Chapter 16. Laboratory: Electrochemistry
Electrical work is similar to physical work, but instead of the physical work of moving a box from the floor to a shelf, electrical work is required to move electrons through a conductor or move ions through a solution to an electrode. And, just as the position of the box determines its physical potential energy, the type and state of charge of ions determine their electric potential energy (or electric potential for short).
Just as air moves (as wind) from a point of higher air pressure to a point of lower air pressure, an electric charge moves (as an electric current) from a point of higher electric pressure to a point of lower electric pressure. Electric pressure is denominated in volts, so an electric charge moves from an area at higher voltage to an area at lower voltage. To determine the electric work needed to move a charge through a conductor, multiply the charge by the potential difference:
Charge (C) · Potential Difference (V) = Electric work (J)
Electric charge is specified in Coulombs (C), the potential difference in volts (V), and electric work in Joules (J).
In this chapter, we’ll examine both types of electrochemical processes by using an electric current to initiate and sustain a chemical reaction, and by using chemical reactions to produce electric current.
Laboratory 16.1: Produce Hydrogen and Oxygen by Electrolysis of Water
Electrolysis is the process of forcing a redox reaction to occur by supplying an external electric current. For example, electrolysis can be used to plate an iron object with chromium by reducing chromium ions from a solution, replacing them with iron ions. Ordinarily, this reaction could not occur, because chromium is higher in the activity series of metals than iron. The application of an external electric current overcomes the difference in activity (potentials) between these two metals, allowing the chromium ions to be reduced to chromium metal.
Electrolysis is commonly used industrially and in laboratories to produce pure hydrogen and oxygen by splitting otherwise stable water molecules into their component gases, a process that requires providing an external electrical current at 1.23V or higher. This voltage is applied to two inert electrodes immersed in a dilute aqueous solution of an ionic compound. (Pure water cannot be used, because it is a very poor conductor of electricity.) In an electrolytic cell (as opposed to a galvanic cell, where the direction is reversed), the negative electrode, or cathode, provides the electrons needed to reduce H+ ions to hydrogen gas. Conversely, the positive electrode, or anode, accepts the electrons needed to oxidize O– ions to oxygen gas.
We can balance this redox reaction using half-reactions. To do so, we must identify which species is being oxidized and which reduced by looking at the change in oxidation states:
2 H+12O–2(l) → 2 H02(g) + O02(g)
From this balanced equation, we see that hydrogen is being reduced and oxygen is being oxidized, which allows us to set up the following two half-reactions:
(oxidation) 2 O–2 → O2 + 4 e–
(reduction) 2 H+ + 2 e– → H2
Doubling the reduction half-reaction to put the same number of electrons on each side—everything, including electrons, must balance—and then adding the two half-reactions gives us the final balanced reaction:
[2 O–2 → O2 + 4 e–] + [4 H+ + 4 e– → 2 H2] = 2 H2O → 2 H2 + O2
In this laboratory session, we’ll use electrolysis to produce hydrogen and oxygen from water.
Procedure
If you have not already done so, put on your splash goggles, gloves, and protective clothing.
Weigh about 15 g of magnesium sulfate heptahydrate and transfer it to the beaker.
Fill the beaker nearly full with distilled water and stir until the magnesium sulfate dissolves completely.
Fill one of the test tubes completely with the magnesium sulfate solution, place your finger or thumb over the tube, invert it, and immerse it in the beaker. Make sure the tube is completely filled with the solution and contains no air bubble. Repeat this procedure for the second test tube, and then use the rubber band to secure the two tubes together. Make sure the mouths of the test tubes are at least several centimeters below the surface of the solution.
Repeat step 4 with the second pair of test tubes.
Place the beaker on the base of the ring stand, with its center under the clamp.
Gently lower the 9V transistor battery into the beaker, with its electrodes up. You’ll see a trail of bubbles begin to rise immediately from each electrode.
Clamp the first pair of test tubes and position them over the electrodes, placed so that each tube catches the trail of bubbles from one of the electrodes.
Note which tube is over the cathode of the battery and which is over the anode. Use the marking pen to make a minus sign on the tube over the cathode and a plus sign on the tube over the anode.
As the reaction proceeds, you’ll notice that the gas in one tube accumulates about twice as fast as the gas in the other tube. When most of the water has been displaced from one of the tubes, which may require 10 to 30 minutes, depending on the size of the test tubes, insert a stopper into each of the two tubes while keeping their mouths below the surface of the solution.
