CHAPTER THREE
Telomerase, the Enzyme That Replenishes Telomeres
Not long after I (Liz) had read the X-ray revealing the DNA of telomeres, I was hired by the University of California, Berkeley, where in 1978 I set up my own laboratory to continue my research into telomeres. There I began to notice something that shocked me. I was still growing Tetrahymena, that hairy, Muppet-like pond scum, and now I was able to tell the sizes of their telomeres from the length of their DNA. And mysteriously, under some conditions, the Tetrahymena’s telomeres would sometimes grow.
This was a shock because I expected that if telomeres were going to change at all, they were going to get shorter, not longer: with each cell division, the number of DNA sequences in the telomeres would more likely shrink. Yet it looked to me as if Tetrahymena was creating new DNA. But this was not supposed to happen. DNA is not supposed to change. You’ve probably heard that the DNA we are born with is the DNA we die with, and that DNA is produced solely through a kind of biochemical photocopying. I checked, and double-checked, and confirmed that what was supposed to be impossible was, in fact, happening. Next, we saw the same thing happening in yeast cells, too. (“We” here included my student Janice Shampay in my lab, working on the experiments that Harvard researcher Jack Szostak and I had dreamed up together.) Then reports from other scientists trickled in that suggested these changes might happen in other tiny, Tetrahymena-like creatures, too. The organisms were, in fact, producing new DNA at the ends of their telomeres. Their telomeres were growing.
No other element of DNA behaves in this way. For decades, genetic scientists believed that any stretch of chromosomal DNA existed only because it had been copied from preexisting DNA. The accepted wisdom was that DNA could not be created from whole cloth where there had not been DNA before. The discovery of this odd behavior told me there was something going on here that no one had seen before. For scientists, that’s one of the most exciting kinds of discoveries to make. It’s thrilling when a strange finding suggests that there are new, unknown street corners of the universe, ripe for exploration. As it turned out, this behavior of telomeres led to more than just a new street corner of the universe; this was a whole new neighborhood, one that no one had known existed.
TELOMERASE: THE SOLUTION TO TELOMERE SHRINKAGE
I kept pondering this strange behavior of the telomere, its apparent ability to grow. I wanted to look for an enzyme in a cell that might add DNA onto telomeres—an enzyme that might replenish telomeres after they’d lost some of their pairs of letters. It was time for me to roll up my sleeves and make more Tetrahymena cell extracts. Why Tetrahymena? Because it’s such a good source of plentiful telomeres. I reasoned that it might be a good source of enzymes that could form telomeres, if such an enzyme existed.
In 1983 I was joined in this quest by Carol Greider, a new graduate student in my lab. We began devising experiments, and then refining those experiments, and on Christmas Day in 1984, Carol developed an X-ray film called an autoradiograph. The patterns on that film showed the first clear signs of a new enzyme at work. Carol went back home and danced with excitement in her living room. The next day, her face alight with suppressed glee in her anticipation of my reaction, she showed me the X-ray film. We looked at each other. Each of us knew this was it. Telomeres could add DNA by attracting this previously undiscovered enzyme, which our lab named telomerase. Telomerase creates new telomeres patterned on its own biochemical sequence.
But science does not work only by the exhilaration of one single eureka moment. We had to be sure. As the weeks stretched into months, we experienced surges of doubt followed by thrills of joy as we painstakingly conducted follow-up experiments. Step by step, we ruled out every possible reason that our exciting first moments in 1984 could have been just a false lead. Eventually, a deeper understanding of telomerase emerged: Telomerase is the enzyme responsible for restoring the DNA lost during cell divisions. Telomerase makes and replenishes telomeres.
Here’s how telomerase works. It includes both protein and RNA, which you can think of as a copy of DNA. That copy includes a template of the telomere’s DNA sequence. Telomerase uses that sequence in the RNA as its own inbuilt biochemical guide to create the right sequence of brand-new DNA. The right sequence is needed to make a DNA scaffold perfectly shaped to attract a sheath of telomere-protective proteins that cover the telomeric DNA. This new segment of DNA is added by telomerase to the end of the chromosome, guided by the RNA template sequence and the DNA’s buddy partner system of pairing up its letters. This ensures that the right sequence of building blocks of telomeric DNA is added. In this way, telomerase re-creates new endings at the chromosome’s tips and replaces ones that have been worn down.
