CHAPTER 9
HYDROGEN AND HYDROGEN FUEL CELLS
Imagine a transportation fuel that could be made from water, the most abundant chemical compound on the planet. Imagine, too, that this fuel, when burned or processed, would regenerate the very same material used to make it: water. Imagine, too, that when this fuel is used to power trucks, cars, and buses, it produces no pollutants whatsoever. The fuel, of course, is hydrogen.
Not only does hydrogen come from an abundant natural resource, it has the highest energy content of any fuel known to humankind (based on weight). It has become one of the shining stars of green transportation fuels. In fact, many clean-car advocates and world leaders are pinning their hopes on the successful development of this seemingly perfect fuel. But is hydrogen all that it’s cracked up to be? Will it become a major source of transportation fuel in the future? Or will the laws of physics banish hydrogen to the scrap heap of exciting but unrealistic pipe dreams?
What is Hydrogen?
Hydrogen is the most common element in the universe, but there’s one hitch. While the element hydrogen is found in great abundance, hydrogen gas (H2) is quite rare. Sure, it is produced by a number of natural processes and is found in minute quantities in natural gas and even biogas. But because it is highly reactive, hydrogen gas is present in very limited quantity. There are no deposits of it sitting around waiting to be tapped.
Hydrogen gas is non-polluting and as safe as gasoline — maybe safer. It can also be produced just about anywhere, either from hydrogen atoms stripped from hydrocarbons like methane or from water molecules. Because hydrogen gas can be derived from water, which covers two thirds of our planet’s surface, it could be the perfect fuel.
As a gas, hydrogen is considered the ultimate “clean energy carrier.” (It’s more properly referred to as a “carrier,” rather than a fuel, because it is not naturally occurring, like coal or natural gas or oil.) It can be burned directly, for example in a furnace or a stove or even a car. Hydrogen gas can also be fed into a device known as a fuel cell, a device that, through a bit of chemical wizardry, produces electricity.
Hydrogen is currently generated in massive quantities for industry. It is used in many disparate industrial processes, such as hydrogenating vegetable oils to produce margarine and making fertilizer. Even the rockets that NASA sends into outer space rely on hydrogen. In addition, hydrogen fuel cells on the space shuttle consume hydrogen to produce the electricity that powers the vehicle’s lights, pumps, and instruments. The only emission of the onboard fuel cells is water, which is consumed by the crew.
How Hydrogen is Made into Fuel
Making hydrogen gas to use as a fuel involves stripping hydrogen atoms from various molecules, such as water. Unfortunately, it takes a lot of energy to separate hydrogen atoms from other atoms to which they are tightly bound. Once they are pried loose, hydrogen atoms quickly react with one another to form hydrogen gas.
Hydrogen can be extracted from a variety of substances besides water, including fossil fuels and some forms of organic matter. Today, manufacturers generate 40 million tons of hydrogen worldwide. Most of it comes from natural gas (methane, CH4) via reforming. In this process, natural gas is combined with steam — superhot water vapor. The reaction yields hydrogen gas and carbon dioxide.
Methane reforming is the cheapest of the half dozen or so manufacturing processes that generate hydrogen. Some consider it the most likely candidate for generating the hydrogen needed to power cars, buses, and trucks powered by fuel cells. Hydrogen can also be generated from methanol, coal, and other hydrogen-rich fuels. Unfortunately, all these processes produce significant amounts of carbon dioxide. From an environmental standpoint, extracting hydrogen from hydrocarbons is a much less desirable option than extracting it from water.
Electrolysis is the process used to strip hydrogen gas from water molecules. During electrolysis, an electric current is passed through water. This causes water molecules to split into hydrogen and oxygen gases. These molecules can then be fed into a fuel cell to generate electricity to power an electric car equipped with an onboard fuel cell.
Unfortunately, the electricity required to split water into oxygen and hydrogen has to be generated somewhere else. One simple and inexpensive way (if you ignore the environmental impacts) is to generate electricity by burning coal or natural gas. Proponents of nuclear power assert that the intense heat generated at nuclear power plants could be used to split water to make hydrogen. This option, however, is fraught with problems, among them the extremely high construction costs of nuclear power plants, the exorbitant liability in case of an accident, waste disposal, and security.
Recognizing the pitfalls of producing electricity from fossil fuels and nuclear power plants, many proponents of hydrogen have pinned their hopes on generating electricity from renewable energy technologies, such as solar electricity, wind, hydro power, geothermal, and biomass. The use of these renewable energy technologies could help render hydrogen production carbon-free — or at least dramatically reduce its carbon footprint.
Is There Enough Water to Make Hydrogen Fuel?