Unclamp the first set of tubes, remove them from the beaker, rinse them with tap water, and place them in the test tube rack.
Clamp the second set of tubes into position, and allow gas to begin accumulating in them.
Remove the tube that was over the anode from the rack. Strike a match, allow it to catch fire completely, and then blow it out. While the burned end of the match is still glowing, remove the stopper from the test tube and thrust the glowing end of the match into the tube. Record your observations on line A of Table 16-1.
Remove the tube that was over the cathode from the rack. Strike a match, hold the burning match over the mouth of the tube, and remove the stopper. Record your observations on line B of Table 16-1.
Pour the solution that remains in the anode test tube into the graduated cylinder, and record the volume of the solution to 0.1 mL on line C of Table 16-1.
Pour the solution that remains in the cathode test tube into the graduated cylinder, and record the volume of the solution to 0.1 mL on line D of Table 16-1.
Fill one of the test tubes to the brim with water, and use the graduated cylinder to measure the volume of water the test tube contains when full. (You will probably have to fill and empty the graduated cylinder two or three times.) Record the volume of the test tube to 0.1 mL on line E of Table 18–1.
Note the level of the gases in the second set of test tubes. When the tube with the larger volume of gas has had about two-thirds of the solution displaced by the gas, reposition the test tubes so that the tube that was initially over the anode is now over the cathode and vice versa.
Allow the reaction to proceed until both tubes are nearly full of gas and the volume of gas in each tube is about equal.
Stopper the tubes while keeping their mouths under the surface of the solution.
Unclamp the second set of tubes, remove them from the beaker, rinse them with tap water, and place them in the test tube rack.
Light a match, remove the stopper from one of the tubes, and hold the lit match just over the mouth of the tube. Record your observations on line H of Table 16-1.
Item | Result |
A. Glowing match + anode gas | |
B. Burning match + cathode gas | |
C. Volume of solution remaining in anode tube | ______.______ mL |
D. Volume of solution remaining in cathode tube | ______.______ mL |
E. Volume of test tube | ______.______ mL |
F. Volume of anode gas (E – C) | ______.______ mL |
G. Volume of cathode gas (E – D) | ______.______ mL |
H. Burning match + mixed anode and cathode gases |
Review Questions
Q: | Q1: From looking at the balanced equation and your own observations during this lab, which gas was produced at the anode and which at the cathode? What evidence supports your conclusions? ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ |
Q: | Q2: Using the values you recorded on lines F and G of Table 16-1, calculate the volume ratio of the two gases produced and the percentage error from the theoretical yield. ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ |
Q: | Q3: Comparing your observations for igniting the cathode gas versus igniting the mixed cathode and anode gases, why was the combustion of the mixed gases so much more vigorous? ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ |
Q: | Q4: At standard temperature and pressure, one mole of a gas occupies about 22.4 L. The density of water is 1.00 g/mL and its formula weight is 18.02 g/mol. If your test tube of mixed anode and cathode gases contains a total of 25.0 mL of gases in a 2:1 hydrogen:oxygen ratio, how much liquid water is produced when they combust? ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ |
Laboratory 16.2: Observe the Electrochemical Oxidation of Iron
In the preceding lab session, we used an external electrical current to force a non-spontaneous redox reaction to occur. But many redox reactions occur spontaneously, without requiring an external energy source to initiate or sustain them. In this lab, we’ll examine a very familiar spontaneous redox reaction, the oxidation of iron-to-iron oxide, otherwise known as rusting.
Although rusting occurs spontaneously, it happens only when specific conditions exist; namely, the presence of oxygen and water. The balanced equation for this electrochemical reaction shows why:
4 Fe(s) + 6 H2O(l) + O2(g) → 2 Fe2O3 · 3H2O(s)
In the absence of oxygen or water, the reaction cannot proceed. And, although these conditions are both necessary and sufficient for the reaction to occur, the reaction rate varies with the presence or absence of electrolytes. Because the current flow inherent to an electrochemical reaction involves the migration of ions, the presence of an electrolyte, such as sodium chloride (common salt) or another ionic salt, increases the reaction rate. That’s why, for example, iron and steel rust much faster near the ocean than they do in drier areas far from salt air, and why automotive rust is a major problem in areas where roads are salted during winter weather.
In this lab session, we’ll examine the rusting of iron. We’ll expose clean iron surfaces to oxygen, water, and salt, alone or in any combination, and observe the effects of each of these environments on the reaction rate.
