CHAPTER FOUR
Unraveling: How Stress Gets into Your Cells
We explore the stress-telomere connection, explain toxic stress versus typical stress, and show how stress and short telomeres affect the immune system. People who respond to stress by feeling overly threatened have shorter telomeres than people who face stress with a rousing sense of challenge. Here, you’ll learn how to move from harmful stress responses to helpful ones.
Nearly fifteen years ago, my husband and I (Elissa) were driving across the country. We had just finished graduate school at Yale and were taking on postdoctoral fellowships in the Bay Area. San Francisco is an expensive city, and so we had arranged to live with my sister and her family. We expected that when we arrived in San Francisco, we would meet our new nephew, who was supposed to be born at any moment. In fact, he was quite overdue. I called every day for news, but I’d had trouble reaching anyone in the family for days.
About halfway through the trip, just after we’d passed Wall Drug Store in South Dakota, my cell phone finally rang. Tearful voices wavered on the other end. The baby had been born, but something had gone terribly wrong during an induced delivery. The baby was on life support and being fed through a gastric tube to his stomach. He was a beautiful healthy boy, but an MRI showed his brain had been profoundly damaged. He was paralyzed, blind, and wracked with seizures.
Eventually, after several months, the baby left the intensive care ward and came home. We joined the family team to help take care of this little guy, who had extraordinary needs. We became intimate with both the demands and sorrows that come with a life of caregiving. We were accustomed to pressure and hard work, but this had nothing in common with the types of stresses we had known. Now there were new feelings of constant vigilance, intermittent urgency, worry about the future, and most of all, a heavy weight on the heart. One of the hardest parts was seeing and feeling the pain my sister and brother-in-law were experiencing every day. On top of the emotional suffering there was, all of a sudden, a new, unexpected, and demanding life centered around medical caregiving.
Caregiving is one of the most profound stresses a person can experience. Its tasks are emotionally and physically demanding, and one reason caregivers get so worn out is that they don’t get to go home from their caregiving “jobs” and recover. At night, when we all need to biologically check out and refresh body and mind, caregivers are on call. They may be repeatedly woken from sleep to respond to someone in need. Caregivers rarely have time to take care of themselves. They skip their own doctor appointments as well as opportunities for exercise and going out with friends. Caregiving is an honorable role that is taken on out of love, loyalty, and responsibility, but it is not supported by society or recognized for its value. In the United States alone, family caregivers perform an estimated $375 billion in unpaid services each year.1
Caregivers often feel unappreciated and become isolated. Health researchers have identified them as one of the most chronically stressed groups of people. This is why we often ask caregivers to volunteer for our studies on stress. Their experiences can tell us a lot about how telomeres react to serious stress. In this chapter, you’ll learn what our groups of caregivers have taught us—that chronic, long-lasting stress can erode telomeres. Fortunately for all of us who cannot escape chronic stress (and for all of us who scored higher than 12 on the stress assessment here), we’ve also learned that we can protect our telomeres from some of stress’s worst damage.
“LIKE THERE IS AN ASSAILANT, WAITING FOR ME”: HOW STRESS HURTS YOUR CELLS
In our very first study together we looked at some of the most highly stressed caregivers of all: mothers who were taking care of their chronically ill children. This is the study we’ve told you about. It’s the one that first revealed a relationship between stress and shorter telomeres. Now we want to show you a close-up look at the extent of that damage. More than ten years later we still find it sobering.
We learned that the years of caregiving had a profound effect, grinding down the women’s telomeres. The longer a mother had been looking after her sick child, the shorter her telomeres. This held true even after we took into account other factors that might affect telomeres, like the mother’s age and body mass index (BMI), which are related to shorter telomeres themselves.
There was more. The more stressed out the mothers felt, the shorter their telomeres. This was true not just for the caregivers of sick children, but also for everyone in the study, including the control group of mothers who had healthy children at home. The high-stress mothers also had almost half the levels of telomerase than the low-stress mothers, so their capacity to protect their telomeres was lower.
People experience stress in many different ways: “like a fifty-pound weight on my chest,” “like a knot in my stomach,” “like a vacuum in my lungs that doesn’t let me take a full breath,” “my heart pounds like there is an assailant, waiting for me.” These metaphors are grounded in the body, because stress is as present in the body as in the head. When the stress-response system is on high alert, the body produces more of the stress hormones cortisol and epinephrine. The heart beats faster and blood pressure increases. The vagus nerve, which helps modulate the physiological reaction to stress, withdraws its activity. That’s why it’s harder to breathe, harder to stay in control, harder to imagine that the world is a safe place. When you suffer from chronic stress, these responses are on a low but constant alert, keeping you in a state of physiological vigilance.
