 intravesical pressure during voiding, causing ischaemia and leading to ischaemic damage to neurons within the bladder (i.e. denervation). Symptomatically, many patients with BOO develop frequency, urgency, and urge incontinence.
intravesical pressure during voiding, causing ischaemia and leading to ischaemic damage to neurons within the bladder (i.e. denervation). Symptomatically, many patients with BOO develop frequency, urgency, and urge incontinence.Regulation of prostate growth and development of benign prostatic hyperplasia
Pathophysiology and causes of bladder outlet obstruction and BPH
Benign prostatic obstruction: symptoms and signs
Diagnostic tests in men with LUTS thought to be due to BPH
The management of LUTS in men: NICE 2010 guidelines
Watchful waiting for uncomplicated BPH
Medical management of BPH: alpha blockers
Medical management of BPH: 5-alpha-reductase inhibitors
Medical management of BPH: combination therapy
Medical management of BPH: alternative drug therapy
Minimally invasive management of BPH: surgical alternatives to TURP
Invasive surgical alternatives to TURP
Acute urinary retention: definition, pathophysiology, and causes
Acute urinary retention: initial and definitive management
Indications for and technique of urethral catheterization
Technique of suprapubic catheterization
Management of nocturia and nocturnal polyuria
High-pressure chronic retention
BPH is characterized by an increase in epithelial and stromal cell numbers (hyperplasia) in the peri-urethral area of the prostate. New epithelial gland formation is normally only seen during fetal development. The development of new glands in the adult prostate has given rise to the concept of ‘reawakening’ of the inductive effect of the prostatic stroma on the prostatic epithelium.
The increase in prostate cell number could reflect proliferation of epithelial and stromal cells, impairment of programmed cell death, or a combination of both. During the early phases of development of BPH, cell proliferation occurs rapidly. In established BPH, cell proliferation slows down and there is impairment of programmed cell death (androgens and oestrogens actively inhibit cell death).
Testosterone can bind directly to the androgen receptor or may be converted to a more potent form—dihydrotestosterone (DHT)—by the enzyme 5α-reductase (5AR). There are two isoforms of 5AR—type I or ‘extraprostatic’ 5AR (which is absent in prostatic tissue and present in, for example, the skin and liver) and type II or ‘prostatic’ 5AR (which is found exclusively on the nuclear membrane of stromal cells, but not within prostatic epithelial cells). Type I 5AR is not inhibited by finasteride, whereas type II 5AR is. Dutasteride inhibits both type I and II 5AR. Finasteride reduces serum DHT by about 70%, and dutasteride by about 95%. Finasteride reduces prostatic DHT (type II) by about 80%, and dutasteride by about 94%. We do not know whether these differences translate into differences in clinical efficacy, since neither drug has been compared against the other.
Testosterone diffuses into prostate and stromal epithelial cells. Within epithelial cells, it binds directly to the androgen receptor. In prostate stromal cells, a small proportion binds directly to the androgen receptor, but the majority binds to 5AR (type II) on the nuclear membrane, is converted to DHT, and then binds (with greater affinity, and therefore greater potency, than testosterone) to the androgen receptor in the stromal cell. Some of the DHT formed in the stromal cells diffuses out of these cells and into nearby epithelial cells (a paracrine action). The androgen receptor/testosterone or androgen receptor/DHT complex then binds to specific binding sites in the nucleus, thereby inducing transcription of androgen-dependent genes and subsequent protein synthesis.
It is thought that stromal/epithelial interactions may be mediated by soluble growth factors—small peptides that stimulate or inhibit cell division and differentiation. Growth-stimulating factors include basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), keratinocyte growth factor (KGF), and insulin-like growth factor (IGF). Transforming growth factors (e.g. TGF-β) normally inhibit epithelial cell proliferation, and it is possible that in BPH, TGF-β is downregulated.
The principal cause of BOO in men is BPH. Less common causes are urethral stricture and malignant enlargement of the prostate. BOO in women is altogether less common, with causes including pelvic prolapse (cystocele, rectocele, uterine) with the prolapsing organ directly compressing the urethra, urethral stricture, urethral diverticulum, post-surgery for ‘stress’ incontinence, Fowler’s syndrome (impaired relaxation of the external sphincter occurring in premenopausal women, often in association with polycystic ovaries), and pelvic masses (e.g. ovarian masses). In either sex, neurological disease [SCI, spina bifida, multiple sclerosis (MS)] can cause failure of relaxation of the external sphincter during voiding [detrusor sphincter dyssynergia (DSD)].
The pathophysiological basis of BOO due to BPE secondary to BPH (BPO) has been studied more than any other type of obstruction. BPO has dynamic and static components:
•Dynamic component of BPO: 1-adrenoceptor-mediated prostatic smooth muscle contraction. Smooth muscle accounts for ~40% of the area density of the hyperplastic prostate, and the human prostate contracts following administration of α-adrenergic agonists. This effect is the rationale for α-adrenoceptor blocker treatment for symptomatic BPO.
•Static component of BPO: mediated by the volume effect of BPE.
John Hunter (1786), who founded the Royal College of Surgeons of England, noted that ‘The disease of the bladder arising from obstruction alone is increased irritability and its consequences, by which it admits of little distension, becomes quick in its action, and thick and strong in its coats’. BOO causes thickening of the wall of the bladder. Microscopically, smooth muscle cells enlarge and there is an increase in connective tissue (collagen and elastin) between the smooth muscle bundles. In some cases, this may lead to poor compliance, with development of high bladder and intrarenal pressures. Progressive hydronephrosis can develop, with impairment of renal function and even renal failure (HPCR).
Experimentally created BOO causes development of bladder overactivity (unstable bladder contractions during bladder filling). This may be due to prolonged  intravesical pressure during voiding, causing ischaemia and leading to ischaemic damage to neurons within the bladder (i.e. denervation). Symptomatically, many patients with BOO develop frequency, urgency, and urge incontinence.
intravesical pressure during voiding, causing ischaemia and leading to ischaemic damage to neurons within the bladder (i.e. denervation). Symptomatically, many patients with BOO develop frequency, urgency, and urge incontinence.
Developed to standardize the approach to diagnosis (and treatment) of men presenting with symptoms suggestive of BPH.1 Every guideline agrees that a history should be taken and an examination performed, and that the severity of urinary symptoms should be formally assessed using the IPSS. This includes a measure of the ‘bother’ caused by the patient’s symptoms (i.e. the degree to which the symptoms are troubling).
During the 1990s, the classic ‘prostatic’ symptoms of frequency, urgency, nocturia, hesitancy, poor flow, intermittent flow, and terminal dribbling—traditionally said to indicate the presence of BOO due to BPE—were shown to bear little relationship to prostate size, flow rate, residual urine volume, or indeed urodynamic evidence of BOO. Age-matched elderly men and women have similar symptom scores (IPSS), despite the fact that women have no prostate and rarely have BOO.
‘Prostatism’ has therefore been replaced by the expression ‘LUTS’, which avoids any implication about the cause of these symptoms. More recently, the expression ‘LUTS/BPH’ has been used to describe the symptoms of BPH. It does not really matter whether you use ‘prostatism’, ‘LUTS’, or ‘LUTS/BPH’, as long as you remember that urinary symptoms may have non-prostatic causes. Try to avoid treating the prostate when the problem may lie elsewhere.
Ask specifically about the presence of:
•Bedwetting: suggests the presence of HPCR (look for distension of the abdomen due to a grossly enlarged bladder that is tense on palpation and dull to percussion).
•Marked frequency and urgency, particularly when also combined with bladder pain: look for CIS of the bladder (urine cytology, flexible cystoscopy, and bladder biopsy).
•Macroscopic haematuria: sometimes due to a large, vascular prostate, but exclude other causes (bladder and kidney cancer and stones) by flexible cystoscopy and upper tract imaging.
•Back pain and neurological symptoms (sciatica, lower limb weakness, or tingling): rarely, LUTS can be due to neurological disease.
Reference
1Irani J, Brown CT, van der Meulen J, Emberton M (2003). A review of guidelines on benign prostatic hyperplasia and lower urinary tract symptoms: are all guidelines the same? BJU Int 92:937–42.
Developed as an attempt to standardize the approach to diagnosis and treatment of men presenting with symptoms suggestive of BPH.1 All agree that a history should be taken and an examination performed, and all recommend the assessment of symptom severity using the IPSS. This includes a measure of the ‘bother’ caused by the patient’s symptoms. There is considerable variation between guidelines in terms of recommended diagnostic tests. High-quality guidelines (e.g. based on results of randomized trials) recommend few diagnostic tests2—urine analysis, completion of a voiding diary (frequency–volume chart) to detect the presence of polyuria and NP (which may be the cause of a patient’s  frequency or nocturia), and measurement of serum creatinine. They regard flow rate measurement and assessment of residual urine volume as optional tests.
frequency or nocturia), and measurement of serum creatinine. They regard flow rate measurement and assessment of residual urine volume as optional tests.
Done to detect nodules that may indicate an underlying prostate cancer and to provide a rough indication of prostate size. Size alone is not an indication for treatment, but if surgical treatment is contemplated, marked prostatic enlargement can be confirmed by TRUS scan (prostate volume in the order of 100mL or more increases the likelihood of an open prostatectomy). Discuss the pros and cons of PSA testing with the patient.
Baseline measure of renal function and to detect renal failure secondary to high-pressure urinary retention.
Varies considerably (by as much as 600mL between repeat measurements) on the same or different days.3 It cannot predict symptomatic outcome from TURP. Along with serum creatinine, it indicates whether watchful waiting (WW) is safe. It is safe not to operate where the PVR volume is <350mL,4,5 since the majority of men show no worsening of creatinine, no increase in PVR and no worsening of symptoms, and do not require TURP.
This is variously regarded as optional, recommended, and obligatory prior to undertaking surgical treatment for BPH. Like PVR, measurement of flow rate varies substantially on a given day,6 cannot distinguish between BOO and a poorly contractile bladder, and is not good at predicting the likelihood of a good symptomatic outcome after TURP.
Reasonably good at predicting symptomatic outcome after TURP. However, most patients without obstruction have a good outcome, and the time, cost, and invasiveness of pressure flow studies are perceived by most urologists as not justifying their routine use. The AUA guidelines on the management of BPH ( http://www.auanet.org) regards pressure flow studies as optional, since they are unable to reliably predict treatment failure in the individual patient (treatment failure is somewhat higher in the absence of obstruction, but unobstructed individuals still have a reasonable chance of improvement with TURP). The AUA guidelines specifically state that ‘If interventional therapy is planned without clear evidence of the presence of obstruction, the patient needs to be informed of possible higher failure rates of the procedure’.
http://www.auanet.org) regards pressure flow studies as optional, since they are unable to reliably predict treatment failure in the individual patient (treatment failure is somewhat higher in the absence of obstruction, but unobstructed individuals still have a reasonable chance of improvement with TURP). The AUA guidelines specifically state that ‘If interventional therapy is planned without clear evidence of the presence of obstruction, the patient needs to be informed of possible higher failure rates of the procedure’.
To detect hydronephrosis if serum creatinine is elevated. The percentage of patients having upper tract dilatation on ultrasound according to serum creatinine is: creatinine <115mmol/L: 0.8%; creatinine 115–130mmol/L: 9%; and creatinine >130mmol/L: 33%.7
References
1Roehrborn CG, Bartsch G, Kirby R, et al. (2001). Guidelines for the diagnosis and treatment of benign prostatic hyperplasia: a comparative international overview. Urology 58:642–50.
2Irani J, Brown CT, van der Meulen J, Emberton M (2003). A review of guidelines on benign prostatic hyperplasia and lower urinary tract symptoms: are all guidelines the same? Br J Urol Int 92:937–42.
3Dunsmuir WD, Feneley M, Corry DA, et al. (1996). The day-to-day variation (test–retest reliability) of residual urine measurement. Br J Urol 77:192–3.
4Bates TS, Sugiono M, James ED, et al. (2003). Is the conservative management of chronic retention in men ever justified? Br J Urol Int 92:581–3.
5Wasson JH, Reda DJ, Bruskewitz RC, et al. (1995). A comparison of transurethral surgery with watchful waiting for moderate symptom of benign prostatic hyperplasia. The Veterans Administration Cooperative Study Group on Transurethral Resection of the Prostate. N Engl J Med 332:75–9.
6Reynard JM, Peters TJ, Lim C, Abrams P (1996). The value of multiple free-flow studies in men with lower urinary tract symptoms. Br J Urol 77:813–18.
7Koch WF, Ezz el Din KE, De Wildt MJ, et al. (1996). The outcome of renal ultrasound in the assessment of 556 consecutive patients with benign prostatic hyperplasia. J Urol 155:186–9.
( http://www.nice.org.uk/CG97)
http://www.nice.org.uk/CG97)
For those practising in the UK, the NICE 2010 LUTS guidelines provide a helpful summary of the diagnostic and treatment options for men with LUTS. Like guidelines in general, they are not written in stone—there is no absolute requirement to follow them to the letter; you may ‘step outside’ the guidelines, as long as your rationale for doing so has a logical (reasonable) basis. Differences exist between those aspects of the NICE guidelines that cover BPH-related LUTS and the AUA 2010 guidelines on the management of BPH ( http://www.auanet.org), and these differences are highlighted where relevant.
http://www.auanet.org), and these differences are highlighted where relevant.