Caution
This experiment uses flame. Be careful with the flame, have a fire extinguisher readily available, and use care in handling hot objects (a hot beaker looks exactly like a cold beaker). Wear splash goggles, gloves, and protective clothing.
Procedure
If you have not already done so, put on your splash goggles, gloves, and protective clothing.
Label six clean, dry test tubes A through F and place them in the test tube rack.
Use the steel wool or sandpaper to polish six iron or steel nails until they are bright and shiny. Make sure to clean the entire nail, including the head, shank, and tip.
Place one of the nails in test tube A and return the test tube to the rack.
Fill test tube B with tap water to just below the rim and insert the stopper. The goal is to determine the water level needed to allow the stopper to seat, leaving only water in the test tube, with no air bubble. When you have determined how much water is needed, mark the level on the outside of the tube and empty the tube.
Repeat step 5 for test tube E.
Set up the beaker on the tripod stand or ring stand, using the wire gauze to protect the beaker from direct flame.
Use test tube B to transfer about two test tubes worth of distilled or deionized water to the beaker.
Light the alcohol lamp or burner, and bring the water to a gentle boil. Continue boiling it gently for several minutes. The goal is to drive off all of the oxygen dissolved in the water.
Place the second clean nail in test tube B.
Use the beaker tongs to fill test tube B with the boiled water to the line you previously made on the test tube and insert the stopper gently. You needn’t force the stopper into place. The idea is simply to prevent the contents of the test tube from being exposed to atmospheric oxygen.
Place the third clean nail in test tube C, and fill the tube to near the rim with distilled or deionized water that has not been boiled. Leave this test tube unstoppered.
Place the fourth clean nail in test tube D, and fill the tube with solid sodium chloride until about half the length of the nail is covered by the sodium chloride.
Use test tube E to transfer about two test tubes worth of 1 M sodium chloride solution to the beaker. Bring the sodium chloride solution to a gentle boil and continue boiling it gently for several minutes to drive out dissolved oxygen.
Place the fifth clean nail in test tube E, use the beaker tongs to fill test tube E with the boiled sodium chloride solution to the line you previously made on the test tube, and insert the stopper gently. Again, seat the stopper gently. All we want to do is prevent the contents of the test tube from being exposed to atmospheric oxygen.
Place the sixth clean nail in test tube F, fill that test tube with unboiled 1M sodium chloride solution, and return the test tube to the rack. Leave it unstoppered.
Set the rack of six test tubes aside; check it under a strong light after several hours or overnight to see if the nail in one or more of the test tubes has begun rusting. Continue checking the tubes periodically until it is evident that rusting is or is not occurring in each of the tubes. Record your observations for each of the tubes in Table 16-2.
Test tube | O2 | H2O | NaCl | Amount of oxidation |
A | • | º | º | none···light···moderate···heavy···extreme |
B | º | • | º | none···light···moderate···heavy···extreme |
C | • | • | º | none···light···moderate···heavy···extreme |
D | • | º | • | none···light···moderate···heavy···extreme |
E | º | • | • | none···light···moderate···heavy···extreme |
F | • | • | • | none···light···moderate···heavy···extreme |
Review Questions
Laboratory 16.3: Measure Electrode Potentials
In the first lab of this chapter, you may have wondered how we knew that the electrolysis of water would require at least 1.23V. A glance at the standard reduction potentials listed in Table 16-3 provides the answer. (Table 16-3 lists standard reduction potentials for only a handful of half-reactions. Comprehensive tables that list reduction potentials for thousands of half-reactions are available online and in printed reference works.) Of the half-reactions involved in the electrolysis of water, the reduction of oxygen gas and hydrogen ions (protons) to water:
O2(g) + 4 H+(aq) + 4 e– → 2 H2O(l)
has the greatest reduction potential, +1.23V. Of course, oxidation and reduction are two sides of the same coin, so the reverse reaction is equally valid:
2 H2O(l) → O2(g) + 4 H+(aq) + 4 e–
So, looking at the oxidation reaction rather than the reduction reaction, the oxidation of water to oxygen gas and hydrogen ions has the greatest oxidation potential, which means that external current must be supplied at a minimum voltage of –1.23V for the half-reaction to occur.
Half-reactions that appear near the top of the table—those with high negative values—represent strong reducers. Those listed near the bottom of the table—those with high positive values—represent strong oxidizers. It’s important to understand that a species in isolation cannot be said to be a reducer or an oxidizer, because the species with which it reacts determines whether the first species functions as a reducer or an oxidizer.
