floral or faunal dispersal across a major geographical barrier. For the ancestral thrushes, the crossing of the Atlantic Ocean around 6 million years ago seems to have been a matter of luck, a chance event that was never to be repeated. No other New World oscine reached the Americas via the transatlantic route – a fact that emphasises the extraordinary nature of this aspect of the early thrushes’ story. In contrast, all other extant songbirds of Central America and the Caribbean evolved from ancestors that reached America from Asia, via the Beringian land bridge.
What factors facilitated the thrush’s unique east–west crossing remains unclear. The fact that the Atlantic Ocean was narrower 6 million years ago, however, can be ignored. Indeed, although continental drift is constantly pushing America away from Europe and Africa, it is doing so only at the rate of growth of a human fingernail, approximately 3–5 centimetres a year. At the time of the Turdus dispersal, the journey would have been only 300–400 kilometres, or approximately 10 per cent, shorter. Voelker, however, believes that a freak storm may have been a key factor. Indeed, modern Atlantic storm systems are often of such intensity that dust from the Sahara has been documented in the New World.
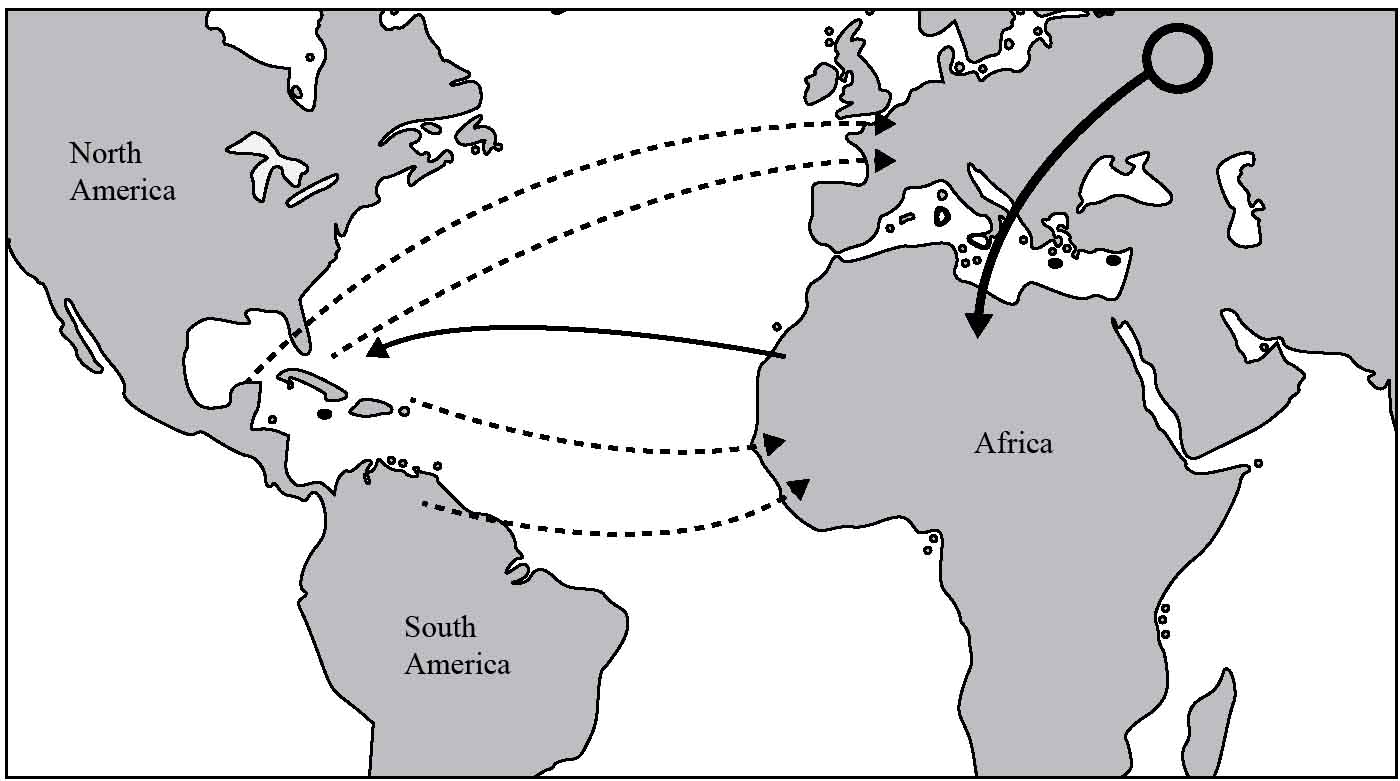
Figure 22.1 Five transatlantic sweepstake dispersals of the thrush family: (solid line) original east–west dispersal, (dotted lines) four reverse dispersals. The black circle indicates the origin of the ancestral thrushes, with the attached black arrow indicating the first intercontinental movement to Africa. Modified from Voelker et al. (2009).3
During the next million years, the early Caribbean thrush population spread out over all of America, from Alaska to Tierra del Fuego, to produce many species, including the American Robin in North America and the Austral Thrush in Argentina and Chile. Crucially, four separate populations then re-crossed the Atlantic Ocean during a narrow temporal window – between 4.7 and 5.7 million years ago – and returned to the Old World (Figure 22.1). It was from these four ‘reverse sweepstake dispersals’ that most of the extant species in Europe and Africa evolved. They include the Common Blackbird, a species widespread across Europe and the Middle East, and the Ring Ouzel, an unusual thrush for being an upland specialist. The migratory Fieldfare and Redwing of the western Palaearctic, as well as the non-migratory African Thrush, also have Caribbean ancestries. However, as highlighted previously, the Song Thrush and Mistle Thrush evolved from a population of early thrushes that remained in Eurasia.