LUTS are classified according to the IPSS as mild (0–7), moderate (8–19), and severe (20–35).
Assess the general medical history to identify possible causes of LUTS and comorbidities; examine the abdomen, genitals, and DRE; dipstick urine for blood, glucose, protein, leucocytes, and nitrites; complete FVC; serum creatinine and eGFR only if suspected renal impairment.
Offer information, advice, and time to decide if they wish to have PSA testing if LUTS are suggestive of BOO secondary to BPE or abnormal feeling of prostate on DRE or patient concerned about prostate cancer.
Do not routinely offer cystoscopy, flow rate, or residual urine volume measurement.
Offer lifestyle advice (e.g. advice on fluid intake); mild or moderate bothersome LUTS—discuss active surveillance* (reassurance, lifestyle advice, no immediate treatment, regular follow-up) or active intervention (conservative management, drugs, surgery).
•Storage symptoms: if OAB is suspected, offer supervised bladder training, advice on fluid intake, lifestyle advice, and, if needed, containment products, i.e. pads or sheaths; offer supervised pelvic floor exercises for stress incontinence caused by prostatectomy—continue for at least 3 months before considering other options.
•Voiding symptoms: offer intermittent self-catheterization (ISC) before indwelling or suprapubic catheterization if less invasive means fail to correct LUTS; tell men with proven BOO that bladder training is less effective than surgery; for post-micturition dribbling, explain how to do urethral milking.
•Offer drug treatment where conservative options are unsuccessful or inappropriate; take account of comorbidities and current treatments; do not offer homeopathy, phytotherapy, or acupuncture (Table 4.1).
•NP: exclude other medical causes—diabetes mellitus and DI, adrenal insufficiency; hypercalcaemia; liver failure; polyuric renal failure; chronic heart failure; OSA, dependent oedema; chronic venous stasis; calcium channel blockers; diuretics; selective serotonin reuptake inhibitor (SSRI) antidepressants.
•Consider a late-afternoon loop diuretic. Consider offering oral desmopressin—measure serum sodium 3 days after the first dose; stop if sodium falls below the normal reference range.
Table 4.1 Drug treatment
| Indication | Treatment | Review |
| Moderate to severe LUTS | Offer an α-blocker (alfuzosin, doxazosin, tamsulosin, terazosin) | At 4–6wk (AUA guidelines 2–4wk), then every 6–12 months |
| OAB | Offer an anticholinergic | At 4–6wk until stable, then every 6–12 months |
| LUTS, prostate estimated >30g or PSA >1.4ng/mL and high risk of progression | Offer 5α-reductase inhibitor (5ARI) | At 3–6 months, then every 6–12 months |
| Bothersome moderate to severe LUTS and prostate estimated >30g or PSA >1.4ng/mL | Consider an α-blocker plus 5ARI | At 4–6wk, then every 6–12 months for the α-blocker; at 3–6 months, then every 6–12 months for the 5ARI |
| Storage LUTS despite α-blocker treatment alone | Consider adding an anticholinergic | At 4–6wk until stable, then every 6–12 months |
If bothersome LUTS that fails to respond to conservative management or drugs; LUTS complicated by recurrent or persistent UTI; retention; renal impairment suspected to be caused by LUT dysfunction; suspected urological cancer; stress incontinence.
(In other words, secondary care—‘health-care professional with specific training in managing LUTS in men’.) A summary of surgical treatment options, based on prostatic size, for voiding symptoms is shown in Table 4.2 and for storage symptoms in Table 4.3.
Table 4.2 Voiding symptoms
| Prostate size | Type of surgery |
| All | TURP (monopolar or bipolar); transurethral vaporization of the prostate (TUVP) (monopolar); holmium laser enucleation of the prostate (HoLEP)* |
| Estimated <30g | Transurethral incision of the prostate (TUIP) [bladder neck incision (BNI)] as an alternative to the above |
| Estimated >30g | TURP, TUVP, HoLEP*, open prostatectomy |
* At a centre specializing in the technique or with mentorship arrangement in place.
•Offer the following only as part of a randomized controlled trial (RCT): prostatic Botox injection; laser vaporization techniques; bipolar TUVP; transurethral vapour resection of the prostate (TUVRP) (monopolar or bipolar).
•Do not offer any of the following as an alternative to TURP, TUVP, or HoLEP:
•Transurethral needle ablation of the prostate (TUNA); transurethral microwave thermotherapy of the prostate (TUMT); high-intensity focused ultrasound (HIFU); laser coagulation; transurethral ethanol ablation of the prostate (TEAP) (AUA 2010 guidelines include TUNA and TUMT as treatment options for the patient with moderate to severe LUTS, i.e. IPSS 8 or more).
Table 4.3 Storage symptoms
| Indication | Type of surgery |
| Detrusor overactivity | Consider offering bladder wall Botox injection (must be willing and able to do ISC); SNS*; cystoplasty (must be willing and able to do ISC) |
| Stress incontinence | Consider artificial urinary sphincter (AUS) (intramural injectables, implanted adjustable compression devices, male slings—only as part of an RCT) |
| Intractable LUTS if cystoplasty or SNS not clinically appropriate or unacceptable to patient | Consider offering urinary diversion |
* SNS, sacral nerve stimulation (the InterStim).
Men seek treatment for their LUTS for several reasons:
•The symptoms may be bothersome.
•They may fear that the symptoms are a warning that acute urinary retention will develop.
•They may be concerned that their symptoms indicate that they have prostate cancer.
Establish what the patient wants from his consultation with you. Once reassured that the likelihood of urinary retention and prostate cancer is low, he may not want treatment for symptoms that, on the surface, may appear quite bad, and he may be happy to adopt a policy of WW.
•To improve bothersome symptoms.
•To prevent symptom progression.
•To reduce long-term complications (urinary retention, renal insufficiency).
•Management options include WW, lifestyle modification, drug treatments (α-adrenergic blockers, 5ARIs, anticholinergics, plant extracts), minimally invasive surgery, TURP, and open prostatectomy. The choice of treatment is determined by the patient, based on his perception of how bad (bothersome) his symptoms are, balanced against the perceived benefit and risks of the various options. Drug treatments have the least impact on symptoms but are generally safe. Minimally invasive surgery has a somewhat greater impact, with a higher risk of side effects. TURP and open prostatectomy have the greatest impact on symptoms, but at the risk of potentially serious complications.
Bothersomeness does not necessarily equate with symptom severity as assessed by symptom scores. Thus, a man with a low symptom score may find his symptoms very bothersome and may want treatment, whereas another man with a high symptom score may not be bothered and may want no treatment. If one symptom is particularly bad, but the other six symptoms in the 7-symptom score are minimal, the overall symptom score will obviously be relatively low, but the patient may find that one symptom very bothersome (e.g. urgency and nocturia tend to be more bothersome than hesitancy or poor flow).
No particular LUTS are specific for prostate cancer. Even if it later turns out that he does have prostate cancer, a patient’s symptoms might be due to coexisting BPH or some other LUT pathology. If he is concerned about the possibility of prostate cancer, counsel him with regard to PSA testing and prostate biopsy.
Many patients are understandably concerned that their urinary symptoms may be a harbinger for the development of acute urinary retention. This may influence their decision to seek help for symptoms, which they may perceive as indicating a risk of subsequent retention, and it may affect the type of treatment they choose. Table 4.4 can help give the patient some idea of his risk of developing urinary retention.
Table 4.4 Yearly risk of retention according to age and symptom score (i.e. number of men experiencing an episode of retention every year)
| Age (y) | Mild symptoms (AUA symptom score 7 or less) | Moderate or severe symptoms (AUA symptom score >7) |
| 40–49 | 3 men in every 1000 | 3 men in every 1000 |
| 70–79 | 9 men in every 1000 | 34 men in every 1000 |
* This table is taken from Jacobsen’s report, a 4-year prospective study of a cohort of >2000 men.1 The presence of LUTS, a low flow rate, an enlarged prostate, and old age were associated with an ↑ risk of urinary retention.
Adjusting for age and flow rate, those with an AUA symptom score of 8 or more had a 2.3-fold ↑ risk of going into urinary retention, when compared with those with an AUA score of 7 or less. Those men with a peak flow rate of <12mL/s had a 4-fold ↑ risk of urinary retention, when compared with those with a flow rate of >12mL/s. Prostate volume of >30mL was associated with a 3-fold ↑ risk of urinary retention, compared with those with prostate volumes of <30mL.
Data sourced from Jacobsen SJ, Jacobson DJ, Girman CJ, et al. (1997) Natural history of prostatism: risk factors for acute urinary retention. J Urol 158:481–7.
Reference
1Jacobsen SJ, Jacobson DJ, Girman CJ, et al. (1997). Natural history of prostatism: risk factors for acute urinary retention. J Urol 158:481–7.
A number of studies have shown that in a substantial proportion of men, symptoms do not progress, even for those with severe symptoms.
•Ball:1 a total of 107 men followed with WW over 5y. In none was there an absolute indication for surgery. Half of the patients were obstructed on urodynamic testing. A third of the patients got better; just under a half stayed the same; a quarter got worse (of whom eight underwent TURP); 2% went into retention.
•PLESS study (Proscar Long-term Efficacy and Safety Study):2 a total of 1500 men with moderate to severe symptoms were randomized to placebo (and a similar number to active drug). Those on placebo had an average fall in symptom score of 1 point at 4y.
•Wasson’s study of WW vs TURP:3 for men with moderate symptoms, the risk of progression to retention, worsening symptoms, or need for TURP was relatively low in those who chose WW; 40% noticed an improvement in their symptoms, 30% got worse, and TURP was required in about a quarter.
•Five centres’ study:4 a total of 500 men referred by their family doctors for consideration for TURP were managed non-operatively after viewing an educational programme. Over the following 4y period, a proportion of the men chose drug treatment or surgery. For men with mild, moderate, or severe symptoms, 10%, 24%, and 39%, respectively, had undergone surgery at the end of 4y. For the same symptom categories, 63%, 45%, and 33% were still not receiving any treatment at the end of 4y. Almost a quarter of men who initially presented with severe symptoms noted an improvement in their symptoms to mild or moderate.
On the basis of these studies, we can say that symptoms, even if severe, do not necessarily get worse, even over fairly long periods of time. This forms the foundation of WW as an option for many patients, even if the symptoms at baseline are severe. The IPSS measures both symptom ‘severity’, but more importantly, the bother that the symptoms cause the patient. Thus, if a patient has a high symptom score (severe symptoms) but is not bothered by these symptoms, there is no indication for treatment. Some patients, on the other hand, have a low symptom score but may find even this degree of symptoms very bothersome. Treatment is indicated in such cases (usually starting with medical therapy such as an α-blocker or a 5ARI).
References
1Ball AJ, Feneley RC, Abrams PH (1981). Natural history of untreated ‘prostatism’. Br J Urol 53:613–16.
2McConnell JD, Bruskewitz R, Walsh PC, et al. (1998). The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia (PLESS). N Engl J Med 338:557–63.
3Wasson JH, Reda DJ, Bruskewitz RC, et al. (1995). A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate. N Engl J Med 332:75–9.
4Barry MJ, Fowler FJJ, Bin L, et al. (1997). The natural history of patients with benign prostatic hyperplasia as diagnosed by North American urologists. J Urol 157:10–14.
As described earlier, BPO is caused partly by α1-adrenoceptor-mediated prostatic smooth muscle contraction, and this is the rationale for α-adrenoceptor blocker treatment for symptomatic BPO.
There are two broad subtypes of α-adrenoceptor (AR)—α1 and α2. Molecular cloning studies have identified three α1-AR subtypes—α1a (predominant in human stroma and therefore mediates prostate smooth muscle contraction), α1b (predominant in human prostate epithelium), and α1L (believed to be a conformational state of the α1a-AR). The AR subtypes mediating the efficacy and side effects of α-adrenoceptor-blocking drugs are unknown.
α-blockers are categorized by their selectivity for the AR and by their elimination half-life.
•Non-selective: phenoxybenzamine—effective symptom control, but high side effect profile.
•α1: prazosin, alfuzosin, indoramin.
•Long-acting α1: terazosin, doxazosin, alfuzosin sustained release (SR).
•Subtype selective: tamsulosin—relatively selective for the α1a-AR subtype, compared to the α1b subtype.
No study has directly compared one α-blocker with another in terms of efficacy or side effects. Terazosin and doxazosin require dose titration to minimize dizziness and syncope at the start of treatment.
Bothersome LUTS where WW has failed or the patient wishes to have treatment.
Patients are able to perceive a 4-point improvement in IPSS. If ‘response’ is defined as >25% improvement in symptoms, relative to placebo, most studies describe response rates of 30–40%.1 The mean probability for improvement in symptom score after TURP is in the order of 80% (i.e. eight out of ten men will notice an improvement in their symptoms after TURP). For those men who respond, α-blockers have a much more rapid onset than do 5ARIs. Their effect will be maximal within a month of starting treatment.