In our caregivers, several aspects of the physiological stress response, including lower vagus activity during stress, and higher stress hormones while sleeping, were linked to shorter telomeres or to less telomerase.2 These responses to stress appeared to be accelerating the biological aging process. We had discovered a new reason that stressed-out people look haggard and get sick: their heavy stresses and cares are wearing down their telomeres.
HOW MUCH STRESS IS TOO MUCH?
Stress is unavoidable. How much of it can we handle before our telomeres are damaged? A consistent lesson from the past decade of studies—and a lesson that echoes what the caregivers taught us—is that stress and telomeres have a dose-response relationship. If you drink alcohol, you’re familiar with dose and response. An occasional glass of wine with dinner is rarely harmful to your health and may even be beneficial, as long as you’re not drinking and driving. Drink several glasses of wine or whiskey, night after night, and the story changes. As you “dose” yourself with more and more alcohol, the poisonous effects of alcohol take over, damaging your liver, heart, and digestive system and putting you at risk for cancer and other serious health problems. The more you drink, the more damage you do.
Stress and telomeres have a similar relationship. A small dose of stress does not endanger your telomeres. In fact, short-term, manageable stressors can be good for you, because they build your coping muscles. You develop skills and confidence that you can handle challenges. Physiologically, short-term stress can even boost your cells’ health (a phenomenon called hormesis, or toughening). The ups and downs of daily life are usually not wearing to your telomeres. But a high dose of chronic stress that wears on for years and years will take its toll.
We now have evidence that links particular kinds of stress to shorter telomeres. These include long-term caregiving for a family member and burnout from job stress. As you may imagine, more serious traumas, both recent and in childhood, have also been linked to damaged telomeres. These traumas include rape, abuse, domestic violence, and prolonged bullying.4
Of course, it’s not the situations themselves that produce the short telomeres; it’s the stress responses that many people feel when they’re in these situations. And even under these stressful circumstances, dose matters. A monthlong crisis at work can be stressful, but there’s no reason to think your telomeres will take a hit. They are more robust than that; otherwise, we’d all be falling apart. (A recent review showed that there is a relationship between short-term stress and shorter telomeres, but that effect is so tiny that we don’t think it will have a meaningful effect on an individual person.5 And even if short-term stress shortens your telomeres, the effect is likely temporary, with telomeres quickly recovering their lost base pairs.) But when stress is an enduring, defining feature of your life, it can act as a slow drip of poison. The longer the stress lasts, the shorter your telomeres. It is vitally important to get out of long-term, psychologically toxic situations if it’s at all possible.
But fortunately for the many of us who live with stressful situations we cannot change, that’s not the whole story. Our studies have shown that being under chronic stress does not inevitably lead to telomere damage. Some of the caregivers we’ve studied were weathering enormous burdens without losing telomere length. These stress-resistant outliers have helped us understand that you do not necessarily have to escape difficult situations to protect your telomeres. Incredible as it sounds, you can learn to use stress as a source of positive fuel—and as a shield that can help protect your telomeres.
DON’T THREATEN YOUR TELOMERES—CHALLENGE THEM
When we looked at the data for our first caregiver study, we realized we had a mystery on our hands. Some of the caregiving mothers in the group reported less stress, and these mothers had longer telomeres. We wondered: Why would they feel less stress? After all, they had been caregiving for just as long as the other mothers in the group. They had a similar number of daily duties and spent just as many hours in the day performing those duties (appointments, administering injections and other treatments, managing tantrums, having to hand- or tube-feed, diaper, and bathe older disabled children).
To understand what was protecting these mothers’ telomeres, we wanted to see people respond to stress in real time, before our eyes. We decided to bring more women into the lab and, essentially, stress them out. Research volunteers who arrive at our stress lab are told something like, “You’re going to perform some tasks in front of two evaluators. We want you to try hard to do your best. You are going to prepare a five-minute speech and then deliver it, and perform some mental arithmetic. You can make some notes for your speech, but you will have to do all the math in your head.” Sound easy? Not really, and especially not in front of an audience.
One by one, the volunteers are escorted into a testing room. Each study volunteer stands at the front of the room and faces two researchers sitting at a desk. The researchers look at the volunteer in a manner best described as stony-faced. No smiling, no nodding, no encouragement. Technically, a stony-faced expression is neutral, neither positive nor negative, but most of us are used to seeing other people smile at us, nod as we talk, or at least make an effort to seem pleasant. When compared to our usual interactions, a stony expression can come across as disapproving or strict.
The researchers explain the task, saying something like, “Please take the number 4,923 and subtract the number 17 from it, out loud. Then take your answer and subtract 17 from it, and so on, as many times as you can in the next five minutes. It is important that you perform this task quickly and accurately. We will judge you on various aspects of your performance. The clock starts now.”