The mystery of the growing telomeres was solved. Telomerase replenishes telomeres by adding telomeric DNA to them. Each time a cell divides, telomeres gradually shorten until they reach a crisis point that signals cell division to stop. But telomerase counteracts this telomere shortening by adding DNA and building back the chromosome end each time a cell divides. This means that the chromosome itself is protected, and an accurate copy of it is made for the new cell. The cell can continue to renew itself. Telomerase can slow, prevent, or even reverse the shortening of telomeres that comes with cell division. Telomeres can, in a sense, be renewed by telomerase. We had found a way to get around the Hayflick limit of cell division… in pond scum.
TELOMERASE: NO ELIXIR OF IMMORTALITY
After these discoveries, both the scientific world and the global media buzzed with hopeful speculation. What if we could increase our supply of telomerase? Could we be like Tetrahymena, with cells that renew forever? (This may have been the first recorded instance of humans fervently wishing to be more like pond scum.)
People wondered if telomerase could be distilled and served up as an elixir of immortality. In this wishful scenario, we’d visit our local telomerase bar every now and then for a hit of the enzyme, which would let us live healthy lives all the way to the very end of the known maximum human life span—or beyond it.
These dreams are perhaps not as ridiculous as they might seem. Telomeres and telomerase form a crucial biological foundation for cell aging. The demonstration of the relationship between telomerase and cell aging first came from Tetrahymena. Guo-Liang Yu, then a graduate student in my Berkeley lab, performed a simple but surgically precise experiment. He replaced the normal telomerase in Tetrahymena cells with a precisely inactivated version. If you feed them properly, Tetrahymena cells are normally immortal in the laboratory. Like the Energizer Bunny, Tetrahymena’s cell divisions normally just keep going and going and going. But this inactivated telomerase caused the telomeres to become shorter and shorter as the Tetrahymena cells divided. Then when the telomeres had become too short to protect the genes inside the chromosome, the cells stopped dividing. Think again of a shoelace. It’s as though the shoelace tip wore down and the shoelace—with all that vital genetic material—became frayed. Inactivating telomerase made the Tetrahymena cells mortal.