The average improvement in symptom score after TURP is about 85%.2 While some of this may represent a placebo response, this improvement is considerably better than that seen with the α-blockers, which result in a 10–30% improvement in symptom score, relative to placebo.3 This equates to a 4- to 5-point improvement in symptom score over placebo.
A substantial proportion of men stop taking their medication either because of side effects (15–30% report some constellation of side effects) or because of a perceived lack of effectiveness (~50% of men stop taking an α-blocker within 3y because of a perception that it has not worked).4 Side effects include asthenia (weakness in 5%), dizziness (2–14%), headache (2%) and postural hypotension (1%), and retrograde ejaculation (8%). There are little data on the safety of concomitant use of α-blockers with drugs for erectile dysfunction.1
A triad of progressive intraoperative miosis (constriction of the pupil) despite preoperative dilation, billowing of a flaccid iris, and iris prolapse towards the incision site during cataract surgery leads to complications such as posterior capsule rupture with vitreous loss and post-operative intraocular pressure spikes (visual acuity outcomes appeared preserved). The original report linked this condition with the preoperative use of tamsulosin; iris dilator smooth muscle inhibition has been suggested as a potential mechanism.5,6
The risk of intraoperative floppy iris syndrome (IFIS) amongst men taking tamsulosin is substantial (43–90% in ten retrospective and prospective studies).7 The risk of IFIS appears to be lower with older generic α-blockers such as terazosin and doxazosin (0–25%).7
Whether stopping α-blocker treatment at any time before surgery mitigates the risk of IFIS is unclear. The AUA 2010 BPH Guidelines7 recommend that men with LUTS secondary to BPH where α-blocker therapy is planned should be asked about planned cataract surgery. Those with planned cataract surgery should avoid the initiation of α-blockers until their cataract surgery is completed.
References
1Lowe F (1999). Alpha-1-adrenoceptor blockade in the treatment of benign prostatic hyperplasia. Prostate Cancer and Prostatic Diseases 2:110–19.
2McConnell JD, Barry MD, Bruskewitz RC, et al. (1994). The BPH Guideline Panel. Benign Prostatic Hyperplasia: Diagnosis and Treatment. Clinical Practice Guideline. Agency for Health Care Policy and Research, publication No. 94–0582. Rockville, MD: Public Health Service, US Department of Health and Human Sciences.
3Boyle P, Robertson C, Manski R, et al. (2001). Meta-analysis of randomized trials of terazosin in the treatment of benign prostatic hyperplasia. Urology 58:717–22.
4de la Rosette, Kortmann B, Rossi C, et al. (2002). Long term risk of retreatment of patients using alpha blockers for lower urinary tract symptoms. J Urol 167:1734–9.
5Chang D, Campbell J (2005). Intraoperative floppy iris syndrome associated with tamsulosin. J Cataract Refract Surg 31:664.
6Bell C, Hatch WV, Fischer HD, et al. (2009). Association between tamsulosin and serious ophthalmic adverse events in older men following cataract surgery. JAMA 301:1991–6.
7American Urological Association (2010). The AUA 2010 BPH Guidelines [online]. Available from:  http://www.auanet.org.
http://www.auanet.org.
5ARIs inhibit the conversion of testosterone to DHT, the more potent androgen in the prostate. This causes shrinkage of the prostatic epithelium, and therefore a reduction in prostate volume, thereby reducing the ‘static’ component of BPE. This takes some months to occur, so urinary symptoms will not improve initially. Finasteride is a competitive inhibitor of the enzyme 5AR (type II isoenzyme), which converts testosterone to DHT. Finasteride therefore lowers serum and intraprostatic DHT levels. Epristeride is a dual inhibitor of 5AR. Whether it has any clinically significant advantages over finasteride remains to be established.
•Finasteride: a number of large studies have shown symptom improvement over placebo in the order of 2–3 points on the IPSS and improvements in flow rate in the order of 1–2mL/s [SCARP1 (Scandinavian BPH Study Group), PROSPECT2 (PROScar Safety Plus Efficacy Canadian Two-year study), PROWESS Study Group,3 and more recently PLESS4 (Proscar Long-term Efficacy and Safety Study)]. The PLESS data also show a small reduction in the risk of urinary retention.
•Dutasteride: evidence for its efficacy is derived from a 2y RCT with an open-label extension;6 SMART 1, which evaluated the effect of a placebo-controlled withdrawal of an α-blocker from a combination therapy arm;7 and from the CombAT study (comparison of dutasteride vs tamsulosin vs dutasteride + tamsulosin).8
Generally speaking, fairly mild. Principally centre around sexual problems (e.g. loss of libido, 5%; impotence, 5%; reduced volume of ejaculate in a few per cent).
The PLESS data5 have been widely publicized as showing a substantial reduction in the risk of urinary retention. In this 4y follow-up study, 42 of 1471 men on finasteride went into urinary retention (3%), while 99 of 1404 men on placebo experienced an episode of retention (7%). This represents an impressive 43% relative reduction in risk in those taking finasteride. However, the absolute risk reduction over a 4y period is a less impressive 4%. So finasteride does reduce the risk of retention, but it is reducing the risk of an event which is actually quite rare, as suggested by the fact that 93% of men on placebo in this study did not experience retention over a 4y period. Put another way, to prevent one episode of retention, 25 men would have to continue treatment with finasteride for 4y.
Finasteride suppresses vascular endothelial growth factor (VEGF). Shrinking large vascular prostates probably helps reduce the frequency of haematuria in men with BPH.5
References
1Andersen JT, Ekman P, Wolf H, et al. (1995). Can finasteride reverse the progress of benign prostatic hyperplasia? A two-year placebo-controlled study. The Scandinavian BPH Study Group. Urology 46:631–7.
2Nickel J, Fradet Y, Boake RC, et al. (1995). Efficacy and safety of finasteride therapy for benign prostatic hyperplasia: results of a 2-year randomised controlled trial (the PROSPECT study). PROscar Safety Plus Efficacy Canadian Two Year Study. Can Med Assoc J 155:1251–9.
3Marberger MJ (1998). Long-term effects of finasteride in patients with benign prostatic hyperplasia: a double-blind, placebo-controlled, multicenter study. PROWESS Study Group. Urology 51:677–86.
4McConnell JD, Bruskewitz R, Walsh PC, et al. (1998). The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia (PLESS). N Engl J Med 338:557–63.
5Foley SJ, Soloman LZ, Wedderburn AW, et al. (2000). Finasteride for haematuria due to BPH. A prospective study of the natural history of hematuria associated with BPH and the effect of finasteride. J Urol 163:496–8.
6Roehrborn C, Lukkarinen O, Mark S, et al. (2005). Long-term sustained improvement in symptoms of benign prostatic hyperplasia with the dual 5alpha-reductase inhibitor dutasteride: results of 4-year studies. BJU Int 96:572–7.
7Barkin J, Guimaraes M, Jacobi G, et al. (2003). Alpha-blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5alpha-reductase inhibitor dutasteride. Eur Urol 44:461–6.
8Roehrborn C, Siami P, Barkin J, et al. (2008). The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT study. J Urol 179:616.
A combination of an α-blocker and a 5ARI. The studies include:
•MTOPS study (Medical Therapy of Prostatic Symptoms):1 3047 men; mean prostate volume 36mL. This combination prevented progression of BPH, when compared with either drug alone (progression being defined as a worsening of symptom score by 4 or more or the development of complications such as UTI or acute urinary retention).
•Veterans Affairs Combination Therapy Study:2 1200 men randomized to placebo, finasteride, terazosin, or both terazosin and finasteride. At 1y follow-up, relative to placebo, finasteride had reduced the symptom score by an average of 3 points, whereas terazosin alone or in combination with finasteride had reduced the symptom score by an average of 6 points.
•PREDICT study (Prospective European Doxazosin and Combination Therapy):3 randomized >1000 men to placebo, finasteride, doxazosin, or both finasteride and doxazosin. Difference in symptom score at baseline and 1y were: placebo –5.7, finasteride –6.6, doxazosin –8.3, and combination therapy –8.5.
•ALFIN study (alfuzosin, finasteride, and combination in the treatment of BPH):4 1000 men randomized to alfuzosin, finasteride, or both. At 6 months, improvement in the IPSS was not significantly different in the alfuzosin vs the combination group.
•CombAT trial:5 4844 men; mean prostate volume 55mL. Compared tamsulosin, dutasteride, and a combination of both. Prostate volume was >30mL (TRUS). Only 2y data are available as of 2011. Combination therapy resulted in significantly greater improvements in symptoms, compared to dutasteride, from month 3 and tamsulosin from month 9 and significantly greater improvement in peak urinary flow from month 6. There was a significant increase in drug-related adverse events with combination therapy. Analyses of the primary endpoints (4y progression of LUTS, urinary retention, and need for prostate surgery) are awaited.
Thus, most studies, except for MTOPS, suggest that combination therapy is no more useful than an α-blocker alone. Disadvantages of combination therapy: greater risk of side effects, no additional benefit over α-blockers alone in most men, need for treatment for >1y before an improvement in symptoms is seen, sexual side effects.
In the Prostate Cancer Prevention Trial,6 18 000 men were randomized to finasteride or placebo over a 7y period. Those in the finasteride group had a lower prevalence of prostate cancer detected on prostate biopsy (26.5% of men receiving finasteride had a positive biopsy vs 29.5% in the placebo group). However, higher-grade tumours (i.e. biologically more aggressive than low-grade cancers) were commoner in the finasteride group (there was a 1.3% increase in high-grade cancers in the finasteride group). The jury is out on whether finasteride causes higher-grade cancers or whether these findings are a histological or sampling artefact. Finasteride increases the ability ( sensitivity) of both PSA, DRE, and prostate biopsy to diagnose high-grade prostate cancer7,8—so-called cytoreduction of the prostate, leading to a greater likelihood of finding high-grade cancer (the argument is that finasteride has less of an effect on PSA reduction in men with high-grade than low-grade cancers, so men with high-grade cancer are more likely to have an elevated PSA, and therefore to undergo prostate biopsy and thus cancer detection).
sensitivity) of both PSA, DRE, and prostate biopsy to diagnose high-grade prostate cancer7,8—so-called cytoreduction of the prostate, leading to a greater likelihood of finding high-grade cancer (the argument is that finasteride has less of an effect on PSA reduction in men with high-grade than low-grade cancers, so men with high-grade cancer are more likely to have an elevated PSA, and therefore to undergo prostate biopsy and thus cancer detection).
References
1McConnell JD, Roehrborn CG, Bautista OM, et al. (2003). The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 349:2387–98.
2Lepor H, Williford WO, Barry MJ, et al. (1996). The efficacy of terazosin, finasteride, or both in benign prostatic hypertrophy. N Engl J Med 335:533–39.
3Kirby RS, Roerborn C, Boyle P, et al. (2003). Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the Prospective European Doxazosin and Combination Therapy (PREDICT) trial. Urology 61:119–26.
4Debruyne FM, Jardin A, Colloi D, et al. (1998). Sustained-release alfuzosin, finasteride and the combination of both in the treatment of benign prostatic hyperplasia. Eur Urol 34:169–75.
5Roehrborn C, Siami P, Barkin J, et al. (2008). The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT study. J Urol 179:616.
6Thompson IM, Goodman PJ, Tangen CM, et al. (2003). The influence of finasteride on the development of prostate cancer. N Engl J Med 349:215–24.
7Thompson IM, Chi C, Ankerst DP, et al. (2006). Effect of finasteride on the sensitivity of PSA for detecting prostate cancer. J Natl Cancer Inst 98:1128.
8Thompson IM, Tangen CM, Goodman PJ, et al. (2007). Finasteride improves the sensitivity of digital rectal examination for prostate cancer detection. J Urol 177:1749.
For a man with frequency, urgency, and urge incontinence—symptoms suggestive of an OAB—consider prescribing an anticholinergic (e.g. oxybutynin, tolterodine, trospium chloride, or flavoxate). There is the concern that these drugs could precipitate urinary retention in men with BOO (because they block parasympathetic/cholinergic-mediated contraction of the detrusor), but the risk of this occurring is probably very low, even in men with urodynamically proven BOO.1
An alternative drug treatment for BPH symptoms, and one which is widely used in Europe and increasingly in North America, is phytotherapy. Fifty per cent of all medications consumed for BPH symptoms are phytotherapeutic ones.2
Examples include the Saw palmetto plant (Serenoa repens) and extracts from the stinging nettle (Urtica dioica), amongst several others. While previous editions of this book quoted studies, including a meta-analysis, that suggested similar efficacy to 5ARIs in terms of improvements in symptoms and flow rates,2,3 more recent studies have generally failed to confirm a clinically important role for Saw palmetto in the management of BPH.4,5
NICE in the UK does not recommend phytotherapy for LUTS in men ( www.nice.org.uk/CG97) and similarly, in the United States, phytotherapy is no longer recommended by the AUA 2010 BPH Guidelines (
www.nice.org.uk/CG97) and similarly, in the United States, phytotherapy is no longer recommended by the AUA 2010 BPH Guidelines ( http://www.auanet.org).
http://www.auanet.org).
