CHAPTER 6
THE PROMISE AND PERILS OF ETHANOL
Now that we’ve examined green vehicles, let’s take a look at green transportation fuels. Of all the clean, green transportation fuels, ethanol is by far the most widely used alternative to gasoline. If you live in North America, chances are you are burning ethanol in your car right now. That’s because many filling stations in the US and Canada sell a blend of 10% ethanol and 90% gasoline, at least during the winter, to help reduce carbon monoxide pollution. If you live in a Midwestern city, chances are there’s a local gas station or two selling an 85% blend of alcohol and gasoline, commonly referred to as E85. If you want to make a switch to a renewable biofuel, you may be able to do it right now, provided your vehicle can run on this mix of ethanol and gasoline.
Unlike biodiesel and straight vegetable oil, which are produced on an extremely small scale, ethanol has become big business. In 2007, the US and Brazil, the undisputed world leaders when it comes to ethanol fuel production, generated 6.5 and 5 billion liquid gallons, respectively. Brazil’s ethanol industry has created about 1 million jobs. Brazil has attained energy self-sufficiency thanks in part to domestic ethanol production from sugars extracted from the nation’s vast fields of sugar cane, but also to domestic oil production. Brazil devotes nearly 9 million acres of cropland — about 1% of its total arable land — to sugar cane for ethanol production. In the US, nearly 25 million acres, or 3.7% of the total arable land, are devoted to corn production to generate ethanol.
Ethanol Stations
As of January 2009, there were around 1,900 gasoline stations in the US that sold E85. Most of these stations are located in the corn belt states, led by Minnesota with 378 stations. Minnesota is followed by Illinois with 223, Wisconsin with 133 and Missouri with 116. Some states like Alaska, Maine, New Hampshire, Rhode Island and Vermont have no E85 stations at all. Why don’t more gas stations sell E85? Unfortunately, E85 must be stored in a separate tank. Installation of a new tanks costs about $60,000.
Ethanol is not a newcomer among the renewable transportation fuels. In fact, ethanol use as a transportation fuel dates back to Henry Ford. In 1896, Ford designed his first car, the Quadricycle, to run on 100% ethanol. In 1908, Ford’s Model T was released. It was capable of running on gasoline, ethanol, or a combination of the two. Henry Ford proclaimed that “ethyl alcohol is the fuel of the future,” and he continued to advocate for ethanol as a transportation fuel during Prohibition.
Like other transportation fuels, ethanol has its pluses and minuses. In fact, ethanol has become highly controversial. While farmers and government officials support its production, some scientists and prominent environmental groups have cast doubt on the highly touted benefits of this fuel. Before I cover the objections to ethanol, I’ll explore what ethanol is, how it is made, and which crop plants yield this green fuel. Later in this chapter, I’ll discuss the flexible-fuel vehicles that burn ethanol and conclude with a summary of the pros and cons of ethanol.
What is Ethanol?
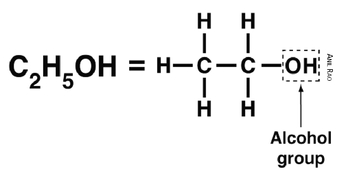
Ethanol is a two-carbon compound with the chemical formula C
2H
5OH (
Figure 6.1). The OH at the end of the molecule is an alcohol group. It consists of an oxygen atom chemically bonded to a hydrogen atom. As shown in
Figure 6.1, the OH group is chemically bonded to one of ethanol’s two carbon atoms.
Ethanol is also known as ethyl alcohol or grain alcohol. Alcohols are water-soluble organic compounds. They evaporate fairly readily and burn cleanly, in part because they contain no impurities like the sulfur contained in gasoline and diesel fuel.
Most readers are familiar with ethanol as the “active ingredient” in beer, wine, and hard liquor. This small molecule has a huge impact on the human body. It alters nerve function in the brain, giving us that feeling of high while reducing coordination and reaction time. It also damages liver cells and is responsible for tens of thousands of highway accidents and about half of all traffic fatalities each year. In sufficient quantities, alcohol can kill a human being outright by suppressing neural activity, especially that of nerves that control breathing and heart beat.
Fig. 6.1:
Ethanol is a two-carbon alcohol that burns inside internal combustion engines.