For example, consider the pairs of ions and elements, Zn2+/Zn, Fe2+/Fe, and Cu2+/Cu, which have reduction potentials of –0.76V, –0.44V and +0.34V, respectively. If you place solid iron in a solution of Cu2+ ions, those ions are reduced to solid copper while the solid iron is oxidized to Fe2+ ions. With this combination of species, iron serves as the reducer and copper as the oxidizer. Conversely, if you place solid zinc in a solution of Fe2+ ions, those ions are reduced to solid iron while the solid zinc is oxidized to Zn2+ ions. With this combination, iron serves as the oxidizer rather than the reducer.
Half-Reaction | E0 (V) |
Li+(aq) + e– → Li(s) | –3.05 |
K+(aq) + e– → K(s) | –2.93 |
Ba2+(aq) + 2 e– → Ba(s) | –2.90 |
Ca2+(aq) + 2 e– → Ca(s) | –2.87 |
Na+(aq) + e– → Na(s) | –2.71 |
Mg2+(aq) + 2 e– → Mg(s) | –2.37 |
Al3+(aq) + 3 e– → Al(s) | –1.66 |
2 H2O(l) + 2 e– → H2(g) + 2 OH-(aq) | –0.83 |
Zn2+(aq) + 2 e– → Zn(s) | –0.76 |
Cr3+(aq) + 3 e– → Cr(s) | –0.73 |
Fe2+(aq) + 2 e– → Fe(s) | –0.44 |
Cd2+(aq) + 2 e– → Cd(s) | –0.40 |
Co2+(aq) + 2 e– → Co(s) | –0.28 |
Ni2+(aq) + 2 e– → Ni(s) | –0.25 |
Sn2+(aq) + 2 e– → Sn(s) | –0.14 |
Pb2+(aq) + 2 e– → Pb(s) | –0.13 |
2 H+(aq) + 2 e– → H2(g) | 0.00 |
Cu2+(aq) + 2 e– → Cu(s) | +0.34 |
O2(g) + 2 H2O(l) + 4 e– → 4 OH–(aq) | +0.40 |
Ag+(aq) + e– → Ag(s) | +0.80 |
Hg2+(aq) + 2 e– → Hg(l) | +0.85 |
O2(g) + 4 H+(aq) + 4 e– → 2 H2O(l) | +1.23 |
Au3+(aq) + 3 e– → Au(s) | +1.50 |
H2O2(aq) + 2 H+(aq) + 2 e– → 2 H2O(l) | +1.78 |
F2(g) + 2 e– → 2 F-(aq) | +2.87 |
Standard reduction potential values can also be used to predict the voltage that will be produced by a cell that uses two different metals as electrodes, simply by calculating the difference in reduction potential between the two metals. For example, if you build a cell that uses electrodes of copper (+0.34V) and zinc (–0.76V), that cell will, in theory, provide current at 1.10V, because:
–0.76V – (+0.34V) = –1.10V
In practice, the actual voltage produced by such cells is always somewhat lower than the theoretical voltage produced by an ideal cell, because real-world physical cells have inefficiencies in ion migration and other issues that reduce the voltage somewhat. Still, using reduction potential values makes it easy to estimate the approximate voltage that will be produced by a cell that uses any two arbitrarily selected metals as electrodes.
But what exactly is a cell? When we drop an iron nail into a solution of copper ions, the iron is oxidized and the copper reduced, but these two half-reactions occur with the reactants in physical contact. Electrons are exchanged, but we have no way to access that electron flow and use it to do useful work. If we separate the reactants physically, the two half-reactions cease—unless, that is, we join them electrically by using a conductor. An arrangement in which the two reactants are physically separated but electrically joined is called a cell, and each of the separate reactants is called a half-cell.
In a cell, electrons released by the reducing agent in its half-cell travel from its electrode as an electric current through a conductor (such as a wire) to the electrode of the half-cell that contains the oxidizing agent, which is reduced by those electrons. That electric current can be used to do useful work, such as lighting a bulb or running a motor. The electrode at which oxidation occurs is called the anode, and the electrode at which reduction occurs is called the cathode.