References
1Reynard J (2004). Does anticholinergic medication have a role for men with lower urinary tract symptoms/benign prostatic hyperplasia either alone or in combination with other agents? Curr Opin Urol 14:13–16.
2Wilt T, Ishani A, Stark G, et al. (1998). Saw palmetto extracts for treatment of benign prostatic hyperplasia: a systematic review. JAMA 280:1604–8.
3Wilt T, Ishani A, Rutks I, et al. (2000). Phytotherapy for benign prostatic hyperplasia. Public Health Nutr 3:459.
4Bent S, Kane C, Shinohara K, et al. (2006). Saw palmetto for benign prostatic hyperplasia. N Engl J Med 354:557–66.
5Shi R, Xie Q, Gang X, et al. (2008). Effect of saw palmetto soft gel capsule on lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized trial in Shanghai, China. J Urol 179:610.
In 1989, Roos reported a seemingly higher mortality and reoperation rate after TURP, when compared with open prostatectomy.1 This, combined with other studies suggesting that symptomatic outcome after TURP was poor in a substantial proportion of patients and that TURP was associated with substantial morbidity, prompted the search for less invasive treatments.
The two broad categories of alternative surgical techniques are minimally invasive and invasive. All are essentially heat treatments, delivered at variable temperature and power and producing variable degrees of coagulative necrosis (minimally invasive) of the prostate or vaporization of prostatic tissue (invasive).
Low-level radiofrequency is transmitted to the prostate via a transurethral needle delivery system; the needles which transmit the energy are deployed in the prostatic urethra once the instrument has been advanced into the prostatic urethra. It is done under local anaesthetic (LA), with or without IV sedation. The resultant heat causes localized necrosis of the prostate.
Improvements in symptom score and flow rate are modest. Side effects include bleeding (one-third of patients), UTI (10%), and urethral stricture (2%). No adverse effects on sexual function have been reported.2 Concerns remain with regard to long-term effectiveness.
The UK NICE does not recommend TUNA for symptoms associated with prostatic enlargement ( https://www.nice.org.uk/guidance/cg97/chapter/1-recommendations).
https://www.nice.org.uk/guidance/cg97/chapter/1-recommendations).
Microwave energy can be delivered to the prostate via an intraurethral catheter (with a cooling system to prevent damage to the adjacent urethra), producing prostatic heating and coagulative necrosis. Subsequent shrinkage of the prostate and thermal damage to adrenergic neurons (i.e. heat-induced adrenergic nerve block) relieves obstruction and symptoms.
Many reports of TUMT treatment are open studies, with all patients receiving treatment (no ‘sham’ treatment group where the microwave catheter is inserted, but no microwave energy is given—this results in 10-point symptom improvements in ~75% of men). Compared with TURP, TUMT results in symptom improvement in 55% of men, and TURP in 75%. Sexual side effects after TUMT (e.g. impotence, retrograde ejaculation) are less frequent than after TURP, but the catheterization period is longer and UTI and irritative urinary symptoms are commoner.3 EAU guidelines state that TUMT ‘should be reserved for patients who prefer to avoid surgery or who no longer respond favourably to medication’. TUMT is still a popular treatment in the United States.
The UK NICE guideline on prostatic enlargement is found here:  https://www.nice.org.uk/guidance/cg97/chapter/1-recommendations.
https://www.nice.org.uk/guidance/cg97/chapter/1-recommendations.
A focused ultrasound beam can be used to induce a rise in temperature in the prostate or indeed in any other tissue to which it is applied. For HIFU treatment of the prostate, a transrectal probe is used. A general anaesthetic (GA) or heavy IV sedation is required during the treatment. It is regarded as an investigational therapy.
The UK NICE does not recommend HIFU for symptoms associated with prostatic enlargement ( https://www.nice.org.uk/guidance/cg97/chapter/1-recommendations).
https://www.nice.org.uk/guidance/cg97/chapter/1-recommendations).
UroLift® aims to open up the prostatic urethra by retracting the lateral lobes using anchoring implants inserted transurethrally. This treatment is suitable for prostates of <100g without a median lobe. The median lobe cannot be anchored posteriorly due to the risk of rectal injury. Its advantages over TURP are that it is simpler to learn, teach, and perform and is quicker to perform and blood loss and hospital stay are less. It is usually done as a day case. It is indicated in men with voiding LUTS and small to moderate-sized prostates (<100g) with no median lobe.
Insert implants at 2 and 10 o’clock in the prostatic urethra, about 1cm distal to the bladder neck. It is important the metal clips are not within the bladder, else they might encrust. Repeat at intervals towards the veru to create good retraction of the lateral lobes. Between two and six implants may be needed, depending on the size and length of the prostate.
UroLift® has been shown to improve LUTS without compromising sexual function.4,5 UroLift® is supported by NICE guidance.6
References
1Roos NP, Wennberg J, Malenka DJ, et al. (1989). Mortality and reoperation after open and transurethral resection of the prostate for benign prostatic hyperplasia. N Engl J Med 320:1120–4.
2Fitzpatrick JM, Mebust WK (2002). Minimally invasive and endoscopic management of benign prostatic hyperplasia. In: Walsh PC, Retik AB, Vaughan ED, Wein AJ (eds). Campbell’s Urology, 8th edn. Philadelphia, PA: WB Saunders.
3D’Ancona FCH, Francisca EAE, Witjes WPJ, et al. (1998). Transurethral resection of the prostate vs high-energy thermotherapy of the prostate in patients with benign prostatic hyperplasia: long-term results. Br J Urol 81:259–64.
4Sønksen J, Barber NJ, Speakman MJ, et al. (2015). Prospective, randomized, multinational study of prostatic urethral lift versus transurethral resection of the prostate: 12-month results from the BPH6 study. Eur Urol 68:643–52.
5Roehrborn CG, Rukstalis DB, Barkin J, et al. (2015). Three-year results of the prostatic urethral L.I.F.T. study. Can J Urol 22:7772–82.
6National Institute for Health and Care Excellence (2015). UroLift for treating lower urinary tract symptoms of benign prostatic hyperplasia. Medical technologies guidance [MTG26]. Available from:  https://www.nice.org.uk/guidance/mtg26.
https://www.nice.org.uk/guidance/mtg26.
Vaporizes and dessicates the prostate. TUVP seems to be as effective as TURP for symptom control and relief of BOO, with durable (5y) results. Operating time and inpatient hospital stay are equivalent. Requirement for blood transfusion may be slightly less after TUVP.1,2 TUVP does not provide tissue for histological examination, so prostate cancers cannot be detected. NICE in the UK has endorsed TUVP as a surgical treatment option for prostatic symptoms.3
Several different techniques of ‘laser prostatectomy’ evolved during the 1990s. Essentially, in the year 2012, we are left with just holmium laser prostatectomy (endorsed by NICE 2010 Guidelines) and the green light laser (NICE 2010 Guidelines recommending its use only in the context of RCTs).3
Performed using a probe consisting of a Nd:YAG laser adjacent to an ultrasound transducer.
This side-firing system used a mirror to reflect, or a prism to refract, the laser energy at various angles (usually 90°) from a laser fibre located in the prostatic urethra onto the surface of the prostate. The principal tissue effect was one of coagulation, with subsequent necrosis.
Produces a greater degree of vaporization than VLAP, allowing the immediate removal of tissue.
Performed by transurethral placement of a laser fibre directly into the prostate that produces a zone of coagulative necrosis some distance from the prostatic urethra.
TULIP, VLAP, contact laser prostatectomy, and ILP have been succeeded by holmium laser prostatectomy.
Also known as ‘greenlight’ photoselective vaporization of the prostate (PVP). An yttrium-aluminium-garnet (YAG) laser light is shone through a potassium titanyl phosphate (KTP) crystal, doubling the frequency and halving the emitted light wavelength to 532nm. This is in the green part of the visible spectrum and is strongly absorbed by Hb, producing efficient prostate tissue vaporization (Fig. 4.1). KTP energy is poorly absorbed by water/saline (the irrigant), and therefore, non-contact vaporization is possible. The benefits include less heating of the delivery fibre, which can last for a longer period of time. Laser systems of 80 and 120W are available. In the 80W system, ~100kJ will be delivered to the average prostate in 30min by rapid pulses of ‘quasi-continuous’ energy. Laser heat is concentrated over a small area, which allows rapid vaporization of tissue, with minimal coagulation of underlying structures (2mm rim of coagulated tissue is left), but creating effective haemostasis. It can be used for larger prostates (>100mL)4 and higher-risk patients on anticoagulants.5
The 2010 NICE Guidelines on the management of LUTS in men state that laser vaporization techniques, of which greenlight laser is one, should be offered only as part of an RCT.3
Using a KTP/532 80W laser (Laserscope®), a 6F side-firing fibre is placed through a 24F continuous irrigation cystoscope, with normal saline irrigation. Generally, the median lobe is treated first, then the lateral lobes, using a sweeping movement of the laser fibre across the prostate, starting at the bladder neck and working distally to the level of the verumontanum. No tissue is available for histology.
KTP laser prostatectomy can be performed safely as a day surgery operation, and in selected cases, a catheter may not be needed post-operatively or can be removed within 24h. It provides a virtually bloodless operation, with no reported need for blood transfusion, even in anticoagulated patients. Irrigation with saline or water avoids the risk of transurethral resection (TUR syndrome). The incidence of retrograde ejaculation is lower than TURP (8.3–52%),5,6 with no reported cases of new erectile dysfunction. When directly compared to TURP, equivalent short-term efficacies are seen, but with significantly shorter catheterization times and inpatient stays in the laser group.7,9
Short- and medium-term outcomes (up to 5y follow-up) demonstrate sustained and statistically significant improvements in symptom scores (IPSS/AUA), flow rate, and PVR volumes.5,6,7,8,9,10
A randomized study comparing greenlight and TURP (the GOLIATH study) with 2y follow-up11 indicated longer operating time, shorter catheter dwell time, and shorter hospital stay with greenlight laser but showed no difference in voiding metrics, compared to TURP. There were fewer reported adverse events with greenlight.
Haematuria (1–11%); dysuria (2–21%); acute urinary retention (1–11%); reoperation rate (0–5% at 1y).
Prostate artery embolization (PAE) is an interventional radiology technique which involves injecting small particles into the blood vessels that supply the prostate. This blocks the blood supply, with the aim of shrinking the prostate through necrosis.
•Perfomed under LA and sedation.
•May be performed on patients not fit for anaesthesia.
The evidence for PAE remains limited at this time. Data available suggest that it might be useful for men with larger vascular prostates that are not suitable for surgical intervention. As many as 25% of patients may not have an improvement in IPSS.
Following CT angiographic planning, a percutaneous transfemoral approach is used to super-selectively catheterize the small prostate arteries. These are embolized with polyvinyl alcohol (PVA), gelatin sponge, and other synthetic biocompatible materials. It is a technically challenging procedure.
Small RCTs12,13 suggest good improvements in voiding parameters. PAE had higher technical and clinical failures. However, long-term data are not available at this time, and there is a paucity of RCT data. Currently, the UK NICE guidance recommends PAE in a research setting with the involvement of a multidisciplinary team (MDT).
The holmium laser is a pulsed, solid-state laser with a wavelength of 2140nm, which is strongly absorbed by water. It is absorbed into prostate tissue to a depth of 0.4mm, and the heat created (>100°C) causes good tissue vaporization, while causing coagulation of small to medium-sized blood vessels. The coagulative depth is about 2–3mm beyond the tissue that has been vaporized. The irrigant is normal saline, so the risk of TUR syndrome is avoided.
HoLEP (endorsed by 2010 NICE Guidelines on the management of LUTS in men, available from:  http://www.nice.org.uk/CG97) is particularly useful for treating larger prostates. An end-firing laser fibre is used to cut grooves into the prostate down to the level of the capsule. The prostate lobes are then dissected off and pushed into the bladder where a mechanical morcellator is used to fragment and aspirate the tissue. HoLEP is technically more difficult to master than laser vaporization and has a longer learning curve, but the overall results are at least equivalent to TURP, with fewer associated risks.
http://www.nice.org.uk/CG97) is particularly useful for treating larger prostates. An end-firing laser fibre is used to cut grooves into the prostate down to the level of the capsule. The prostate lobes are then dissected off and pushed into the bladder where a mechanical morcellator is used to fragment and aspirate the tissue. HoLEP is technically more difficult to master than laser vaporization and has a longer learning curve, but the overall results are at least equivalent to TURP, with fewer associated risks.