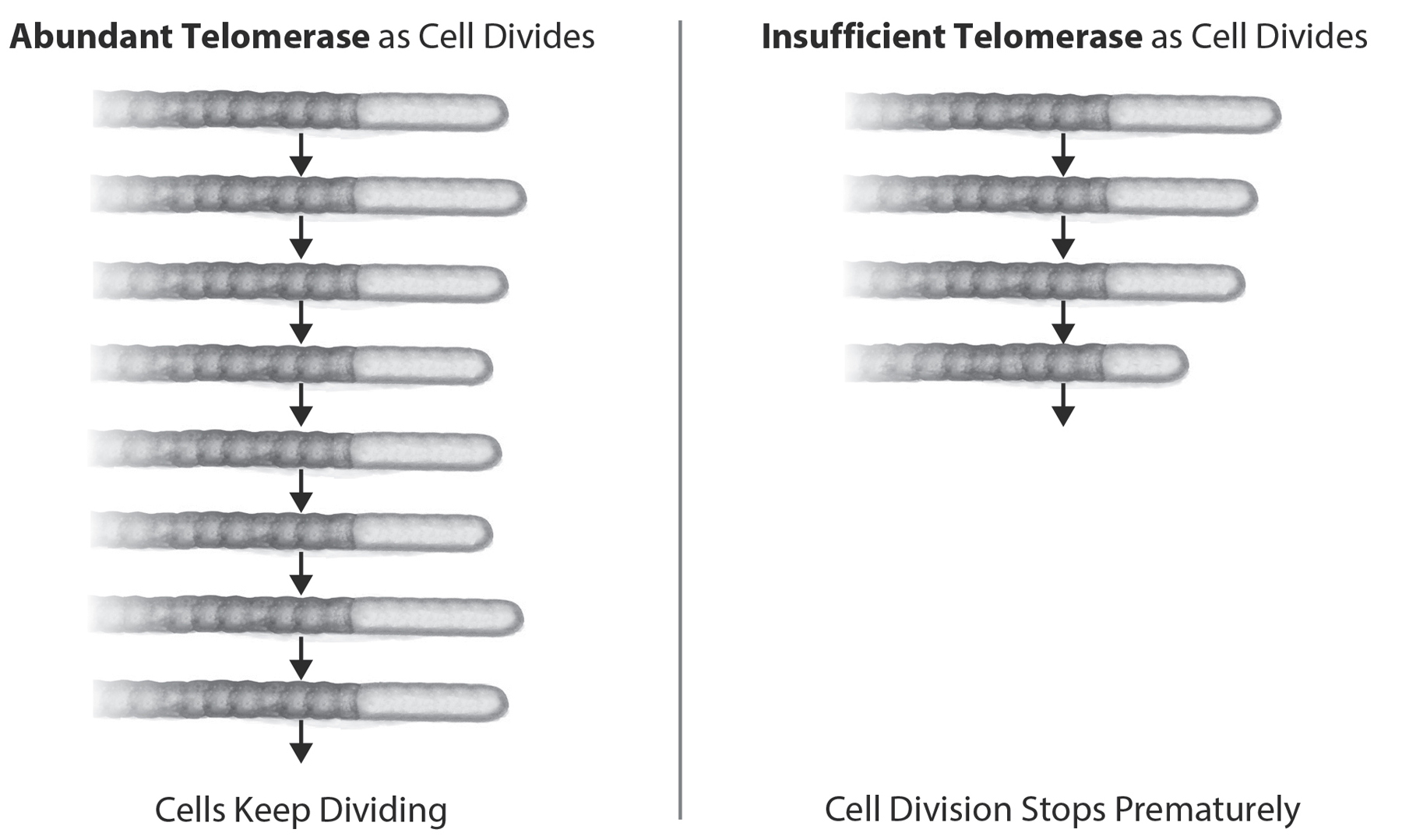
Figure 11: Consequences of Enough, or Not Enough, Telomerase Action. Telomere DNA shortens because the enzymes to duplicate the DNA don’t work at the telomere ends (incomplete DNA replication). Telomerase elongates telomeres and thus counterbalances the inexorable attrition of telomeric DNA. With abundant telomerase, telomeres are maintained and cells can keep dividing. With insufficient telomerase (due to genetics, lifestyle, or other causes) telomeres shorten rapidly, cells stop dividing, and senescence soon follows. Reprinted with permission from AAAS (Blackburn, E., E. Epel, and J. Lin., “Human Telomere Biology: A Contributory and Interactive Factor in Aging, Disease Risks, and Protection,” Science (New York) 350, no. 6265 (December 4, 2015): 1193–98).
Without telomerase, the cells stop renewing.
And then, at other labs around the world, the same was found for nearly all cells, except bacteria (whose chromosomes are circles of DNA instead of lines and thus have no ends to protect). Longer telomeres and more telomerase delayed premature cellular aging, and shortened telomeres and less telomerase sped it up. The telomerase-health connection was nailed when clinician Inderjeet Dokal and his colleagues in the United Kingdom and the United States first discovered that when people have a genetic mutation that slashes telomerase levels in half, they develop severe inherited telomere syndromes.1 This is the same category of disease that was diagnosed in Robin Huiras. Without sufficient telomerase, the telomeres quickly shorten, and the body succumbs to early disease.
Tetrahymena cells have telomerase in sufficient quantities so that they can constantly rebuild their telomeres. This allows Tetrahymena to perpetually renew itself and to forever avoid cell aging. But we humans normally don’t have enough telomerase to accomplish this feat. We are very miserly when it comes to telomerase. Our cells are reluctant to hand out telomerase willy-nilly to their telomeres all the time. We produce telomerase in sufficient quantities to rebuild telomeres… but only up to a point. As we age, the telomerase in most of our cells generally becomes less active, and telomeres get shorter.