Reverse dispersals
It has been argued that sweepstake dispersals rarely lead to successful colonisations, since they usually involve single, or just a few, individuals. In such a situation, the most likely outcome would be death before a self-sustaining population could be established.5 Throughout the evolution of the genus Turdus, however, there has been a tendency to move in flocks, a fact that would make successful colonisation after a sweepstake dispersal more likely.3 Recent evidence suggests that this can still happen, as the Fieldfare, which breeds in woodland and scrub in northern Europe and Asia, colonised Greenland after a flock reached the island early in the last century.6
In contrast to the initial east–west dispersal, the four return crossings could have been aided by the palaeoclimatic conditions that prevailed before the South and North American continents joined. Storms that formed in the eastern Pacific and Caribbean tended to move eastwards across the Atlantic, a factor that would have favoured the return dispersals to the Old World. This weather system, however, changed dramatically when the gap between the two continents closed.
Beneath the waterway that linked the Pacific and Atlantic Oceans – the Central American seaway – two tectonic plates collided, forcing the Pacific plate slowly beneath the Caribbean plate. The pressure and heat generated by this collision led to the formation of submarine volcanoes, some of which grew tall enough to break through the ocean surface and form islands approximately 15 million years ago. Over the next 10 million years, more and more volcanic islands formed, while the movement of the tectonic plates pushed up the sea floor, eventually forcing some areas above sea level. At the same time, the continents’ coasts were eroded by strong ocean currents, and vast amounts of sediment – sand, soil and mud – were deposited between the newly emerged islands, filling in all the gaps. The resultant Panamanian isthmus linked the two continents and ended what George Simpson famously described as South America’s ‘splendid isolation’.7 Indeed, the loss of the Central American seaway was one of the most important geological events to happen during the last 60 million years, since it not only played a major role in promoting the region’s biodiversity but also greatly affected the Earth’s climate.