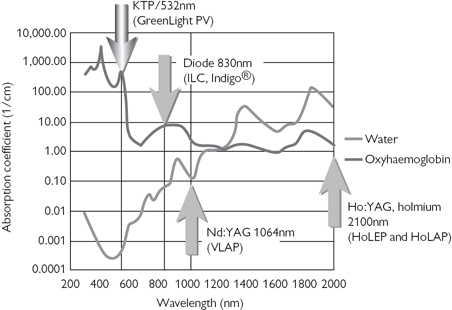
Fig. 4.1 Absorption curve of water and oxyhaemoglobin. From Laserscope® Physician training manual 2006.
Reproduced with permission from the American Medical Systems Inc, Minnesota.
In a randomized trial comparing holmium laser enucleation with TURP for prostates of >40g, HoLEP was equivalent to TURP, but with those in the HoLEP group having a shorter catheterization time and hospital stay. A larger volume of prostatic tissue was removed.14 Long-term follow-up (7y) demonstrates sustained significant improvements in symptom scores and flow rates.15 In a direct comparison with open prostatectomy, HoLEP has also demonstrated equivalent improvement in symptom scores and flow rates at 3y follow-up.16
A side-firing dual-wavelength fibre is used in a near-contact mode to vaporize prostatic tissue circumferentially to produce a satisfactory channel. Original techniques used 60W lasers; however, lasers of up to 100W are now available. Symptom improvements are sustained in the long term,17 and when directly compared with TURP, similar efficacy was seen in the short term, but with shorter hospital stay and catheter times in the HoLAP group and less bleeding than for TURP.18 Studies suggest overall, it is most effective for smaller prostate glands.
This technique copies that of TURP, whereby the precise cutting ability of the holmium laser is used to remove pieces of prostate down to the capsule to create a large and relatively bloodless channel. It can be used on prostate glands of all sizes. Again, it has short catheterization times and hospital stays and is associated with minimal post-operative dysuria.19
References
1Hammadeh MY, Madaan S, Hines J, Philp T (2000). Transurethral electrovaporization of the prostate after 5 years; is it effective and durable? BJU Int 86:648–51.
2Mc Allister WJ, Karim O, Plail RO, et al. (2003). Transurethral electrovaporization of the prostate: is it any better than conventional transurethral resection of the prostate? BJU Int 91:211–14.
3National Institute for Health and Care Excellence (2010). Lower urinary tract symptoms in men: management. Clinical Guideline [CG97]. Available from:  http://www.nice.org.uk/CG97.
http://www.nice.org.uk/CG97.
4Sandhu JS, Ng C, Vanderbrink BA, et al. (2004). High-power potassium-titanyl-phosphate photoselective laser vaporisation of prostate for treatment of benign prostatic hyperplasia in men with large prostates. J Urol 64:1155–9.
5Sandhu JS, Ng CK, Gonzalez RR, et al. (2005). Photoselective laser vaporization prostatectomy in men receiving anti-coagulants. J Endourol 19:1196–8.
6Sarica K, Alkan E, Lüleci H, et al. (2005). Photoselective vaporization of the enlarged prostate with KTP laser: long-term results in 240 patients. J Endourol 19:1199–202.
7Bachmann A, Schürch L, Ruszat R, et al. (2005). Photoselective vaporisation (PVP) versus transurethral resection of the prostate (TURP): a prospective bi-centre study of perioperative morbidity and early functional outcome. Eur Urol 48:965–72.
8Bouchier-Hayes DM, Anderson P, Van Appledorn S, et al. (2006). KTP laser versus transurethral resection: early results of a randomised trial. J Endourol 20:580–5.
9Sandhu JS, Ng C, Vanderbrink BA, et al. (2004). High-power potassium-titanyl-phosphate photoselective laser vaporisation of prostate for treatment of benign prostatic hyperplasia in men with large prostates. J Urol 64:1155–9.
10Malek RS, Kuntzman RS, Barrett DM (2005). Photoselective potassium-titanyl-phosphate laser vaporisation of the benign obstructive prostate: observations on long-term outcomes. J Urol 174:1344–8.
11Thomas JA, Tubaro A, Barber N, et al. (2016). A Multicenter Randomized Noninferiority Trial Comparing GreenLight-XPS Laser Vaporization of the Prostate and Transurethral Resection of the Prostate for the Treatment of Benign Prostatic Obstruction: Two-yr Outcomes of the GOLIATH Study. Eur Urol 69:94–102.
12Gao Y.A., Huang Y., Zhang R., et al. (2014). Benign prostatic hyperplasia: prostatic arterial embolization versus transurethral resection of the prostate: a prospective, randomized, and controlled clinical trial. Radiology 270:920–8.
13Carnevale FC, Iscaife A, Yoshinaga EM, et al. (2016). Transurethral Resection of the Prostate (TURP) Versus Original and PErFecTED Prostate Artery Embolization (PAE) Due to Benign Prostatic Hyperplasia (BPH): preliminary results of a single center, prospective, urodynamic-controlled analysis. Cardiovasc Intervent Radiol 39:44–52.
14Wilson LC, Gilling PJ, Williams A, et al. (2006). A randomised trial comparing holmium laser enucleation versus transurethral resection in the treatment of prostates larger than 40 grams: results at 2 years. Eur Urol 50:569–73.
15Elzayat EA, Habib EI, Elhilali MM (2005). Holmium laser enucleation of the prostate: a size-independent new ‘gold standard’. Urology 66:108–13.
16Kuntz RM, Ahyai S, Lehrich K (2006). Transurethral holmium laser enucleation of the prostate compared with transvesical open prostatectomy: 3 years follow-up of a randomised trial. Proc SPIE 6078:11.
17Tan AHH, Gilling PJ, Kennett KM, et al. (2003). Long-term results of high-power holmium laser vaporization (ablation) of the prostate. BJU Int 92:707–9.
18Mottet N, Anidjar M, Bourdon O, et al. (1999). Randomised comparison of transurethral electroresection and holmium:YAG laser vaporization for symptomatic benign prostatic hyperplasia. J Endourol 13:127–30.
19Gilling PJ, Cass CB, Cresswell MD, et al. (1996). The use of holmium laser in the treatment of benign prostatic hyperplasia. J Endourol 5:459–61.
Removal of the obstructing tissue of BPH or obstructing prostate cancer from within the prostatic urethra, leaving the compressed outer zone intact (the ‘surgical capsule’). An electrically heated wire loop is used, through a resectoscope, to cut the tissue and diathermy bleeding vessels. The cut ‘chips’ of the prostate are pushed back into the bladder by the flow of irrigating fluid and, at the end of resection, are evacuated using specially designed ‘evacuators’—a plastic or glass chamber attached to a rubber bulb which allows fluid to be flushed in and out of the bladder.
•Bothersome LUTS that fail to respond to changes in lifestyle or medical therapy.
•Recurrent acute urinary retention.
•Renal impairment due to BOO (high-pressure chronic urinary retention).
•Recurrent haematuria due to BPE.
•Bladder stones due to prostatic obstruction.
•TURP not technically possible (e.g. limited hip abduction).
•Failed TURP (e.g. because of bleeding).
•Urethra too long for the resectoscope to gain access to the prostate.
•Presence of bladder stones which are too large for endoscopic cystolitholapaxy, combined with marked enlargement of the prostate.
•Prior prostatectomy in which most of the gland has been resected or removed; this obliterates the tissue planes.
The preferred operation if enlargement of the prostate involves mainly the middle lobe. The bladder is opened, the mucosa around the protruding adenoma is incised, and the plane between the adenoma and the capsule is developed to enucleate the adenoma. A 22Ch urethral catheter and a suprapubic catheter (SPC) are left, together with a retropubic drain. The urethral catheter is removed in 3 days, and the SPC is clamped at 6 days, with its removal 24h later. The drain can be removed 24h after this (day 8).
Popularized by Terence Millin (Ireland, 1947). Compared with the suprapubic (transvesical) approach, it allows more precise anatomic exposure of the prostate, thus giving better visualization of the prostatic cavity, which allows more accurate removal of the adenoma, better control of bleeding points, and more accurate division of the urethra, so reducing the risk of incontinence.
As well as the contraindications noted, the retropubic approach should not be employed when the middle lobe is very large because it is difficult to get behind the middle lobe and so to incise the mucosa (safely) distal to the ureters.
The prostate is exposed by a Pfannenstiel or lower midline incision. Haemostasis is achieved before enucleating the prostate by ligating the dorsal vein complex with sutures placed deeply through the prostate. The prostatic capsule and adenoma are incised transversely with the diathermy just distal to the bladder neck. The plane between the capsule and adenoma is found with scissors and developed with a finger. Sutures are used for haemostasis. A wedge of the bladder neck is resected. A catheter is inserted and left for 5 days, and the transverse capsular incision is closed. A large tube drain (30Ch Robinson’s) is left for 1–2 days.
•Rectal perforation (close and cover with a colostomy).
Painful inability to void, with relief of pain following drainage of the bladder by catheterization.
The combination of reduced or absent urine output with lower abdominal pain is not, in itself, enough to make a diagnosis of acute retention. Many acute surgical conditions cause abdominal pain and fluid depletion, the latter leading to reduced urine output, and this reduced urine output can give the erroneous impression that the patient is in retention when, in fact, they are not. Thus, central to the diagnosis is the presence of a large volume of urine which, when drained by catheterization, leads to resolution of the pain. What represents ‘large’ has not been strictly defined, but volumes of 500–800mL are typical. Volumes <500mL should lead one to question the diagnosis. Volumes >800mL may be defined as acute-on-chronic retention.
Normal micturition requires:
•Afferent input to the brainstem and cerebral cortex.
•Coordinated relaxation of the external sphincter.
•Sustained detrusor contraction.
•The absence of an anatomic obstruction in the outlet of the bladder.
Four broad mechanisms can lead to urinary retention:
• urethral resistance (i.e. BOO).
urethral resistance (i.e. BOO).
•Low bladder pressure (i.e. impaired bladder contractility).
•Interruption of sensory or motor innervation of the bladder.
•Central failure of coordination of bladder contraction with external sphincter relaxation.
•Malignant enlargement of prostate.
•Urethral stricture; prostatic abscess.
Urinary retention in men is either spontaneous or precipitated by an event. Precipitated retention is less likely to recur once the event which caused it has been removed. Spontaneous retention is more likely to recur after a trial of catheter removal and therefore to require definitive treatment (e.g. TURP). Precipitating events include anaesthetic and other drugs (anticholinergics, sympathomimetic agents such as ephedrine in nasal decongestants), non-prostatic abdominal or perineal surgery, and immobility following surgical procedures.
Advancing age is a strong predictor of the risk of urinary retention in men. Other factors that predict risk of urinary retention are the presence of LUTS (higher symptom scores), previous episodes of spontaneous retention, low Qmax (though there is some debate), and larger prostate volume. Elevated PVR does not seem to predict the risk of retention and nor does treatment with anticholinergic medication.1
Causes of acute urinary retention in either sex
•Haematuria, leading to clot retention.
•Drugs (as mentioned in the text).
•Pain (adrenergic stimulation of the bladder neck).
•Post-operative retention (see  p. 107).
p. 107).
•Sacral (S2–4) nerve or compression or damage, resulting in detrusor areflexia—cauda equina compression (due to prolapsed L2–L3 disc or L3–L4 intervertebral disc pressing on sacral nerve roots of the cauda equina, trauma to vertebrae, benign or metastatic tumours).
•Suprasacral SCI [results in loss of coordination of external sphincter relaxation with detrusor contraction—so-called detrusor sphincter dyssynergia (DSD)—so the external sphincter contracts when the bladder contracts).
•Radical pelvic surgery damaging the pelvic parasympathetic plexus (radical hysterectomy, abdominoperineal resection): unilateral injury to the pelvic plexus (preganglionic parasympathetic and post-ganglionic sympathetic neurons) denervates motor innervation of the detrusor muscle.
•Pelvic fracture rupturing the urethra (more likely in men than women).
•Neurotropic viruses involving sensory dorsal root ganglia of S2–4 (herpes simplex or zoster).
•MS [can affect any part of the central nervous system (CNS) (Fig. 4.2)]; retention caused by detrusor areflexia or DSD.
•Diabetic cystopathy (causes sensory and motor dysfunction).
•Damage to dorsal columns of the spinal cord, causing loss of bladder sensation (tabes dorsalis, pernicious anaemia).
•Pelvic prolapse (cystocele, rectocele, uterine); urethral stricture; urethral diverticulum.
•Post-surgery for ‘stress’ incontinence.
•Pelvic masses (e.g. ovarian masses).
•Fowler’s syndrome:  electromyographic (EMG) activity can be recorded in the external urethral sphincters of these women (which, on ultrasound, is of
electromyographic (EMG) activity can be recorded in the external urethral sphincters of these women (which, on ultrasound, is of  volume) and is hypothesized to cause impaired relaxation of the external sphincter; occurs in premenopausal women, often in association with polycystic ovaries.
volume) and is hypothesized to cause impaired relaxation of the external sphincter; occurs in premenopausal women, often in association with polycystic ovaries.
Instrumentation of the LUT; surgery to the perineum or anorectum; gynaecological surgery; bladder overdistension; reduced sensation of bladder fullness; pre-existing prostatic obstruction; epidural anaesthesia. Post-partum retention is not uncommon, particularly with epidural anaesthesia and instrumental delivery.