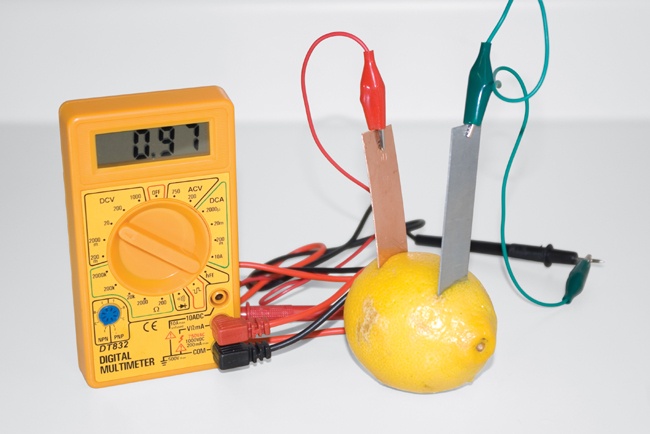
In this laboratory session, we’ll build such an electrochemical cell, also called a galvanic cell or voltaic cell, using electrodes of various metals. We’ll embed those electrodes in a lemon. The semipermeable membranes in the lemon will function as physical barriers to isolate the two half-cells from each other, while the hydronium and citrate ions produced by the citric acid in the lemon juice provide internal electric connectivity between the half-cells. that current to establish that an electrochemical cell exists, and compare our actual measured voltages with the voltage that an ideal cell using those electrodes would produce.
If we stopped at that point, no reactions would occur and no current would be produced, because the semipermeable membranes in the lemon prevent internal migration of metal ions between the electrodes. In other words, no return path for the current exists. But if we provide a return path by connecting the two electrodes directly together with a copper wire, the reaction proceeds and current is produced. We’ll measure the voltage of that current to establish that an electrochemical cell exists, and compare our actual measured voltages with the voltage that an ideal cell using those electrodes would produce.
Caution
Although none of the materials used in this lab session is particularly hazardous, make sure to dispose of the food items after use. DO NOT EAT ANY FOOD ITEM used in this session. The food items will be contaminated with metal ions, including toxic heavy-metal ions. Wear splash goggles, gloves, and protective clothing.
Procedure
If you have not already done so, put on your splash goggles, gloves, and protective clothing.
Use steel wool or sandpaper to polish each of the electrodes to remove surface oxidation, oils, and other contaminants.
Use the knife to make two slits in the lemon, spaced as far apart as possible, and large enough to accept the electrodes. It’s important that the two electrodes do not touch once they are inserted into the lemon.
Insert the copper electrode in one of the slits and insert an electrode made of a different metal in the other slit.
Touch or clamp one of the leads from the DMM to each of the two electrodes and observe the reading on the DMM. If necessary, reverse the leads to obtain a positive voltage reading. Record the voltage reading to 0.01V in the appropriate cell of Table 16-4.
Leaving the copper electrode in position, remove the second electrode and replace it with another electrode of a different metal.
Use the DMM to record the voltage with this second pair of metals, and record that voltage to 0.01V in the appropriate cell of Table 16-4.
Repeat steps 6 and 7 until you have tested all combinations of the copper electrode with all of the other electrodes you have available.
Based on the voltage readings you obtained for copper with each of the other metals, choose one of those metals to replace the copper electrode. Test that metal in combination with all of the other metals. (But remember you’ve already tested it with copper.) Record the voltages you observe to 0.01V in the appropriate cells of Table 16-4.
Repeat step 9 until you have tested every metal paired with every other metal.
Electrode | Mg | Al | Zn | Fe | Ni | Sn | Pb | Cu |
Mg | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | |
Al | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | |
Zn | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | |
Fe | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | |
Ni | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | |
Sn | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | |
Pb | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | |
Cu | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V | ___.____V |
Review Questions
Q: | Q1: Of the eight metals shown in Table 16-4, why did we first use copper as the reference electrode for each of the other metals? __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ |
Q: | Q2: Using copper as the reference electrode, rank the cell voltage for each of the other anodes that you tested. __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ |
Q: | Q3: Using the standard reduction potentials listed in Table 16-3, calculate the theoretical voltage for each of the cells you tested and recorded in Table 16-4. How do the actual voltages you measured compare to the theoretical voltages you calculated? Propose an explanation for any differences. ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ |
Laboratory 16.4: Observe Energy Transformation (Voltage and Current)
The law of conservation of energy says that energy can neither be created nor destroyed, but can be transformed from one form to another. For example, an electric generator converts mechanical energy into electric energy, a motor converts electric energy into mechanical energy, and an incandescent light bulb converts electric energy into light energy and heat energy. Chemical energy is another form of energy and—as is true of all forms of energy—can be converted to other forms of energy.