On the plus side, ethanol can be burned in vehicles in place of gasoline, but only in vehicles with properly modified engines (higher compression engines with special gaskets and hoses). More commonly, alcohol is mixed with gasoline at concentrations ranging from 5-95%. As noted above, in the US, many gas stations sell a mix of 10% ethanol and 90% gasoline either throughout the year or just during the winter months. This mix helps reduce carbon monoxide pollution from cars. (Ethanol oxygenates the fuel and reduces carbon monoxide emissions.) No engine modifications are required to burn ethanol at this concentration. As of January 2009, approximately 1,900 filling stations in the US offered gasoline containing 85% ethanol (E85). Many of these are located in the Midwest, close to the source.
E85 Fueling Station Statistics
You can tell a lot about a country’s commitment to ethanol by the number of ethanol fueling stations per million people in the population. In Brazil, there are 176 stations for every million people. In Sweden, there are 130. In the US, however, there are only 6 stations for every million inhabitants.
You can also tell a lot by the percentage of registered flex-fuel vehicles and the amount of gasoline ethanol “displaces.” In Brazil, 12% of all registered vehicles are flex-fuel. In Sweden, it’s 2.9%, and in the US it’s 2.8%. Unfortunately, most people who drive flex-fuel vehicles in the US don’t fill their tanks with E85. Many are not even aware they own a flex-fuel vehicle. As for market share, ethanol’s share of the gasoline market is about 50% in Brazil, but only 4% in the US.
E85 can be burned only in vehicles with specially modified engines, known as flexible-fuel vehicles. Flexible-fuel vehicles burn two or more fuels and are also commonly referred to as flex-fuel or, occasionally, as dual-fuel vehicles. Those that operate on E85 are sometimes referred to as E85 vehicles. E85 vehicles can operate on mixtures of gasoline and ethanol, ranging from 10-85% alcohol or 100% gasoline, as noted in the sidebar.
In cold regions, the amount of alcohol in the fuel is often reduced to 50-70% during winter months to avoid cold-start problems. Reducing the ethanol content also reduces the emission of pollutants during cold starts (discussed below).
In Brazil, all gasoline contains 20-25% ethanol. Over 33,000 filling stations, however, offer a 95% alcohol-gasoline blend (known as E95) for use in flex-fuel vehicles. Some stations also sell 100% ethanol for Brazil’s large fleet of ethanol-only vehicles. These cars are equipped with high-compression engines designed to operate on pure alcohol.
Ethanol is also popular in Europe. European nations produce about 90% of the fuel they consume. The largest consumers are Germany, Sweden, France, and Spain. No country is more fond of ethanol fuel than Sweden, which boasts approximately 1,200 filling stations that dispense ethanol.
How is Ethanol Made?
Ethanol is a byproduct of the fermentation of glucose. Glucose is a six-carbon simple sugar with the chemical formula C6H12O6. It is also known as a monosaccharide. Glucose is present in fruit such as grapes. When fermented, grape juice is turned into delicious wine. The alcohol in the wine is produced from the glucose in the fruit. Glucose is also found in various seeds such as corn, wheat, and barley, which can be used to make beer and hard liquor. Commercially, corn and sugar cane are the two main sources of glucose used to make ethanol fuel. This process also relies on fermentation.
Fermentation is the partial breakdown of glucose by certain species of yeast. This reaction occurs in the absence of oxygen. During fermentation, glucose is broken down to two molecules of ethanol and two molecules of carbon dioxide. For chemically inclined readers, the reaction is this: C6H12O6 ➛ 2 C2H5OH + 2 CO2.
Ethanol can then be burned — in the chemical reaction we call combustion. During the combustion reaction, ethanol is broken down into two molecules of carbon dioxide. The reaction also releases heat, which powers vehicles. The released carbon dioxide in this reaction enters the atmosphere, where it can then be taken up by plants grown to produce more glucose used to make ethanol. In essence, then carbon dioxide produced during the combustion of ethanol is continually recycled. It’s for this reason that ethanol and other biofuels help reduce carbon dioxide emissions. In contrast, the combustion of fossil fuels releases carbon that has been stored in the Earth’s crust for millions of years, producing carbon dioxide. Carbon dioxide released during the combustion of coal, oil, oil byproducts such as gasoline, and natural gas steadily increases in the atmosphere. Rising levels of carbon dioxide, in turn, contribute to global warming.