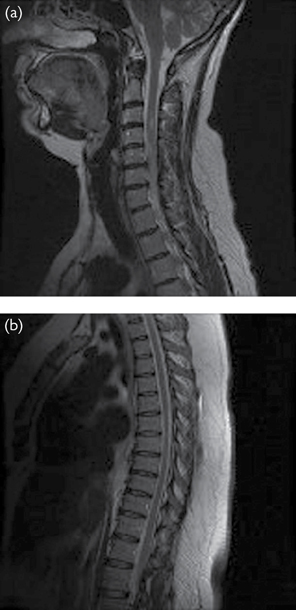
Fig. 4.2 MRI of the cervical and sacral cord in a young patient presenting with urinary retention. The patient had undiagnosed multiple sclerosis. Signal changes are seen in the cervical, thoracic, and lumbosacral cord.
Reference
1Kaplan SA, Wein AJ, Staskin DR, Roehrborn CG, Steers WD (2008). Urinary retention and post-void residual urine in men: separating truth from tradition. J Urol 180:47–54.
Urethral catheterization to relieve pain (suprapubic catheterization if the urethral route not possible). Record the volume drained—this confirms the diagnosis, determines subsequent management, and provides prognostic information with regard to outcome from this treatment.
Discuss trial without catheter (TWOC) with the patient. Precipitated retention often does not recur; spontaneous retention often does. Fifty per cent with spontaneous retention will experience a second episode of retention within the next week or so, and 70% within the next year. A maximum flow rate (Qmax) of <5mL/s and low voiding detrusor pressure predict subsequent retention. Thus, while most will require definitive treatment (e.g. TURP), a substantial minority will get away without needing surgery.
In men, mortality in the first year after acute urinary retention is 2–3 times higher than the general ♂ population. Not surprisingly, it increases with age (Table 4.5). A substantial proportion of this  mortality seems to be linked to comorbidity in these men.1 Thus, when deciding whether to ‘subject’ a man to TURP for retention, remember that acute retention represents a harbinger of severe systemic disease. A careful assessment for comorbidity (cardiovascular disease, diabetes, chronic pulmonary disease) should be made, and referral for appropriate specialist advice on management of this comorbidity should be considered.
mortality seems to be linked to comorbidity in these men.1 Thus, when deciding whether to ‘subject’ a man to TURP for retention, remember that acute retention represents a harbinger of severe systemic disease. A careful assessment for comorbidity (cardiovascular disease, diabetes, chronic pulmonary disease) should be made, and referral for appropriate specialist advice on management of this comorbidity should be considered.
Table 4.5 One-year mortality rates in men with acute retention
| Age (y) | Spontaneous acute retention (%) | Precipitated acute retention (%) |
| 45–54 | 4 | 10 |
| 85or over | 33 | 45 |
| All ages | 15 | 25 |
•Prostate-shrinking drugs [5ARIs in those with benign-feeling prostates, luteinizing hormone-releasing hormone (LHRH) agonists in those with malignant-feeling prostates on DRE, confirmed by TRUS-guided prostate biopsy), followed by a TWOC several months later.
•Long-term urethral or suprapubic catheter.
•Clean intermittent self-catheterization (CISC)—not a realistic option for most men, but some will be able and happy to do this.
CISC, either until normal voiding function recovers or permanently if it does not. Fowler’s syndrome—sacral neuromodulation (e.g. Medtronic InterStim).
•Relative risks of TURP for retention vs TURP for LUTS: post-operative complications, 26:1; blood transfusion, 2.5:1; in-hospital death, 3:1.1,2
•Failure to void after initial catheter removal: high retention volume, greater age, and low maximum detrusor pressure are predictive for failure to void after TURP. Ten per cent in those with acute retention of urine and 40% in those with acute-on-chronic retention fail to void after initial post-TURP TWOC. Overall, 1% of men will fail to void after subsequent TWOCs and will require long-term catheterization.3
References
1Armitage JN, Sibanda N, Cathcart P, et al. (2008). Mortality in men admitted to hospital with acute urinary retention: database analysis. BMJ 335:1199–202.
2Pickard R, Emberton M, Neal D (1998). The management of men with acute urinary retention. Br J Urol 81:712–20.
3Reynard JM (1999). Failure to void after transuretural resection of the prostate and mode of presentation. Urology 53:336–9.
•Prevention of urinary retention—a period of post-operative catheterization is commonly employed after many operations where limited mobility makes normal voiding difficult.
•Monitoring of urine output (e.g. post-operatively); prevention of damage to the bladder during a Caesarean section.
•Bladder drainage following surgery to the bladder, prostate, or urethra (e.g. TURP, TURBT, open bladder stone removal, radical prostatectomy).
•Bladder drainage following injuries to the bladder.
Explain the need for, and the method of, catheterization to the patient. Use the smallest catheter—in practical terms, usually a 12Ch, with a 10mL balloon. For longer catheterization periods (weeks), use a silastic catheter to limit tissue ‘reaction’, thereby reducing the risk of a catheter-induced urethral stricture. If clot retention, use a 3-way catheter (20Ch or greater) to allow evacuation of clots and bladder irrigation to prevent subsequent catheter blockage.
The technique is aseptic. One gloved hand is sterile, and the other is ‘dirty’. The dirty hand holds the penis or separates the labia to allow cleansing of the urethral meatus; this hand should not touch the catheter. Use sterile water or sterile cleaning solution to ‘prep’ the skin around the meatus.
Apply lubricant jelly to the urethra. Traditionally, this contains LA (e.g. 2% lidocaine), which takes between 3 and 5min to work. However, a randomized, placebo-controlled trial showed that 2% lidocaine was no more effective for pain relief than anaesthetic-free lubricant,1 suggesting that it is the lubricant action that prevents urethral pain. If using an LA lubricant, warn the patient that it may ‘STING’. An LA lubricant is contraindicated in patients with allergies to LAs and in those with urethral trauma where there is a (theoretical) risk of complications arising from systemic absorption of lidocaine. When instilling jelly, do so gently—a sudden, forceful depression of the plunger of the syringe can rupture the urethra! In ♂, ‘milk’ the gel towards the posterior urethra, while squeezing the meatus, to prevent it from coming back out of the meatus.
Insert the catheter using the sterile hand until the flow of urine confirms it is in the bladder. Failure of urine flow may indicate that the catheter balloon is in the urethra. Intraurethral inflation of the balloon can rupture the urethra. If no urine flows, attempt aspiration of urine using a 50mL bladder syringe (lubricant gel can occlude the eye-holes of the catheter). Absence of urine flow indicates either the catheter is not in the bladder or, if the indication for catheterization is retention, that the diagnosis is wrong (there will usually be a few millilitres of urine in the bladder, even in cases where the absence of micturition is due to oliguria or anuria, so complete absence of urine flow usually indicates the catheter is not in the bladder). If the catheter will not pass into the bladder, and you are sure that the patient is in retention, proceed with suprapubic catheterization.
Reference
1Birch BR (1994). Flexible cystoscopy in men: is topical anaesthesia with lignocaine gel worthwhile? Br J Urol 73:155.
•Failed urethral catheterization in urinary retention.
•Preferred site for long-term catheters, e.g. intractable urinary incontinence where other methods have failed; neurological disease (long-term bladder management in SCI or MS). Long-term urethral catheters commonly lead to acquired hypospadias in ♂ (ventral splitting of the glans penis) and patulous urethra in ♀ (leading to frequent balloon expulsion and bypassing of urine around the catheter); hence, the suprapubic site is preferred for long-term catheters.
•Used in the initial management of urethral trauma where urethral catheterization has failed.
Insertion of suprapubic catheterization is best avoided in:
•Patients with clot retention, the cause of which may be an underlying bladder cancer (the cancer could be ‘spread’ along the catheter track to involve the skin).
•Patients with known carcinoma of the bladder.
•Patients with lower midline incisions (the bowel may be ‘stuck’ to the deep aspect of the scar, leading to the potential for bowel perforation).
•Pelvic fractures where the catheter may inadvertently enter the large pelvic haematoma which always accompanies severe pelvic fracture. This can lead to infection of the haematoma, and the resulting sepsis can be fatal. Failure to pass a urethral catheter in a patient with a pelvic fracture usually indicates a urethral rupture (confirmed by urethrography) and is an indication for formal open suprapubic cystotomy.
•Patients on anticoagulation and antiplatelet treatment.
•Patients with subcutaneous vascular graft in the suprapubic region, e.g. femoro-femoral cross-over graft.
•Ask if previous abdominal surgery in the suprapubic or pelvic region.
•Enquire if there is neurological disease (bladder capacity often small and urethral incompetence in women is common, which makes bladder distension difficult; SCI autonomic dysreflexia is common in bladder distension, so a spinal or general anaesthetic may be required).
•Abdominal examination: inspect for lower abdominal scars; palpate and percuss the lower abdomen to confirm the bladder is distended.
•Consider stopping anticoagulation (or modifying with heparin ‘bridging’ therapy) and antiplatelet treatment if safe to do so.
British Association of Urological Surgeons (BAUS) SPC practice guidelines. Risks include:
•Haemorrhage, including haematuria and intra-abdominal bleeding.
•Infection, including UTI and infection of track site or wound.
•General risks of long-term catheterization.
LA or spinal or general anaesthetic if:
•At risk of autonomic dysreflexia (or at patient’s request).
•The bladder cannot be distended sufficiently to allow safe SPC insertion.
Give antibiotic prophylaxis if there is likely to be urine bacterial colonization.
Prior to insertion of the trocar, be sure to confirm the diagnosis by:
•Abdominal examination (palpate and percuss the lower abdomen to confirm the bladder is distended).
•Ultrasound: BAUS SPC practice guidelines state that ultrasound can be used to determine if there is interposing bowel in the planned suprapubic track ‘if carried out by a competent, trained practitioner’. They do not define ‘competent’ or ‘trained’. They admit that ‘the reliability of ultrasonography in excluding the presence of a loop of intestine in the suprapubic region has not been formally evaluated’.
•Aspiration of urine using a 21G (green) needle, usually 2cm above the pubic symphysis.
Use a wide-bore trocar if you anticipate that the catheter will be in place for >24h (small-bore catheters will block within a few days). Aim to place the catheter about 2cm above the pubis symphysis. Placement too close to the symphysis will result in difficult trocar insertion (the trocar will hit the symphysis). Instil a few millilitres of LA into the skin of the intended puncture site and down to the rectus sheath. Confirm the location of the bladder by drawing back on the needle to aspirate urine from the bladder. This helps guide the angle of trocar insertion. Make a 1cm incision with a sharp blade through the skin. Hold the trocar handle in your right hand, and steady the needle end with your left hand (this hand helps prevent insertion too deeply). Push the trocar in the same direction in which you previously aspirated urine. As soon as urine issues from the trocar, withdraw the latter, holding the attached sheath in place. Push the catheter in as far as it will go. Inflate the balloon. Peel away the side of the sheath, and remove it.
•Bowel perforation that accounts for the reported mortality of SPC insertion of 1–2%.1,2 Try to avoid the temptation to describe SPC insertion as a ‘minor’ procedure. BAUS SPC practice guidelines state the patient and their carers should be given written guidance, including instructions for ‘prompt referral to the team who inserted the catheter if the patient is unexpectedly unwell and/or has marked abdominal pain. The instructions should include contact numbers and clear instructions to make contact in the event of bleeding that is either heavy or persistent, symptoms to suggest infection, or the presence of lower abdominal pain that is failing to improve or spreading away from the immediate catheter site’.
•Persistent haematuria (may require bladder washouts and even, very occasionally, return to theatres for cystoscopic diathermy of the bleeding point if it can be found). Pass a urethral catheter to assist bladder washouts and irrigation. Pull the SPC balloon back against the anterior bladder wall to tamponade the bleeding.
•Track site or wound infection. Antibiotics, and if an abscess, incision and drainage. The author has never seen a case of cellulitis, ‘track infection’, or abscess in over 500 SPC insertions in patients at high risk of these potential complications, i.e. SCI patients with urine bacterial colonization. He advises patients of the very common occurrence of granulation tissue around the SPC track and that this does not represent track infection and should not be treated with antibiotics. The track often takes many months to ‘mature’, i.e. for skin to grow down the track. BAUS SPC practice guidelines state ‘systemic antibiotics should not be used to treat uncomplicated pericatheter discharge or asymptomatic bacteriuria’.