In this lab session, we’ll transform chemical energy into electric energy and use that electric energy to do work; specifically, to light an LED. Electric energy has two key metrics, which can best be understood by analogy with plumbing. Just as a differential in water pressure forces water to flow through a pipe, a differential in electrical pressure—called voltage, denominated in volts, and abbreviated V—forces electricity to flow through a wire or other conductor. And, just as the amount of water transferred can be specified as a flow rate—denominated in gallons/minute, liters/second, or some other unit of measure—the flow rate of electricity is called current, and denominated in amperes or amps (abbreviated A), where one ampere represents a current flow of one coulomb per second. In this lab session, we’ll measure voltage and current in a voltaic cell.
Caution
Hydrochloric acid is corrosive and emits strong irritating fumes. Wear splash goggles, gloves, and protective clothing.
Procedure
If you have not already done so, put on your splash goggles, gloves, and protective clothing.
Use steel wool or sandpaper to polish each of the electrodes to remove surface oxidation, oils, and other contaminants.
Transfer about 200 mL of 2 M hydrochloric acid to the beaker.
Clamp one of the patch cables to the copper electrode and the other patch cable to the magnesium electrode.
Clamp the remaining end of each patch cable to one of the wires that extends from the base of the LED.
Immerse both electrodes in the beaker of hydrochloric acid. If the LED does not light, reverse the leads. The light emitted by the LED represents work done by the electric current produced by an electrochemical reaction.
Leaving the copper electrode in position, remove the magnesium electrode, disconnect it from the patch cable, and rinse it thoroughly with water.
Connect the iron electrode to the patch cable, and immerse the iron electrode in the beaker of hydrochloric acid. The LED should not illuminate.
Disconnect both leads from the LED and reconnect them to the DMM. Verify that the voltage across the copper/iron electrode pair is approximately 0.78V.
Remove the iron electrode from the acid bath and rinse it thoroughly with water.
Immerse only 1 cm of the iron electrode in the acid bath, and use the DMM to record the voltage and current (amperage) on the first line of Table 16-5.
Increase the distance the iron electrode is immersed in the acid bath centimeter by 1 centimeter, recording the voltage and current at each immersion level in Table 16-5.
Review Questions
Laboratory 16.5: Build a Voltaic Cell with Two Half-Cells
If you immerse magnesium metal in a solution of copper sulfate, a spontaneous redox reaction occurs. An atom of magnesium releases two electrons and is oxidized to an Mg2+ ion. Those two electrons are captured by a Cu2+ ion, reducing it to copper metal, which is deposited on the magnesium electrode as a thin plating. This reaction occurs spontaneously, because magnesium ions are less attractive to electrons than are copper ions.
We can write this redox reaction as two half-reactions, with the standard reduction potentials shown in parentheses:
Mg(s) → Mg2+(aq) + 2e– (+2.37V)
Cu2+(aq) + 2e– → Cu(s) (+0.34V)
In the combined reaction, magnesium atoms serve as the reducing agent, which means that they are oxidized to magnesium ions. Conversely, copper ions serve as the oxidizing agent, which means that they are reduced to metallic copper. Adding up the reduction potentials tells us that this cell should be able to provide an electron flow at 2.71V. There’s a problem with that, though.
When magnesium metal is in direct contact with a solution of copper ions, electrons are transferred directly from the magnesium metal to the copper ions, so those electrons are not accessible to do work. In effect, both half-reactions are occurring in the same reaction vessel, so there is no externally accessible flow of electrons that can be captured to do work.
But what if we could split the two half-reactions into separate vessels and somehow intercept that electron flow? As it turns out, we can do just that by putting the copper and magnesium parts of the cell in separate containers. That physically isolates the magnesium atoms and ions from the copper atoms and ions, but we still somehow have to provide an external path for the flow of electrons and an electrical connection between the copper and magnesium segments of the cell.
The first part of that circuit is no problem. We can use ordinary copper wire clipped to the electrodes as a way to route electricity outside the cell, where it can do useful work such as lighting a light bulb. But we still need the internal connection between the two half-cells. The answer is a device called a salt bridge, which is a tube filled with a conducting solution, with one end immersed in each of the half-cells. The salt bridge conducts electricity while preventing ions from migrating from one half-cell to the other.