Glucose used to make ethanol can be extracted from several larger molecules — chemists call them polymers (a polymer is a chemical made up of many smaller molecules). Two of the most common sources of glucose are starch and cellulose, both of which are produced by plants. Starch and cellulose are complex carbohydrates, known as polysaccharides. They consist of many molecules of glucose linked by chemical bonds to form long chains (
Figure 6.2). The major structural difference between starch and cellulose is the type of bonds that link the glucose molecules together. In starch, the bond is quite simple to break down. You do it all the time after ingesting starchy foods such as bread, crackers, and pasta. Enzymes in your digestive system break the bonds, releasing glucose that’s absorbed into your bloodstream. Starch can also be broken down by heat in an ethanol refinery.

Fig. 6.2:
Many glucose molecules are chemically bonded to form long chains, known as polymers. The most common source of glucose for ethanol production is starch (shown here). Efforts are now underway to tap into the glucose stored in cellulose, a much more common polymer in the plant world.
In cellulose, the bonds joining glucose molecules in the long-chain molecules are much more difficult to break down. Because you lack the enzymes required to break cellulose down, cellulose molecules in foods like celery and carrots are indigestible. We can extract nutrients from such foods, but the glucose molecules in starch aren’t available to us. (Cellulose isn’t useless, however. It forms the insoluble fiber in our diets that helps reduce colon cancer.) Cows and horses do have the enzymes to break cellulose down to produce glucose, which is why they can subsist on a diet of grasses and grains. As you may know, however, these animals don’t produce the enzymes themselves. Rather, they have bacteria in their stomachs that produce the enzymes that can digest cellulose. This results in the release of glucose molecules, which nourishes cows and horses.
Because it is easier to break starch down, very little glucose is currently produced from cellulose. Interestingly however, plants produce a lot more cellulose than glucose. Because cellulose is produced in such abundance, scientists are currently working on ways to extract glucose from this abundant resource as well as the other, smaller plant sugars, notably fructose and sucrose.
Corn vs. Sugar Cane Ethanol
In the US, 97% of all ethanol is produced from corn starch extracted from the kernels. Much of the corn is grown in the Great Plains states like Kansas, Iowa, and Nebraska.
In Brazil, ethanol is produced exclusively from sucrose extracted from sugar cane. Because sugar cane requires a tropical or subtropical climate, very little sugar cane is grown in the US. In fact, only four states have regions suitable for growing this crop: Florida, Louisiana, Texas, and Hawaii. (Louisiana recently opened its first commercial sugar cane ethanol production facility.)
Producing ethanol from sucrose extracted from sugar cane is considerably easier and more efficient than producing it from corn starch. The conversion of sucrose to ethanol primarily requires the addition of yeast, which converts the sugars to ethanol. Making ethanol from corn, in contrast, requires additional cooking and the use of special enzymes. As a result, the energy required to produce ethanol from corn is about twice that required to produce ethanol from sugar cane. The result of this is that sugar cane ethanol production has a much higher net energy efficiency than corn ethanol production — about five times higher.
As noted in Chapter 1, net energy is the amount of energy released from a fuel during combustion minus the energy required to produce it. In the case of biofuels, for example, energy is required to grow and fertilize the crop, transport it to the processing facility, produce ethanol, and ship the fuel to market.
Net energy can be thought of as energy out minus energy in. Fuels are typically compared on a closely related metric net energy efficiency basis, often expressed as the ratio of energy in to energy out — which is just another way of looking at the same thing. According to a number of studies, for instance, the net energy efficiency of corn ethanol is 1:1.3 to about 1:1.7. What this means is that every unit of energy invested in the production of corn-based ethanol yields 1.3-1.7 units of usable energy. (Although this doesn’t sound impressive, the ratio is actually higher than that of gasoline and diesel fuel.) The net energy efficiency of sugar cane ethanol, depending on the process, is a very impressive 1:8-1:10. In other words, for every unit of energy invested in sugar cane ethanol production, you get 8-10 units of energy out. If it only takes about half as much energy to produce ethanol from sugar cane, why is the net energy efficiency over five times greater?
One reason is that Brazil’s sugar cane is a high-yield crop. In fact, sugar cane is one of the most efficient photosynthesizers in the plant kingdom. (It can convert up to 2% of the incident solar energy into biomass. Although that sounds low, the figure is higher than that for most plants, which are around 1% efficient.) As a result, sugar cane yields 700-830 gallons per acre of ethanol, compared to around 400 gallons per acre from corn.