TELOMERASE AND THE CANCER PARADOX
It’s natural to wonder if we could extend human life through artificial methods of increasing telomerase. Ads for telomerase-boosting supplements abound on the Internet claiming that we can. Telomerase and telomeres have wonderful properties that can allow us to avoid horrible diseases and feel more youthful. But they are not magical life extenders—they don’t let us live past the normal human life span as we know it. In fact, if you try to extend your life by using artificial methods of increasing telomerase, you are putting yourself in danger.
That’s because telomerase has a dark side. Think of Dr. Jekyll and Mr. Hyde—they are the same person, but one with a drastically different character depending on whether it is day or night. We need our good Dr. Jekyll telomerase to stay healthy, but if you get too much of it in the wrong cells at the wrong time, telomerase takes on its Mr. Hyde persona to fuel the kind of uncontrolled cell growth that is a hallmark of cancer. Cancer is, basically, cells that won’t stop dividing; it’s often defined as “cell renewal run amok.”

Figure 12: Telomere-Related Genes and Disease. Telomere maintenance genes can protect us from common diseases, but can put us at risk for some cancers. Having gene variants for more telomerase and telomere proteins means longer telomeres. This natural genetic way of making telomeres longer lowers risks for most diseases of aging, including heart disease and Alzheimer’s disease, but the high telomerase also means that cells that are prone to become cancerous can keep dividing unchecked, causing a greater risk for certain types of cancer (brain cancers, melanoma, and lung cancers). Bigger isn’t always better!
You don’t want to bomb your cells with artificial telomerase that may goad them into taking the road toward becoming cancerous. Unless the telomerase supplement field comes up with more thorough demonstrations of safety in large—and long-term—clinical trials, in our view it’s sensible to skip any pill, cream, or injection that claims it will increase your telomerase. Depending on your individual propensity for different types of cancer, you may be potentially increasing the chance of developing any of a number of different cancers (such as melanomas or brain and lung cancers). Knowing this, it comes as no surprise that our cells keep their telomerase on a tight rein.
Given these scary-sounding findings, you may be wondering, why are we suggesting activities that boost telomerase? The answer is that there is a big difference between the body’s normal physiological responses to the lifestyle suggestions we make for your health in this book and taking an artificial substance (no matter how “natural” its plant source—remember that plants are some of nature’s biggest chemical warfarers, having evolved an armamentarium of strong chemicals to fend off hungry animals and marauding pathogens). The suggestions we include in this book for increasing your telomerase action are gentle and natural—and they increase telomerase in safe amounts. You do not need to worry about an increased cancer risk with these strategies. They simply don’t increase telomerase to the levels or in the ways that would be harmful.
Paradoxically, we do need to keep our telomeres healthy to ward off cancer, too. Some types of cancers are more likely to develop when too little available telomerase makes telomeres too short—blood cancers like leukemia, skin cancers besides melanoma, and some gastrointestinal cancers such as pancreatic cancer. This was proved with the discovery that people born with a mutation that precisely inactivated a telomerase gene had much higher risk for these cancers. Such cancers arise because losing telomere protection allows our genes to become more easily damaged—and altered genes can eventually lead to cancers. Furthermore, too little telomerase weakens the telomeres in our immune cells. Our immune system usually keeps a sharp eye out for anything perceived as “foreign,” and that includes harmful cancer cells as well as pathogenic invaders from the outside such as bacteria and viruses. Without telomeres long enough to act as buffers, the cells of the immune system will eventually become senescent.
Some of these immune cells are like surveillance cameras posted at every corner of the body. If they become senescent, their lenses then act as though they are steamed up, and they miss the “foreign” cancer cells. So the teams of immune cells that would normally be called up fail to leap into action. The result of weakened telomeres is that the immune defenses of the body are more likely to lose the fight against a cancer (or a pathogen).
The key is to well regulate the action of telomerase on telomeres—in the right cells and at the right times, because only that will keep telomeres and us healthy. The body knows how to do this, and we can help it with a lifestyle full of renewal strategies.
YOU CAN INFLUENCE YOUR TELOMERES AND TELOMERASE
By the turn of the millennium, scientists had become accustomed to thinking about both telomeres and telomerase as foundations of cell renewal. But the telomere syndromes, starting with the shocking finding that cutting telomerase by merely half could have such drastic effects, had galvanized everyone into thinking only in terms of genes that determined whether our telomeres were long or short, and whether we had enough telomerase to replenish worn-down telomeres.