•Recurrent UTIs: the definition of what represents a ‘UTI’ is a source of much confusion and the cause of much inappropriate prescribing of antibiotics, which inevitably leads to the development of resistant bacteria in the urine. Inevitably, any foreign body in the bladder will become colonized with bacteria very rapidly. We do not regard the mere presence of bacteria or pus cells (of whatever number) as indicative of a UTI (the presence of bacteria in the absence of constitutional symptoms of feeling unwell, fever, and cloudy, smelly urine is not regarded as ‘active’ infection, but rather is better termed ‘colonization’). Avoid the temptation to prescribe antibiotics for mere colonization. Symptomatic UTIs (fever, feeling generally unwell, smelly and cloudy urine) can be a very difficult problem to manage. Not infrequently, patients (particularly those with SCI managed with long-term catheter drainage) report such symptoms in the absence of any bacterial growth in the urine. Others report feeling perfectly well, for months on end, in the face of urine that is full of bacteria! Remember, although short courses of antibiotics (7–10 days) may resolve what we think may be the symptoms of UTI, no amount of antibiotics will, over the long term, be able to sterilize the urine of a patient with a foreign body such as a catheter in it. Low-dose antibiotics (a quarter of the normal daily treatment dose) may keep the symptoms at bay or reduce the frequency of ‘infective’ episodes (but long-term use of nitrofurantoin or trimethoprim—two popular low-dose antibiotics—is associated, albeit rarely, with severe side effects such as blood dyscrasias or pulmonary fibrosis). In some patients, the only solution is to change bladder management. There is a greater risk of pyelonephritis in the chronically catheterized patient.
•Catheter blockages: due to encrustation of the lumen of the catheter with bacterial biofilms. Proteus mirabilis, Morganella, and Providencia species secrete a polysaccharide matrix. Within this, urease-producing bacteria generate ammonia from nitrogen in urine, raising urine pH and precipitating magnesium and calcium phosphate crystals. The matrix–crystal complex blocks the catheter. Catheter blockage causes bypassing, which soils the patient’s clothes. Bladder distension can cause autonomic dysreflexia in patients with thoracic or cervical SCIs, leading to extreme rises in BP that, believe it or not, can cause stroke and death! Regular bladder washouts and  catheter size sometimes help. There is a suggestion, based on in vitro experiments on catheters in the laboratory, that intermittent catheter drainage (by the use of a valve inserted between the catheter and the drainage bag) can reduce the likelihood of catheter blockages. Whether this holds true in patients remains to be documented.
catheter size sometimes help. There is a suggestion, based on in vitro experiments on catheters in the laboratory, that intermittent catheter drainage (by the use of a valve inserted between the catheter and the drainage bag) can reduce the likelihood of catheter blockages. Whether this holds true in patients remains to be documented.
•Bladder stone formation: necessitating surgical removal (endoscopic or open cystolithotomy), occurs in one in four patients followed over a 5y period.3
•‘Track’ problems at the time of catheter changes: difficultly removing the catheter (some catheter balloons have a ‘memory’, retaining an awkward shape such that they resist removal); difficulty reinserting the catheter (may require repositioning of the SPC site).
•In ♀ patients managed by a long-term urethral catheter, the pressure of the catheter can cause urethral and bladder neck erosion, leading to a so-called patulous urethra. In the ♂, a long-term urethral catheter can lead to pressure atrophy of the meatus of the penis, leading to an acquired hypospadias (‘kippering’ of the glans penis and even the shaft of the penis). While a mild acquired hypospadias has no great functional effect, cosmetically it does.
•Catheter bypassing: either around the suprapubic site or per urethra. Management is empirical. Try as small a balloon size as possible. If the leakage is due to bladder spasms, then a smaller balloon may possibly reduce their intensity and frequency. Anticholinergics may help, as may intravesical Botox injections. Other options include condom sheath drainage (in men) or bladder neck closure. This is not the minor operation (‘just a few stitches’) that patients are sometimes led to believe, and often—30% of cases—the closure breaks down so the leak persists. Bladder neck closure is irreversible, and access to the bladder via the suprapubic track is not always easy, particularly if access to the ureteric orifices is required for upper tract endoscopy.
•Bladder cancer (SCC of the bladder): there is conflicting evidence regarding the incidence of bladder cancer in SCI patients, some studies suggesting an  risk and others suggesting the risk is the same as in the non-spinal injured population.4 The author feels that the risk of SCC is greater than in the ambulant, non-catheterized population, but that the risk is still low. The pathogenesis is likely to involve chronic bacterial colonization of the bladder of spinal patients, whether managed with indwelling catheters, ISC, or sheath drainage, and so the presence of the catheter per se is not enough to induce development of a cancer. Screening cystoscopy studies have either failed to result in a downstaging of bladder cancer, when compared with non-screened patients, or simply not detected any cases of bladder cancer. Screening cystoscopy remains a subject of debate.5
risk and others suggesting the risk is the same as in the non-spinal injured population.4 The author feels that the risk of SCC is greater than in the ambulant, non-catheterized population, but that the risk is still low. The pathogenesis is likely to involve chronic bacterial colonization of the bladder of spinal patients, whether managed with indwelling catheters, ISC, or sheath drainage, and so the presence of the catheter per se is not enough to induce development of a cancer. Screening cystoscopy studies have either failed to result in a downstaging of bladder cancer, when compared with non-screened patients, or simply not detected any cases of bladder cancer. Screening cystoscopy remains a subject of debate.5
•When should the first change be done, and who should do it? BAUS SPC practice guidelines state ‘It is not necessary for the first catheter change to be carried out by the team who inserted the catheter; the first change is generally deferred for at least 2 weeks (and typically 6–12 weeks) after catheter insertion to allow the track to ‘mature’.
•How often should the catheter be changed? Usually 6-weekly to 3-monthly, but there is no hard and fast rule that a catheter must be changed at a given interval.
•To maintain bladder capacity over the long term, should the catheter be clamped and should long-term anticholinergic medication be given? Nice idea in theory, but no evidence of efficacy of either method.
•Can a flip-flow valve be used to allow intermittent bladder emptying? Yes, as long as the patient does not get autonomic dysreflexia with bladder distension and does not leak urethrally with a full bladder.
•Can the patient or carer change the SPC? Yes, if adequately trained.
•What should I do for bypassing of urine (leakage of urine either per urethra or around the SPC)? Try anticholinergics, and if this fails, bladder Botox injections. If all else fails, a bladder neck or urethral closure may be necessary.
•The catheter keeps blocking. What can I do? BAUS SPC practice guidelines state that ‘Repeated catheter blockages are frequently related to the development of bladder calculi’. The author agrees that blockages are ‘related to’ the same process that causes bladder stones, but catheter blockages are not caused by bladder stones, for in the author’s experience of managing many, many hundreds of patients with bladder stones, such stones are often far too large to block the catheter eye-holes. Catheter blockages and bladder stones are caused by the same process—chronic bacterial colonization of the bladder and any artificial device left within the bladder such as a catheter, followed by the development of a biofilm around the colonies of bacteria, followed by infiltration of this biofilm with calcium and phosphate. This is the process of catheter encrustation (which blocks catheters), and it is also the process of bladder stone formation. Where blockages become problematic, increase the SPC size, increase fluid intake, and consider bladder washouts (daily or every few days)—the evidence base for the efficacy of all of this is weak. The evidence base for in vivo use of a flip-flow valve to stop blockages is non-existent. In the author’s experience, full-dose courses of antibiotics or low-dose prophylactic antibiotics only very occasionally resolve this problem and, more often than not, lead to the emergence of multiresistant bacteria in the urine.
References
1Ahluwalia RS, Johal N, Kouriefs C, et al. (2006). The surgical risk of suprapubic catheter insertion and long-term sequelae. Ann R Coll Surg Engl 88:171–6.
2Sheriff MK, Foley S, McFarlene J, et al. (1998). Long-term suprapubic catheterization: clinical outcome and satisfaction survey. Spinal Cord 36:171–6.
3Ord J, Lunn D, Reynard J (2003). Bladder management and risk of bladder stone formation in spinal cord injured patients. J Urol 170:1734–7.
4Subramonian K, Cartwright RA, Harnden P, Harrison SCW (2004). Bladder cancer in patients with spinal cord injuries. Br J Urol Int 93:739–43.
5Hamid R, Bycroft J, Arya M, Shah PJ (2003). Screening cystoscopy and biopsy in patients with neuropathic bladder and chronic suprapubic indwelling catheters: is it valid? J Urol 170 (2 Pt 1):425–7.
Further reading
Harrison SCW, Lawrence WT, Morley R, Pearce I, Taylor J (2011). British Association of Urological Surgeons’ (BAUS suprapubic catheter practice guidelines (BAUS) suprapubic catheter practice guidelines. Br J Urol Int 107:77–85.
Ord J, Lunn D, Reynard J (2003). Bladder management and risk of bladder stone formation in spinal cord injured patients. J Urol 170:1734–7.
Sabbuba NA, Stickler DJ, Long M, et al. (2005). Does the valve-regulated release of urine from the bladder reduce the encrustation and blockage of indwelling catheters by crystalline Proteus mirabilis biofilms? J Urol 173:262–6.
Stickler DJ, Zimakoff J (1994). Complications of urinary tract infections associated with devices for long-term bladder management. J Hosp Infect 28:177–94.
Warren JW, Muncie HL, Hebel JR, Hall-Craggs M (1994). Long-term urethral catheterisation increases risk of chronic pyelonephritis and renal inflammation. J Am Geriatr Soc 42:1286–90.
Nocturia is often particularly resistant to treatment.
First, establish whether the patient is polyuric (>3L of urine/24h) by getting them to complete an FVC. If they are polyuric, this may account for their daytime and night-time voiding frequency. Establish whether they have a solute or water diuresis and the causes thereof (Box 4.1).
If non-polyuric (<3L urine output/24h), determine the distribution of urine output over the 24h period. If more than one-third of urine output is between the hours of midnight and 8 a.m., then the patient has NP.
If there is NP, exclude other medical causes: diabetes mellitus and DI, adrenal insufficiency; hypercalcaemia; liver failure; polyuric renal failure; chronic heart failure; OSA, dependent oedema; chronic venous stasis; calcium channel blockers; diuretics; and SSRI antidepressants.
The impact of α-blockers, 5ARIs, and anticholinergics on nocturia is modest.
Nocturia persists in 20–40% of men after TURP.
Patients preselected on the basis of a favourable symptomatic response to a test stimulation can experience a reduction in nocturia,1 but not all patients respond to the test stimulation and the treatment is expensive and not yet widely available in all countries.
The evidence base for NP treatments is limited (very few randomized, placebo-controlled trials).
Many patients have reduced their afternoon and evening fluid intake in an attempt to reduce their night-time diuresis.
Diuretics, taken several hours before bedtime, reduce nocturnal voiding frequency in some patients.2,3
A synthetic analogue of arginine vasopressin (endogenous ADH) which, if taken at night, can reduce urine flow by its antidiuretic action. It has been suggested that NP may be caused by a lack of endogenous production of ADH in elderly people. However, adults both with and without NP have no rise in ADH at night (i.e. ADH secretion remains remarkably constant throughout the day in adults with and without NP). Furthermore, the diuresis in adults with NP is a solute diuresis due to nocturnal natriuresis.4 Thus, lack of ADH secretion at night is not the cause of the diuresis in nocturnal polyuric adults, and therefore, from a theoretical perspective, there is no logical basis for using desmopressin in NP.5 There is limited evidence that it reduces night-time voiding frequency (at least in responder enrichment studies) and increases sleep duration in a proportion of patients with NP.6
Hyponatraemia (sodium <130mmol/L) in 5% of patients. Measure serum sodium 3 days after starting DDAVP, and stop if hyponatraemia develops.3
Box 4.1 Investigation of the polyuric patient (urine output >3L per 24h)
•>250mOsm/kg = solute diuresis.
•<250mOsm/kg = water diuresis.
•Solute diuresis: poorly controlled diabetes mellitus, saline loading (e.g. post-operative diuresis), diuresis following relief of HPCR.
•Water diuresis: primary polydipsia, DI (nephrogenic, e.g. lithium therapy; central—ADH deficiency).
OSA is highly prevalent in those over 65y of age. It is often manifested by snoring. There is a strong association between OSA symptoms and nocturia.7 Large negative intrathoracic pressure swings may trigger cardiac-mediated natriuresis and hence cause NP.
References
1Spinelli M (2003). New sacral neuromodulation lead for percutaneous implantation using local anesthesia: description and first experience. J Urol 170:1905–7.
2Reynard JM, Cannon A, Yang Q, Abrams P (1998). A novel therapy for nocturnal polyuria: a double-blind randomized trial of frusemide against placebo. Br J Urol 81:215–18.
3National Institute for Health and Care Excellence (2010). Lower urinary tract symptoms in men: management. Clinical guideline [CG97]. Available from:  http://www.nice.org.uk/CG97.
http://www.nice.org.uk/CG97.
4Matthiesen TB, Rittig S, Norgaard JP, Pedersen EB, Djurhuus JC (1996). Nocturnal polyuria and natriuresis in male patients with nocturia and lower urinary tract symptoms. J Urol 156:1292–9.
5McKeigue P, Reynard J (2000). Relation of nocturnal polyuria of the elderly to essential hypertension. Lancet 355:486–8.
6Mattiasson A (2002). Efficacy of desmopressin in the treatment of nocturia: a double-blind placebo-controlled study in men. Br J Urol 89:855–62.
7Umlauf M (1999). Nocturia and sleep apnea symptoms in older patients: clinical interview. Sleep 22:S127.