Brazil also enjoys a very long growing season, which further increases output. In addition, Brazilian producers often burn waste material from the cane fields to generate electricity to power ethanol facilities. This lowers the demand for outside energy in ethanol production facilities. (Interestingly, some of these facilities produce excess electricity that is fed into the nation’s electrical grid.) Finally, much of the labor is done by hand, too, which boosts the net energy efficiency. (However, there are rumors that slave labor is exploited to produce sugar cane in remote areas of the Amazon basin.) As a side note, other byproducts from the production of sugar cane ethanol are used to fertilize fields prior to planting, further reducing energy requirements.
Because the net energy efficiency of sugar cane ethanol is much higher than that of corn ethanol, Brazilian companies produce ethanol much more cheaply than facilities in the US. The cost to produce a gallon of ethanol from sugar cane is about $0.83 in Brazil; it costs about $1.14 to produce a gallon of ethanol from corn in the US.
Higher net energy efficiency also translates into lower greenhouse gas emissions. It is estimated that the greenhouse gas emission reduction from the use of sugar cane ethanol produced in Brazil is 86-90% when compared to gasoline. The use of corn-based ethanol in the US results in a 10-30% decrease in carbon dioxide emissions. (As you shall soon see, these estimates are pretty controversial.)
What is more, Brazilian officials are looking for ways to increase yields and cut costs even more. For example, new higher-yield strains of sugar cane are being developed. Strains resistant to pests and drought are also in the works. In 2007, Brazilian scientist reported a promising new way to increase ethanol production from yeast. They found that exposing yeast to low-frequency magnetic waves increased the amount of ethanol produced during fermentation by 17%. This treatment also speeds up the chemical reactions, lopping two hours off the standard fermentation times. (Time is money!) Although this discovery took place in the lab, scientists believe that this technique could easily be implemented in large-scale facilities.
Research is also being carried out in the US to increase the output and reduce the cost and emissions of corn-based ethanol. Companies are working on such techniques as cold starch fermentation to reduce energy requirements and boost net energy efficiency — and save money. US companies are also using waste generated during the production of ethanol to produce methane gas that can be burned at the facility to produce process heat and electricity. This could boost the net energy efficiency substantially.
Perhaps an even more important effort is the development of cellulosic ethanol, that is, producing ethanol from cellulose rather than from starch. This technique promises to achieve a much higher net energy efficiency than traditional corn-based ethanol production. (More on this shortly.)
Although ethanol production in Brazil is much less expensive, imports have been limited, in large part because of America’s powerful corn lobby — specifically those interested in bolstering domestic ethanol production. To protect their growing and still young industry, lobbyists from the corn-growing states convinced the US Congress to impose a tariff on imported Brazilian ethanol of $0.54 per gallon. Congress also provides the ethanol producers (not the farmers) a $0.51 tax credit for each gallon they produce, which is intended to stimulate domestic production.
Besides the tariff of $0.54 a gallon on imported ethanol, which limits import, Congress also passed legislation that places a 7% maximum limit on imported Brazilian ethanol. In other words, Brazil cannot export to the US more than 7% of the ethanol that was produced in the US in the previous year. Before you chafe at these seemingly unfair policies, say proponents, bear in mind that they are designed to help American farmers and manufacturers who produce our ethanol from an abundant crop: corn. Industry officials, farmers, and government officials are concerned, and rightly so, that an influx of inexpensive ethanol produced from sugar cane and other high-sugar crops might cripple or destroy America’s corn ethanol production, which is in an early stage of development.
According to supporters of US policies, Brazil still has huge amounts of land that could be converted to growing sugar cane. Were it to convert this land to sugar cane production, it’s conceivable that inexpensive Brazilian ethanol could flood the US market, driving domestic ethanol producers out of business. It’s feared that American farmers and manufacturers would be hard-pressed to compete in the ethanol market if restrictions were removed. The Brazilian government has also poured billions, some estimate about $7 billion, into the sugar cane ethanol industry since the mid 1970s — immediately after the first energy crisis. (Brazil took action to achieve energy self-sufficiency and never took its eye off the prize!)
Another reason behind the constraints on Brazilian ethanol imports is that the US is reluctant to trade its long-standing dependence on foreign oil for a new reliance on foreign renewable fuels.“We are not interested in becoming the Saudi Arabia of ethanol,” counters Eduardo Carvalho, director of the National Sugarcane Agro-Industry Union, a producers’ group. “It’s not our strategy because it doesn’t produce results. As a large producer and user, we need to have other big buyers and sellers in the international market if ethanol is to become a commodity, which is our real goal.”