As each volunteer begins the math task, the researchers stare at her, pencils poised to record her answer. If she fumbles (and almost everyone fumbles), the researchers turned toward each other and whisper.
Then the volunteer goes on to her five-minute speech with the same researchers evaluating her and behaving in a similar way. If she finishes before the five minutes are up, the researchers point to the timer and say, “Please continue!” As she talks, the researchers glance at each other and slightly furrow their brows and shake their heads.
This lab stressor test, developed by Clemens Kirschbaum and Dirk Hellhammer, is a staple of psychology research, and its point is definitely not to test math and speech skills. Instead, it’s designed to induce stress. What makes it so stressful? Mental math and on-the-fly public speaking are tricky to perform well. The most stressful element, though, is what’s called social evaluative stress. Anyone who tries to perform a task in front of an audience will probably feel increased stress about their performance. When that audience appears judgmental, the stress is intensified. Even though our volunteers’ physical survival was not at risk and they were safe in a clean, well-lighted university lab, this test was capable of eliciting a full-blown stress response.
We’ve put caregivers and noncaregivers through this protocol. We assessed their thoughts at two different times during the lab stressor: just after they’d learned what they were going to do, and just after they’d finished the two tasks. What we found was that although all the women felt some stress, not everyone had the same type of stress response. And only one kind of stress response went hand in hand with unhealthy telomeres.6
The Threat Response: Anxious and Ashamed—and Aging
Some of the women had what’s known as a threat response to the lab stressors. The threat response is an old, evolutionary response, a kind of switch to be flipped in case of dire emergency. Basically, the threat response was designed to surge when we are face-to-face with a predator who is probably going to eat us. The response prepares our body and mind for the trauma of being attacked. As you might guess, if it keeps on happening without letup, this is not the response associated with telomere health.
If you already suspect that you have an exaggerated threat response to stress, don’t worry. In a moment, we’ll show you some lab-tested ways to convert a habitual threat response into one that is healthier for your telomeres. First, though, it’s important to know what a threat response looks and feels like. Physically, the threat response causes your blood vessels to constrict so that you’ll bleed less if you’re wounded, but also less blood flows to your brain. Your adrenal gland releases cortisol, which gives you energizing glucose. Your vagus nerve, which runs a direct line from your brain to your viscera and normally helps you feel calm and safe, withdraws its activity. As a result, your heart rate accelerates, and your blood pressure increases. You may faint or even release your bladder. A branch of the vagus innervates the muscles of facial expression, and when that nerve isn’t active, it becomes even harder for someone to interpret your facial expressions accurately. If others are wearing a similarly ambiguous expression, one that leaves lots of room for your interpretation, you in turn may view them as more hostile. You tend to freeze, you are unable to run or fight—and your hands and feet get colder, making movement more difficult.
A full-throttle threat response unleashes some uncomfortable physical reactions, but there are psychological ones, too. As you might expect, the threat response is associated with fear and anxiety. Shame, too, if you’re worried about failing in front of other people. People with a strong habitual threat response tend to suffer from anticipatory worry; they imagine a bad outcome to an event that hasn’t happened yet. That was exactly what happened to many of the caregivers in our lab. They felt high levels of threat—not just after they had finished the tasks but before the tasks had even begun. This group of caregivers became fearful and anxious when they heard the somewhat vague information about having to give a speech and do mental math. They anticipated a bad outcome, and they felt failure and shame.
As a group, our caregivers had a stronger threat response. The chronic stress of being a caregiver had made them more sensitive to a lab stressor. The ones with the strongest threat responses also had the shortest telomeres. The noncaregivers were less likely to have an exaggerated threat response, but those who did had shorter telomeres, too. Having a large anticipatory threat response—meaning that they felt threatened at the mere thought of the lab stressor before it even happened—was what mattered most.7 Here was some vital information about how stress gets into our cells. It’s not just from experiencing a stressful event, it’s also from feeling threatened by it, even if the stressful event hasn’t happened yet.
Excited and Energized: The Challenge Response
Feeling threatened is not the only way to respond to stress. It’s also possible to feel a sense of challenge. People with a challenge response may feel anxious and nervous during a lab stressor test, but they also feel excited and energized. They have a “bring it on!” mentality.