In this lab session, we’ll build a voltaic cell comprising a magnesium half-cell and a copper half-cell, and measure the voltage and current produced by the cell.
Procedure
If you have not already done so, put on your splash goggles, gloves, and protective clothing.
Transfer 50 mL of 1.0 M magnesium sulfate solution to one beaker, and 50 mL of 1.0 M copper sulfate solution to the other beaker.
Use steel wool or sandpaper to polish each of the electrodes to remove surface oxidation, oils, and other contaminants.
Clip one end of each patch cable to an electrode and the other ends of the patch cables to the DMM terminals.
Immerse the magnesium electrode in the beaker of magnesium sulfate solution, and the copper electrode in the beaker of copper sulfate solution.
Observe the voltage reading on the DMM. No voltage is detected, because no circuit yet exists.
Plug one end of the plastic tubing tightly with cotton. Use the Beral pipette to fill the plastic tubing with 1 M sodium chloride solution, making sure that there are no air bubbles in the tubing. (If the solution runs out of the plugged end as you fill the tubing, the plug isn’t tight enough.) When the tubing is full, plug the open end tightly with cotton and add a few more drops of sodium chloride solution to make sure that the plug is saturated with solution.
Disposal
The magnesium sulfate solution can be flushed down the drain with copious water. Precipitate the copper ions by adding sodium carbonate solution and filtering the solid copper carbonate precipitate, which may be disposed of with household waste; the supernatant fluid may be flushed down the drain. The electrodes can be retained for later use.
Check again to make sure that there are no air bubbles in the tubing, and then immerse one end of the tubing in the beaker of magnesium sulfate solution and the other end in the beaker of copper sulfate solution.
Record the voltage and current readings on the DMM on line A of Table 16-6.
Add 50 mL of water to the beaker of magnesium sulfate, stir to mix thoroughly, and record the voltage and current readings on the DMM on line B of Table 16-6.
Add 50 mL of water to the beaker of copper sulfate, stir to mix thoroughly, and record the voltage and current readings on the DMM on line C of Table 16-6.
Review Questions
Laboratory 16.6: Build a Battery
In everyday life, the words “cell” and “battery” are often used interchangeably. For example, most people refer to AA cells as “AA batteries.” But in chemistry the terms “cell” and “battery” have specific meanings. In the preceding experiments, we’ve been working with cells, which are self-contained or separated containers that use an electrochemical reaction to produce electricity. A battery is an interconnected group of two or more cells.
A battery may be constructed by connecting cells in series, which is to say, connecting the anode of one cell to the cathode of the next cell in a daisy chain. In a series battery, the voltages are additive. For example, if you construct three cells, each of which provides 1.2V at a current of 50 mA, and connect those three cells in series, the resulting battery provides 3.6V (1.2V · 3) at a current of 50 mA. The advantage of a series battery is that it provides higher voltage than an individual cell can provide.
Conversely, a battery may be constructed by connecting cells in parallel, which is to say, connecting the anodes of all of the member cells together and the cathodes of all of the member cells together. A battery connected in parallel has the same voltage as any of the individual cells, but the current is additive. For example, if you construct three cells, each of which provides 1.2V at a current of 50 mA, and connect those three cells in parallel, the resulting battery provides 1.2V at a current of 150 mA (50 mA · 3). The advantage of a parallel battery is that it provides higher current than an individual cell can provide.
In theory, it’s also possible to construct a hybrid series/parallel battery that contains a group of series batteries connected in parallel (or, another way of looking at it, a group of parallel batteries connected in series). Such hybrid batteries provide both higher voltage and higher current than the individual cells provide. In practice, though, when both higher voltage and higher current are needed, it’s more common to construct a series battery (to boost voltage) whose electrodes are physically large with high surface areas and can therefore provide higher current directly. A 12V car battery is an excellent example of such a battery.
Another important aspect of a battery is its internal resistance. Voltage, current, and resistance are interrelated, as stated by Ohm’s Law: E = I · R
where E is voltage in volts (V), I is current in amps (A), and R is resistance in ohms (Ω). At a particular voltage, the amount of current in a circuit is determined by the resistance of the circuit, including the internal resistance of the battery. Higher resistance means lower current, and vice versa. For that reason, battery designers take great pains to minimize internal resistance.
In this laboratory session, we’ll examine the voltage, resistance, and current of a single cell. We’ll then use multiple cells to construct batteries in series and parallel, and examine the characteristics of those batteries.