That was when I (Elissa) began a postdoctoral fellowship in health psychology at the University of California, San Francisco. Susan Folkman, the now-retired director of the Osher Center for Integrative Medicine and a pioneer in the study of stress and coping, invited me to join a team that was interviewing mothers of children with chronic conditions, a group under tremendous psychological strain.
I felt a profound empathy for these caregiving mothers, who seemed extraordinarily worn out and older than their chronological ages. By then, Liz had moved to the San Francisco campus of the University of California, and I was aware of her work on biological aging. I approached Liz and told her about the caregiving mothers we were studying. If I could come up with the funds, would it be possible to test the mothers’ telomeres and telomerase? Was it worth investigating whether stress could shorten telomeres and lead to early cell aging?
Like most other molecular biologists at the time, I (Liz) was peering down at telomeres from one particular mountaintop. I was thinking about our telomere maintenance in terms of the cellular molecules specified by the genes that control telomeres. When Elissa asked me about studying caregivers, however, it was as though I suddenly saw telomeres from a whole new viewpoint, from a completely different mountain. I responded as both a scientist and a mother. “We need another ten years just to more fully understand the genetics of telomeres,” I mused, somewhat doubtfully, but I could also well imagine the tremendous stresses these women were under. I thought about the way we describe exhausted, stressed people: careworn. Mothers of chronically sick children are women who are worn down. Was it possible that their telomeres were worn down, too? “Yes,” I agreed. “Let’s do this study, if we can find a scientist in my lab who will help with the measurements.” My postdoctoral fellow, Jue Lin, raised her hand. She proceeded to refine a way to sensitively and carefully measure telomerase in healthy human cells, and the work began.
We selected a group of mothers who were each caring for a chronically ill biological child. A research subject who might have an outside “issue” could warp the results, so any mother with a major health problem was screened out of the study. We used a similar process to select a control group of mothers whose children were healthy. This process took several years of careful selection and assessments.
We took a sample of each woman’s blood and measured the telomeres in the white blood cells. We recruited the help of Richard Cawthon at the University of Utah, who had recently devised a new easier way to measure the length of telomeres in white blood cells (applying a method called polymerase chain reaction).
One day in 2004, the assay results came in. I (Elissa) was sitting in my office as the numerical analysis came out of the printer. I looked down at the scatter plot and gasped. There was a pattern to the data, the exact gradient we thought might exist indeed was right there, on the page. It showed that the more stress you are under, the shorter your telomeres and the lower your telomerase levels.
I immediately picked up the phone and called Liz. “The results are in,” I said, “and the findings are even more striking than we’d thought they might be.”
We’d asked the question Can the way we live change our telomeres and telomerase? Now we had an answer.
Yes.
Yes, the mothers who perceived themselves to be under the most stress were the ones with the lowest telomerase.
Yes, the mothers who perceived themselves to be under the most stress were the ones with the shortest telomeres.
Yes, mothers who had been caregiving for the longest time had shorter telomeres.
This triple yes meant that our results weren’t just a coincidence or a statistical blip. It also meant that our life experiences, and the way we respond to those events, can change the lengths of our telomeres. In other words, we can change the way that we age, at the most elemental, cellular level.