The Ethanol Corridor
On October 7, 2008, the first “biofuels corridor” was officially opened along I-65, a major interstate highway in the central US. I-65 runs from northern Indiana all the way to southern Alabama. There are now more than 200 fueling stations that carry E85 along I-65. Because of this, it is possible to drive a flex-fuel vehicle from Lake Michigan to the Gulf of Mexico without being farther than a quarter tank’s worth of fuel from an E85 gas station.
Despite the lopsided playing field, Brazil still exports 160 million gallons of ethanol per year to the US.
Cellulosic Ethanol — The Rising Star
As noted earlier, cellulose is produced in abundance by plants. It’s the bulk of a tree’s trunk, for instance, and makes up a large portion of corn plants and various seed crops. In fact, cellulose is the most common organic compound on the face of the Earth. Tapping into this abundant compound could dramatically expand the amount of glucose that’s available for ethanol production in places where sugar cane production is limited by climate. Because cellulose is not digestible, cellulosic ethanol production shouldn’t threaten world food supplies either!
Cellulosic ethanol, combined with conventional starch-based ethanol, could enable manufacturers to produce fuel from the entire plant — from the stalks, leaves, and seeds of a corn plant, for instance. This would greatly increase the yields of glucose and dramatically improve the net energy efficiency of production from seed-bearing crops. In fact, it is conceivable that the net energy efficiency of ethanol production from this combined production process could rival, or even exceed, that of sugar cane ethanol.
To preserve food supplies, we could harvest seeds for consumption by humans and livestock, while the cellulosic component would be sent to ethanol plants. This would alleviate concerns over the impact of ethanol production on food supplies and rising food prices, a topic discussed in more detail in the last section of this chapter.
Some experts argue that cellulosic ethanol will simply augment, not replace, corn-based ethanol in the US. However, I believe that the future of ethanol fuel production hinges on the successful development of economical, energy-efficient cellulose ethanol production. Corn should, in my view, be primarily devoted to food production.
In the US, ethanol could be made from cellulose in “stover” — the stalks and leaves of corn plants left over after the corn cobs are removed. Cellulose from wood and straw, which is the leftover stalks of grain crops such as wheat, oats and barley, is another abundant, untapped resource. Several fast-growing and highly productive grasses, switch grass and miscanthus among them, produce enormous amounts of organic material (mostly cellulose) per acre. Hemp, kenaf, and cotton could also be good sources of cellulose. With aggressive and environmentally sensitive development of these and other sources, cellulose could surely augment, or even replace, the production of ethanol from corn. Ethanol production could also be supplemented by production from sorghum, potatoes, sweet potatoes, sugar beets, cassava, sunflowers, fruit, and molasses.
To be sustainable, ethanol production from cellulose will require careful attention to the health and vitality of the soils on which fuel crops are grown. As any agronomist will tell you, soil nutrients and organic matter are vital to the long-term health and productivity of soil. So farmers must take care to return soil nutrients and organic matter to the soil. Without careful replacement strategies, we’re simply mining our soils of their natural wealth. Scientists at the National Renewable Energy Laboratory in Golden, Colorado, estimate that two thirds of the biomass of a corn crop could be harvested without damaging the soil. One third would need to be plowed under to ensure an adequate replacement of organic matter. Inorganic nutrients could be replaced by wastes from livestock facilities or perhaps human waste treatment plants, although that can be quite a challenge.
In tropical and subtropical countries, cellulosic ethanol could supplement sugar cane-based ethanol production. The organic materials left over after sugar juices are squeezed from sugar cane stalks, known as bagasse, are currently burned to produce electricity at many refineries, as noted earlier. However, some could be used as a feedstock for cellulosic ethanol production. A dry ton of sugar cane bagasse yields 80 gallons of ethanol, which compares favorably with corn starch, which yields 98 gallons per ton.
Because cellulosic ethanol is in its embryonic stage, it’s far behind corn and sugar cane ethanol. In fact, it has only recently left the laboratory and is now being field-tested in a new facility in Louisiana. Stay tuned.
Switching to Ethanol
As noted earlier, you may be burning ethanol in your car, truck, or SUV right now because it’s frequently added to the gasoline we buy. Check the gas pump the next time you fill up. There’s usually a sticker on the pump that indicates whether the fuel contains ethanol, and how much.