Our colleague, Wendy Mendes, a health psychologist at the University of California, San Francisco (UCSF), has spent over a decade examining the body’s responses to different types of stressors in the lab, and has mapped out the differences that occur in the brain, in the body, and in behavior during “good stress” compared to “bad stress.” Whereas the threat response prepares you to shut down and tolerate the pain, the challenge response helps you muster your resources. Your heart rate increases, and more of your blood is oxygenated; these are positive effects that allow more blood to flow where it’s needed, especially to the heart and brain. (This is the opposite of what happens when you’re threatened. Then, the blood vessels constrict.) During the challenge response, your adrenal gland gives you a nice shot of cortisol to increase your energy—but then your brain quickly and firmly shuts off cortisol secretion when the stressful event is over. This is a robust, healthy kind of stress, similar to the kind you may have when you exercise. The challenge response is associated with making more accurate decisions and doing better on tasks, and is even associated with better brain aging and a reduced risk of developing dementia.8 Athletes who have a challenge response win more often, and a study of Olympic athletes has shown that these highly successful folks have a history of seeing their life problems as challenges to be surmounted.9
The challenge response creates the psychological and physiological conditions for you to engage fully, perform at your best, and win. The threat response is characterized by withdrawal and defeat, as you slump in your seat or freeze, your body preparing for wounding and shame as you anticipate a bad outcome. A predominant habitual threat response can, over time, work itself into your cells and grind down your telomeres. A predominant challenge response, though, may help shield your telomeres from some of the worst effects of chronic stress.
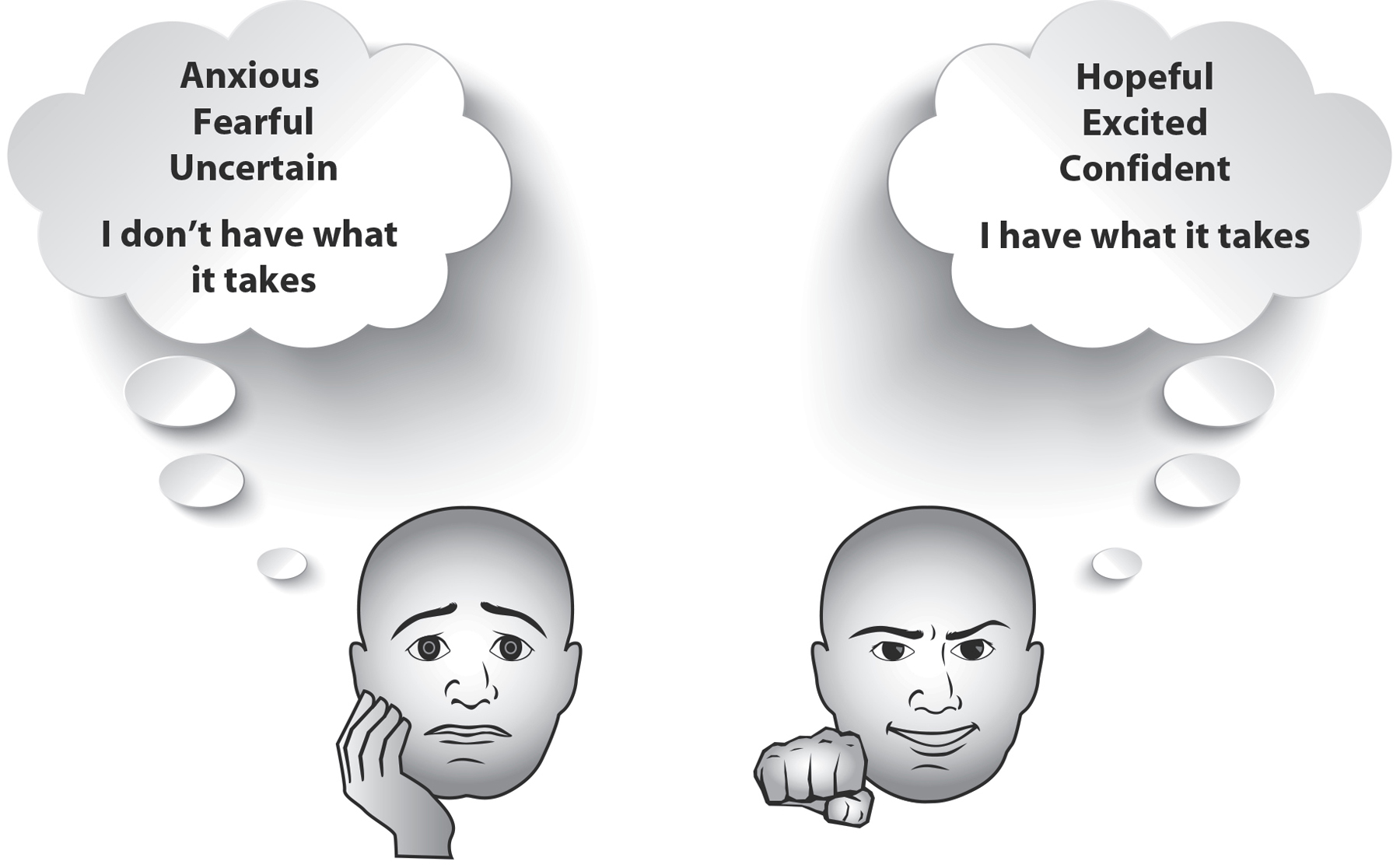
Figure 14: Threat versus Challenge Responses. People tend to have many thoughts and feelings when facing a stressful situation. Here are two different types of responses: One is characterized by feeling threatened, by a fear of losing, or possibly being shamed. The other is characterized by feeling challenged and confident about achieving a positive outcome.