The obvious effect of the seaway’s closure on the region’s biota was the increased migration of animals and plants between the two continents – what has been termed the Great American Interchange. In North America today, the many species of opossum, armadillo and porcupine can all be traced back to ancestors that crossed the land bridge from the south, while the ancestors of llamas and raccoons trekked in the opposite direction. As we have discussed, the direction of traffic for birds was primarily from the south to the north – an event that greatly transformed the tropical avifauna of the New World.8 However, the gradual shoaling and ultimate closure of the Central American seaway also significantly altered the Earth’s climate. The lack of water exchange between the Pacific and Atlantic Oceans around 4.7 million years ago resulted in altered salinities as evaporation in the tropical Atlantic and the Caribbean left their waters saltier and put freshwater vapour into the atmosphere.9 The Trade Winds developed and transported the water vapour westwards across the isthmus, where it fell as rain in the Pacific. As a result, the Pacific became relatively fresher while salinity increased in the Atlantic. All these changes, together with the re-routing of currents to form the Gulf Stream, effectively eliminated the favourable storm patterns for reverse transatlantic dispersals. It is significant, therefore, that no subsequent intra-Caribbean or west–east Atlantic lineage diversification occurred after the formation of the Panamanian isthmus.
But how did Voelker’s team deduce that most of the members of the genus Turdus evolved from Caribbean ancestors? And how can they be so sure that the initial, as well as the subsequent dispersals, were not the result of an interchange via the Beringian route? As unlikely as it may seem, the answers to these questions were deduced by analysing the family’s genetic material. By comparing homologous sequences of DNA from 65 species of the genus Turdus, Voelker and his colleagues were able to construct a robust phylogeny. The resultant tree showed that four of the Old World clades are more closely related to extant Central American species than they are to each other. In other words, these four clades must have evolved independently from early Caribbean species, rather than descending directly from African or Eurasian ancestors. Furthermore, using the Beringian connection hypothesis to explain the relationships between Old and New World species would require a minimum of seven continental extinctions.3 Given the long-term persistence of thrushes in central and eastern Eurasia, it seems improbable that no lineage was able to survive the varied North American habitats that existed during the last 6 million years. According to Voelker, therefore, the transatlantic route is a far more parsimonious explanation than the Beringian path. Indeed, the latter would require repeated extinctions across both Europe and North America, for which there is no current evidence.
The conclusion that palaeoclimatic changes played a significant role in the sweepstake dispersals of thrushes is dependent on the accuracy of the dates assigned to the various nodes or divergence points. So how were these derived? In practice, such dates are best obtained by calibrating against a reliable fossil. However, for the genus Turdus this approach is not possible, as the few existing specimens cannot be attributed to any extant species. An alternative method is to apply a molecular clock: one that relies on a fixed rate of DNA mutation, usually a 2 per cent divergence per million years for the commonly used avian genes. The universality of this method, however, has now been questioned.10 Voelker’s team, as a result, calibrated their time tree against a vicariant event, one that led to the speciation of two African thrushes. The tropical forest of central Africa attained its maximum eastern extension, reaching the coast of Kenya, between 5 and 3 million years ago. It then underwent a rapid retraction to the west that resulted in the separation of an ancestral population of thrushes. One group became restricted to the high-altitude mountains in Kenya, the Taita Thrush, while the other population was limited to the lowland forests of the rift system and became the Abyssinian Thrush. Since both species are sedentary, it is reasonable to assume that their speciation took place no later than 3 million years ago. Using this fixed date, Voelker then calibrated the rest of the thrushes’ phylogenetic tree and translated the relative divergence times into absolute ones.
As a youngster, I was unaware that the Common Blackbird’s low-frequency song provides a clue to its evolutionary origins. Foliage absorbs high-frequency sound waves, so the Blackbird’s deep, baritone notes are much better at penetrating dense vegetation. This characteristic trait is now known to have been honed millions of years ago in the tropics to facilitate the defence of territories and the winning of mates. As I ponder this amazing fact, it seems appropriate that a Song Thrush should be singing from a nearby tree, while out of my study window I spot a Blackbird hopping about on our lawn. Although well aware that both species belong to the genus Turdus, I hadn’t realised the remarkably different evolutionary pathways that have led each to my tended patch of suburbia.