Anyone who retains a certain volume of urine in their bladder after voiding or attempted voiding can be said to be in chronic retention. For those who retain the ability to pass urine, such a situation can be termed ‘chronic retention’, and this may be low pressure (normal creatinine and absence of hydronephrosis on ultrasound) or high pressure (raised creatinine which falls post-catheterization, usually with hydronephrosis on ultrasound). Those with chronic retention who suddenly become unable to pass urine (and this is usually painful) can be said to have developed acute-on-chronic retention. Again, this can be low pressure or high pressure. A workable definition (one that is related to the outcome of TURP) for the acute setting of painful inability to void is that acute retention (non-chronic) is a painful inability to void with a catheterization volume of <800mL, and acute-on-chronic retention is a painful inability to void with a catheterization volume of >800mL.1
In the context of chronic retention, precisely what ‘a certain volume’ means is variably defined. Some say a patient has chronic retention when they consistently leave 300mL behind post-void, others 800mL2,3, and NICE4 describes it as a residual volume of >1000mL or the presence of a palpable/percussable bladder (though the bladder can certainly be palpated or percussed when containing <1000mL, so this definition is not strictly consistent).
The 2010 NICE guidelines on management of LUTS in men provide a useful algorithm for the management of chronic retention (Fig. 4.3). A creatinine and renal ultrasound are done.
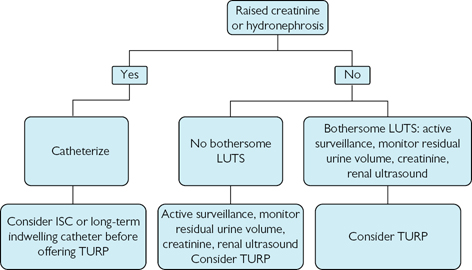
Fig. 4.3 NICE guidelines for management of LUTS in men—modified version.
Data sourced from National Institute for Health and Clinical Excellence (2010) Lower urinary tract symptoms in men: management. NICE guideline (CG97).
References
1Reynard JM (1999). Failure to void after transuretural resection of the prostate and mode of presentation. Urology 53:336–9.
2Mitchell JP (1984). Management of chronic urinary retention. BMJ 289:515–16.
3Abrams P, Dunn M, George N (1978). Urodynamic findings in chronic retention of urine and their relevance to results of surgery. BMJ 2:1258–60.
4National Institute for Health and Care Excellence (2010). Lower urinary tract symptoms in men: management. Clinical guideline [CG97]. Available from:  http://www.nice.org.uk/CG97.
http://www.nice.org.uk/CG97.
In the 2nd edition of this book, this was defined as maintenance of voiding, with a bladder volume of >800mL and an intravesical pressure above 30cmH2O, accompanied by hydronephrosis1,2 and since this definition has been shown to be helpful in predicting the outcome of the commonest surgical treatment for urinary retention,1 it is one that I have decided to keep for this 4th edition. Over time, this leads to renal failure. When the patient is suddenly unable to pass urine, acute-on-chronic high-pressure retention of urine has occurred.
A man with high-pressure retention who continues to void spontaneously may be unaware that there is anything wrong. He will often have no sensation of incomplete emptying, and his bladder seems to be insensitive to the gross distension. Often, the first presenting symptom is that of bedwetting. This is such an unpleasant and disruptive symptom that it will cause most people to visit their doctor. Visual inspection of the patient’s abdomen may show marked distension due to a grossly enlarged bladder. The diagnosis of chronic retention can be confirmed by palpation of the enlarged, tense bladder which is dull to percussion.
Catheterization relieves the pressure on the kidneys and allows normalization of renal function. A large volume of urine is drained from the bladder (often in the order of 1–2L and sometimes much greater). The serum creatinine is elevated, and an ultrasound will show hydronephrosis, with a grossly distended bladder if the scan is done before relief of retention.
Anticipate profound diuresis following drainage of the bladder due to:
•Excretion of salt and water that has accumulated during the period of renal failure.
•Loss of the corticomedullary concentration gradient, due to continued perfusion of the kidneys with diminished flow of urine through the nephron (this washes out the concentration gradient between the cortex and medulla).
•An osmotic diuresis caused by elevated serum urea concentration.
A small percentage of patients have a postural drop in BP. It is wise to admit patients with HPCR for a short period of observation until the diuresis has settled. A few will require IV fluid replacement if they experience a symptomatic fall in BP when standing.
TURP or a long-term catheter. In those unable to void who have been catheterized, a TWOC is clearly not appropriate in cases where there is back pressure on the kidneys. Rarely, a patient who wants to avoid TURP and does not want an indwelling catheter will be able to empty their bladder by ISC.
References
1Reynard JM (1999). Failure to void after transuretural resection of the prostate and mode of presentation. Urology 53:336–9.
2Mitchell JP (1984). Management of chronic urinary retention. BMJ 289:515–16.
Relatively rare (75% of women undergoing pressure flow studies have BOO, compared with 60% of unselected men with LUTS).1,2
It may be symptom-free and present with LUTS or as acute urinary retention. In broad terms, the causes are related to obstruction of the urethra (e.g. urethral stricture, compression by a prolapsing pelvic organ such as the uterus, post-surgery for stress incontinence) or have a neurological basis (e.g. injury to the sacral cord or parasympathetic plexus, degenerative neurological disease, e.g. MS, diabetic cystopathy).
Women have a higher Qmax, for a given voided volume than do men. Women with BOO have a lower Qmax than those without BOO. There are no universally accepted urodynamic criteria for diagnosing BOO in women.
Treat the cause (e.g. dilatation of a urethral stricture; repair of a pelvic prolapse). Where this it is not possible (because of a neurological cause such as MS or SCI), the options are:
•ISC or intermittent catheterization by a carer.
•Indwelling catheter (preferably suprapubic rather than urethral).
•Mitrofanoff catheterizable stoma.
Where urethral ISC is technically difficult, a catheterizable stoma can be constructed between the anterior abdominal wall and the bladder, using the appendix, Fallopian tube, or a narrowed section of the small intestine. This is the Mitrofanoff procedure. It is simply a new urethra which has an abdominal location, rather than a perineal one, and is therefore easier to access for ISC.
For women with a suprasacral SCI with preserved detrusor contraction and urinary retention due to DSD, sacral deafferentation, combined with a Brindley stimulator, can be used to manage the resulting urinary retention.
A primary disorder of sphincter relaxation (as opposed to secondary to, for example, SCI). Increased EMG activity (repetitive discharges on external sphincter EMG) can be recorded in the external urethral sphincters of these women (which, on ultrasound, are of  volume) and is hypothesized to cause impaired relaxation of the external sphincter. Occurs in premenopausal women, typically aged 15–30, often in association with polycystic ovaries (50% of patients), acne, hirsutism, and menstrual irregularities. May also be precipitated by childbirth or gynaecological or other surgical procedures. They report no urgency with bladder volumes of >1000mL, but when attempts are made to manage their retention by ISC, they experience pain, especially on withdrawing the catheter.
volume) and is hypothesized to cause impaired relaxation of the external sphincter. Occurs in premenopausal women, typically aged 15–30, often in association with polycystic ovaries (50% of patients), acne, hirsutism, and menstrual irregularities. May also be precipitated by childbirth or gynaecological or other surgical procedures. They report no urgency with bladder volumes of >1000mL, but when attempts are made to manage their retention by ISC, they experience pain, especially on withdrawing the catheter.
•Pathophysiology: may be due to a channelopathy of the striated urethral sphincter muscle, leading to involuntary external sphincter contraction.
•Treatment: ISC, sacral neuromodulation with Medtronic InterStim (90% void post-implantation and 75% are still voiding at 3y follow-up). The mechanism of action of sacral neuromodulation in urinary retention is unknown.
References
1Madersbascher S, Pycha A, Klingler CH, et al. (1998). The aging lower urinary tract: a comparative urodynamic study of men and women. Urology 51:206–12.
2Swinn MJ, Wiseman OJ, Lowe E, Fowler CJ (2002). The cause and treatment of urinary retention in young women. J Urol 167:151–6.
A urethral stricture is a scar in the subepithelial tissues of the corpus spongiosum which constricts the lumen of the urethra. Since it is only the anterior urethra that is surrounded by the corpus spongiosum, by consensus, urethral strictures are said only to affect the anterior urethra.1 A narrowing of the calibre of the posterior urethra is termed a stenosis.
The process of scar formation occurs in the spongy erectile tissue (corpus spongiosum) of the penis that surrounds the urethra—spongiofibrosis.
•Inflammation [e.g. balanitis xerotica obliterans (BXO)], gonococcal infection leading to gonococcal urethritis (less common nowadays because of prompt treatment of gonorrhoea).
•Straddle injuries—blow to the bulbar urethra (e.g. cross-bar injury).
•Iatrogenic—instrumentation (e.g. traumatic catheterization, traumatic cystoscopy, TURP, BNI).
The role of non-specific urethritis (e.g. Chlamydia) in the development of anterior urethral strictures has not been established.
Fibrosis of the tissues around the urethra results from trauma—pelvic fracture or surgical (radical prostatectomy, TURP, urethral instrumentation). By consensus, they are now described as stenosis and are no longer described as strictures. These are essentially distraction injuries (leading to a stenosis of the urethra) where the posterior urethra has been pulled apart and the subsequent healing process results in the formation of a scar which contracts and thereby narrows the urethral lumen.
•Voiding symptoms—hesitancy, poor flow, post-micturition dribbling.
•Urinary retention—acute, or high-pressure acute-on-chronic.
•UTI—prostatitis, epididymitis.
Where the patient presents with urinary retention, the diagnosis is usually made following a failed attempt at urethral catheterization. In such cases, avoid the temptation to ‘blindly’ dilate the urethra. Dilatation may be the wrong treatment option for this type of stricture—it may convert a short stricture, which could have been cured by urethrotomy or urethroplasty, into a longer and denser stricture, thus committing the patient to more complex surgery and a higher risk of recurrent stricturing. Place an SPC instead, and image the urethra with retrograde and antegrade urethrography to establish the precise position and length of the stricture.
Similarly, avoid the temptation to inappropriately dilate a urethral stricture diagnosed at flexible cystocopy (urethroscopy). Arrange retrograde urethrography, so appropriate treatment can be planned.
•Urethral dilatation: designed to stretch the stricture without causing more scarring; bleeding post-dilatation indicates tearing of the stricture (i.e. further injury has been caused), and re-stricturing is likely.
•Internal (optical) urethrotomy: stricture incision with an endoscopic knife or laser. Divides the stricture, followed by epithelialization of the incision. If deep spongiofibrosis is present, the stricture will recur. Best suited for short (<1.5cm) bulbar urethral strictures with minimal spongiofibrosis.2 A catheter is left for 3–5 days (what evidence there is suggests keeping a catheter for 3 days reduces the risk of extravasation of urine and infective complications that may result;1 longer catheterization does not reduce long-term re-stricturing). Consider ISC for 3–6 months, starting several times daily, reducing to once or twice a week towards the end of this period. For strictures in other parts of the anterior urethra or where there has been a previous optical urethrotomy or dilatation, an optical urethrotomy will almost certainly fail to cure the stricture. Avoid optical urethrotomy for sphincter strictures (e.g. post-TURP) because the sphincter may be rendered incompetent—dilatation is a safer option.
•Excision and re-anastomosis or tissue transfer: best chance of cure; excises the area of spongiofibrosis with primary re-anastomosis or closure of the defect with buccal mucosa or a pedicled skin flap. Stricturotomy and buccal mucosal grafting, rather than transecting the entire urethra and then re-anastomosing it, is becoming increasingly popular (but not for strictures that obliterate the entire urethral lumen).
A stepwise progression up this ‘reconstructive ladder’ (the process of starting with a simple procedure and moving onto the next level of complexity when this fails) is not appropriate for every patient. For the patient who wants the best chance of long-term cure, offer excision and re-anastomosis or tissue transfer upfront. For the patient who is happy with lifelong ‘management’ of his stricture (with repeat dilatation or optical urethrotomy), offer dilatation or optical urethrotomy.
Genital lichen sclerosis and atrophicus in the ♂. Hyperkeratosis is seen histologically. Appears as a white plaque on the foreskin or the glans of the penis, or within the urethral meatus. Commonest cause of stenosis of the meatus. The foreskin becomes thickened and adheres to the glans, leading to phimosis (a thickened, non-retractile foreskin). Patients with long-standing BXO and meatal stenosis often have more proximal urethral strictures.
References
1Mundy AR, Andrich DE (2010). Urethral strictures. BJU Int 107:6–26.
2Pansadoro V, Emiliozzi P (1996). Internal urethrotomy in the management of anterior urethral strictures: long term follow-up. J Urol 156:73–5.
* AUA 2010 guidelines use the terms ‘watchful waiting’ and ‘active surveillance’ interchangeably; it defines ‘watchful waiting’ as ‘a management strategy in which the patient is monitored by his physician, but currently receives no active intervention’. WW is recommended for patients with mild symptoms (AUA-SI <8) and patients with AUA-SI of 8 or more who are not bothered by their LUTS drug treatment.