People don’t generally show responses that are all threat or all challenge. Most experience some of both. In one study, we found that it was the proportion of these responses that mattered most for telomere health. The volunteers who felt more threat than challenge had shorter telomeres. Those who saw the stressful task as more of a challenge than a threat had longer telomeres.10
What does this mean for you? It means you have reason to be hopeful. We do not mean to trivialize or underestimate the potential that very tough, difficult, or intractable situations have for harm to your telomeres. But when you can’t control the difficult or stressful events in your life, you can still help protect your telomeres by shifting the way you view those events.
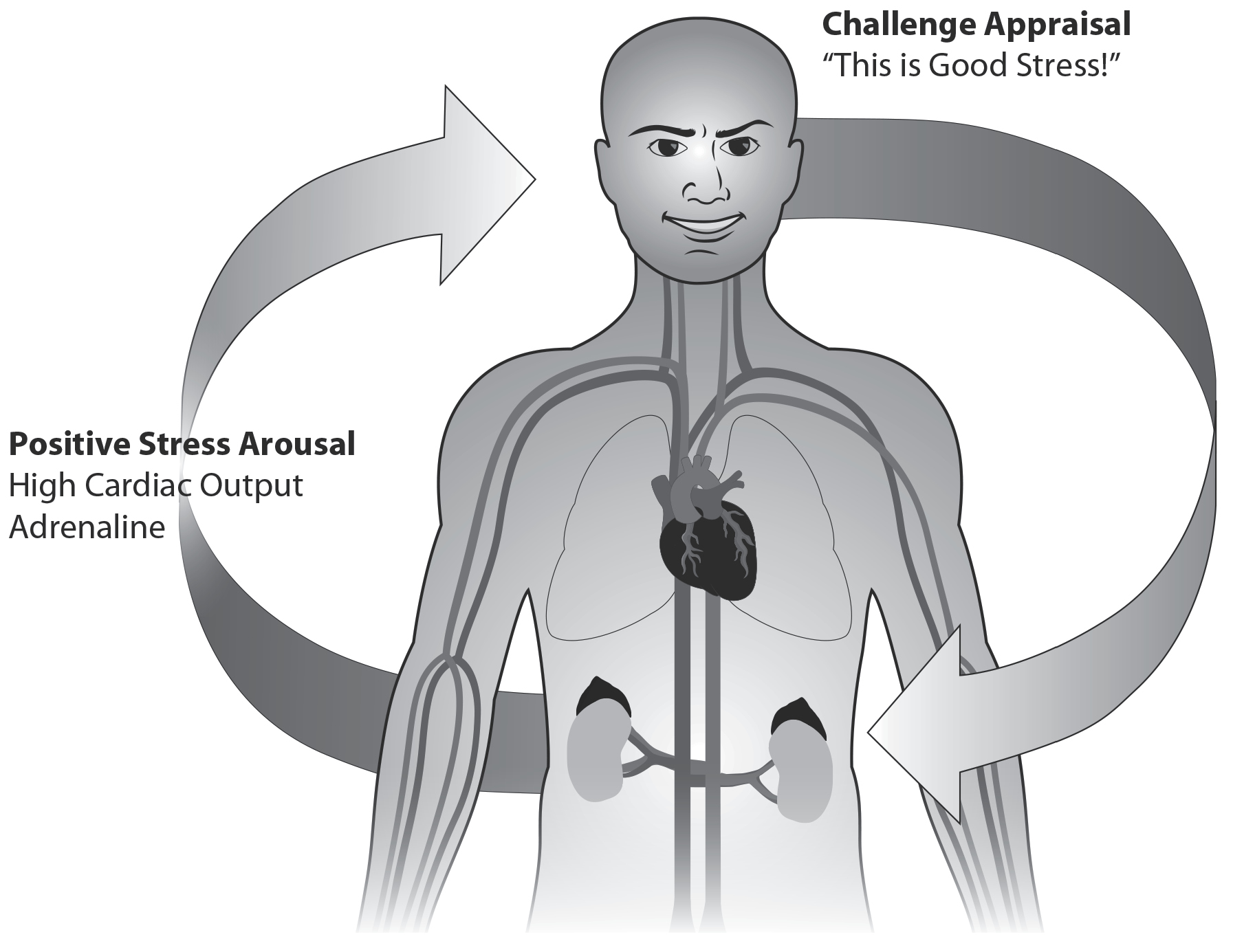
Figure 15: Positive Stress (Challenge Stress) Energizes. Our body automatically reacts to a stressful event within seconds and also reacts to our thoughts about the event. When we start to notice the stress response in our muscle tension, heart rate, and breathing, we can relabel it by saying, “This is good stress, energizing me so I can perform well!” This can help shape the body’s response to be more energizing, bringing more dilation to the vessels and more blood to the brain.