If you would like to burn E85, be sure your car or truck is a flex-fuel vehicle (FFV). One of the main differences between a flex-fuel vehicle and a vehicle that burns gasoline or E10 or E15 is that in the former, hoses are made of materials that can withstand corrosive ethanol. FFVs also come with a special sensor in the fuel line. It analyzes the fuel mixture and controls the fuel injection and timing, automatically adjusting for different fuel ratios (for example, if you fill up on E10 one week, and on E85 the next).
Unfortunately, as noted earlier, many people who drive flex-fuel vehicles are unaware of the fact. In a 2005 survey, for instance, researchers found that 68% of all Americans were not aware that they owned a flex-fuel vehicle, largely because manufacturers haven’t done much to distinguish their FFVs from regular vehicles. How do you know if your car or truck is a flex-fuel vehicle?
One way is to check the gas cap. If your car is a flex-fuel car, the gas cap will tell you so. It might even be yellow, indicating that the car can be operated on E85. (Since 2006, many new flex-fuel vehicles in the US are equipped with bright yellow gas caps to signal to their drivers that theirs is an E85 vehicle.) Some gas caps clearly indicate that E85 is
not to be added to the tank. You can also call the dealership or check the owner’s manual for guidance. For a list of flex-fuel vehicles by manufacturer in the US, Europe, and Brazil, visit
e85.whipnet.net/flex.cars/.
The First FFV
The first flexible-fuel vehicle produced in the US was the 1996 Ford Taurus. It could run on E85 or methanol — M85.
In 2008, there were over 7.3 million E85 flex-fuel vehicles running on US roads, up from 5 million in 2005. Flex-fuel vehicles are becoming common, especially in the Midwest, where corn is grown and ethanol production is booming. The US government has also been purchasing flex-fuel vehicles for use in its massive fleet of 650,000 vehicles. Currently, nearly 120,000 government vehicles are flex-fuel.
If you car isn’t designed to operate on E85, when the time comes to purchase a new vehicle, consider this option. You’ll find that most automobiles and light-duty vehicles, such as vans, SUVs, and pickup trucks, are available with a flex-fuel option. Before you purchase a new vehicle, however, be sure that E85 is available in your area. To locate a gas station near you, go to the National Ethanol Vehicle Coalition’s website at
e85refueling.com/ and click on your state. You may want to check the price, too.
Interestingly, the retail price of E85 varies widely across the US. As you might expect, the lowest prices are found at gas stations in the Midwest — close to the raw materials (corn) and many ethanol refineries. In August 2008, the average spread between the price of E85 and gasoline was about 17%. But national averages are deceptive. In Indiana, the price differential was 35%. That is, ethanol was 35% cheaper than gasoline. In Minnesota and Wisconsin, two pro-renewable energy states, the price differential was 30%. In Maryland, however, the difference was 19%, and in California ethanol was 12-15% cheaper. In Utah, it was just 3% cheaper.
Price difference is important because ethanol contains about 30% less energy per gallon. So when you fill up your car with E85, you can expect to get lower mileage. If E85 is 25- 30% less expensive, you’re effectively paying the same amount as you would for gasoline. If the price differential is less, you’re paying more per mile traveled.
The Pros and Cons of Ethanol
Like any alternative fuel ethanol has its pros and cons. Before you switch to ethanol, you may want to consider these very carefully. Let’s start with the pros.
One of ethanol’s biggest advantages is that it is a renewable fuel. Moreover, it burns relatively cleanly and could help reduce the emission of the greenhouse gas carbon dioxide, helping reduce the costly social, economic, and environmental impacts of climate change. Ethanol is also produced domestically, which reduces our dependence on foreign oil and helps support farmers and rural economies. In addition, use of this fuel requires very little modification of an engine. Ethanol is also widely available in some locations and reasonably priced. Ethanol is also biodegradable. In an accident, ethanol fires, unlike gasoline ones, are easily put out with water.
On the downside, ethanol production from corn requires massive amounts of land. Corn requires lots of nutrients and, without sustainable fertilization, successive corn crops leave the soil impoverished. If it is not sustainably grown and harvested, widespread corn production to make ethanol could further deplete the valuable agricultural soils of the world.
Corn production requires massive inputs of fertilizer made from natural gas as well as pesticides made from chemicals extracted from oil. Pesticides can pollute ground water and surface waters and kill birds and other beneficial species. Sugar cane requires fewer inputs and is, reportedly, less damaging. However, habitat loss could be a problem if farmers expand their operations to feed the growing biofuels industry.