Caution
Although none of the materials used in this lab session is particularly hazardous, make sure to dispose of the food items after use. DO NOT EAT ANY FOOD ITEM used in this session. The food items will be contaminated with metal ions, including toxic heavy-metal ions. Wear splash goggles, gloves, and protective clothing.
Procedure
If you have not already done so, put on your splash goggles, gloves, and protective clothing.
Use steel wool or sandpaper to polish each of the electrodes to remove surface oxidation, oils, and other contaminants.
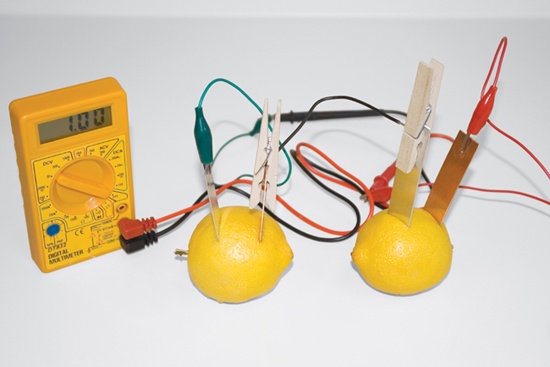
Use the knife to make two slits in the one of the lemons, spaced as far apart as possible, and large enough to accept the electrodes. It’s important that the two electrodes do not touch once they are inserted into the lemon.
Insert the copper electrode in one of the slits and the magnesium electrode in the other slit.
Touch or clamp one of the leads from the DMM to each of the two electrodes and observe the voltage reading on the DMM. If necessary, reverse the leads to obtain a positive voltage reading. Record the voltage reading to 0.01V on line A of Table 16-7.
Switch the DMM to resistance mode and record the resistance reading on line A of Table 16-7.
Switch the DMM to current mode and record the current reading on line A of Table 16-7.
Repeat steps 3 and 4 to prepare the other two lemons.
Use a patch cable to connect the copper electrode on the first lemon to the magnesium electrode on the second lemon.
Use patch cables to connect the magnesium electrode on the first lemon to one of the DMM terminals and the copper electrode on the second lemon to the other DMM terminal. If necessary, reverse the cable connections on the DMM to obtain a positive voltage reading. Record that voltage reading on line B of Table 16-7.
Switch the DMM to resistance mode and record the resistance reading on line B of Table 16-7.
Switch the DMM to current mode and record the current reading on line B of Table 16-7.
Add the third lemon to the circuit by disconnecting the copper electrode on the second lemon from the DMM and reconnecting it to the magnesium electrode on the third lemon. Then connect the copper electrode on the third lemon to the DMM terminal. Record the voltage reading on line C of Table 16-7.
Switch the DMM to resistance mode and record the resistance reading on line C of Table 16-7.
Switch the DMM to current mode and record the current reading on line C of Table 16-7.
Disconnect all of the patch cables.
Use a patch cable to connect the copper electrode of the first lemon to the copper electrode of the second lemon.
Use a second patch cable to connect the magnesium electrode of the first lemon to the magnesium electrode of the second lemon.
Use patch cables to connect the magnesium electrode on the first lemon to one of the DMM terminals and the copper electrode on the first lemon to the other DMM terminal. If necessary, reverse the cable connections on the DMM to obtain a positive voltage reading. Record that voltage reading on line D of Table 16-7.
Switch the DMM to resistance mode and record the resistance reading on line D of Table 16-7.
Switch the DMM to current mode and record the current reading on line D of Table 16-7.
Use a patch cable to connect the copper electrode on the third lemon to the copper electrode on the second lemon, and a second patch cable to connect the magnesium electrode on the third lemon to the magnesium electrode on the second lemon. Record the voltage reading on line E of Table 16-7.
Switch the DMM to resistance mode and record the resistance reading on line E of Table 16-7.
Switch the DMM to current mode and record the current reading on line E of Table 16-7.
Configuration | Voltage | Resistance | Current |
A. Cell | ___.____V | ____.___ Ω | ____.___ mA |
B. Series battery (2-cell) | ___.____V | ____.___ Ω | ____.___ mA |
C. Series battery (3-cell) | ___.____V | ____.___ Ω | ____.___ mA |
D. Parallel battery (2-cell) | ___.____V | ____.___ Ω | ____.___ mA |
E. Parallel battery (3-cell) | ___.____V | ____.___ Ω | ____.___ mA |