WHY DO SOME PEOPLE FEEL MORE THREAT THAN OTHERS?
Reflect on incidents in your life that have been difficult. Ask yourself: Do you tend to respond by feeling more threatened or challenged? Do you borrow trouble, feeling anticipatory threat about events that haven’t happened yet—and that may not ever happen? When you’re stressed, do you feel ready for action, or do you feel like diving under the covers and hiding?
If you tend to feel more of a threat response, don’t waste your time feeling bad about it. Some of us are simply wired to be more stress reactive. It has been critical to human survival for some of us to respond in a robust way to changes in our environment, and for others to be more sensitive. After all, someone’s got to alert the tribe to dangers and warn the more gung-ho members against taking foolhardy risks.
Even if you weren’t strongly wired at birth to feel threat, conditions in your life may have altered your natural response. Teenagers who were exposed to maltreatment when they were children respond to stressful tasks with blood-flow patterns characteristic of a threat response, experiencing vasoconstriction rather than strong blood flow out of the heart.11 (On the other hand, people who experienced moderate adversity in childhood tend to show more of a challenge response than people who had it easy as children—more evidence that small doses of stress can be healthy, provided that resources are available to help you cope.) As we described earlier, prolonged stress can wear down emotional resources, making people more prone to feeling threatened.12
Either by birth or by the circumstances of your life, you may have a strong threat response. The question is: Can you learn to feel challenged instead? Research says the answer is yes.
DEVELOPING A CHALLENGE RESPONSE
What happens as an emotion arises? Scientists used to believe it was a more linear process—that we experience events in the world, our limbic system reacts with an emotion, like anger or fear, which causes the body to respond with an increased heart rate or sweaty palms. But it’s more complicated than that. The brain is wired to predict things ahead of time, not just react after things have happened.13 The brain uses memories of past experiences to continually anticipate what will happen next, and then corrects those predictions with both the current incoming information from the outside world, and from all the signals within our body. Then our brain comes up with an emotion to match all of this. Within seconds, we patch all this information together, without our awareness, and we feel some emotion.
If our “database” of past experience has a lot of shame in it, we are more likely to expect shame again. For example, if you feel high arousal and jittery, maybe from that morning’s strong coffee, and if you see two people who could be talking about you, your mind may quickly cook up the emotions of shame and threat. Our emotions are not pure reactions to the world; they are our own fabricated constructions of the world.14
Knowing how emotions are created is powerful. Once you know this, you can have more choice over what you experience. Instead of feeling your body’s stress responses and viewing them as harmful, a common experience in your brain’s database, you can think about your body’s arousal as a source of fuel that will help your brain work quickly and efficiently. And if you practice this enough, then eventually your brain will come to predict feelings of arousal as helpful. Even if you’re one of those people whose brain is hardwired to feel more threat, you can feel that immediate instinctive survival response—and then revise the story. You can choose to feel challenged.
Sports psychologist Jim Afremow, PhD, who consults with professional and Olympic athletes, was once approached by a sprinter who was struggling with her hundred-meter time. She had already diagnosed the reason she wasn’t running as well as she wanted to. “It’s the stress,” she said. “Before every race, my pulse races. My heart is about to jump out of my chest. You’ve got to help me stop it!”
Afremow laughed. “Do you really want to stop your heart?” The worst thing athletes can do, he says, is try to get rid of their stress. “They need to think of stress as helping them get ready to perform. They need to say, ‘Yes! I need this!’ Instead of trying to make the butterflies in their stomach go away, athletes need to make those butterflies line up and fly in formation.” In other words, they need to make the stress work for them.
The sprinter took Afremow’s advice. By viewing her physical responses as tools that would help her rise to the challenge of a race, she was able to shave milliseconds off her time (a big deal for a hundred-meter runner) and set a personal record.
It sounds unbelievably simple, but research backs up this efficient method of converting threat to challenge. When research volunteers are told to interpret their body’s arousal as something that will help them succeed, they have a greater challenge response. One study found that students who are encouraged to view stress in this way score higher on their GREs.15 And when researchers put people through lab stressors, the ones who are told to think of stress as useful are able to maintain their social equilibrium. Instead of looking away, playing with their hair, or fidgeting—all signs of feeling somewhat threatened—the challenge participants make direct eye contact. Their shoulders are relaxed, and their bodies move fluidly. They feel less anxiety and shame.16 All these benefits happened simply because people were told to think of their stress as good for them.