As noted in previous chapters, the production of biodiesel, straight vegetable oil, and ethanol is hampered — at least now — by a lack of sufficient feedstock. Put another way, the world’s farmers don’t grow enough fuel crops to generate a sufficient amount of these fuels. And even if they did, the economic repercussions on food prices could be serious. Especially vulnerable are the world’s poor, who depend on cheap grain crops to meet a good part of their nutritional needs.
To produce sufficient amounts of the biological feedstocks needed to meet the world’s astronomical transportation fuel needs, nations would need to convert vast acreages of cropland and wildlands to the cultivation of fuel crops — a move that could have serious ecological impacts. Even then, we may not be able to produce enough fuel to replace the massive quantities of gasoline, diesel, and jet fuel currently consumed by humankind’s millions of motorized vehicles. What about hydrogen? Is there enough water to yield enough hydrogen to replace the vast quantities of fossil fuel used by the transportation sector?
Actually, yes.
According to the folks at
fuelcellsworks.com, if the US converted all of its 230 million cars, pickups, vans, and SUVs to hydrogen, they could all be fueled from 310 billion gallons of water each year. While that is a huge quantity of water, it’s a drop in the bucket (pun intended) compared to our current consumption. In fact, Americans presently consume over 15 times that amount in their homes — or about 4,800 billion gallons per year — to cook, drink, shower, bathe, flush toilets, and wash clothes. US farmers consume three times
that amount to irrigate their crops each year. Coal, natural gas, and nuclear power plants use more than 200 times that amount — about 70,000 billion gallons a year. Refineries that extract gasoline from crude oil consume about 300 billion gallons of water.
Clearly, water availability wouldn’t limit hydrogen production. What is more, water wouldn’t be“consumed” in the process because, as previously noted, hydrogen recombines with oxygen to form water in fuel cells.
What is a Fuel Cell?
Although hydrogen can be burned directly — and cleanly — many experts and government officials believe that the greatest hope for the transportation sector rests on the use of fuel cells. But just what is a fuel cell?
A fuel cell is a rather simple-looking device that combines hydrogen and oxygen to produce electricity. The electricity is then fed to an electric motor that propels a car forward (
Figure 9.1).
A fuel cell is an electrochemical device. That is, it produces electricity from fuel, specifically hydrogen and oxygen. The typical fuel cell consists of hundreds of smaller individual fuel cells. They are connected to one another in series, a type of electrical connection that increases voltage. (Each cell puts out about 0.5 to 7.0 volts, depending on current draw). Larger currents are produced by increasing the area of the individual cells.
Fig. 9.1:
See text below for an explanation of how a fuel cell works.
Although there are several types of fuel cells, they all contain an electrolyte sandwiched between two thin electrodes: an anode and cathode. An anode is a negatively charged electrode; a cathode is a positively charged electrode.
In a hydrogen fuel cell, hydrogen is piped into the fuel cell at the anode, which is made of a catalyst, usually a platinum group metal or alloy. (Catalysts are chemicals that speed up chemical reactions without undergoing any chemical change themselves.) Oxygen, which is usually supplied from air, enters at the cathode, which is also made from a catalyst.
At the anode, hydrogen atoms split into negatively charged electrons and positively charged protons. (Remember, a hydrogen atom consists of a single positively charged proton in the nucleus and a negatively charged electron that orbits the nucleus.)
In polymer electrolyte membrane fuel cells (PEM), one common type of fuel cell, positively charged protons move from the anode through the electrolyte to the cathode. When the electrons, protons, and oxygen meet at the cathode side, they combine, producing water and heat.
The movement of the electrons is an important part of the process. As shown in
Figure 9.1, electrons are forced to pass from the anode through an external circuit, rather than travelling through the electrolyte. They travel directly from the anode to the cathode by an electrical connection. This flow of electrons creates an electrical current. The energy these electrons “carry” is used to power loads connected to the circuit. (An electrical load is any electrical device that uses electricity.) Because much of the energy the electrons carry is dissipated in loads, the electrons that make it to the cathode are largely de-energized. At the cathode, these electrons recombine with protons and oxygen to produce water. It’s all pretty simple — if you have a PhD in electrochemistry, that is.
To summarize, in a hydrogen fuel cell vehicle the anode catalyzes the dissociation (splitting) of hydrogen molecules. This reaction releases the electrons and protons. The electrons flow via an external circuit to the cathode, creating the electrical current. It’s this current that’s used to power the electric motor that propels the vehicle forward as well as all the smaller loads, such as lights and radio. At the cathode, the electrons, protons, and oxygen react to reform water. Thus direct hydrogen fuel cells produce pure water as their only emission. (For information on other types of fuel cells, see
fuelcellsworks.com.)