Corn ethanol also diverts food crops to fuel production and has been blamed for rising prices in the US and less developed countries like Mexico. Some analysts believe that a good part of the price increase is attributable to rising oil prices as well as a rise in the price of corn caused by rich oil sheiks in the Middle East. They reportedly drove up the price of ethanol by buying corn commodities at inflated prices in an effort to cripple the corn ethanol industry.
High ethanol blends also create problems with cold starts — starting a car on a cold day. That’s because alcohol doesn’t achieve enough vapor pressure for the fuel to evaporate and spark ignition during cold weather. To avoid this problem, which occurs at temperatures below 59°F, US and European markets have adopted E85. This is the maximum blend that can be used in flexible-fuel vehicles. In regions where temperatures drop even lower, the ethanol content is reduced to 70%. In places where temperatures drop below 10°F, it is recommended to install engine heaters (block heaters) or switch to regular gasoline during cold weather.
Brazilian flex vehicles are built with a small secondary gasoline reservoir to combat this problem. This tank is located near the engine and provides fuel to start the vehicle in cold weather. This is important for people in Brazil’s central and southern regions where temperatures drop below 59°F in the winter. Improvements in engine design launched in 2009 could eliminate the need for secondary gas storage tanks.
As noted earlier, ethanol also decreases the fuel mileage in flex-fuel vehicles because fuel economy is directly proportional to the energy content of the fuel. For E10, the effect is quite small. Studies show a very slight decrease in fuel mileage — about 3%. For E85, however, the decline becomes significant, although the performance varies depending on the vehicle. Based on EPA tests run on all 2006 E85 models, the average fuel economy for E85 vehicles dropped 25.6% compared to unleaded gasoline. If you’re paying 26% less for E85, there’s no economic tradeoff.
Engines that run on 100% ethanol, which could be used to power automobiles, trucks, tractors, and even airplanes, could help solve this problem. Ethanol-only engines have much higher compression ratios, which increase power output and improve fuel economy compared to lower-compression ratio engines in FFVs. These engines, however, are not suitable for gasoline. When ethanol is widely available, high-compression ethanol-only vehicles could be a practical alternative to FFVs. Such vehicles could achieve the same as or slightly greater fuel economy than gasoline engines.
Some studies suggest that ethanol is not as environmentally beneficial as once thought. When ethanol is burned in a car, for example, nitrogen in the air combines with oxygen in the combustion chamber to produce nitrogen oxides. Nitrogen oxides react with water in the presence of sunlight to form nitric acid, which is deposited on the land and water as acid rain and snow. Nitrogen oxides also enter a series of complex reactions that result in the formation of photochemical smog, a toxic brew of chemicals. That said, let’s not lose track of the fact that gasoline- and diesel-powered cars and trucks also produce nitrogen oxides, so the lack of other pollutants (like particulates) probably makes ethanol the better fuel.
MIT Develops Ethanol Injection System
Researchers at MIT have discovered an ingenious way to power ethanol cars and trucks. They have devised a dual-fuel direct injection system. One fuel injector injects pure alcohol into the cylinder, the other injects pure gasoline. The fuel mix is controlled by a computer based on engine performance. The fuel is injected into a turbocharged, high-compression ratio, small displacement engine. Though they have fewer cc’s, these engines perform like engines with twice the displacement.
In this design, each fuel is delivered to the engine via a separate fuel line. Rather than blending the fuel, there’s a tank for gasoline and a much smaller tank for alcohol. The engine runs on gasoline under low-power cruise conditions. Alcohol is injected into the cylinders with gasoline when required, for example when accelerating rapidly. The researchers found that this system reduces gasoline consumption and carbon dioxide emissions by 30%, producing better fuel economy than a turbo diesel or hybrid. It also solves the cold-weather starting problem.
As for global warming, studies suggest that plowing up new land to grow crops that are used to make ethanol destroys wildlife habitat and reduces the ability of the Earth’s ecosystems to incorporate carbon dioxide. (Carbon dioxide is incorporated by vegetation, such as trees and grasses.) Growing fuel crops could therefore increase global warming risks.
If crops are grown on existing farmland, however, and are generated from abundant cellulose, including various types of organic wastes, ethanol could help combat global warming by reducing atmospheric carbon dioxide levels. Stay tuned for more as we move forward.