A challenge response doesn’t make you less stressed. Your sympathetic nervous system is still highly aroused, but it is a positive arousal, putting you in a more powerful, more focused state. To channel your stress so that it gives you more good energy for an event or performance, say to yourself, “I’m excited!” or “My heart is racing and my stomach is doing cartwheels. Fantastic—those are the signs of a good, strong stress response.” Of course, if you are under the kind of emotionally depleting stress that our caregiving mothers experienced, this language could feel too glib. Instead, talk to yourself in a gentler way. You could say, “My body’s responses are trying to help me. They’re designed to help me focus on the tasks at hand. They’re a sign that I care.” The challenge response is not a falsely chipper, gee-I’m-so-happy-that-stressful-things-are-happening-to-me attitude. It is the knowledge that even though times may be very difficult, you can shape stress to your purpose.
For those who feel addicted to “good stress”—the achievement stress involved in the constant excitement of, for example, working in a start-up company and never having downtime, know that even good stress can be overdone. It’s healthy to have times when your cardiovascular system is mobilized and your psyche is primed for action. But our bodies and minds aren’t built to sustain this kind of high stimulation on a consistent basis. Being able to relax, although it’s been overrated as a sole source of stress management, is still necessary. We recommend that you regularly engage in an activity that brings you deep restoration. There is high-quality evidence that meditation, chanting, and other mindfulness practices can reduce stress, stimulate telomerase, and perhaps even help your telomeres to grow. See here to learn more about these cell-protecting strategies.
Even in chronically stressful situations like caregiving, the stress is not a monolith or a blanket of darkness that cannot be lifted. Stress and stressful events do not live in each little moment, although they can visit. There is some freedom in each moment, because we can have a choice about how we spend this moment. We can’t rewrite the past and we can’t dictate what happens in the future, but we can choose where to place our attention in the moment. And although we can’t always choose our immediate reactions, we can shape our subsequent responses.
Some clever studies have shown that merely anticipating a stressful event has almost the same effect on the brain and body as experiencing the stressful event.17 When you worry about events that haven’t happened yet, you’re letting stress flow over its time boundaries the way a river can overflow its banks, flooding the minutes, hours, and days that could otherwise be more enjoyable. It is almost always possible to find something to worry about and therefore possible to keep the stress response engaged on an almost constant basis. When you anticipate a bad outcome before an event has even begun, you increase your dose of threat stress, and that’s the last thing you need. But rather than avoiding thinking about stressful things, it’s how we think about them that matters.
A SHORT PATH TO A LONG DISEASESPAN: STRESS, AGING IMMUNE CELLS, AND INFLAMMATION
It never fails. Just after you’ve met an important work deadline, or as you’re boarding a plane for a long-overdue beach vacation, you come down with the mother of all colds: sneezing, runny nose, sore throat, fatigue. Coincidence? Probably not. While your body is actively fighting stress, your immune system can be bolstered for a time. But that effect can’t last forever. Chronic stress suppresses aspects of the immune system, leaving us more vulnerable to infections, causing us to produce fewer antibodies in response to vaccinations, and making our wounds heal more slowly.20
There is an unsavory relationship between stress, immune suppression, and telomeres. For years, scientists were unsure just how stress, which lives in the mind, could damage the immune system. Now we have an important part of the answer: telomeres. People with chronic stress have shorter telomeres, and short telomeres can lead to prematurely aging immune cells, which means worse immune function.
Shorter Telomeres, Weaker Immune System
Certain immune cells are like SWAT teams that fight viral infections. These cells are known as T-cells, because they are stored in the thymus gland, which sits under the sternum bone in the chest. Once T-cells mature, they leave the thymus and circulate continuously throughout the body. Each T-cell has a unique receptor on its surface. The receptor acts like a searchlight on a police helicopter, sweeping the body and looking for “criminals”—cells that are either infected or cancerous. Of particular interest to aging is the type of T-cell called a CD8 cell.
But it isn’t enough for the T-cell to simply spot a villainous cell. In order to complete the job, the T-cell needs to receive a second signal from a surface protein, called CD28. When the T-cell kills its target, the cell develops “memory” so that if the same virus infects the body again in the future, the T-cell can multiply into thousands and thousands of progeny cells just like itself. Together they can mount a rapid, efficient immune response against that specific virus. This is the basis of vaccination. The vaccine is typically a piece of a viral protein or a killed virus; the immunity lasts for years, since the T-cells that have responded to the initial vaccination remain in the body for a long time (sometimes for life) and are available to fight off an infection if the virus should work its way into the body again.