Hydrogen Cars, Trucks, Vans, and SUVs
You may be surprised to learn that numerous vehicles — including buses, trains, golf carts, bicycles, motorcycles, ships, planes, and even submarines — have been built to run on hydrogen. There’s even a hydrogen-powered wheelchair! Hydrogen vehicles can be powered directly by hydrogen by burning it in an internal combustion engine similar to those in conventional gasoline-powered vehicles or indirectly by hydrogen via fuel cells, as just described.
In fuel cell vehicles powered by hydrogen, hydrogen is fed into a fuel tank at a special filling station (there are only a few in the US, and they’re only for experimental vehicles now on the road). Here, the hydrogen is stored under pressure. When the car is turned on, hydrogen flows from the fuel tank to the fuel cell. The fuel cell generates electricity that is fed to the electric motor.
The Pros and Cons of Fuel Cell Vehicles
Fuel cells and fuel cell vehicles offer many advantages over conventional gasoline or diesel-powered vehicles, many of which have been discussed or mentioned earlier in the chapter. Here’s a quick summary:
• Fuel cells are powered by the most abundant element on Earth: hydrogen. Hydrogen can be extracted from one of the most abundant chemical compounds: water. Either fresh or salt water can be used. (The electrolysis of seawater is currently a source of chlorine used in industry; the hydrogen produced by this process could also be captured and put to use.)
• The amount of water required is only a small portion of the water already consumed by society.
• Hydrogen could be generated anywhere, so long as there is a supply of electricity and water. (Solar-powered electrolysis units at filling stations could make hydrogen widely available.)
• Hydrogen fuel cells “burn” cleanly and emit only water. (Fuel cells powered by hydrocarbons such as methanol produce water and carbon dioxide.)
• With proper safety measures, hydrogen can be safely stored.
• Pound for pound hydrogen is the most energy-dense fuel available to humankind.
On the downside, hydrogen gas does not appear naturally in great quantities. Large supplies of it would have to be manufactured.
Although pound for pound hydrogen is the most energy-dense fuel available, hydrogen is a gas, so, volumetrically, it is a low-density fuel. Therefore, it is difficult to store enough hydrogen in a tank to generate the same amount of energy available from conventional liquid fuels, such as diesel and gasoline. This poses a problem for drivers who want to travel 300-500 miles on a single tank of fuel. Researchers currently store hydrogen in vehicles in high-pressure tanks.
Another problem is the lack of infrastructure — for example, fueling stations. For hydrogen to be feasible, some argue that we’d need to create a massive delivery system throughout the world. This, in turn, would require a significant investment in time and money. Costs for large-scale deployment would be substantial, although hydrogen could be generated locally at filling stations by small-scale electrolyzers. (Storing significant amounts of hydrogen at local stations, however, could be problematic.)
Another downside of hydrogen fuel cell vehicles is that they can’t operate at temperatures below freezing (32°F). Water in the fuel cell solidifies if the cell and its contents are not kept above the freezing point. However, Honda and GM claim they have developed a fuel system that can operate at -20°F.
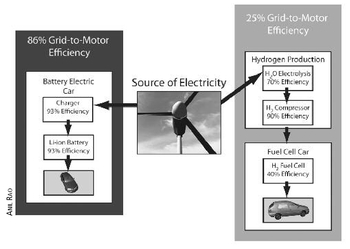
The most significant downside of hydrogen — and the factor mostly likely to eliminate it from the mix of green fuels — has to do with efficiency. According to several studies, it is three to four times more efficient to run a vehicle on electricity directly than it is to use electricity to generate hydrogen gas from water to feed a fuel cell to make electricity to power a vehicle. As you can see in
Figure 9.2, the grid-to-motor efficiency of an electric car is 86%. The grid-to-motor efficiency of a hydrogen fuel cell car is only about 25%.
More than anything else, this fundamental problem is likely to limit hydrogen as a fuel source. Why go through all the steps of making hydrogen when you can power a car directly from electricity at a much higher efficiency? And why bother, when we already have the technology to meet 90% of all Americans’ daily transportation needs with 60-mile range electric vehicles? And why spend millions developing hydrogen, when hybrid or plug-in hybrid technology can satisfy practically all other transportation needs?
In summation, while hydrogen-powered vehicles may seem like a wonderful idea, chances are the technology will never gain prominence unless scientists find ways to generate hydrogen with little or no energy input. Those damn laws of physics could banish hydrogen and hydrogen fuel cell vehicles to the scrap heap of exciting but unrealistic pipe dreams.
Fig. 9.2:
The most fundamental problem with hydrogen fuel cell vehicles is their inefficiency, as shown here. Bottom line: unless we can find a way to produce hydrogen that requires little energy (good luck!), it makes much more sense to use electricity directly to power a vehicle than to use it to split water to make hydrogen.