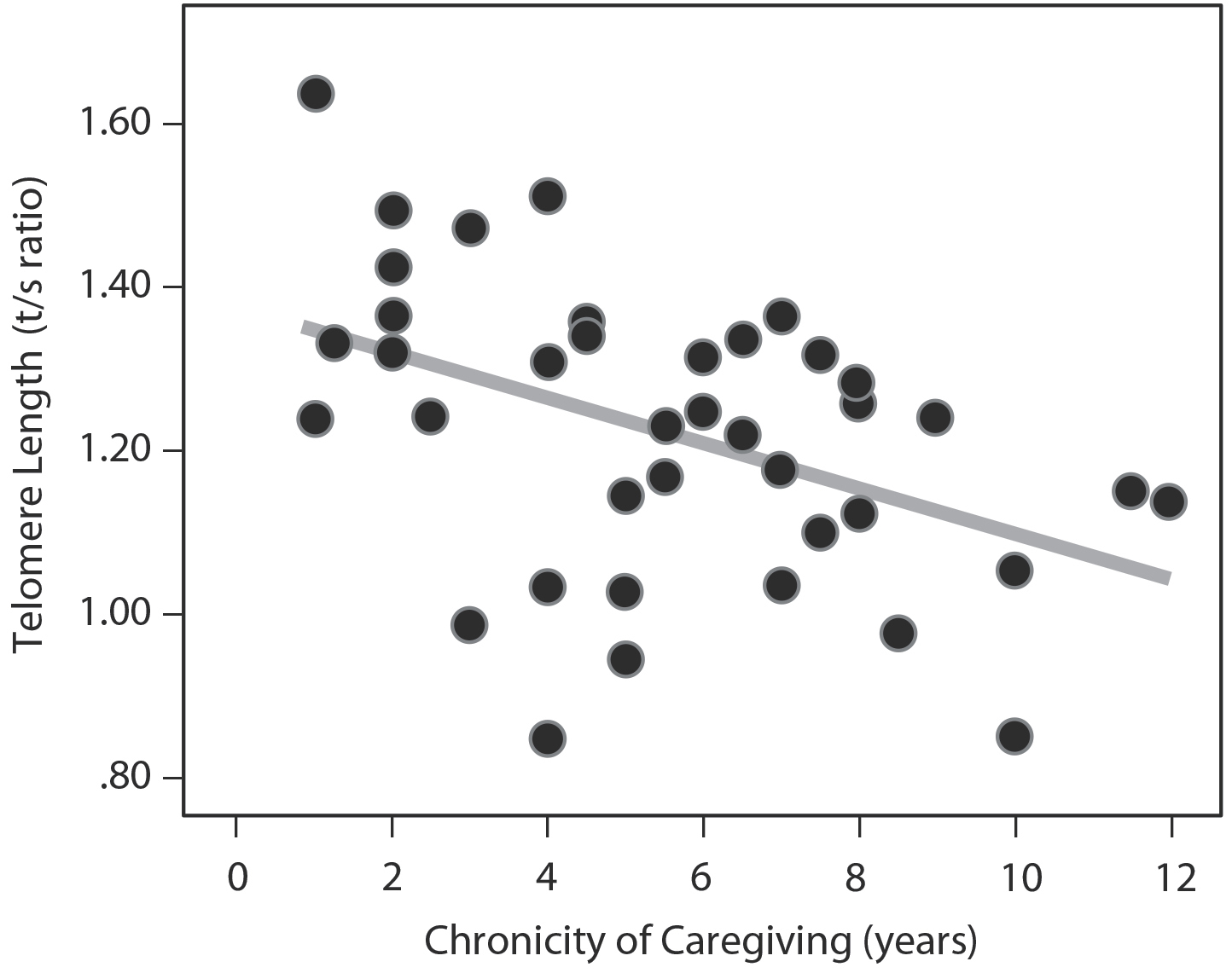
Figure 13: Telomere Length and Chronic Stress. The more years since the child had been diagnosed (thus the more years of chronic stress), the shorter the telomeres.2
Whether aging can be sped up, slowed, or reversed has been a topic of medical debate for centuries. What we have learned since this first study of caregivers is wholly new. We, as a field, have learned that by our actions, we can keep our telomeres—and hence our cells—from aging prematurely. We may even be able to partly reverse the cellular aging process caused by telomere wear and tear. Over the years, the results of our initial study have held up, and many additional studies that you will read about here have taken this first finding much further, showing that many different life factors can affect our telomeres.
In the rest of this book you will hear us talking about how you can increase telomerase and protect your telomeres. Our recommendations are based on studies, some that measure telomeres, some that measure telomerase activity, and some that do both. You can join us on the journeys of exploration that have followed from those first mountaintop views. Use this research as a North Star to help you change the way you use your mind, take care of your body, and even how you interact with your community, to protect your telomeres and enjoy your healthspan.