We have a tremendously large repertoire of T-cells, each with the capacity to recognize just one particular antigen or virus. Because we have such a huge variety of different T-cells, when we become infected with a particular virus, the few T-cells that have the correct receptor for the virus must create many progeny in order to combat the infection. During this massive process of cell division, telomerase is ratcheted up to high levels. However, it simply can’t keep up with the speedy rate of telomere shortening, and eventually the telomerase response weakens to a whisper, and the telomeres in those responding T-cells keep getting shorter. So they pay for those heroic responses. When a T-cell’s telomeres grow short, the cell becomes old, and it loses the CD28 surface marker that is necessary for mounting a good immune response. The body becomes like a city that’s lost its budget for police helicopters and searchlights. The city looks normal from the outside, but lies vulnerable to criminal infestation. The antigens on bacteria, viruses, or cancerous cells are not cleared from the body. That’s a reason people with aging cells—including the elderly and the chronically stressed—are so vulnerable to sickness, and why it’s hard for them to weather diseases like the flu or pneumonia. It’s partly why HIV progresses to AIDS.21
When telomeres in these aging T-cells are too short, even young people are more vulnerable. Sheldon Cohen, a psychologist at Carnegie Mellon University, asked young, healthy volunteers to live isolated in hotels so he could study the effects of giving them a noseful of the virus that causes the common cold. First, he measured their telomeres. The people with shorter telomeres in their immune cells, and especially in their near-senescent CD8 cells, developed colds faster, with more severe symptoms (which were measured by weighing their used tissues).22
What’s Stress Got to Do with It?
Our CD8 T-cells (the fighters in the immune system) appear to be especially vulnerable to stress. In another of our family caregiver studies, we took blood samples from mothers who had a child with autism living at home. We found that these caregiving mothers had lower telomerase in their CD8 cells that had lost the critical CD28 surface marker, suggesting they would be in danger of developing critically short telomeres over the years. Rita Effros, an immunologist from University of California, Los Angeles, and a pioneer in understanding aging immune cells, has created “stress in a dish”—she has shown that exposing immune cells to the stress hormone cortisol dampens their levels of telomerase.23 A compelling reason to learn how to respond to stress in a healthier way.
Shorter Telomeres, More Inflammation
Unfortunately, the news gets worse. When the telomeres of aging CD8 cells wear down, the aging cells send out proinflammatory cytokines, those protein molecules that create systemic inflammation. As the telomeres continue to shorten and the CD8 cells become fully senescent, they refuse to die and they accumulate in the blood over time. (Normally CD8 T-cells gradually die by a natural type of cell death called apoptosis. Apoptosis rids the body of old or damanged immune cells so they do not overwhelm the body or develop into the types of blood cancers called leukemias.) These senescent T-cells are the rotten apples in the barrel, with their bad effects spreading outward. They pump out slightly more inflammatory substances each year like a slow drip. If you have too many of these aging cells in your bloodstream, you’re at risk for rampant infections and all the diseases of inflammation. Your heart, your joints, your bones, your nerves, and even your gums can become diseased. When stress makes your CD8 cells grow old, you grow old, too—no matter what your chronological age is.
Experiencing stress and pain is unavoidable. It is part and parcel of being involved with life, of loving and caring for people, caring about issues, and taking risks. Use the challenge response to protect your cells while you engage fully with life. The Renewal Lab at the end of this chapter offers some specific techniques to help you cultivate this response. The challenge response is not the only tool in your box, though. For powerful stress-relievers that are great for your telomeres, check out “Stress-Reducing Techniques Shown to Boost Telomere Maintenance” at the end of Part Two. And if stress tends to lead you into destructive thinking patterns—maybe you suppress painful thoughts or ruminate excessively about them, or perhaps you begin to anticipate negative responses from other people—turn to the next chapter. We’ll help you protect your telomeres from this harmful thinking.
TELOMERE TIPS
 Your telomeres don’t sweat the small stuff. Toxic stress, on the other hand, is something to watch for. Toxic stress is severe stress that lasts for years. Toxic stress can dampen down telomerase and shorten telomeres.
Your telomeres don’t sweat the small stuff. Toxic stress, on the other hand, is something to watch for. Toxic stress is severe stress that lasts for years. Toxic stress can dampen down telomerase and shorten telomeres.
 Short telomeres create sluggish immune function and make you vulnerable even to catching the common cold.
Short telomeres create sluggish immune function and make you vulnerable even to catching the common cold.
 Short telomeres promote inflammation (particularly in the CD8 T-cells), and the slow rise of inflammation leads to degeneration of our tissues and diseases of aging.
Short telomeres promote inflammation (particularly in the CD8 T-cells), and the slow rise of inflammation leads to degeneration of our tissues and diseases of aging.
 We cannot rid ourselves of stress, but approaching stressful events with a challenge mentality can help promote protective stress resilience in body and mind.
We cannot rid ourselves of stress, but approaching stressful events with a challenge mentality can help promote protective stress resilience in body and mind.